Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1202
Peer-review started: March 18, 2021
First decision: June 3, 2021
Revised: June 17, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: October 27, 2021
Processing time: 222 Days and 5.2 Hours
Immunoinflammatory markers such as the peripheral blood neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) have gained considerable attention as prognostic markers in gastrointestinal stromal tumors (GISTs).
To assess the prognostic value of Onodera’s Prognostic Nutritional Index (OPNI) for GISTs.
All patients who had undergone surgical resection for a primary, localized GIST from 2009 to 2016 at our cancer center were initially and retrospectively identified. Recurrence-free survival (RFS) was calculated by the Kaplan-Meier method and compared by the log-rank test. We used multivariate Cox proportional hazard regression models to identify associations with outcome variables.
A total of 235 GISTs were identified and included for analysis under our inclusion criteria. Univariate and multivariate analyses both identified the OPNI as an independent prognostic marker, and the OPNI was associated with the primary site, tumor size, mitotic index, tumor rupture, necrosis, and modified NIH risk classification. Low OPNI (< 51.30; hazard ratio = 5.852; 95% confidence interval: 1.072–31.964; P = 0.0414) was associated with worse RFS. The 2- and 5-year RFS rates of the patients with a low OPNI were 92.83% and 76.22%, respectively, whereas 100% and 98.41% were achieved by the patients with a high OPNI.
The preoperative OPNI is a novel and useful prognostic marker for GISTs.
Core Tip: Immunoinflammatory markers such as the peripheral blood neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio have gained considerable attention as prognostic markers in gastrointestinal stromal tumors (GISTs). Here we conducted the first investigation of the prognostic value of Onodera’s Prognostic Nutritional Index (OPNI) for GISTs. A total of 235 GISTs were identified and included for analysis under our inclusion criteria. Our study shown that the 2- and 5-year recurrence-free survival rates of the patients with a low OPNI were 92.83% and 76.22%, respectively, whereas 100% and 98.41% were achieved by the patients with a high OPNI, which demonstrated that the preoperative OPNI is a novel and useful prognostic marker for GISTs.
- Citation: Wang H, Xu YY, You J, Hu WQ, Wang SF, Chen P, Yang F, Shi L, Zhao W, Zong L. Onodera's Prognostic Nutritional Index is a novel and useful prognostic marker for gastrointestinal stromal tumors. World J Gastrointest Surg 2021; 13(10): 1202-1215
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1202.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1202
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal (GI) tract; their estimated clinical incidence is nearly 1 per 100000 individuals per year[1,2]. The driving force of GISTs is thought to be mutation in c-Kit and minimally in the PDGFRA oncogene (platelet derived growth factor receptor alpha)[3,4]. GISTs can be malignant tumors arising anywhere in the GI tract or abdominal cavity[5]. Surgery remains the standard treatment for primary GISTs, and it has been the only potentially curative therapy.
GIST relapse is common even when the tumor undergoes R0 resection. The disease-free survival (DFS) of patients with GISTs has been markedly improved by the use of the molecularly-specific oral anticancer agent imatinib mesylate (IM), but its adverse reaction and resistance have some hindrance in the treatment of GISTs. Systemic adjuvant IM therapy needs more assurance to be beneficial for target patients. The four most important prognostic factors for GISTs are the tumor location, tumor size, mitotic index, and presence/absence of tumor rupture as suggested by the U.S. famous institutes (NIH, AFIP)[6-8]. Despite the use of these guidelines, even the latest risk stratification system should be improved[9-11].
One of the components of the tumor microenvironment is tumor-associated inflammatory cells. These cells have important roles in both tumor development and progression, which can promote the proliferation, invasion, and metastasis of tumor cells[12]. Immunoinflammatory factors were shown to be associated with the oncogenesis, progression, and prognosis of GISTs. The peripheral blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR; an easily measured, reproducible and cost-effective systemic inflammatory marker) have been investigated as prognostic markers in patients with multiple solid tumors such as non-small-cell lung cancer, colorectal cancer, and gastric cancer[13-15].
Onodera’s Prognostic Nutritional Index (OPNI) was useful for GI surgery patients to evaluate immune nutritional status[16]. The OPNI has been reported to be a useful prognostic marker in esophageal cancer[17], gastric cancer[18], colorectal cancer[19], and pancreatic cancer[20], but the prognostic value of the OPNI for GISTs has not been determined. We conducted the present study to evaluate the prognostic value of the OPNI for GIST.
We retrospectively retrieved the data of the patients with GISTs treated at Northern Jiangsu People’s Hospital (Yangzhou, China) from 2009 to 2016. The inclusion criteria were as follows: (1) R0 resection in GIST; (2) absence of coeval tumors; (3) no treatment or therapies (chemotherapy, radiotherapy, or imatinib); and (4) without signs of infection. A final total of 235 GISTs were included. This study was approved by the Ethics Committee of Northern Jiangsu People’s Hospital, and written informed consent for their data to be used was obtained from all the patients.
All the patients’ preoperative peripheral blood routine tests had been performed within 7 d before surgery. The NLR value was calculated as the neutrophil count (109/L) divided by the lymphocyte count (109/L). The value of the PLR was calculated by the same method as the NLR. The OPNI was calculated as the serum albumin (g/L) + 5 × total lymphocyte count (109/L).
All specimens were diagnosed as GI mesenchymal (non-epithelial) tumors by hematoxylin and eosin (H&E) staining, and further confirmed by positive immunohistochemical staining for CD117 and discovered on GIST 1(DOG-1) with or without CD 34, desmin, SMA, and S-100 positive expression. If the result was negative for both staining, then c-Kit gene exons 9, 11, 13, and 17 or PDGFRA gene exons 12 and 18 were analyzed for DNA mutation.
We obtained the patients’ clinical data from their medical records: Age, gender, and basic clues like primary tumor location, tumor diameter, and rupture of tumor (preoperative/intraoperative). Pathologists measured tumor diameter before specimen fixation. The cell type, mitotic index, and necrosis of tumor were the histopathological markers for analysis. Tumor shape and size, mitotic index, tumor location, and rupture of tumor are four risk stratification factors. And the mitotic index was counted per 50 randomly selected high-power fields by two pathologists.
After their surgeries, the patients were followed by endoscopy and computed tomography examinations every 6 mo to evaluate the presence/absence of tumor recurrence and distant metastasis. We obtained the patients’ follow-up information from the hospital’s records and tumor registry, or by contacting directly with the patients or their family member.
Patients with GISTs can live with the tumor for a relatively long time even if they recur/metastasize. We speculated that the most suitable event for survival analysis was relapse or metastasis, and use of IM treatment for relapse and metastasis of GISTs can affect overall survival. We calculated the duration of a patient’s relapse free survival (RFS) from the surgery date for GIST, which was the study’s primary outcome. And the study’s secondary endpoints were receiver operator characteristic (ROC) analysis of NLR, PLR, OPNI, and Ki-67 index, and correlation between tumor size and NLR, PLR, OPNI, and Ki-67 index.
IBM SPSS Statistics were used to calculate all statistical analyses. Continuous variables are presented as the mean ± SD, and count data are summarized using frequencies and percentages. We calculated the correlation of continuous variables by obtaining the Pearson correlation coefficient, and we calculated the correlation of discrete variables by obtaining Spearman’s correlation coefficient. ROC analysis was used to determine the cut-off points of the NLR, PLR, OPNI, and Ki-67 index. Univariate analysis was performed using the Kaplan-Meier method, and the results were compared by the log-rank test. We conducted a multivariate analysis with the Cox proportional hazards model. A P value < 0.05 was accepted as significant.
The median age of the 235 patients (118 men and 117 women) was 62 years (range, 30–86 years), along with 125 patients (53%) aged more than 60 years. The basic symptoms of the GIST patients were abdominal discomfort/pain (n = 104), GI bleeding and obstruction (n = 63 and 8), rupture of tumor (n = 2), weight loss (n = 7), and being asymptomatic (n = 51). The GISTs can be found in the stomach (n = 183), small intestine (n = 41), colorectum (n = 10), and intraperitoneum with unknown etiology. The tumor sizes varied from 0.4 to 20 cm (median, 4.3 cm). Histologically, the spindle-cell type was most common (n = 206), followed by the epithelioid-cell type (n = 16) and the mixed type (n = 13). The mitotic index, necrosis, and more detailed clinicopathological variables are summarized in Table 1.
| Characteristic | n (%) |
| Gender | |
| Male | 118 (50.2) |
| Female | 117 (49.8) |
| Age (yr, mean SD) | 60.09 ± 10.12 |
| ≤ 60 | 110 (46.8) |
| > 60 | 125 (53.2) |
| Clinical manifestation | |
| Abdominal discomfort or pain | 104 (44.3) |
| Gastrointestinal bleeding | 63 (26.8) |
| Obstruction | 8 (3.4) |
| Perforation or rupture | 2 (0.9) |
| Weight loss | 7 (3.0) |
| Asymptomatic | 51 (21.7) |
| Preoperative laboratory variables | |
| Hemoglobin (g/L, mean SD) | 122.69 ± 29.94 |
| White blood cell (109 /L, mean SD) | 6.52 ± 2.70 |
| Neutrophil count (109 /L, mean SD) | 4.40 ± 2.35 |
| Lymphocyte count (109 /L, mean SD) | 1.42 ± 0.53 |
| Platelet count (109 /L, mean SD) | 230.11 ± 100.76 |
| Albumin (g/L, mean SD) | 44.19 ± 6.66 |
| NLR (mean SD) | 3.80 ± 3.95 |
| PLR (mean SD) | 184.83 ± 109.06 |
| OPNI (mean SD) | 51.27 ± 7.12 |
| Primary tumor site | |
| Stomach | 183 (77.9) |
| Small intestine | 41 (17.4) |
| Colorectum | 10 (4.3) |
| Intraperitoneally with unknown origin | 1 (0.4) |
| Tumor size (cm, mean SD) | 5.003 ± 3.5458 |
| ≤ 2.0 | 55 (23.4) |
| 2.1-5.0 | 93 (39.6) |
| 5.1-10.0 | 67 (28.5) |
| > 10.0 | 20 (8.5) |
| Predominant cell type | |
| Spindle | 206 (87.7) |
| Epithelioid | 16 (6.8) |
| Mixed | 13 (5.5) |
| Mitotic index (per 50 HPFs) | |
| ≤ 5 | 182 (77.4) |
| 6-10 | 43 (18.3) |
| > 10 | 10 (4.3) |
| Necrosis | |
| Yes | 66 (28.1) |
| No | 169 (71.9) |
| Tumor rupture | |
| Yes | 11 (4.7) |
| No | 224 (95.3) |
| Risk classification | |
| Very low risk | 58 (24.7) |
| Low risk | 77 (32.8) |
| Intermediate risk | 41 (17.4) |
| High risk | 59 (25.1) |
| CD117 | |
| (–) | 4 (1.7) |
| (+) | 169 (71.9) |
| (++) | 18 (7.7) |
| (+++) | 44 (18.7) |
| CD34 | |
| (–) | 11 (4.7) |
| (+) | 165 (70.2) |
| (++) | 12 (5.1) |
| (+++) | 47 (20.0) |
| DOG-1 | |
| (–) | 3 (1.3) |
| (+) | 211 (89.8) |
| (++) | 12 (5.1) |
| (+++) | 9 (3.8) |
| Ki-67 index (%, mean SD) | 4.65 ± 6.37 |
| Follow-up time (months, mean SD) | 40.20 ± 20.18 |
| Follow-up status | |
| Relapse-free survival | 215 (91.5) |
| Relapse | 15 (6.4) |
| Metastasis | 5 (2.1) |
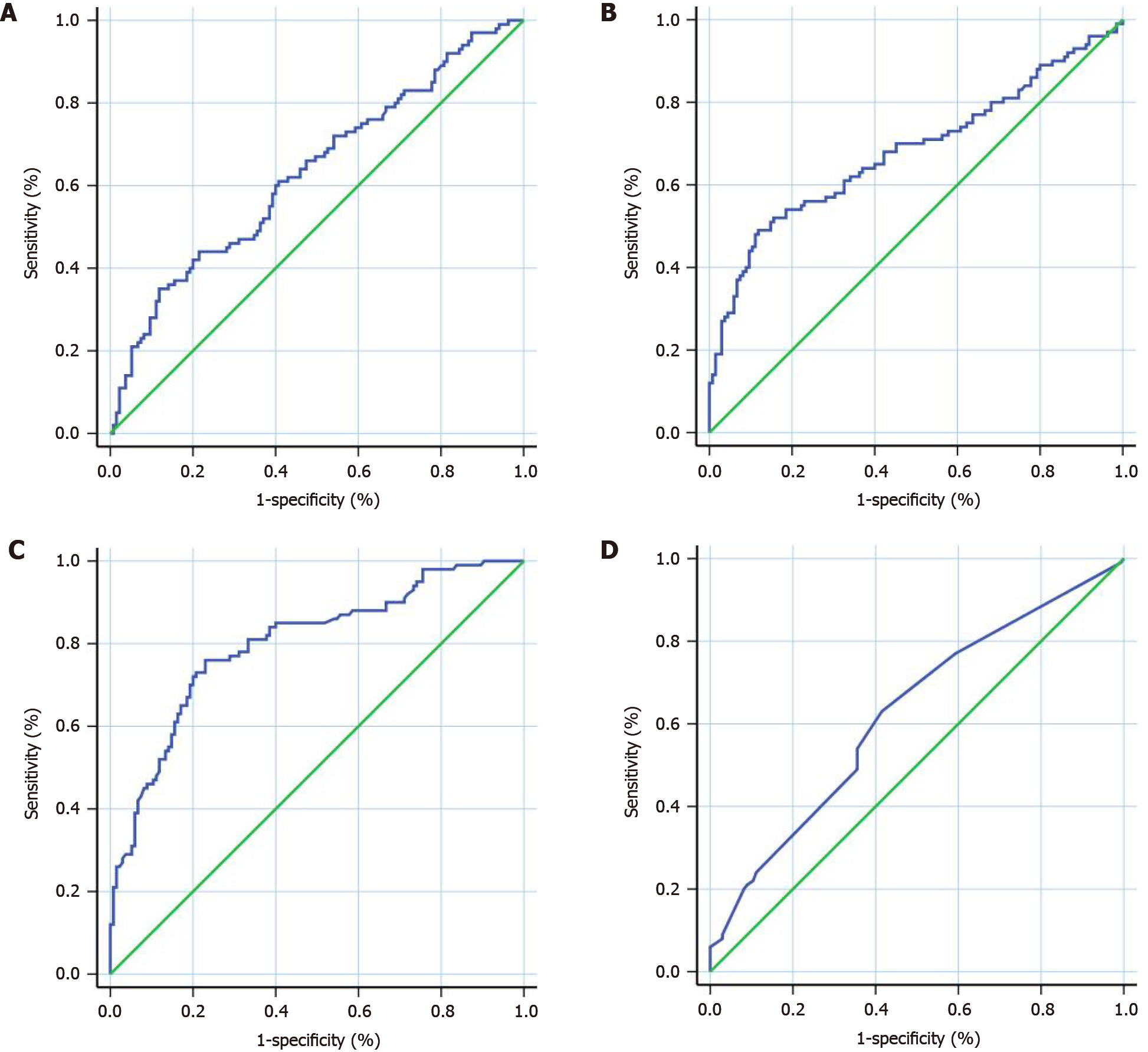
We used the continuous variables of NLR, PLR, OPNI, and the Ki-67 index as test variables, and the RFS as the state variable. The areas under the ROC curves, cut-off points, sensitivities, specificities, and Youden indexes of the NLR, PLR, OPNI, and Ki-67 index are provided in Table 2 and Figure 1.
| NLR | PLR | OPNI | Ki-67 index | |
| Cut-off point | 4.34 | 220.76 | 51.30 | 2.5% |
| Sensitivity% (95%CI) | 35.00 (25.73-45.19) | 49.00 (38.86-59.20) | 76.00 (66.43-83.98) | 63.00 (52.76-72.44) |
| Specificity% (95%CI) | 88.15 (81.47-93.07) | 88.15 (81.47-93.07) | 77.04 (69.02-83.83) | 58.52 (49.73-66.93) |
| Youden Index | 0.2315 | 0.3715 | 0.5304 | 0.2152 |
| AUC (95%CI) | 0.6308 (0.5584-0.7031) | 0.6820 (0.6096-0.7545) | 0.7999 (0.7420-0.8578) | 0.6237 (0.5514-0.6960) |
| P value | 0.0006 | < 0.0001 | < 0.0001 | 0.0012 |
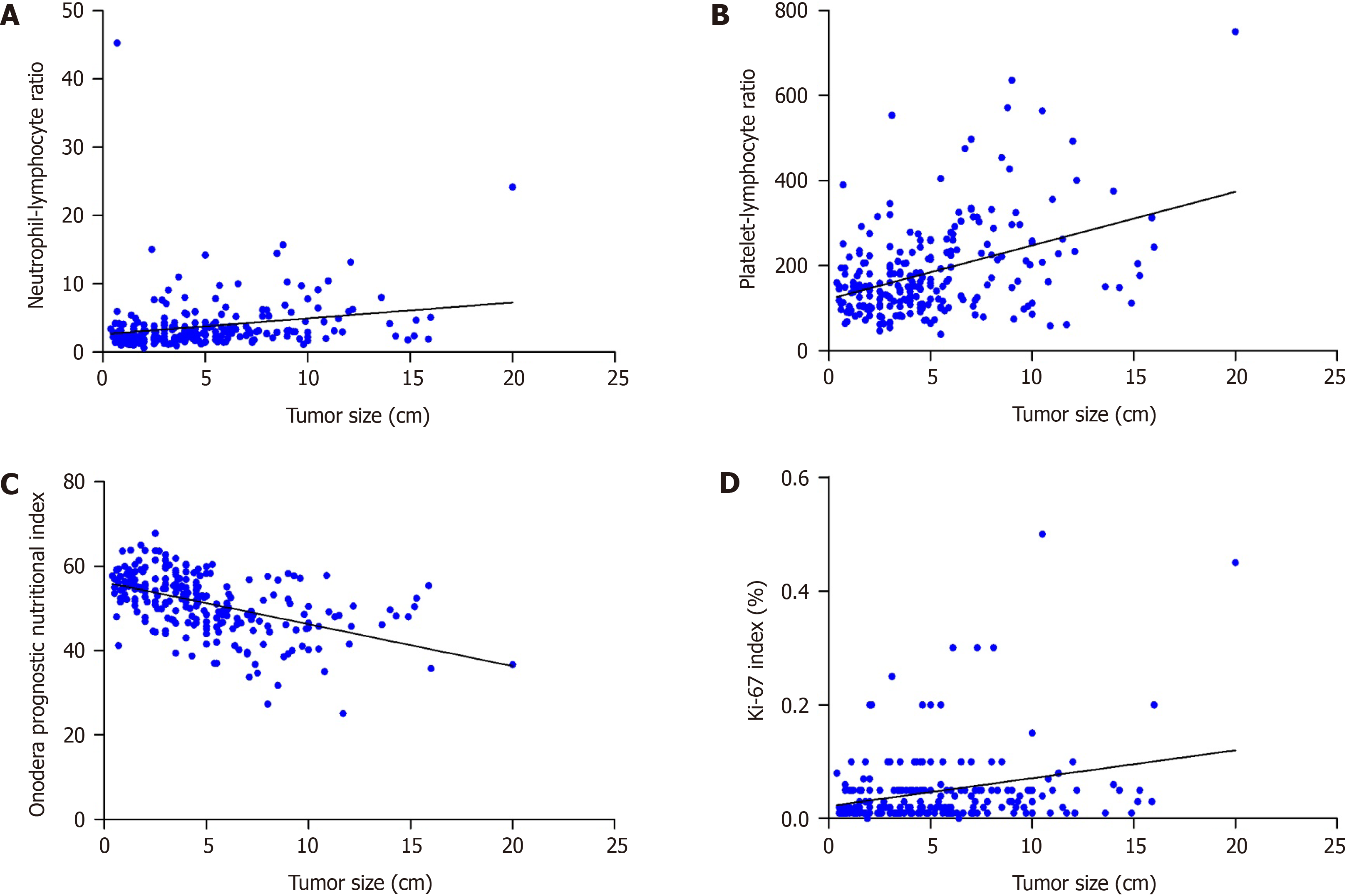
A lower OPNI was associated with the primary tumor location (P = 0.0004), tumor diameter (P < 0.0001), mitotic index (P < 0.0001), rupture of tumor (P = 0.0030), necrosis (P < 0.0001), and risk stratification by the modified NIH (P < 0.0001). A significant correlation was observed between the NLR and tumor size [Pearson correlation coefficient (r) = 0.2082, P = 0.0013]. Similarly, the PLR, OPNI, and Ki-67 index were each correlated strongly with tumor size (Table 3). There was a negative correlation between the OPNI and GIST tumor size, whereas the NLR, PLR, and Ki-67 index were positively correlated with GIST tumor size (Figure 2). Spearman’s correlation test revealed that the PLR (Rs =0.2045, P = 0.0016), OPNI (Rs = −3.048, P < 0.0001), and Ki-67 index (Rs =0.2551, P < 0.0001) were correlated with the mitotic index (Table 3). Correlation analysis of clinicopathologic parameters with OPNI, NLR, PLR, and Ki-67 index are shown in the Supplementary Tables 1-4, which showed no signi
| Tumor size | Mitotic index | |||
| Pearson r | P value | Rs | P value | |
| NLR | 0.2082 | 0.0013 | 0.1021 | 0.1185 |
| PLR | 0.4098 | < 0.0001 | 0.2045 | 0.0016 |
| OPNI | -0.4955 | < 0.0001 | -3.048 | < 0.0001 |
| Ki-67 index | 0.2727 | < 0.0001 | 0.2551 | < 0.0001 |
Patients were followed for a median of 35 mo (range 7–90 mo), and 9.79% (23/235) of the patients were lost to follow-up. The number of relapse patients was, including 5.96% (14/235) with local recurrence in the abdominopelvic cavity and 3.83% (9/235) with liver metastasis (n = 9), and lymph metastasis was not seen. The Kaplan-Meier 1-, 2-, and 5-year RFS rates were 99.15% (95%CI: 96.64–99.7), 96.61% (95%CI: 92.97–98.38), and 86.87% (95%CI: 78.73–92.04), respectively.
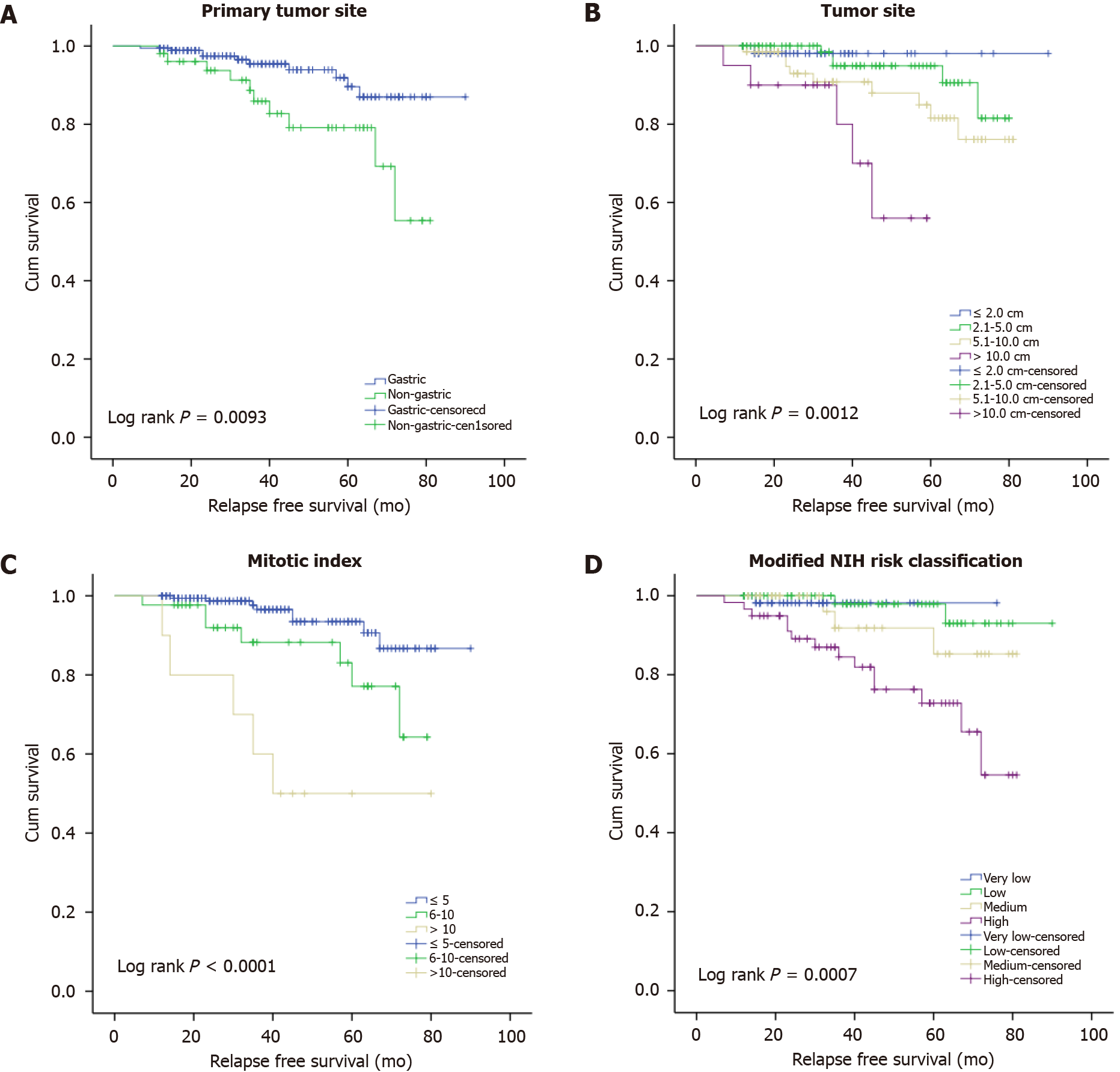
The results of our univariate survival analysis demonstrated that the primary site (log-rank P = 0.0093), tumor size (log-rank P = 0.0012), mitotic index (log-rank P < 0.0001), modified NIH risk stratification (log-rank P = 0.0007), NLR (log-rank P = 0.0224), PLR (log-rank P = 0.0069), and OPNI (log-rank P = 0.0002) were specific prognostic markers for RFS of our GIST patient series. The correlations of clinicopathological factors with the RFS are shown in Table 4 and Figure 3. The univariate survival analysis shows no significance association between recurrence and albumin and lymphocyte count. And the results of ROC analysis for albumin and lymphocyte count are shown in Supplementary Table 5.
| Factor | 1-year RFS rate (95%CI) | 2-year RFS rate (95%CI) | 5-year RFS rate (95%CI) | Log-rank P value |
| Age (yr) | 0.5441 | |||
| ≤ 60 | 99.09% (93.72-99.87) | 96.92% (90.66-99.01) | 91.20% (80.62-96.14) | |
| > 60 | 99.20% (94.46-99.89) | 96.35% (90.49-98.62) | 82.93% (69.26-90.91) | |
| Gender | 0.2889 | |||
| Male | 98.31% (93.39-99.57) | 95.19% (88.74-97.99) | 84.07% (71.68-91.35) | |
| Female | 100% | 98.03% (92.30-99.51) | 84.96% (66.88-93.61) | |
| GI bleeding | 0.1877 | |||
| Yes | 98.41% (89.26-99.77) | 98.41% (89.26-99.77) | 82.02% (63.00-91.85) | |
| No | 99.42% (95.94-99.92) | 95.84% (90.90-98.12) | 89.37% (80.44-94.36) | |
| Primary site | 0.0093 | |||
| Gastric | 99.45% (96.18-99.92) | 97.47% (93.30-99.04) | 89.62% (79.32-94.94) | |
| Non-gastric | 98.08% (87.12-99.73) | 93.75% (81.78-97.95) | 79.12% (61.86-89.21) | |
| Tumor size | 0.0012 | |||
| ≤ 2.0 cm | 100% | 98.10% (87.12-99.73) | 98.10% (87.12-99.73) | |
| 2.1-5.0 cm | 100% | 100% | 94.90% (84.98-98.33) | |
| 5.1-10.0 cm | 98.51% (89.87-99.79) | 92.93% (82.17-97.30) | 81.55% (65.45-90.65) | |
| > 10.0 cm | 95.00% (69.46-99.28) | 90.00% (65.59-97.40) | 56.00% (20.71-80.77) | |
| Predominant cell type | 0.7759 | |||
| Spindle | 99.51 % (96.60-99.93) | 97.22 % (93.41-98.84) | 88.47 % (79.83-93.55) | |
| Epithelioid | 93.75 % (63.22-99.10) | 93.75 % (63.22-99.10) | 84.38 % (49.30-96.00) | |
| Mixed | 100% | 100% | 76.39 % (30.91-94.01) | |
| Mitotic index | < 0.0001 | |||
| ≤ 5 per 50 HPFs | 100% | 98.67% (94.75-99.67) | 93.47% (85.43-97.15) | |
| 6-10 per 50 HPFs | 97.67% (84.61-99.67) | 91.93% (76.88-97.34) | 77.13% (53.86-89.67) | |
| >10 per 50 HPFs | 100% | 80.00% (40.86-94.59) | 50.00% (18.35-75.32) | |
| Necrosis | 0.2676 | |||
| Yes | 98.48% (89.72-99.79) | 98.48% (89.72-99.79) | 83.69% (66.12-92.63) | |
| No | 100% | 95.79% (90.79-98.10) | 89.58% (81.22-94.34) | |
| Tumor rupture | 0.0695 | |||
| Yes | 100% | 100% | 63.49% (23.81-86.61) | |
| No | 99.11% (96.48-99.78) | 96.43% (92.62-98.29) | 88.40% (79.94-93.44) | |
| Risk classification | 0.0007 | |||
| Very low risk | 100% | 98.18% (87.78-99.74) | 98.18% (87.78-99.74) | |
| Low risk | 100% | 100% | 97.92% (86.11-99.70) | |
| Intermediate risk | 100% | 100% | 85.27% (59.66-95.20) | |
| High risk | 96.61% (87.11-99.14) | 89.10% (77.27-94.97) | 72.82% (56.21-83.98) | |
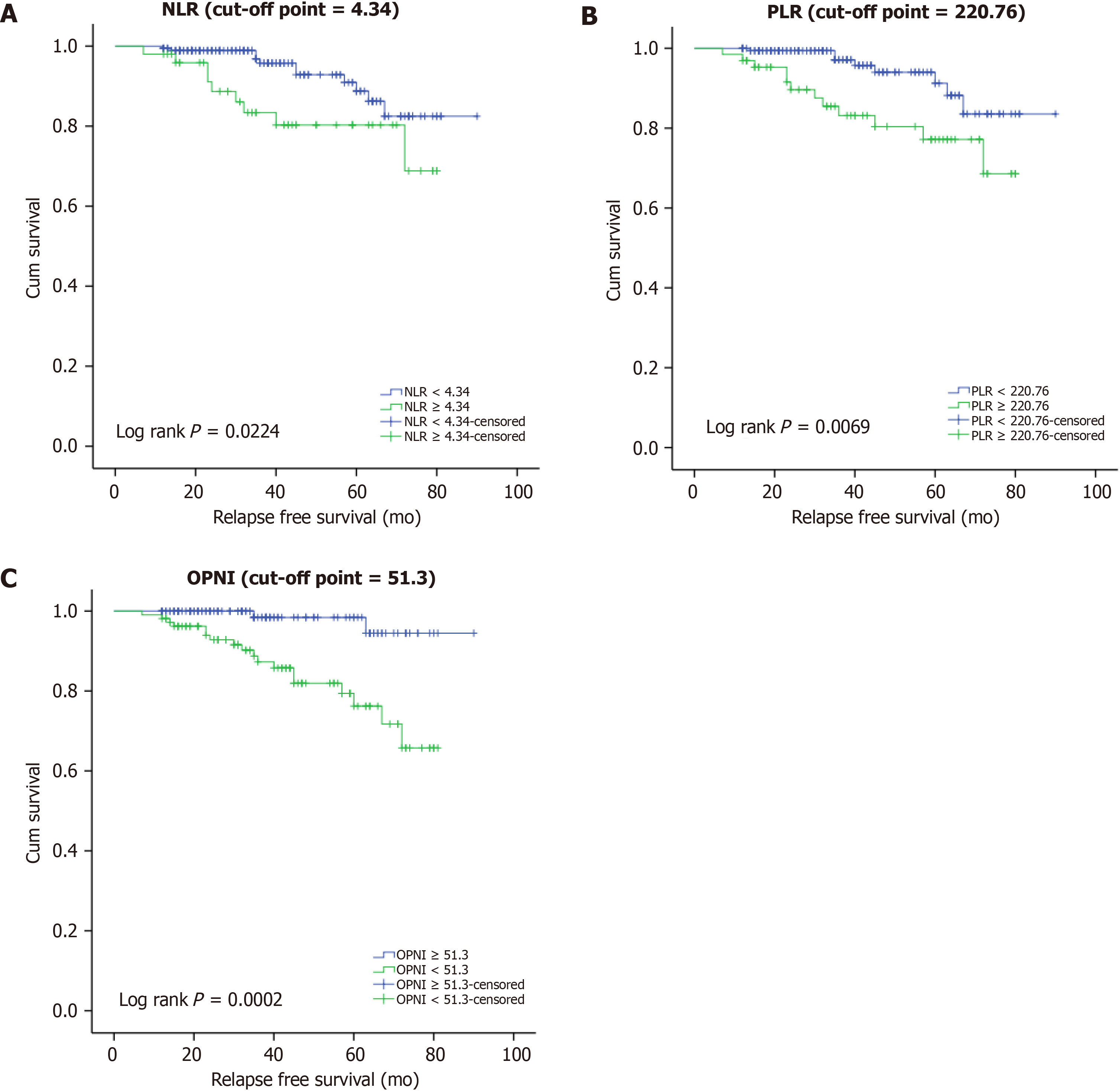
| NLR | 0.0224 | |||
| < 4.34 | 99.46% (96.22-99.92) | 98.89% (95.65-99.72) | 88.76% (78.31-94.35) | |
| ≥ 4.34 | 98.00% (86.63-99.72) | 88.68% (74.82-95.15) | 80.29% (64.11-89.73) | |
| PLR | 0.0069 | |||
| < 220.76 | 100% | 99.39% (95.75-99.91) | 91.24% (80.00-96.31) | |
| ≥ 220.76 | 96.92% (88.25-99.22) | 89.64% (78.27-95.23) | 77.17% (61.76-86.99) | |
| OPNI | 0.0002 | |||
| ≥ 51.30 | 100% | 100% | 98.41% (89.26-99.77) | |
| < 51.30 | 98.13% (92.73-99.53) | 92.83% (85.49-96.53) | 76.22% (62.51-85.48) | |
| Ki-67 index | 0.0592 | |||
| < 2.5% | 100% | 98.88% (92.29-99.84) | 88.03% (68.96-95.72) | |
| ≥ 2.5% | 98.29% (93.34-99.57) | 94.34% (87.79-97.43) | 84.39% (74.22-90.79) | |
| Albumin | 0.0589 | |||
| < 38.95 | 99.86% (96.42-99.91) | 98.79% (96.65-98.72) | 89.74% (76.31-93.35) | |
| ≥ 38.95 | 99.01% (89.63-99.82) | 90.68% (86.52-96.45) | 87.23% (75.11-89.63) | |
| Lymphocyte count | 0.0524 | |||
| < 0.975 | 99.46% (96.22-99.82) | 96.89% (95.15-99.02) | 88.76% (78.11-94.05) | |
| ≥ 0.975 | 98.70% (89.93-99.62) | 90.68% (86.82-95.15) | 87.29% (74.11-93.53) |
The collinearity diagnostics of all the explanatory variables was performed to exclude the internal correlation. We selected only the factors that showed a significant correlation with RFS in the univariate survival analysis for inclusion in the Cox proportional hazards model in entry strategies. The results of the study are listed in Table 5. The only significant independent negative prognostic indicators for RFS were high mitotic index (HR6–10/50 HPFs vs 5/50 HPFs = 1.896, 95%CI: 0.518–6.949; HR> 10/50 HPFs vs 5/50 HPFs = 6.791, 95%CI: 1.554–29.672; overall P = 0.0365) and low OPNI (HR = 5.852, 95%CI: 1.072–31.964; P = 0.0414) (Figure 4).
| Factor | Hazard ratio | 95%CI | P value |
| Primary tumor site | 0.0878 | ||
| Gastric | 1.000 | - | - |
| Non-gastric | 2.641 | 0.866-8.053 | - |
| Tumor size (cm) | 0.4749 | ||
| ≤ 2.0 | 1.000 | - | - |
| 2.1-5.0 | 1.318 | 0.006-292.720 | 0.9201 |
| 5.1-10.0 | 1.612 | 0.006-445.888 | 0.8678 |
| > 10.0 | 4.765 | 0.015-1515.961 | 0.5953 |
| Mitotic index (/50 HPFs) | 0.03651 | ||
| ≤ 5 | 1.000 | - | - |
| 6-10 | 1.896 | 0.518-6.949 | 0.3341 |
| >10 | 6.791 | 1.554-29.672 | 0.01091 |
| Tumor rupture | 0.5202 | ||
| No | 1.000 | - | - |
| Yes | 0.589 | 0.117-2.957 | - |
| NIH risk classification | 0.9763 | ||
| Very low risk | 1.000 | - | - |
| Low risk | 0.283 | 0.001-64.779 | 0.6491 |
| Intermediate risk | 0.282 | 0.001-91.515 | 0.6681 |
| High risk | 0.277 | 0.001-101.508 | 0.6702 |
| NLR | 0.7613 | ||
| < 4.34 | 1.000 | - | - |
| ≥ 4.34 | 0.838 | 0.268-2.620 | - |
| PLR | 0.6958 | ||
| < 220.76 | 1.000 | - | - |
| ≥ 220.76 | 1.259 | 0.397-3.995 | - |
| OPNI | 0.04141 | ||
| ≥ 51.30 | 1.000 | - | - |
| < 51.30 | 5.852 | 1.072-31.964 | - |
More precise risk classification criteria that can be used to predict the postoperative prognosis of patients with GIST - especially criteria that can be simply and feasibly measured and calculated by using clinicopathological data - have been required. Herein, we evaluated the prognostic value of the OPNI for patients with GISTs, and our analyses demonstrated that the OPNI was an independent prognostic marker that was associated with the GIST primary site, tumor size, mitotic index, tumor rupture, necrosis, and modified NIH risk classification in our patient series.
The AFIP criteria[7] and the modified NIH consensus criteria[8], which encompass the four factors tumor diameter, mitotic index, location, and rupture of tumor, are the most widely used criteria to evaluate the post-surgery or intra-surgery risk in GIST cases, and the accuracy of these four factors is generally similar for prognosis. A nomogram that can be used to estimate the RFS at 2 and 5 years after surgery for a primary GIST was developed by the Memorial Sloan-Kettering Cancer Center sarcoma team[22]. And more recently, a novel prognostic contour map was generated using the pooled data of 920 GIST patients who received no adjuvant therapy[21].
The OPNI, as a nutrition index, was initially established by Onodera and his colleagues in 1984. The OPNI has been used to divide patients with higher and lower OPNI values for prognostic evaluation, and it was reported that the prognoses of the patients with lower OPNI values were significantly worse than those of the patients with higher OPNI values[22]. Similar results regarding gastric carcinoma have also been reported[23]. In the present study, however, the cut-off value of the PNI was shown to be 51.30 in the ROC analysis. Our further analysis demonstrated that a lower OPNI was associated with the primary tumor site, tumor size, mitotic index, tumor rupture, necrosis, and the modified NIH risk classification. In the multivariate survival analysis, the OPNI was an independent prognostic indicator for GISTs.
A low OPNI may be the result of hypoproteinemia and/or lymphopenia, which can be explained by several potential phenomena: (1) The nutritional supplementation of branched-chain amino acids can improve a patient’s hypoproteinemia and reduce tumor recurrence[24]; and (2) Lymphocytes have an important role in the host immune response, counteracting tumor formation and progression[25].
Because OPNI consists of albumin and lymphocyte count levels, low OPNI means hypoalbuminemia and lymphocytopenia, which may contribute to tumor development and progression[24]. Lower albumin levels in patients with lower OPNI reflect malnutrition and impaired protein synthesis ability especially those with large tumor size and high mitotic index. Lymphocytes have an important role in the host immune response, counteracting tumor formation and progression[25]. The present study also examined lymphocyte-related markers, such as NLR and PLR, but these markers were not identified as independent prognostic factors in the multivariate analysis. OPNI predicted the prognosis of GIST patients more precisely than NLR and PLR because the OPNI contains albumin and lymphocyte levels as nutritional and immune factors.
Our study has several limitations to address. This was a single-center retrospective study, and a multicenter study is needed to expand the sample size to compensate for this deficiency. The best cut-off value was determined by the highest Youden index by plotting the ROC curve, but it is still unclear what cut-off value is the best for the clinical diagnosis of GISTs. An exploration of the best cut-off value and studies of its intrinsic molecular mechanism are future research topics.
In conclusion, our analyses demonstrated an association between immunoinflammatory and nutritional factors and the recurrence-free survival and clinicopathological features of patients with primary GISTs. The OPNI was shown to be an independent indicator for progression-free survival in GISTs, and it may be a valuable parameter for predicting a tumor’s biological behavior using peripheral blood samples.
Prognostic markers have gained considerable attention in gastrointestinal stromal tumors (GISTs).
To improve the prognostic prediction of GISTs, we designed this study.
We conducted the first investigation of the prognostic value of Onodera’s Prognostic Nutritional Index (OPNI) for GISTs.
In this study, the recurrence-free survival, and the receiver operator characteristic analysis of neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), OPNI, and Ki-67 index, and the correlation between tumor size and NLR, PLR, OPNI and Ki-67 index were detected.
Univariate and multivariate analyses both identified the OPNI as an independent prognostic marker.
The preoperative OPNI could be a prognostic marker for GISTs.
We hope that we could find a valuable parameter for predicting the prognosis of GISTs using peripheral blood samples.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Palacios Huatuco RM, Takata T S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006;114:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Nishida T, Goto O, Raut CP, Yahagi N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. 2016;122:3110-3118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 4. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 5. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 482] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 6. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 7. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 8. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 9. | Zhou Y, Hu W, Chen P, Abe M, Shi L, Tan SY, Li Y, Zong L. Ki67 is a biological marker of malignant risk of gastrointestinal stromal tumors: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e7911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Zong L, Chen P, Jiang J, Wang L, Li QG. Predictive value of p53 expression in the risk of malignant gastrointestinal stromal tumors: Evidence from 19 studies. Exp Ther Med. 2012;3:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Zong L, Chen P, Jiang J, Wang H, Wang L. Correlation between p16 expression and malignant risk of gastrointestinal stromal tumor: evidence from nine studies. Hepatogastroenterology. 2012;59:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8326] [Article Influence: 489.8] [Reference Citation Analysis (0)] |
| 13. | Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 338] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 15. | Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 421] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 17. | Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 19. | Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Hubbard TJ, Lawson-McLean A, Fearon KC. Nutritional predictors of postoperative outcome in pancreatic cancer (Br J Surg 2011; 98: 268-274). Br J Surg. 2011;98:1032; author reply 1032-1032; author reply 1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 22. | Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, Donohue JH, DeMatteo RP. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Nishikawa H, Osaki Y. Clinical significance of therapy using branched-chain amino acid granules in patients with liver cirrhosis and hepatocellular carcinoma. Hepatol Res. 2014;44:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47141] [Article Influence: 3367.2] [Reference Citation Analysis (5)] |