Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1463
Peer-review started: April 28, 2021
First decision: June 17, 2021
Revised: June 30, 2021
Accepted: October 22, 2021
Article in press: October 22, 2021
Published online: November 27, 2021
Processing time: 211 Days and 22.8 Hours
Gastric cancer is an aggressive disease with frequent lymph node (LN) involvement. The NCCN recommends a D2 lymphadenectomy and the harvesting of at least 16 LNs. This threshold has been the subject of great debate, not only for the extent of surgery but also for more appropriate staging. The reclassification of stage IIB through IIIC based on N3b nodal staging in the eighth edition of the American Joint Committee on Cancer (AJCC) staging system highlights the efforts to more accurately discriminate survival expectancy based on nodal number. Furthermore, studies have suggested that pathologic assessment of 30 or more LNs improve prognostic accuracy and is required for proper staging of gastric cancer.
To evaluate the long-term survival of advanced gastric cancer patients who deviated from expected survival curves because of inadequate nodal evaluation.
Eligible patients were identified from the Surveillance, Epidemiology, and End Results database. Those with stage II–III gastric cancer were considered for inclusion. Three groups were compared based on the number of analyzed LNs. They were inadequate LN assessment (ILA, < 16 LNs), adequate LN assessment (ALA, 16-29 LNs), and optimal LN assessment (OLA, ≥ 30 LNs). The main outcomes were overall survival (OS) and cancer-specific survival. Data were analyzed by the Kaplan-Meier product-limit method, log-rank test, hazard risk, and Cox proportional univariate and multivariate models. Propensity score matching (PSM) was used to compare the ALA and OLA groups.
The analysis included 11607 patients. Most had advanced T stages (T3 = 48%; T4 = 42%). The pathological AJCC stage distribution was IIA = 22%, IIB = 18%, IIIA = 26%, IIIB = 22%, and IIIC = 12%. The overall sample divided by the study objective included ILA (50%), ALA (35%), and OLA (15%). Median OS was 24 mo for the ILA group, 29 mo for the ALA group, and 34 mo for the OLA group (P < 0.001). Univariate analysis showed that the ALA and OLA groups had better OS than the ILA group [ALA hazard ratio (HR) = 0.84, 95% confidence interval (CI): 0.79–0.88, P < 0.001 and OLA HR = 0.73, 95%CI: 0.68–0.79, P < 0.001]. The OS outcome was confirmed by multivariate analysis (ALA HR = 0.68, 95%CI: 0.64–0.71, P < 0.001 and OLA: HR = 0.48, 95%CI: 0.44–0.52, P < 0.001). A 1:1 PSM analysis in 3428 patients found that the OLA group had better survival than the ALA group (OS: OLA median = 34 mo vs ALA median = 26 mo, P < 0.001, which was confirmed by univariate analysis (HR = 0.81, 95%CI: 0.75–0.89, P < 0.001) and multivariate analysis: (HR = 0.71, 95%CI: 0.65–0.78, P < 0.001).
Proper nodal staging is a critical issue in gastric cancer. Assessment of an inadequate number of LNs places patients at high risk of adverse long-term survival outcomes.
Core Tip: A large database was analyzed to investigate survival outcomes related to lymph node assessment in locally advanced gastric cancer patients with radical gastrectomy. Independent of TNM-stage, the group with assessment of < 16 lymph nodes (LNs) had significantly worse survival than two other groups, 16-29 LNs and ≥ 30 LNs. Stage migration because of inadequate specimen analysis and improper lymphadenectomy was the main root cause.
- Citation: Desiderio J, Sagnotta A, Terrenato I, Garofoli E, Mosillo C, Trastulli S, Arteritano F, Tozzi F, D'Andrea V, Fong Y, Woo Y, Bracarda S, Parisi A. Long-term survival of patients with stage II and III gastric cancer who underwent gastrectomy with inadequate nodal assessment. World J Gastrointest Surg 2021; 13(11): 1463-1483
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1463.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1463
Lymph node (LN) involvement in gastric cancer is one of the most significant prognostic factors for survival. Starting with the fifth edition of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) cancer staging manual, the N category has been evaluated based on the total number of metastatic LNs detected in a surgical specimen, independent of their retrieved locations[1]. Until recently, the N3 category required the identification of at least 15 positive LNs. However, the seventh edition revisions of nodal classification introduced the N3a (7–15 positive LNs) and N3b (≥ 16 positive LNs) substages[2], with the updates having a significant impact on the eighth edition updates to stages IIB through IIIA-C[3]. Consequently, the current guidelines recommend the analysis of at least 16 LNs[4].
Despite the national guidelines, many studies particularly those from Western countries continue to show high rates of inadequate nodal assessment[5-7]. To mitigate the effects of stage migration and survival inaccuracies in patients with locally advanced gastric cancer, use of the ratio of positive to total LN has been proposed[8]. However, the utility of existing prognostic methods remains limited in patients with an insufficient total number of assessed LNs. This study aimed to evaluate the survival impact of inadequate LN assessment (ILA) in patients with advanced gastric cancer disease (stages II and III) compared with that of patients receiving adequate and optimal nodal evaluation (≥ 30 LNs), as defined in the latest AJCC cancer staging manual[9].
Eligible patients were identified in the Surveillance, Epidemiology, and End Results (SEER) database[10] and detailed data were retrieved with SEER*Stat 8.3.5 software https://seer.cancer.gov/seerstat/. Patients 18 years of age or older with a diagnosis of stage II–III gastric cancer, according to the eighth edition of the AJCC cancer staging manual[9], were included in the study. Patients with cardiac tumors, without resective surgery, without available LN assessment information, and patients without follow-up duration data were excluded from the study. Tumor location was identified using the “primary site labeled” variable (C16.1: fundus of stomach; C16.2: body of stomach; C16.3: gastric antrum; C16.4: pylorus; C16.5: lesser curvature of stomach, NOS; C16.6: greater curvature of stomach, NOS; C16.8: overlapping lesion of stomach; and C16.9: stomach, NOS). Histology was evaluated by the International Classification of Disease for Oncology (ICD-O-3; M-8010/3-M-8015/3, M-8020/3-M-8022/3, M-8030/3-M-8035/3, M-8041/3, M-8043/3, M-8050/3-M-8052/3, M-8070/3-M-8078/3, M-8140/3-M-8145/3, M-8147/3, M-8210/3-M-8211/3, M-8214/3, M-8220/3, M-8221/3, M-8230/3, M-8231/3, M-8255/3, M-8260/3-M-8263/3, M-8310/3, M-8323/3, M-8480/3, M-8481/3, M-8490/3, M-8510/3, M-8560/3, M-8562/3, M-8570/3-M-8576/3, and M-8980/3-M-8982/3).
The study population was divided into three groups based on the number of retrieved and analyzed LNs, which were inadequate LN assessment (ILA), < 16 LNs, adequate LN assessment (ALA), 16-29 LNs, and optimal LN assessment (OLA), ≥ 30 LNs. The type of gastrectomy was identified using cancer-specific codes (40–42, 50, 52, and 62 indicated total or near-total gastrectomy and 30–33, 51, 60, 61, and 63 indicated partial gastrectomy). “CHT recode” and “radiation recode” were used to determine whether single or combined treatments were administered. The “CS Tumor Size/Ext Eval (2004 +)” and “CS Reg Node Eval (2004 +)” codes were used to identify patients who received neoadjuvant treatment.
Patient characteristics were summarized by descriptive statistics. The study groups were compared using Pearson’s chi square test or Student’s t-test, as appropriate. Overall survival (OS) was defined as the duration from the date of diagnosis to death or last follow-up, with no restriction on the cause of death. Cancer-specific survival (CSS) was defined as the duration from the date of diagnosis to death from gastric cancer other than other causes. Patients with a follow-up of less than 1 mo and patients without data on their alive or dead status were excluded from the survival analysis. OS and CSS were calculated using the Kaplan-Meier product-limit method.
The log-rank test was used to assess potential differences between subgroups. The hazard ratio (HR) and its relative 95% confidence interval (CI) were estimated for each parameter of interest using the Cox proportional univariate model while adopting the most suitable prognostic category as the referent group. In addition, a multivariate Cox proportional hazard model was developed by stepwise regression (forward selection). The enter and remove limits were P = 0.05 and P = 0.10, respectively. Significance was defined at the P < 0.05 level.
To control for potential confounders that could affect the outcomes of interest, propensity score matching (PSM)[11,12] was employed to generate two treatment groups with a balanced distribution of baseline features. Propensity scores were obtained from logistic regression, and the dependent variable was the choice to undergo surgery. The retrieval of 16–29 LNs was the control. The selected covariates were diagnosis period, age at diagnosis, sex, race, primary site, eighth edition N, and T stage, histology, and grading. To ensure good matches, patients were matched 1:1 using the nearest neighbor method and a caliper distance of 0.25 of the standard deviation of the logit of the estimated propensity score. Balance between the two groups was assessed using the relative multivariate imbalance measure, L1, as proposed by Iacus et al[13,14]. All analyses were carried out with SPSS v. 21.0. The statistical methods were reviewed by one of the authors of this manuscript (Terrenato I).
Based on the inclusion criteria, we studied 11,607 patients with stage II–III gastric carcinoma diagnosed between 2004 and 2015 (Table 1). There were 6697 men (58%) in the sample population, and the mean age at diagnosis was 69 years of age. In 4626 patients (40%), the tumor was located at the antrum/pylorus, and a poorly/undifferentiated adenocarcinoma was reported in 8524 patients (73%). Neoadjuvant chemotherapy was administered in 11% of cases, and a partial gastrectomy was performed in 72%. Most patients had advanced T stages (T3 = 5,569, 48%; T4a = 3,551, 31%; T4b = 1,254, 11%), while T1–T2 stages accounted for only 10% of the total sample. The patient distribution based on the N stages reported in the SEER registry was N0 = 2,863, 25%; N1 = 2,422, 21%; N2 = 2,757, 24%; N3a = 2,498, 21%; and N3b = 1,067, 9%. The patient distribution based on gastric cancer stage was IIA = 2,585, 22%; IIB = 2,129, 18%; IIIA = 3,049, 26%; IIIB = 2,511, 22%; and IIIC = 1,333, 12%.
| Characteristic | n (%) |
| Year of diagnosis | |
| 2004-2006 | 3142 (27) |
| 2007-2009 | 3028 (26) |
| 2010-2012 | 2850 (25) |
| 2013-2015 | 2587 (22) |
| Age at diagnosis (yr) | |
| Median (range) | 69 (12-99) |
| Sex | |
| Male | 6697 (58) |
| Female | 4910 (42) |
| Race | |
| White | 7045 (61) |
| Black | 2076 (18) |
| Asian/Pacific | 2486 (21) |
| Marital status | |
| Single/divorced | 2539 (22) |
| Married | 6805 (58) |
| Widowed | 1837 (16) |
| NA | 426 (4) |
| Insurance status | |
| Insured | 8033 (69) |
| Uninsured | 432 (4) |
| NA | 3142 (27) |
| Site of tumor | |
| Fundus/body | 1866 (16) |
| Antrum/pylorus | 4626 (40) |
| Overlapping lesion | 1299 (11) |
| Stomach, NOS | 3816 (33) |
| Tumor size (cm) | |
| ≤ 5 | 5431 (47) |
| 5.1-10 | 4203 (36) |
| ≥ 10.1 | 1135 (10) |
| NA | 838 (7) |
| Histology | |
| ADC, NOS | 4481 (39) |
| Signet ring cell carcinoma | 2726 (23) |
| ADC, instestinal type | 1943 (17) |
| Carcinoma, diffuse type | 958 (8) |
| ADC with mixed subtypes | 424 (4) |
| Other | 1075 (9) |
| Grade | |
| Well/moderately differentiated | 2674 (23) |
| Poorly/undifferentiated | 8524 (73) |
| NA | 409 (4) |
| T stage, 8th ed. | |
| T1 | 290 (2) |
| T2 | 943 (8) |
| T3 | 5569 (48) |
| T4a | 3551 (31) |
| T4b | 1254 (11) |
| N stage, 8th ed. | |
| N0 | 2863 (25) |
| N1 | 2422 (21) |
| N2 | 2757 (24) |
| N3a | 2498 (21) |
| N3b | 1067 (9) |
| Stage, 8th ed. | |
| IIA | 2585 (22) |
| IIB | 2129 (18) |
| IIIA | 3049 (26) |
| IIIB | 2511 (22) |
| IIIC | 1333 (12) |
| Chemotherapy | |
| Yes | 6473 (56) |
| No | 5134 (44) |
| Neoadjuvant chemotherapy | |
| Yes | 1255 (11) |
| No | 10352 (89) |
| Radiotherapy | |
| Yes | 4285 (37) |
| No | 7322 (63) |
| Type of surgery | |
| Partial gastrectomy | 8320 (72) |
| Total gastrectomy | 3287 (28) |
| Number of retrieved lymphnodes | |
| < 16 LNs (ILA) | 5806 (50) |
| 16-29 LNs (ALA) | 4085 (35) |
| ≥ 30 LNs (OLA) | 1716 (15) |
Based on the overall number of retrieved LNs, patients were divided into three groups, ILA (< 16 LNs = 5806, 50%), ALA (16–29 LNs = 4085, 35%), and OLA, 30 + LNs = 1716, 15%). Clinicopathologic characteristics are reported in Table 2. In the last study period, a distribution trend for the total sample population was identified and determined to be in favor of the OLA group (30% vs 19% in the ILA and ALA groups, respectively). The median age was higher in the ILA group (71 years) than in the other two groups, 68 years in the ALA group and 65 years in the OLA group. No differences were found in the T1, T2, and T4b stage rates, and only slight differences were found in the T3 (50% vs 47% vs 46%) and T4a (29% vs 32% vs 33%) stage rates (P < 0.001).
| Characteristic | ILA | ALA | OLA | P value |
| n = 5806 | n = 4085 | n = 1716 | ||
| Year of diagnosis | < 0.001 | |||
| 2004-2006 | 1861 (32) | 947 (23) | 334 (20) | |
| 2007-2009 | 1563 (27) | 1071 (26) | 394 (23) | |
| 2010-2012 | 1299 (22) | 1080 (27) | 471 (27) | |
| 2013-2015 | 1083 (19) | 987 (19) | 517 (30) | |
| Age at diagnosis (yr) | < 0.001 | |||
| Median (range) | 71 (12-99) | 68 (14-98) | 65 (18-93) | |
| Sex | 0.218 | |||
| Male | 3365 (58) | 2318 857) | 1014 (59) | |
| Female | 2441 (42) | 1767 (43) | 702 (41) | |
| Race | < 0.001 | |||
| White | 3695 (64) | 2377 (58) | 973 (57) | |
| Black | 1061 (18) | 747 (18) | 268 (15) | |
| Asian/Pacific | 1050 (18) | 961 (24) | 475 (28) | |
| Marital status | < 0.001 | |||
| Single/divorced | 1261 (22) | 900 (22) | 378 (22) | |
| Married | 3282 (57) | 2439 (60) | 1084 (63) | |
| Widowed | 1059 (18) | 595 (15) | 183 (11) | |
| NA | 204 (3) | 151 (4) | 71 (4) | |
| Insurance status | < 0.001 | |||
| Insured | 3721 (64) | 2998 (73) | 68 (4) | |
| Uninsured | 224 (4) | 140 (4) | 1314 (77) | |
| NA | 1861 (32) | 924 (23) | 334 (20) | |
| Primary site | < 0.001 | |||
| Fundus/body | 835 (14) | 696 (17) | 335 (19) | |
| Antrum/pylorus | 2562 (44) | 1511 (37) | 553 (32) | |
| Overlapping lesion | 549 (10) | 515 (13) | 235 (14) | |
| Stomach, NOS | 1860 (32) | 1363 (33) | 593 (35) | |
| Tumor size (cm) | < 0.001 | |||
| ≤ 5 | 2977 (51) | 1808 (44) | 646 (38) | |
| 5.1-10 | 1906 (33) | 1573 (39) | 724 (42) | |
| ≥ 10.1 | 438 (8) | 448 (11) | 249 (15) | |
| NA | 485 (8) | 256 (6) | 97 (6) | |
| Hystology | < 0.001 | |||
| ADC, NOS | 2426 (42) | 1517 (37) | 538 (31) | |
| Signet ring cell carcinoma | 1248 (22) | 997 (24) | 481 (28) | |
| ADC, instestinal type | 928 (16) | 702 (17) | 313 (18) | |
| Carcinoma, diffuse type | 422 (7) | 360 (9) | 176 (10) | |
| ADC with mixed subtypes | 170 (3) | 166 (4) | 88 (5) | |
| Other | 612 (10) | 343 (9) | 120 (7) | |
| Grade | < 0.001 | |||
| Well/moderately differentiated | 1493 (26) | 862 (21) | 319 (19) | |
| Poorly/undifferentiated | 4072 (70) | 3106 (76) | 1346 (78) | |
| NA | 241 (4) | 117 (3) | 51 (3) | |
| T stage, 8th ed. | < 0.001 | |||
| T1 | 111 (2) | 131 (3) | 48 (3) | |
| T2 | 490 (8) | 321 (8) | 132 (8) | |
| T3 | 2877 (50) | 1897 (47) | 795 (46) | |
| T4a | 1658 (29) | 1321 (32) | 572 (33) | |
| T4b | 670 (11) | 415 (10) | 169 (10) | |
| N stage, 8th ed. | < 0.001 | |||
| N0 | 1810 (31) | 794 (19) | 259 (15) | |
| N1 | 1528 (26) | 671 (16) | 223 (13) | |
| N2 | 1517 (26) | 900 (22) | 340 (20) | |
| N3a | 951 (16) | 1167 (29) | 380 (22) | |
| N3b | 0 | 553 (14) | 514 (30) | |
| Stage, 8th ed. | < 0.001 | |||
| IIA | 1557 (27) | 775 (19) | 253 (15) | |
| IIB | 1293 (22) | 621 (15) | 215 (13) | |
| IIIA | 1754 (30) | 942 (23) | 353 (20) | |
| IIIB | 1055 (18) | 1091 (27) | 365 (21) | |
| IIIC | 147 (3) | 656 (16) | 530 (31) | |
| Chemotherapy | < 0.001 | |||
| Yes | 2814 (48) | 2490 (61) | 1169 (68) | |
| No | 2992 (52) | 1595 (39) | 547 (32) | |
| Neoadjuvant chemotherapy | < 0.001 | |||
| Yes | 408 (7) | 536 (13) | 311 (18) | |
| No | 5398 (93) | 3549 (87) | 1405 (82) | |
| Radiotherapy | < 0.001 | |||
| Yes | 1993 (34) | 1630 (40) | 662 (39) | |
| No | 3813 (66) | 2455 (60) | 1054 (61) | |
| Type of surgery | < 0.001 | |||
| Partial gastrectomy | 4623 (80) | 2742 (67) | 955 (56) | |
| Total gastrectomy | 1183 (20) | 1343 (33) | 761 (44) |
As expected, significant differences were identified for the N stage variable. In particular, most patients in the ILA group were classified in the N0 and N1 stages (31% and 26%, respectively). However, that was not the case in the ALA (19% and 16%, respectively) and OLA (15% and 13%, respectively) groups. Regarding staging, no patients in the ILA group were staged as N3b, and 16% were staged as N3a. The findings affected the attribution of the condensed stage. Most patients in the ILA group were in stage II or IIIA, while only 18% and 3% were in stages IIIB and IIIC, respectively. In contrast, 27% and 16% of the patients in the ALA group were in these stages, respectively, and 21% and 31% of the patients in the OLA group were in these stages, respectively (P < 0.001). Differences were also seen in the treatments administered; most patients in the ILA group received a partial gastrectomy, and few received neoadjuvant therapy (7%).
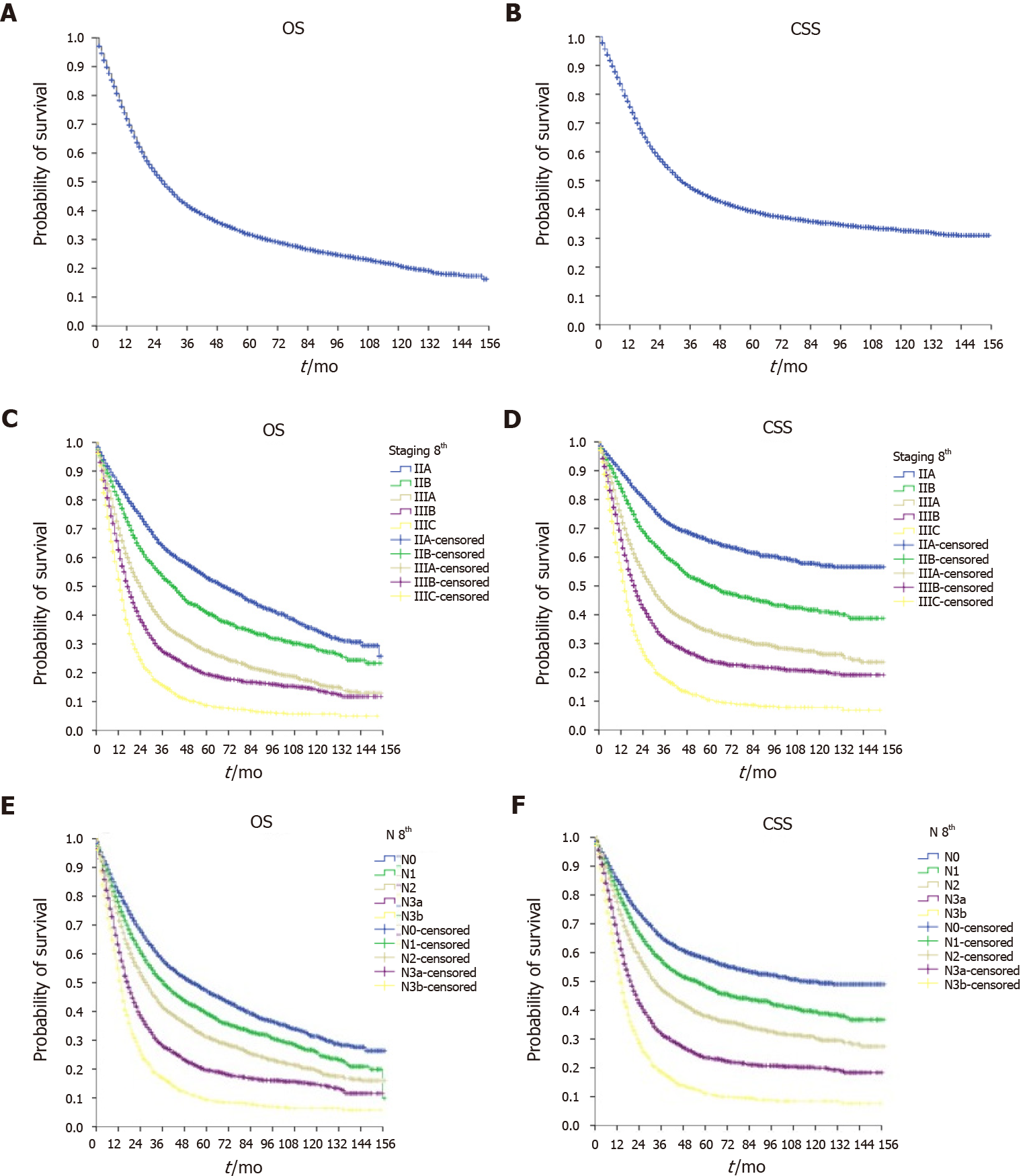
Figure 1 shows the survival curves of the overall sample. The median OS was 27 mo (95%CI: 26.1–27.9), and the median CSS was 33 mo (95%CI: 31.5–34.5). OS in each disease stage (Figure 1) was stage IIA = 69 mo (95%CI: 63.1–74.9), stage IIB = 42 mo (95%CI: 38.6–45.4), stage IIIA = 24 mo (95%CI: 22.5–25.5), stage IIIB = 17 mo (95%CI: 16.1–17.9), and stage IIIC = 13 mo (95%CI: 12.2–13.8). OS in each N stage was N0 = 51 mo (95%CI: 46.2–55.8), N1 = 36 mo (95%CI: 33.0–39.0), N2 = 27 mo (95%CI: 25.2–28.8), N3a = 17 mo (95%CI: 16.0–18.0), and N3b = 14 mo (95%CI: 13.1–14.9).
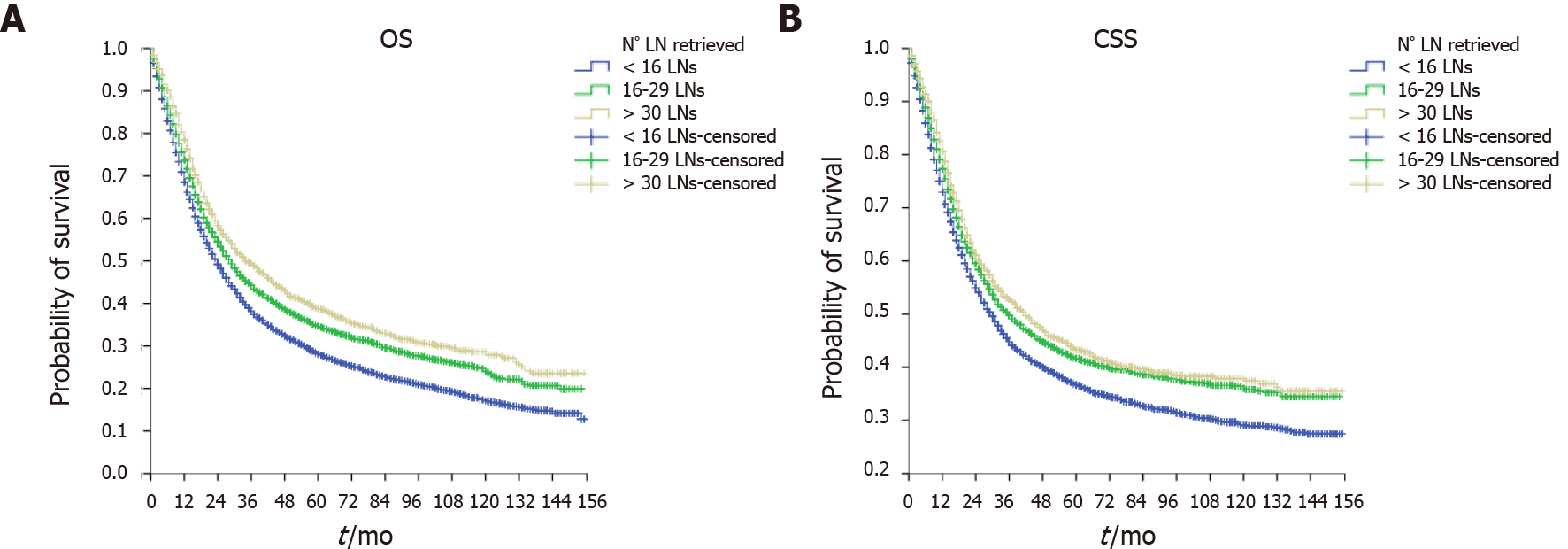
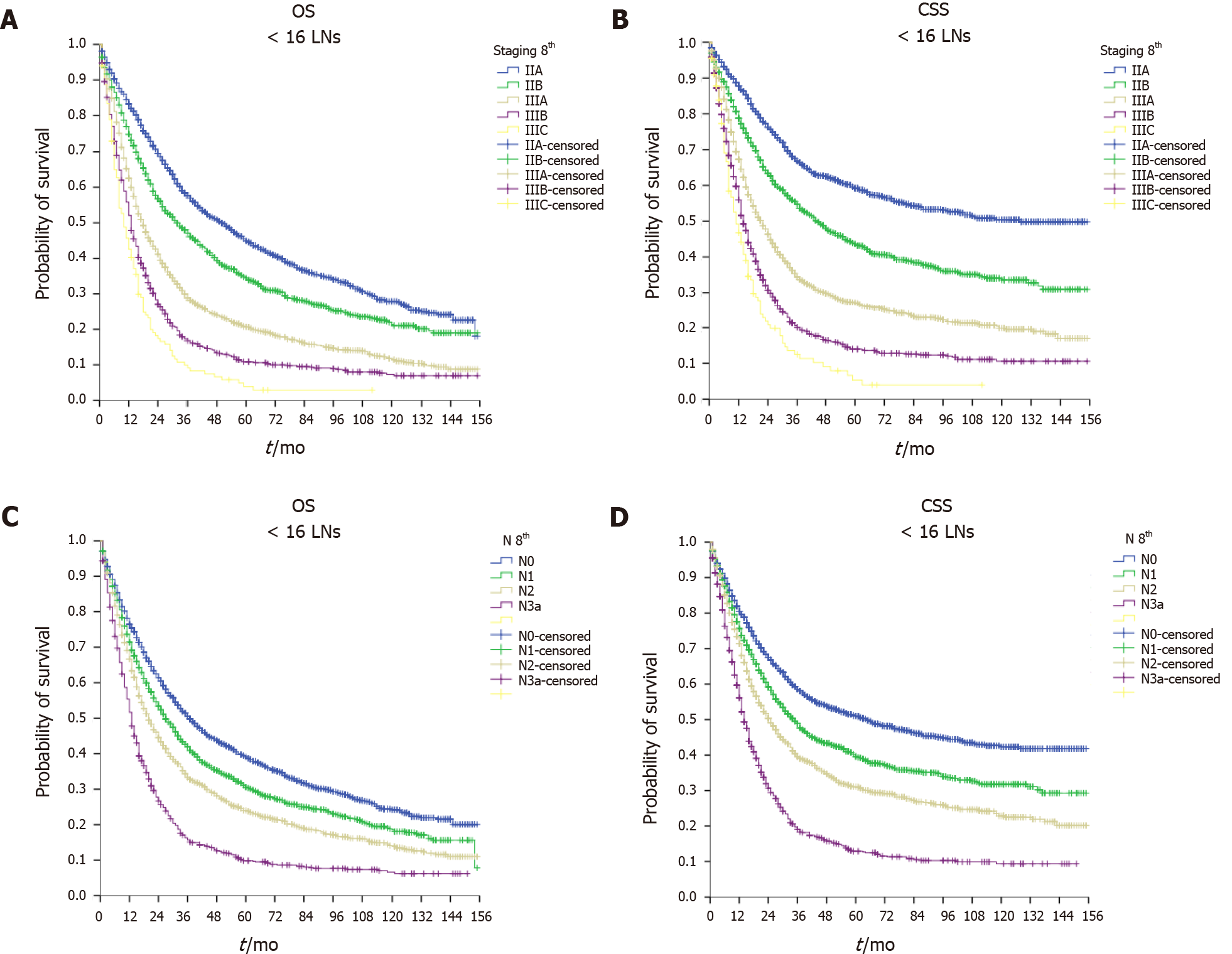
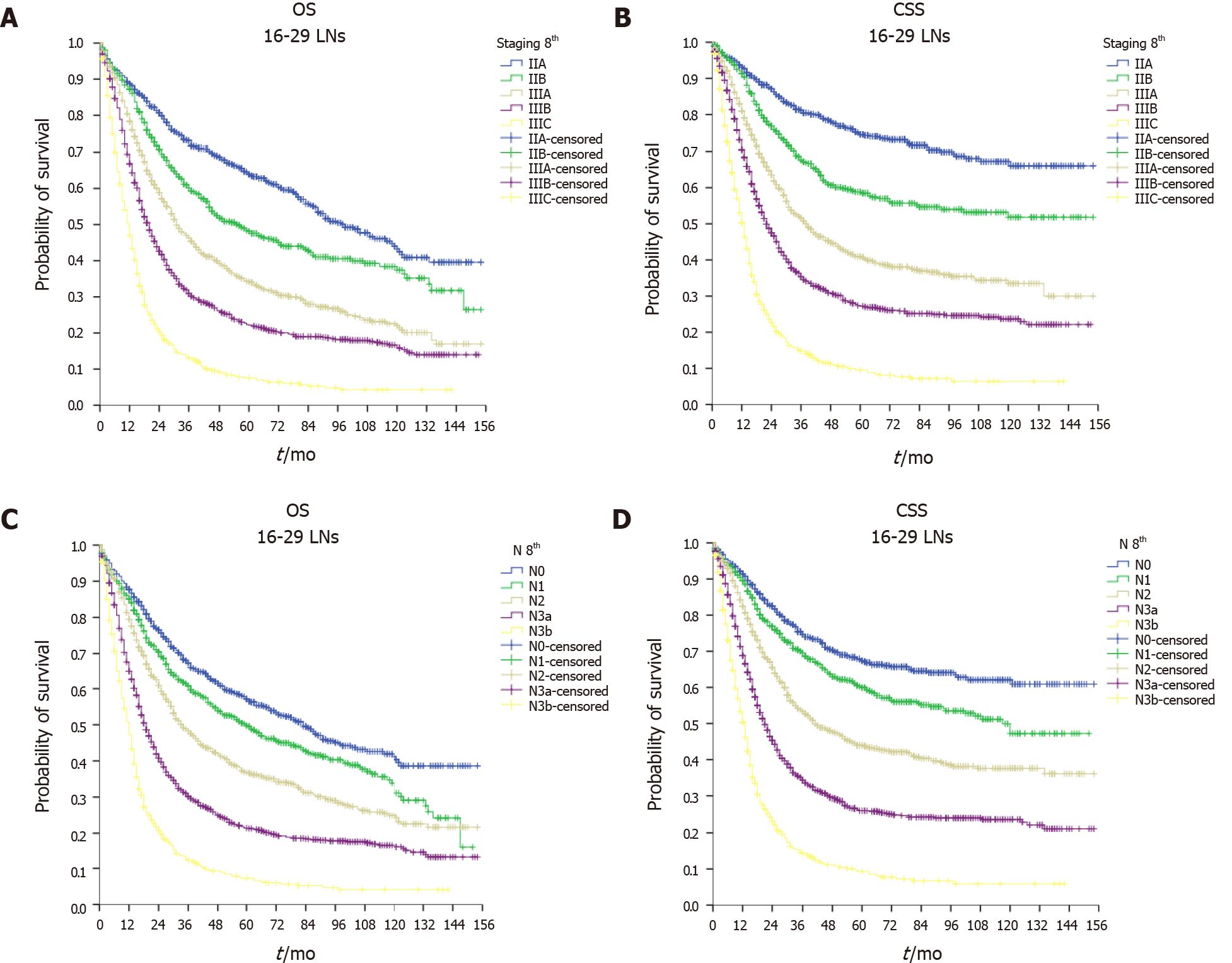
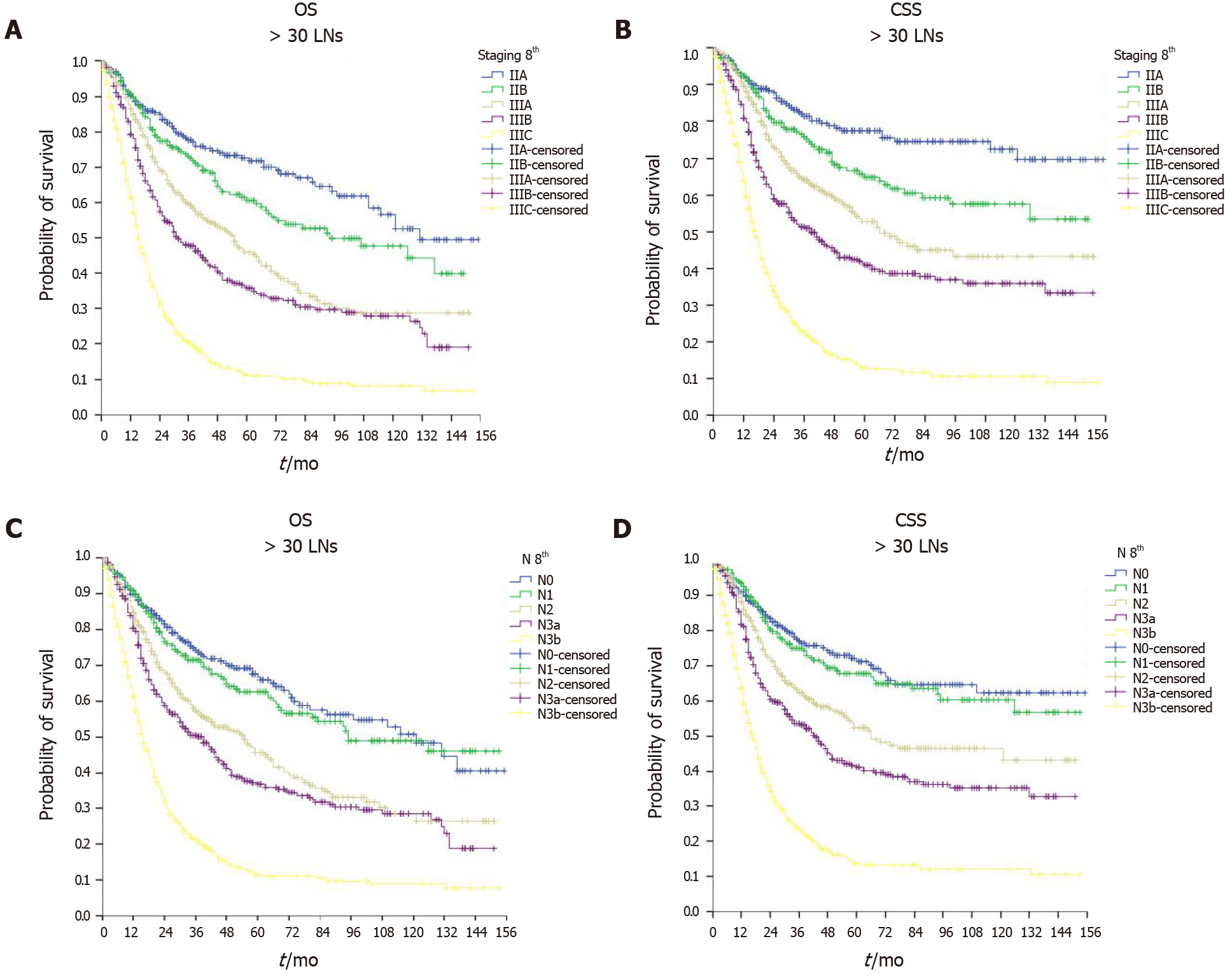
As shown in Figure 2, the ILA group had the worst median OS (24, 95%CI: 22.9–25.1 mo) and median CSS (30, 95%CI: 28.4–31.6 mo) compared with the ALA group (median OS = 29, 95%CI: 27.2–30.8 mo and median CSS = 36, 95%CI: 32.9–39.1 mo, P < 0.001) and the OLA group (median OS = 34, 95%CI: 30.0–38.0 mo and median CSS = 42, 95%CI: 35.9–48.1 mo, P < 0.001). Of note, when comparing the ALA and OLA groups, the difference was significant for OS (P < 0.001) but not for CSS (P < 0.078). Figures 3-5 show OS and CSS by the stage of disease and are arranged by study group. The actual survival curves for ILA group within stage IIA revealed significantly worse outcomes for ILA and ALA compared with OLA. The three substages of stage III in the ILA group did not have the expected distribution, as found in the ALA and OLA groups.
The findings were confirmed after evaluating OS and CSS by the N stage (Figures 3-5). The OLA group had the best discrimination profile among the survival curves (Figure 5). In contrast, the ILA group did not have a survival curve for the N3b substage, and the difference between the N0 and N+ patients in that group was not as consistent as in the other two groups (Figure 3). Of note, the ALA group had an adequate patient distribution (Figure 4). However, the mean difference between the N3a and N3b substages was only 7 mo in the ALA group compared with 23 mo in the OLA group.
As shown in Table 3, the Cox regression model univariate analysis clearly showed that the ALA and OLA groups had better OS (ALA HR = 0.84, 95%CI: 0.79–0.88, P < 0.001 and OLA HR = 0.73, 95%CI: 0.68–0.79, P < 0.001) and CSS (ALA HR = 0.85, 95%CI: 0.81–0.90, P < 0.001 and OLA HR = 0.80, 95%CI: 0.74–0.86, P < 0.001) than the ILA group. Other prognostic factors related to OS and CSS included age, race, site of tumor, histology, grade, T stage, N stage, stage of disease, type of gastrectomy, chemotherapy, neoadjuvant therapy, and radiotherapy. After adjusting for other variables in the multivariate Cox analysis (Table 3), the ALA and OLA groups still had significantly better OS (ALA HR = 0.68, 95%CI: 0.64–0.71, P < 0.001 and OLA HR = 0.48, 95%CI: 0.44–0.52, P < 0.001) and CSS (ALA HR = 0.64, 95%CI: 0.60–0.68, P < 0.001 and OLA HR = 0.47, 95%CI: 0.43–0.51, P < 0.001) than the ILA group. Age, race, histology, T stage, N stage, stage of disease, type of gastrectomy, chemotherapy, and radiotherapy were confirmed as significant prognostic factors in the multivariate model for both OS and CSS.
| Overall survival | Cancer-specific survival | |||||||
| Variable | Univariable | Multivariable1 | Univariable | Multivariable1 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | ||||||||
| Male | Reference | Reference | ||||||
| Female | 1.03 (0.99-1.08) | 0.171 | 1.07 (1.02-1.13) | 0.006 | ||||
| Age, yr | ||||||||
| < 70 | Reference | Reference | Reference | Reference | ||||
| ≥ 70 | 1.57 (1.50-1.64) | < 0.001 | 1.51 (1.43-1.59) | < 0.001 | 1.32 (1.26-1.39) | < 0.001 | 1.33 (1.26-1.41) | < 0.001 |
| Race | ||||||||
| White | Reference | Reference | Reference | |||||
| Black | 0.97 (0.91-1.03) | 0.283 | 1.11 (1.05-1.18) | 0.001 | 0.97 (0.90-1.03) | 0.317 | 1.10 (1.03-1.18) | 0.005 |
| Asian/Pacific | 0.80 (0.75-0.85) | < 0.001 | 0.82 (0.77-0.87) | < 0.001 | 0.82 (0.77-0.87) | < 0.001 | 0.83 (0.78-0.89) | < 0.001 |
| Insurance status | ||||||||
| NA | Reference | Reference | ||||||
| Insured | 0.92 (0.88-0.97) | 0.001 | 1.05 (0.91-1.21) | 0.550 | ||||
| Uninsured | 0.96 (0.84-1.09) | 0.501 | 0.95 (0.83-1.09) | 0.459 | ||||
| Site of tumor | ||||||||
| Fundus-body | Reference | Reference | ||||||
| Antrum-pylorus | 1.08 (1.01-1.15) | 0.030 | 1.08 (0.99-1.16) | 0.055 | ||||
| Overlapping lesion of the stomach | 1.30 (1.19-1.42) | < 0.001 | 1.35 (1.23-1.49) | < 0.001 | ||||
| Stomach, NOS | 1.05 (0.98-1.13) | 0.167 | 1.04 (0.96-1.12) | 0.373 | ||||
| Histology | ||||||||
| ADC, NOS | Reference | Reference | Reference | Reference | ||||
| Signet ring cell carcinoma | 1.16 (1.09-1.23) | < 0.001 | 1.14 (1.07-1.21) | < 0.001 | 1.28 (1.20-1.37) | < 0.001 | 1.16 (1.09-1.25) | < 0.001 |
| ADC, instestinal type | 0.87 (0.81-0.93) | < 0.001 | 0.98 (0.91-1.05) | 0.547 | 0.79 (0.73-0.86) | < 0.001 | 0.93 (0.86-1.01) | 0.086 |
| Carcinoma, diffuse type | 1.15 (1.06-1.26) | 0.001 | 1.12 (1.03-1.23) | 0.011 | 1.22 (1.11-1.34) | < 0.001 | 1.11 (1.00-1.22) | 0.045 |
| ADC with mixed subtypes | 1.06 (0.94-1.20) | 0.349 | 1.09 (0.96-1.24) | 0.169 | 1.12 (0.98-1.28) | 0.103 | 1.08 (0.94-1.24) | 0.281 |
| Other | 0.92 (0.85-1.00) | 0.062 | 0.97 (0.89-1.06) | 0.543 | 0.95 (0.86-1.04) | < 0.001 | 1.01 (0.92-1.11) | 0.862 |
| T stage, 8th ed. | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 1.13 (0.93-1.36) | 0.217 | 1.08 (0.88-1.32) | 0.455 | 1.12 (0.89-1.41) | 0.322 | 1.01 (0.79-1.28) | 0.963 |
| T3 | 1.52 (1.28-1.80) | < 0.001 | 1.37 (1.09-1.73) | 0.008 | 1.69 (1.37-2.07) | < 0.001 | 1.34 (1.02-1.76) | 0.033 |
| T4a | 2.34 (1.98-2.78) | < 0.001 | 1.72 (1.33-2.22) | < 0.001 | 2.89 (2.35-3.55) | < 0.001 | 1.70 (1.27-2.28) | < 0.001 |
| T4b | 2.83 (2.37-3.38) | < 0.001 | 2.08 (1.48-2.93) | < 0.001 | 3.54 (2.86-4.39) | < 0.001 | 2.06 (1.40-3.01) | < 0.001 |
| N stage, 8th ed. | ||||||||
| N0 | Reference | Reference | Reference | Reference | ||||
| N1 | 1.22 (1.14-1.31) | < 0.001 | 1.30 (1.16-1.46) | < 0.001 | 1.31 (1.21-1.43) | < 0.001 | 1.26 (1.10-1.43) | 0.001 |
| N2 | 1.49 (1.39-1.59) | < 0.001 | 1.49 (1.28-1.73) | < 0.001 | 1.70 (1.57-1.84) | < 0.001 | 1.44 (1.22-1.70) | < 0.001 |
| N3a | 2.05 (1.92-2.19) | < 0.001 | 2.10 (1.61-2.74) | < 0.001 | 2.51 (2.32-2.71) | < 0.001 | 2.06 (1.55-2.76) | < 0.001 |
| N3b | 2.90 (2.66-3.15) | < 0.001 | 3.22 (2.17-4.80) | < 0.001 | 3.68 (3.36-4.03) | < 0.001 | 3.29 (2.14-5.05) | < 0.001 |
| Stage, 8th ed. | ||||||||
| IIA | Reference | Reference | Reference | Reference | ||||
| IIB | 1.34 (1.24-1.45) | < 0.001 | 1.17 (1.05-1.31) | 0.005 | 1.62 (1.47-1.78) | < 0.001 | 1.38 (1.22-1.57) | < 0.001 |
| IIIA | 1.96 (1.83-2.10) | < 0.001 | 1.43 (1.21-1.70) | < 0.001 | 2.53 (2.33-2.75) | < 0.001 | 1.78 (1.48-2.15) | < 0.001 |
| IIIB | 2.47 (2.30-2.66) | < 0.001 | 1.44 (1.08-1.92) | 0.012 | 3.38 (3.11-3.68) | < 0.001 | 1.87 (1.37-2.55) | < 0.001 |
| IIIC | 3.68 (3.39-3.99) | < 0.001 | 1.59 (1.04-2.44) | 0.034 | 5.19 (4.73-5.70) | < 0.001 | 2.05 (1.29-3.26) | 0.003 |
| Grade | ||||||||
| Well/moderately differentiated | Reference | Reference | Reference | |||||
| Poorly/undifferentiated | 1.35 (1.28-1.43) | < 0.001 | 1.19 (1.12-1.26) | < 0.001 | 1.59 (1.49-1.70) | < 0.001 | ||
| Type of surgery | ||||||||
| Partial gastrectomy | Reference | Reference | Reference | Reference | ||||
| Total gastrectomy | 1.25 (1.19-1.32) | < 0.001 | 1.23 (1.17-1.30) | < 0.001 | 1.32 (1.25-1.40) | < 0.001 | 1.22 (1.15-1.29) | < 0.001 |
| Chemotherapy | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.62 (0.59-0.64) | < 0.001 | 0.68 (0.64-0.72) | < 0.001 | 0.71 (0.67-0.74) | < 0.001 | 0.73 (0.68-0.78) | < 0.001 |
| Neoadjuvant chemotherapy | ||||||||
| No | Reference | Reference | ||||||
| Yes | 0.76 (0.71-0.83) | < 0.001 | 0.82 (0.75-0.89) | < 0.001 | ||||
| Radiotherapy | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.65 (0.62-0.68) | < 0.001 | 0.77 (0.73-0.82) | < 0.001 | 0.70 (0.66-0.73) | < 0.001 | 0.75 (0.71-0.80) | < 0.001 |
| Number of retrieved lymph nodes | ||||||||
| ILA | Reference | Reference | Reference | Reference | ||||
| ALA | 0.84 (0.79-0.88) | < 0.001 | 0.68 (0.64-0.71) | < 0.001 | 0.85 (0.81-0.90) | < 0.001 | 0.64 (0.60-0.68) | < 0.001 |
| OLA | 0.73 (0.68-0.79) | < 0.001 | 0.48 (0.44-0.52) | < 0.001 | 0.80 (0.74-0.86) | < 0.001 | 0.47 (0.43-0.51) | < 0.001 |
ALA and OLA were compared in in 3428 PSM 1:1 patient pairs (n = 1,714 per group) out of a total of 5801 patients. The L1 test measure was larger in the unmatched sample (0.989) than in the matched sample (0.964), indicating that the two groups were well-balanced across all considered variables. Successful matching (Table 4) was confirmed during the analysis because there were no differences between the two groups in the year of diagnosis, patient characteristics (age, sex, race, marital status, and insurance status), and tumor characteristics (site, grade, T stage, and N stage). As shown in Table 5, the OLA group had better OS and CSS than the ALA group (OLA median OS = 34 mo vs ALA median = 26 mo, P < 0.001, respectively; CSS: OLA median = 42 mo vs ALA median = 31 mo, P < 0.001, respectively). The Cox analysis conducted after PSM (Table 6) confirmed that OLA was associated with significantly improved OS (univariable HR = 0.81, 95%CI: 0.75–0.89, P < 0.001 and multivariable HR = 0.71, 95%CI: 0.65–0.78, P < 0.001) and CSS (univariable HR = 0.84, 95%CI: 0.76–0.92, P < 0.001 and multivariable HR = 0.74, 95%CI: 0.67–0.82, P < 0.001).
| Characteristic | ALA, n = 1714 | OLA, n = 1714 | P value |
| Year of diagnosis | 0.978 | ||
| 2004-2006 | 339 (20) | 334 (19) | |
| 2007-2009 | 385 (23) | 394 (23) | |
| 2010-2012 | 468 (27) | 471 (28) | |
| 2013-2015 | 522 (30) | 515 (30) | |
| Age at diagnosis (yr) | 0.861 | ||
| Median (range) | 66 (14-98) | 66 (18-93) | |
| Sex | 0.945 | ||
| Male | 1011 (59) | 1013 (59) | |
| Female | 703 (41) | 701 (41) | |
| Race | 0.181 | ||
| White | 972 (57) | 973 (57) | |
| Black | 303 (18) | 268 (16) | |
| Asian/Pacific | 439 (25) | 473 (28) | |
| Marital status | 0.234 | ||
| Single/divorced | 389 (23) | 378 (22) | |
| Married | 1035 (60) | 1082 (63) | |
| Widowed | 218 (13) | 183 (71) | |
| NA | 72 (4) | 71 (4) | |
| Insurance status | 0.958 | ||
| Insured | 1305 (76) | 1312 (77) | |
| Uninsured | 339 (20) | 334 (19) | |
| NA | 70 (4) | 68 (4) | |
| Primary site | 0.926 | ||
| Fundus/body | 327 (19) | 333 (19) | |
| Antrum/pylorus | 571 (33) | 553 (32) | |
| Overlapping lesion | 235 (14) | 235 (14) | |
| Stomach, NOS | 581 (34) | 593 (35) | |
| Tumor size (cm) | 0.016 | ||
| ≤ 5 | 737 (43) | 645 (38) | |
| 5.1-10 | 663 (39) | 724 (42) | |
| ≥ 10.1 | 222 (13) | 248 (15) | |
| NA | 92 (5) | 97 (6) | |
| Hystology | 0.046 | ||
| ADC, NOS | 557 (32) | 538 (31) | |
| Signet ring cell carcinoma | 492 (29) | 480 (28) | |
| ADC, Instestinal type | 289 (17) | 313 (18) | |
| Carcinoma, diffuse type | 177 (10) | 175 (10) | |
| ADC with mixed subtypes | 69 (4) | 88 (5) | |
| Other | 130 (8) | 110 (7) | |
| Grade | 0.892 | ||
| Well/moderately differentiated | 310 (18) | 319 (19) | |
| Poorly/undifferentiated | 1351 (79) | 1345 (79) | |
| NA | 53 (3) | 50 (3) | |
| T stage, 8th ed. | 0.553 | ||
| T1 | 59 (3) | 48 (3) | |
| T2 | 137 (8) | 132 (8) | |
| T3 | 769 (45) | 793 (46) | |
| T4a | 597 (35) | 572 (33) | |
| T4b | 152 (9) | 169 (10) | |
| N stage, 8th ed. | 0.659 | ||
| N0 | 250 (15) | 259 (15) | |
| N1 | 252 (15) | 223 (13) | |
| N2 | 342 (20) | 340 (20) | |
| N3a | 380 (22) | 380 (22) | |
| N3b | 490 (29) | 512 (30) | |
| Stage, 8th ed. | 0.808 | ||
| IIA | 263 (15) | 253 (15) | |
| IIB | 226 (13) | 215 (13) | |
| IIIA | 351 (21) | 353 (21) | |
| IIIB | 377 (22) | 365 (21) | |
| IIIC | 497 (29) | 528 (30) | |
| Chemotherapy | 0.025 | ||
| Yes | 1105 (65) | 1167 (32) | |
| No | 609 (35) | 1167 (68) | |
| Neoadjuvant chemotherapy | < 0.001 | ||
| Yes | 225 (13) | 310 (18) | |
| No | 1499 (87) | 1404 (82) | |
| Radiotherapy | 0.051 | ||
| Yes | 718 (42) | 662 (39) | |
| No | 996 (58) | 1052 (61) | |
| Type of surgery | < 0.001 | ||
| Partial gastrectomy | 1099 (64) | 955 (56) | |
| Total gastrectomy | 615 (36) | 759 (44) |
| Median OS, mo(95%CI) | P value | Median CSS, mo(95%CI) | P value | |
| ALA | 26 (23.5-28.4) | < 0.001 | 31 (27.3-34.7) | < 0.001 |
| OLA | 34 (30.0-38.0) | 42 (35.9-48.1) |
| Overall survival | Cancer-specific survival | |||||||
| Variable | Univariable | Multivariable1 | Univariable | Multivariable1 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | ||||||||
| Male | Reference | Reference | ||||||
| Female | 0.98 (0.90-1.07) | 0.662 | 1.03 (0.94-1.14) | 0.517 | ||||
| Age | ||||||||
| < 70 | Reference | Reference | ||||||
| ≥ 70 | 1.56 (1.43-1.71) | < 0.001 | 1.35 (1.23-1.49) | < 0.001 | ||||
| Race | ||||||||
| White | Reference | Reference | Reference | |||||
| Black | 0.96 (0.85-1.08) | 0.528 | 1.10 (0.97-1.24) | 0.147 | 0.94 (0.83-1.08) | 0.383 | 1.08 (0.94-1.23) | 0.274 |
| Asian/pacific | 0.80 (0.72-0.89) | < 0.001 | 0.84 (0.75-0.93) | 0.001 | 0.81 (0.72-0.90) | < 0.001 | 0.87 (0.77-0.97) | 0.014 |
| Insurance status | ||||||||
| NA | Reference | Reference | ||||||
| Insured | 0.87 (0.79-0.97) | 0.009 | 0.87 (0.78-0.97) | 0.013 | ||||
| Uninsured | 0.99 (0.78-1.27) | 0.972 | 0.96 (0.74-1.25) | 0.754 | ||||
| Site of tumor | ||||||||
| Fundus-body | Reference | Reference | ||||||
| Antrum-pylorus | 1.07 (0.95-1.22) | 0.275 | 1.11 (0.97-1.27) | 0.140 | ||||
| Overlapping lesion of the stomach | 1.33 (1.15-1.55) | < 0.001 | 1.39 (1.18-1.64) | < 0.001 | ||||
| Stomach, NOS | 1.04 (0.92-1.18) | 0.516 | 1.03 (0.90-1.19) | 0.631 | ||||
| Histology | ||||||||
| ADC, NOS | Reference | Reference | Reference | |||||
| Signet ring cell carcinoma | 1.26 (1.13-1.41) | < 0.001 | 1.37 (1.22-1.54) | < 0.001 | 1.22 (1.08-1.38) | 0.001 | ||
| ADC, instestinal type | 0.89 (0.79-1.03) | 0.114 | 0.81 (0.70-0.95) | 0.008 | 0.91 (0.78-1.06) | 0.238 | ||
| Carcinoma, diffuse type | 1.23(1.06-1.44) | 0.008 | 1.29 (1.09-1.52) | 0.003 | 1.18 (0.99-1.40) | 0.054 | ||
| ADC with mixed subtypes | 1.15 (0.93-1.43) | 0.193 | 1.28 (1.02-1.60) | 0.032 | 1.13 (0.89-1.42) | 0.318 | ||
| Other | 0.93 (0.77-1.13) | 0.472 | 0.99 (0.81-1.21) | 0.913 | 1.15 (0.93-1.41) | 0.199 | ||
| T stage, 8th ed | ||||||||
| T1 | Reference | Reference | ||||||
| T2 | 1.18 (0.83-1.67) | 0.356 | 1.43 (0.91-2.24) | 0.121 | ||||
| T3 | 1.85 (1.37-2.51) | < 0.001 | 2.60 (1.74-3.87) | < 0.001 | ||||
| T4a | 2.84 (2.10-3.86) | < 0.001 | 4.28 (2.87-6.39) | < 0.001 | ||||
| T4b | 3.44 (2.49-4.75) | < 0.001 | 5.13 (3.39-7.77) | < 0.001 | ||||
| N stage, 8th ed | ||||||||
| N0 | Reference | Reference | ||||||
| N1 | 1.09 (0.89-1.34) | 0.380 | 1.08 (0.86-1.36) | 0.518 | ||||
| N2 | 1.56 (1.31-1.87) | < 0.001 | 1.63 (1.33-1.99) | < 0.001 | ||||
| N3a | 2.28 (1.93-2.69) | < 0.001 | 2.61 (2.16-3.16) | < 0.001 | ||||
| N3b | 4.58 (3.91-5.37) | < 0.001 | 5.50 (4.59-6.58) | < 0.001 | ||||
| Stage, 8th ed | ||||||||
| IIA | Reference | Reference | Reference | Reference | ||||
| IIB | 1.38 (1.12-1.70) | 0.003 | 1.47 (1.18-1.81) | < 0.001 | 1.70 (1.33-2.18) | < 0.001 | 1.77 (1.38-2.29) | < 0.001 |
| IIIA | 1.96 (1.63-2.35) | < 0.001 | 2.21 (1.83-2.66) | < 0.001 | 2.39 (1.92-2.98) | < 0.001 | 2.66 (2.12-3.32) | < 0.001 |
| IIIB | 2.85 (2.39-3.40) | < 0.001 | 3.31 (2.76-3.96) | < 0.001 | 3.75 (3.04-6.64) | < 0.001 | 4.22 (3.40-5.23) | < 0.001 |
| IIIC | 5.73 (4.85-6.77) | < 0.001 | 6.32 (5.31-7.52) | < 0.001 | 8.06 (6.58-9.88) | < 0.001 | 8.58 (6.97-10.57) | < 0.001 |
| Grade | ||||||||
| Well/moderately differentiated | Reference | Reference | Reference | |||||
| Poorly/undifferentiated | 1.52 (1.35-1.72) | < 0.001 | 1.19 (1.05-1.35) | 0.007 | 1.70 (1.49-1.96) | < 0.001 | ||
| Type of surgery | ||||||||
| Partial gastrectomy | Reference | Reference | Reference | Reference | ||||
| Total gastrectomy | 1.40 (1.29-1.53) | < 0.001 | 1.28 (1.17-1.40) | < 0.001 | 1.43 (1.31-1.58) | < 0.001 | 1.24 (1.13-1.37) | < 0.001 |
| Chemotherapy | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.56 (0.51-0.61) | < 0.001 | 0.63 (0.56-0.70) | < 0.001 | 0.61 (0.55-0.67) | < 0.001 | 0.66 (0.89-0.74) | < 0.001 |
| Neoadjuvant chemotherapy | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.71 (0.62-0.81) | < 0.001 | 1.48 (1.35-1.63) | < 0.001 | 0.76 (0.66-0.87) | < 0.001 | 1.33 (1.20-1.48) | < 0.001 |
| Radiotherapy | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.64 (0.59-0.70) | < 0.001 | 0.77 (0.69-0.86) | < 0.001 | 0.67 (0.61-0.73) | < 0.001 | 0.76 (0.68-0.85) | < 0.001 |
| Number of retrieved lymph nodes | ||||||||
| ALA | Reference | Reference | Reference | Reference | ||||
| OLA | 0.81 (0.75-0.89) | < 0.001 | 0.71 (0.65-0.78) | < 0.001 | 0.84 (0.76-0.92) | < 0.001 | 0.74 (0.67-0.82) | < 0.001 |
In this study evaluating the impact of nodal assessment in patients who underwent resection for locally advanced gastric cancer in the United States between 2004 and 2015, we found significant discrepancies between expected and actual survival differences in stage II and III gastric cancer patients who had a minimum of 30 LNs assessed compared with those who had <16 or 16-29 LNs. The adverse impact of insufficient nodal analysis was found to be significant in both II and III stage disease. The results suggest that proper assessment of nodal status requires at least 16 LNs, and optimally 30 LNs. Optimization of gastric cancer care across Eastern and Western countries continues to make substantial progress. The updated eighth edition of the TNM-staging system incorporated survival data from additional Eastern nations to provide a more accurate prognosis of all patients diagnosed with gastric cancer worldwide. One of the most important unresolved issues is understanding the true impact of surgical resection and extent of nodal assessment[15,16].
Gastrectomy, including LN dissection, has a major role in optimizing the treatment strategy for locally advanced gastric cancer. Improper LN dissection not only increases the risk of residual tumor and disease recurrence, but also compromises the patient’s stage attribution[17] and more important may affect the choice of adjuvant therapies. The AJCC cancer staging system has been developed over the years to improve pathology assessment, facilitate comparisons, and increase compliance among centers. Nodal status is a relevant prognostic factor, and assessing an adequate number of LNs enables proper staging and, consequently leads to optimal treatment management[18,19].
Significant variation has been seen in different series across the East and West[7]. Asian countries generally have a median number of harvested LNs that is three or four times higher than those in other regions. The variation affects staging accuracy and long-term patient survival. Meanwhile, insufficient LN assessment is often apparent in the current literature[20]. For example, in his review of 15 studies, which included 27,942 patients, Khanjani et al[5] showed that only 52.2% of the patients received an adequate nodal evaluation, given the AJCC’s current recommendation to assess at least 16 LNs[9].
In this study, we focused on patients whose staging was expected to have the greatest impact on their treatment pathway, namely patients with potentially curable advanced disease that was formally classified as stage II or III. This issue is particularly relevant in Western countries, where most patients are belatedly diagnosed with gastric cancer because of a lack of screening programs and where gastric cancer treatments vary greatly by center. As the SEER database is one of the largest cancer databases in the West, it is particularly representative of the current management of patients with gastric cancer. We selected 11,607 patients who had undergone radical gastrectomy. In total, 50% of the patients did not reach the AJCC criteria for correct staging (< 16 LNs). Moreover, only 15% had an analysis of ≥ 30 LNs, which is considered the optimal assessment of N status. However, if we only considered the last study period, awareness of the complexity of disease treatment, and the development of referral centers seemed to result in more attention and more patients with correct management. For example, in the last study period, 30% of the overall sample population was in the OLA group.
For pathological staging, two factors are interrelated the depth of tumor invasion of the gastric wall (T stage) and the number of positive nodes among all retrieved nodes (N stage). T stage evaluation is not subject to significant surgical or pathological issues, but N stage evaluation is strongly influenced by surgical skill and pathologist interpretation. Regarding the latter, a difference in the analysis can be easily detected if the specimen is sent to the pathologist in a single piece or already divided by LN stations by the surgeon. Therefore, an inadequate assessment reflects a process bug that is generated at some point between the surgical procedure and the final specimen analysis. There is a need for a dedicated multidisciplinary team to manage gastric cancer patients. Interestingly, in our analysis, no significant differences between the three study groups were seen in the T stage distribution, meaning that the number of retrieved LNs was not influenced by the primary site extension.
As expected, we found a large disparity in patients classified as N0 and N1 in the ILA and OLA groups, with 30% vs 15% N0 and 26% vs 13% N1, respectively. If an inadequate number of nodes is assessed, a patient may be inappropriately considered node negative or assigned to a lower N stage. Consequently, the patient is assigned to a lower overall stage. Moreover, while the current recommendation to analyze at least 16 LN allows for N3b substage classification, which requires ≥ 16 positive LNs, the likelihood that a patient would be classified as N3b with only 16 analyzed nodes is extremely low. Therefore, a larger number of nodes is needed for this evaluation[21]. Based on this classification requirement, the N3b substage could not be assessed in the ILA group. However, 30% of patients in the OLA group were in that substage. The N3a and N2 categories can also be influenced by the overall number of analyzed nodes, and patients can therefore be subject to a stage migration effect. As a consequence, patients in the ILA group were formally assigned to the earlier II and IIIA stages, and very few patients fell within the more advanced stages. There was a 10-fold difference in the percentage of patients in the IIIC category in the ILA and OLA groups (3% vs 31%, respectively).
Three main questions can be answered by the present study: (1) Does this have an overall impact on long-term survival? (2) How beneficial is the correct staging of patients? and (3) Given the same stage conditions and patient characteristics, is there a survival difference between ALA and OLA? The answer to the first question is yes. The ILA group had the worst OS (median = 24 mo) and CSS (median = 30 mo) compared with the ALA (median OS = 29 mo, median CSS = 36 mo, P < 0.001) and OLA (median OS = 34 mo, median CSS = 42 mo, P < 0.001) groups. Our findings clearly show that the stage-specific survival curves of the ILA group do not follow the expected trend. In particular, there were 49-month and 81-month mean differences between patients in stage IIA in the ILA group and in the ALA and OLA groups, respectively. Regarding the second question, correct staging requires the efforts of surgeons and pathologists. Of course, several other factors may influence survival in this context. Therefore, we included patient and tumor characteristics and treatment variables in the Cox regression analysis. The multivariate model confirmed that the ALA and OLA groups significantly improved OS (ALA HR = 0.68 and OLA HR = 0.48, P < 0.001) and CSS (ALA HR = 0.64 and OLA HR = 0.47, P < 0.001). Regarding the third question, PSM in the ALA and OLA groups (3428 matched patients) demonstrated that optimal assessment was key for better survival (univariable OS HR = 0.81, P < 0.001; multivariable HR = 0.71, P < 0.001).
This study evaluated patients included in a population registry who were selected by both direct and indirect variables related to a code system. One major study limitation was the use of a population registry based on a coding system of direct and indirect variables, which reduced the availability of more detailed information of the patient characteristics and treatment details. For example, the extent of lymphadenectomy performed or the type of neoadjuvant and adjuvant chemotherapy regimens were not known. In particular, standard D2 LN dissection may not have been performed in the elderly or in high-risk patients who were included in the analysis. As a result, it is presumed that the prognosis in these categories was poor independent of the inadequate staging effect. Despite the limitations, the strength of the study is the large sample of patients analyzed, which allowed statistical rigor. Moreover, the SEER database contains rigorous, standardized information and the guarantee of a high-quality data collection process.
Inadequate staging is an important issue in gastric cancer management that adversely impacts the survival of a large proportion of patients undergoing radical resection. Our study findings demonstrate that analyzing < 16 LNs is insufficient for accurate staging and prognostically misleading. In contrast, analyzing 16–29 LNs improves the accuracy of staging, and evaluation of ≥ 30 LNs offers the most consistent chance of correctly classifying patients into the appropriate N3 substages. Therefore, surgeons and pathologists should make concerted efforts to analyze as many LNs as possible beyond the current NCCN recommendations. That may require a D2 lymphadenectomy as recommended by experienced surgeons in patients without restrictive surgical risk. Moreover a more thorough reassessment of the surgical specimen may be required if an inadequate number of LNs is initially found after a radical gastrectomy. Most important, all patients with inadequate LNA should be considered at high risk for stage migration and can expect a survival rate significantly worse than their formally assigned TNM-stage.
Lymphadenectomy in gastric cancer remains a relevant issue because of its impact on survival. The SEER database is one of the largest Western cancer databases. Patients were assigned to three groups depending on the number of analyzed lymph nodes (LNs) to evaluate survival differences and the stage migration effect.
Gastric cancer should be treated in dedicated centers to offer the patient both optimal surgery and a correct pathological assessment and to avoid improper staging.
We aimed to analyze the survival of patients with inadequate numbers of assessed LNs and to quantify the effect vs correctly staged patients, based on the stage definitions in the AJCC staging manual.
Eligible gastric cancer patients were identified in the SEER database and assigned of three groups, inadequate LN assessment (< 16 LNs), adequate LN assessment (16-29 LNs), and optimal LN assessment (≥ 30 LNs).
The ILA group had the worst survival. The finding was confirmed in by univariate and multivariate analysis. OLA gave the best chance of both correct staging and proper surgery performed as demonstrated after propensity score matching.
Inadequate staging led to a significant reduction in the expected survival associated with the formally attributed stage. An analysis of at least > 16 LNs should be offered to all patients treated with curative intent.
The role of referral centers for gastric cancer should be strengthened to obtain optimal treatment and accurate patient staging.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: An T, Kocazeybek B, Ojima T S-Editor: Liu M L-Editor: Filipodia P-Editor: Liu M
| 1. | Klein Kranenbarg E, Hermans J, van Krieken JH, van de Velde CJ. Evaluation of the 5th edition of the TNM classification for gastric cancer: improved prognostic value. Br J Cancer. 2001;84:64-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 3. | Kim SG, Seo HS, Lee HH, Song KY, Park CH. Comparison of the Differences in Survival Rates between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: a Single-Institution Study of 5,507 Patients in Korea. J Gastric Cancer. 2017;17:212-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 3. 2021 [cited 30 January 2021]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. |
| 5. | Khanjani N, Mirzaei S, Nasrolahi H, Hamedi SH, Mosalaei A, Omidvari S, Ahmadloo N, Ansari M, Sobhani F, Mohammadianpanah M. Insufficient lymph node assessment in gastric adenocarcinoma. J Egypt Natl Canc Inst. 2019;31:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Baxter NN, Tuttle TM. Inadequacy of lymph node staging in gastric cancer patients: a population-based study. Ann Surg Oncol. 2005;12:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, Xu Y, Li DM, Wang ZN, Xu HM. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | The American Joint Committee on Cancer. Cancer Staging Manual. 8th ed. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LRE, editor. Springer International Publishing, 2017. |
| 10. | SEER Program SEER*Stat Database. Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973-2015 varying) - Linked To County Attributes - Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. [cited 20 April 2021] Available from: www.seer.cancer.gov. |
| 11. | Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011;30:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Hansen BB, Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Statist Sci. 2008;23:219-236. [RCA] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Iacus S, King G, Porro, G. CEM: Coarsened exact matching software. J Statist Software. 2009;30:1-27. |
| 14. | Iacus S, King G, Porro, G. Causal Inference without Balance checking: coarsened exact matching. Political Analysis. 2011;20:1-24. [DOI] [Full Text] |
| 15. | Troian M, Nagliati C, Balani A. Esophagogastric premalignant conditions. A literature review. J Gastric Surg. 2020;2:79-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Zemni I, Mansouri H, Ben Safta I, Ayadi MA, Ben Dhiab T, Chargui R, Rahal K. Resectable gastric signet ring cell carcinoma: clinicopathological characteristics and survival outcomes. J Gastric Surg. 2020;2:71-78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Woo Y, Goldner B, Ituarte P, Lee B, Melstrom L, Son T, Noh SH, Fong Y, Hyung WJ. Lymphadenectomy with Optimum of 29 Lymph Nodes Retrieved Associated with Improved Survival in Advanced Gastric Cancer: A 25,000-Patient International Database Study. J Am Coll Surg. 2017;224:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Coco D, Leanza S. Assessment of the Completeness of Lymph Node Dissection Using Indocyanine Green in Laparoscopic and Robotic Gastrectomy for Gastric Cancer-A Review. J Gastric Surg. 2021;3. [DOI] [Full Text] |
| 19. | Zhong Q, Huang CM, Chen QY, Lin JX, Xie JW, Li P, Zheng CH. Current Status of Indocyanine Green Tracer-Guided Lymph Node Dissection in Minimally Invasive Surgery for Gastric Cancer. J Gastric Surg. 2021;3. [DOI] [Full Text] |
| 20. | Woo Y, Son T, Song K, Okumura N, Hu Y, Cho GS, Kim JW, Choi SH, Noh SH, Hyung WJ. A Novel Prediction Model of Prognosis After Gastrectomy for Gastric Carcinoma: Development and Validation Using Asian Databases. Ann Surg. 2016;264:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Seevaratnam R, Bocicariu A, Cardoso R, Yohanathan L, Dixon M, Law C, Helyer L, Coburn NG. How many lymph nodes should be assessed in patients with gastric cancer? Gastric Cancer. 2012;15 Suppl 1:S70-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |