Copyright
©The Author(s) 2023.
World J Gastrointest Surg. Jun 27, 2023; 15(6): 1149-1158
Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1149
Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1149
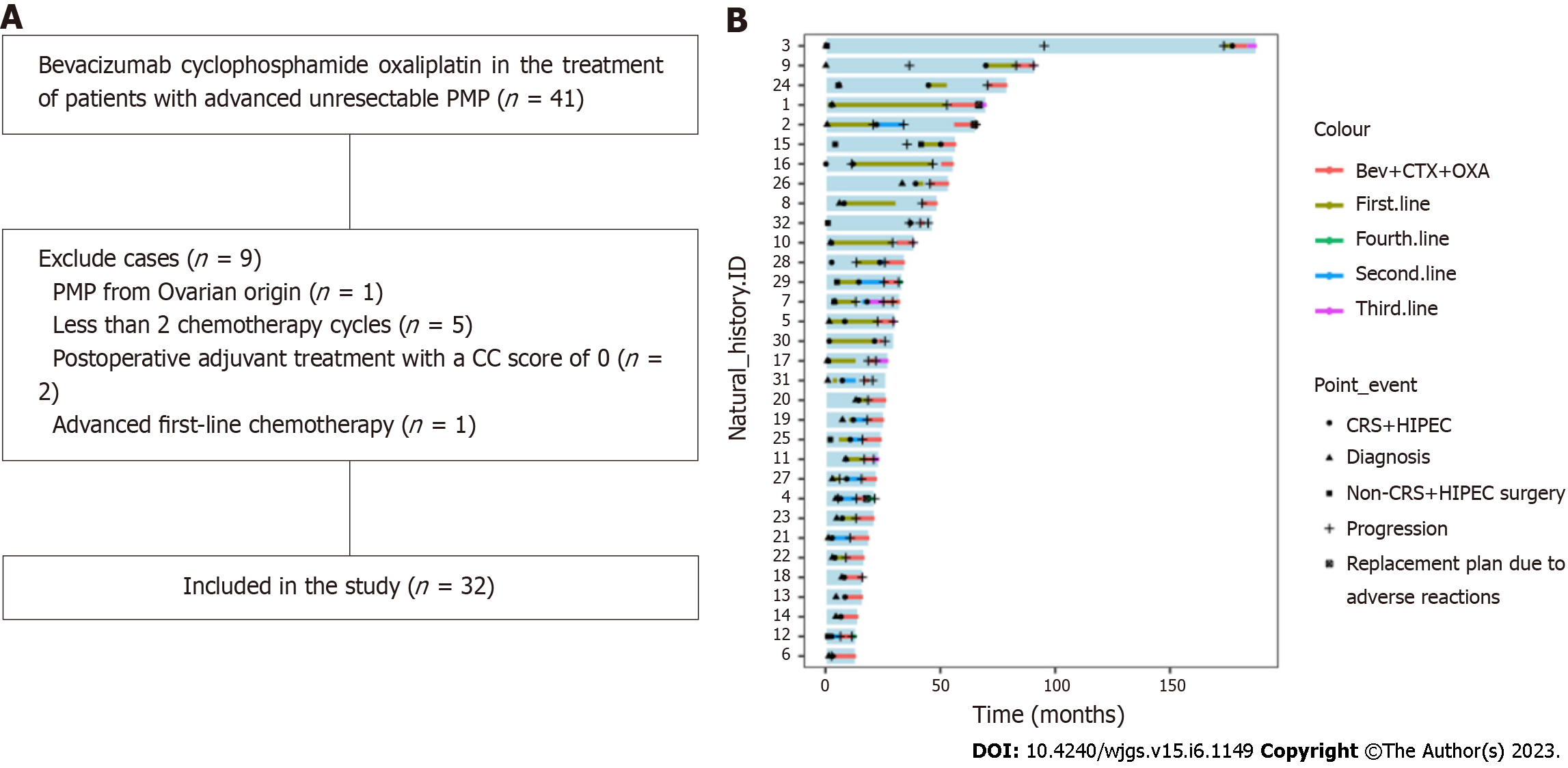
Figure 1 Study design.
A: Flow chart; B: Swimmer plot of the 32 patients. PMP: Pseudomyxoma peritonei; CC: Completeness of cytoreduction; Bev+CTX+OXA: Bevacizumab combined with cyclophosphamide and oxaliplatin; CRS: Cytoreductive surgery; HIPEC: Hyperthermic intraperitoneal chemotherapy.
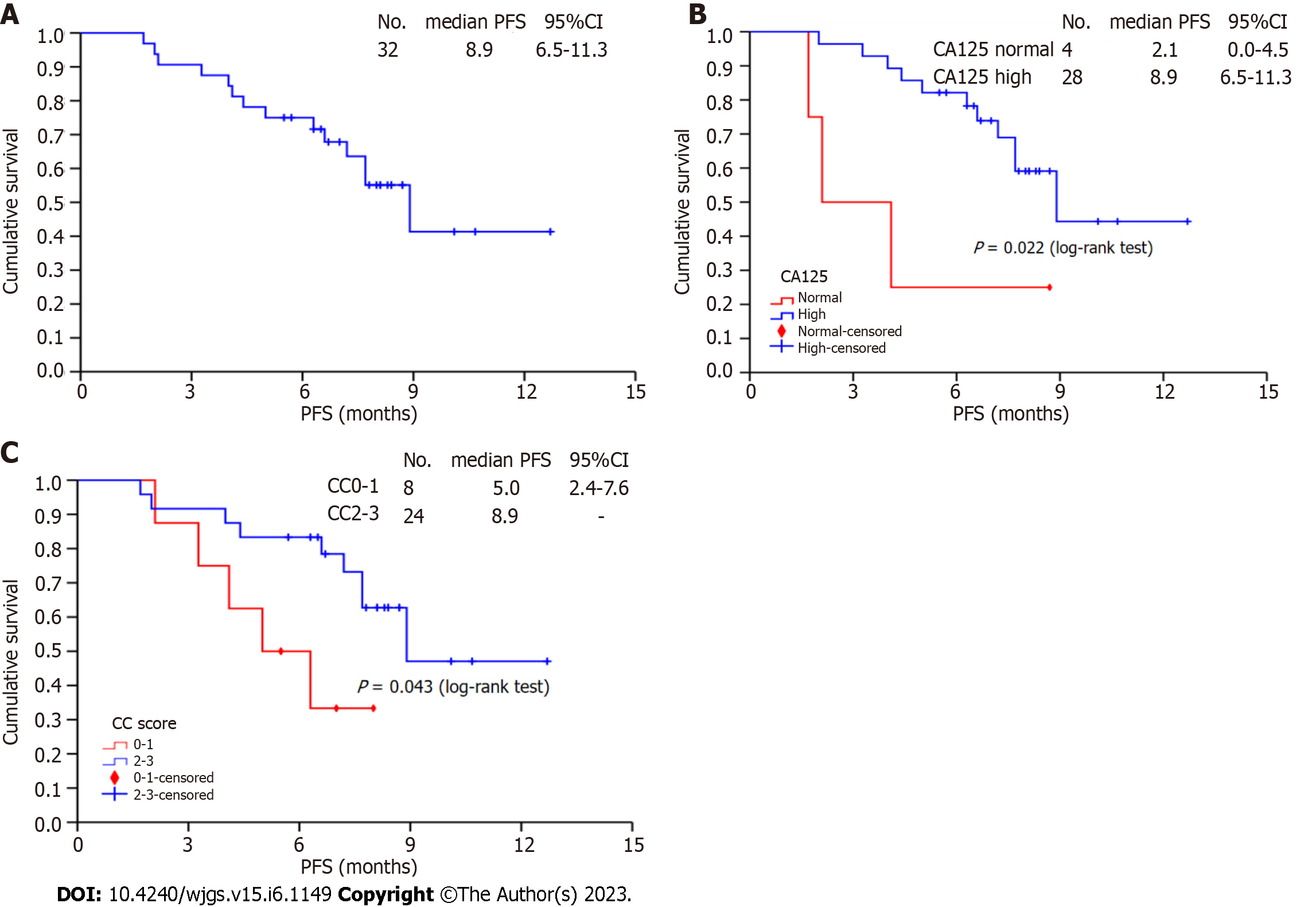
Figure 2 Progression-free survival for 32 patients.
A: Total progression-free survival; B: Stratified analysis of preoperative CA125; C: Stratified analysis of completeness of cytoreduction score. PFS: Progression-free survival; CC: Completeness of cytoreduction.
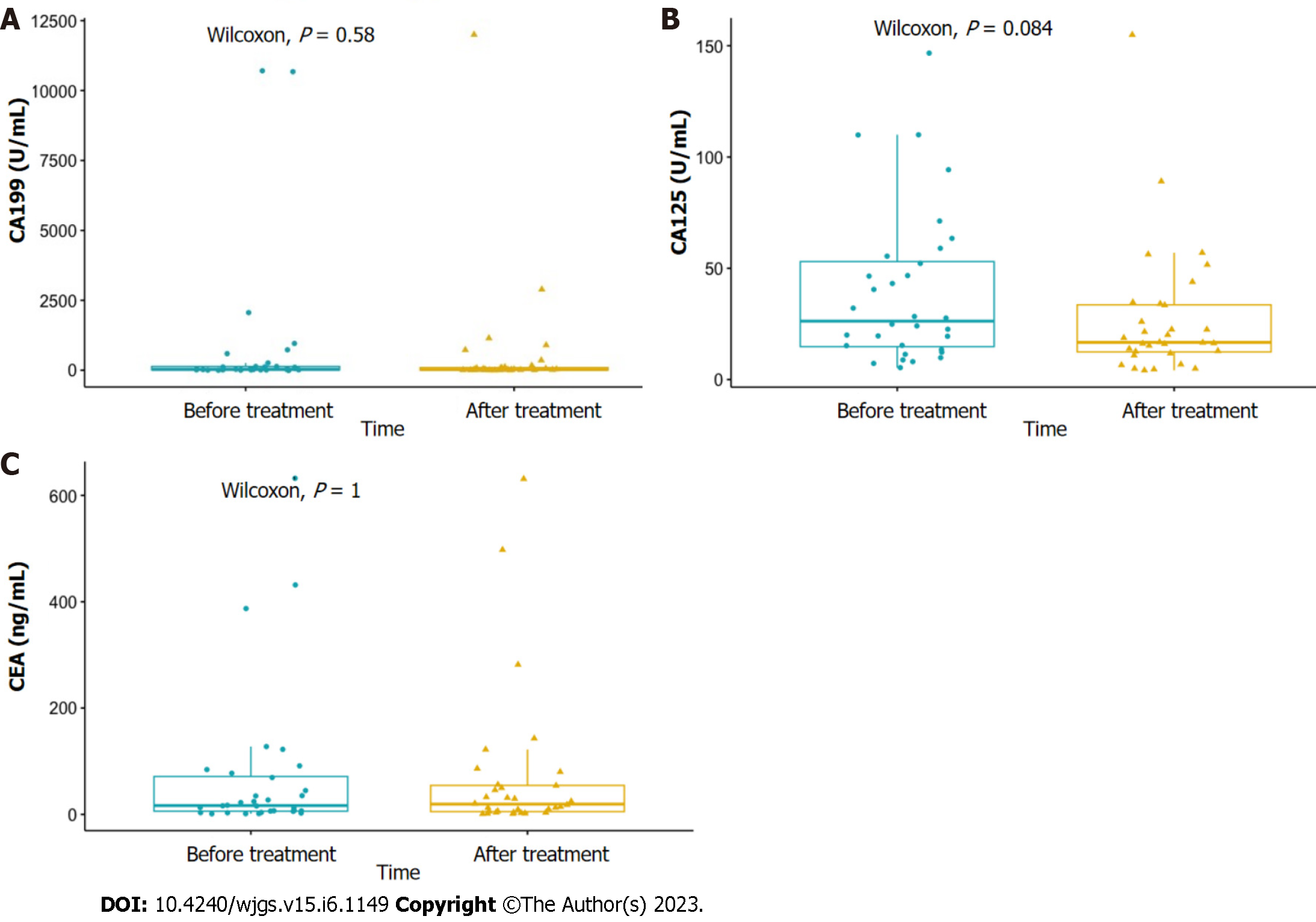
Figure 3 Changes in serum tumor markers in 32 patients before and after treatment.
A: Changes in CA199 before and after treatment; B: Changes in CA125 before and after treatment; C: Changes in carcinoembryonic antigen before and after treatment. CEA: Carcinoembryonic antigen.
- Citation: Zhang Y, Zhao X, Gao C, Lin LY, Li Y. Treatment outcome analysis of bevacizumab combined with cyclophosphamide and oxaliplatin in advanced pseudomyxoma peritonei. World J Gastrointest Surg 2023; 15(6): 1149-1158
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1149.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1149