Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1226
Peer-review started: March 20, 2021
First decision: June 3, 2021
Revised: June 15, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: October 27, 2021
Processing time: 220 Days and 2.8 Hours
Nonoperative management (NOM) is a promising therapeutic modality for patients with perforated peptic ulcer (PPU). However, the risk factors for poor efficacy and adverse events of NOM are a concern.
To investigate the factors predictive of poor efficacy and adverse events in patients with PPU treated by NOM.
This retrospective case-control study enrolled 272 patients who were diagnosed with PPU and initially managed nonoperatively from January 2014 to December 2018. Of these 272 patients, 50 converted to emergency surgery due to a lack of improvement (surgical group) and 222 patients were included in the NOM group. The clinical data of these patients were collected. Baseline patient characteristics and adverse outcomes were compared between the two groups. Logistic regression analysis and receiver operating characteristic curve analyses were conducted to investigate the factors predictive of poor efficacy of NOM and adverse outcomes in patients with PPU.
Adverse outcomes were observed in 71 patients (32.0%). Multivariate analyses revealed that low serum albumin level was an independent predictor for poor efficacy of NOM and adverse outcomes in patients with PPU.
Low serum albumin level may be used as an indicator to help predict the poor efficacy of NOM and adverse outcomes, and can be used for risk stratification in patients with PPU.
Core Tip: Risk factors are associated with a poor efficacy in patients with perforated peptic ulcer (PPU) treated by nonoperative management (NOM), and can be used for risk stratification in patients with PPU. Serum albumin level is an important predictor of the poor efficacy of NOM.
- Citation: Liang TS, Zhang BL, Zhao BB, Yang DG. Low serum albumin may predict poor efficacy in patients with perforated peptic ulcer treated nonoperatively. World J Gastrointest Surg 2021; 13(10): 1226-1234
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1226.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1226
Perforation is a serious complication of peptic ulcer disease (PUD) with a morbidity rate between 6.2% and 27%[1-3]. Patients with perforated peptic ulcer (PPU) tend to be young male smokers residing in developing countries, while patients in developed countries tend to be elderly with associated use of steroid or non-steroidal anti-inflammatory drugs and multiple comorbidities[4]. The incidence of PPU has significantly decreased worldwide, especially in high-income countries[5], and only 2%-14% of PUD patients present with an acute abdominal perforation[6]. The reason for this overall progress is the introduction of new drugs (H2 receptor antagonists and proton pump inhibitors [PPIs]) and the diagnosis and management of Helicobacter pylori infection[4,7,8].
PPU is still one of the most common causes of abdominal pain in the emergency department and requires prompt diagnosis and treatment. Nonoperative treatment should be considered in patients with uncomplicated PPU, which prevents surgery and its resultant morbidity. Studies have demonstrated that approximately 40%-80% of patients with PPU will heal spontaneously, and most patients with uncomplicated PPU can benefit from nonoperative management (NOM)[5,9-11]. Prognostic factors that can enhance recovery, and reduce morbidity and mortality should be identified and investigated further.
The aim of this study was to evaluate the relationship between risk factors and clinical outcome, and identify which factors can be used for risk stratification in patients with PPU.
This was a single-center retrospective case-control study. Patients who were diagnosed with PPU by computed tomography (CT) scan and treated by NOM on admission between January 2014 and December 2018 at Liaocheng People’s Hospital (Shandong, China) were enrolled in the study. The following patients were considered suitable for NOM: Patients with an empty stomach at the time of perforation and who were in good general condition, patients with tolerable abdominal pain, limited peritonitis with no manifestations of shock on admission, or a CT scan of the abdomen revealed that free air or liquid was limited to 1-2 zones. Those who were accepted for emergency surgery on admission or had suspected gastric cancer were excluded. Patients with severe liver disease or renal disease were also excluded. The patients were divided into two groups based on whether vital parameters are normal and the findings of peritonitis or septic shock: The nonoperative management group (NOM group) and the surgical management group (surgical group). This study was approved by the Ethics Committee of Liaocheng People’s Hospital. As it was a retrospective study, signed informed consent was not necessary.
All patient data were obtained from electronic charts. Demographic data such as gender and age were collected. A medical history of hypertension, diabetes mellitus, and smoking status was recorded. Clinical variables such as duration of abdominal pain, physical examinations, and vital signs were evaluated. Laboratory variables including leukocyte count, hemoglobin, serum albumin, procalcitonin (PCT) concentration, and C-reactive protein (CRP) were collected.
Nonoperative treatment of patients with PPU consisted of fasting, hemodynamic resuscitation, nasogastric suction, appropriate antibiotics, and antisecretory therapy with PPIs and somatostatin and repeated clinical assessment. If there was no significant improvement in the patient’s condition within 12 h, operative treatment was considered. Clinical improvement was defined as a composition of improvements in vital signs and abdominal signs. They were managed by an experienced surgeon. Water-soluble contrast imaging was performed in all patients to determine whether the perforation had sealed. Gastroscopy and Helicobacter pylori examination were recommended within 1 mo after the patient had completely recovered.
Continuous variables are expressed as the mean ± SD or median (interquartile range) as appropriate. Categorical variables are expressed as the number and percentage. The Student's t-test or Mann–Whitney U test was used to compare the continuous data as appropriate. The χ2 or Fisher’s exact test was used to compare the categorical data. Logistic regression analyses were used to identify clinical data, which were independent predictors for clinical failure of NOM or adverse outcomes in patients with PPU. Unadjusted variables with a P value < 0.05 in the univariate analyses were subsequently included in the multivariate logistic regression model. To assess the predictive ability of clinical data, a receiver operating characteristic (ROC) curve was performed and the area under the curve (AUC) was calculated. All statistical tests were two-tailed, and differences were considered significant when P < 0.05.
Between January 2014 and December 2018, 306 patients with PPU were admitted to the Gastrointestinal Surgery Department of our hospital. A total of 272 patients with PPU who were initially managed nonoperatively were included in the analysis, and 50 of them were converted to surgery. Finally, 222 patients received nonoperative treatment. The baseline characteristics of the patients are summarized in Table 1. The proportion of patients older than 70 years, with pain duration prior to admission ≥ 12 h and body temperature ≥ 38 °C was higher in the surgical group than in the NOM group. The levels of PCT and CRP and the proportion of patients with serum albumin < 30 g/L were higher in the surgical group than in the NOM group.
| Variables | Surgical group | NOM group | P value |
| n = 50 | n = 222 | ||
| Age in yr, average (median) | 66.5 (15.8) | 58.0 (21.3) | < 0.001 |
| ≥ 70 yr | 19 (38.0) | 44 (19.8) | 0.006 |
| Male, n (%) | 32 (64.0) | 162 (73.0) | 0.205 |
| Hypertension | 16 (32.0) | 45 (20.3) | 0.072 |
| DM | 11 (22.0) | 28 (12.6) | 0.087 |
| Smoking | 26 (52.0) | 83 (37.4) | 0.057 |
| Alcohol consumption | 18 (36.0) | 56 (25.2) | 0.122 |
| NSAIDs use | 16 (32.0) | 50 (22.5) | 0.158 |
| Pain duration prior to admission (median) | 8.0 (9.0) | 6.0 (6.0) | 0.001 |
| ≥ 12 h | 16 (32.0) | 33 (14.9) | 0.004 |
| Heart rate (bpm) (median) | 92.0 (24.0) | 86.0 (18.0) | 0.116 |
| Body temperature (C) (median) | 36.7 (1.2) | 36.7 (0.7) | 0.826 |
| ≥ 38 C | 9 (18.0) | 19 (8.6) | 0.047 |
| Hemoglobin (g/L) | 116.8 22.7 | 126.5 22.2 | 0.006 |
| < 90 g/L | 7 (14.0) | 15 (6.8) | 0.090 |
| WBC count (× 109/L) (median) | 9.5 (6.6) | 10.5 (3.3) | 0.479 |
| ≥ 12 × 109/L | 18 (36.0) | 77 (34.7) | 0.860 |
| Procalcitonin (ng/mL) (median) | 5.14 (10.03) | 0.88 (3.96) | < 0.001 |
| CRP (mg/L) (median) | 151.28 (151.16) | 68.46 (119.35) | < 0.001 |
| Serum albumin (g/L) | 27.5 4.65 | 33.7 6.79 | < 0.001 |
| < 30 g/L | 32 (64.0) | 54 (24.3) | < 0.001 |
In this study, the incidence of adverse outcomes was 30% in the surgical group and 25.2% in the NOM group; there were no significant differences between the two groups (P = 0.487). However, the length of hospital stay in the surgical group was longer than that in the NOM group (P < 0.001; Table 2).
| Complications | Surgical group, n = 50 | NOM group, n = 222 | P value |
| Wound infection | 3 (6.0) | 0 | 0.006 |
| Respiratory infection | 2 (4.0) | 11 (5.0) | 1.000 |
| Urinary infection | 4 (8.0) | 9 (4.1) | 0.415 |
| Ascites | 3 (6.0) | 24 (10.8) | 0.304 |
| Pleural effusion | 3 (6.0) | 7 (3.2) | 0.582 |
| Abdominal abscess | 0 (0) | 5 (2.3) | 0.588 |
| Total complications | 15 (30) | 56 (25.2) | 0.487 |
| Length of hospital stay in d | 12 (7) | 9 (3) | < 0.001 |
For the prediction of poor efficacy of NOM, variables including age ≥ 70 years, pain duration prior to admission ≥ 12 h, and serum albumin < 30 g/L were entered into the multivariate logistic regression model. The results showed that serum albumin < 30 g/L was an independent indicator for poor efficacy of NOM (adjusted odds ratio [OR]: 5.073, 95%CI: 2.527-10.184, P < 0.001). In addition, pain duration prior to admission ≥ 12 h independently predicted poor efficacy of NOM (Table 3).
| Variable | OR | 95%CI | P value | Adjusted OR | 95%CI | P value |
| Age ≥ 70 yr | 2.479 | 1.282-4.795 | 0.007 | 1.278 | 0.605-2.698 | 0.521 |
| Male | 0.658 | 0.344-1.260 | 0.207 | |||
| Hypertension | 1.851 | 0.939-3.648 | 0.075 | |||
| Diabetes mellitus | 1.954 | 0.898-4.253 | 0.091 | |||
| Smoking status | 1.814 | 0.978-3.365 | 0.059 | |||
| Alcohol consumption | 1.667 | 0.869-3.201 | 0.124 | |||
| NSAIDs use | 1.619 | 0.826-3.171 | 0.160 | |||
| Pain duration prior to admission ≥ 12 h | 2.695 | 1.339-5.427 | 0.005 | 2.495 | 1.163-5.352 | 0.019 |
| Heart rate | 1.018 | 0.998-1.037 | 0.071 | |||
| Body temperature ≥ 38 C | 2.345 | 0.991-5.549 | 0.052 | |||
| Hemoglobin < 90 g/L | 0.445 | 0.171-1.157 | 0.097 | |||
| WBC count ≥ 12 × 109/L | 1.059 | 0.058-2.009 | 0.860 | |||
| Procalcitonin | 1.027 | 1.000-1.056 | 0.052 | |||
| CRP | 1.001 | 1.000-1.002 | 0.198 | |||
| Serum albumin < 30 g/L | 5.331 | 2.876-10.635 | < 0.001 | 5.073 | 2.527-10.184 | < 0.001 |
With regard to adverse outcomes, variables including age ≥ 70 years and serum albumin < 30 g/L were entered into the multivariate logistic regression model. The results showed that serum albumin < 30 g/L was also an independent indicator of adverse outcomes (adjusted OR: 2.945, 95%CI: 1.625-5.339, P <0.001) (Table 4). Thus, serum albumin < 30 g/L was an independent risk factor for predicting the poor efficacy of NOM and adverse outcomes.
| Variables | OR | 95%CI | P value | Adjusted OR | 95%CI | P value |
| Age ≥ 70 yr | 2.331 | 1.277-4.254 | 0.006 | 1.630 | 0.853-3.114 | 0.139 |
| Male | 1.390 | 0.777-2.488 | 0.268 | |||
| Hypertension | 1.008 | 0.528-1.928 | 0.980 | |||
| Diabetes mellitus | 1.729 | 0.842-3.550 | 0.136 | |||
| Smoking status | 0.757 | 0.432-1.328 | 0.331 | |||
| Alcohol consumption | 0.970 | 0.527-1.785 | 0.922 | |||
| NSAIDs use | 0.977 | 0.519-1.839 | 0.941 | |||
| Pain duration prior to admission ≥ 12 h | 1.316 | 0.667-2.594 | 0.428 | |||
| Heart rate | 1.005 | 0.988-1.023 | 0.568 | |||
| Body temperature ≥ 38 C | 0.586 | 0.214-1.606 | 0.299 | |||
| Hemoglobin < 90 g/L | 0.590 | 0.236-1.471 | 0.257 | |||
| WBC count ≥ 12 × 109/L | 0.787 | 0.441-1.405 | 0.418 | |||
| Procalcitonin | 1.021 | 0.994-1.048 | 0.126 | |||
| CRP | 1.000 | 0.999-1.001 | 0.933 | |||
| Serum albumin < 30 g/L | 3.376 | 1.917-5.946 | < 0.001 | 2.945 | 1.625-5.339 | < 0.001 |
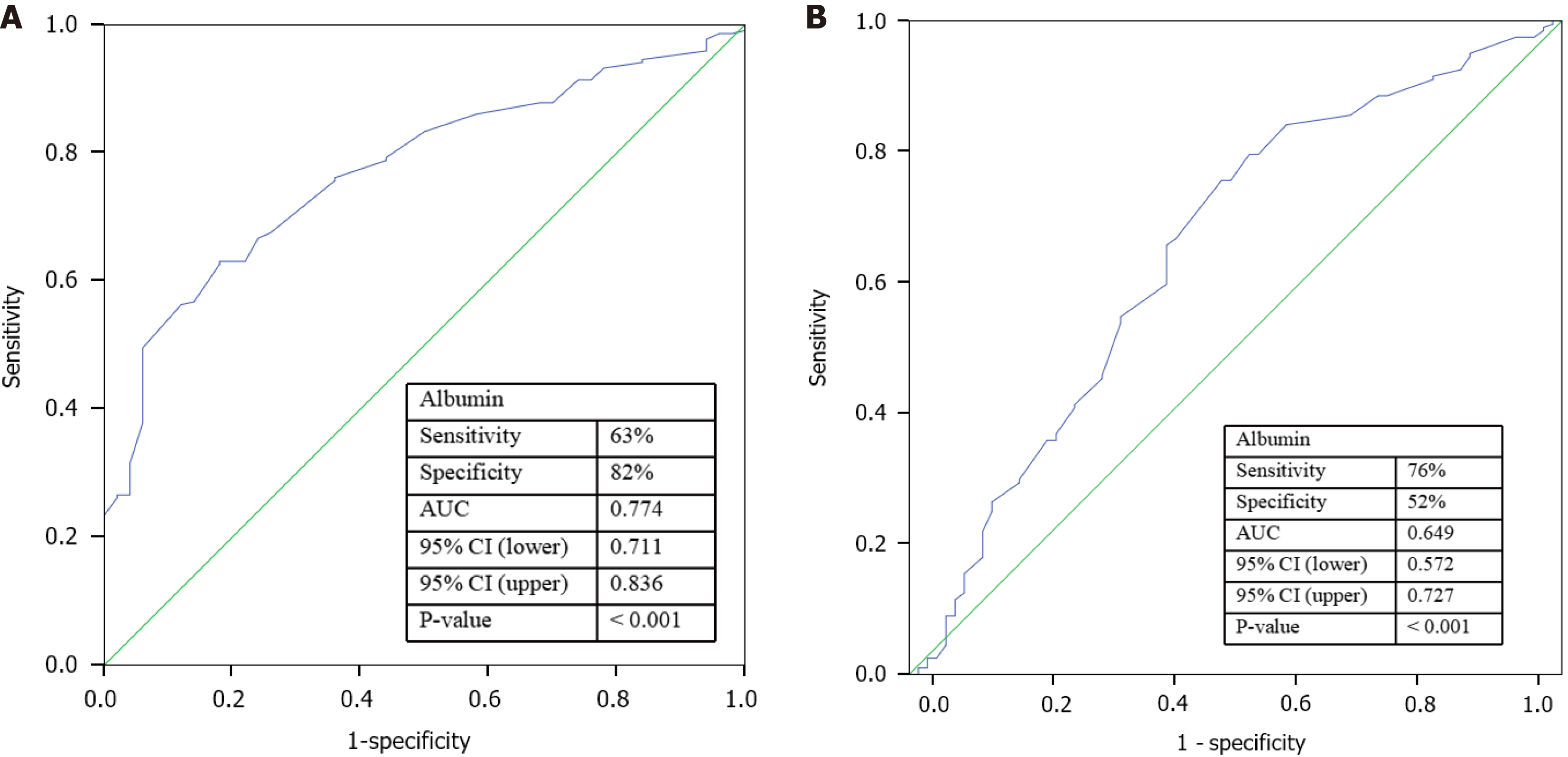
The ROC curves for serum albumin in predicting poor efficacy of NOM and adverse outcomes are shown in Figure 1. The optimal cut-off value of serum albumin for predicting poor efficacy of NOM was 31.8 g/L, with 63% sensitivity and 82% specificity. The optimal cut-off value of serum albumin for predicting adverse outcomes was 29.9 g/L, with 76% sensitivity and 52% specificity. The AUC values for serum albumin for predicting poor efficacy of NOM and adverse outcomes was (0.774, 95%CI: 0.711–0.836) and (0.649, 95%CI: 0.572–0.727) (P < 0.001, respectively).
Our prediction models demonstrated the risk factors for poor efficacy of NOM and adverse outcomes in patients with PPU, and the AUC values verified their significance. Accumulating evidence has shown that serum albumin is not only a parameter of nutritional status but also a marker of acute inflammation and is associated with disease severity. Patients in the surgery group represented relatively serious infections. Therefore, the proportion of patients with serum albumin < 30 g/L was higher in the surgical group. Our results showed that serum albumin was an excellent risk predictor, not only for predicting poor efficacy of NOM but also for adverse outcomes. In addition, pain duration prior to admission ≥ 12 h was an independent risk factor for predicting poor efficacy of NOM.
In 1946, Taylor proposed the famous “Taylor method” in the NOM of PPU, and concluded that 28 PPU patients receiving NOM showed a lower mortality rate than patients receiving direct simple closure with an omental patch[12]. The first randomized trial performed by Crofts et al[10] revealed that 72% of patients treated by NOM had lower morbidity and mortality compared to the surgical group. Several retrospective studies have reported that the NOM technique has a higher success rate in well-selected patients[13]. Moreover, surgical treatment did not show an advantage with regard to morbidity and mortality compared to NOM[5,9,10]. According to World Society of Emergency Surgery guidelines, patients with PPU were suggested to avoid endoscopic treatment such clipping, fibrin glue sealing, or stenting. This approach needs further validation, as it may not be effective in perforated ulcer cases due to fibrotic tissue with loss of compliance. In our study, approximately 81.6% (222/272) of patients received NOM, and the incidence of non-fatal complications was similar to that for those who converted to surgery. These data are in accordance with previous studies and indicate that NOM is a feasible approach[9,14]. However, NOM for PPU is still controversial and has not been widely adopted. In many hospitals, surgical treatment is the preferred choice, and NOM is just an alternative for patients who are not suitable or unwilling to undergo surgery[6]. This study tried to determine the risk factors that will help clinicians select patients with PPU who will experience poor efficacy. Based on logistic regression analysis, two parameters were significantly correlated with poor efficacy of NOM: serum albumin < 30 g/L and pain duration prior to admission ≥ 12 h. With regard to adverse outcomes, only serum albumin < 30 g/L was an independent risk predictor. Furthermore, the AUC values showed that serum albumin had moderate power in predicting clinical outcomes.
Serum albumin has been used as a diagnostic marker for malnutrition in clinical practice for several years as it can reflect the nutritional status of patients[15]. The current evidence shows that serum albumin is not only a parameter of nutritional status, but also a marker of acute inflammation and is associated with disease severity[16]. In a prospective study including 2465 patients who were admitted to the emergency department, the mortality rate was higher in patients with low levels of serum albumin than those with normal serum albumin levels[17]. A previous study showed that PPU patients with low levels of serum albumin at presentation may predict the need for gastric resection, and elevated serum albumin levels can increase the success rate of NOM[18]. Consistent with a previous study, our findings showed that serum albumin < 30 g/L can predict the need for surgical management in patients with PPU who were initially treated nonoperatively. This study is the first to demonstrate that serum albumin is also an independent risk factor for adverse outcomes in patients with PPU. In patients with perforations, the production of acute phase proteins and inflammatory factors will lead to a further decline in serum albumin. Fluids leak slowly from intravascular to interstitial spaces causing local swelling, which induce difficult healing in patients with low levels of serum albumin. Routine measurement of serum albumin on admission, can be used for risk stratification in patients with PPU.
When the onset time of abdominal pain prior to admission is more than 12 h, pyrexia, hypotension and abdominal distension with acute circulatory collapse may be evident[19]. In our study, pain duration prior to admission ≥ 12 h was an independent risk factor for predicting poor efficacy of NOM. The data from our study were consistent with those observed in a previous study[20].
In our analysis, 81.6% of cases (222/272) received NOM with a complication rate of 32%, and patients who converted to surgery had a morbidity rate of 30%. In addition, our study also demonstrated that hospital stay was shorter in the NOM group than in the surgical group. Taken together, these findings show that NOM was safe and effective in patients with PPU. In addition, several risk factors have been confirmed to be significantly associated with poor efficacy of NOM and can be used for risk stratification in patients with PPU.
This study had several limitations. First, this was a single-center retrospective study, and the patients were treated by different doctors. Second, relevant indicators were analyzed only when the patient was admitted to the hospital, and the various indicators during hospitalization were not included. Third, there is currently no uniform standard for uncomplicated upper gastrointestinal perforation; thus, biases in patient selection may exist.
The use of NOM for PPU may be debated for some time. The advantages of NOM are obvious. It is important to stratify patients into high and low risk on admission. NOM is recommended in patients who are in good general condition with an empty stomach at the time of perforation. Low serum albumin is an independent risk factor that may predict adverse consequences of NOM for PPU.
Nonoperative management (NOM) is a promising therapeutic modality for patients with perforated peptic ulcer (PPU). However, the risk factors for poor efficacy and adverse events of NOM are a concern.
Prognostic factors that could enhance recovery, and reduce morbidity and mortality should be identified and investigated further in patients with PPU.
The aim of this study was to evaluate the relationship between risk factors and clinical outcome, and identify which factors can be used for risk stratification in patients with PPU.
Total 272 patients who were diagnosed with PPU and initially managed nonoperatively from January 2014 to December 2018 were enrolled. The clinical data of these patients were collected. Baseline patient characteristics and adverse outcomes were compared between the two groups.
Multivariate analyses revealed that low serum albumin level was an independent predictor for poor efficacy of NOM and adverse outcomes in patients with PPU.
Low serum albumin level may be used as an indicator to help us predict poor efficacy of NOM and adverse outcomes, and can be used for risk stratification in patients with PPU.
Low serum albumin is an independent risk factor that may predict adverse consequences of NOM for PPU.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Esmat SM, Homan M S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Anbalakan K, Chua D, Pandya GJ, Shelat VG. Five year experience in management of perforated peptic ulcer and validation of common mortality risk prediction models - are existing models sufficient? Int J Surg. 2015;14:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 2. | Kim JM, Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, Jung EJ, Ju YT, Jeong CY, Ha WS. Analysis of risk factors for postoperative morbidity in perforated peptic ulcer. J Gastric Cancer. 2012;12:26-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Møller MH, Engebjerg MC, Adamsen S, Bendix J, Thomsen RW. The Peptic Ulcer Perforation (PULP) score: a predictor of mortality following peptic ulcer perforation. A cohort study. Acta Anaesthesiol Scand. 2012;56:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Kang JY, Elders A, Majeed A, Maxwell JD, Bardhan KD. Recent trends in hospital admissions and mortality rates for peptic ulcer in Scotland 1982-2002. Aliment Pharmacol Ther. 2006;24:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Zittel TT, Jehle EC, Becker HD. Surgical management of peptic ulcer disease today--indication, technique and outcome. Langenbecks Arch Surg. 2000;385:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 6. | Bertleff MJ, Lange JF. Perforated peptic ulcer disease: a review of history and treatment. Dig Surg. 2010;27:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 8. | Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T, Søreide K. Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19:347-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 139] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (4)] |
| 9. | Bucher P, Oulhaci W, Morel P, Ris F, Huber O. Results of conservative treatment for perforated gastroduodenal ulcers in patients not eligible for surgical repair. Swiss Med Wkly. 2007;137:337-340. [PubMed] |
| 10. | Crofts TJ, Park KG, Steele RJ, Chung SS, Li AK. A randomized trial of nonoperative treatment for perforated peptic ulcer. N Engl J Med. 1989;320:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 158] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Buck DL, Vester-Andersen M, Møller MH; Danish Clinical Register of Emergency Surgery. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg. 2013;100:1045-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | BIRKS PM. Perforated peptic ulcer treated without operation. Lancet. 1947;1:467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 43] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Songne B, Jean F, Foulatier O, Khalil H, Scotté M. [Non operative treatment for perforated peptic ulcer: results of a prospective study]. Ann Chir. 2004;129:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kocer B, Surmeli S, Solak C, Unal B, Bozkurt B, Yildirim O, Dolapci M, Cengiz O. Factors affecting mortality and morbidity in patients with peptic ulcer perforation. J Gastroenterol Hepatol. 2007;22:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Clark LN, Helm MC, Higgins R, Lak K, Kastenmeier A, Kindel T, Goldblatt M, Gould JC. The impact of preoperative anemia and malnutrition on outcomes in paraesophageal hernia repair. Surg Endosc. 2018;32:4666-4672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Allison SP, Lobo DN, Stanga Z. The treatment of hypoalbuminaemia. Clin Nutr. 2001;20:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, Neeser O, Huber A, Stanga Z, Mueller B, Schuetz P. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am J Med. 2020;133:713-722.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 18. | Seow JG, Lim YR, Shelat VG. Low serum albumin may predict the need for gastric resection in patients with perforated peptic ulcer. Eur J Trauma Emerg Surg. 2017;43:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Chung KT, Shelat VG. Perforated peptic ulcer - an update. World J Gastrointest Surg. 2017;9:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 183] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (6)] |
| 20. | Cao F, Li J, Li A, Fang Y, Wang YJ, Li F. Nonoperative management for perforated peptic ulcer: who can benefit? Asian J Surg. 2014;37:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |