Published online Sep 15, 2017. doi: 10.4239/wjd.v8.i9.429
Peer-review started: January 28, 2017
First decision: May 11, 2017
Revised: May 31, 2017
Accepted: June 19, 2017
Article in press: June 20, 2017
Published online: September 15, 2017
Processing time: 230 Days and 10.5 Hours
To determine the clinical features of diabetes in children and adolescents in Ghana.
Retrospective review of clinical features of all children and adolescents with new-onset diabetes seen at the paediatric endocrinology clinic of Komfo Anokye Teaching Hospital in Kumasi, from February 2012 to Auguest 2016.
One hundred and six subjects presented with diabetes. Ninety (84.9%) were diagnosed by clinical features and family history as type 1, and 16 (15.1%) type 2. For type 1 subjects, age range at diagnosis was 0.9-19.9 year (y), peak age of onset 12-13 year, and 3.3% were < 5 year, 21.1% 5- < 10 year, 45.6% 10- < 15 year and 30.0% 15- < 20 year. Seventy-one point one percent were female. Common clinical features were polyuria (100%), polydipsia (98.9%), and weight loss (82.2%). Mean BMI SD was -0.54, range -3.84 to 2.47. 60.0% presented in diabetic ketoacidosis (DKA). Nine had infections at onset (skin, abscess, leg ulcer). Mean ± SD HbA1c at diagnosis was 12.7% ± 1.9% (115 ± 21 mmol/mol). Four have since died: Hypoglycaemia (2), recurrent DKA (1), osteosarcoma (1). Two other type 1 cases died of DKA at presentation in emergency before being seen by the paediatric endocrinologist. Crude mortality rate including these 2 cases was 32.2/1000 patient years. Type 2 cases were 81% female, age of onset 9-19 year. Mean BMI SD was 1.49, range -0.87 to 2.61. Forty-three point eight percent presented in DKA. All type 2 cases had acanthosis nigricans. Overall, 9.8% did not have home refrigeration, most using clay pot evaporative cooling for insulin storage.
Type 1 occurs with a female preponderance and high DKA rates. Type 2 also occurs. Typology based on clinical features is difficult. Community and professional awareness is warranted.
Core tip: In this study of 106 consecutive new diagnoses of diabetes in young people < 20 years in a tertiary referral centre in Ghana, type 1 predominated (85%) with the remaining cases clinically diagnosed as type 2. Both types had a female preponderance. Type 1 peak age of onset was 12-13 years. All type 2 subjects had acanthosis nigricans. Most presented in ketoacidosis signifying a lack of awareness of presentation features. Clinic numbers quickly rose due to availability of supplies and expertise. Further typology studies are indicated to further define diabetes type.
- Citation: Ameyaw E, Asafo-Agyei SB, Thavapalan S, Middlehurst AC, Ogle GD. Clinical profile of diabetes at diagnosis among children and adolescents at an endocrine clinic in Ghana. World J Diabetes 2017; 8(9): 429-435
- URL: https://www.wjgnet.com/1948-9358/full/v8/i9/429.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i9.429
Understanding the presentation and types of diabetes in children and youth in any particular country is essential in improving awareness and care. Ghana is a less-resourced country in West Africa. There is no published data on clinical features of young Ghanaians with diabetes and, as with many low-income countries[1], there is little public health sector support and also lack of awareness amongst both health workers and the general society[2]. Insulin is only intermittently available from the government health service, and blood glucose meters and strips and HbA1c testing are not provided by the Ghana National Health Insurance Scheme. The families must often buy these supplies, often at premium prices[3], which many cannot afford to do[2].
The lack of awareness leads to misdiagnosis and mismanagement. Ketoacidosis is very common at initial presentation in Africa[2,4-6], and can mimic infections and acute medical conditions[7-10].
This study determined the clinical features of children and adolescents presenting with diabetes at the Paediatric Endocrine Clinic, Komfo Anokye Teaching Hospital (KATH) at Kumasi, a tertiary referral centre for northern Ghana. This clinic has been supported since 2012 by the International Diabetes Federation (IDF) Life for a Child Program[11] with provision of insulin, blood glucose meters and strips, insulin syringes, HbA1c testing, education materials, and mentoring.
A total of 106 subjects were enrolled, all < 20 years of age at diagnosis. They included all subjects being followed at the Paediatric Endocrine Clinic on 24/02/2012 as well as all new diagnoses until 31/08/2016. During this period, two other subjects < 20 years old (both female, aged 12 and 15 years old respectively) presented with diabetic ketoacidosis (DKA) and died in the emergency department. They were not seen by the pediatric endocrinologist or in the clinic, and no further information is available. Therefore, they were included in the mortality rate calculation, but excluded from the remainder of the analysis. The study was approved by the institutional ethics board and subjects gave informed consent.
Date of birth and sex was recorded, as well as date of diagnosis.
Diabetes was diagnosed according to standard World Health Organization criteria[12]. Determination of the type of diabetes was made by the local investigators according to available clinical features and history. Type 1 patients generally had lower body mass index (BMI), more rapid symptom onset, and were more sensitive to insulin. Type 2 patients had higher BMI, acanthosis nigricans, and needed more insulin with time, with insulin requirements falling sharply in those started on metformin. The presence of polyuria, polydipsia, weight loss, malnutrition and ketoacidosis at the time of diagnosis were recorded. Body weight and height were measured by electronic scales and stadiometer respectively with subjects wearing light-weight clothing and without shoes. BMI was then calculated. BMI SD scores were calculated using World Health Organization standards[13,14].
Ketoacidosis was defined by clinical features along with an elevated blood glucose and ketonuria (blood gas measurements are generally not available). Family history of type 1 diabetes, and history of other medical conditions were also recorded.
Blood glucose was measured in a laboratory via venous sample. HbA1c was measured using a Clover analyzer (Infopia, Anyang, South Korea).
The following information was collected for each subject: Whether the mother or father was living with the subject, mother’s and father’s educational level, who was the primary caregiver, whether the primary caregiver was literate, time spent travelling to clinic, and average weekly household income. It was also recorded whether the subject was at school, whether diabetes was limiting school attendance, and whether they were in the appropriate grade for age, and how well overall the young person was psychologically coping with their diabetes (rated as poor, average or good). Finally, the method of insulin storage was recorded.
Crude mortality rate was calculated as the total number of deaths divided by the sum of the periods from the commencement of the study, or from the date of diagnosis if they were diagnosed after the study commenced. It is expressed as mortality per 1000 patient years.
Data and descriptive statistics were managed in Excel. Unpaired t-test and χ2 tests were done using the Social Science Statistics on-line calculators[14]. Significance was set as < 0.05.
One hundred and six subjects with diabetes were seen at the paediatric endocrine clinic. Ninety (84.9%) were diagnosed by clinical features and family history as type 1, and 16 (15.1%) type 2.
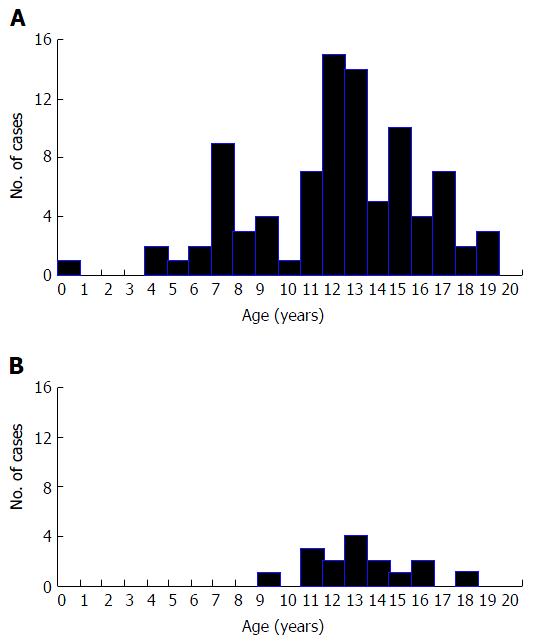
Table 1 shows age of onset and gender of the 90 type 1 subjects, as well as BMI, BMI SD score, presence of DKA at diagnosis, and blood glucose and HbA1c at diagnosis. Figure 1A shows the distribution of age of onset. Three point three percent were < 5 years, 21.1% 5- < 10 years, 45.6% 10- < 15 years and 30.0% 15- < 20 years. Common clinical features at diagnosis were polyuria (100.0%), polydipsia (98.9%), and weight loss (82.2%). Nine (10%) had infections at onset (tinea capitis, abscess, leg ulcer, vaginal candidiasis).
| Type 1 | Type 2 | Difference | |
| Number (%) | 90 (84.9) | 16 (15.1) | P < 0.001 |
| Male: Female ratio | 1:2.5 | 1:4.3 | Not significant |
| Age at diagnosis (range), yr | 0.9-19.9 | 9.0-18.7 | - |
| Age at diagnosis (mean ± SD), yr | 12.6 ± 3.8 | 13.6 ± 2.3 | Not significant |
| Peak age at diagnosis, yr | 12-13 | 13-14 | - |
| Diabetic ketoacidosis at onset (%) | 54 (60.0) | 7 (43.8) | Not significant |
| BMI at onset (mean; range) | 18.1; 12.5-34.7 | 27.8; 17.6-38.2 | - |
| BMI SD score at onset (mean; range) | -0.54, -3.84-2.47 | 1.49, -0.87-2.61 | P < 0.001 |
| HbA1c at diagnosis (mean ± SD) (%) (mmol/mol) | 12.7 ± 1.9 (115 ± 21) | 12.8 ± 1.5 (116 ± 16) | Not significant |
Nine type 1 subjects had a first-degree relative with type 1: Sister (two subjects), brother (three), sister and brother (two), two brothers (one), mother (one), with one other subject having a grandmother with type 1. The number of insulin injections each day was two for 17 (18.9%) subjects, three for 24 (26.7%), five for 47 (52.2%) and unknown for two (2.2%). The type of insulin was pre-mixed for 11 (12.2%) subjects, and short-acting combined with long-acting for 79 (87.8%).
Four of the 106 patients have since died: One from metastatic osteosarcoma (diagnosed well after onset of type 1), two from hypoglycemia at home (2 years after diagnosis), and one from a recurrent episode of DKA (2 years after diagnosis). Two others died in emergency department during treatment of DKA at diagnosis, and were not seen by the paediatric endocrinologist (see Methods). Crude mortality rate for the type 1 patients was six deaths per 186 patient years (i.e., 32.2 deaths per 1000 patient years).
For the 16 type 2 cases, Table 1 shows age of onset and gender, as well as BMI, BMI SD score, presence of DKA at diagnosis and blood glucose and HbA1c at diagnosis. Figure 1B shows age of onset. Six point three percent were 5- < 10 years, 68.7% 10- < 15 years and 25.0% 15- < 20 years. Common clinical features at diagnosis were polyuria (100.0%), polydipsia (100.0%), and weight loss (93.8%). All type 2 subjects had acanthosis nigricans. None had infections at onset. One had substantial visual loss at diagnosis, of uncertain aetiology. Three subjects had first degree relatives with type 2, and two others had a second-degree relative. Four subjects (25.0%) were treated with metformin only, six (37.5%) with insulin only, five (31.3%) with metformin together with insulin and one (6.3%) also with glibenclamide. No subject with type 2 died.
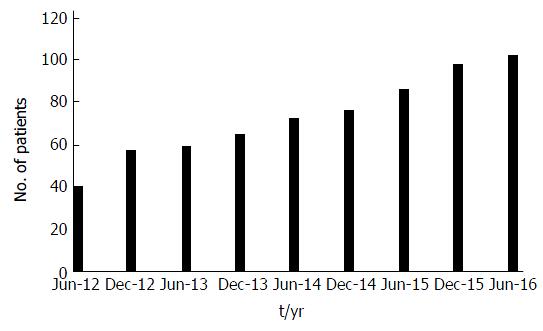
Figure 2 shows the rapid increase in clinic numbers in the 4 years from June 2012 to June 2016 - clinic numbers were censused at the end of every half-year.
The mother was living with the subject in 83 (78.3%) cases and the father in 78 (73.6%). The mother’s educational level was primary school in 31 (29.2%) cases, high school in 26 (24.5%), tertiary in 10 (9.4%), no schooling in 38 (35.8%) and unknown in 1 (0.9%). The father’s educational level was primary school in 26 (24.5%) cases, high school in 31 (29.2%), tertiary in 23 (21.7%), no schooling in 21 (19.8%) and unknown in 5 (4.7%). The primary caregiver was the mother in 75 (70.8%) cases, father in 15 (14.2%), sister in 3 (2.8%), brother in 2 (1.9%), grandmother in 4 (3.8%), aunt in 6 (5.7%) and self in 1 (0.9%). The primary caregiver was literate in 79 (74.5%) cases. Twenty-four (22.6%) families had to travel long distances (> 2 h travelling time each way) for supplies and review. The average weekly household income was 63 USD and the range was 5-625 USD. Ninety-six (90.6%) subjects were attending school. Diabetes was limiting attendance at school for 44 (45.8%) subjects, not limiting attendance for 51 (53.1%) and unknown for 1 (1.0%). In addition, 18 (18.8%) were not in the appropriate grade for their age, 76 (79.2%) were in the appropriate grade, and 2 (2.1%) unknown. Diabetes coping abilities were assessed as poor for 12 (11.3%) subjects, average for 37 (34.9%), good for 55 (51.9%) and unknown for 2 (1.9%). Ninety-five (89.6%) subjects were literate or learning at school, 8 (7.5%) were not literate and 3 (2.8%) unknown. Insulin storage method was a refrigerator at the family home for 92 subjects (90.2%), for two a refrigerator outside the home (2.0%) and for eight clay pot evaporative cooling (7.8%).
There are very limited published data on diabetes in young people in Ghana. The International Diabetes Federation Diabetes Atlas estimates an incidence of type 1 diabetes of 2.9 per 100000 children < 15 years per annum and a prevalence of 18.0 per 100000 children < 15 years: An estimated 1800 children in the country[15,16]. This is however based on a small study in Nigeria in 1992[17]. It is possible that the current Ghanaian incidence is different from this estimate, and the prevalence/incidence ratio is likely to be substantially lower as the Atlas estimates do not assume any mortality[16]. In Ghana, it is likely that many children and young adults with diabetes die before they are diagnosed, or die during the first episode of DKA or early in ongoing management. DKA is frequently misdiagnosed at first as another condition - with a legion of alternatives including pneumonia, gastroenteritis, malaria, typhoid, appendicitis and a number of other conditions[1,7-10]. At a training workshop organised by Ghana Society of Pediatric Endocrinology and Diabetes (GSPED) in August 2016, some participants from district and regional hospitals admitted that most of their patients with DKA die. Indeed, two centres admitted that all such patients have died during management. The rate of DKA at onset in type 1 subjects was high at 60.0%, consistent with rates of 69.8% reported from South Africa[4], 75% from Tanzania[5], and 77.1% from Nigeria[6]. Community and health professional awareness on the presentation of diabetes in young people is warranted given this late presentation and the likely substantial numbers of deaths where the correct diagnosis is not made at all. Type 1 patients were generally lean or underweight at diagnosis, and presented with classic symptoms. There was a female preponderance as is often observed in low-incidence countries[18].
There was also a female preponderance in the type 2 population, consistent with data in adults in Kumasi[19]. Type 2 subjects were often overweight. This is of concern, as overweight is now not uncommon in Ghanaian children and youth[20,21]. All had acanthosis nigricans - a physical marker suggestive of insulin resistance[22]. Interestingly, seven of 16 type 2 subjects presented in DKA, suggesting a diagnosis of ketosis-prone type 2 diabetes, which is well-reported in populations in Africa and of African descent[23,24].
The youngest child was 10 mo of age at diagnosis. Development of diabetes at a young age can indicate a monogenic cause, and genetic testing is indicated if the onset is < 6 mo of age or if there are syndromal features of known single-gene defects (which were not present in the infant in this series)[25]. In some of these cases, alternate non-insulin therapy may be possible[25].
Even with accurate diagnosis, mortality has been high in studies in sub-Saharan Africa[26-28], but there are indications it is falling - for instance in Rwanda it was found to be between 13.9-40.2 per 1000 patient years, depending on the fate of those lost to follow-up[29]. The figure of 32/1000 from this study is in this range - and in Rwanda like in Ghana, care is improving as supplies are made available[30]. This improvement in survival is seen in the dramatic increase in the clinic population from 23 to 102 cases over the five years - “if you build it they will come” - and not just come but survive and thrive. Such rapid increases in numbers in clinics that are able to provide standard care (also seen in Tanzania[31]) indicate the strain that will be on resources as survival improves as insulin and other critical supplies are provided by programs such as IDF Life for a Child, and paediatric endocrinologists, trained in Kenya[32] and elsewhere, return to their home countries to establish clinics.
Patient education is critical in improving care. At this study clinic, all patients are called on the telephone to come in for education every fortnight. They are taught at an appropriate educational level about the pathophysiology of diabetes, how to appropriately store and administer insulin, injection sites, adjust doses, manage diet and exercise, detect acute complications, etc.
The study results demonstrate the socio-economic challenges faced by many subjects, and the necessity for support with supplies, consistent with past reports[1-3,28]. A number of young people were also facing challenges with continuing their education, as demonstrated in the study by Kratzer[2].
Some families do not have access to home refrigeration for insulin storage, and so place the insulin in a clay pot using evaporative cooling. Such methods do substantially reduce storage temperatures unless humidity is very high[33].
The major limitation of this study is the lack of ability and resources to measure autoantibodies and C-peptide to confirm the diagnosis of type 1 or type 2, or an atypical form. Such assistance with typology would not only be interesting scientifically, but would be helpful to individualise management. However, the presence or absence of autoantibodies alone may not be categorical in this population. Agyei-Frempong et al[34] in a study of autoimmunity in a population of adults with diabetes in Kumasi found that glutamic acid decarboxylase (GAD) antibody and/or insulinoma antibody (IA2) were present in 35% of those on insulin and 16.5% of those not requiring insulin.
In summary, both type 1 and type 2 diabetes occur in young people in northern Ghana, with high rates of DKA at onset, and a female preponderance. Deaths in the first few years are still not uncommon. Community and health professional awareness is indicated to achieve prompt and accurate diagnosis and prevent deaths at onset. Although not assessed in this study, it is reasonable to conclude that further health professional and patient education is needed to continue to improve management, and therefore reduce the risk of long-term complications. Improvements in the availability of diagnostic technology (particularly blood glucose meters and strips) is also indicated. A patient support group would also be very beneficial.
We thank the staff at the statistics department of KATH and Jean-Pierre Chanoine for helpful comments on the manuscript, and Ms Jane Aquaye for data entry.
Limited information is available on types of diabetes in young people in Africa, nor on prognosis.
The epidemiology and prognosis of diabetes in young people in sub-Saharan Africa is of importance as services are developed to look after these young people.
This study shows that both type 1 and type 2 diabetes are occurring in young people in Ghana, with some phenotypic overlap. Mortality was found to be 32.2 per 1000 patient years.
The study shows how numbers of children and young people in a clinic in a less-resourced country quickly grow as care is given in a paediatric endocrine clinic.
This study offers a valuable insight in the clinical profile of diabetes in population of children and adolescents in Ghana. The subject is interesting and worth investigating, since the data regarding diabetes burden in Africa are still scarce and the study population is particularly vulnerable.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Ghana
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alamgir MA, Guerrero-Romero F, Lovrencic MV, Zhao J S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Ogle GD, Middlehurst AC, Silink M. The IDF Life for a Child Program Index of diabetes care for children and youth. Pediatr Diabetes. 2016;17:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Kratzer J. Structural barriers to coping with type 1 diabetes mellitus in Ghana: experiences of diabetic youth and their families. Ghana Med J. 2012;46:39-45. [PubMed] |
| 3. | Ogle GD, Kim H, Middlehurst AC, Silink M, Jenkins AJ. Financial costs for families of children with Type 1 diabetes in lower-income countries. Diabet Med. 2016;33:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Reddy Y, Ganie Y, Pillay K. Characteristics of children presenting with newly diagnosed type 1 diabetes. S Afr J Child Health. 2013;7:46-48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Majaliwa ES, Munubhi E, Ramaiya K, Mpembeni R, Sanyiwa A, Mohn A, Chiarelli F. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar es Salaam, Tanzania. Diabetes Care. 2007;30:2187-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Onyiriuka AN, Ifebi E. Ketoacidosis at diagnosis of type 1 diabetes in children and adolescents: frequency and clinical characteristics. J Diabetes Metab Disord. 2013;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Rwiza HT, Swai AB, McLarty DG. Failure to diagnose diabetic ketoacidosis in Tanzania. Diabet Med. 1986;3:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Durai R, Hoque H, Ng P. The Acute Abdomen - Commonly missed and mis-diagnosed conditions: Review. Webmed Central Surgery. 2010;1:1-14. |
| 9. | Murunga AN, Owira PM. Diabetic ketoacidosis: an overlooked child killer in sub-Saharan Africa? Trop Med Int Health. 2013;18:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Makani J, Matuja W, Liyombo E, Snow RW, Marsh K, Warrell DA. Admission diagnosis of cerebral malaria in adults in an endemic area of Tanzania: implications and clinical description. QJM. 2003;96:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 11. | International Diabetes Federation Life for a Child Program. [accessed 2017 Jan 21]. Available from: https://www.lifeforachild.org/. |
| 12. | WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: WHO, 2006. . |
| 13. | de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4196] [Cited by in RCA: 5402] [Article Influence: 317.8] [Reference Citation Analysis (0)] |
| 14. | Social Science Statistics. Statistical Calculators. [accessed 2017 May 20]. Available from: http://www.socscistatistics.com. |
| 15. | International Diabetes Federation. IDF Diabetes Atlas, 7th ed. Brussels, Belgium, 2015. . |
| 16. | Patterson C, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103:161-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (2)] |
| 17. | Afoke AO, Ejeh NM, Nwonu EN, Okafor CO, Udeh NJ, Ludvigsson J. Prevalence and clinical picture of IDDM in Nigerian Igbo schoolchildren. Diabetes Care. 1992;15:1310-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Karvonen M, Pitkäniemi M, Pitkäniemi J, Kohtamäki K, Tajima N, Tuomilehto J. Sex difference in the incidence of insulin-dependent diabetes mellitus: an analysis of the recent epidemiological data. World Health Organization DIAMOND Project Group. Diabetes Metab Rev. 1997;13:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Danquah I, Bedu-Addo G, Terpe KJ, Micah F, Amoako YA, Awuku YA, Dietz E, van der Giet M, Spranger J, Mockenhaupt FP. Diabetes mellitus type 2 in urban Ghana: characteristics and associated factors. BMC Public Health. 2012;12:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Kumah DB, Akuffo KO, Abaka-Cann JE, Affram DE, Osae EA. Prevalence of Overweight and Obesity among Students in the Kumasi Metropolis. J Nutr Metab. 2015;2015:613207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Mohammed H, Vuvor F. Prevalence of childhood overweight/obesity in basic school in Accra. Ghana Med J. 2012;46:124-127. [PubMed] |
| 22. | Guran T, Turan S, Akcay T, Bereket A. Significance of acanthosis nigricans in childhood obesity. J Paediatr Child Health. 2008;44:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Smiley D, Chandra P, Umpierrez GE. Update on diagnosis, pathogenesis and management of ketosis-prone Type 2 diabetes mellitus. Diabetes Manag (Lond). 2011;1:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Lontchi-Yimagou E, Nguewa JL, Assah F, Noubiap JJ, Boudou P, Djahmeni E, Balti EV, Atogho-Tiedeu B, Gautier JF, Mbanya JC. Ketosis-prone atypical diabetes in Cameroonian people with hyperglycaemic crisis: frequency, clinical and metabolic phenotypes. Diabet Med. 2017;34:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Rubio-Cabezas O, Hattersley AT, Njølstad PR, Mlynarski W, Ellard S, White N, Chi DV, Craig ME; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2014;15 Suppl 20:47-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Sidibé AT, Traoré HA, Litman-Ali IT, Dembélé M, Traoré AK, Cissé I. Le diabète juvénile au Mali. Rev Franç Endocrinol Clin. 1999;40:514-520. |
| 27. | Beran D, Yudkin JS, de Courten M. Access to care for patients with insulin-requiring diabetes in developing countries: case studies of Mozambique and Zambia. Diabetes Care. 2005;28:2136-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Piloya-Were T, Sunni M, Ogle GD, Moran A. Childhood diabetes in Africa. Curr Opin Endocrinol Diabetes Obes. 2016;23:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Marshall SL, Edidin D, Arena VC, Becker DJ, Bunker CH, Gishoma C, Gishoma F, LaPorte RE, Kaberuka V, Ogle G. Mortality and Natural Progression of Type 1 Diabetes Patients Enrolled in the Rwanda LFAC Program from 2004-2012. Int J Diabetes Dev Countries. 2016;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Marshall SL, Edidin DV, Arena VC, Becker DJ, Bunker CH, Gishoma C, Gishoma F, LaPorte RE, Kaberuka V, Ogle G. Glucose control in Rwandan youth with type 1 diabetes following establishment of systematic, HbA1c based, care and education. Diabetes Res Clin Pract. 2015;107:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Muze KC, Majaliwa ES. Type 1 diabetes care updates: Tanzania. Indian J Endocrinol Metab. 2015;19:S12-S13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Odundo GO, Ngwiri T, Otuoma O, Laigong P, Mukhwana R, Limbe MS, Chanzu NM. The Impact and Successes of a Paediatric Endocrinology Fellowship Program in Africa. Int J Endocrinol. 2016;2016:1560248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Ogle GD, Abdullah M, Mason D, Januszewski AS, Besançon S. Insulin storage in hot climates without refrigeration: temperature reduction efficacy of clay pots and other techniques. Diabet Med. 2016;33:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Agyei-Frempong MT, Titty FV, Owiredu WK, Eghan BA. The prevalence of autoimmune diabetes among diabetes mellitus patients in Kumasi, Ghana. Pak J Biol Sci. 2008;11:2320-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (1)] |