Published online Jan 27, 2021. doi: 10.4240/wjgs.v13.i1.76
Peer-review started: October 11, 2020
First decision: November 3, 2020
Revised: November 12, 2020
Accepted: December 1, 2020
Article in press: December 1, 2020
Published online: January 27, 2021
Processing time: 94 Days and 11.4 Hours
Xanthogranulomatous inflammation is characterized histologically by a collection of lipid-laden macrophages admixed with lymphocytes, plasma cells, neutrophils, and often multinucleated giant cells with or without cholesterol clefts.
To review the medical literature on xanthogranulomatous appendicitis (XGA).
We present a patient with XGA and review published articles on XGA accessed via the PubMed, MEDLINE, Google Scholar, and Google databases. Keywords used were “appendix vermiformis,” “appendectomy,” “acute appendicitis,” and “XGA.” The search included articles published before May 2020, and the publication language was not restricted. The search included letters to the editor, case reports, review articles, original articles, and meeting presentations. Articles or abstracts containing adequate information about age, sex, clinical presentation, white blood cells, initial diagnosis, surgical approach, histopathological and immunohistochemical features of appendectomy specimens were included in the study.
A total of 29 articles involving 38 patients with XGA, were retrospectively analyzed. Twenty (52.6%) of the 38 patients, aged 3 to 78 years (median: 34; IQR: 31) were female, and the remaining 18 (47.4%) were male. Twenty-five patients were diagnosed with acute appendicitis, ruptured appendicitis, or subacute appendicitis, and the remaining 13 patients underwent surgery for tumoral lesions of the ileocecal region. Twenty-two of the patients underwent urgent or semi-urgent surgery, and the remaining 16 patients underwent interval appendectomy.
Xanthogranulomatous inflammation rarely affects the appendix vermiformis. It is associated with significant diagnostic and therapeutic dilemmas due to its variable presentation. It is often associated with interval appendectomies, and a significant number of patients require bowel resection due to the common presentation of a tumoral lesion. XGA is usually identified retrospectively on surgical pathology and has no unique features in preoperative diagnostic studies.
Core Tip: Xanthogranulomatous inflammation is characterized histologically by a collection of lipid-laden macrophages admixed with lymphocytes, plasma cells, neutrophils, and often multinucleated giant cells with or without cholesterol clefts. Xanthogranulomatous appendicitis (XGA) has rarely been reported to date. In this review article, we present a patient with XGA, and review data from all articles published on this rare situation. This review study shows that XGA is associated with significant diagnostic and therapeutic dilemmas due to its variable presentation. It is often associated with interval appendectomies, and a significant number of patients require bowel resection due to the common presentation of a tumoral lesion.
- Citation: Akbulut S, Demyati K, Koc C, Tuncer A, Sahin E, Ozcan M, Samdanci E. Xanthogranulomatous appendicitis: A comprehensive literature review. World J Gastrointest Surg 2021; 13(1): 76-86
- URL: https://www.wjgnet.com/1948-9366/full/v13/i1/76.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i1.76
Xanthogranulomatous inflammation is a well-known form of inflammation, characterized histologically by a collection of lipid-laden macrophages admixed with lymphocytes, plasma cells, neutrophils, and often multinucleated giant cells with or without cholesterol clefts[1]. Xanthogranulomatous inflammation was first described in the urogenital system by Osterlind in 1944[2]. Since then, it has been reported in other organs, such as the gallbladder, stomach, colon, anorectal area, endometrium, ovary, epididymis, vagina, testis, prostate, skin, urinary bladder, bone, thyroid, lung and adrenal glands, fallopian tubes, and the appendix vermiformis[3-9].
Xanthogranulomatous appendicitis (XGA) is rarely reported in the appendix vermiformis. Some studies stated that the first XGA case was reported by Cozzuto and colleagues[1,3]. Other studies stated that Birch and colleagues[4,10-13] were the first to report this entity. However, according to our literature search, the first case of XGA was described by Dymock and colleagues in 1977[14]. Its clinical significance includes the significant diagnostic challenge it causes because it can mimic clinically, radiologically, and even pathologically malignant tumors as well as other inflammatory processes of the appendix vermiformis. It is usually found retrospectively on surgical pathology and has no unique features on imaging studies, including abdominopelvic computed tomography. Little information has been written in the literature regarding this entity. Moreover, its clinical implications remain to be evaluated. In this review article, we present a case of XGA, and review data from all articles published on XGA.
The primary aim of this study was to review the articles published in the medical literature on XGA. To achieve this objective, a literature search was conducted on PubMed, MEDLINE, Google Scholar, and Google databases using the following keywords: “Appendix vermiformis,” “acute appendicitis,” “XGA,” “interval appendectomy,” and “appendectomy.” All documents published on XGA before May 2020 were reviewed. The corresponding authors of the articles with substantially large amounts of missing information were e-mailed to obtain information on their cases. As a result, articles without an accessible full-text version, those that did not provide adequate information in their abstracts, and those that did not include comprehensive information as that provided in other studies were excluded. As some enrolled articles were published in the form of a literature review, their tables were also used. The following information was collected: Reference list, publication year, paper type (full text, abstract, poster), age, sex, clinical presentation, white blood cell (WBC) count, radiological tools, surgical approach, histopathological features (giant cells, plasma cells, foamy histiocytes, CD68 stain), and follow-up. The secondary aim of this study was to present a 66-year-old woman with XGA.
Although a total of 36 article titles[3-38] matched as a result of the literature review conducted in accordance with the criteria specified in the methodology section, seven articles[1,14,33-37] were excluded from the study due to the absence of demographic and clinical data of the patients. A total of 38 patients, 20 (52.6%) female and 18 (47.4%) male, aged from 3 to 78 years (median: 34; IQR: 31) were included in this study. Fifteen patients' WBC values were reported, and 13 (86.7%) of them had leukocytosis. Treatment was planned for 25 patients with a pre-diagnosis of acute appendicitis, ruptured appendicitis, or subacute appendicitis. On the other hand, treatment was planned for nine patients due to a mass in the ileocecal region. After appropriate medical treatment for a total of 16 patients, interval appendectomy was performed. Of the 29 articles, 27 were published in English, one in Japanese, and one in Spanish. The full text was obtained for 28 of the 29 articles, whereas only the abstract was available for one paper. The details of the demographic and clinical characteristics of the patients are given in Tables 1 and 2.
| No. | Ref. | Year | Country | Language | Article type | Article type | Age | Sex | Clinical presentation | WBC |
| 1 | Quadri et al[15] | 2019 | United States | English | Case series | Full text | 64 | M | RLQ pain + palpable mass | NA |
| 2 | Yang et al[16] | 2018 | South Korea | English | Congress present | Full text | 69 | M | NA | NA |
| 3 | Al-Zaidi et al[36] | 2018 | India | English | Case report | Full text | 48 | M | RLQ pain | 16000 |
| 4 | Adhikari et al[4] | 2019 | India | English | Case report | Full text | 49 | F | RLQ pain + fever | 12200 |
| 5 | Kaushik et al[17] | 2017 | India | English | Case report | Full text | 47 | F | Abdominal pain, vomiting, fever | 14000 |
| 6 | Hoabam et al[10] | 2017 | India | English | Case report | Full text | 56 | F | RLQ pain | 14000 |
| 7 | Mehrotra et al[18] | 2017 | India | English | Case report | Full text | 30 | F | RLQ pain | Normal |
| 8 | Laiphrakpam | 2017 | India | English | Case report | Full text | 36 | M | RLQ Pain | Normal |
| 9 | Nam et al[20] | 2016 | South Korea | English | Case report | Full text | 23 | F | Low abdominal pain | NA |
| 10 | Cavusoglu et al[21] | 2016 | Turkey | English | Case report | Full text | 12 | M | NA | NA |
| 11 | M | NA | NA | |||||||
| 11 | Jusoh et al[5] | 2016 | Malaysia | English | Case report | Full text | 16 | M | RLQ pain | NA |
| 12 | Thapa et al[6] | 2016 | Nepal | English | Case report | Full text | 19 | F | RLQ pain | NA |
| 13 | Singh et al[7] | 2015 | India | English | Case report | Full text | 21 | F | RLQ pain | NA |
| 14 | Altay et al[22] | 2015 | Turkey | English | Case report | Full text | 73 | F | RLQ pain | Leukocytosis |
| 15 | Chandanwale | 2015 | India | English | Case report | Full text | 40 | F | RLQ pain | NA |
| 16 | Montazer et al[23] | 2014 | Iran | English | Case report | Full text | 29 | F | RLQ pain | 13000 |
| 17 | Kochhar et al[24] | 2014 | India | English | Case report | Full text | 50 | M | RLQ pain + fever | 24000 |
| 18 | Al-Rawabdeh et al[12] | 2013 | United States | English | Case report | Full text | 11 | M | RLQ pain | 4900 |
| 19 | Mado et al[25] | 2013 | Japan | English | Image in surgery | Full text | 78 | M | RLQ pain | NA |
| 20 | Martinez-Garza et al[26] | 2011 | Spain | Spanish | Case report | Full text | 30 | F | RLQ pain | 13700 |
| 21 | Omer et al[8] | 2011 | Sudan | English | Case report | Full text | 49 | M | RLQ pain | NA |
| 22 | Omori et al[27] | 2011 | Japan | Japanese | Case report | Full text | 57 | F | RLQ pain | NA |
| 23 | Young et al[28] | 2009 | United States | English | Case report | Full text | 32 | F | RLQ pain | 22000 |
| 24 | Chuang et al[29] | 2005 | Taiwan | English | Case report | Abstract | 39 | M | RLQ pain | NA |
| 25 | Guo et al[30] | 2003 | United States | English | Original article | Full text | 4 | F | NA | NA |
| 12 | M | NA | NA | |||||||
| 13 | M | NA | NA | |||||||
| 3 | M | NA | NA | |||||||
| 9 | M | NA | NA | |||||||
| 29 | F | NA | NA | |||||||
| 29 | F | NA | NA | |||||||
| 27 | M | NA | NA | |||||||
| 26 | Munichor | 2000 | Israel | English | Case report | Full text | 37 | F | RLQ pain | 12000 |
| 27 | McVey et al[32] | 1994 | United States | English | Letter | Full text | 40 | F | RLQ pain | 12100 |
| 28 | Birch et al[13] | 1993 | United Kingdom | English | Brief report | Full text | 51 | M | Perineal pain | NA |
| 66 | F | Right flank pain | 20000 | |||||||
| 29 | Rogers et al[9] | 1992 | United Kingdom | English | Case report | Full text | 56 | F | RLQ pain | NA |
| No. | Preoperative Diagnosis | Surgical approach | Giant Cells | Plasma Cells | Eosinophils | CD68 Stain | Foamy Histiocytes |
| 1 | Mass | Right hemicolectomy | NA | NA | NA | NA | NA |
| 2 | Perforated App | Appendectomy (Interval) | NA | NA | NA | NA | NA |
| 3 | Perforated App | Right hemicolectomy | Yes | Yes | NA | Yes | Yes |
| 4 | AAp | Appendectomy | Yes | Yes | Yes | NA | Yes |
| 5 | Neoplastic mass | Limited colon resection | Yes | Yes | Yes | NA | Yes |
| 6 | AAp | Appendectomy | Yes | Yes | NA | NA | Yes |
| 7 | AAp | Appendectomy | Yes | NA | NA | NA | Yes |
| 8 | AAp | Appendectomy | Yes | Yes | NA | NA | Yes |
| 9 | Chronic Ap or mucocele | Appendectomy | NA | NA | NA | Yes | Yes |
| 10 | Mass | Appendectomy (Interval) | Yes | Yes | NA | Yes | NA |
| AAp | Appendectomy (Interval) | Yes | Yes | NA | NA | NA | |
| 11 | AAp | Appendectomy (Interval) | Yes | NA | NA | NA | Yes |
| 12 | AAp | Appendectomy (Interval) | Yes | Yes | Yes | NA | Yes |
| 13 | AAp | Appendectomy | Yes | Yes | Yes | Yes | Yes |
| 14 | Mass | Appendectomy | NA | NA | NA | Yes | NA |
| 15 | Mass | Right hemicolectomy | Yes | Yes | NA | NA | Yes |
| 16 | AAp | Appendectomy | Yes | NA | NA | Yes | Yes |
| 17 | AAp | Right hemicolectomy + ileostomy | Yes | Yes | NA | NA | Yes |
| 18 | AAp | Appendectomy | Yes | Yes | NA | NA | NA |
| 19 | Mucocele | Ileocecal resection | NA | NA | NA | NA | Yes |
| 20 | AAp | Appendectomy | NA | NA | NA | NA | Yes |
| 21 | Mass | Appendectomy (Interval) | Yes | Yes | NA | NA | NA |
| 22 | Mass | Right hemicolectomy + right nephrectomy + oophorectomy | NA | Yes | NA | NA | Yes |
| 23 | AAp | Appendectomy (Interval) | NA | NA | NA | NA | NA |
| 24 | Colitis of cecum | Right hemicolectomy | NA | NA | Yes | NA | NA |
| 25 | AAp | Appendectomy (Interval) | Yes | NA | NA | NA | NA |
| AAp | Appendectomy (Interval) | No | NA | NA | NA | NA | |
| AAp | Appendectomy (Interval) | No | NA | NA | NA | NA | |
| AAp | Appendectomy (Interval) | No | NA | NA | NA | NA | |
| AAp | Appendectomy (Interval) | Yes | NA | NA | NA | NA | |
| AAp | Appendectomy (Interval) | No | NA | NA | NA | NA | |
| Subacute AAp. | Appendectomy (Interval) | No | NA | NA | NA | NA | |
| Subacute AAp. | Appendectomy (Interval) | Yes | NA | NA | NA | NA | |
| 26 | AAp | Appendectomy | NA | Yes | Yes | Yes | Yes |
| 27 | Mass | Appendectomy (Interval) | NA | Yes | NA | NA | Yes |
| 28 | AAp | Appendectomy | NA | Yes | NA | NA | Yes |
| Mass | Appendectomy | NA | Yes | NA | NA | Yes | |
| 29 | Fistula | Appendectomy | NA | NA | NA | NA | NA |
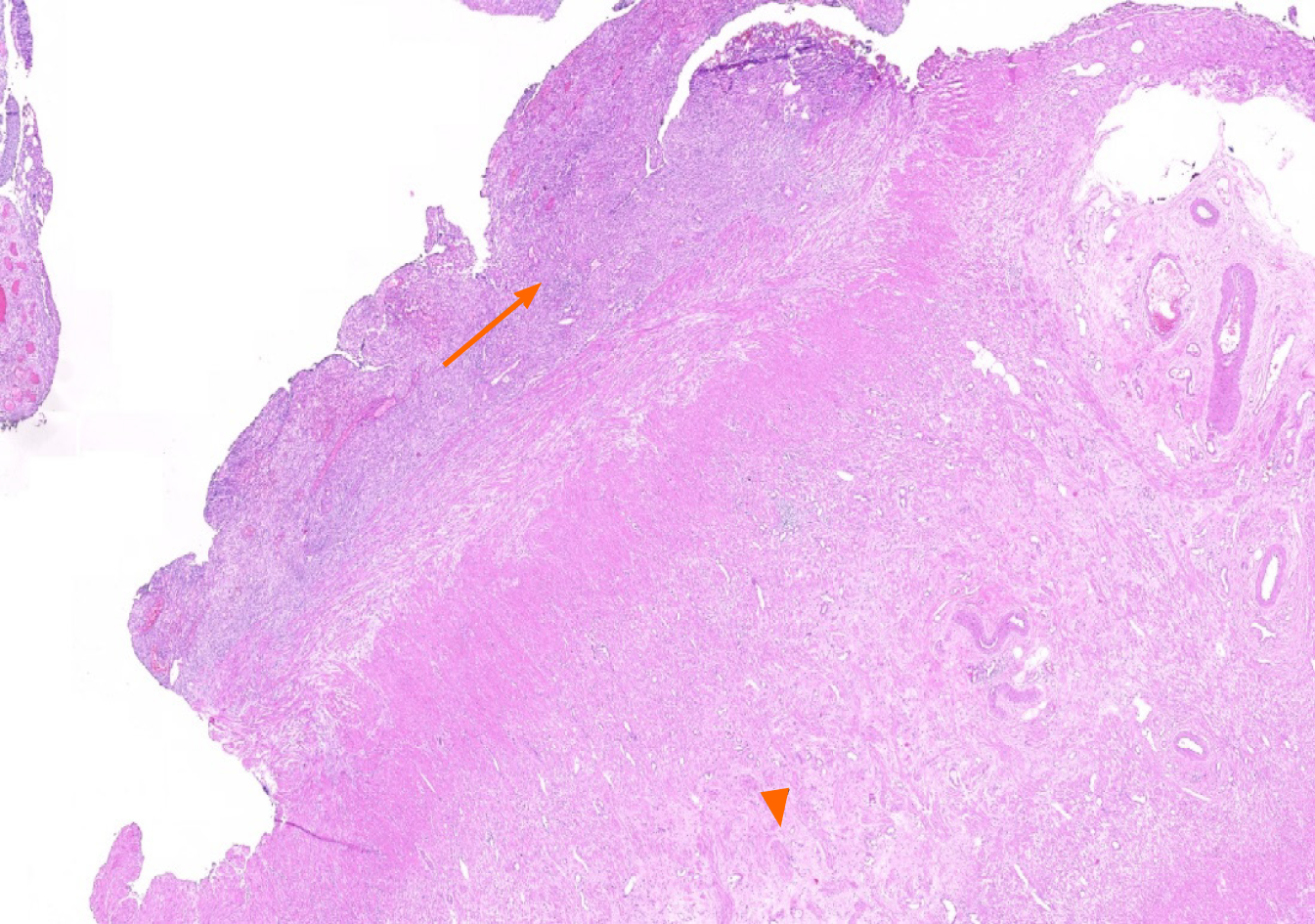
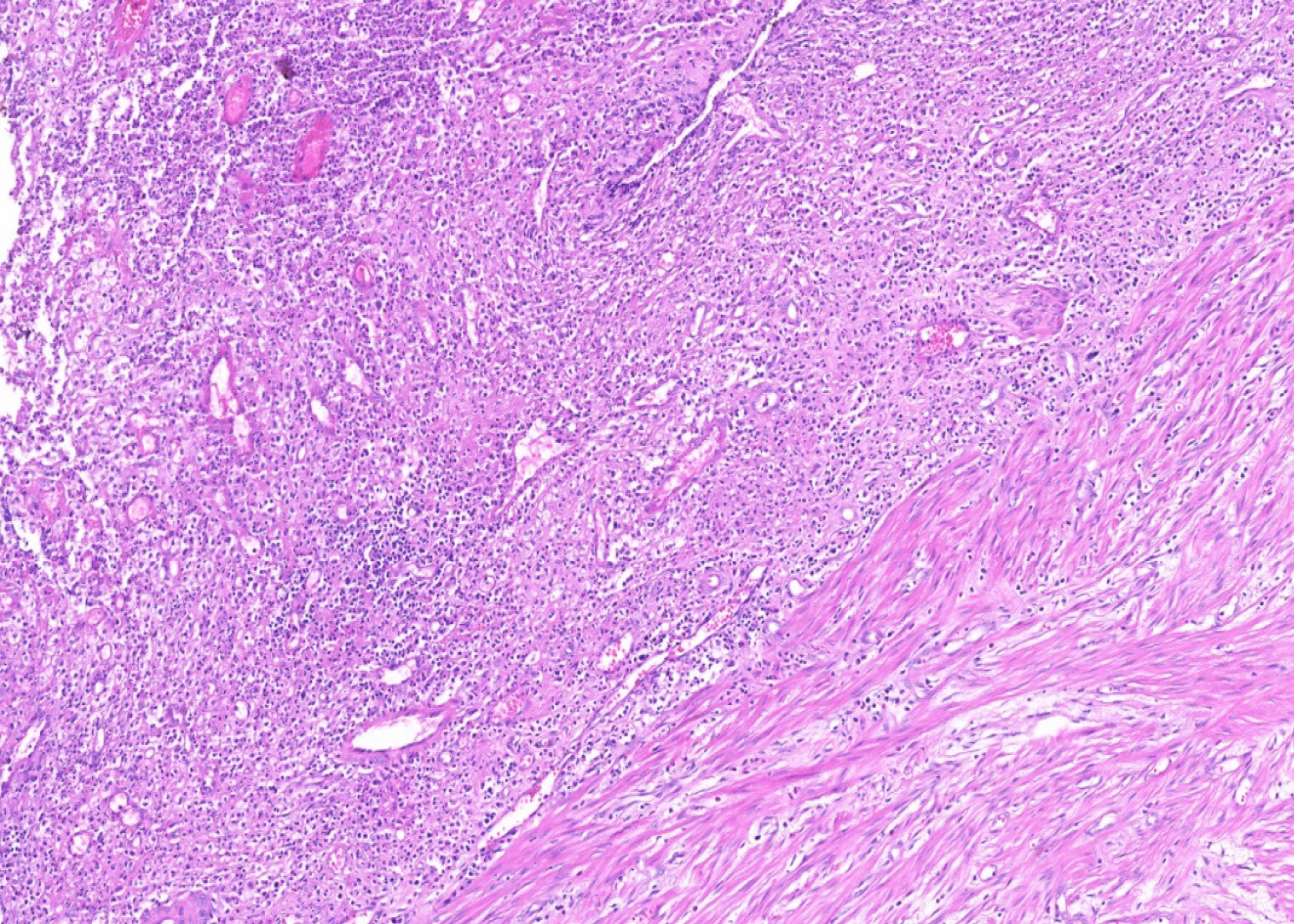
A 66-year-old female patient was admitted to our emergency unit because of right lower quadrant pain, which started 3 d prior to admission. She had a medical history of left hemiparesis secondary to cerebrovascular events, mitral valve stenosis, and atrial fibrillation. As the patient had a speech disorder, the history was taken from her husband and children who lived in the same house. Physical examination revealed significant rebound tenderness in the right lower quadrant. Biochemical analyses were as follows: WBC count 12.900/mL (4.300-10.300), platelets 337000 mL (156000-373000), neutrophils 80.7%, CRP 13.7 mg/dL (0-0.35), and international normalized ratio 1.7 (0.8-1.2). Ultrasonography revealed an edematous and aperistaltic tubular structure with a diameter of 2 cm in the right lower quadrant of the abdomen that was not compressible with external pressure. In addition, free fluid was detected around the defined structure, suggesting ruptured appendicitis. After evaluating the clinical, radiological, and biochemical blood parameters of the patient, she was diagnosed with ruptured appendicitis, and underwent surgery under emergency conditions. In view of the logistic problems with laparoscopic equipment at the time of surgery, laparotomy was performed using an infraumbilical midline incision. The exploration showed that the ileocecal region was completely surrounded by the omentum, and the sigmoid colon was attached to this defined area. After careful dissection of the omentum and sigmoid colon, a ruptured appendix with distal necrosis was observed, and the surrounding pus was then aspirated. As the stump of the appendix was very large and inflamed, appendectomy was performed after a clamp was placed at the junction with the cecum. The stump was closed with 3/0 polypropylene sutures using the transfixion suture technique. One drain was placed in the pelvis, and the operation was terminated. The pathology report was prepared as ruptured appendicitis secondary to xanthogranulomatous inflammation (Figures 1-3). Antibiotic treatment consisting of ceftriaxone and metronidazole was administered for 5 d postoperatively. The patient was discharged without any postoperative complications.
Acute appendicitis is the most common acute surgical condition of the abdomen. Most of the resected appendectomy specimens have been reported to have marked cellular infiltration, predominantly by neutrophils. In contrast, the occurrence of xanthogranulomatous inflammation is extremely rare. In a 2-year study that was performed to determine the incidence of various non-neoplastic and neoplastic lesions of the appendix, only one case of this entity was identified (0.22%)[34]. Similarly, Laishram and colleagues[37] reported that the incidence of XGA was 0.25% in 4298 appendectomy specimens. On the other hand, Shaik and colleagues[35] stated that the incidence of XGA among patients who underwent appendectomy was 0.64%.
Grossly, the typical findings are bright yellow or golden yellow mass-like lesions associated with abscess cavities[1,36]. Kaushik and colleagues[17] studied the cytological evaluation of the touch imprint preparation for intraoperative diagnosis of XGA. The smears revealed benign glandular epithelial cell groups and sheets of xanthoma cells along with multinucleate histiocytic giant cells in the background of neutrophils and mononuclear inflammatory cells. Microscopic examination of XGA usually reveals a nodular or diffuse mucosal to transmural collection of macrophages, including foamy histiocytes (xanthoma-type cells), intermixed with varying amounts of other inflammatory cells[7].
Although the histopathological features of the xanthogranulomatous process have been defined, the exact etiopathogenesis of XGA is still unknown. Proposed theories include defective lipid transport, immunologic disturbances of leukocyte and macrophage chemotaxis, infection by lowvirulence organisms, such as Proteus and Escherichia species, and lymphatic obstruction[7,13,17,25,31,34].
Cozzutto and colleagues[1] performed an extensive review of all cases from various organs. In that study, the authors noted that the xanthogranulomatous process is usually associated with inflammation, hemorrhage, and necrosis. They suggested that hemorrhage may play a major role in the development of foamy macrophages, postulating that the ingested erythrocytes and platelets at the bleeding site overwhelm the lysosomal system of the macrophages, causing deposition of phospholipids, which results in a foamy appearance of the macrophages. Other authors have suggested that there are several factors that may precipitate XGA, including lumen obstruction, suppurative inflammation, hemorrhage, and local tissue hypoxia, with no single pathophysiological factor that can possibly cause XGA[5,30,37].
Other lesions with granulomatous inflammation and foam cells can be seen in the differential diagnoses, such as Crohn’s disease and malakoplakia. The absence of transmural involvement by granulomas can exclude Crohn’s disease, and the absence of Michaelis Gutmann bodies can rule out malakoplakia. Furthermore, it can be very challenging to differentiate XGA from an infiltrative cancer because XGA might present as a mass lesion with extension of fibrosis and inflammation to the surrounding tissues, mimicking an infiltrative cancer[4,7,11,24,30,36].
Most XGA cases reported were in the adult age group, with the median age of presentation in this review being 34 years. The mean age (35.9 years) identified in this review was lower than the previously reported mean age of 47.9 years (83%, 21-78 years)[36]. This appears to be caused by the recent, more XGA pediatric reports published. However, this disease remains more common in adults, with only 6 out of 38 patients in this review belonging to the pediatric age group (15.8%). The oldest patient diagnosed with XGA in this review was 78 years old, who presented with a mucocele of the appendix[25], and the youngest affected patient was 3 years old, diagnosed with interval appendectomy[30]. No sex predilection was reported for XGA[34,36], and in this review, there was no significant difference in the number of cases reported between females (52.6%) and males (47.4%).
Patients with XGA usually present with right lower abdominal quadrant pain, fever, nausea, and vomiting. However, the clinical presentation of XGA is variable, which seems to vary with the spread of the disease. While some authors suggested an association of the xanthogranulomatous response with long-standing inflammation of the appendix and formation of the appendiceal mass[34], others have reported cases of XGA with typical signs and symptoms of acute appendicitis[31]. In this review, 22 of the 38 reported cases were diagnosed with acute appendicitis (57.9%), two of which were found to be ruptured.
XGA showed a higher incidence in interval appendectomies[6,16,20]. Guo et al[30] reviewed the histopathology of all interval appendectomy specimens within a four-year period, and compared them with a control group of patients who had acute appendicitis and underwent routine acute appendectomy. The study revealed that xanthogranulomatous inflammation is common in interval appendectomy specimens. They represented 36% of the interval appendectomy cases in their series, but they did not occur in the emergency appendectomy group.
Due to the destructive nature of the disease, XGA can occasionally present with a mass lesion that can mimic locally advanced cancer, but it has a benign course and can be cured surgically. Altay and colleagues reported uterine and right adrenal involvement, presenting as a complicated pelvic abscess on radiological imaging[22]. In this review, 13 of the 38 patients had a mass (34%), and two patients had a mucocele. Eight patients required bowel resection ranging from limited ileocecal resection to formal right hemicolectomy. Of the 38 patients, 30 underwent appendectomy, 16 of which as an interval appendectomy.
The variable presentation of XGA requires the consideration of acute appendicitis, a mucinous epithelial neoplasm, a non-mucinous epithelial neoplasm, and a range of chronic infectious diseases. Atypical appendiceal pathologies ranging from neoplasms to inflammatory conditions can mimic and even cause a superimposed acute appendicitis, making them difficult to differentiate from typical inflammation. Contrast-enhanced multidetector computed tomography is the gold standard and the most cost-effective diagnostic test for appendicitis in non-pregnant adults with right lower quadrant pain[15,38,39]. However, radiological findings are non-specific, and XGA is usually found retrospectively on surgical pathology and has no unique features on abdominopelvic contrast-enhanced computed tomography[15].
In summary, xanthogranulomatous inflammation is an unusual, destructive, chronic inflammatory process that involves various organs. While it rarely affects the appendix vermiformis, it is associated with significant diagnostic and therapeutic dilemmas due to its variable presentation. It is more often associated with interval appendectomies, and a significant number of patients require bowel resection due to the common presentation of a mass lesion. XGA is usually identified retrospectively on pathological examination of the appendiceal specimen, and has no unique features on imaging studies including contrast-enhanced computed tomography.
Xanthogranulomatous inflammation is characterized histologically by a collection of lipid-laden macrophages admixed with lymphocytes, plasma cells, neutrophils, and often multinucleated giant cells with or without cholesterol clefts
Although a limited number of case reports on xanthogranulomatous appendicitis (XGA) have been published to date, no systematic literature analysis has been conducted.
The main objective of this study was to review the articles published in the medical literature on XGA. A secondary objective of this study was to present the medical history of a female patient diagnosed with XGA.
A systematic literature search was conducted on PubMed, Medline, Google Scholar, and Google databases using the following keywords: Appendix vermiformis, acute appendicitis, XGA, interval appendectomy, and appendectomy. The search included articles published before May 2020, and the publication language was not restricted.
A total of 29 articles involving 38 patients with XGA, were retrospectively analyzed. A total of 38 patients, 20 (52.6%) female and 18 (47.4%) male, aged from 3 to 78 years were included in this study. Fifteen patients' WBC values were reported, and 13 (86.7%) of them had leukocytosis. Twenty-five patients were diagnosed with acute appendicitis, ruptured appendicitis, or subacute appendicitis, and the remaining 13 patients underwent surgery for tumoral lesions of the ileocecal region. Twenty-two of the patients underwent urgent or semi-urgent surgery, and the remaining 16 patients underwent interval appendectomy.
Xanthogranulomatous inflammation rarely affects the appendix vermiformis. It is associated with significant diagnostic and therapeutic dilemmas due to its variable presentation. It is often associated with interval appendectomies, and a significant number of patients require bowel resection due to the common presentation of a tumoral lesion.
A review of the literature and our experience of appendiceal diseases suggest that XGA is usually identified after histopathological examination of the appendectomy specimen and XGA has no unique features in preoperative diagnostic studies. Therefore, the most important factors regarding the preliminary diagnosis of XGA are surgeon's experience, clinical suspicion and intraoperative findings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aranda-Narvaez J S-Editor: Fan JR L-Editor: Webster JR P-Editor: Li JH
| 1. | Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Levy M, Baumal R, Eddy AA. Xanthogranulomatous pyelonephritis in children. Etiology, pathogenesis, clinical and radiologic features, and management. Clin Pediatr (Phila). 1994;33:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Munichor M, Kerner H, Cohen H, Bickel A, Iancu TC. Xanthogranulomatous appendicitis--an incidental finding of localized pathology. Ultrastruct Pathol. 2000;24:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Adhikari A, Ray RN, Minz RS, Nayek BC. Xanthogranulomatous appendicitis: Entity of surprise. Arch Med Health Sci. 2018;6:120-121. |
| 5. | Jusoh AC, Ghani SA. Xanthogranulomatous lesion in recurrent appendicitis. Formosan J Surg. 2016;49:114-118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Thapa R, Gurung P, Kafle N, Pradhanang S, Lakhey M, Singh DR. Xanthogranulomatous appendicitis: A case report. J Institute of Med. 2017;38:126-128. |
| 7. | Singh V, John KM, Malik A, Pareek T, Dutta V. Xanthogranulomatous appendicitis: Uncommon histological variant of a common entity. Med J Armed Forces India. 2015;71:S19-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Omer TA, El Hassan LAM, El Hassan AM, Fahal AH. A rare presentation of xanthogranulomatous appendicitis and caecal angiolipoma in the same patient. Sudan Med J. 2011;47:165-168. |
| 9. | Rogers S, Slater DN, Anderson JA, Parsons MA. Cutaneous xanthogranulomatous inflammation: a potential indicator of internal disease. Br J Dermatol. 1992;126:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Hoabam S, Devi KS, Thiyam U. Xanthogranulomatous appendicitis- An uncommon entity. J Evolution Med Dent Sci. 2017;6(13):1061-1063. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Chandanwale SS, Dey I, Kaur S, Nair R, Patil AA. Xanthogranulomatous appendicitis mimicking appendicular lump: An uncommon entity. Clin Cancer Investig J. 2015;4:769-771. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Al-Rawabdeh SM, Prasad V, King DR, Kahwash SB. Xanthogranulomatous appendicitis in a child: report of a case and review of the literature. Case Rep Med. 2013;2013:498191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Birch PJ, Richmond I, Bennett MK. Xanthogranulomatous appendicitis. Histopathology. 1993;22:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Dymock RB. Pathological changes in the appendix: a review of 1000 cases. Pathology. 1977;9:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Quadri R, Vasan V, Hester C, Porembka M, Fielding J. Comprehensive review of typical and atypical pathology of the appendix on CT: cases with clinical implications. Clin Imaging. 2019;53:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Yang DM, Kim HC, Kim SW. A Pictorial review of xanthogranulomatous inflammation of various organs in the abdomen and pelvis. European Congress of Radiology February 28–March 4 2018 Vienna, Austria. [DOI] [Full Text] |
| 17. | Kaushik R, Gulati A, Vedant D, Kaushal V. Cytological diagnosis of xanthogranulomatous appendicitis. J Cytol. 2017;34:48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Mehrotra N, Arakeri SU. Xanthogranulomatous Appendicitis: A Rare Case Report. JKIMSU. 2017;6:125-128. |
| 19. | Laiphrakpam A, Singh KL, Devi LS, Moirangthem G S. Xanthogranulomatous appendicitis: A rare histopathological entity. J Med Soc. 2017;31:208-210. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Nam S, Kang J, Choi SE, Kim YR, Baik SH, Sohn SK. Xanthogranulomatous Appendicitis Mimicking Residual Burkitt's Lymphoma After Chemotherapy. Ann Coloproctol. 2016;32:83-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Cavusoglu YH, Ardicli B, Apaydin S, Avsarlar CE, Yilmaz E. Xanthogranulomatous appendicitis in interval appendectomy specimens of children. J Pediatric Surg Case Rep. 2016;8:27-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Altay C, Yavuz E, Egeli T, Canda EA, Sarioglu S, Secil M. Xanthogranulomatous appendicitis causing an endometrial abscess: radiological findings. Wien Klin Wochenschr. 2015;127:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Montazer F, Emadian O, Torabizadeh Z, Majlesi M, Bahari M. Xanthogranulomatous appendicitis an incidental finding. Int J Med Invest. 2014;4:37-39. |
| 24. | Kochhar G, Saha S, Andley M, Kumar A, Kumar A. Xanthogranulomatous appendicitis with a fulminant course: report of a case. J Clin Diagn Res. 2014;8:ND01-ND02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Mado K, Mazaki T, Henmi A, Masuda H, Takayama T. Xanthogranulomatous appendicitis. Indian J Surg. 2013;75:405-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Martinez-Garza PA, Robles LLPA, Visag CVJ, Reyes EL. Xanthogranulomatous appendicitis: report of a case and literature review. Cir Gen. 2011;33:262-265. |
| 27. | Omori I, Kohashi T, Matsugu Y, Nakahara H, Nishisaka T. A case of xanthogranulomatous appendicitis difficult to differentiate from appendiceal cancer. J Japan Surgical Assoc. 2011;72:409-413. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Young BC, Hamar BD, Levine D, Roqué H. Medical management of ruptured appendicitis in pregnancy. Obstet Gynecol. 2009;114:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Chuang YF, Cheng TI, Soong TC, Tsou MH. Xanthogranulomatous appendicitis. J Formos Med Assoc. 2005;104:752-754. [PubMed] |
| 30. | Guo G, Greenson JK. Histopathology of interval (delayed) appendectomy specimens: strong association with granulomatous and xanthogranulomatous appendicitis. Am J Surg Pathol. 2003;27:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Khalyl-Mawad J, Greco MA, Schinella RA. Ultrastructural demonstration of intracellular bacteria in xanthogranulomatous pyelonephritis. Hum Pathol. 1982;13:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | McVey RJ, McMahon RF. Xanthogranulomatous appendicitis. Histopathology. 1994;24:198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Carr NJ, Path MRC, Montgomery E. Patterns of Healing in the Appendix. The Morphologic Changes in Resolving Primary Acute Appendicitis and a Comparison With Crohn’s Disease. Int J Surg Pathol. 1994;2:23-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Kulkarni MP, Sulhyan KR, Barodawala SM, Yadav DH. Histopathological study of lesions of the appendix. Int J Health Sci Res. 2017;7:90-95. |
| 35. | Shaik S, Jayakumar NM, Manikyam UK. Should every appendicectomy specimen be subjected to histopathological examination? J Evid Based Med Health. 2017;4:5772-5775. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Al-Zaidi RS. Xanthogranulomatous Appendicitis: an Unusual Pattern of Appendiceal Inflammation. Saudi J Pathol Microbiol. 2018;3:115-120. [DOI] [Full Text] |
| 37. | Laishram S, Shimray R, Pukhrambam GD, Sarangthem B, Sharma AB. Xanthogranulomatous inflammatory lesions: a 10-year clinicopathological study in a teaching hospital. Bangladesh J Med Sci. 2014;13:302-305. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Rosen MP, Ding A, Blake MA, Baker ME, Cash BD, Fidler JL, Grant TH, Greene FL, Jones B, Katz DS, Lalani T, Miller FH, Small WC, Spottswood S, Sudakoff GS, Tulchinsky M, Warshauer DM, Yee J, Coley BD. ACR Appropriateness Criteria® right lower quadrant pain--suspected appendicitis. J Am Coll Radiol. 2011;8:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Drake FT, Flum DR. Improvement in the diagnosis of appendicitis. Adv Surg. 2013;47:299-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |