Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1651
Peer-review started: April 27, 2021
First decision: July 14, 2021
Revised: July 14, 2021
Accepted: November 14, 2021
Article in press: November 14, 2021
Published online: December 27, 2021
Processing time: 240 Days and 10.3 Hours
Liver cirrhosis is the main cause of portal hypertension. The leading cause of death in patients with liver cirrhosis is its most common complication, esophageal variceal bleeding (EVB). Endoscopic variceal ligation (EVL) is recommended by many guidelines to treat EVB and prevent rebleeding; however, esophageal ulcers occur after treatment. Delayed healing of ulcers and unhealed ulcers lead to high rebleeding and mortality rates. Thus, the prevention of early postoperative rebleeding is of great significance in improving the quality of life and prognosis of patients.
To evaluate the efficacy of aluminum phosphate gel (APG) plus a proton pump inhibitor (PPI) in the prevention of early rebleeding after EVL in patients with EVB.
The medical records of 792 patients who were diagnosed with EVB and in whom bleeding was successfully stopped by EVL at Shenzhen People’s Hospital, Guangdong Province, China from January 2015 to December 2020 were collected. According to the study inclusion and exclusion criteria, 401 cases were included in a PPI-monotherapy group (PPI group), and 377 cases were included in a PPI and APG combination therapy (PPI + APG) group. We compared the incidence rates of early rebleeding and other complications within 6 wk after treatment between the two groups. The two-sample t-test, Wilcoxon rank-sum test, and chi-squared test were adopted for statistical analyses.
No significant differences in age, sex, model for end-stage liver disease score, coagulation function, serum albumin level, or hemoglobin level were found between the two groups. The incidence of early rebleeding in the PPI + APG group (9/337; 2.39%) was significantly lower than that in the PPI group (30/401; 7.48%) (P = 0.001). Causes of early rebleeding in the PPI group were esophageal ulcer (3.99%, 16/401) and esophageal varices (3.49%, 14/401), while those in the PPI + APG group were also esophageal ulcers (5/377; 1.33%) and esophageal varices (4/377; 1.06%); such causes were significantly less frequent in the PPI + APG group than in the PPI group (P = 0.022 and 0.024, respectively). The early mortality rate within 6 wk in both groups was 0%, which was correlated with the timely rehospitalization of all patients with rebleeding and the conduct of emergency endoscopic therapy. The incidence of adverse events other than early bleeding in the PPI + APG group (28/377; 7.43%) was significantly lower than that in the PPI group (63/401; 15.71%) (P < 0.001). The incidence of chest pain in the PPI + APG group (9/377; 2.39%) was significantly lower than that in the PPI group (56/401; 13.97%) (P < 0.001). The incidence of constipation in the PPI + APG group (16/377; 4.24%) was significantly higher than that in the PPI group (3/401; 0.75%) (P = 0.002) but constipation was relieved after patients drank more water or took lactulose. In the PPI and PPI + APG groups, the incidence rates of spontaneous peritonitis within 6 wk after discharge were 0.50% (2/401) and 0.53% (2/377), respectively, and those of hepatic encephalopathy were 0.50% (2/401) and 0.27% (1/377), respectively, presenting no significant difference (P > 0.999).
PPI + APG combination therapy significantly reduces the incidence of early rebleeding and chest pain in patients with EVB after EVL.
Core Tip: Esophageal variceal bleeding (EVB) is a common disease with a high mortality rate. Esophageal variceal ligation (EVL) is an effective means of hemostasis; however, ulcer foci of the esophagus can form after treatment. Patients with delayed healing of ulcers and unhealed ulcers are prone to experiencing early rebleeding. No studies have reported on the promotion of ulcer healing or prevention of rebleeding in EVB patients after EVL. This study showed that the application of aluminum phosphate gel in combination with a proton pump inhibitor after EVL significantly reduced the incidence of early rebleeding following endoscopic surgery in EVB patients.
- Citation: Zhang ZL, Peng MS, Chen ZM, Long T, Wang LS, Xu ZL. Effect of aluminum phosphate gel on prevention of early rebleeding after ligation of esophageal variceal hemorrhage. World J Gastrointest Surg 2021; 13(12): 1651-1659
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1651.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1651
Rupture and bleeding of esophageal gastric varices is a common complication in patients with liver cirrhosis, with an annual incidence of approximately 10% to 15%[1]. Some research has found that the mortality of esophageal gastric variceal bleeding within 6 wk after treatment is 15% to 25%[2-4]. Esophageal variceal bleeding (EVB) is more common than gastric variceal bleeding, and taking steps to prevent early rebleeding after treating EVB is of significant importance.
Many guidelines suggest endoscopic variceal ligation (EVL) as an effective means to treat EVB and prevent rebleeding[5-8]. EVL ligates varicose veins to form fibrosis after venous ischemia, stenosis, and vascular occlusion at the ligation site to achieve a hemostatic effect. The ulcer surface left by the ligation usually takes 2 to 3 wk to fully heal[9]. Necrosis of the mucosa and submucosa occurs 24 h after EVL, while the onset of acute inflammation usually happens 3 to 7 d after the operation. On postoperative day 7, the local tissues that have been ligated evolve from a necrotic state to one of crusting and shedding to form ulcers. This process is the main period in which postoperative bleeding may occur and a key window during which to prevent early bleeding. Early rebleeding after EVL often occurs within 3-14 d after endoscopic surgery[10]. During the healing window, high-risk factors, such as consumption of an improper diet by the patient, may cause early rebleeding of varicose veins, with an incidence rate of 4.6% to 5.1% and a mortality rate as high as 22% to 23.8%[11,12]. The possible causes of rebleeding after EVL include the presence of varicose veins that have not completely disappeared during the operation; ulcers caused by ischemia after EVL; the presence of portal hypertension, rupturing the fragile ulcers; the ferrule falling off prematurely; and the existence of tissue fibrosis that is not firm enough[13].
Proton pump inhibitors (PPIs) inhibit gastric acid secretion, increase the gastric pH value, mitigate damage to the mucosa from gastric acid, and promote postoperative ulcer healing. A randomized controlled clinical trial has shown that PPIs reduce the size of esophageal ulcers after EVL and the incidence of rebleeding[14]. Studies have also found that treatment with PPIs for 10 d to 30 d after EVL is safe and reduces the incidence of early postoperative bleeding[15,16]. Aluminum phosphate gel (APG) is usually used as a protective agent for the mucous membrane of the digestive tract. It has a specific viscosity, forming a large contact area with the ulcer[17]; thus, it is closely integrated with the ulcer surface formed after EVL, exhibiting a protective effect at the wound’s surface. This study retrospectively analyzed the efficacy of APG combined with PPI to prevent early rebleeding after EVL in patients with EVB.
This retrospective analysis selected a total of 792 patients who were diagnosed with EVB at Shenzhen People’s Hospital, Guangdong Province, China from January 2015 to December 2020 and included 778 patients for final analysis according to the screening criteria. These 778 patients were stratified into a PPI-monotherapy group (PPI group) and a PPI and APG combination therapy (PPI + APG) group according to their treatment plans, and their medical records were collected for the analysis. This study was reviewed and approved by the ethics committee of Shenzhen People’s Hospital. All study participants provided informed consent.
Male or female patients aged 18 to 75 years old, diagnosed with EVB by gastroscopy and treated with EVL, were eligible for inclusion in this study.
Patients with the following were excluded from this study: (1) Incomplete clinical data or (2) other serious diseases, such as coronary heart disease, chronic renal insufficiency, or advanced liver cancers, at the time of hospital admission that may affect the patient’s prognosis.
In the PPI group, patients after endoscopic therapy were treated with a conventional dose of PPI (20 mg rabeprazole, 40 mg pantoprazole, or 40 mg esomeprazole daily) for 4 consecutive weeks. In the PPI + APG group, in addition to the same PPI treatment, patients were given 20 g of APG administered orally three times daily (Boryung Pharmaceutical Co., Ltd., Seoul, South Korea) for 2 consecutive weeks, starting on the same day after endoscopic therapy. All patients were evaluated, and those with no contraindications were given nonselective beta-blocker therapy (propranolol) to prevent rebleeding[5-8]. All patients were closely followed, and those with suspected rebleeding were rehospitalized and immediately underwent endoscopy and treatment.
Early postoperative rebleeding in the EVB patients was identified based on the occurrence[8] of active bleeding events (i.e., hematemesis, melena, or hematochezia; reduction in systolic blood pressure > 20 mmHg or increase in the heart rate > 20 beats/min; or > 30 g/L of hemoglobin in the absence of blood transfusion) at 72 h to 6 wk after the first bleeding was under control. Rebleeding was the main outcome indicator of this study; secondary outcome indicators included deaths, infection, and other adverse events.
The Statistical Package for the Social Sciences version 25.0 software (IBM Corporation, Armonk, NY, United States) was used for statistical analyses in this study. Normally distributed measurement data were presented as the mean ± SD and were compared between the two groups using a two-sample t-test. Skewed measurement data were presented as the median (lower quartile, upper quartile) and were compared between the two groups using the Wilcoxon rank-sum test. Count data are presented as the number of cases and percentages and were compared between the two groups using the chi-squared test. P values of less than 0.05 were considered statistically significant.
Table 1 summarizes the statistical analysis of the general data of the two groups of study participants recorded at the time of hospital discharge. No significant differences in age, sex, model for end-stage liver disease score, prothrombin activity, fibrinogen, platelet count, serum albumin level, or hemoglobin level were found between the two groups.
| Characteristic | PPI group (n = 401) | PPI + APG group (n = 377) | P value |
| Age (yr) | 53.55 ± 12.55 | 52.73 ± 13.35 | 0.376 |
| Female/Male | 83/318 | 73/304 | 0.642 |
| MELD score | 14.94 ± 3.05 | 15.19 ± 3.30 | 0.275 |
| Prothrombin activity (%) | 65.30 ± 15.26 | 65.70 ± 16.94 | 0.731 |
| Fibrinogen (g/dL) | 1.94 ± 0.68 | 2.00 ± 0.73 | 0.211 |
| Platelet (109/L) | 104.43 ± 69.88 | 97.67 ± 70.20 | 0.179 |
| Albumin (g/dL) | 3.39 ± 0.51 | 3.40 ± 0.52 | 0.682 |
| Hemoglobin (g/dL) | 8.92 ± 0.68 | 8.86 ± 0.69 | 0.229 |
Table 2 summarizes the incidence of early rebleeding in the two groups. The incidence of early rebleeding in the PPI + APG group (2.39%, 9/337) was significantly lower than that in the PPI group (30/401; 7.48%) (P = 0.001). Considering the causes of early rebleeding, the incidence rates of esophageal ulcers (6/377; 1.33%) and esophageal varices (1.06%, 4/377) in the PPI + APG group were significantly lower than those in the PPI group (16/401; 3.99%; P = 0.022 and 14/401; 3.49%; P = 0.024, respectively). PPI + APG combination therapy reduced the early incidence rates of esophageal ulcer and bleeding as well as esophageal varices and bleeding after EVL. The early mortality rate within 6 wk after surgery was 0% in both groups; this low rate of mortality was related to the timely rehospitalization of all patients with rebleeding and the conduct of emergency endoscopic therapy.
| Characteristic | PPI group (n = 401) | PPI + APG group (n = 377) | P value |
| Early rebleeding | 30 (7.48) | 9 (2.39) | 0.001 |
| Source of rebleeding | |||
| Esophageal ulcer | 16 (3.99) | 5 (1.33) | 0.022 |
| Esophageal varices | 14 (3.49) | 4 (1.06) | 0.024 |
| 6-wk mortality | 0 | 0 | 1.000 |
Table 3 summarizes the statistical analysis of other complications and events except for bleeding that occurred in the two groups of patients. In the PPI + APG group, the incidence of other complications was 7.43% (28/377), which was significantly lower than that in the PPI group (63/401; 15.71%; P < 0.001), while the incidence of chest pain in the PPI + APG group was 2.39% (9/377), which was also significantly lower than that in the PPI group (56/401; 13.97%; P < 0.001). In contrast, the incidence of constipation in the PPI + APG group was 4.24% (16/377), which was significantly higher than that in the PPI group (3/401; 0.75%; P = 0.002). Nevertheless, all cases of constipation in the PPI + APG group were relieved after drinking water for hydration and the oral administration of lactulose. The incidence rates of spontaneous peritonitis within 6 wk after discharge were 0.50% (2/401) and 0.53% (2/377), respectively, and those of hepatic encephalopathy were 0.50% (2/401) and 0.27% (1/377), respectively, showing no significant difference between the two groups (P > 0.999).
| Characteristic | PPI group (n = 401) | PPI + APG group (n = 377) | P value |
| Total complications | 63 (15.71) | 28 (7.43) | < 0.001 |
| Chest pain | 56 (13.97) | 9 (2.39) | < 0.001 |
| Constipation | 3 (0.75) | 16 (4.24) | 0.002 |
| Spontaneous peritonitis | 2 | 2 | 1.000 |
| Hepatic encephalopathy | 2 | 1 | 1.000 |
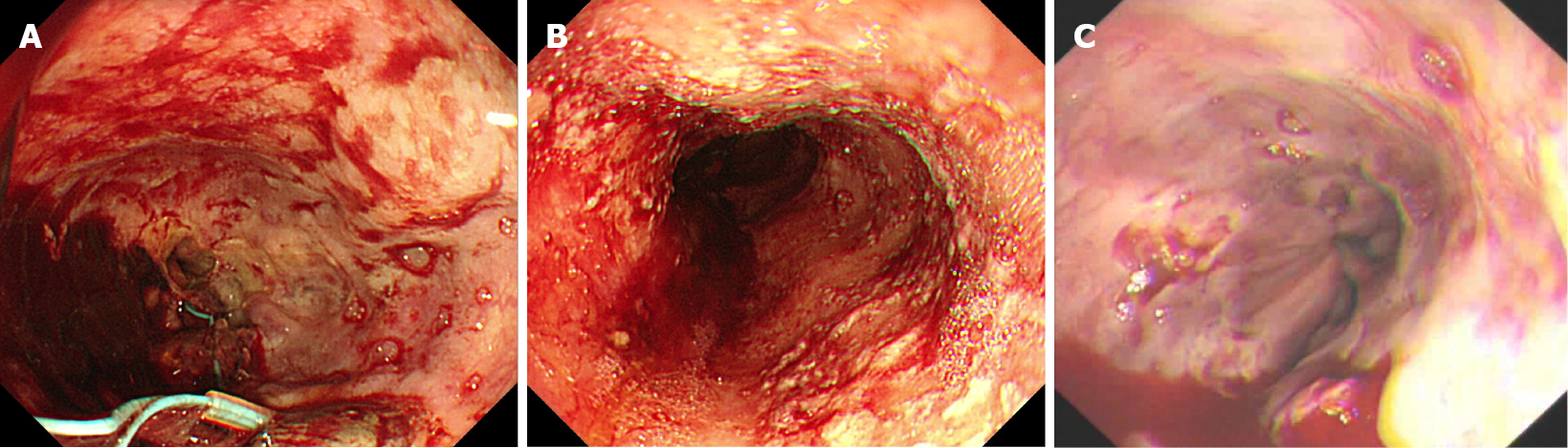
Rebleeding frequently occurs in patients with EVB after EVL treatment. The cause of early rebleeding is currently considered to primarily relate to postoperative ulcer formation and varicose veins (Figure 1). However, no relevant clinical guidelines offering a definition or treatment advice are currently available. One study reported that the polyps formed by banding began to exhibit mucosal and submucosal necrosis and dissolution of the bands 5 to 7 d after EVL, following which the necrotic tissues were shed and ulcers were formed[18]. As compared with ordinary peptic ulcers, patients with portal hypertension experience a poorer esophageal mucosal blood supply and a lower mucosal regeneration rate and self-defense ability, and their ulcer wounds are more susceptible to gastric acid damage, resulting in bleeding. In our study, early esophageal ulcer bleeding was found in 16 cases in the PPI group and 5 cases in the PPI + APG group, accounting for 53.85% (21/39) of the early rebleeding cases.
PPIs inactivate NA+-K+-ATPase, thereby inhibiting gastric acid secretion, reducing the injury caused by gastric acid to the mucosa, and promoting postoperative ulcer healing[19]. Although reports have suggested that long-term application of PPIs might increase the occurrence of spontaneous peritonitis and hepatic encephalopathy in patients with liver cirrhosis[20-22], a recent clinical study determined that routine application of PPIs for 30 d after EVL was safe and could significantly reduce the upper gastrointestinal rebleeding and mortality rates of patients within 30 d of hospitalization[16]. Meanwhile, a recent meta-analysis also showed that PPI reduced the rebleeding rate of patients with liver cirrhosis and esophagogastric variceal bleeding by nearly 50% after endoscopic therapy. This meta-analysis recommended treating patients with bleeding from esophageal varices with PPIs for at least 4 wk after endoscopic therapy. As a mucosal-protection agent, APG boasts the capabilities to neutralize and buffer gastric acid; it increases the pH value in the upper gastrointestinal tract, promotes the formation of blood clots, and forms a colloidal protective film to closely combine with the ulcer formed after the operation. Thus, APG blocks the invasion of stomach acid, stimulates mucosal epithelial cells to secrete mucus, and promotes the self-repair of epithelial cells. The formed protective film also provides good drug-attachment sites for orally administered PPIs to facilitate drug absorption. Theoretically, therefore, APG has a complementary effect with PPI preparations. The viscous nature of APG facilitates its attachment to the surface of esophageal ulcers. Recently, several studies on the treatment of postoperative esophageal stenosis using APG as an adhesive for the esophagus demonstrated a good therapeutic effect[17,23,24].
The present retrospective study revealed that the incidence of early rebleeding after EVL in EVB patients in the PPI + APG group was 2.39% (9/377), which was significantly lower than that in the PPI group (30/401; 7.48%; P = 0.001). In addition, PPI + APG combination therapy decreased the early rebleeding rate of esophageal ulcer and esophageal varices after EVL and significantly reduced the incidence of chest pain after EVL (2.39% vs 13.97%; P < 0.001). Although APG triggered a significant increase in the incidence of constipation (4.24% vs 0.75%; P = 0.002), the patients who experienced this complication achieved relief after drinking more water or consuming lactulose, without serious adverse consequences. Cases of spontaneous peritonitis and hepatic encephalopathy within 6 wk after EVL occurred after early rebleeding in both groups of patients. However, after 4 wk of PPI therapy in combination with 2 wk of APG administration, no significant increase in the number of cases of spontaneous peritonitis or hepatic encephalopathy was found. There was also no significant difference in the mortality between the two groups of patients within 6 wk of EVL, possibly due to the close follow-up performed by physicians, that is, all patients with suspected early rebleeding were hospitalized in time and subjected to emergency endoscopic therapy.
However, this study still has certain limitations. First, although the inclusion and exclusion criteria of this retrospective study were formulated, this study lacked good control over some related variables (patient's diet, movement, some chronic medication, etc.), so the level of evidence is not high. Second, this single-center study only included residents of the hospital’s service region, thus lacking representativeness and thereby limiting the applicability of the experimental results. In addition, the follow-up period of this study was 6 wk, and only the incidence rates of early rebleeding and related secondary outcome indicators were studied. Some clinical data and long-term indicators, such as mortality, survival, and the number of hospitalizations, were not further examined in this study. Also, no reports on whether double-dose PPI therapy can reduce the incidence of early rebleeding after EVL endoscopic therapy in EVB patients are available, and this study similarly did not test this theory. All bleeding and rebleeding patients achieved the goal of hemostasis through endoscopy and drug therapy, and no cases required transjugular intrahepatic portosystemic shunt treatment in this study. Further research via prospective, multicenter, large-sample, long-term follow-up randomized controlled clinical trials is necessary.
In conclusion, the application of APG in combination with PPI therapy for the treatment of EVP after endoscopic EVL promotes the rapid healing of postoperative esophageal ulcers and relieves chest pain symptoms in the patients. Importantly, this combination therapy regimen significantly reduces the incidence of early rebleeding from postoperative esophageal ulcer and esophageal varices, with relatively few adverse reactions.
Endoscopic variceal ligation (EVL) is a common treatment for esophageal variceal bleeding (EVB), but early rebleeding may occur after endoscopic therapy. And most patients are released from the hospital by the time they bleed again, which can be life-threatening.
How to reduce the early rebleeding after EVL is very important. Oral medication is accessible and convenient for patients. Therefore, we wanted to study oral drugs to reduce the rate of early rebleeding after EVL.
This study aimed to investigate oral medications to reduce early rebleeding after EVL. It was found that the combination of proton pump inhibitor (PPI) and aluminum phosphate gel (APG) could significantly reduce the incidence of early rebleeding. This oral treatment regimen is clinically worthwhile.
The patients were divided into two groups. One group was treated with oral PPI after EVL. The other group was treated with oral PPI combined with APG. A retrospective study was conducted to compare and analyze the therapeutic effects of the two groups of patients.
We found that PPI combined with APG therapy could significantly reduce the rate of early rebleeding after EVL, and reduce the incidence of chest pain after EVL. But this is a retrospective study and it would be nice to do a prospective multicenter study.
To prevent early rebleeding after EVL, the combination of PPI and APG can be considered.
Oral medications are used to reduce the risk of postoperative rebleeding in EVL patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Estremera-Arevalo F, Savarino V S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1428] [Article Influence: 178.5] [Reference Citation Analysis (3)] |
| 2. | Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412-19.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refifining shortterm prognosis and risk factors. Am J Gastroenterol. 2012;107:1872-1878. [RCA] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Fortune BE, Garcia-Tsao G, Ciarleglio M, Deng Y, Fallon MB, Sigal S, Chalasani NP, Lim JK, Reuben A, Vargas HE, Abrams G, Lewis MD, Hassanein T, Trotter JF, Sanyal AJ, Beavers KL, Ganger D, Thuluvath PJ, Grace ND, Groszmann RJ; Vapreotide Study Group. Child-Turcotte-Pugh Class is Best at Stratifying Risk in Variceal Hemorrhage: Analysis of a US Multicenter Prospective Study. J Clin Gastroenterol. 2017;51:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2277] [Article Influence: 227.7] [Reference Citation Analysis (3)] |
| 6. | Karstensen JG, Ebigbo A, Bhat P, Dinis-Ribeiro M, Gralnek I, Guy C, Le Moine O, Vilmann P, Antonelli G, Ijoma U, Anigbo G, Afiheni M, Duduyemi B, Desalegn H, De Franchis R, Ponchon T, Hassan C, Aabakken L. Endoscopic treatment of variceal upper gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Cascade Guideline. Endosc Int Open. 2020;8:E990-E997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: Varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol. 2020;26:83-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery; Chinese Medical Association. [Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension (2019 edition)]. Zhonghua WaiKe ZaZhi. 2019;57:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 9. | Kapoor A, Dharel N, Sanyal AJ. Endoscopic diagnosis and therapy in gastroesophageal variceal bleeding. Gastrointest Endosc Clin N Am. 2015;25:491-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 10. | Hou MC, Lin HC, Lee FY, Chang FY, Lee SD. Recurrence of esophageal varices following endoscopic treatment and its impact on rebleeding: comparison of sclerotherapy and ligation. J Hepatol. 2000;32:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Dueñas E, Cachero A, Amador A, Rota R, Salord S, Gornals J, Xiol X, Castellote J. Ulcer bleeding after band ligation of esophageal varices: Risk factors and prognosis. Dig Liver Dis. 2020;52:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Sinclair M, Vaughan R, Angus PW, Gow PJ, Parker F, Hey P, Efthymiou M. Risk factors for band-induced ulcer bleeding after prophylactic and therapeutic endoscopic variceal band ligation. Eur J Gastroenterol Hepatol. 2015;27:928-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Cho E, Jun CH, Cho SB, Park CH, Kim HS, Choi SK, Rew JS. Endoscopic variceal ligation-induced ulcer bleeding: What are the risk factors and treatment strategies? Medicine (Baltimore). 2017;96:e7157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Shaheen NJ, Stuart E, Schmitz SM, Mitchell KL, Fried MW, Zacks S, Russo MW, Galanko J, Shrestha R. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology. 2005;41:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Lo EA, Wilby KJ, Ensom MH. Use of proton pump inhibitors in the management of gastroesophageal varices: a systematic review. Ann Pharmacother. 2015;49:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Ghoz H, Patel P, Stancampiano F, Patel S, Fox EA, Yousaf MB, Omer M, Heckman MG, Spiegel MR, Palmer WC. Proton-pump-inhibitor use associated with lower short-term rebleeding and mortality in patients receiving esophageal variceal band ligation: a retrospective cohort study. Eur J Gastroenterol Hepatol. 2020;32:1571-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Nie D, Yan X, Huang Y. Efficacy of hydrocortisone sodium succinate and aluminum phosphate gel for stricture prevention after ≥3/4 circumferential endoscopic submucosal dissection. J Int Med Res. 2020;48:300060519894122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Polski JM, Brunt EM, Saeed ZA. Chronology of histological changes after band ligation of esophageal varices in humans. Endoscopy. 2001;33:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ward RM, Kearns GL. Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 2013;15:119-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Alaniz C, Mohammad RA, Welage LS. High-dose PPIs in patients with variceal hemorrhage. Arch Intern Med. 2010;170:1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Chavez-Tapia NC, Tellez-Avila FI, Garcia-Leiva J, Valdovinos MA. Use and overuse of proton pump inhibitors in cirrhotic patients. Med Sci Monit. 2008;14:CR468-CR472. [PubMed] |
| 22. | Li DK, Chung RT. Use of proton pump inhibitors in chronic liver diseases. Clin Liver Dis (Hoboken). 2017;10:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Yan X, Huang Y, Nie D, Wang Y, Chang H, Zhang Y, Yao W, Li K. Efficacy of oral steroid gel in preventing esophageal stricture after extensive endoscopic submucosal dissection: a randomized controlled trial. Surg Endosc. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Yan X, Nie D, Zhang Y, Chang H, Huang Y. Effectiveness of an orally administered steroid gel at preventing restenosis after endoscopic balloon dilation of benign esophageal stricture. Medicine (Baltimore). 2019;98:e14565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |