Published online Mar 10, 2016. doi: 10.4239/wjd.v7.i5.89
Peer-review started: September 5, 2015
First decision: November 24, 2015
Revised: December 24, 2015
Accepted: January 16, 2016
Article in press: January 19, 2016
Published online: March 10, 2016
Processing time: 188 Days and 12 Hours
Approximately 30%-50% of people are recognized to have low levels of vitamin D, and insufficiency and deficiency of vitamin D are recognized as global health problems worldwide. Although the presence of hypovitamin D increases the risk of rickets and fractures, low vitamin D levels are also associated with hypertension, cancer, and cardiovascular disease. In addition, diabetes mellitus (DM) and chronic kidney disease (CKD) are also related to vitamin D levels. Vitamin D deficiency has been linked to onset and progression of DM. Although in patients with DM the relationship between vitamin D and insulin secretion, insulin resistance, and β-cell dysfunction are pointed out, evidence regarding vitamin D levels and DM is contradictory, and well controlled studies are needed. In addition, vitamin D influences the renin-angiotensin system, inflammation, and mineral bone disease, which may be associated with the cause and progression CKD. There is increasing evidence that vitamin D deficiency may be a risk factor for DM and CKD; however, it remains uncertain whether vitamin D deficiency also predisposes to death from DM and CKD. Although at this time, supplementation with vitamin D has not been shown to improve glycemic control or prevent incident DM, clinical trials with sufficient sample size, study periods, and optimal doses of vitamin D supplementation are still needed. This review focuses on the mechanism of vitamin D insufficiency and deficiency in DM or CKD, and discusses the current evidence regarding supplementation with vitamin D in patients with these diseases.
Core tip: Vitamin D plays an essential role in diabetes mellitus (DM) and chronic kidney disease (CKD). The relationship between vitamin D and insulin secretion, insulin resistance, and β-cell dysfunction are pointed out. Vitamin D deficiency has been linked with the renin-angiotensin system and inflammation, which may be associated with the cause and progression CKD. There is increasing evidence that vitamin D deficiency may be a risk factor for DM and CKD. Clinical trials with sufficient sample size, study periods, and optimal doses of vitamin D supplementation are still needed.
- Citation: Nakashima A, Yokoyama K, Yokoo T, Urashima M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes 2016; 7(5): 89-100
- URL: https://www.wjgnet.com/1948-9358/full/v7/i5/89.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i5.89
Diabetes mellitus (DM) and chronic kidney disease (CKD) are common diseases worldwide, and their prevalence continues to increase[1,2]. Vitamin D deficiency is also recognized as a worldwide health problem[3], and is associated with rickets and fracture. In addition, hypovitamin D has recently been considered a responsible factor in the onset and progression of DM and CKD. There has been increasing evidence suggesting that an inverse vitamin D status is prevalent in patients with DM or CKD[4]. Furthermore, supplementation of vitamin D in patients with DM or CKD has been reported in several trials and a meta-analysis[5]. In this review, we provide current clinical data on the mechanism of vitamin D deficiency and the effects of vitamin D on patients with DM or CKD.
Vitamin D is a fat-soluble steroid hormone derived from dietary intake as well as synthesis through the skin via exposure to sunlight (Figure 1). Vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are produced through solar ultraviolet B radiation (UVB; wavelength 290 to 315 nm). Vitamin D3 is manufactured from previtamin D3, which is changed through UVB irradiation from provitamin D3[6]. Most 25-hydroxyvitamin (25[OH]D) is derived from skin conversion. An alternative source is from dietary intake, mainly from foods of plant or animal origin. In general, animals and fish contain vitamin D3, and mushrooms contain vitamin D2[7]. Vitamin D from the skin and diet is either stored in adipose tissue or converted to 25(OH)D in the liver. Vitamin D metabolism requires two hydroxylations to form its active metabolite. The first hydroxylation of vitamin D takes place in the liver where vitamin D is metabolized to 25(OH)D by cytochrome P 2R1 (CYP2R1). 25(OH)D binds to vitamin D-binding protein (DBP) and can flow into the blood in a stable form. 25(OH)D-DBP complex is excreted into the urine and reabsorbed through megalin, a multiligand scavenger receptor in the proximal tubules[8,9], where the complex is converted by 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) and changed to its active form 1,25-dihydroxyvitamin (OH)2D, although other tissues have 1α-hydroxylase enzymatic activity[10]. CYP27B1 gene expression in the kidney is mediated by various factors. Parathyroid hormone (PTH), hypocalcemia, hypophosphatemia, and calcitonin affect the activation of CYP27B1 and can increase 1,25-(OH)2D levels. On the other hand, 1,25-(OH)2D and fibroblast growth factor-23 (FGF-23) inhibit CYP27B1 and can decrease 1,25-(OH)2D levels[11].
The binding of 1,25(OH)2D to the vitamin D receptor (VDR) in the nuclear receptor affects gene transcription. In general, 1,25(OH)2D promotes dietary calcium and phosphorus absorption in the intestine and regulates reabsorption of calcium in the renal tubules. Because VDR is expressed in a variety of organs, such as the heart, liver, blood vessels, and the central nervous system, 25-hydroxyvitamin D-1α-hydroxylase is also expressed in these tissues[12].
It is widely believed that 25(OH)D is the only precursor of 1,25(OH)2D and does not influence individual tissues. However, recent reports revealed that 25(OH)D has a weak binding capacity for VDR and affects several tissues in the autocrine or paracrine system[13,14]. In addition, extrarenal 1α-hydroxylase enzymatic activity is controlled in different ways that that in renal tubular cells[15].
Because 1,25(OH)2D has a short half-life (approximately 15 h), 1,25(OH)2D levels are not considered a good indicator of vitamin D levels. As 25(OH)D is more stable in the blood than 1,25(OH)2D, blood concentrations of 25(OH)D are 500 to 1000 times higher than 1,25(OH)2D concentrations. Therefore, to evaluate vitamin D deficiency and insufficiency, serum 25(OH)D concentrations are considered an adequate biomarker. The United States Institute of Medicine defines vitamin D deficiency as 25(OH)D levels less than 20 ng/mL and greater than 20 ng/mL is sufficient upon evidence related to bone health[16]. Several studies reported that people with 25(OH)D levels less than 20 ng/mL is the risk factor of fracture[17] and have greater subsequent rates of bone loss[18]. On the other hand, the Endocrine Society’s guidelines, which are based on patients with endocrine disorders, define vitamin D insufficiency as 25(OH)D levels of 21-29 ng/mL[19,20]. Despite these different definitions, both guidelines agree that vitamin D insufficiency and deficiency are common problems in certain populations.
About 1 billion people worldwide lack vitamin D[21,22]. Vitamin D deficiency and insufficiency are prevalent conditions not only in elderly people but also in adolescents[23] and children[24]. One study reported that almost one half of participants had 25(OH)D levels less than 40 nmol/L during the winter and spring[25]. In this study, 7437 people from a British birth cohort study who were 45 years old had 25(OH)D levels measured. Although the prevalence of hypovitamin D, defined as levels below 40 nmol/L, was 15.4% during the spring and summer, the proportion was 46.6% during the winter and autumn. Other studies showed that vitamin D deficiency was especially common in older persons (67-95 years)[26,27], and more than 50% of postmenopausal women taking medication for osteoporosis had 25(OH)D levels below 30 ng/mL[28]. Various factors, including age, sex, location, nutrition status, and physical fitness, affect vitamin D status[29]. In addition, diabetes, renal function, hypoalbuminemia, and albuminuria are also risk factors for vitamin D deficiency[30,31].
Recently, the relationship between 25(OH)D levels and genetic polymorphisms of DBP were reported[32]. It was previously known that 25(OH)D concentrations differed between black Americans and whites[33]. Although it was generally thought that nutritional, environmental, and hormonal factors affected racial differences[34], the detailed mechanisms behind these differences are unknown. Powe et al[32] reported that although total 25(OH)D and DBP were lower in black subjects than in white subjects, concentrations of estimated bioavailable 25(OH)D were similar between black and white subjects. In addition, because the affinity of DBP to 25(OH)D differs in the DBP gene polymorphism, genetic polymorphisms of DBP genes (rs7041 and rs4588) provide a likely explanation for racial variations in levels of DBP and 25(OH)D[35]. The combination of rs7041 and rs4588 produces amino acid changes resulting in variant DBPs (Gc1F, Gc1S, and Gc2). The phenotype of Gc1F, which is common in black homozygotes, was associated with the lowest levels of DBP (Gc1F/Gc1F homozygotes). On the other hand, Gc1S, which is common in white subjects, was associated with the highest DBP levels (Gc1S/Gc1S homozygotes). The Gc2/Gc2 homozygotes and Gc1F/Gc1S heterozygotes were associated with intermediate DBP levels. These findings suggest that racial differences in the distribution of DBP and total 25(OH)D are caused by DBP polymorphisms, and low total 25(OH)D levels do not indicate vitamin D deficiency. For purposes of cross-racial evaluations of vitamin D deficiency, it might be appropriate to estimate serum total 25(OH)D concentrations using DBP polymorphisms and DBP.
Associations between vitamin D levels and mortality have been shown by several observational studies[36,37]. Low vitamin D levels have also been shown to be associated with obesity, fractures, and infections[38]. Several observational studies have revealed potential links between low vitamin D levels and cardiovascular disease[39]. It is well known that people who live at high altitudes are at higher risk for hypertension and cardiovascular disease[40,41]. In a study of patients with hypertension who were exposed to UVB radiation three times a week for 3 mo, 25(OH)D concentrations increased by about 180%, and blood pressure became normal[42]. A prospective, nested, case-control study of 1484 women without hypertension and with low 25(OH)D levels showed that women with lower 25(OH)D levels had a higher rate of incident hypertension than controls. Low 25(OH)D concentrations have been shown to be inversely related to developing hypertension[43]. A recent Mendelian randomization study of vitamin D status and blood pressure concluded that increased plasma concentrations of 25(OH)D might reduce the risk for hypertension[44]. Cardiovascular disease such as coronary arterial disease[45], myocardial infarction[46], heart failure[47], and stroke[48] are also associated with vitamin D deficiency. However, a recent study showed that high levels of 25(OH)D were also associated with cardiovascular disease mortality[49]. This prospective, observational, cohort study analyzed 247574 citizens from Denmark and showed that a 25(OH)D level below 12.5 nmol/L was associated with a higher risk for mortality [hazard ratio (HR) = 1.59] compared with the reference range (50-75 nmol/L); however, those with levels higher than 125 nmol/L had the highest mortality risk (HR = 1.95). There is a possibility that maintaining adequate vitamin D levels is essential for human health.
As mentioned above, vitamin D status and cardiovascular disease are strongly associated. Animal models offer several mechanisms to explain this association. Activation of the renin-angiotensin-aldosterone system (RAAS) has been seen in VDR knockout mice[50], and vitamin D has been shown to regulate the nuclear factor kappa beta pathway in renal failure model mice[51]. In vascular endothelial cells, transcription of nitric oxide synthase has been shown to be inhibited by vitamin D in mice[52]. In addition, vitamin D has been shown to activate the Keap1-Nrf pathway, which opposes oxidative stress, in renal failure model mice[53].
Type 1 DM is caused by a complex autoimmune destruction of pancreatic islet β-cells, leading to absolute insulin deficiency. The autoimmune nature of type 1 DM has been clarified with the detection of auto-antibodies against islet β-cells and their infiltration by T cells, B cells, and macrophages[54]. Vitamin D has been shown to have immunomodulatory properties as well. Many immunomodulatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and inflammatory bowel disease have been reported to be associated with vitamin D deficiency[55,56]. Type 1 DM is also said to be related to vitamin D deficiency[57]. As VDR are expressed in human T and B lymphocytes, vitamin D is thought to modify the Th1/Th2 cytokine profile[58]. In addition, vitamin D is also thought to be associated with the immune system via its inhibition of lymphocyte proliferation[59]. Non-obese diabetic (NOD) mice with vitamin D deficiency showed an increased incidence and severity of diabetes[60]. Using 1,25(OH)2D reduced the manifestation of diabetes in NOD mice by decreasing the number of effector T cells[61,62]. Another study reported that 1,25(OH)2D also counteracted cytokine-induced expression of Fas, which regulates cell death in human islet cells[63].
The relationship between sunlight exposure and the incidence of type 1 DM has been reported[64]. One study showed that providing vitamin D supplements to infants in North Europe, where daylight hours are shorter than in other countries, decreased the risk for new-onset type 1 DM[65]. Although children suspected of having rickets during the study period had a relative risk (RR) of 3.0 (1.0-9.0) for type 1 DM, children who had taken 2000 IU vitamin D daily had a RR = 0.22 (0.05-0.89). Some studies were designed to clarify the effect of vitamin D on the preservation of β-cell function after the onset of type 1 DM[66]. Two studies found no significant effects of administration on vitamin D in protecting β-cell function[67,68]. However, another study reported significant effects of vitamin D administration on maintaining β-cell function after the development of type 1 DM. Thirty-eight patients with new-onset type 1 DM were randomly assigned to receive daily oral therapy with cholecalciferol, 2000 IU, or placebo[69]. The cumulative incidence of progression to undetectable (≤ 0.1 ng/mL) fasting C-peptide and stimulated C-peptide levels was lower in the cholecalciferol group than in the placebo group. In another study, alfacalcidol (0.25 μg/d) preserved β-cell function in children with newly diagnosed type 1 DM[70]. Further studies are needed to clarify whether the administration of 25(OH)D or 1,25(OH)2D can inhibit the onset of type 1 DM.
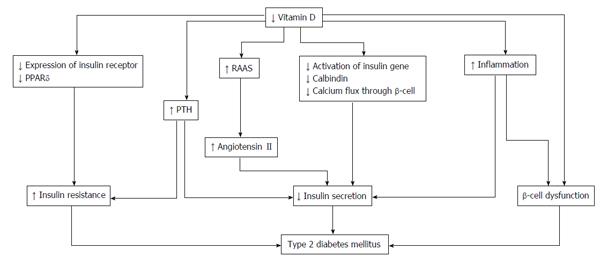
As VDRs in pancreatic β-cells play an important role in the progression of type 2 DM[71], vitamin D deficiency is related to insulin secretion, insulin resistance, and β-cell dysfunction in the pancreas[72] (Figure 2). The secretion of pancreatic insulin is inhibited by vitamin D deficiency in the diabetic animal model[73,74]. Administration of vitamin D restores glucose-stimulated insulin secretion and promotes β-cell survival by modulating the generation and effects of cytokines[75,76]. Insulin secretion is also influenced by calcium concentration and flux through the β-cells[77]. Vitamin D regulates the function of calbindin, a systolic calcium-binding protein found in pancreatic β-cells, and acts as a modulator of depolarization-stimulated insulin secretion via regulation of intracellular calcium[78]. PTH, which has its concentration regulated by vitamin D, is associated with insulin synthesis and secretion in the pancreas[79].
Insulin sensitivity is also associated with vitamin D. By stimulating the expression of insulin receptors, vitamin D regulates insulin sensitivity[80,81]. In addition, vitamin D enhances insulin sensitivity by promoting the expression of peroxisome proliferator-activated receptor (PPAR) delta, which is a widely expressed member of the PPAR family of nuclear receptor fatty acid sensors and regulates fatty acids in skeletal muscle and adipose tissue[82]. Intracellular calcium is a key factor of peripheral insulin resistance via an impaired signal transduction pathway leading to decreased glucose transporter activity[83,84].
The indirect effect of vitamin D is exerted by regulating calcium flux through the cell membrane and intracellular calcium. While low vitamin D induces secondary hyperparathyroidism, increased PTH levels are also associated with diabetes. A recent observational study of 494 women undergoing serial metabolic characterization revealed that hypovitamin D levels with increased PTH levels were an independent predictor of β-cell dysfunction, insulin resistance, and glycemia[85]. Vitamin D affects insulin resistance through the RAAS. One animal study demonstrated that vitamin D negatively regulated expression of renin genes in a mice model[86]. Furthermore, low levels of 1,25(OH)2D increased renal renin production and activated the RAAS system in an animal model[87]. Finally, angiotensin II inhibited the action of insulin in vascular and skeletal muscle tissues, leading to impaired glucose uptake[88].
Systemic inflammation has an important role in insulin resistance and cardiovascular events in patients with type 2 DM[89]. As β-cells in the pancreas are affected via cytokine-induced apoptosis, high levels of inflammation cause worsening glycemic control. Vitamin D could decrease the effects of systemic inflammation and protect against β-cell cytokine-induced apoptosis by directly modulating the expression and activity of cytokines, as has been shown in animal models[90]. In patients with type 2 DM, incubation of isolated monocytes with 1,25(OH)2D decreased the expression of inflammatory cytokines affecting insulin resistance, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α[91].
A prospective cohort study designed in the United Kingdom showed that baseline 25(OH)D concentrations in patients without diabetes were inversely related with the risk for hyperglycemia and insulin resistance at 10 years of follow-up visits[92]. Moreover, a similar study reported that low 25(OH)D levels were a risk factor for type 2 DM[93]. This prospective, cohort study was conducted over 29 years among 9841 subjects without diabetes. Lower vitamin D levels were a risk factor for incident type 2 DM. However, a recent Mendelian randomization approach study found that low 25(OH)D levels were not genetically associated with the risk for type 2 DM[94]. This result suggests that the association between 25(OH)D concentrations and type 2 DM is not causal. A meta-analysis of 16 studies reported that the odds ratio for type 2 DM was 1.5 (1.33-1.70) for the bottom vs top quartile of 25(OH)D levels[95]. Numerous randomized controlled studies have investigated whether vitamin D supplementation influences glycemic homeostasis[96,97]. As described above, vitamin D is thought to improve insulin resistance and promote insulin secretion. Therefore, clinical trials often use outcomes such as homeostasis model assessment of insulin resistance, fasting plasma glucose levels, and hemoglobin A1c levels. Some clinical trials have assessed the combined effects of vitamin D and calcium supplementation on glucose homeostasis of patients with diabetes[98,99] and without diabetes[100]. These studies suggest that vitamin D plus adequate calcium levels might be needed for an improvement in glycemic status. However, a recent meta-analysis concluded that vitamin D supplementation given to address concerns with glycemic control and insulin resistance in patients with diabetes is not recommended, although the doses of vitamin D supplementation may not have been optimal; almost all of the included trials used vitamin D doses of at least 2000 IU/d[101]. Because most trials focused on glycemic status and insulin resistance over short durations (12 mo or less), we should await the results of ongoing trials with longer follow-up periods to provide new evidence regarding the potential role of vitamin D supplementation in type 2 DM[102].
One study was designed to examine the protective effect of vitamin D against the development of type 2 DM[103]. A total of 2447 older people (mean age, 77 years) were allocated to 800 IU daily vitamin D3 and 1000 mg calcium both, or placebo for 24-62 mo. Vitamin D in combination with calcium was not able to prevent the development of diabetes or an increase in the need for medication in patients with diabetes. The Women’s Health Initiative Calcium/Vitamin D Study, a randomized, placebo-controlled trial of 33951 postmenopausal women, followed participants receiving 1000 mg elemental calcium plus 400 IU of vitamin D3 daily, or placebo for 7 years. Calcium plus vitamin D3 supplementation did not reduce the risk for developing diabetes over 7 years[104]. These results suggest that vitamin D supplementation at doses of 400 to 800 IU/d, with or without calcium, does not prevent new-onset type 2 DM.
Although at this time, supplementation with vitamin D has not been shown to improve glycemic control or prevent incident type 2 DM, clinical trials with sufficient sample size, study periods, and optimal doses of vitamin D supplementation are still needed. In addition, it is important that future studies of vitamin D use primary outcomes, such as all-cause mortality and cardiovascular disease, as endpoints.
One study revealed that paricalcitol diminished residual albuminuria in patients with diabetic nephropathy[105]. In this study, patients were randomly assigned (1:1:1) to receive placebo, 1 μg/d paricalcitol, or 2 μg/d paricalcitol for 24 wk to investigate the effect on mean urinary albumin-to-creatinine ratio (UACR). Patients receiving 2 μg paricalcitol showed a nearly sustained reduction in UACR, ranging from -18% to -28% (P = 0.014 vs placebo). However, few trials have used a vitamin D receptor antagonist (VDRA) for patients with diabetes, and none has a sufficient number of patients or follow-up period. The effect of vitamin D3 and VDRA on hard outcomes, such as progression of diabetes, cardiovascular disease, and all-cause mortality, requires larger and longer-term trials.
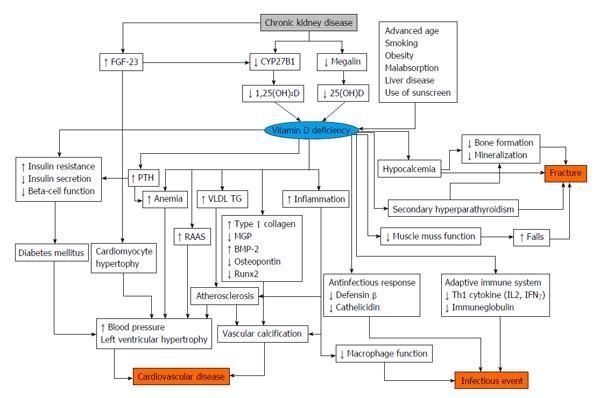
Some studies indicate that 1,25(OH)2D levels decrease in patients with CKD[106]. There are the several theories about the pathogenesis of vitamin D deficiency in CKD. Megalin, which is present in endocytic receptors in proximal tubule cells, is involved in the reabsorption of DBP from glomerular ultrafiltrates[107]. In addition, megalin also mediates the subsequent intracellular conversion of 25(OH)D to its active form. As kidney function declines, megalin expression in the proximal tubule decreases[108]. Megalin function is also attenuated with reduced kidney function, because of damages from low molecular weight proteinuria. The activity of CYP27B1 is also associated with decreasing kidney function[109]. As FGF-23 reduces expression of cotransporters NaPi-IIa and NaPi-IIc, of the brush border in the proximal tubules, these mechanisms inhibit phosphorus absorption and CYP27B1 activity.
In addition to the decline of 1,25(OH)2D levels, 25(OH)D levels also decrease in patients with CKD. There are the several plausible mechanisms that explain the decreases in 25(OH)D. The complex of 25(OH)D and DBP leaks with proteinuria. Uptake of 25(OH)D decreases due to down-regulation of megalin levels. One study showed that 25(OH)D concentrations in patients with CKD were low[110]. The prevalence of vitamin D deficiency is 35% among about 4000 patients with CKD in the United States[111].
There is some evidence that vitamin D status is associated with poor clinical outcomes in patients with CKD[112] (Figure 3). Low 25(OH)D levels are associated with all-cause mortality and cardiovascular disease in patients with CKD[113]. The risk for end stage renal disease is higher in patients with low vitamin D status. Among patients undergoing hemodialysis and peritoneal dialysis, low 25(OH)D levels are also associated with cardiovascular disease[114].
There is some evidence regarding restitution of vitamin D in patients with CKD[115,116] and as well as in patients undergoing dialysis [117,118]. As previously described, patients with kidney failure usually have insufficient 1,25(OH)2D levels, and a VDRA is used for these patients. One study revealed that paricalcitol diminished albuminuria in patients with diabetic nephropathy[105]. In the VITAL study, which was designed to compare the effectiveness between paricalcitol and placebo, the paricalcitol group showed a decreased UACR of -16% compared with placebo[105]. However, as of yet, no other studies have investigated the effectiveness of vitamin D supplementation for protection of kidney function; thus, future studies are needed. Another study showed that paricalcitol led to decreases in levels of brain natriuretic peptide (BNP) in patients with CKD[119]. On the other hand, a recent study reported that treatment with paricalcitol did not improve left ventricular mass and function in patients with CKD[120]. There is controversial evidence regarding the role of VDRA to cardiovascular disease and surrogate makers. It is thought that as 1,25(OH)2D inhibits activation of the RAAS, it leads to organ protection[121]. In addition, there is some evidence regarding VDRAs in patients undergoing hemodialysis. A retrospective cohort study showed that VDRA users had a lower mortality rate than non-VDRA users[122]. However, the Dialysis Outcomes and Practice Patterns Study revealed that taking vitamin D agents did not improve clinical outcome in patients undergoing dialysis. In addition, a recent study reported that pharmacological doses of alfacalcidol were associated with accelerated progression of aortic stiffness in patients undergoing hemodialysis[123]. To date, various discussions have taken place regarding the use of VDRA in patients undergoing dialysis, but adequate clinical studies are needed before any recommendations can be made.
According to the Kidney Disease Improving Global Outcomes guidelines, 25(OH)D levels should be determined in patients with CKD stage 3-5, and if levels are low, physicians should consider vitamin D supplementation[124]. Low 25(OH)D levels are associated with all-cause mortality and cardiovascular disease in patients with CKD as well as in patients undergoing dialysis[125]. Another study showed that among these patient groups, those with low levels of 25(OH)D and high levels of FGF-23 have worse outcomes[38]. However, there is not sufficient evidence regarding vitamin D supplementation for patients with CKD and those undergoing dialysis[126]. Although studies have reported that cholecalciferol decreases albuminuria[127,128] and improves PTH levels[129] in patients with CKD, there is no study with set clinical outcomes such as all-cause mortality or cardiovascular disease. In patients undergoing dialysis, cholecalciferol decreases BNP levels and reduces left ventricular hypertrophy[130]. As VDRAs increase calcium and phosphorus levels in patients undergoing dialysis, it is usually recommended that physicians only need to monitor calcium and phosphorus levels when using a VDRA[131]. On the other hand, vitamin D3, such as cholecalciferol, does not increase calcium and phosphorus levels[132,133]. As with patients with CKD, there is no evidence with hard endpoints regarding the use of vitamin D3 supplementation in patients undergoing hemodialysis.
Emerging evidence is accumulating on the important role of vitamin D in the pathogenesis of diabetes and CKD. Many prospective studies have shown associations between vitamin D status and chronic disease, including diabetes and CKD. However, there are contradictory findings regarding whether restitution of normal vitamin D levels modifies the occurrence or clinical course of these diseases. Although there is a concern that vitamin D may be a surrogate marker for poor health status, further well-designed clinical trials are needed in this area.
P- Reviewer: Nechifor G, Panchu P, Tamemoto H, Tomkin GH S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care. 2006;29:2114-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 435] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 2. | Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, Mallamaci F, Massy ZA, Rossignol P, Vanholder R. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1232] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 4. | Husemoen LL, Thuesen BH, Fenger M, Jørgensen T, Glümer C, Svensson J, Ovesen L, Witte DR, Linneberg A. Serum 25(OH)D and type 2 diabetes association in a general population: a prospective study. Diabetes Care. 2012;35:1695-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29:e142-e150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 888] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 7. | Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S-1688S. [PubMed] |
| 8. | Christensen EI, Willnow TE. Essential role of megalin in renal proximal tubule for vitamin homeostasis. J Am Soc Nephrol. 1999;10:2224-2236. [PubMed] |
| 9. | Verroust PJ, Birn H, Nielsen R, Kozyraki R, Christensen EI. The tandem endocytic receptors megalin and cubilin are important proteins in renal pathology. Kidney Int. 2002;62:745-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Dusso AS. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int Suppl (2011). 2011;1:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Silver J, Naveh-Many T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat Rev Nephrol. 2013;9:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets. 2006;10:735-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Segersten U, Correa P, Hewison M, Hellman P, Dralle H, Carling T, Akerström G, Westin G. 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab. 2002;87:2967-2972. [PubMed] |
| 14. | van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium 2011 Dietary Reference Intakes for Calcium and Vitamin D. Final appraisal determination, laparoscopic surgery for inguinal hernia repair. Washington (DC): National Academies Press 2011; . |
| 17. | Buchebner D, McGuigan F, Gerdhem P, Malm J, Ridderstråle M, Akesson K. Vitamin D insufficiency over 5 years is associated with increased fracture risk-an observational cohort study of elderly women. Osteoporos Int. 2014;25:2767-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6974] [Cited by in RCA: 6845] [Article Influence: 488.9] [Reference Citation Analysis (0)] |
| 20. | DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S-1696S. [PubMed] |
| 21. | Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 744] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 22. | Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28. [PubMed] |
| 23. | Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 551] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 24. | Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105:971-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860-868. [PubMed] |
| 26. | Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66:929-936. [PubMed] |
| 27. | Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1070] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 28. | Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 585] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 29. | Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1049] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 30. | Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71:134-139. [PubMed] |
| 31. | Tanaka H, Hamano T, Fujii N, Tomida K, Matsui I, Mikami S, Nagasawa Y, Ito T, Moriyama T, Horio M. The impact of diabetes mellitus on vitamin D metabolism in predialysis patients. Bone. 2009;45:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 802] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 33. | Kohli NR, Van Valkengoed IG, Nicolaou M, Brewster LM, Van Der A DL, Stronks K, Snijder MB. Vitamin D status partly explains ethnic differences in blood pressure: the ‘Surinamese in the Netherlands: study on ethnicity and health’. J Hypertens. 2012;30:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [PubMed] |
| 35. | Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet. 1993;92:183-188. [PubMed] |
| 36. | Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 486] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 37. | Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot Ld, Streppel M, Gardiner J, Ordóñez-Mena JM, Perna L. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 38. | Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associate with Infectious and Cardiac Deaths in the HEMO Study. J Am Soc Nephrol. 2016;27:227-237. [PubMed] |
| 39. | Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 776] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 40. | Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39-48. [PubMed] |
| 41. | Brock KE, Ke L, Tseng M, Clemson L, Koo FK, Jang H, Seibel MJ, Mpofu E, Fraser DR, Mason RS. Vitamin D status is associated with sun exposure, vitamin D and calcium intake, acculturation and attitudes in immigrant East Asian women living in Sydney. J Steroid Biochem Mol Biol. 2013;136:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709-710. [PubMed] |
| 43. | Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 44. | Vimaleswaran KS, Cavadino A, Berry DJ; LifeLines Cohort Study investigators, Jorde R, Dieffenbach AK, Lu C, Alves AC,Heerspink HJ, Tikkanen E, Eriksson J, Wong A, Mangino M,Jablonski KA, Nolte IM, Houston DK, Ahluwalia TS, van der Most PJ, Pasko D, Zgaga L, Thiering E, Vitart V, Fraser RM, Huffman JE, de Boer RA, Schöttker B, Saum KU, McCarthy MI, Dupuis J,Herzig KH, Sebert S, Pouta A, Laitinen J, Kleber ME, Navis G,Lorentzon M, Jameson K, Arden N, Cooper JA, Acharya J, Hardy R, Raitakari O, Ripatti S, Billings LK, Lahti J, Osmond C, Penninx BW, Rejnmark L, Lohman KK, Paternoster L, Stolk RP,Hernandez DG, Byberg L, Hagström E, Melhus H, Ingelsson E,Mellström D, Ljunggren O, Tzoulaki I, McLachlan S, Theodoratou E, Tiesler CM, Jula A, Navarro P, Wright AF, Polasek O;International Consortium for Blood Pressure (ICBP); Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium; Global Blood Pressure Genetics (Global BPGen) consortium; Caroline Hayward, Wilson JF, Rudan I, Salomaa V,Heinrich J, Campbell H, Price JF, Karlsson M, Lind L, Michaëlsson K, Bandinelli S, Frayling TM, Hartman CA, Sørensen TI,Kritchevsky SB, Langdahl BL, Eriksson JG, Florez JC, Spector TD, Lehtimäki T, Kuh D, Humphries SE, Cooper C, Ohlsson C,März W, de Borst MH, Kumari M, Kivimaki M, Wang TJ, Power C,Brenner H, Grimnes G, van der Harst P, Snieder H, Hingorani AD,Pilz S, Whittaker JC, Järvelin MR, Hyppönen E. Association of vitamin D status with arterial blood pressure andhypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:719-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 45. | Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliövaara M, Impivaara O, Reunanen A. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 811] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 47. | di Giuseppe R, Buijsse B, Hirche F, Wirth J, Arregui M, Westphal S, Isermann B, Hense HW, Dierkes J, Boeing H. Plasma fibroblast growth factor 23, parathyroid hormone, 25-hydroxyvitamin D3, and risk of heart failure: a prospective, case-cohort study. J Clin Endocrinol Metab. 2014;99:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. 2013;73:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 49. | Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, Halkjær J, Lind B, Heegaard AM, Schwarz P. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J Clin Endocrinol Metab. 2015;100:2339-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 50. | Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229-238. [PubMed] |
| 51. | Li YC. Renoprotective effects of vitamin D analogs. Kidney Int. 2010;78:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Andrukhova O, Slavic S, Zeitz U, Riesen SC, Heppelmann MS, Ambrisko TD, Markovic M, Kuebler WM, Erben RG. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol. 2014;28:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 53. | Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, Hirata M, Shinohara M, Fukagawa M, Nishi S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014;27:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 54. | Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 924] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 55. | Lin CH, Kadakia S, Frieri M. New insights into an autoimmune mechanism, pharmacological treatment and relationship between multiple sclerosis and inflammatory bowel disease. Autoimmun Rev. 2014;13:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Gatenby P, Lucas R, Swaminathan A. Vitamin D deficiency and risk for rheumatic diseases: an update. Curr Opin Rheumatol. 2013;25:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 58. | Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 59. | Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 427] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 60. | Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Zella JB, DeLuca HF. Vitamin D and autoimmune diabetes. J Cell Biochem. 2003;88:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Takiishi T, Ding L, Baeke F, Spagnuolo I, Sebastiani G, Laureys J, Verstuyf A, Carmeliet G, Dotta F, Van Belle TL. Dietary supplementation with high doses of regular vitamin D3 safely reduces diabetes incidence in NOD mice when given early and long term. Diabetes. 2014;63:2026-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, Muharram G, Kerr Conte J, Pattou F. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes Epidemiology Research International Group. Diabetes. 1988;37:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1156] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 66. | Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia. 1999;42:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 321] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 67. | Bizzarri C, Pitocco D, Napoli N, Di Stasio E, Maggi D, Manfrini S, Suraci C, Cavallo MG, Cappa M, Ghirlanda G. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33:1962-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Walter M, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1alpha,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care. 2010;33:1443-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Gabbay MA, Sato MN, Finazzo C, Duarte AJ, Dib SA. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β-cell function in new-onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2012;166:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 70. | Ataie-Jafari A, Loke SC, Rahmat AB, Larijani B, Abbasi F, Leow MK, Yassin Z. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clin Nutr. 2013;32:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 72. | Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820-825. [PubMed] |
| 73. | Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 497] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 74. | Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Cade C, Norman AW. Rapid normalization/stimulation by 1,25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology. 1987;120:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Clark SA, Stumpf WE, Sar M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes. 1981;30:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol. 2011;347:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Kadowaki S, Norman AW. Pancreatic vitamin D-dependent calcium binding protein: biochemical properties and response to vitamin D. Arch Biochem Biophys. 1984;233:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Fadda GZ, Akmal M, Lipson LG, Massry SG. Direct effect of parathyroid hormone on insulin secretion from pancreatic islets. Am J Physiol. 1990;258:E975-E984. [PubMed] |
| 80. | Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct. 2002;20:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Maestro B, Dávila N, Carranza MC, Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 241] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 82. | Dunlop TW, Väisänen S, Frank C, Molnár F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005;349:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Zemel MB. Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem. 1998;188:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 84. | Reusch JE, Begum N, Sussman KE, Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology. 1991;129:3269-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R. Prospective associations of vitamin D status with β-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes. 2014;63:3868-3879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125-E132. [PubMed] |
| 87. | Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008;74:1577-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Cheng Q, Boucher BJ, Leung PS. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia. 2013;56:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65:S152-S156. [PubMed] |
| 90. | Riachy R, Vandewalle B, Kerr Conte J, Moerman E, Sacchetti P, Lukowiak B, Gmyr V, Bouckenooghe T, Dubois M, Pattou F. 1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20. Endocrinology. 2002;143:4809-4819. [PubMed] |
| 91. | Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47-57. [PubMed] |
| 92. | Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 93. | Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 94. | Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F, Langenberg C, Wareham NJ, Forouhi NG. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 95. | Parekh D, Sarathi V, Shivane VK, Bandgar TR, Menon PS, Shah NS. Pilot study to evaluate the effect of short-term improvement in vitamin D status on glucose tolerance in patients with type 2 diabetes mellitus. Endocr Pract. 2010;16:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 97. | Oosterwerff MM, Eekhoff EM, Van Schoor NM, Boeke AJ, Nanayakkara P, Meijnen R, Knol DL, Kramer MH, Lips P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: a randomized placebo-controlled trial. Am J Clin Nutr. 2014;100:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 98. | Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 99. | Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: a randomised controlled clinical trial. Diabetologia. 2014;57:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 100. | Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 433] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 101. | Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3551-3560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 102. | Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 405] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 103. | Avenell A, Cook JA, MacLennan GS, McPherson GC. Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2009;38:606-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 104. | de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 105. | de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 494] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 106. | Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. Am J Kidney Dis. 2012;60:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/”megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci USA. 1994;91:9725-9729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 421] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 108. | Hosaka K, Takeda T, Iino N, Hosojima M, Sato H, Kaseda R, Yamamoto K, Kobayashi A, Gejyo F, Saito A. Megalin and nonmuscle myosin heavy chain IIA interact with the adaptor protein Disabled-2 in proximal tubule cells. Kidney Int. 2009;75:1308-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, Schoonjans K, Verstuyf A. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 110. | Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 111. | Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 999] [Cited by in RCA: 920] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 112. | Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 113. | Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ, Simon JF, Srinivas TR, Nally JV. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis. 2011;58:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, Sea MM, Woo J. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87:1631-1638. [PubMed] |
| 115. | Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis. 2009;53:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, Wells D, Aitkenhead H, Manickavasagar B, van’t Hoff W. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol. 2012;7:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 117. | Jean G, Souberbielle JC, Chazot C. Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant. 2009;24:3799-3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 118. | Armas LA, Zena M, Lund R, Heaney RP. Calcium absorption response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2013;8:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, Pritchett Y, Chang Y, Agarwal R, Wanner C. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164:902-909.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 120. | Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, Lo G, Lai KN, Lo WK, Lam CW. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014;25:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 121. | de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 122. | Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115-1125. [PubMed] |
| 123. | Charitaki E, Davenport A. Aortic pulse wave velocity in haemodialysis patients is associated with the prescription of active vitamin D analogues. J Nephrol. 2014;27:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1076] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 125. | Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Tonelli M. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004-1013. [PubMed] |
| 126. | Kandula P, Dobre M, Schold JD, Schreiber MJ, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 127. | Molina P, Górriz JL, Molina MD, Peris A, Beltrán S, Kanter J, Escudero V, Romero R, Pallardó LM. The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant. 2014;29:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 128. | Kim MJ, Frankel AH, Donaldson M, Darch SJ, Pusey CD, Hill PD, Mayr M, Tam FW. Oral cholecalciferol decreases albuminuria and urinary TGF-β1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int. 2011;80:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 129. | Alvarez JA, Law J, Coakley KE, Zughaier SM, Hao L, Shahid Salles K, Wasse H, Gutiérrez OM, Ziegler TR, Tangpricha V. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 130. | Bucharles S, Barberato SH, Stinghen AE, Gruber B, Piekala L, Dambiski AC, Custodio MR, Pecoits-Filho R. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 131. | Warn PA, Sharp A, Morrissey G, Denning DW. Activity of aminocandin (IP960; HMR3270) compared with amphotericin B, itraconazole, caspofungin and micafungin in neutropenic murine models of disseminated infection caused by itraconazole-susceptible and -resistant strains of Aspergillus fumigatus. Int J Antimicrob Agents. 2010;35:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Armas LA, Andukuri R, Barger-Lux J, Heaney RP, Lund R. 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2012;7:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Massart A, Debelle FD, Racapé J, Gervy C, Husson C, Dhaene M, Wissing KM, Nortier JL. Biochemical parameters after cholecalciferol repletion in hemodialysis: results From the VitaDial randomized trial. Am J Kidney Dis. 2014;64:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |