Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.136
Peer-review started: April 24, 2014
First decision: May 20, 2014
Revised: November 11, 2014
Accepted: November 27, 2014
Article in press: December 1, 2014
Published online: February 15, 2015
Processing time: 282 Days and 4.9 Hours
Sodium-glucose cotransporter 2 (SGLT2) inhibition induces glucosuria and decreases blood glucose levels in diabetic patients and lowers hypoglycemic risk. SGLT1 is expressed in the kidney and intestine; SGLT1 inhibition causes abdominal symptoms such as diarrhea and reduces incretin secretion. Therefore, SGLT2 selectivity is important. Ipragliflozin is highly selective for SGLT2. In type 2 diabetes mellitus (T2DM), urinary glucose excretion increased to 90 g/24 h after 28 d of treatment with ipragliflozin 300 mg/d. Twelve weeks of ipragliflozin 50 mg/d vs placebo reduced glycated hemoglobin and body weight by 0.65% and 0.66 kg, respectively, in Western T2DM patients, and by 1.3% and 1.89 kg, respectively, in Japanese patients. Ipragliflozin (highly selective SGLT2 inhibitor) improves glycemic control and reduces body weight and lowers hypoglycemic risk and abdominal symptoms. Ipragliflozin can be a novel anti-diabetic and anti-obesity agent.
Core tip: Ipragliflozin is highly selective for sodium-glucose cotransporter 2 (SGLT2) inhibitor. Twelve weeks of ipragliflozin 50 mg/d vs placebo decreased HbA1c and body weight by 0.65% and 0.66 kg, respectively, in Western patients, and by 1.3% and 1.89 kg, respectively, in Japanese patients. The highly selective SGLT2 inhibitor ipragliflozin improves glycemic control and reduces body weight, and lowers hypoglycemic risk and abdominal symptoms. Ipragliflozin has potential as a novel anti-diabetic and anti-obesity agent.
- Citation: Ohkura T. Ipragliflozin: A novel sodium-glucose cotransporter 2 inhibitor developed in Japan. World J Diabetes 2015; 6(1): 136-144
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/136.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.136
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and defective insulin secretion[1]. Hyperglycemia is caused by glucose influx exceeding glucose outflow from the plasma compartment[2]. In the fasting state, hyperglycemia is related to increased hepatic glucose production[2]. In the postprandial state, further glucose excursions result from insufficient glucose output suppression and defective insulin stimulation of glucose disposal in target tissues[2]. Once the renal tubular transport maximum for glucose exceeds, glycosuria curbs, but does not prevent further hyperglycemia[2].
Oral hypoglycemic agents include insulin secreta-gogues [sulfonylureas, meglitinides, and dipeptidyl peptidase-4 (DPP-4) inhibitors] and insulin sensitizers [metformin and thiazolidinediones (TZDs)][3]. α-gluco-sidase inhibitors decrease glucose absorption. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes recom-mend metformin as the first-line oral therapy[2,3]. If the glycated hemoglobin (HbA1c) target is not achieved by 3 mo, either sulfonylurea, TZD, DPP-4 inhibitor, GLP-1 receptor agonist, or basal insulin should be combined with metformin[2].
The ADA recommends lowering HbA1c to < 7.0% to reduce microvascular disease incidence[4]. However, only approximately half of T2DM patients achieve this[3,5]. Oral hypoglycemic agents have side effects: hypoglycemia and weight gain (sulphonylureas)[6]; peripheral edema, weight gain, and fractures (TZDs)[7]; a possible increased risk of bladder cancer (pioglitazone)[8]; and abdominal symptoms (metformin and α-glucosidase inhibitors). Metformin can also cause lactate acidosis.
Few insulin sensitizers and anti-obesity agents exist. Mazindol maintains body weight after obesity therapy and treats obesity-related diseases such as diabetes, hypertension, and hyperlipidemia[9], but has side effects including tremor, nausea, vomiting, and diarrhea. Therefore, novel anti-diabetic and anti-obesity agents are required.
The kidney is important in glucose metabolism; it is a target for therapeutic intervention[10]. Sodium-glucose cotransporter 2 (SGLT2) mediates glucose reabsorption from the proximal renal tubule[10]. SGLT2 inhibition induces glucosuria and lowers blood glucose in diabetes, and a lowers hypoglycemic risk[10].
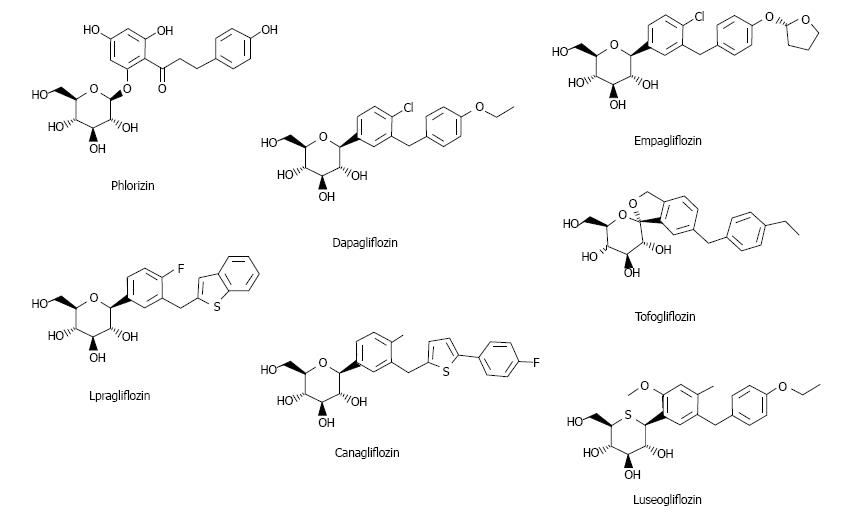
Ipragliflozin is an SGLT2 inhibitor first released in Japan (Figure 1)[3]. Here studies on ipragliflozin and other SGLT2 inhibitors are reviewed.
Two types of SGLT exist: SGLT1 and SGLT2. SGLT1 is expressed in the kidney and intestine; intestinal SGLT1 inhibition causes abdominal symptoms such as diarrhea. It is pivotal for intestinal mass absorption of d-glucose and triggers glucose-induced secretion of gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1)[11]. Therefore, SGLT1 inhibition reduces incretin secretion. Miglitol (α-glucosidase inhibitor) suppresses GIP and increases GLP-1, reducing body weight and improving glycemic control[12], but suppression of GLP-1 reduces insulin secretion[13]. Therefore, SGLT2 selectivity is important. The selectivity of currently available SGLT2 inhibitors is presented in Table 1[3,14-19].
| Company | IC50 for human SGLT1/SGLT2 (nmol/L) | SGLT2 selectivity (fold) | |
| Phlorizin | 210/34.6 | 6 | |
| Ipragliflozin | Astellas | 1876/7.38 | 254 |
| canagliflozin | Johnson and Johnson Mitsubishi Tanabe | 684/4.4 | 155 |
| Dapagliflozin | Bristol-Myers Squibb AstraZeneca | 1391/1.12 | 1242 |
| Empagliflozin | Boehringer Ingelheim | 8300/3.1 | 2680 |
| Tofogliflozin | Chugai | 8444/2.9 | 2912 |
| luseogliflozin | Taisho | 3990/2.26 | 1770 |
Healthy Japanese subjects receiving ipragliflozin excreted approximately 70 and 50 g of glucose/24 h after a single 300 mg dose or after multiple 50 or 100 mg doses, respectively[20]. In healthy European subjects, ipragliflozin dose-dependently increased urinary glucose excretion (UGE) to a maximum of approximately 59 g/24 h (327 mmol/24 h) (dose: 5-600 mg/d) without affecting plasma glucose levels[21]. In T2DM, ipragliflozin increased UGE to a maximum of approximately 90 g/24 h after 28 d of treatment with 300 mg/d[22]. Therefore, SGLT2 inhibitors increased UGE more in T2DM patients compared with healthy subjects[3]. Human exfoliated proximal tubular epithelial cells (HEPTECs) from T2DM patients expressed significantly more SGLT2 and the facilitative glucose transporter GLUT2 than cells from healthy individuals[23]. Renal glucose uptake in HEPTECs isolated from T2DM patients was markedly increased compared with that in healthy controls[23]. Therefore, renal glucose transporter expression and activity is increased in T2DM[23]. In T2DM patients, ipragliflozin increases glycosuria directly proportional to the glomerular filtration rate (GFR) and degree of hyperglycemia, so it can be reliably predicted for individuals[24]. Although absolute glycosuria decreases with declining GFR, ipragliflozin efficiency is maintained in patients with severe renal impairment[24].
AUCinf or Cmax of single doses of sitagliptin, piog-litazone, or glimepiride[25] were unaffected by multiple doses of ipragliflozin; the combination was well tolerated in healthy subjects[25]. Ipragliflozin (300 mg qd) and metformin together were well tolerated in T2DM patients; the addition of ipragliflozin did not result in a clinically relevant change in the pharmacokinetic properties of metformin[26]. Dose adjustments may not be required when ipragliflozin is administered with other glucose-lowering drugs[25].
Moderate hepatic impairment had no clinically relevant effects on the single-dose pharmacokinetics of iprag-liflozin and its major metabolite[27]. A single oral dose of ipragliflozin 100 mg was well tolerated in healthy subjects and those with moderate hepatic impairment[27].
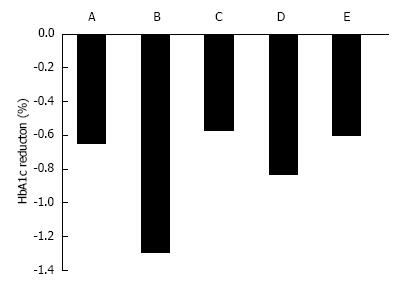
In Western T2DM patients, a 12-wk treatment with ipragliflozin 12.5, 50, 150, and 300 mg/d reduced HbA1c by 0.49%, 0.65%, 0.73%, and 0.81%, respectively, compared with placebo treatment (Figure 2)[28]. In Japanese patients, 12-wk treatment with ipragliflozin 12.5, 25, 50, and 100 mg/d reduced HbA1c by 0.61%, 0.97%, 1.29%, and 1.31%, respectively, compared with placebo treatment[29].
Canagliflozin 50, 100, 200, 300 mg/d and 300 mg twice daily for 12 wk significantly reduced HbA1C by 0.79%, 0.76%, 0.70%, 0.92%, and 0.95%, respectively, compared with reductions of 0.22% for placebo (all P < 0.001), and 0.74% for sitagliptin[30]. The adjusted mean difference in HbA1c between placebo and 100 mg canagliflozin was -0.54%[30] (Figure 2). Dapagliflozin 2.5, 5, and 10 mg reduced HbA1c by 0.67%, 0.70%, and 0.84%, respectively[31]. Empagliflozin 5, 10, and 25 mg for 12 wk reduced HbA1c by 0.4%, 0.5%, and 0.6% compared with placebo (+0.09%)[32]. Ipragliflozin reduced HbA1c levels when added to metformin (-0.87 ± 0.66), pioglitazone (-0.64 ± 0.609), or sulfonylurea (-0.83 ± 0.717)[3,33].
In Western T2DM patients, 12-wk of ipragliflozin treatment at 12.5, 50, 150, and 300 mg/d decreased fasting plasma glucose (FPG) by 0.84, 1.10, 1.30, and 1.68 mmol/L, respectively compared with placebo[28]. In Japanese T2DM patients, 12.5, 25, 50, and 100 mg ipragliflozin decreased FPG from baseline by 15.6, 23.7, 34.1, and 46.9 mg/dL (0.87, 1.32, 1.89 and 2.60 mmol/L) compared with +12.0 mg/dL for placebo[29].
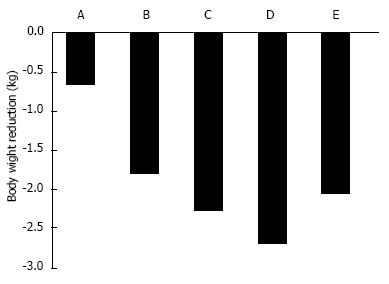
In T2DM patients, SGLT2 inhibitors ipragliflozin, dapagliflozin, and canagliflozin reduced body weight by approximately 2 kg[3] (Figure 3). In Western individuals, the standard dose of 50-mg ipragliflozin for 12 wk reduced body weight by 0.66 kg[28]. In Japanese T2DM patients, 12-wk of placebo or 12.5-100 mg ipragliflozin treatment reduced body weight by 0.39 kg and 1.46-2.10 kg, respectively[29]. Twelve-weeks of canagliflozin 100 mg[30], dapagliflozin 10 mg[34], or empagliflozin 25 mg[32] reduced body weight by 2.28, 2.7, and 2.06 kg, respectively.
Most weight loss in patients receiving dapagliflozin is related to visceral and subcutaneous fat loss[3,35]. After 24-wk of dapagliflozin treatment at 10 mg/d, placebo-corrected changes were -2.08 kg body weight, -1.52 cm waist circumference, -1.48 kg total body fat mass, -258.4 cm3 visceral adipose tissue, and -184.9 cm subcutaneous adipose tissue[3,35]. Compared with placebo, 26.2% more patients achieved weight reduction of at least 5%[3,35].
SGLT2 inhibitors decrease blood pressure via osmotic diuresis induced by glucose in the urine earlier during treatment[3]. Ipragliflozin 50 mg for 16 wk reduced systolic blood pressure by 3.2 mmHg and diastolic blood pressure by 2.5 mmHg, without hypotension[36]. Dapagliflozin for 12 wk reduced systolic blood pressure by 2.6-6.4 mmHg, with no clear dose-dependent relationship, but changes in diastolic blood pressure and heart rate were small and inconsistent[34]. Small dose-related increases in 24-h urine volumes were observed (107-470 mL above baseline volumes of 1.8-2.2 L)[34].
Canagliflozin 100 and 300 mg for 26 wk significantly reduced systolic BP by 3.7 and 5.4 mmHg, respectively, compared with placebo (both P < 0.001)[37]. Diastolic BP was also reduced by 1.6 and 2.0 mmHg, respectively[37]. Minimal changes in heart rate were observed with canagliflozin 100 and 300 mg compared with placebo (-1.6, -0.5, and +1.4 beats/min, respectively)[37]. Empagliflozin 25 mg for 12 wk decreased systolic blood pressure by 3.4 mmHg, and diastolic blood pressure by 1.7 mmHg, but there was no significant difference compared with placebo[32]. Overall, SGLT2 inhibitors reduced blood pressure by approximately 2-6 mmHg.
Chronic hyperglycemia induces β-cell dysfunction and insulin resistance[38]. SGLT2 inhibitors improve glucose toxicity and glycemic control[3]. There are no clinical reports on effect of ipragliflozin on β-cell function but ipragliflozin increased insulin content in the pancreas and suppressed the loss of insulin-positive cells in islets of db/db mice, an animal model of T2DM[3,39].
Compared with placebo, canagliflozin 100 mg/d for 12 wk significantly improved β-cell function as assessed by homeostasis model assessment 2 (HOMA2)-%B ( measure of fasting insulin secretion)[30]. Another study reported improvements in β-cell function following 26-wk treatment with canagliflozin 100 and 300 mg compared with placebo, with increases in HOMA2-%B of 12.4 and 22.8, respectively[37].
Proinsulin/insulin (PI/I) ratio reflects β-cell dys-function associated with the onset and progression of T2DM[40,41]. Mitiglinide improved the postprandial insulin secretion profile, suppressed the postprandial glucose spike, and improved the PI/I ratio in T2DM patients with low insulin resistance and low triglyceride levels[42]. Dose-related decreases in proinsulin/insulin ratio of 0.5 and 0.8 pmol/mIU were observed with canagliflozin at 100 and 300 mg, respectively, compared with placebo, and decreases in proinsulin/C peptide ratio were also seen with both doses of canagliflozin[37]. These results suggest that SGLT2 inhibitors improve β-cell function.
To date, there are no clinical reports on effect of ipra-gliflozin on insulin resistance. However, reductions in HOMA2 insulin resistance after dapagliflozin treatment at 2.5 and 10 mg for 12 wk were significantly larger compared with placebo[43]. The most precise method to assess insulin resistance is the glucose clamp technique[44]. Results of a hyperinsulinemic-euglycemic clamp study demonstrated that within 3 d of completing 2-wk of dapagliflozin treatment, Zucker diabetic fatty rats displayed improved glucose utilization accompanied by reduced glucose production and enhanced glucose influx into liver tissue[16]. In a clamp study of T2DM patients, 12-wk of dapagliflozin treatment increased glucose disposal rates[3,45]. There are few glucose clamp studies of SGLT2 inhibitors because the method is complex and expensive[46]. Recently, a novel insulin resistance index “20/(fasting C-peptide × fasting plasma glucose),” to estimate the insulin resistance index was derived from the glucose clamp method[46]. This index will evaluate insulin resistance in clinical studies.
A meta-analysis of 45 clinical trials indicated that SGLT2 inhibitors increased the risk of urinary and genital tract infections [odds ratios, 1.42 (95%CI: 1.06-1.90) and 5.06 (95%CI: 3.44-7.45)], respectively, probably a result of glucosuria[47].
In ipragliflozin phase 3 trial, treatment-emergent urinary tract infections (UTIs) were reported in 32/412 patients across all treatment groups, including placebo[28]. Infections were symptomatic and asymptomatic in 9 and 23 patients, respectively[28]. A total of 14 patients experienced treatment-emergent genital tract infections but there was no evidence that the frequency was related to the dose of ipragliflozin[28]. All events were treated with antifungal or antibacterial agents and were resolved prior to the final study visit (except three)[28]. In canagliflozin phase 3 trial, the incidence of genital mycotic infections, UTIs, and osmotic diuresis-related adverse events was higher in the treatment group[37]. UTIs were observed in 5%-12% of dapagliflozin-treated patients (with no clear dose relationship) compared with 6% of placebo-treated patients and 9% of metformin-treated patients[34]. Genital infections were observed in 2%-7% of dapagliflozin treated patients, 0% of placebo-treated patients, and 2% of metformin-treated patients[34]. Therefore, STLT2 inhibitors might increase the risk of UTIs.
In a multi-center Japanese study of 361 patients rando-mized to receive either ipragliflozin (12.5, 25, 50, or 100 mg/d) or a placebo for 12 wk, a single mild symptomatic hypoglycemic event (not confirmed by plasma glucose measurement) occurred in one patient in the 100-mg ipragliflozin group[29]. In ipragliflozin phase 3 trial, only one patient in each of the ipragliflozin 50 mg (67 patients) and 300 mg (68 patients) dose groups experienced treatment-emergent hypoglycemia[29]. In T2DM patients, ipragliflozin did not significantly increase the incidence of hypoglycemic events compared to placebo, even in combination with other hypoglycemic agents[3,48]. Hypoglycemic events were reported in 6%-10% of patients treated with dapagliflozin, with no dose-dependent relationship, compared with 4% and 9% for placebo and metformin, respectively[34]. There were no symptomatic hypoglycemic events with a fingerstick glucose of ≤ 50 mg/dL[34]. In canagliflozin phase 3 trial, the incidence of hypoglycemia was similar for canagliflozin 100 and 300 mg and placebo (3.6%, 3.0%, and 2.6%, respectively), with no report of severe hypoglycemia[37]. Therefore, these data suggest that SLGT2 inhibitors lowers hypoglycemic risk.
Ipragliflozin caused a mild 1.5%-2.0% increase in hema-tocrit at all doses[29]. Similarly, blood urea nitrogen (BUN) was also mildly increased by 1.0-2.2 mg/dL compared with placebo[29].
An increased incidence of bladder and breast cancer was indicated in patients receiving dapagliflozin compared with controls[47]. Data on bladder and breast cancer were retrieved from regulatory databases and other sources to produce a pool of 5501 patients (at least 5000 patient-years of exposure to dapagliflozin), and a total of 3184 patients (at least 2350 patient-years of exposure to placebo or an active comparator)[49,50]. Nine cases of bladder cancer were identified in patients treated with dapagliflozin compared with one case in patients receiving placebo[49,50]. The number of observed cases exceeds the expected number in the general diabetic population[47]. UTIs may increase the risk of bladder cancer. However, early detection after short exposure and potential detection bias related to frequent urinalysis mitigate against a causative relationship[47]. Therefore, no robust conclusions can be drawn, pending accumulation of long-term data[47].
There were 9 cases of breast cancer in the dapagliflozin group (2223 patients) compared with one case in the placebo group (1053 patients), diagnosed within the first year of the study[47]. These figures were higher than the predicted number of 7.1 cases based on the Surveillance Epidemiology and End Results (SEER) program[51]. It remains uncertain whether the use of dapagliflozin is associated with an increased risk of breast cancer and further studies are needed[47]. There are no reports indicating that other SGLT2 inhibitors are associated with an increased risk of cancer[3].
A meta-analysis of 20 randomized, double-blind studies demonstrated SGLT2 inhibitors administered with metformin significantly decreased the incidence of diarrhea[52]. However, the addition of SGLT2 inhibitors increased the risk of genital infection[52]. Despite some limitations, SGLT2 inhibitors have a favorable safety profile, and combination therapy with metformin is well tolerated[52].
One study investigated effect of ipragliflozin on quality of life[28]. Outcomes were assessed using the European Quality of Life-5 Dimensions (EQ-5D)[53], Audit of Diabetes-Dependent Quality of Life (ADDQoL)[54], and Diabetes Medication Satisfaction (Diab-MedSat) questionnaires[55]. No differences were observed in EQ-5D domains or ADDQoL scores at week 12[28]. However, mean changes in EQ-5D visual analogue scale scores from baseline to week 12 showed positive changes in the treatment groups, suggesting improvements in perceived health status[28]. Changes in Diab-MedSat scores for burden and symptoms were small and similar across all treatment groups, but changes in the efficacy score from baseline to week 12 were greater for the ipragliflozin groups[28]. Another study reported that changes from baseline to week 12 in EQ-5D domains and ADDQoL scores were small across all treatment groups but with a non-statistically significant trend for improvement in the ipragliflozin treatment groups[33]. These results suggest that the SGLT2 inhibitor ipragliflozin may improve the quality of life in T2DM patients.
There are no clinical reports on ethnic differences in effects of ipragliflozin. However, past reports imply that ipragliflozin reduces HbA1c more in Japanese patients compared with Western patients (Figure 2)[28,29]. The mechanism is unclear, but a meta-analysis reported that DPP-4 inhibitors were associated with a reduction in HbA1c of 0.65% in non-Japanese randomized, controlled trials (RCTs; 55 patients), compared with 1.67% in Japanese RCTs[56]. There may be pharmacogenetic or cultural lifestyle differences that contribute to the larger reduction in HbA1c in Japanese patients. Japanese people have a greater amount of abdominal visceral fat relative to abdominal subcutaneous fat compared with Caucasians[57]. Dapagliflozin reduced visceral adipose tissue more than subcutaneous adipose tissue[35]. Therefore, the difference in visceral adipose tissue between Japanese and Western T2DM patients may contribute to the difference in effect of SGLT2 inhibitors.
Japanese and Asian patients often show reduced β-cell function[46] and East Asians may have a limited innate capacity for insulin secretion[58,59]. The body mass index (BMI) of Japanese T2DM patients was significantly correlated with insulin secretion ability in a meal tolerance test; the insulin secretion ability diminished in patients with BMI < 20 kg/m2[60]. Other reported complications associated with familial renal glucosuria include episodes of ketosis, UTIs, and natriuresis[61]. SGLT2 inhibitors increase blood ketone bodies[62]. Low insulin secretion ability and lean stature in Asian patients receiving SGLT2 inhibitors may increase the risk of ketosis; therefore, caution is required.
SGLT2 inhibitor ipragliflozin improves glycemic control and reduces body weight, especially in Japanese T2DM patients. Furthermore, ipragliflozin lowers hypoglycemic risk and abdominal symptoms and can be safely used with sulphonylureas, metformin, pioglitazone, and DPP4 inhibitors. SGLT2 inhibitors are likely to improve β-cell function and insulin sensitivity. They offer great potential as novel anti-diabetic and anti-obesity agents. Ipragliflozin is particularly effective for Japanese T2DM patients with a greater abdominal visceral fat relative to abdominal subcutaneous fat than Caucasians. Ipragliflozin is a highly selective SGLT2 inhibitor, and lower hypoglycemic risk and abdominal symptoms.
P- Reviewer: Mansour AA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1664] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 2. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2563] [Cited by in RCA: 2609] [Article Influence: 200.7] [Reference Citation Analysis (4)] |
| 3. | Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013;139:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1908] [Cited by in RCA: 1918] [Article Influence: 137.0] [Reference Citation Analysis (1)] |
| 5. | Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999-2004. Ann Epidemiol. 2008;18:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Gallwitz B, Häring HU. Future perspectives for insulinotropic agents in the treatment of type 2 diabetes-DPP-4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2010;12:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf. 2010;9:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 9. | Inoue S, Egawa M, Satoh S, Saito M, Suzuki H, Kumahara Y, Abe M, Kumagai A, Goto Y, Shizume K. Clinical and basic aspects of an anorexiant, mazindol, as an antiobesity agent in Japan. Am J Clin Nutr. 1992;55:199S-202S. [PubMed] |
| 10. | Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:313-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 556] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 12. | Sumi K, Ohkura T, Yamamoto N, Fujioka Y, Matsuzawa K, Izawa S, Shiochi H, Kinoshita H, Ohkura H, Kato M. Long-term miglitol administration suppresses postprandial glucose-dependent insulinotropic polypeptide secretion. Diabetology International. 2013;4:190-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101:1421-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 200] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H. Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:423-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, Du F, Liu Y, Xu J, Conway B. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012;7:e30555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57:1723-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 453] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 18. | Ohtake Y, Sato T, Kobayashi T, Nishimoto M, Taka N, Takano K, Yamamoto K, Ohmori M, Yamaguchi M, Takami K. Discovery of tofogliflozin, a novel C-arylglucoside with an O-spiroketal ring system, as a highly selective sodium glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2012;55:7828-7840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Kakinuma H, Oi T, Hashimoto-Tsuchiya Y, Arai M, Kawakita Y, Fukasawa Y, Iida I, Hagima N, Takeuchi H, Chino Y. (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem. 2010;53:3247-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Kadokura T, Saito M, Utsuno A, Kazuta K, Yoshida S, Kawasaki S, Nagase I, Kageyama S. Ipragliflozin (ASP1941), a selective sodium-dependent glucose cotransporter 2 inhibitor, safely stimulates urinary glucose excretion without inducing hypoglycemia in healthy Japanese subjects. Diabetol Int. 2011;2:172-182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Veltkamp SA, Kadokura T, Krauwinkel WJ, Smulders RA. Effect of Ipragliflozin (ASP1941), a novel selective sodium-dependent glucose co-transporter 2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Drug Investig. 2011;31:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Schwartz SL, Akinlade B, Klasen S, Kowalski D, Zhang W, Wilpshaar W. Safety, pharmacokinetic, and pharmacodynamic profiles of ipragliflozin (ASP1941), a novel and selective inhibitor of sodium-dependent glucose co-transporter 2, in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 597] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 24. | Ferrannini E, Veltkamp SA, Smulders RA, Kadokura T. Renal glucose handling: impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013;36:1260-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Smulders RA, Zhang W, Veltkamp SA, van Dijk J, Krauwinkel WJ, Keirns J, Kadokura T. No pharmacokinetic interaction between ipragliflozin and sitagliptin, pioglitazone, or glimepiride in healthy subjects. Diabetes Obes Metab. 2012;14:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Veltkamp SA, van Dijk J, Collins C, van Bruijnsvoort M, Kadokura T, Smulders RA. Combination treatment with ipragliflozin and metformin: a randomized, double-blind, placebo-controlled study in patients with type 2 diabetes mellitus. Clin Ther. 2012;34:1761-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Zhang W, Krauwinkel WJ, Keirns J, Townsend RW, Lasseter KC, Plumb L, Kadokura T, Ushigome F, Smulders R. The effect of moderate hepatic impairment on the pharmacokinetics of ipragliflozin, a novel sodium glucose co-transporter 2 (SGLT2) inhibitor. Clin Drug Investig. 2013;33:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Fonseca VA, Ferrannini E, Wilding JP, Wilpshaar W, Dhanjal P, Ball G, Klasen S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Invest. 2013;5:382-391. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 31. | Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 32. | Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013;15:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 517] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 35. | Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 650] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 36. | Kashiwagi A, Takinami Y, Kazuta K, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improved glycaemic control with additional benefits of reductions of body weight and blood pressure in Japanese patients with type 2 diabetes mellitus: BRIGHTEN study. Portugal, Lisbon: The European Association for the Study of Diabetes, 47 Annual Meeting 2011; . |
| 37. | Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 487] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 38. | Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53 Suppl 1:S119-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 634] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 39. | Takasu T, Tahara A, Yokono M, Hayashizaki Y, Kurosaki E, Imamura M, Funatsu T, Li Q. ASP1941, a novel, potent and selective SGLT2 inhibitor, improves hemoglobin A1c and symptoms of diabetes in animal models. American Diabetes Association. Orlando (FL), 2010; 70th Scientific Sessions. Available from: http: //professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79510. |
| 40. | Reder ME, Porte D Jr, Schwartz RS, Kahn SE. Disprop-ortionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:604-608. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Røder ME, Dinesen B, Hartling SG, Houssa P, Vestergaard H, Sodoyez-Goffaux F, Binder C. Intact proinsulin and beta-cell function in lean and obese subjects with and without type 2 diabetes. Diabetes Care. 1999;22:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Ohkura T, Inoue K, Fujioka Y, Nakanishi R, Shiochi H, Sumi K, Yamamoto N, Matsuzawa K, Izawa S, Ohkura H. The proinsulin/insulin (PI/I) ratio is reduced by postprandial targeting therapy in type 2 diabetes mellitus: a small-scale clinical study. BMC Res Notes. 2013;6:453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, Tokudome T, Langkilde AM, Parikh S. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013;15:432-440. [RCA] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:214-223. |
| 45. | Mudaliar S, Henry RR, Boden G, Smith S, Chalamandaris AG, Duchesne D, Iqbal N, List J. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol Ther. 2014;16:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Ohkura T, Shiochi H, Fujioka Y, Sumi K, Yamamoto N, Matsuzawa K, Izawa S, Kinoshita H, Ohkura H, Kato M. 20/(fasting C-peptide × fasting plasma glucose) is a simple and effective index of insulin resistance in patients with type 2 diabetes mellitus: a preliminary report. Cardiovasc Diabetol. 2013;12:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 48. | Goto K, Kashiwagi A, Kazuta K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin reduces A1C and body weight in type 2 diabetes patients who have inadequate glycemic control on metformin alone: ILLUMINATE study. Philadelphia (PA): American Diabetes Association, 72th Scientific Sessions 2012; . |
| 49. | Available from: http: //www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm262994.pdf. |
| 50. | European Medicines Agency. Assessment Report: Forxiga (Dapagliflozin). Procedure no. EMEA/H/C/002322. London: European Medicines Agency, 2012. [updated 2013; April 1] Available from: http: //www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002322/WC500136024.pdf. |
| 51. | Bhartia M, Tahrani AA, Barnett AH. SGLT-2 inhibitors in development for type 2 diabetes treatment. Rev Diabet Stud. 2011;8:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Kawalec P, Mikrut A, Łopuch S. The safety of dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter 2 (SGLT-2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30:269-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | EuroQol 5 Dimensions. [Accessed 13 July 2006]. Available from: http: //www.euroqol.org. |
| 54. | McMillan CV, Honeyford RJ, Datta J, Madge NJ, Bradley C. The development of a new measure of quality of life for young people with diabetes mellitus: the ADDQoL-Teen. Health Qual Life Outcomes. 2004;2:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Brod M, Skovlund SE, Wittrup-Jensen KU. Measuring the impact of diabetes through patient report of treatment satisfaction, productivity and symptom experience. Qual Life Res. 2006;15:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46:1453-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40 Suppl 1:S302-S304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Puech A, Monleaud-Dupy M, Jacob M, Jean M. [Stability studies of aqueous solutions of hyoscyamine sulphate (author’s transl)]. J Pharm Belg. 1996;32:117-127. [PubMed] |
| 59. | Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789-1796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 60. | Ohkura T, Fujioka Y, Izawa S, Sumi K, Yamamoto N, Shiochi H, Matsuzawa K, Kinoshita H, Ohkura H, Kato M. Endogenous insulin secretion ability in meal tolerance test correlated with body mass index (BMI) in Japanese type 2 diabetes patients. Int J Diabetes Dev Ctries. 2014;Published online. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010;5:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 62. | Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |