INTRODUCTION
The incidence of osteoporosis and type 2 diabetes mellitus is known to increase in prevalence with aging. However, both diseases were traditionally viewed as separate entities. Previous studies have shown that patients with type 1 and type 2 diabetes have an increased risk of fractures, and that hyperglycemia and insulin affects bone metabolism. In addition, there is a positive association between bone mineral density (BMD) and fat mass[1,2], suggesting that accumulation of fat mass influences bone metabolism. Adipose tissue secretes a variety of biological active molecules such as leptin, adiponectin, and resistin, all of which play important roles in glucose metabolism. Previously, these adipokines are reported to regulate bone metabolism[3-5].
On the other hand, a previous excellent study using gene mutant mice has shown that osteocalcin, which is one of the osteoblast-specific proteins and has several hormonal features, regulates glucose metabolism[6]. Osteocalcin knockout (Ocn-/-) mice were previously generated to examine the role of osteocalcin in bone tissue[7]. Although it was not reported at that time, the authors found that Ocn-/- mice were obese and had an abnormal accumulation of visceral fat. In 2007, it was reported that Ocn-/- mice displayed hyperglycemia and impaired glucose tolerance due to insulin insufficiency and resistance[6]. In the mice, pancreatic β-cell proliferation and insulin secretion were significantly decreased, and insulin resistance by reduced adiponectin expression in adipocytes was observed. Osteocalcin has 46-50 amino acids and undergoes γ-carboxylation of glutamyl residues at three positions 17, 21, and 24, which facilitates binding of osteocalcin to hydroxyapatite in bone matrix. Further examinations have shown that undercarboxylated form of osteocalcin (ucOC) is an active form in glucose metabolism[6,8]. In this review, I describe the role of osteocalcin in glucose metabolism and the association between serum osteocalcin level and parameters of glucose homeostasis in humans.
OSTEOCALCIN REGULATES GLUCOSE METABOLISM
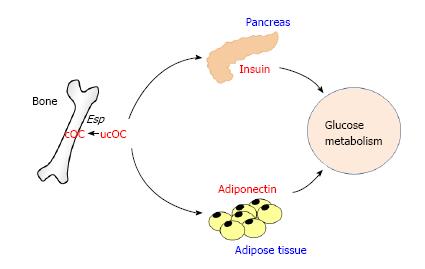
The Karsenty group reported for the first time an interesting and excellent animal study using gene mutant mice models showing that osteocalcin secreted from bone might be involved in whole body glucose homeostasis (Figure 1)[6]. In the study, Ocn-/- mice and Esp knockout (Esp-/-) mice were used to examine the function of osteocalcin in β-cell and adipocytes. Esp encodes osteotesticular protein tyrosine phosphatase (OST-PTP), which is restricted to osteoblasts, sertoli cells and embryonic stem cells[9]. OST-PTP is a transmembrane tyrosine phosphatase and can not directly affect distant tissues. Since it stimulates carboxylation of osteocalcin and decreases osteocalcin bioactivity, Esp-/- mice was examined as a model of gain of osteocalcin bioactivity[6]. Ocn-/- mice showed hyperglycemia and glucose intolerance, decreased β-cell and insulin secretion, decreased insulin sensitivity and adiponectin expression, and increased fat mass and serum triglyceride level. Moreover, when Ocn expression vector-transfected COS cells were cocultured with islets or adipocytes, the expression of insulin and adiponectin was significantly increased. In addition, recombinant osteocalcin injection improved the glucose intolerance and increased insulin expression in β-cells. In contrast to Ocn-/- mice, Esp-/- mice showed hypoglycemia and low blood glucose level after glucose injection, increased insulin expression and secretion, as well as increased insulin sensitivity and adiponectin expression in adipose tissue. Furthermore, Esp-/- mice displayed decreased fat mass and serum triglyceride level, a resistance of high fat diet-induced obesity and diabetes, as well as resistance to streptozotocin-induced diabetes. Namely, the metabolic phenotype of Ocn-/- mice is the mirror image of the one seen in Esp-/- mice. To examine whether metabolic abnormalities of Esp-/- mice could be corrected by inhibition of osteocalcin expression, Esp-/- mice were crossed with Ocn+/- mice. In Esp-/-;Ocn+/- mice, the metabolic abnormalities such as hypoglycemia, hyperinsulinemia and increased serum adiponectin level were completely reversed. Several in vitro experiments were performed by using carboxylated osteocalcin (cOC) and ucOC. UcOC significantly stimulated the expression of cyclin D1 and insulin in islets as well as of adiponectin in adipocytes, whereas cOC showed no effects on them. However, studies by other groups have suggested that both cOC and ucOC can stimulate the response to insulin in adipocytes and myoblasts[10].
