Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1536
Peer-review started: January 29, 2021
First decision: July 29, 2021
Revised: August 1, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: December 27, 2021
Processing time: 328 Days and 22.8 Hours
Crohn’s disease (CD) is a complex and relapsing gastrointestinal disease with mesenteric alterations. The mesenteric neural, vascular, and endocrine systems actively take part in the gut dysbiosis-adaptive immunity-mesentery-body axis, and this axis has been proven to be bidirectional. The abnormalities of morphology and function of the mesenteric component are associated with intestinal inflammation and disease progress of CD via responses to afferent signals, neuropeptides, lymphatic drainage, adipokines, and functional cytokines. The hypertrophy of mesenteric adipose tissue plays important roles in the pathogenesis of CD by secreting large amounts of adipokines and representing a rich source of proinflammatory or profibrotic cytokines. The vascular alteration, including angiogenesis and lymphangiogenesis, is concomitant in the disease course of CD. Of note, the enlarged and obstructed lymphatic vessels, which have been described in CD patients, are likely related to the early onset submucosa edema and being a cause of CD. The function of mesenteric lymphatics is influenced by endocrine of mesenteric nerves and adipocytes. Meanwhile, the structure of the mesenteric lymphatic vessels in hypertrophic mesenteric adipose tissue is mispatterned and ruptured, which can lead to lymph leakage. Leaky lymph factors can in turn stimulate adipose tissue to proliferate and effectively elicit an immune response. The identification of the role of mesentery and the crosstalk between mesenteric tissues in intestinal inflammation may shed light on understanding the underlying mechanism of CD and help explore new therapeutic targets.
Core Tip: Crohn’s disease (CD) is a complex autoimmune disease with increasing incidence worldwide, especially in Asian countries in recent years. There has been excellent progress in understanding the role of the mesentery in the pathogenesis and disease progress of CD. The crosstalk between components and intestinal inflammation has aroused many researchers’ interests. Herein, we will discuss the basic function and the alteration under inflammatory state of mesenteric nerves, blood vessels, lymphatics, and fat mass. Existing therapeutic strategies associated with mesentery components will also be summarized.
- Citation: Yin Y, Zhu ZX, Li Z, Chen YS, Zhu WM. Role of mesenteric component in Crohn’s disease: A friend or foe? World J Gastrointest Surg 2021; 13(12): 1536-1549
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1536.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1536
Crohn’s disease (CD) is a chronic relapsing autoimmune disease that can affect the entire gastrointestinal tract and is mainly characterized by segmental intestinal inflammation[1]. The mesentery is now well recognized as the collection of tissues that maintains all abdominal digestive organs in position and in continuity with other systems. The mesentery is made up of adipose tissue, a connective tissue matrix, nerve tissue, lymphatics, blood vessels, and immune cells[2-4]. The macroscopic lesions of mesentery including thickening, stiff, and hypertrophy are hallmarks of CD[5,6]. The histopathological findings of the mesentery from patients with CD demonstrates fibrosis, dilated lymphatic vessels (LV), perivascular inflammation, perineuronal chronic inflammation, and small-sized adipocytes[7,8]. However, the role and the involvement of the mesentery in the pathogenesis and clinical course of CD is still unclear and controversial. Some research points to the mesentery as a protective organ, able to mount a controlled inflammatory response following abnormal intestinal bacterial translocation[9,10]. On the opposing side, there is evidence suggesting that the participation and involvement of the mesentery in the setting of CD is negative, fueling the pathogenesis of the disease[11]. This review aims to describe the role of mesenteric nerves, lymphatics, blood vessels, and adipose tissue in the systemic and local inflammation in CD. Recent studies and progress on this topic will be reviewed to investigate the relationship between the mesentery and disease course of CD and the potential therapeutic target for CD treatment.
There have been several studies indicating the involvement of the neuroendocrine and enteric nervous system in CD[12]. However, the role of mesenteric nerves in the pathogenesis and prognosis of CD is still unclear. In fact, as a vital part of the brain-gut axis, the mesenteric nerves provide a physiological link between the central nerve system and gastrointestinal tract[13]. Based on anatomical considerations, the mesenteric nerves include the vagal and sympathetic nerves. The vagus nerve (VN) is the main component of the parasympathetic nerve system, which is composed of afferent and efferent fibers[14]. Peripheral sensations can be integrated into the central autonomic network via vagal afferents, and then the efferent response of the VN is able to modulate gastrointestinal nociception and inflammation[15]. The sympathetic nerve enters the intestinal tract along with the artery and terminates in the enteric nervous system, innervating the intestinal layers and intestinal associated lymphoid tissue[16,17].
Previous studies have confirmed that vagal and sympathetic nerves play an important role in regulating inflammation[18]. In trinitrobenzene sulfonic acid–induced colitis and acetic acid-induced colitis mice models, hyperexcitable visceromotor neurons were observed in the inferior mesenteric ganglia[19]. A recent animal experiment also confirmed that vagotomy increased the susceptibility to colitis in mice, mainly by inhibiting the alpha7 nicotinic acetylcholine receptors-mediated cholinergic anti-inflammatory pathway[20], whereas treatment with nicotine (alpha7 nicotinic acetylcholine receptors agonist) and galantamine (cholinesterase inhibitors) was shown to reverse the severity of colitis induced by dextran sulfate sodium[21,22]. In addition, another study found that vagal innervation was involved in the formation of tertiary lymphoid tissue in colitis, which is lymphoid tissue that forms as a result of chronic inflammation in a tissue or organ[23]. Unfortunately, the role of this lymphoid tissue in inflammatory bowel disease (IBD) remains unclear. Similarly, sympathectomy aggravated colitis (induced by dextran sulfate sodium or via T cell transfer) in mice. It was also observed in this experiment that intestine-specific vagal nerve denervation had no effect in dextran sulfate sodium-induced colitis[24]. Meanwhile, some researchers proved that the sympathetic nerve played a pivotal role in inhibiting innate immune cells against microorganism, likely via the adrenergic β2 receptor[25], which not only inhibited the secretion of tumor necrosis factor alpha (TNFα) but also drove rapid interleukin (IL)-10 secretion from innate cells[26]. In addition, several studies have shown that anxiety and depression can interact with intestinal inflammation through the bidirectionality of the brain-gut axis in patients with IBD[27]. The positive implementation of psychological intervention in patients with CD can alleviate the changes of their condition[28]. Therefore, we have reasons to believe that the pathogenesis of CD is closely related to the changes of mesenteric nerves.
Indeed, the tone of the vagus system is altered in patients with CD[29]. A matched cohort study for nearly 60 years found a positive correlation between vagotomy and IBD, especially in CD patients, which indirectly highlighted the beneficial role of vagal tone in intestinal inflammation[30]. A study has also confirmed that the sympathetic innervation of intestinal mucosa and the catecholamine neurotransmitters released by sympathetic nerve in CD patients decreased[31]. Interestingly, as a form of IBD, ulcerative colitis (UC) was not associated with the loss of sympathetic nerve fibers. By contrast, increased density of the sympathetic nerve network was found in UC patients[32]. Thus, the underlying mechanism of CD and UC seems different in intestinal immunity regulated by sympathetic nerves. Based on these studies, a research group conducting a clinical trial of VN stimulation in patients with active CD reported clinical, biological, and endoscopic remission in 5 of 7 patients treated with VN stimulation and restored vagal tone[33].
In summary, the mesenteric nerves have been proven to be involved in the bidirectional regulation of inflammation and emotion of the brain-gut axis and in the pathogenesis of CD. The clinical trials with VN stimulation intervention provide a neo-target for CD treatment. Meanwhile, drugs targeting neurotransmitter receptors also seem promising and worth exploring. Anti-depression treatment helps decrease the mesenteric afferent nerve activity and further ameliorates intestinal inflammation, which can be a potential therapeutic target for CD treatment.
The abnormality of mesenteric blood supply in CD has been confirmed, although the underlying mechanism is not well clarified. Histopathological features of injured blood vessels, including vascular injury, focal arteritis, fibrin deposition, arterial occlusion, and even granulomatous vasculitis, are observed in diseased segment in CD[34,35]. Meanwhile, the microvascular dysfunction was found to be correlated with disease activity and relapse of CD[36,37]. Radiological evidence of mesenteric hypervascularity (also known as the “comb sign”) coupled with radiological evidence of nodal enlargement is associated with endoscopic evidence of mucosal ulceration[38]. The association between splanchnic hemodynamics and disease activity of CD has also been investigated by Doppler sonography[39]. Of note, the superior mesenteric artery flow has been accessed for Crohn’s ileitis diagnosis and for disease activity monitoring[40,41]. The velocity of blood flow in the superior mesenteric artery was markedly higher in CD patients compared to controls. By contrast, the resistance index of the superior mesenteric artery was lower in active CD than controls[42,43]. The cumulative clinical evidence suggests that the function of vasculature is altered in CD.
Angiogenesis is an important component of CD pathogenesis. Molecular studies have confirmed that angiogenesis is crucial to inflammation and is associated with activation and proliferation of endothelial cells and capillary and venule remodeling, resulting in an expansion of the tissue microvascular bed[44-46]. A potential consequence of this expansion is notable promotion of inflammation through various cytokines, chemokines, and matrix metalloproteinases[47,48]. The involvement of hypoxia inducible factor (HIF) has been extensively studied. Increased expression of HIF-1 and HIF-2 has been detected in inflamed tissue of IBD patients[49]. Importantly, HIF stimulates angiogenesis via vascular endothelial growth factor (VEGF) induction[50]. Of note, VEGF-A is markedly increased in the tissue and serum of patients with CD[51-53] and is implicated in angiogenesis in experimental colitis[54]. The importance of the VEGF family proteins in the pathogenesis and disease course of IBD has also been demonstrated in studies assessing the efficacy of different therapeutic regimens for IBD. Recently, Algaba et al[55] found that circulating levels of VEGF-A significantly decreased after anti-TNF-α therapy and that elevated VEGF-A levels at baseline might predict a poor response to TNF-α inhibitors.
Endothelial cell adhesion molecules also play an important role in vascular proliferation through recruitment of inflammatory cells to the site of inflamed intestine. The activated vascular endothelial cells express several cell adhesion molecules, which are essential for the regulation of leukocyte trafficking and migration[56]. Three main families of cell adhesion molecules and their ligands (selectins, integrins, and immunoglobulin superfamily) are engaged in the process. The binding of the integrins α4β7 and α4β1 on leukocytes to their ligands on the endothelial cells, mucosal addressin cell adhesion molecule-1 (MadCAM-1) and vascular CAM-1, seem to be one of the most important interaction[57]. Previous studies have proved that mucosal addressin CAM-1 is overexpressed on intestinal high endothelial venules during active IBD, which promotes homing and tethering of inflammatory cells[57,58]. Anti-integrin therapeutics, including gut-selective antibodies against the β7 integrin subunit (etrolizumab) and the α4β7 integrin heterodimer (vedolizumab and abrilumab), the non-gut selective anti-α4 integrin (natalizumab), as well as small molecules (AJM300) were developed for IBD treatment. Among which, vedolizumab and etrolizumab demonstrate similar inhibition of dynamic adhesion of lymphocytes from IBD patients to mucosal addressin CAM-1.
The abnormal upregulation of endothelial cell adhesion molecules and increased adhesion of leukocytes likely result in coagulation abnormalities. In fact, CD patients are at high risk of developing mesenteric thrombosis[59,60]. Among patients with CD, mesenteric venous thrombosis is associated with bowel stenosis and CD-related intestinal surgery[60]. Purposed risk factors also include the use of conjugated estrogens, surgery-associated trauma, intestinal stricture, pregnancy, and history of blood clot[61]. As aforementioned, anti-adhesion molecule therapy, which deters leukocyte recruitment, has been shown to be effective in the treatment of CD. The clinical evidence has confirmed angiogenesis as a component of CD[62] and angiogenesis blockade as a new therapeutic approach to experimental colitis[63].
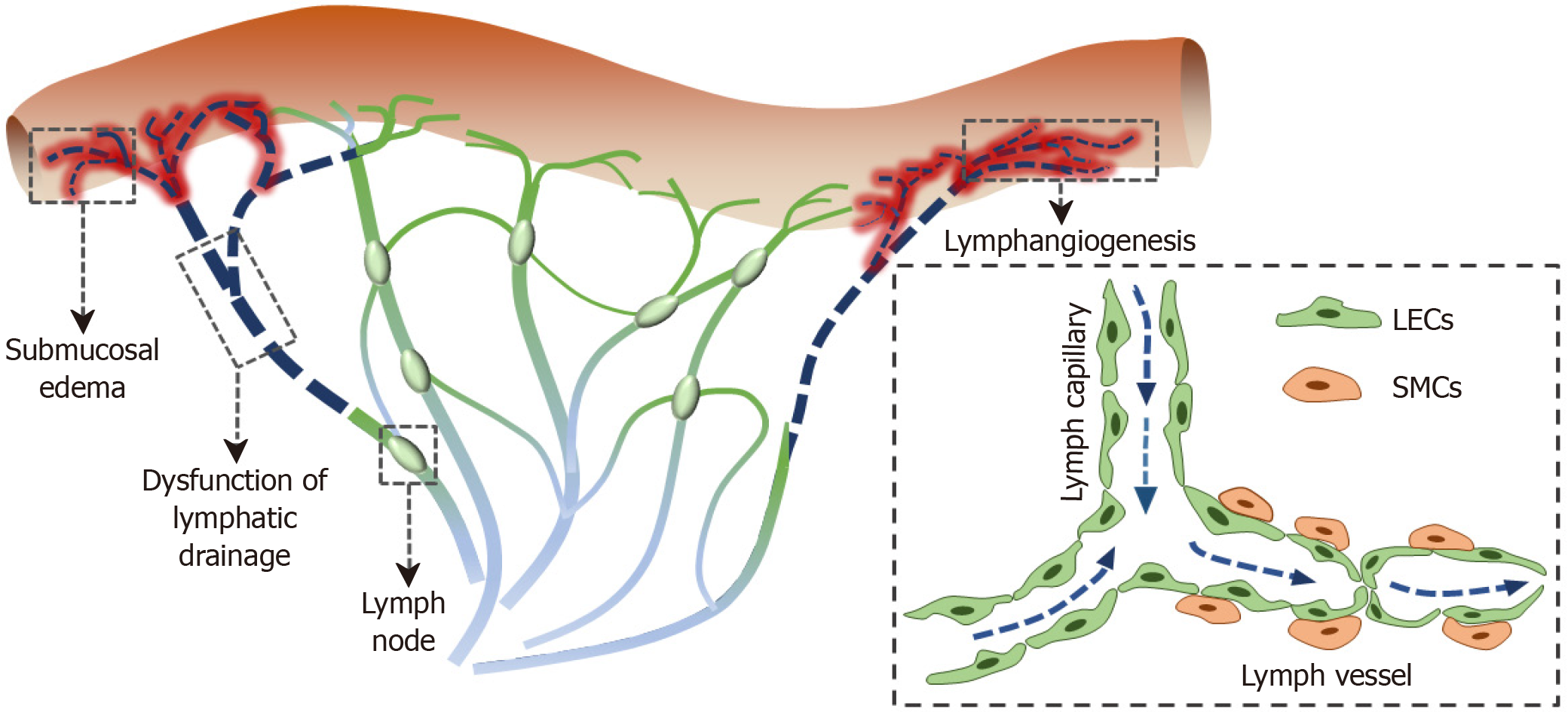
Although the pathophysiology of CD remains unknown, the involvement of the lymphatic system in CD has long been suggested. Abnormal lymphatics, such as lymphangiogenesis and enlarged and obstructed LVs, has been described in CD patients and is likely related to early onset submucosa edema (Figure 1)[64]. It is reported that intestinal granulomas[65], granulomas in the mesenteric lymph nodes, decreased intestinal, and mesenteric LV density[66] are associated with the postoperative recurrence of CD.
Lymph flow plays an important role in transporting antigens, dendritic cells, and macrophages[67,68]. Many studies have reported that lymphatic dysfunction can lead to immunosuppression[69,70]. It is believed that lymph flow is enhanced during an inflammatory state. However, inflammation may in turn impair lymphatic pumping with lymphatic obstruction and impaired lymphatic contraction, leading to a poor drainage of interstitial fluid[71,72]. It is well-known that inflammatory mediators, such as prostaglandins and cytokines, can increase vascular permeability, causing submucosal edema. These inflammatory mediators play a potential role in altering LV contractions and lymph flow during their transport from inflammatory tissues to draining lymph nodes, impairing immune response[72]. Rahier et al[73] reported that the LV density increased in inflammatory bowel disease. One possible reason for the lymphangiogenesis may be contributing to improved lymphatic drainage in response to mesenteric lymphatic obstruction, marked lacteal dilatation, and extensive submucosal edema[72].
The molecular underlying mechanism of lymphangiogenesis in CD patients remains largely unknown. Many factors are involved in lymphangiogenesis, such as members of the VEGF family, hepatocyte growth factor, insulin-like growth factor-2, platelet-derived growth factor-BB, and fibroblast growth factor-2[74-77]. VEGF-C and VEGF-D are members of the VEGF family, which mediate lymphangiogenesis via their receptor VEGFR3[78]. The blockade of the VEGFR3 signaling pathway can suppress lymphangiogenesis and further aggravate intestinal inflammation. Of note, lymphangiogenic factor VEGF-C has shown promising therapeutic effects in experimental colitis, both clinically and histologically[79]. These studies suggest that mesenteric lymphatics may be a promising potential target for CD treatment. Recently, we found that intestinal inflammation was significantly improved by the application of lymphatics-targeting drug release in the IL-10-/- spontaneous experimental colitis, suggesting that mesenteric LVs are potential targets for CD treatment[80].
The lymphoid aggregates resembling tertiary lymphoid organs, composed of CD3+ T cells surrounding CD20+ B cell clusters, have been observed in the mesentery of CD patients[81-83]. Guedj et al[81] recently proposed a notion that mesenteric adipose cells can participate in the process of tertiary lymphoid organ formation in the creeping fat of CD-affected mesentery. In addition, lymphoid cells invade the LV wall in CD-affected mesentery, suggesting the involvement of tertiary lymphoid organs in the lymphatic remodeling[82]. The lymphatic remodeling includes lymphangiogenesis, LV dilation, and lymph leakage. Interestingly, the lymph leakage in surrounding mesenteric adipose tissue can stimulate the growth of adipose tissue. The leaky antigens, lipids, and cytokines released from adipose cells can effectively promote immune response[84].
As described above, increased LV density in the intestinal wall has been found in CD patients. Recently, a study has found that decreased LV density in intestinal mucosa is associated with higher risk of endoscopic recurrence after surgical intervention[85], suggesting that increased LV density may contribute to reduced recurrence of CD, which was consistent with the notion that increased lymphangiogenesis could be a compensatory response to lymphatic dysfunction. By contrast, the results reported by Li et al[66] showed that increased mesenteric LV density in the proximal margin was associated with higher risk of early clinical recurrence after surgery in CD patients. One possible reason for the difference is that the locations of the LV densities were different.
Granulomas are observed only in some patients with CD (less than 13%), and they are associated with a more aggressive disease phenotype of CD[86]. In this case, patients with granulomas, who have undergone surgery for CD, have a higher risk for reoperation[86]. Of note, Li et al[87] reported that the presence of granulomas in mesenteric lymph nodes instead of the granulomas in the intestine is an independent risk factor for postoperative recurrence in CD patients. In conclusion, accumulating studies have demonstrated the involvement of the lymphatic system in CD. Although the underlying mechanism of the alterations of mesenteric lymphatics is not well clarified, promoting lymphatic function in CD patients could improve prognosis.
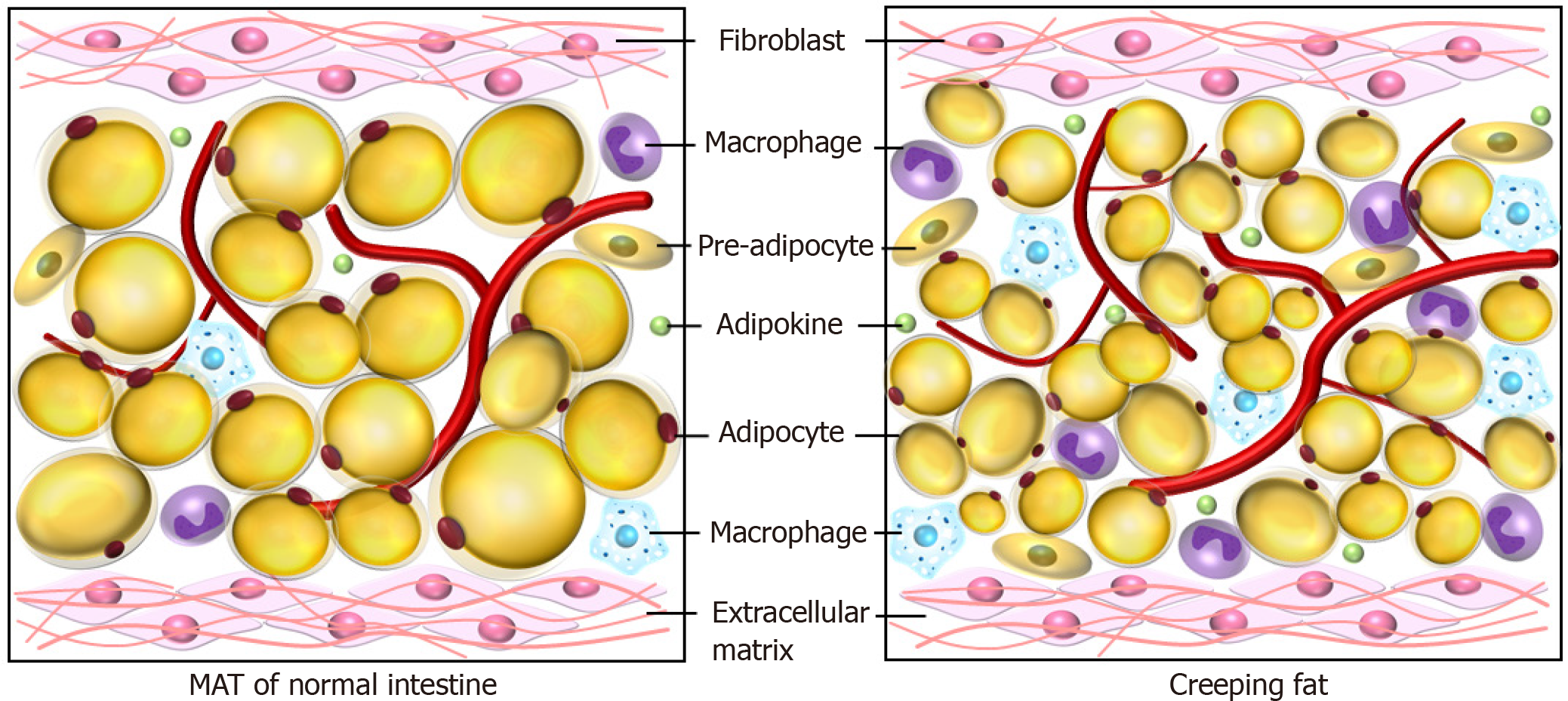
Mesenteric adipose tissue hypertrophy is regarded as a feature of CD and was firstly reported by Dr. Burrill B. Crohn himself to be a consistent symptom of the disease[8]. The pathologically altered mesenteric fat tissue is called “creeping fat,” defined as expansion of mesenteric adipose tissue around the inflamed and fibrotic intestine (Figure 2)[5]. The creeping fat takes place at the mesenteric transition zone, where the intestinal wall and mucosa change synchronizing with the mesentery[88]. Additionally, creeping fat has been used as an anatomical marker for surgeons to determine the margin of resection during surgery[89]. Meanwhile, a number of studies revealed that creeping fat might play an important role in the pathogenesis of CD, by secreting large amounts of adipokines and representing a rich source of TNF, IL-6, IL-10, and other proinflammatory or profibrotic cytokines[90].
It has been demonstrated that adipokines are strongly associated with severity of intestinal inflammation. However, their exact role in the pathogenesis and disease course of IBD has not been concluded. Herein, we are discussing three important adipokines (adiponectin, leptin, and apelin) and their roles in the crosstalk with intestinal inflammation.
Adiponectin is a well-explored adipokine and plays a key role in regulating insulin sensitivity[91]. According to previous studies, adiponectin is markedly upregulated in the creeping fat of CD compared to the non-creeping fat of CD, UC, and healthy controls[92]. Its molecular architecture is strikingly similar to that of TNF-α in the terminal structure of the globular domain, despite lacking homology in the primary sequence[93]. Therefore, adiponectin presents an anti-inflammatory effect based on the antagonistic effect of TNF-α[94]. On the other hand, it is demonstrated that adiponectin inhibits the expression of adhesion molecules, metalloproteinases, and proinflammatory mediators[95].
Leptin is mainly secreted by white adipose tissue and regulates the differentiation, function, and metabolism of a variety of immune cell subpopulations and intestinal epithelial cells[96-98]. Previous studies described that leptin expression was upregulated in the mesenteric tissue of CD patients[99]. It has been shown that leptin modulates intestinal inflammation in experimental colitis[100]. Moreover, several studies have demonstrated that leptin deficiency and the pharmacologic blockade of the leptin receptor notably ameliorate colitis[101]. Leptin promotes T cell proliferation, resulting in an increased production of type 1 T helper cell-related cytokines[98]. A recent study revealed that leptin was crucial to human immune homeostasis and contributed to autoimmunity in a TNFα-dependent manner[102].
Apelin induces proliferation of intestinal epithelial cells[103]. Meanwhile, it was revealed that apelin plays a significant role in the development and stabilization of LVs[104,105]. Ge et al[106] reported that apelin was highly expressed in the mesenteric fat and in colon tissues of CD patients, which strongly suggested that apelin may ameliorate intestinal inflammation by enhancing lymphatic drainage. Han et al[103] indicated that the intraperitoneal injection of apelin-13 decreased mucosal inflammation, inhibited the infiltration of inflammatory cells, and decreased expression of proinflammatory cytokine mRNA levels in the murine colonic tissue. Exogenous apelin can also enhance tissue repair by increasing the colonic epithelial cell proliferation[103].
As aforementioned, leptin promotes the M2 macrophage subtype and subsequently enhances fibrosis by secreting large amounts of profibrotic factors such as tumor growth factor-β[107,108]. Meanwhile, Rieder et al[109] observed that creeping fat derived mediators such as free fatty acids (FFAs), induced a differential and selective proliferative response by human intestinal fibroblast and human intestinal muscle cells. FFA can promote the proliferation of human intestinal muscle cells and human intestinal fibroblasts rather than increase the proliferation of epithelial cells, endothelial cells, or adipocytes. This suggests that the proliferation induced by FFAs is intestinal mesenchymal cell specific. The proliferation induced by long-chain FFAs is dependent on the kinases p38 mitogen-activated protein kinase, protein kinase C, and phosphoinositide 3-kinase[109]. These studies suggest that creeping fat correlates with the stricture formation.
Bacteria translocate from the intestine to the mesentery through transmural inflammation in CD, largely resulting from impaired epithelial integrity[110]. Adipocytes and pre-adipocytes in the mesenteric fat express functional pattern recognition receptors, such as toll-like receptors and nucleotide oligomerization domain receptor-1[111-114]. These receptors respond to the translocated bacteria by sensing microbe-derived molecules[10]. The downstream signaling cascade leads to activation of transcription factors (such as nuclear factor-κB) and induction of proinflammatory cytokines and chemokines[115]. Moreover, pre-adipocytes can differentiate into macrophages and then modulate the inflammatory reaction, including phagocytic activity and proinflammatory cytokine release[116].
It is revealed the visceral adipose tissue presents a microbiome signature enriched in Proteobacteria of patients with CD[117]. Meanwhile, the abundance of bacteria in visceral adipose tissue can be altered with the clinical status of CD patients. Patients with active CD showed a higher abundance of common mucosal bacteria (i.e. Bacteroidetes). Additionally, the formation of creeping fat is associated with translocation of gut bacteria[118]. The creeping fat seems to be a protective response to prevent systemic dissemination of potentially harmful bacterial antigens. The crosstalk between mesentery adipose tissue and microbiota needs further investigation, and the results may provide a new perspective for the management of CD patients.
Mesenteric nerves, blood vessels, lymphatics, and adipose tissue are not only associated with intestinal inflammation but also influence other parts of the mesentery[70,119]. The function of mesenteric lymphatics is influenced by endocrine of mesenteric nerves and adipocytes. Nerve fibers around submucosal arteries and mesenteric LVs markedly increase in CD patients, suggesting that neurogenic inflammation is likely associated with early onset lymphatic vascular dilation and submucosa edema. Meanwhile, the structure of the mesenteric LV in hypertrophic mesenteric adipose tissue is mispatterned and ruptured, which can lead to lymph leakage. Leaky lymph factors stimulate adipose tissue to proliferate and effectively elicit an immune response. LVs mediate lipid absorption and transport, share an intimate spatial association with adipose tissue, and regulate the traffic of immune cells[120,121]. Adipokines such as apelin can in turn ameliorate chronic colitis in IL-10-/- mice by promoting intestinal lymphatic function[106]. The neuropeptides, such as vasoactive intestinal peptide, alter lymphatic pumping by decreasing the frequency of lymphatic contractions and hyperpolarizing the lymphatic muscle membrane potential in a concentration-dependent manner[122]. The complex crosstalk between mesenteric nerves, blood vessels, lymphatics, and adipose tissue suggests dysregulation of mesenteric homeostasis in patients with CD. The interaction is likely to play a role in the pathogenesis and disease course of inflammation and remodeling in mesenteric adipose tissue in CD.
Accumulating evidence has shown that mesenteric organs including mesenteric nerves, blood vessels, lymphatics, and adipose tissue play a crucial role in the pathogenesis and progress of CD. Existing and emerging clinical evidence strongly suggests that the gut-mesentery axis is bidirectional. The intestinal inflammation and the dysregulation of the crosstalk among mesenteric components interact with each other and contribute to disease aggravation. The mesenteric inflammation may be an independent clinical risk factor associated with surgical outcomes. Recently, Coffey et al[88] reported that inclusion of the mesentery in ileocolic resection for CD is associated with reduced recurrence requiring reoperation, which suggests a more radical resection of mesenteric tissue along with the diseased bowel leads to better surgical outcomes, especially postoperative disease recurrence.
The evaluation of changes in morphology and function of mesenteric nerves, vasculature, lymphatics, and fat mass provide more potential targets for CD treatment. Our group has shown that apelin can ameliorate chronic colitis in Il-10-/- mice by promoting intestinal lymphatic functions[106]. Moreover, a chylomicrons-simulating strategy has been developed, fulfilling sustained drug release in mesenteric lymphatics and enhancing the therapeutic effect on intestinal inflammation by increasing lymphatic drainage[80]. We do believe that more and more agents and strategies targeting mesenteric content will be developed and bring more alternative therapies for CD patients. Mucosal healing has been emphasized as the current dominant standard for disease remission, whereas the changes in morphology and function of mesenteric nerves, vasculature, lymphatics, and adipose tissue can also be monitored during treatment. The improvement or resolution of inflammation of the submucosa, regulation of angiogenesis, enhancement of lymphatic drainage, and amelioration of adipose tissue-associated inflammation could be the next therapeutic goals for CD patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fonseca-Alves CE S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1799] [Article Influence: 224.9] [Reference Citation Analysis (111)] |
| 2. | Coffey JC, O'Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol. 2016;1:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Coffey JC, O'leary DP. Defining the mesentery as an organ and what this means for understanding its roles in digestive disorders. Expert Rev Gastroenterol Hepatol. 2017;11:703-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Coffey JC, Walsh D, Byrnes KG, Hohenberger W, Heald RJ. Mesentery - a 'New' organ. Emerg Top Life Sci. 2020;4:191-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: evidence for a possible causative link. J Pathol. 2000;190:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Herlinger H, Furth EE, Rubesin SE. Fibrofatty proliferation of the mesentery in Crohn disease. Abdom Imaging. 1998;23:446-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | CROHN BB, GINZBURG L, OPPENHEIMER GD. Regional ileitis; a pathologic and clinical entity. Am J Med. 1952;13:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Batra A, Heimesaat MM, Bereswill S, Fischer A, Glauben R, Kunkel D, Scheffold A, Erben U, Kühl A, Loddenkemper C, Lehr HA, Schumann M, Schulzke JD, Zeitz M, Siegmund B. Mesenteric fat - control site for bacterial translocation in colitis? Mucosal Immunol. 2012;5:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Kredel L, Batra A, Siegmund B. Role of fat and adipokines in intestinal inflammation. Curr Opin Gastroenterol. 2014;30:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, Cherbut C, Galmiche JP. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol. 2003;27:987-991. [PubMed] |
| 12. | Villanacci V, Bassotti G, Nascimbeni R, Antonelli E, Cadei M, Fisogni S, Salerni B, Geboes K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol Motil. 2008;20:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 2787] [Article Influence: 464.5] [Reference Citation Analysis (2)] |
| 14. | Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl). 1990;181:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 207] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Powley TL, Phillips RJ. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes? Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217-G1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Chiocchetti R, Mazzuoli G, Albanese V, Mazzoni M, Clavenzani P, Lalatta-Costerbosa G, Lucchi ML, Di Guardo G, Marruchella G, Furness JB. Anatomical evidence for ileal Peyer's patches innervation by enteric nervous system: a potential route for prion neuroinvasion? Cell Tissue Res. 2008;332:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kulkarni-Narla A, Beitz AJ, Brown DR. Catecholaminergic, cholinergic and peptidergic innervation of gut-associated lymphoid tissue in porcine jejunum and ileum. Cell Tissue Res. 1999;298:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Brinkman DJ, Ten Hove AS, Vervoordeldonk MJ, Luyer MD, de Jonge WJ. Neuroimmune Interactions in the Gut and Their Significance for Intestinal Immunity. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Linden DR. Enhanced excitability of guinea pig inferior mesenteric ganglion neurons during and following recovery from chemical colitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1067-G1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Di Giovangiulio M, Bosmans G, Meroni E, Stakenborg N, Florens M, Farro G, Gomez-Pinilla PJ, Matteoli G, Boeckxstaens GE. Vagotomy affects the development of oral tolerance and increases susceptibility to develop colitis independently of the alpha-7 nicotinic receptor. Mol Med. 2016;22:464-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Wazea SA, Wadie W, Bahgat AK, El-Abhar HS. Galantamine anti-colitic effect: Role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-κB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Sci Rep. 2018;8:5110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Seyedabadi M, Rahimian R, Ghia JE. The role of alpha7 nicotinic acetylcholine receptors in inflammatory bowel disease: involvement of different cellular pathways. Expert Opin Ther Targets. 2018;22:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Olivier BJ, Cailotto C, van der Vliet J, Knippenberg M, Greuter MJ, Hilbers FW, Konijn T, Te Velde AA, Nolte MA, Boeckxstaens GE, de Jonge WJ, Mebius RE. Vagal innervation is required for the formation of tertiary lymphoid tissue in colitis. Eur J Immunol. 2016;46:2467-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Willemze RA, Welting O, van Hamersveld HP, Meijer SL, Folgering JHA, Darwinkel H, Witherington J, Sridhar A, Vervoordeldonk MJ, Seppen J, de Jonge WJ. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterol Motil. 2018;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Willemze RA, Welting O, van Hamersveld P, Verseijden C, Nijhuis LE, Hilbers FW, Meijer SL, Heesters BA, Folgering JHA, Darwinkel H, Blancou P, Vervoordeldonk MJ, Seppen J, Heinsbroek SEM, de Jonge WJ. Loss of intestinal sympathetic innervation elicits an innate immune driven colitis. Mol Med. 2019;25:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Ağaç D, Estrada LD, Maples R, Hooper LV, Farrar JD. The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun. 2018;74:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 27. | Fournier A, Mondillon L, Luminet O, Canini F, Mathieu N, Gauchez AS, Dantzer C, Bonaz B, Pellissier S. Interoceptive Abilities in Inflammatory Bowel Diseases and Irritable Bowel Syndrome. Front Psychiatry. 2020;11:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Bernstein CN. The Brain-Gut Axis and Stress in Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2017;46:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Pellissier S, Dantzer C, Mondillon L, Trocme C, Gauchez AS, Ducros V, Mathieu N, Toussaint B, Fournier A, Canini F, Bonaz B. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn's disease and irritable bowel syndrome. PLoS One. 2014;9:e105328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Liu B, Wanders A, Wirdefeldt K, Sjölander A, Sachs MC, Eberhardson M, Ye W, Ekbom A, Olén O, Ludvigsson JF. Vagotomy and subsequent risk of inflammatory bowel disease: a nationwide register-based matched cohort study. Aliment Pharmacol Ther. 2020;51:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Magro F, Vieira-Coelho MA, Fraga S, Serrão MP, Veloso FT, Ribeiro T, Soares-da-Silva P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Kyösola K, Penttilä O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. 1977;12:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski JL, Pellissier S. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 34. | Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, Pounder RE. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989;2:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 405] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Wakefield AJ, Pittilo RM, Sim R, Cosby SL, Stephenson JR, Dhillon AP, Pounder RE. Evidence of persistent measles virus infection in Crohn's disease. J Med Virol. 1993;39:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Ludwig D, Wiener S, Brüning A, Schwarting K, Jantschek G, Stange EF. Mesenteric blood flow is related to disease activity and risk of relapse in Crohn's disease: a prospective follow-up study. Am J Gastroenterol. 1999;94:2942-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | McLaren WJ, Anikijenko P, Thomas SG, Delaney PM, King RG. In vivo detection of morphological and microvascular changes of the colon in association with colitis using fiberoptic confocal imaging (FOCI). Dig Dis Sci. 2002;47:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Sakurai T, Katsuno T, Saito K, Yoshihama S, Nakagawa T, Koseki H, Taida T, Ishigami H, Okimoto KI, Maruoka D, Matsumura T, Arai M, Yokosuka O. Mesenteric findings of CT enterography are well correlated with the endoscopic severity of Crohn's disease. Eur J Radiol. 2017;89:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Andrade TG, Fogaça HS, Elia CC, Pitrowsky MT, Souza HS. Crohn's disease activity assessed by Doppler sonography: the role of aortic flow parameters. Clinics (Sao Paulo). 2013;68:457-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | van Oostayen JA, Wasser MN, van Hogezand RA, Griffioen G, Biemond I, Lamers CB, de Roos A. Doppler sonography evaluation of superior mesenteric artery flow to assess Crohn's disease activity: correlation with clinical evaluation, Crohn's disease activity index, and alpha 1-antitrypsin clearance in feces. AJR Am J Roentgenol. 1997;168:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | van Oostayen JA, Wasser MN, Griffioen G, van Hogezand RA, Lamers CB, de Roos A. Diagnosis of Crohn's ileitis and monitoring of disease activity: value of Doppler ultrasound of superior mesenteric artery flow. Am J Gastroenterol. 1998;93:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Bolondi L, Gaiani S, Brignola C, Campieri M, Rigamonti A, Zironi G, Gionchetti P, Belloli C, Miglioli M, Barbara L. Changes in splanchnic hemodynamics in inflammatory bowel disease. Non-invasive assessment by Doppler ultrasound flowmetry. Scand J Gastroenterol. 1992;27:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Kircher PR, Spaulding KA, Vaden S, Lang J, Doherr M, Gaschen L. Doppler ultrasonographic evaluation of gastrointestinal hemodynamics in food hypersensitivities: a canine model. J Vet Intern Med. 2004;18:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 2882] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 46. | Bagli E, Xagorari A, Papetropoulos A, Murphy C, Fotsis T. Angiogenesis in inflammation. Autoimmun Rev. 2004;3 Suppl 1:S26. [PubMed] |
| 47. | Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999;103:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Szekanecz Z, Koch AE. Vascular endothelium and immune responses: implications for inflammation and angiogenesis. Rheum Dis Clin North Am. 2004;30:97-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 360] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 50. | Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. 2001;96:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Kapsoritakis A, Sfiridaki A, Maltezos E, Simopoulos K, Giatromanolaki A, Sivridis E, Koukourakis MI. Vascular endothelial growth factor in inflammatory bowel disease. Int J Colorectal Dis. 2003;18:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Griga T, Gutzeit A, Sommerkamp C, May B. Increased production of vascular endothelial growth factor by peripheral blood mononuclear cells in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Chidlow JH Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB, Kevil CG. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Algaba A, Linares PM, Encarnación Fernández-Contreras M, Figuerola A, Calvet X, Guerra I, de Pousa I, Chaparro M, Gisbert JP, Bermejo F. The effects of infliximab or adalimumab on vascular endothelial growth factor and angiopoietin 1 angiogenic factor levels in inflammatory bowel disease: serial observations in 37 patients. Inflamm Bowel Dis. 2014;20:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Rudolph EH, Woods JM. Chemokine expression and regulation of angiogenesis in rheumatoid arthritis. Curr Pharm Des. 2005;11:613-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn's disease and ulcerative colitis. Inflammopharmacology. 2012;20:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 58. | Lamb CA, O'Byrne S, Keir ME, Butcher EC. Gut-Selective Integrin-Targeted Therapies for Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:S653-S668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | Jackson CS, Fryer J, Danese S, Vanagunas A, Polensky S, Buchman AL. Mesenteric vascular thromboembolism in inflammatory bowel disease: a single center experience. J Gastrointest Surg. 2011;15:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Naïk Vietti N, Vietti Violi N, Schoepfer AM, Fournier N, Guiu B, Bize P, Denys A; Swiss Inflammatory Bowel Disease Cohort Study Group. Prevalence and clinical importance of mesenteric venous thrombosis in the Swiss Inflammatory Bowel Disease Cohort. AJR Am J Roentgenol. 2014;203:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Hatoum OA, Spinelli KS, Abu-Hajir M, Attila T, Franco J, Otterson MF, Telford GL, Binion DG. Mesenteric venous thrombosis in inflammatory bowel disease. J Clin Gastroenterol. 2005;39:27-31. [PubMed] |
| 62. | Knod JL, Crawford K, Dusing M, Collins MH, Chernoguz A, Frischer JS. Angiogenesis and Vascular Endothelial Growth Factor-A Expression Associated with Inflammation in Pediatric Crohn's Disease. J Gastrointest Surg. 2016;20:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Danese S, Sans M, Spencer DM, Beck I, Doñate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD, Mazar AP, Fiocchi C. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut. 2007;56:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Wu TF, MacNaughton WK, von der Weid PY. Lymphatic vessel contractile activity and intestinal inflammation. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Anseline PF, Wlodarczyk J, Murugasu R. Presence of granulomas is associated with recurrence after surgery for Crohn's disease: experience of a surgical unit. Br J Surg. 1997;84:78-82. [PubMed] |
| 66. | Li Y, Ge Y, Gong J, Zhu W, Cao L, Guo Z, Gu L, Li J. Mesenteric Lymphatic Vessel Density Is Associated with Disease Behavior and Postoperative Recurrence in Crohn's Disease. J Gastrointest Surg. 2018;22:2125-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Dongaonkar RM, Nguyen TL, Quick CM, Hardy J, Laine GA, Wilson E, Stewart RH. Adaptation of mesenteric lymphatic vessels to prolonged changes in transmural pressure. Am J Physiol Heart Circ Physiol. 2013;305:H203-H210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Negrini D, Moriondo A. Lymphatic anatomy and biomechanics. J Physiol. 2011;589:2927-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108:18784-18789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 70. | von der Weid PY, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr Opin Gastroenterol. 2011;27:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 71. | Thaunat O, Kerjaschki D, Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Von Der Weid PY, Rehal S. Lymphatic pump function in the inflamed gut. Ann N Y Acad Sci. 2010;1207 Suppl 1:E69-E74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Rahier JF, De Beauce S, Dubuquoy L, Erdual E, Colombel JF, Jouret-Mourin A, Geboes K, Desreumaux P. Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:15593-15598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 75. | Cao R, Björndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531-3536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 395] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 77. | Matsuo M, Yamada S, Koizumi K, Sakurai H, Saiki I. Tumour-derived fibroblast growth factor-2 exerts lymphangiogenic effects through Akt/mTOR/p70S6kinase pathway in rat lymphatic endothelial cells. Eur J Cancer. 2007;43:1748-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 79. | D'Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014;124:3863-3878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 80. | Yin Y, Yang J, Pan Y, Guo Z, Gao Y, Huang L, Zhou D, Ge Y, Guo F, Zhu W, Song Y, Li Y. Chylomicrons-Simulating Sustained Drug Release in Mesenteric Lymphatics for the Treatment of Crohn's-Like Colitis. J Crohns Colitis. 2021;15:631-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Guedj K, Abitbol Y, Cazals-Hatem D, Morvan M, Maggiori L, Panis Y, Bouhnik Y, Caligiuri G, Corcos O, Nicoletti A. Adipocytes orchestrate the formation of tertiary lymphoid organs in the creeping fat of Crohn's disease affected mesentery. J Autoimmun. 2019;103:102281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, Nalbantoglu I, Dubuquoy L, Chau A, Pariente B, Kartheuser A, Zinselmeyer BH, Colombel JF. Lymphoid Aggregates Remodel Lymphatic Collecting Vessels that Serve Mesenteric Lymph Nodes in Crohn Disease. Am J Pathol. 2016;186:3066-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | Sura R, Colombel JF, Van Kruiningen HJ. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn's disease: an immunohistochemical study. Aliment Pharmacol Ther. 2011;33:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 84. | Cao E, Watt MJ, Nowell CJ, Quach T, Simpson JS, De Melo Ferreira V, Agarwal S, Chu H, Srivastava A, Anderson D, Gracia G, Lam A, Segal G, Hong J, Hu L, Phang KL, Escott ABJ, Windsor JA, Phillips ARJ, Creek DJ, Harvey NL, Porter CJH, Trevaskis NL. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat Metab. 2021;3:1175-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 85. | Rahier JF, Dubuquoy L, Colombel JF, Jouret-Mourin A, Delos M, Ferrante M, Sokol H, Hertogh GD, Salleron J, Geboes K, Desreumaux P. Decreased lymphatic vessel density is associated with postoperative endoscopic recurrence in Crohn's disease. Inflamm Bowel Dis. 2013;19:2084-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Johnson CM, Hartman DJ, Ramos-Rivers C, Rao BB, Bhattacharya A, Regueiro M, Schwartz M, Swoger J, Al Hashash J, Barrie A, Pfanner TP, Dunn M, Koutroubakis IE, Binion DG. Epithelioid Granulomas Associate With Increased Severity and Progression of Crohn's Disease, Based on 6-Year Follow-Up. Clin Gastroenterol Hepatol. 2018;16:900-907.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Li Y, Stocchi L, Liu X, Rui Y, Liu G, Remzi FH, Shen B. Presence of Granulomas in Mesenteric Lymph Nodes Is Associated with Postoperative Recurrence in Crohn's Disease. Inflamm Bowel Dis. 2015;21:2613-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 88. | Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA, Shen B, Waldron D, Peirce C, Moloney M, Skelly M, Tibbitts P, Hidayat H, Faul PN, Healy V, O'Leary PD, Walsh LG, Dockery P, O'Connell RP, Martin ST, Shanahan F, Fiocchi C, Dunne CP. Inclusion of the Mesentery in Ileocolic Resection for Crohn's Disease is Associated With Reduced Surgical Recurrence. J Crohns Colitis. 2018;12:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 89. | Althoff P, Schmiegel W, Lang G, Nicolas V, Brechmann T. Creeping Fat Assessed by Small Bowel MRI Is Linked to Bowel Damage and Abdominal Surgery in Crohn's Disease. Dig Dis Sci. 2019;64:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Schäffler A, Fürst A, Büchler C, Paul G, Rogler G, Schölmerich J, Herfarth H. Secretion of RANTES (CCL5) and interleukin-10 from mesenteric adipose tissue and from creeping fat in Crohn's disease: regulation by steroid treatment. J Gastroenterol Hepatol. 2006;21:1412-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2315] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 92. | Choi HM, Doss HM, Kim KS. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 93. | Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 478] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 94. | Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 621] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 95. | Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 96. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 97. | Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667-2676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 98. | Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1519] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 99. | Siegmund B. Mesenteric fat in Crohn's disease: the hot spot of inflammation? Gut. 2012;61:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Weidinger C, Ziegler JF, Letizia M, Schmidt F, Siegmund B. Adipokines and Their Role in Intestinal Inflammation. Front Immunol. 2018;9:1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 101. | Singh UP, Singh NP, Guan H, Busbee B, Price RL, Taub DD, Mishra MK, Fayad R, Nagarkatti M, Nagarkatti PS. Leptin antagonist ameliorates chronic colitis in IL-10⁻/⁻ mice. Immunobiology. 2013;218:1439-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Ziegler JF, Böttcher C, Letizia M, Yerinde C, Wu H, Freise I, Rodriguez-Sillke Y, Stoyanova AK, Kreis ME, Asbach P, Kunkel D, Priller J, Anagnostopoulos I, Kühl AA, Miehle K, Stumvoll M, Tran F, Fredrich B, Forster M, Franke A, Bojarski C, Glauben R, Löscher BS, Siegmund B, Weidinger C. Leptin induces TNFα-dependent inflammation in acquired generalized lipodystrophy and combined Crohn's disease. Nat Commun. 2019;10:5629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Han S, Wang G, Qiu S, de la Motte C, Wang HQ, Gomez G, Englander EW, Greeley GH Jr. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul Pept. 2007;142:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F, Takakura N. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes. 2013;62:1970-1980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 105. | Kim JD, Kang Y, Kim J, Papangeli I, Kang H, Wu J, Park H, Nadelmann E, Rockson SG, Chun HJ, Jin SW. Essential role of Apelin signaling during lymphatic development in zebrafish. Arterioscler Thromb Vasc Biol. 2014;34:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 106. | Ge Y, Li Y, Chen Q, Zhu W, Zuo L, Guo Z, Gong J, Cao L, Gu L, Li J. Adipokine apelin ameliorates chronic colitis in Il-10-/- mice by promoting intestinal lymphatic functions. Biochem Pharmacol. 2018;148:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Kredel LI, Batra A, Stroh T, Kühl AA, Zeitz M, Erben U, Siegmund B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn's disease. Gut. 2013;62:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 108. | Vernon MA, Mylonas KJ, Hughes J. Macrophages and renal fibrosis. Semin Nephrol. 2010;30:302-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 109. | Rieder F, Doyon G, Ouyang Z, West G, Fiocchi C. Adipocyte and Preadipocyte Derived-Mediators Induce a PRO-Fibrogenic Phenotype in Human Intestinal Mesenchymal Cells -A Novel Link Between Fat and Intestinal Fibrosis. Gastroenterology. 2014;146. [DOI] [Full Text] |
| 110. | Sedman PC, Macfie J, Sagar P, Mitchell CJ, May J, Mancey-Jones B, Johnstone D. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 232] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 111. | Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255-24263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 112. | Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 113. | Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Schölmerich J, Schäffler A. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring). 2009;17:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Stroh T, Batra A, Glauben R, Fedke I, Erben U, Kroesen A, Heimesaat MM, Bereswill S, Girardin S, Zeitz M, Siegmund B. Nucleotide oligomerization domains 1 and 2: regulation of expression and function in preadipocytes. J Immunol. 2008;181:3620-3627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 115. | Zhou YJ, Zhou H, Li Y, Song YL. NOD1 activation induces innate immune responses and insulin resistance in human adipocytes. Diabetes Metab. 2012;38:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850-9855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 117. | Serena C, Queipo-Ortuño M, Millan M, Sanchez-Alcoholado L, Caro A, Espina B, Menacho M, Bautista M, Monfort-Ferré D, Terrón-Puig M, Núñez-Roa C, Maymó-Masip E, Rodriguez MM, Tinahones FJ, Espin E, Martí M, Fernández-Veledo S, Vendrell J. Microbial Signature in Adipose Tissue of Crohn's Disease Patients. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 118. | Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, Hendrick G, Caldera JR, Arias C, Moskowitz JE, Ho Sui SJ, Yang S, Underhill D, Brady MJ, Knott S, Kaihara K, Steinbaugh MJ, Li H, McGovern DPB, Knight R, Fleshner P, Devkota S. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell. 2020;183:666-683.e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 119. | Chakraborty S, Zawieja S, Wang W, Zawieja DC, Muthuchamy M. Lymphatic system: a vital link between metabolic syndrome and inflammation. Ann N Y Acad Sci. 2010;1207 Suppl 1:E94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 120. | Harvey NL. The link between lymphatic function and adipose biology. Ann N Y Acad Sci. 2008;1131:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 759] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 122. | von der Weid PY, Rehal S, Dyrda P, Lee S, Mathias R, Rahman M, Roizes S, Imtiaz MS. Mechanisms of VIP-induced inhibition of the lymphatic vessel pump. J Physiol. 2012;590:2677-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |