Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.689
Revised: June 18, 2014
Accepted: July 17, 2014
Published online: October 15, 2014
Processing time: 96 Days and 11.5 Hours
Treatment of type 1 diabetes mellitus has always posed a challenge to balance hyperglycemia control with hypoglycemia episodes. The quest for newer therapies is continuing and this review attempts to outline the recent developments. The insulin molecule itself has got moulded into different analogues by minor changes in its structure to ensure well controlled delivery, stable half-lives and lesser side effects. Insulin delivery systems have also consistently undergone advances from subcutaneous injections to continuous infusion to trials of inhalational delivery. Continuous glucose monitoring systems are also becoming more accurate and user friendly. Smartphones have also made their entry into therapy of diabetes by integrating blood glucose levels and food intake with calculated adequate insulin required. Artificial pancreas has enabled to a certain extent to close the loop between blood glucose level and insulin delivery with devices armed with meal and exercise announcements, dual hormone delivery and pramlintide infusion. Islet, pancreas-kidney and stem cells transplants are also being attempted though complete success is still a far way off. Incorporating insulin gene and secretary apparatus is another ambitious leap to achieve insulin independence though the search for the ideal vector and target cell is still continuing. Finally to stand up to the statement, prevention is better than cure, immunological methods are being investigated to be used as vaccine to prevent the onset of diabetes mellitus.
Core tip: As therapy of type 1 diabetes poses important challenges because of life long insulin dependence,multiple injections, excursions in glucose values and inability to simulate the pancreas, newer modalities of therapy are emerging. Hence, this is the right time to review developments in this front. This review conjures up recent advances in continuous glucose monitors, closed loop systems, insulin analogues, insulin gene therapy, transplantation and immunological vaccination.
- Citation: Aathira R, Jain V. Advances in management of type 1 diabetes mellitus. World J Diabetes 2014; 5(5): 689-696
- URL: https://www.wjgnet.com/1948-9358/full/v5/i5/689.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i5.689
The year 1923 is a watershed in the history of diabetes mellitus when insulin was discovered by Banting and Best[1]. Today the world has come a long way from that, but living with type 1 diabetes still remains akin to a tight rope walk, balancing between hyperglycemia and hypoglycemic episodes. Multiple injections, strict control on food and exercise are herculean tasks to deal with, especially in children. Hence, the need for better therapies is warranted and they have thus evolved from nascent stages to actual usage.
The incidence of type 1 diabetes varies among different countries, which reflects the roles played by genetic and environmental factors in the ultimate expression of the disease. It varies from 57.4 cases/100000 per year in Finland to 0.6 cases/100000 per year in India[2]. The fact that there is a rising trend in the number of children diagnosed to have type 1 diabetes is supported by a number of studies. Whether this can be attributed to an absolute increase in the incidence of the disease is still under speculation because the proportion of children with highest risk human leukocyte antigen haplotypes have decreased and hence, the changing environmental patterns may rather be uncovering the latent genetic factors to cause earlier expression of the disease[3]. The changing epidemiology is bringing more and more children to us to care for. Thus, unveiling newer and better therapies becomes an onus on us.
In this chapter, we shall be presenting a brief overview of the recent advances in the management of type 1 diabetes, including newer insulins, newer insulin delivery options, hypoglycemia prevention through use of technology and lastly, advances in the field of “curing” diabetes through transplant and gene therapy.
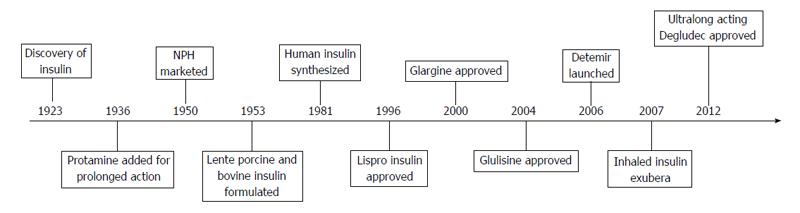
The quest for the ideal insulin has led to the discovery of a variety of analogues to match the mighty pancreas and yet, many lacunae are left to be filled. The timeline of important events in the history of insulin is presented in Figure 1.
Insulin analogues were designed to overcome the problems of poor stability and erratic absorption profile of the preceding generations of insulin.
Insulin lispro: Short acting insulin is necessary to deal with meal time hyperglycemia. Insulin Lispro which was approved in 1996 has rapid onset of action and shorter duration so that post prandial hypoglycaemia can be prevented. The inversion of proline at position 28 with lysine at position 29 allowed insulin to exist more in the monomeric form that is easily absorbed which could counteract meal time hyperglycemia without causing prolonged hypoglycaemia. The modification in the amino acid sequence did not alter the receptor binding and hence, is as effective as regular insulin[4].
Insulin aspart: Substituting proline at position 28 with aspartic acid formed insulin aspart which is also short acting due to absence of hexamer formation. Immunogenicity and teratogenicity profile was similar to regular insulin[5].
Insulin glulisine: This is the newest addition to the list of short acting insulin produced by substituting asparagine at position B3 by lysine and lysine at position B29 by glutamine. It is unique in action by causing phosphorylation of Insulin Receptor Substrate 2. Increased binding to insulin like growth factor (IGF) 1 receptor and mitogenic activity has however, raised concerns over its tumorigenic potential which needs further evaluation[6]. Food and drug administration (FDA) approval has been obtained for use of glulisine in children > 4 years.
Isophane, Lente and Ultralente failed to ensure long time control of glucose with minimum variations and hence, they made way for newer long acting insulins.
Insulin glargine: Amino acid alterations brought about a change in pH from 5.4 to 6.7 that made glargine poorly soluble at physiological pH. The stability of its hexameric structure prevents rapid absorption from subcutaneous tissue and its activity is maintained for 11 to 24 h. Glargine also has affinity to the IGF 1 receptor making it mitogenic, but the clinical significance of this finding is still questionable[7]. Safety in the pediatric age group has been established but due to the acidic pH burning sensation has been reported in some children.
Insulin detemir: Detemir binds reversibly to albumin and undergoes a slow release process as only free detemir is biologically active. Onset of action is within 1 to 2 h and lasts for 24 h. Peakless activity ensures stability[8]. Detemir shows more reproducible pharmacokinetics in children than glargine[9]. The United States FDA has approved the use of Detemir and Glargine only in children > 6 years.
Insulin albulin: As the name suggests, insulin albulin has been developed by directly fusing single human insulin gene to human albumin gene that makes this analogue long acting. The peakless effect makes albulin a potential agent for long term glycemic control. The affinity of albulin to IGF 1 receptors is less compared to other analogues which makes albulin less likely to trigger mitogenesis[10]. Insulin albulin still has to evolve to enter clinical application.
Insulin degludec: Approved in 2012, Insulin degludec shows a flat profile upon injection with a half-life of 25 h, enabling once in 3 d injection. The dihexamers associate with each other to form multi hexamers that slowly form monomers and enter the bloodstream. When compared to other long acting insulins, degludec shows much lower variability in day to day glucose levels. Trials investigating degludec have also included children and adolescents. Nocturnal Hypoglycemia, which is the bottle neck in intensive glucose lowering, is reported to be up to 25% lower with degludec[11]. Increase in adverse cardiovascular events is a concern with degludec and use in pediatric age group is not yet approved.
The search for alternative routes of delivery of insulin paved way to the discovery of inhaled insulin Exubera that was approved in 2006, but withdrawn from the market a year later due to poor sales. It was thought that the large surface area of the lungs would facilitate better absorption. However, bioavailability was found to be only 10% and so higher doses were required. Unpredictable absorption patterns that varied with age, respiratory tract infection and smoking form important hurdles for lungs to be the route of choice[12].
Despite the initial enthusiasm with oral insulin which was considered as the “holy grail” for treating diabetes, it remains an enigmatic target due to enzymatic digestion of insulin and inadequate intestinal absorption.
Buccal and skin patches are also candidate routes for delivering insulin that await further research.
Parallel to the advancements in insulin, the modes of delivery also underwent considerable changes in the last 50 years. The first pump designed by Dr. Arnold Kadish in 1963 was bulky and had to be worn like a backpack as in Figure 2. It was replaced by the “big blue brick” model which again became obsolete due to inaccuracies. All the early models could only provide a single basal delivery rate and had to be programmed frequently. The technological boom that accompanied the dawn of the 20th century brought about further developments and today we have insulin pumps that are convenient, small, accurate and adjustable.
Fear of hypoglycemia is recognised as the most important road block in the path to achieving good glycemic control. Continuous blood glucose monitoring system is an important aid in the management of type 1 diabetes and an essential prerequisite for closed loop systems. The superiority of Continuous glucose monitors (CGMs) over self-monitoring of glucose in reducing the time spent in hypoglycemia has been proven beyond doubt[13].
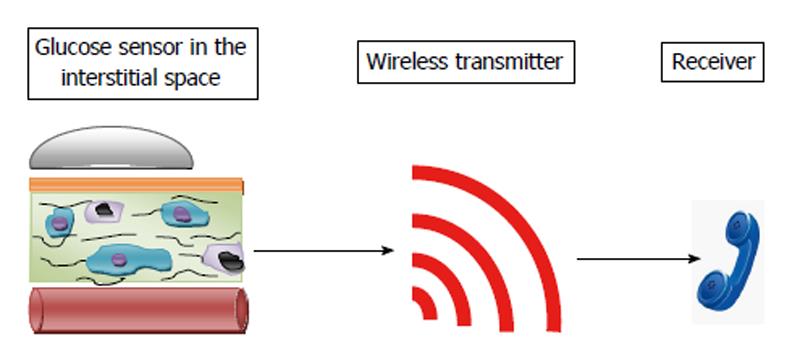
The basic structure of a CGM consists of a sensor, wireless transmitter and a receiver as in Figure 3.
Sensor provides real time blood glucose levels and typically consists of a membrane layer, electrode and enzyme matrix. It works on the same principle as the conventional glucose monitors using the glucose oxidase catalysed oxidation of glucose to produce hydrogen peroxide that generates an electric current at the electrode[14]. The membrane layer forms a barrier between the electrode and the surrounding tissues, which mandates adequate permeability to glucose and oxygen. Sensors are inserted subcutaneously and detect glucose concentration in the interstitial compartment. In the earlier versions, blood glucose values were stored and had to be downloaded to view the level of control retrospectively. The present CGMs have sensors that display the glucose values in real time which enables the user to take appropriate steps in case of skewed values. The CGMs are also equipped with systems that would alert the user when values are above or below the set thresholds. The receiver may either be a display device to be worn like a pager or may be connected to an insulin pump.
A drawback that has emerged with CGMs is bioinstability. Sensors become unstable secondary to inflammatory reaction, granuloma formation, blood clots, etc[15]. This brings about drifts in glucose values and a need for intermittent calibration with conventional blood glucose measurements. Coating of the membrane layer with silicon oxide nanoparticles containing Polyethylene Glycol has been found to prevent bioinstability of sensors[16]. Further research is ongoing to discover the most appropriate material to coat the sensors. Another innovation that has been successful is replacement of electrochemical sensors with fluorescent sensors. When glucose binds to the receptors, the fluorophore fluoresces brightly. These sensors are highly accurate even with extreme values of glucose[17]. Despite these refinements, there are two important shortcomings with the CGMs. First, the interstitial glucose measurement does not exactly reflect the blood glucose concentration. Second is the time lag due to glucose transport to the interstitium and sensor processing. The CGMs lag behind blood glucose by an average of 4 to 10 min[18].
Another method of blood glucose monitoring that had emerged in 1999 was the Glucowatch Biographer. This device was worn like a wristwatch. It used the process of reverse iontophoresis to stimulate the secretion of subcutaneous fluid, and glucose content was measured using a biosensor unit. There was good correlation with the blood glucose monitoring devices[19]. However, skin irritation and false alarms were obstacles to the widespread clinical use of this device.
A recently developed non-invasive CGM device named HG1c uses the principle of Raman spectroscopy where a painless pulse of monochromatic light is transmitted into the skin, and the scattered light is detected for the determination of glucose levels. This device can be worn on the abdomen like a band and measures blood glucose levels every five minutes. The sensor transmits data to a smartphone which is also enabled with alarms during periods of glucose excursion[20]. A similar iPhone operating system-enabled smartphone-based Wireless Smart Gluco-Monitoring system has also been developed[21].
Many smartphone based glucose monitors and applications are helping to make the life of a diabetic patient easier. These allow the user to enter diabetes related data like carbohydrates and water consumed, insulin dose taken, duration of exercise, etc. Based on the information given these apps can also calculate the amount of insulin required. A device named Eyesense is under development which will be able to determine blood glucose level using a small photometer implanted in the interstitial fluid under the conjunctiva[21].
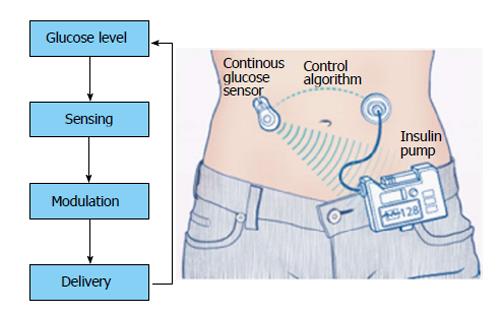
The idea of closed loop systems came into vogue as the repeated discrete subcutaneous doses caused fluctuating insulin and in turn glucose levels. Blood glucose concentration stands on a delicate balance between caloric intake and expenditure which is modified by the insulin doses that necessarily do not mimic the original pancreatic secretion. As the CGMs started providing real time feedback of the glucose levels, the extreme variations were uncovered. The concept of artificial pancreas surfaced when CGMs were linked to insulin pumps as Continuous Subcutaneous Insulin Infusion gained acceptance from the 1990s[22]. The principle of closed loop systems is simple as shown in Figure 4.
In contrast to the pre-programmed insulin pumps, closed loop systems modulate insulin delivery at intervals of 1 to 15 min.
The characteristics that are desired in an ideal closed loop system would be the following[23]: (1) Response to glucose levels in a highly specific way; (2) Response within a timescale of minutes; (3) Monitoring within the visceral region; (4) Pulsatile output to avoid desensitization of insulin receptors; and (5) No chemical modification of insulin.
The backbone of the closed loop system is the control algorithm. Control algorithms direct insulin delivery as per glucose levels and account for measurement errors and kinetic delays.
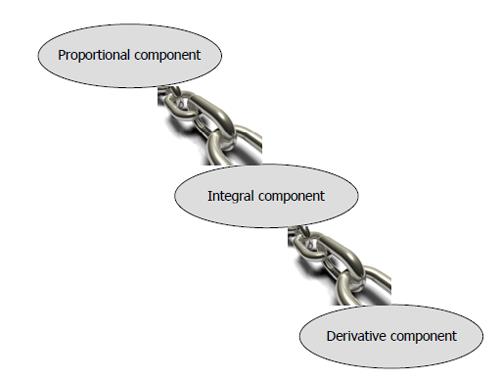
There are two categories of control algorithms: (1) Proportional Integral Derivative (PID); and (2) Model Predictive Control (MPC).
The schema of PID is given in Figure 5. The PID was one of the most initial algorithms developed for artificial pancreas. The proportional component detects deviations from target glucose, integral component measures the area under the curve between the measured and target levels and the derivative component assesses the rate of change of measured glucose levels. However, PID is rather a reactive algorithm which implies that skewed values of glucose cannot be prevented but can only be shortened in duration because the PID responds to observed glucose levels. Adding announced meals to the algorithm or patient directed insulin boluses can overcome hyperglycemia but hypoglycemic episodes may not be prevented.
This is a proactive algorithm because it can forecast the blood glucose values from the current concentration and is designed in such a way that it brings the forecasted glucose closer to the target glucose values. Based on the current glucose levels further insulin delivery is planned but after the first step is executed the system is reassessed and further delivery is planned. This enables a step by step assessment and reaction, yet in a proactive manner. In this way MPC can prevent hypoglycemic episodes and reduce the time spent in hyperglycemia. MPC can efficiently deal with meals and exercise without any additional inputs[24]. MPC also has capabilities to learn the patient’s routine to adjust the insulin delivery based on this information using the run to run control algorithms and also optimize according to circadian fluctuations[25].
The inherent disadvantages of interstitial insulin infusion account for the delay in responding to post prandial hyperglycemia. Hence, systems have been developed for adding meal announcements to cause priming.
Intensification of insulin delivery saw hypoglycemia as the major barrier which induced development of dual hormonal pumps employing glucagon along with insulin. Glucagon has been the choice as it is a fast acting counter regulatory hormone to insulin and is found to be deficient in type 1 diabetes patients. Glucagon has enabled to close the glucose-insulin loop in the initial studies[26].
Intraportal or intraperitoneal insulin infusion to mimic the natural secretory pathway is another gate that has been opened for better control of blood sugar. However, the invasive procedure involved in placing the device and risks of infection are the hurdles to its more widespread usage[27].
“Low Glucose Suspend” is another feature to combat hypoglycemia as the pump would automatically stop insulin infusion for up to 2 h when hypoglycemia is detected which is of benefit especially during nocturnal hypoglycemic episodes[28].
Pramlintide is an amylin analogue that delays gastric emptying and reduces glucagon secretion. Pramlintide infusion along with insulin is found to enhance peripheral tissue sensitivity to insulin[29].
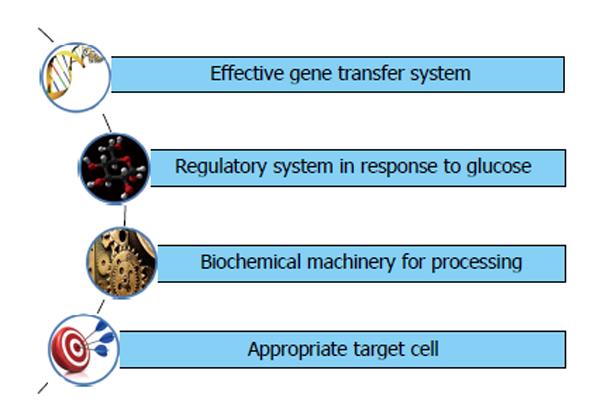
Gene therapy is the fancy word for most diseases without a cure and so it is for diabetes also. Insulin gene therapy envisages introduction of insulin secretory machinery into non beta cells. The requirements for insulin gene transfer are schematically represented in Figure 6.
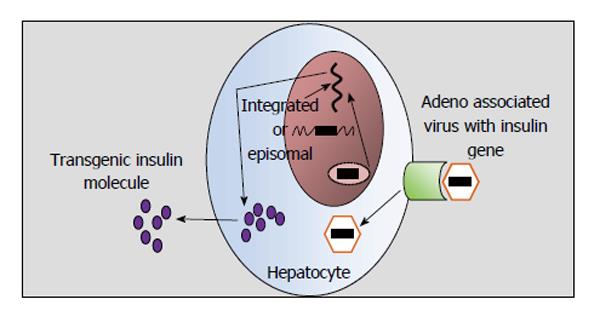
Gene transfer can be achieved by viral or non viral vectors. Among non viral vectors direct injection of DNA, electroporation and gene gun methods were tried but gene expression was transient. Retro virus, adeno virus and adeno associated virus have been looked upon as the living carriers of the insulin gene (Figure 7). Problems are galore even with these viral vectors. Retro viral vectors integrate at random sites, have limited insertion capacity and infect only proliferating cells. Adenoviral vectors remain as extra- chromosomal DNA and sometimes activate cellular immune response to viral proteins.
Under normal circumstances insulin biosynthesis is regulated at the translational level which is rapid enough to react to physiological changes. Transcriptional control supplements the translational regulation. To ensure glucose responsiveness, glucose responsive promoters are linked to the insulin producing gene. However, introducing promoters alone may not be sufficient as translational regulation is difficult to be mimicked in a non-beta cell[30]; and since insulin release is controlled at the transcriptional level the rapidity of the response would be compromised.
Proinsulin is converted to insulin by endoproteases PC1, PC2 and an exopeptidase, carboxypeptidase H which is another example of translational control[31]. In non beta cells the generic proprotein convertase Furin can cleave pro-insulin if appropriate cleavage sites are introduced by mutation but mutated pro- insulin may induce immune attack[32].
An ideal target cell ought to have all beta cell characteristics but has to be free from immune attack. This statement seems utopian as the sophisticated machinery in the beta cell for insulin synthesis and release according to the metabolic needs is not to be easily found in any other cell type. Hepatocyte stood out as a good option as it is enabled with glucose sensing system and glucose regulated promoter. Unfortunately there are no processing enzymes and exocytosis system[33]. The pituitary cell on the other hand, has processing enzymes and exocytosis system but lacks glucose sensing system. Myocytes are also among candidate target cells. K cells, endocrine cells in the gut that secrete incretins, are endowed with glucose sensing system, glucose regulated promoter, exocytosis system and processing enzymes. Genetically engineered K cells have been shown to produce enough insulin in a glucose regulated manner in murine models though tumor cell lines were used. Though the ideal target non beta cell still remains elusive, the K cells form a promising option[34,35].
Despite developments in closed loop systems and encouraging results from insulin gene therapy, completely mimicking the beta cells still remained a distant dream. Thus, pancreas transplant was considered as a viable option. Whole pancreas transplant was tried initially in patients requiring kidney transplant but complications were galore like pseudocyst, fistula, thrombosis and pancreatitis. Moreover, transplanting the whole pancreas when the patients were only in need of the islets of Langerhans which constitute a meagre 2% of the pancreatic mass was like losing the battle for want of a horse shoe nail[36].
In addition to transplanting only the endocrine component, islet cell transplantation is minimally invasive and is associated with lower morbidity. After pancreas retrieval, the islets are isolated and cultured which is the most formidable step in the whole procedure. The most commonly used anatomical site for islet transplant is the liver due to the convenience of access and good entrapment and engraftment in the sinusoids though spleen, renal capsule and the gonads have been tried[37]. Islet cell transplantation done in animals resulted in universal reversal of diabetes but reproduction of these results in human beings was a Himalayan task in the 1990s as only 11% achieved insulin independence. However, in 2009, the Collaborative Islet Transplant Registry reported that the overall incidence of sustained graft function was 77% after first 6 mo, 66% after 1 and 45% at 3 years[38]. Though independence from exogenous insulin can be achieved, extrapolation of results from studies done in adults to children with type 1 diabetes mellitus (T1DM) would be a precocious decision and awaits more research.
The interest stem cell therapy created in almost all chronic diseases is also reverberating in type 1 diabetes. Generation of sufficient mass of beta cells, releasing insulin in response to physiological signals and protection from autoimmunity are the most important challenges. Stem cells can be converted to beta cells by sequential transient activation of specific transcription factors like Pa x 4, Nk x 6.1 and Nk x 2.2[39]. The possibility of teratogenicity with embryonal stem cells makes mesenchyme derived stem cells a better option. An alternative approach is by neogenesis of beta cells from mature beta cells with the use of GLP analogue (Exendin), Epidermal Growth Factor and gastrin. The common endodermal origin of pancreas, liver and small intestine allows trans-differentiation of any of these cell types to beta cells[40]. Trans- differentiation involves reprogramming mature cells by certain transcription factors into alternate developmental lineages.
The principle behind this model is to induce lymphocytes against a specific antigen in such a way that on encountering that particular epitope the lymphocytes would induce cytokines that suppress autoimmunity like interleukin 4 that are produced by Th1 cells. Insulin given orally and subcutaneously in mice models prevented T1DM[41]. Replicating these findings in humans will take time but these provide some light at the end of the tunnel.
Novel therapies are continuing to emerge for the ultimate cure of type 1 diabetes, but emulating the intricate control system of the beta cell that is tailor made for minute to minute control of blood sugar is a difficult goal to attain. We hope that sustained efforts toward this distant goal will provide the elixir for millions of children with T1DM.
Continuous glucose monitors have evolved from retrospective display to real time monitors enabled with alarms connected to smartphones and to more non-invasive methods. Closed loop systems have been undergoing developments to simulate the pancreas by incorporating better sensors, feedback, control algorithms and response. Newer insulin analogues have more predictable half-life and activity. Inhalational, buccal and transdermal delivery routes are awaited for clinical application. Insulin independence is aimed at by incorporating insulin gene into non beta cells with reliable glucose response apparatus. Islet cell transplantation is also continually transforming to reach the point of complete cure. Immunological vaccination is in its nascent stages to prevent the occurrence of type 1 diabetes.
P- Reviewer: Georgescu A, Romani A, Vorobjova T S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Karamitsos DT. The story of insulin discovery. Diabetes Res Clin Pract. 2011;93 Suppl 1:S2-S8. [PubMed] |
| 2. | Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1186] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 3. | Weets I, De Leeuw IH, Du Caju MV, Rooman R, Keymeulen B, Mathieu C, Rottiers R, Daubresse JC, Rocour-Brumioul D, Pipeleers DG. The incidence of type 1 diabetes in the age group 0-39 years has not increased in Antwerp (Belgium) between 1989 and 2000: evidence for earlier disease manifestation. Diabetes Care. 2002;25:840-846. [PubMed] |
| 4. | Anderson JH, Brunelle RL, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Multicenter Insulin Lispro Study Group. Clin Ther. 1997;19:62-72. [PubMed] |
| 5. | Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care. 2000;23:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, Trüb T. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 518] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Home P. Insulin glargine: the first clinically useful extended-acting insulin in half a century? Expert Opin Investig Drugs. 1999;8:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Home P, Bartley P, Russell-Jones D, Hanaire-Broutin H, Heeg JE, Abrams P, Landin-Olsson M, Hylleberg B, Lang H, Draeger E. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care. 2004;27:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Danne T, Datz N, Endahl L, Haahr H, Nestoris C, Westergaard L, Fjording MS, Kordonouri O. Insulin detemir is characterized by a more reproducible pharmacokinetic profile than insulin glargine in children and adolescents with type 1 diabetes: results from a randomized, double-blind, controlled trial. Pediatr Diabetes. 2008;9:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Duttaroy A, Kanakaraj P, Osborn BL, Schneider H, Pickeral OK, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, Renard E, Russell-Jones D, Philotheou A, Francisco AM. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 12. | Mandal TK. Inhaled insulin for diabetes mellitus. Am J Health Syst Pharm. 2005;62:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 370] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 14. | Mizutani F, Yabuki S, Iijima S. Carbon paste electrode incorporated with cobalt(II) octaethoxyphthalocyanine for the amperometric detection of hydrogen peroxide. Electroanalysis. 1995;7:706-709. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Klueh U, Liu Z, Feldman B, Henning TP, Cho B, Ouyang T, Kreutzer D. Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol. 2011;5:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Lane JE, Shivers JP, Zisser H. Continuous glucose monitors: current status and future developments. Curr Opin Endocrinol Diabetes Obes. 2013;20:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Peyser T, Zisser H, Khan U, Jovanovič L, Bevier W, Romey M, Suri J, Strasma P, Tiaden S, Gamsey S. Use of a novel fluorescent glucose sensor in volunteer subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2011;5:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Wentholt IM, Hart AA, Hoekstra JB, Devries JH. Relationship between interstitial and blood glucose in type 1 diabetes patients: delay and the push-pull phenomenon revisited. Diabetes Technol Ther. 2007;9:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Garg SK, Potts RO, Ackerman NR, Fermi SJ, Tamada JA, Chase HP. Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Lyandres O, Yuen JM, Shah NC, VanDuyne RP, Walsh JT, Glucksberg MR. Progress toward an in vivo surface-enhanced Raman spectroscopy glucose sensor. Diabetes Technol Ther. 2008;10:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Joseph Tran BS, Rosanna Tran BS, John R White Jr PA, Smartphone-Based Glucose Monitors and Applications in the Management of Diabetes: An Overview of 10 Salient “Apps” and a Novel Smartphone-Connected Blood Glucose Monitor. Clinical Diabetes. 2012;30:173-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002;25:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Adams G, Clark J, Sahota T, Tanna S, Taylor MJ. Diabetes mellitus and closed-loop insulin delivery. Biotechnol Genet Eng Rev. 2000;17:455-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Man CD, Place J, Facchinetti A. Closed-Loop Artificial Pancreas Using Subcutaneous Glucose Sensing and Insulin Delivery and a Model Predictive Control Algorithm: Preliminary Studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3:1014-1021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Owens C, Zisser H, Jovanovic L, Srinivasan B, Bonvin D, Doyle FJ. Run-to-run control of blood glucose concentrations for people with Type 1 diabetes mellitus. IEEE Trans Biomed Eng. 2006;53:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | El-Khatib FH, Jiang J, Damiano ER. Adaptive closed-loop control provides blood-glucose regulation using dual subcutaneous insulin and glucagon infusion in diabetic Swine. J Diabetes Sci Technol. 2007;1:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, Shaw JA, Pickup JC, Amiel SA. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34:2023-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Huffman DM, McLean GW, Seagrove MA. Continuous subcutaneous pramlintide infusion therapy in patients with type 1 diabetes: observations from a pilot study. Endocr Pract. 2009;15:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Goodge KA, Hutton JC. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic beta-cell. Semin Cell Dev Biol. 2000;11:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Hutton JC. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia. 1994;37 Suppl 2:S48-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994;269:6241-6245. [PubMed] |
| 33. | Nett PC, Sollinger HW, Alam T. Hepatic insulin gene therapy in insulin-dependent diabetes mellitus. Am J Transplant. 2003;3:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Corbett JA. K cells: a novel target for insulin gene therapy for the prevention of diabetes. Trends Endocrinol Metab. 2001;12:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Yao L, Shen K, Xu M, Zhou P, Yang W, Liu X, Qin X. Genetically engineered K cells provide sufficient insulin to correct hyperglycemia in a nude murine model. Acta Biochim Biophys Sin (Shanghai). 2008;40:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Jahansouz C, Kumer SC, Ellenbogen M, Brayman KL. Evolution of β-Cell Replacement Therapy in Diabetes Mellitus: Pancreas Transplantation. Diabetes Technol Ther. 2011;13:395-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witson J, Bansal-Pakala P, Balamurugan AN. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Vantyghem MC, Kerr-Conte J, Arnalsteen L, Sergent G, Defrance F, Gmyr V, Declerck N, Raverdy V, Vandewalle B, Pigny P. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care. 2009;32:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Takeuchi H, Nakatsuji N, Suemori H. Endodermal differentiation of human pluripotent stem cells to insulin-producing cells in 3D culture. Sci Rep. 2014;4:4488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Berneman-Zeitouni D, Molakandov K, Elgart M, Mor E, Fornoni A, Domínguez MR, Kerr-Conte J, Ott M, Meivar-Levy I, Ferber S. The temporal and hierarchical control of transcription factors-induced liver to pancreas transdifferentiation. PLoS One. 2014;9:e87812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252-10256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 392] [Article Influence: 11.5] [Reference Citation Analysis (0)] |