Figure 1 The effects of osteocalcin on pancreas and adipose tissue.
Undercarboxylated osteocalcin (ucOC) is an active form regulating glucose metabolism. Esp inactivate osteocalcin by carboxylating ucOC. ucOC increases insulin expression in pancreas and adiponectin expression in adipose tissue.
In addition to the direct effect of osteocalcin on insulin secretion, it has been shown that osteocalcin indirectly stimulates it through increasing the secretion of glucagon-like peptide-1 (GLP-1), an incretin released by intestinal endocrine cells[11,12]. Mizokami et al[11,12] demonstrated that treatment with ucOC significantly increased GLP-1 expression in STC-1 enteroendocrine cells in vitro, and that administration of ucOC increased serum GLP-1 and insulin levels in mice. These effects were potentiated by an inhibitor of dipeptidyl peptidase-4 and blocked by a GLP-1 receptor antagonist, suggesting that ucOC increases insulin secretion through GLP-1 secretion from intestinal endocrine cells. In contrast, cOC did not affect GLP-1 or insulin secretion.
From the Karsenty group, the effects of recombinant osteocalcin injection on glucose metabolism in wild-type (WT) mice were reported[8]. Continuous intraperitoneal injection of low dose recombinant osteocalcin increased insulin secretion, β-cell proliferation, insulin sensitivity and adiponectin expression as well as decreased fat mass in WT mice. Moreover, recombinant osteocalcin injection prevented high fat diet-induced obesity and diabetes. Further, therapeutic potential of intermittent administration of recombinant osteocalcin was also tested[13]. Daily injection of osteocalcin significantly improved glucose intolerance and insulin resistance in mice fed not only normal diet but also high fat diet. In addition, hepatic steatosis induced by high fat diet was completely recovered in mice treated with osteocalcin daily injection. Of interest, it is reported that oral administration of osteocalcin also improved impaired glucose tolerance in vivo[11,12,14]. Oral administration of ucOC reached small intestine and remained there for at least 24 h as well as entered the general circulation. Daily and long-term (13 wk) intermittent oral administration of ucOC significantly reduced fasting blood glucose level and improved glucose tolerance in mice without affecting insulin sensitivity. Oral administration also increased fating serum insulin level and β-cell area in pancreas. The serum GLP-1 level was increased in accordance with the presence of ucOC in the intestine and systemic circulation. Taken together, these findings suggest that the intermittent injection and oral administration of recombinant osteocalcin may be useful for treatment of type 2 diabetes and obesity. However, it is reported that oral administration of osteocalcin did not affect insulin sensitivity[11,12]; thus further studies are necessary to define the difference between oral administration and intraperitoneal infection of osteocalcin.
AN ENDOCRINE FEEDFORWARD LOOP BETWEEN BONE AND PANCREAS
Previous studies have shown that patients with type 1 diabetes mellitus, which is caused by autoimmune destruction of insulin-producing β-cells, have a significant reduction in BMD with decreased bone formation and an increased risk of fragility fractures[15-17]. These clinical feature suggests that insulin signal in osteoblasts has pivotal roles in bone formation and bone development. Osteoblasts have a functional insulin receptor and that treatment with insulin stimulates the proliferation and differentiation of osteoblasts[18,19]. In addition, osteoblast-specific insulin receptor knockout (Ob-IR-/-) mice displayed reduced bone accumulation due to decreased bone formation and deficient number of osteoblasts, and deletion of insulin receptor in osteoblasts induced decreases in alkaline phosphatase (ALP) activity and osteocalcin expression by inhibiting a Runx2 inhibitor, Twist2[20]. These findings indicate that insulin signaling may be an anabolic factor for bone formation; however, the detail mechanisms are still unclear. FoxO1, which belongs to the Forkhead family of transcription factors, is a major transcriptional mediator of insulin signaling and insulin transmits its signal by inhibiting FoxO1 activity in various cells[21]. Previous in vitro studies showed that FoxO1 physically interacts with Runx2 via its C-terminal region and inhibits Runx2-dependent transcriptional activity as well as osteocalcin expression in osteoblasts, and that insulin and insulin-like growth factor-I signals prevent FoxO1 from inhibiting Rux2 activity by promoting FoxO1 phosphorylation and nuclear exclusion[22]. In contrast, osteoblast-specific FoxO1 knockout (Ob-FoxO1-/-) mice showed marked reduction of bone mass[23]. Moreover, FoxO1 is known to protect osteoblast function against oxidative stress. Therefore, further studies are necessary to understand the underlying mechanism of insulin in osteoblasts.
Based on the action of insulin in bone, with regard to the hormonal loop networks, it is rational to suggest that signals derived from osteoblasts might affect insulin expression and secretion in β-cells. Previously, Fulzele et al[20] and Ferron et al[24] reported that insulin signaling in osteoblasts contributes to whole-body glucose homeostasis by increasing β-cell proliferation and insulin secretion by using Ob-IR-/- mice, indicating a feedforward loop between bone and pancreas. Ob-IR-/- mice developed marked peripheral adiposity and hyperglycemia accompanied by severe glucose intolerance and insulin resistance. Fat mass was significantly greater in Ob-IR-/- mice than that in control mice, and examination of body composition revealed a 40% increase in fat mass and an 8% decrease in lean mass in Ob-IR-/- mice. Moreover, glucose tolerance tests showed that plasma glucose levels after glucose injection was significantly higher in Ob-IR-/- mice than controls, whereas serum insulin level was significantly decreased in Ob-IR-/- mice. Pancreatic β-cell mass and insulin expression were significantly decreased in Ob-IR-/- mice, and insulin tolerance tests and gene expression analysis showed that Ob-IR-/- mice had a severe insulin resistance. Furthermore, Ob-IR-/- mice had decreased rates of oxygen consumption and energy expenditure compared with controls. Serum ucOC level was decreased in Ob-IR-/- mice, and its infusion reversed the glucose intolerance seen in Ob-IR-/- mice, suggesting that an endocrine loop through insulin and ucOC exists between bone and pancreas. On the other hand, Ob-FoxO1-/- mice showed hypoglycemia and hyperinsulinemia with an increase in β-cell mass[25]. Ob-FoxO1-/- mice have a phenotype that mirrors the metabolic phenotype of Ocn-/- mice, thus suggesting a gain of osteocalcin activity in Ob-FoxO1-/- mice. Ferron et al[24] showed that FoxO1 haploinsufficiency rescued the phenotype of Ob-IR-/- mice. These findings suggest that FoxO1 inactivation may be involved in the effects of insulin signal in osteoblasts on systemic glucose homeostasis. Previous studies showed that an interaction of FoxO1 and ATF4 stimulated osteocalcin expression by luciferase assay using osteocalcin promoter gene[23], whereas their interaction inactivated osteocalcin by reducing undercarboxylated form, resulting in glucose intolerance[26].
OSTEOCLASTS REGULATE GLUCOSE METABOLISM BY DECARBOXYLATION OF OSTEOCALCIN
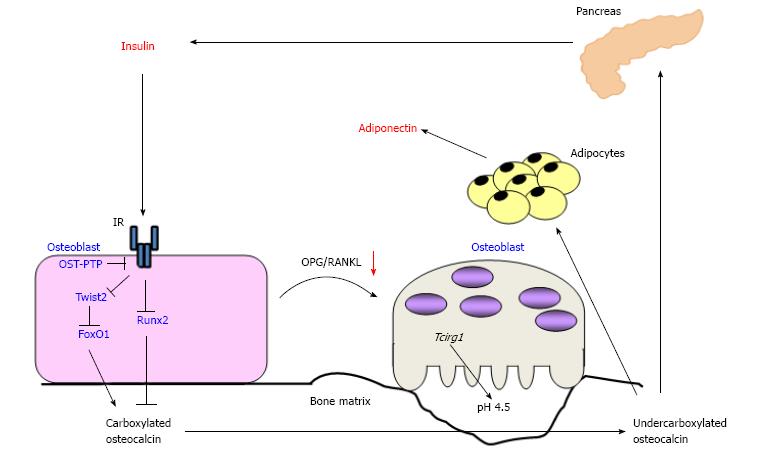
Osteoblasts produce osteocalcin and carboxylate it dependently on vitamin K, resulting in accumulation of bone matrix-embedded cOC. For the function of osteocalcin in glucose metabolism, osteoclasts are reported to be necessary. Ferron et al[24] noticed that serum level of CTx, a marker of bone resorption, was markedly decreased in Ob-IR-/- mice. Co-culture with osteoclast precursor cells and IR null osteoblasts showed that the area covered by resorption pits was significantly decreased. The expression of osteoprotegerin (OPG), a decoy receptor for receptor activator of nuclear factor kappa-B ligand (RANKL), was significantly increased in IR null osteoblasts, and insulin treatment decreased OPG expression and secretion in WT osteoblasts, but not IR null osteoblasts. In addition, the expression of CathepsinK (CtsK) and Tcirg1, both of which play pivotal roles in bone resorption, was decreased in Ob-IR-/- bone. The expression of CtsK and Tcirg1 was also decreased in osteoclast precursor cells cocultured with IR null osteoblasts. These findings suggest that insulin signaling in osteoblasts favors not only osteoblastic differentiation and osteocalcin expression but also the activation of osteoclasts and bone resorption by inhibiting OPG expression (Figure 2).
Figure 2 Schematic representation of the endocrine loops between bone and pancreas.
Insulin signaling stimulates the expression of osteocalcin and osteoblastic differentiation via inhibiting Twist2, an inhibitor of Runx2, as well as FoxO1, resulting in accumulation of carboxylated osteocalcin in bone matrix. Conversely, insulin activates osteoclasts and accelerate bone turnover via increasing the ratio of osteoprotegerin/receptor activator of nuclear factor kappa-B ligand. Activated osteoclasts decarboxylate bone matrix-embedded osteocalcin, and then undercarboxylated osteocalcin (ucOC) is released into the circulation. UcOC stimulates the expression of insulin in pancreas and of adiponectin in adipose tissue. OST-PTP: Osteotesticular protein tyrosine phosphatase; AMPK: AMP-activated protein kinase; OPG/RANKL: Osteoprotegerin/receptor activator of nuclear factor kappa-B ligand; IR: Insulin receptor.
The gene Tcirg1 encodes a vacuolar proton pump subunit essential for acidification of bone matrix, which is essential for bone resorption[27]. Because acidification decarboxylates proteins[28], it was assumed that insulin signaling in osteoblasts may regulate glucose metabolism by decarboxylation of osteocalcin in bone matrix following activation of osteoclasts. To examine whether or not the regulation of glucose metabolism by insulin signaling in osteoblasts depends on the activation of osteoclasts, Ob-IR-/- mice were crossed with oc/oc mice, a model of loss-of-function in Tcirg1[29]. Ob-IR+/-; oc/+ mice showed a significant reduction in insulin secretion as well as impaired glucose tolerance although Ob-IR+/- or oc/+ mice had no phenotype of glucose intolerance. Moreover, Esp-/-; oc/+ mice showed normal bone resorption, normal osteocalcin carboxylation, blood glucose, insulin secretion, and insulin sensitivity, while Esp-/- mice showed hypoglycemia and low blood glucose level after glucose injection, increased insulin expression and secretion, as well as increased insulin sensitivity and adiponectin expression in adipose tissue. Furthermore, treatment with alendronate, a bisphosphonate, in Esp-/- mice showed that the phenotype of glucose abnormality was completely normalized. On the contrary, RANKL treatment induced bone resorption and increased serum level of ucOC, resulting in less glucose intolerance and less fat mass in WT mice fed a high-fat diet than controls. Taken altogether, these findings indicate that bone resorption is essential to activate osteocalcin and regulate glucose homeostasis by bone.
THE CROSS RELATIONSHIPS BETWEEN BONE AND ADIPOSE TISSUE THROUGH OSTEOCALCIN AND ADIPOKINES
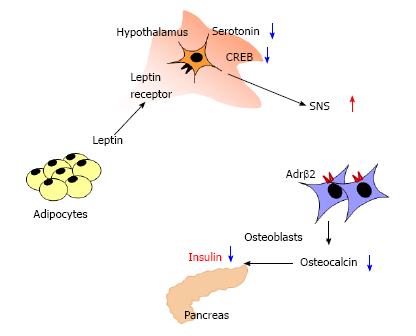
Previous studies have shown that adipose tissue is involved in bone metabolism. Adipocyte is recently known to not only be an energy-storing organ but also secret a variety of biological active molecules, which are named adipokines[30]. Leptin is known to suppress appetite and regulate energy expenditure through its receptor presented in hypothalamus. In leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice, high bone mass with a massive increase in bone formation was observed[31]. Although ob/ob mice had no leptin signal in osteoblasts, intracerebroventicular infusion of leptin decreased bone mass, suggesting that leptin is a potent inhibitor of bone formation acting through the central nervous system. Ultimately, leptin inhibits the production and release of serotonin in brainstem neurons[32], and this decrease in serotonin leads to reduced CREB signaling in the hypothalamus, resulting in an increased activation of the sympathetic nervous system (SNS)[33]. Neurons from the SNS are present in bones and activation of SNS inhibits the proliferation and differentiation of osteoblasts through the β2-adrenergic receptor (Adrβ2)[34]. Of interest, it has been shown that leptin regulates insulin secretion through direct and indirect mechanisms. Morioka et al[35] reported that pancreas-specific leptin receptor knockout mice showed improved glucose tolerance due to enhanced early-phase insulin secretion. Upregulation of SNS by leptin has been shown to indirectly suppress insulin secretion by inhibiting the production of ucOC. Hinoi et al[36] reported that ob/ob and osteoblast-specific deletion of Adrβ2 (Ob-Adrβ2-/-) mice showed increases in serum insulin level and decreases in blood glucose level compared to WT mice. Ob/+; Ob-Adrβ2+/- mice showed an increase in serum insulin level and a decrease in blood glucose level although serum insulin and blood glucose levels were not changed in ob/+ or Ob-Adrβ2+/- mice. Moreover, when ob/ob mice were crossed with Ocn-/- mice, ob/ob; Ocn-/- mice reversed the glucose abnormalities of ob/ob mice. Because insulin is adipogenic, increases body fat mass, and stimulates the production and secretion of leptin[37], it is suggested that negative feedback loops may exist between pancreas, adipose tissue, brain, and bone (Figure 3).
Figure 3 Schematic representation of the regulation of insulin secretion by leptin.
Leptin directly inhibits insulin expression in pancreas. Leptin activates SNS by decreasing serotonin and cAMP response element binding protein in hypothalamus, and then enhanced SNS suppresses osteoblast differentiation and osteocalcin expression through the Adrβ2 in osteoblasts. Thus, leptin indirectly inhibits insulin expression via central nervous system and bone. SNS: Sympathetic nervous system.
On the other hand, resistin acts in insulin target organs such as skeletal muscle, liver, and adipose tissue and reduces insulin sensitivity there[38]; thus, it is suggested to be a molecule linking obesity to type 2 diabetes[39]. Resistin is previously reported to be expressed in osteoblasts and osteoclasts and to increase the number of differentiated osteoclasts as well as the proliferation of osteoblastic cells[40], suggesting that resistin may be involved in bone metabolism. However, previous studies showed that osteocalcin did not affect the expression of leptin or resistin in adipose tissue[6,25]. Therefore, these two adipokines may affect the function of osteocalcin in glucose metabolism but may not have direct cross relationships with osteocalcin.
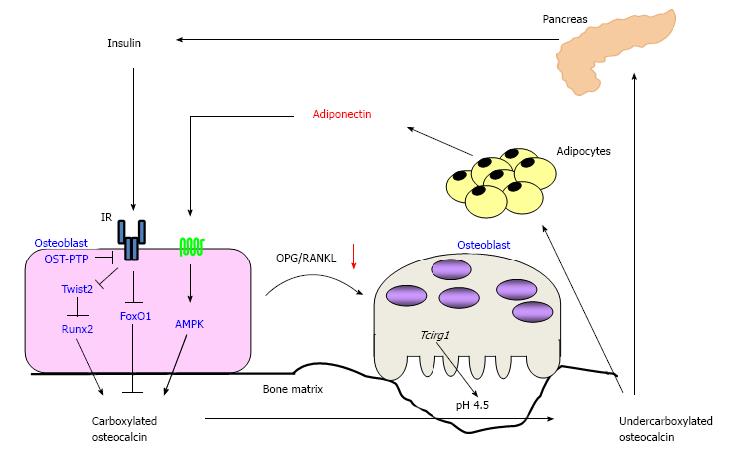
It has been shown that osteoblast has an adiponectin receptor and adiponectin signaling stimulates the differentiation and osteocalcin expression in osteoblasts. Previously, Luo et al[41] showed that recombinant adiponectin increased ALP activity and osteocalcin expression in human osteoblasts. Furthermore, we demonstrated that adiponectin activated AMP-activated protein kinase (AMPK), and that a knockdown of adiponectin receptor by using siRNA induced an inhibition of ALP activity as well as of osteocalcin expression in mouse osteoblastic MC3T3-E1 cells[42]. These findings suggest that adiponectin directly stimulates osteoblastic differentiation and osteocalcin expression in vitro. Moreover, it is also reported that adiponectin stimulated osteoclast activity by increasing RANKL expression and decreasing OPG expression in osteoblasts although adiponectin had no direct effects on osteoclasts[43]. Because adiponectin stimulates osteocalcin expression in osteoblasts and the differentiation of osteoclasts as well as osteocalcin alternatively stimulates the expression of adiponectin in adipocytes, it is reasonable to assume that there is an endocrine loop between bone and adipose tissue through adiponectin and osteocalcin (Figure 4). However, there is no direct evidence showing the endocrine loop so far.
Figure 4 Schematic representation of the endocrine loops between bone and pancreas, and adipose tissue.
Insulin signaling stimulates the expression of osteocalcin and osteoblastic differentiation via inhibiting Twist2, an inhibitor of Runx2, as well as FoxO1, resulting in accumulation of carboxylated osteocalcin in bone matrix. Adiponectin also stimulates osteocalcin expression and the differentiation of osteoblasts via AMP-activated protein kinase (AMPK) signaling pathway. Both insulin and adiponectin activate osteoclasts and accelerate bone turnover via increasing the ratio of RANKL/OPG. Activated osteoclasts decarboxylate bone matrix-embedded osteocalcin, and then undercarboxylated osteocalcin (ucOC) is released into the circulation. UcOC stimulates the expression of insulin in pancreas and of adiponectin in adipose tissue. OST-PTP: Osteotesticular protein tyrosine phosphatase; IR: Insulin resistance.
Taken together, leptin negatively regulates insulin secretion from pancreas directly and indirectly though hypothalamus-SNS and bone, while adiponectin positively regulates insulin secretion through bone. In patients with obesity and metabolic syndrome, increased serum leptin and decreased serum adiponectin levels may lead to a reduction in residual insulin secretion from pancreas and impaired glucose tolerance in part through bone.
THE EFFECTS OF OSTEOCALCIN ON MUSCLE
Previous studies have shown that osteocalcin affects muscle function and myoblastic differentiation. Tsuka et al[44] reported that ucOC treatment activated ERK signaling in a dose-dependent manner in C2C12 myotubes, and that ucOC significantly increased insulin-induced glucose uptake by activating ERK signaling in the cells. Shen et al[45] showed that osteocalcin expression was significantly decreased in osteoblast/osteocyte-specific Connexin43 knockout mice (Ob/Oc-Cx43-/-). Of note, muscle volume and muscle power were significantly reduced in Ob/Oc-Cx43-/- mice compared to WT mice. Treatment with ucOC significantly increased fusion rate of C2C12 cells, and injection of ucOC to Ob/Oc-Cx43-/- mice significantly increased muscle volume and grip strength. On the other hand, recent several studies have shown that muscle-derived factors named myokines affect bone metabolism. For example, Tanaka et al[46] reported that osteoglycin secreted from myoblasts regulated osteoblastic differentiation and osteocalcin expression. Stable overexpression of osteoglycin significantly enhanced the expression of osteocalcin in osteoblastic MC3T3-E1 cells, while a reduction in endogenous osteoglycin decreased it. Treatments with the conditioned medium from osteoglycin-overexpressed or -suppressed myoblastic cells showed the same results mentioned above. Family with sequence similarity 5, member C (FAM5C) is also secreted from muscle, and FAM5C is reported to stimulate the differentiation and osteocalcin expression in osteoblastic cells[47]. Therefore, there may be an endocrine cross relationship between bone and muscle through osteocalcin.
A RECEPTOR FOR OSTEOCALCIN AND ITS SIGNAL TRANSDUCTION
Previous studies suggest that G-protein coupled receptor family C group 6 member A (GPRC6A) is a candidate or mediating the response to osteocalcin in β-cells[48]. GPRC6A is orphan receptor belonging to the G protein-coupled receptors, which is known as seven-transmembrane domain receptors, and is ubiquitously expressed and sense amino acids and extracellular calcium[49,50]. It is reported that GPRC6A knockout mice showed osteopenia, hyperglycemia, impaired glucose tolerance, insulin resistance, and hepatic steatosis[51], suggesting that GPRC6A may participate in the anabolic response of multiple tissues. Based on the metabolic abnormalities of the GPRC6A knockout mice, Pi et al[51] hypothesized that GPRC6A might be involved in the function of ucOC in glucose homeostasis. To examine the role of GPRC6A in osteocalcin function, the effects of osteocalcin on GPRC6A-expressed cells were investigated. Recombinant osteocalcin stimulated ERK activity in HEK-293 cells overexpressing GPRC6A in a dose-dependent manner, while it did not affect untransfected control cells. It was confirmed that mouse pancreatic β-cell TC-6 cell line and pancreas isolated from WT mice expressed GPRC6A, and that recombinant osteocalcin treatment stimulated ERK activity in vitro and in vivo. Moreover, administration of recombinant osteocalcin induced significant increases in insulin expression in pancreas as well as in serum insulin level in WT mice, but not GPRC6A knockout mice. In 3T3 adipocytes, ucOC activated adenylate cyclase to produce cAMP and ERK signaling through GPRC6A, resulting in the expression of adiponectin[14]. On the other hand, it is reported that ucOC did not increase cytosolic cAMP in C2C2 myotubes, and that the increased glucose uptake by ucOC was not blocked by an inhibitor of PKA although GPRC6A was expressed in C2C12[44]. Further, knockdown of GPRC6A using RNA interference did not affect the action of ucOC in the cells. GPRC6A is also expressed in epithelial cells of the small intestine, and colocalized with GLP-1 in the cells[12], suggesting that ucOC may stimulate GLP-1 expression via GPRC6A although there is no direct evidence thus far.
However, there are still several issues on the signaling pathway of osteocalcin to be clarified. No studies on whether ucOC, not cOC, directly binds to GPRC6A are reported thus far. It is also still unclear whether the effects of ucOC are solely mediated by GPRC6A. It is reported that the response of GPRC6A to ucOC was similar to that of calcium and arginine which are known as GPRC6A ligands. It is thus speculated that GPRC6A could sense both nutrient derived factors, such as calcium and amino acids, as well as ucOC and may not only participate in the endocrine function of ucOC. In addition, GPRC6A is widely expressed in multiple tissues and GPRC6A knockout results in multiple metabolic abnormalities. Additional examination using conditional deletion of GPRC6A in specific tissue will be necessary to establish the tissue-specific functions of GPRC6A.
ASSOCIATION BETWEEN OSTEOCALCIN AND GLUCOSE METABOLISM IN HUMANS
Since in vitro and in vivo studies described above have shown that osteocalcin plays crucial roles in glucose metabolism, of particular interest is whether osteocalcin level in the circulation is associated with glucose metabolism in humans. Indeed, the size and some amino acids of osteocalcin are different between mice and humans, and osteocalcin is encoded by a single gene in humans that is highly conserved across species, while mice contain a cluster of three osteocalcin genes[51,52]. Thus, it is quite important to examine the role of osteocalcin in glucose metabolism also in humans. Kindblom et al[53] showed that total osteocalcin level was inversely correlated with plasma glucose level and fat mass in elderly non-diabetic subjects. Fernandez-Real et al[54] also demonstrated that serum total osteocalcin level was associated with insulin sensitivity in non-diabetes subjects. Pittas et al[55] reported cross-sectional and longitudinal studies showing that serum total osteocalcin level was inversely associated with fasting plasma glucose, fasting insulin, a parameter of insulin resistance [homeostasis model assessment for insulin resistance (HOMA-IR)], and fat mass in a cross-sectional analysis, and that total osteocalcin level was associated with changes in fasting plasma glucose in a prospective analysis. We also previously showed that total serum osteocalcin was inversely associated with glucose and visceral fat mass and positively with serum adiponectin level, parameters of insulin secretion and its sensitivity in patients with type 2 diabetes[56,57]. In addition, we reported a longitudinal study showing that changes in osteocalcin was negatively correlated with changes in HbA1c during treatments of type 2 diabetes[58].
To examine the association of ucOC with glucose metabolism, we previously measured serum ucOC levels by using electrochemiluminescence immunoassay and analyzed the association between ucOC and parameters of glucose metabolism in diabetic patients. We firstly reported that serum ucOC level was negatively correlated with %Trunk fat and visceral/subcutaneous fat ratio as well as fasting plasma glucose and HbA1c independent of various confounding factors[59]. However, the correlations of ucOC with the parameters were almost same as those of total osteocalcin. Hwang et al[60] also reported that elevated levels of both cOC and ucOC were associated with improved glucose tolerance and that ucOC was associated with enhanced β-cell function, and that cOC was associated with improved insulin sensitivity in middle-age male healthy subjects. On the contrary, Iki et al[61] showed that ucOC was significantly and inversely correlated with fasting plasma glucose, HbA1c and HOMA-IR after adjusting for total osteocalcin, while total osteocalcin was not associated with these parameters after adjusting for ucOC. These findings suggest that osteocalcin is involved in glucose metabolism not only in rodents but also in humans. However, because it is still controversial whether ucOC is the active form of endocrine factor in humans, further large-scale studies and meta-analysis were necessary in future.
CONCLUSION
Emerging evidence from epidemiological, clinical, and experimental studies have shown that bone interacts with glucose metabolism by regulated insulin secretion from pancreas as well as adipokines from adipose tissue, and that bone is an active tissue involved in energy homeostasis (Figure 5). Previous in vitro and in vivo studies have shown that osteocalcin has an endocrine function regulating systemic glucose homeostasis and plays important roles in the interaction among bone, pancreas, and adipose tissues. Although several clinical studies suggested that osteocalcin might be involved in systemic glucose homeostasis, there are no direct evidence that osteocalcin regulate glucose metabolism in human. This relatively new topic should further be explored to understand the pathophysiology of glucose tolerance and diabetes-related bone disease and develop a new therapy of metabolic syndrome and diabetes mellitus.
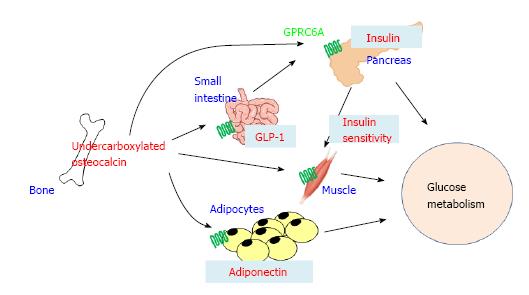
Figure 5 Schematic representation of the mechanisms regulating glucose metabolism by bone.
Undercarboxylated osteocalcin (ucOC) secreted from bone directly stimulates insulin secretion from pancreas and indirectly through increasing the secretion of glucagon-like peptide-1 (GLP-1) from small intestine. UcOC stimulates the expression of adiponectin in adipose tissue, resulting in increasing insulin sensitivity. UcOC also enhances insulin signaling in muscle. G-protein coupled receptor family C group 6 member A (GPRC6A) is a receptor for ucOC and expressed in pancreas β-cells, epithelial cells of small intestine, adipocytes, and myotubes.
P- Reviewer: Chen XZ, Gunther T, Maddaloni E S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