Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.577
Revised: March 19, 2014
Accepted: July 17, 2014
Published online: October 15, 2014
Processing time: 205 Days and 8.3 Hours
Peripheral arterial disease, manifested as intermittent claudication or critical ischaemia, or identified by an ankle/brachial index < 0.9, is present in at least one in every four patients with type 2 diabetes mellitus. Several reasons exist for peripheral arterial disease in diabetes. In addition to hyperglycaemia, smoking and hypertension, the dyslipidaemia that accompanies type 2 diabetes and is characterised by increased triglyceride levels and reduced high-density lipoprotein cholesterol concentrations also seems to contribute to this association. Recent years have witnessed an increased interest in postprandial lipidaemia, as a result of various prospective studies showing that non-fasting triglycerides predict the onset of arteriosclerotic cardiovascular disease better than fasting measurements do. Additionally, the use of certain specific postprandial particle markers, such as apolipoprotein B-48, makes it easier and more simple to approach the postprandial phenomenon. Despite this, only a few studies have evaluated the role of postprandial triglycerides in the development of peripheral arterial disease and type 2 diabetes. The purpose of this review is to examine the epidemiology and risk factors of peripheral arterial disease in type 2 diabetes, focusing on the role of postprandial triglycerides and particles.
Core tip: Peripheral arterial disease is highly prevalent in type 2 diabetes; traditional risk factors contribute to the disease. Interestingly, postprandial lipidaemia is increased in both conditions. However, one study showed that only subjects with both type 2 diabetes and peripheral arterial disease had elevation of postprandial lipids; subjects with type 2 diabetes and a normal ankle-brachial index had a normal postprandial response. Because most of the triglycerides of chylomicrons are extracted in muscle and adipose cells in the legs, the authors speculate on whether arteriosclerosis in the legs may contribute to greater postprandial lipidaemia.
- Citation: Valdivielso P, Ramírez-Bollero J, Pérez-López C. Peripheral arterial disease, type 2 diabetes and postprandial lipidaemia: Is there a link? World J Diabetes 2014; 5(5): 577-585
- URL: https://www.wjgnet.com/1948-9358/full/v5/i5/577.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i5.577
Peripheral arterial disease (PAD) is produced by narrowing of the calibre of the medium-sized arteries and its widest definition encompasses all extracoronary and extracerebral vascular disease. However, the term PAD is usually restricted to involvement of the lower limbs, particularly in the iliac bifurcation, and the iliofemoral and popliteal arteries[1]. The main cause of arterial stenosis in developed countries is atherosclerosis.
The prevalence of PAD in Europe and the United States is estimated to be 27 million persons[2]. The prevalence of PAD increases progressively with age, with most cases starting after the age of 40 years. It is well known that only a very few PAD patients actually have symptoms, around 10%-20%[3]. The use of a standardized questionnaire in the physician’s office can increase the detection of claudicant patients[4,5]. Most patients with PAD are identified from non-invasive tests, such as the ankle-brachial index (ABI). Using this widely extended technique in Spain led to the identification of PAD in 8% of individuals aged 55-85 years[6]. In addition to age, the other cardiovascular risk factors also increase the likelihood of developing PAD. Thus, in persons with a low cardiovascular risk the prevalence of PAD is almost inconsiderable[7], whereas it can reach 27% in persons with type 2 diabetes[8].
The prognosis for patients with PAD, both symptomatic and asymptomatic, is poor[9]. Overall mortality is increased and the risk of death is even greater than that in patients who have angina or acute myocardial infarction[10-13]. Data from Spain confirm these findings. An analysis of the FRENA, REACH and AIRVAG registries showed that patients with PAD have a greater frequency of symptomatic multivessel disease and a worse one-year prognosis than patients with single-vessel involvement or cerebrovascular disease[14].
Diabetes, together with smoking, is the main risk factor for PAD[15]. Of patients who attended an angiology office in Spain due to intermittent claudication and who underwent arterial surgery or had an ABI ≤ 0.9, 67% had diabetes mellitus[16]. Population-based studies in Spain, undertaken in either the general population or at various levels of care, showed that the presence of diabetes mellitus doubled or even tripled the possibility of having PAD (Table 1)[6,17-23]. The prevalence of an ABI < 0.9 in series of Spanish patients with diabetes ranges from 21% to 60% (Table 1)[8,24,25]. In the autonomous communities of Andalusia and the Canary Islands, 72% of all lower-limb amputations between 1996 and 2006 involved patients with diabetes[23,26,27]. In patients with diabetes, for every 1% increase in haemoglobin A1c there is a corresponding 26% increased risk of PAD[28]. The presence of PAD also increases the risk of death in patients with diabetes mellitus[29,30]. The prognosis for PAD is worse in patients with diabetes than those without diabetes[31].
| Study | Number of subjects | Age (yr) | Study population | ABI < 0.9 (%) | |
| HERMEX[17] | 2833 | 51 | General | All | 3.7 |
| Without diabetes | 2.8 | ||||
| With Diabetes | 6.2 | ||||
| ESTIME[6] | 1324 | 68 | General | All | 8 |
| Without diabetes | 6.6 | ||||
| With diabetes | 19 | ||||
| MERITO[19] | 1519 | 66 | Internal medicine outpatient clinic | SCORE ≥ 3 | 26.2 |
| With Diabetes | 26.1 | ||||
| VITAMIN[20] | 493 | 68 | Internal medicine outpatient clinic | Without DM2 | 21 |
| With DM2 | 38 | ||||
| ARPTER[18] | 3171 | 63 | General | All | 6.4 |
| Without diabetes | 5.4 | ||||
| With diabetes | 12.6 | ||||
| REGICOR[21] | 6262 | 56 | General | All | 4.5 |
| Without diabetes | 4 | ||||
| With diabetes | 8.4 | ||||
| FUENCARRAL Health Center[22] | 1360 | 70 | Primary health care centre | Without diabetes | 4.3 |
| With diabetes | 11.3 | ||||
| ALBACETE[23] | 784 | 61 | General | All | 10.5 |
| Without diabetes | 9 | ||||
| With diabetes | 19 | ||||
| RONDA PRIM Health Center[25] | 289 | 65 | Primary health centres | Diabetes | 21.5 |
| CIUDAD JARDIN Health Center[78] | 456 | 61 | Primary health centre | Diabetes | 27 |
| PADiD Study[24] | 1462 | 78 | Internal medicine outpatient clinics | Diabetes | 60 |
| MARINA BAIXA Hospital[89] | 360 | 67 | Internal medicine outpatient clinics | Diabetes | 27 |
The diagnosis of PAD usually depends on the sum of the symptoms, particularly intermittent claudication, plus the physical examination, especially the lack of pulses and the trophic disorders leading to critical limb ischaemia and distal necrosis[32]. However, patients, particularly diabetic patients, commonly have other processes at the same time that can alter the traditional symptoms of PAD, making them much less specific[33]. Accordingly, the measurement of the ratio of the systolic blood pressures in the ankle and the arm, the ABI, has been recommended as the screening method for asymptomatic PAD and as a form of confirmation in symptomatic PAD[2,34,35]. A finding in one limb of an ABI < 0.9 with the measurement taken at rest under standard conditions is considered diagnostic of PAD, with an ABI between 0.9 and 1.0 considered borderline[36].
One limitation of the ABI, especially relevant in patients with diabetes, is arterial media calcification, which can lead to non-compressible arteries (ABI > 1.4) or false normal values. A recent study showed that individuals with an ABI > 1.4 have a worse prognosis than those with a normal ABI and even those with an ABI < 0.9. The prevalence of diabetes in the group with an ABI > 1.4 was 58%, compared with 18% and 48% in those with a normal ABI or those with an ABI < 0.9[37]. It has long been known that the sensitivity of the ABI to correctly diagnose PAD is considerably reduced in the presence of arterial media calcification and that, clinically, this calcification is associated with the presence of peripheral neuropathy[38,39]. Accordingly, in the presence of peripheral neuropathy it is recommended to use an alternative method, such as flow wave analysis using Doppler colour ultrasound[40,41]. In our experience this limitation is not negligible. In a series of 456 patients with type 2 diabetes, 35 were found to have intermittent claudication (7.6%); only 22 of these had an ABI < 0.9. Of the other 13, 12 underwent colour Doppler ultrasound and in 3 (25%) we obtained a monophasic wave, diagnostic of PAD. Thus, a normal ABI does not rule out PAD in patients with type 2 diabetes, and these patients should therefore undergo complementary tests if they have symptoms suggestive of PAD[8].
The resting ABI should be used as the diagnostic technique for PAD when lower limb arteriosclerosis is suspected. This should be done in persons with one or more of the following: symptoms in the lower limbs after exercise, wounds with delayed healing, and individuals older than 65 years of age or older than 50 years with a history of smoking or diabetes[34]. Given the high prevalence of PAD in patients with diabetes, the ADA recommends screening with the ABI in patients with diabetes who are older than 50 years and who have another risk factor (smoking, hypertension, hyperlipidaemia, or diabetes for more than 10 years)[42].
Lipid abnormalities in PAD have received less attention than in other areas, as for example, in coronary anomalies. Very few prospective studies have focused on the relation between triglycerides and peripheral vascular disease. The most common feature of PAD is raised levels of triglycerides and lower levels of high-density lipoprotein (HDL) cholesterol as compared with age- and sex-matched controls without vascular disease, with similar levels of cholesterol and low-density lipoprotein (LDL) cholesterol[43-47]. The frequency of a cluster of lipid abnormalities of the type of raised triglycerides and small and dense LDL and reduced HDL was 20% in persons with PAD vs 0% in the control group[48]. Several studies have also shown that triglyceride levels are a predictive factor for PAD[49-51], though not all[52].
Unlike the carbohydrates, which normally only show transitory increases after a meal, the circulating triglycerides show a pronounced increase (postprandial lipidaemia) one hour after the intake of a fat-rich meal (around 30-60 g), and can remain high for 5-8 h after the meal. As most persons regularly consume fatty meals every 4-5 h, the usual state in humans insofar as their triglyceride metabolism is concerned is clearly a continuous postprandial lipidaemic state[53,54].
The large triglyceride-transporting particles, the chylomicrons and the very low-density lipoprotein (VLDL), are too large to cross the endothelium and they therefore don’t contribute to the atherosclerosis, but the same does not occur with the chylomicron remnants and the intermediate-density lipoprotein (IDL), which are much smaller particles[55]. Evidence exists that the cholesterol in the postprandial particles, originating in the intestine, contribute to the phenomenon of atherosclerosis, both in animals and in humans[56-59].
Since the seminal work of Zilversmit, many case-control studies have found an association between the magnitude of the postprandial lipidaemia and the presence and severity of coronary artery disease[60,61]; these studies have been reviewed by Lopez-Miranda et al[62]. Prospective studies, however, are few and controversial. Reyes-Soffer et al[63] followed 69 patients with type 2 diabetes who were free of coronary disease for a mean of 8.7 years; 33 patients remained disease-free. No differences were found in the postprandial parameters at the initial visit between the groups, and the authors concluded that the postprandial triglycerides do not predict the onset of coronary disease in individuals with diabetes. A more recent study involving 514 survivors of an acute coronary syndrome found that the postprandial triglycerides after the oral intake of 75 g of fat predicted the appearance of new events at 18 mo. In the subgroup of patients without diabetes or oral glucose intolerance the relative increase in postprandial triglycerides was an independent predictor of events[64].
Interest in studying postprandial lipidaemia has increased over recent years as a result of studies showing that serum triglyceride levels measured in a non-fasting state have proved to be better predictors for the risk of vascular disease than fasting triglyceride concentrations, i.e., when they are quantified after 8-10 h of fasting[65-68]. Two meta-analyses also support the association between fasting and postprandial triglycerides and the vascular risk[69,70]. One of the problems encountered when introducing postprandial triglyceride measurements in the clinical setting is the absence of specific recommendations in the clinical practice guidelines and thus the identification of a threshold level above which postprandial hypertriglyceridaemia is recognised. To date, only the American Association of Clinical Endocrinologists has considered the possibility of evaluating the non-fasting triglyceride concentration[71]. Based on evidence from the above mentioned population-based studies, an expert group estimated non-fasting triglyceride levels < 180 mg/dL as desirable[72]. This means that 38% of the men and 20% of the women in the Copenhagen study who had figures above these levels have postprandial hypertriglyceridaemia[73].
The study of postprandial (hyper)lipidaemia has several inconveniences. The most important at present is the poor clinical yield and the great complexity of the fat test; its prolonged time is uncomfortable for both the patient and the medical personnel, not to mention the lack of standardization for the test. A few years ago, using data from a meta-analysis of 113 studies in healthy subjects by Mihas et al[74], an expert group attempted to standardize the test and recommended a fat tolerance test meal consisting of 75 g fat, 25 g carbohydrates and 10 g protein. Furthermore, the fatty test meal should contain mixtures of saturated and unsaturated fatty acids in a digestible form and be easy to prepare. The candidates for the test should have fasting triglycerides of 90-180 mg/dL and the test can be shortened with the measurement of the serum triglycerides at 4 h, with no need to reach a complete postprandial curve of 8 or 12 h[72].
Little attention has been given to the study of postprandial lipidaemia in patients with PAD. Only the elegant paper by Lupattelli et al[75] showed that the magnitude of postprandial lipidaemia, expressed as “the area under the incremental curve for triglycerides,” was higher in 16 non-diabetic normolipidaemic claudicant patients with PAD than in 10 normolipidaemic control subjects, suggesting the relevance of postprandial lipoprotein metabolism in the pathogenesis of peripheral atherosclerosis. However, although normolipidaemic, the patients in Lupattelli’s study had slightly higher fasting triglycerides than their controls.
In recent years our group has studied the relation between lipids and postprandial particles, PAD and type 2 diabetes mellitus. Firstly, the postprandial triglycerides were more strongly associated with PAD in individuals with type 2 diabetes mellitus than were the fasting triglycerides. A group of 119 patients with type 2 diabetes mellitus treated with just diet and/or oral glucose lowering agents, with no lipid-lowering treatment, were analyzed at fasting and 4 h after a mixed breakfast containing 50 g of fat and 40 g of carbohydrates. Although the patients with cardiovascular disease, most of them with asymptomatic PAD and identified by an ABI < 0.9, had lower fasting HDL cholesterol levels and higher triglyceride levels, only the triglycerides at 4 h post-breakfast were associated in the multivariate analysis with cardiovascular disease, together with the duration of the disease and smoking[76].
The postprandial triglycerides include not only those contained in chylomicron particles and their remnants, but also those contained in VLDL and IDL. In an attempt to further understand the role of postprandial fat in PAD, we undertook a second experiment to analyze the serum concentration of apolipoprotein B48, a protein that is only associated with chylomicrons and their remnants and is not interchanged with any other circulating particle. This second study involved 101 patients with type 2 diabetes mellitus and 73 controls without diabetes, both groups with no known cardiovascular disease. Asymptomatic vascular disease was identified from the ABI and as a marker of postprandial particles we used the apolipoprotein B48, measured with a commercial enzyme-linked immunosorbent assay. Of the patients with type 2 diabetes mellitus, 21 had PAD as defined by an ABI < 0.9, though no control had PAD. The levels of triglycerides and apolipoprotein B48, both fasting and postprandial, were significantly higher in the group of diabetic patients with PAD than in those without PAD and the controls. Curiously, no differences were found between the controls and the patients with type 2 diabetes mellitus without PAD. Of all the lipid and non-lipid parameters studied, only apolipoprotein B48 and smoking were associated with the presence of PAD in a binary logistic regression analysis. Likewise, the presence of PAD was an independent predictor of the levels of apolipoprotein B48, both fasting and 4 h after a mixed breakfast[77].
As the patients with type 2 diabetes mellitus in the previous studies did not receive any insulin or lipid-lowering therapy, we decided to confirm the findings in a larger population with type 2 diabetes mellitus without these exclusion criteria. Again, using an ABI < 0.9 as a marker of PAD, we found in 456 patients with type 2 diabetes mellitus that fasting apolipoprotein B48 was a marker of PAD, independently of the other lipid factors, statin treatment or insulin therapy[78]. Identical results have also been reported by another group[79].
Taken together, these studies confirm an association between postprandial particles, measured as triglycerides 4 h after breakfast or as fasting and postprandial apolipoprotein B48, and PAD. In the above-mentioned studies, a diabetic status in itself was not associated with a greater concentration of postprandial triglycerides or apolipoprotein B48 if there was no PAD. As mentioned earlier, the case-control studies show an association between postprandial lipidaemia and cardiovascular disease, particularly coronary disease.
An explication for this association was provided by Lupattelli et al[75]. Somehow, and following the hypothesis of Zilversmit[80], the exposure of the endothelium to greater concentrations of postprandial particles favours the appearance of arteriosclerotic lesions, in our case in the lower limbs. Though this hypothesis is the most plausible, no causality can be deduced from the association studies. Accordingly, it is worth speculating about whether arteriosclerotic disease in the legs could alter chylomicron metabolism, slowing it. With this in mind, consideration should be given to the study by Horton et al[81], who showed that men have higher triglyceride concentrations than women because women posses a greater extractive capacity of triglycerides in adipose and muscle tissues in the lower limbs when they undergo a fatty breakfast. For some reason the catabolism of the chylomicrons in the legs is not negligible and an alteration in the circulation in the legs may worsen or slow this metabolism.
The kinetics of lipoproteins are marked by (1) their intestinal production; (2) hydrolysis of their triglycerides by the action of lipoprotein-lipase anchored in the endothelium (but synthesised in adipose and muscle tissue cells); and (3) removal of chylomicron remnants by hepatic receptors. These steps are all modulated by the levels and genetic variants of the apolipoproteins like C-II, C-III, E, A-5[82,83]. As persons with arteriosclerosis, particularly those with PAD, have a marked endothelial dysfunction[84], it is possible to speculate that the action of an enzyme anchored to the endothelium, as is the case of lipoprotein lipase (LPL), is reduced. Given the great extension of the endothelial surface in the legs (in comparison with coronary arteriosclerosis), established PAD might affect postprandial lipidaemia more intensely than coronary disease.
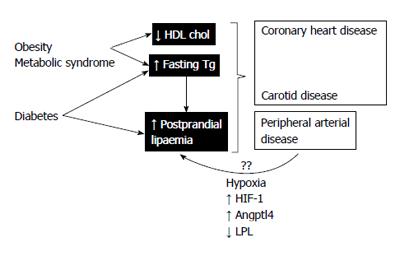
If this hypothesis were true, what would its mechanism of production be? The consequence of arteriosclerosis is tissue ischaemia. This is usually manifested as intermittent claudication, though the tissues may experience hypoxia in earlier stages. Tissue hypoxia leads to changes in the endothelial cells (where the LPL are anchored) or in the production of LPL (or its associated proteins) by adipose or muscle cells[85]. Cells submitted to hypoxia upregulate the expression of hypoxia-inducible factor 1, a transcription factor that induces changes in innumerable target genes that were reviewed some time ago[86]. Of note among these changes is the raised expression of angiopoietin-like 4 protein (Angptl4) and vascular endothelial growth factor (VEGF). VEGF intervenes in the processes of angiogenesis, much related with chronic ischaemia of the lower limbs and the formation of collateral vessels. Angptl4 is a potent inhibitor of LPL, the enzyme that intervenes critically in the first step of the catabolism of triglyceride-rich particles[87]. A recent experimental animal study showed that mice submitted to cyclic hypoxia experienced inhibition of the catabolism of triglyceride-rich lipoproteins as a consequence of a drastic reduction in adipose tissue LPL activity, coupled with a notable increase in Angptl4[88] (Figure 1).
Taken together, these data suggest that postprandial hyperlipidaemia, a recognised vascular risk factor associated with obesity, the metabolic syndrome and type 2 diabetes, could be aggravated by PAD, further exposing other arterial territories to greater concentrations of postprandial atherogenic particles. Finally, if the hypoxia were an underlying mechanism, it could be improved by percutaneous or surgical revascularization.
Authors would like to thank to Ian Johnstone for the English edition of the manuscript.
P- Reviewer: Barzilay JI, Neri V, Tarantino G S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Guijarro C, Mostaza JM, Hernández-Mijares A. [Lower limb arterial disease and renal artery stenosis]. Clin Investig Arterioscler. 2013;25:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4051] [Cited by in RCA: 4117] [Article Influence: 228.7] [Reference Citation Analysis (0)] |
| 3. | Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026-3049. [PubMed] |
| 4. | Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 375] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 744] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Blanes JI, Cairols MA, Marrugat J. Prevalence of peripheral artery disease and its associated risk factors in Spain: The ESTIME Study. Int Angiol. 2009;28:20-25. [PubMed] |
| 7. | Alonso I, Valdivielso P, Josefa Zamudio M, Sánchez Chaparro MA, Pérez F, Ramos H, González Santos P. [Usefulness of the ankle-arm index for detection of peripheral arterial disease in a working population of Junta de Andalucía at Málaga]. Med Clin (Barc). 2009;132:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Mancera-Romero J, Rodríguez-Morata A, Angel Sánchez-Chaparro M, Sánchez-Pérez M, Paniagua-Gómez F, Hidalgo-Conde A, Valdivielso P. Role of an intermittent claudication questionnaire for the diagnosis of PAD in ambulatory patients with type 2 diabetes. Int Angiol. 2013;32:512-517. [PubMed] |
| 9. | Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, Darius H, Burghaus I, Trampisch HJ. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Kieback AG, Lorbeer R, Wallaschofski H, Ittermann T, Völzke H, Felix S, Dörr M. Claudication, in contrast to angina pectoris, independently predicts mortality risk in the general population. Vasa. 2012;41:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Inglis SC, Lewsey JD, Lowe GD, Jhund P, Gillies M, Stewart S, Capewell S, Macintyre K, McMurray JJ. Angina and intermittent claudication in 7403 participants of the 2003 Scottish Health Survey: impact on general and mental health, quality of life and five-year mortality. Int J Cardiol. 2013;167:2149-2155. [PubMed] |
| 12. | Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1889] [Cited by in RCA: 1814] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 13. | Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Guijarro C. Enfermedad arterial oclusiva en los estudios REACH, FRENA y AIRVAG. Anales de Cirugía Vascular. 2009;23:21-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Criqui MH. Peripheral arterial disease--epidemiological aspects. Vasc Med. 2001;6:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Puras-Mallagray E, Gutiérrez-Baz M, Cáncer-Pérez S, Alfayate-García JM, de Benito-Fernández L, Perera-Sabio M, Criado-Galán F, Hernández-Mijares A. Estudio de prevalencia de la enfermedad arterial periférica y diabetes en Espa-a. Angiologia. 2008;60:317-326. |
| 17. | Félix-Redondo FJ, Fernández-Bergés D, Grau M, Baena-Diez JM, Mostaza JM, Vila J. Prevalence and clinical characteristics of peripheral arterial disease in the study population Hermex. Rev Esp Cardiol (Engl Ed). 2012;65:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Baena-Díez JM, Alzamora MT, Forés R, Pera G, Torán P, Sorribes M. Ankle-brachial index improves the classification of cardiovascular risk: PERART/ARTPER Study. Rev Esp Cardiol. 2011;64:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Manzano L, Mostaza JM, Suárez C, Cairols M, Redondo R, Valdivielso P, Monte R, Blázquez JC, Ferreira EM, Trouillhet I. [Value of the ankle-brachial index in cardiovascular risk stratification of patients without known atherotrombotic disease. MERITO study]. Med Clin (Barc). 2007;128:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Manzano L, García-Díaz Jde D, Gómez-Cerezo J, Mateos J, del Valle FJ, Medina-Asensio J, Viejo LF, Fernández-Ballesteros A, Solís J, Herrero Domingo A. [Clinical value of the ankle-brachial index in patients at risk of cardiovascular disease but without known atherothrombotic disease: VITAMIN study]. Rev Esp Cardiol. 2006;59:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ramos R, Quesada M, Solanas P, Subirana I, Sala J, Vila J, Masia R, Cerezo C, Elosua R, Grau M. Prevalence of Symptomatic and Asymptomatic Peripheral Arterial Disease and the Value of the Ankle-brachial Index to Stratify Cardiovascular Risk. Eur J Vasc Endovasc Surg. 2009;38:305-311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Vicente I, Lahoz C, Taboada M, Laguna F, García-Iglesias F, Mostaza Prieto JM. [Ankle-brachial index in patients with diabetes mellitus: prevalence and risk factors]. Rev Clin Esp. 2006;206:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Carbayo JA, Divisón JA, Escribano J, López-Abril J, López de Coca E, Artigao LM, Martínez E, Sanchis C, Massó J, Carrión L. Using ankle-brachial index to detect peripheral arterial disease: prevalence and associated risk factors in a random population sample. Nutr Metab Cardiovasc Dis. 2007;17:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | González-Clemente JM, Piniés JA, Calle-Pascual A, Saavedra A, Sánchez C, Bellido D, Martín-Folgueras T, Moraga I, Recasens A, Girbés J. Cardiovascular risk factor management is poorer in diabetic patients with undiagnosed peripheral arterial disease than in those with known coronary heart disease or cerebrovascular disease. Results of a nationwide study in tertiary diabetes centres. Diabet Med. 2008;25:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Bundó M, Aubà J, Vallés R, Torner O, Pérez AM, Massons J. [Peripheral arteriopathy in type 2 diabetes mellitus]. Aten Primaria. 1998;22:5-11. [PubMed] |
| 26. | Almaraz MC, González-Romero S, Bravo M, Caballero FF, Palomo MJ, Vallejo R, Esteva I, Calleja F, Soriguer F. Incidence of lower limb amputations in individuals with and without diabetes mellitus in Andalusia (Spain) from 1998 to 2006. Diabetes Res Clin Pract. 2012;95:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Aragón-Sánchez J, García-Rojas A, Lázaro-Martínez JL, Quintana-Marrero Y, Maynar-Moliner M, Rabellino M, Hernández-Herrero MJ, Cabrera-Galván JJ. Epidemiology of diabetes-related lower extremity amputations in Gran Canaria, Canary Islands (Spain). Diabetes Res Clin Pract. 2009;86:e6-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Selvin E, Wattanakit K, Steffes MW, Coresh J, Sharrett AR. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2006;29:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Leibson CL, Ransom JE, Olson W, Zimmerman BR, O’fallon WM, Palumbo PJ. Peripheral arterial disease, diabetes, and mortality. Diabetes Care. 2004;27:2843-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Norman PE, Davis WA, Bruce DG, Davis TM. Peripheral arterial disease and risk of cardiac death in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2006;29:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 509] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 32. | Layden J, Michaels J, Bermingham S, Higgins B. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ. 2012;345:e4947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 610] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 34. | Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Vasc Surg. 2011;54:e32-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851-2906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1068] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 36. | Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1162] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 37. | Arain FA, Ye Z, Bailey KR, Chen Q, Liu G, Leibson CL, Kullo IJ. Survival in patients with poorly compressible leg arteries. J Am Coll Cardiol. 2012;59:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care. 2005;28:2206-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Potier L, Halbron M, Bouilloud F, Dadon M, Le Doeuff J, Ha Van G, Grimaldi A, Hartemann-Heurtier A. Ankle-to-brachial ratio index underestimates the prevalence of peripheral occlusive disease in diabetic patients at high risk for arterial disease. Diabetes Care. 2009;32:e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Rodriguez-Morata A, Jiménez-Moleón J, Cuenca-Manteca J, Fernández-Quesada F, Ros-Vidal R, Gómez-Medialdea R, Ros-Díe E. Sensibilidad, especificidad y fiabilidad de la ecografía Doppler arterial en el diagnóstico de la isquemia crítica de los miembros inferiores con relación a la arteriografía. Angiología. 2007;59:121-127. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 42. | American Diabetes Association. Executive summary: Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 Suppl 1:S4-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | O’Neal DN, Lewicki J, Ansari MZ, Matthews PG, Best JD. Lipid levels and peripheral vascular disease in diabetic and non-diabetic subjects. Atherosclerosis. 1998;136:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Seeger JM, Silverman SH, Flynn TC, Bailey JC, Klingman NV, Lawson GA, Borgeson MD, Barratt EJ. Lipid risk factors in patients requiring arterial reconstruction. J Vasc Surg. 1989;10:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Wang T, Elam MB, Forbes WP, Zhong J, Nakajima K. Reduction of remnant lipoprotein cholesterol concentrations by cilostazol in patients with intermittent claudication. Atherosclerosis. 2003;171:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Bainton D, Sweetnam P, Baker I, Elwood P. Peripheral vascular disease: consequence for survival and association with risk factors in the Speedwell prospective heart disease study. Br Heart J. 1994;72:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Sentí M, Nogués X, Pedro-Botet J, Rubiés-Prat J, Vidal-Barraquer F. Lipoprotein profile in men with peripheral vascular disease. Role of intermediate density lipoproteins and apoprotein E phenotypes. Circulation. 1992;85:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Rizzo M, Pernice V, Frasheri A, Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis. 2008;197:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344-353. [PubMed] |
| 50. | Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyörälä K. 5-year incidence of atherosclerotic vascular disease in relation to general risk factors, insulin level, and abnormalities in lipoprotein composition in non-insulin-dependent diabetic and nondiabetic subjects. Circulation. 1990;82:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 167] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 52. | Wattanakit K, Folsom AR, Selvin E, Weatherley BD, Pankow JS, Brancati FL, Hirsch AT. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005;180:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Roche HM, Gibney MJ. Postprandial triacylglycerolaemia--nutritional implications. Prog Lipid Res. 1995;34:249-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Roche HM, Zampelas A, Jackson KG, Williams CM, Gibney MJ. The effect of test meal monounsaturated fatty acid: saturated fatty acid ratio on postprandial lipid metabolism. Br J Nutr. 1998;79:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31:1716-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Fogelstrand P, Borén J. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr Metab Cardiovasc Dis. 2012;22:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Stalenhoef AF, de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr Opin Lipidol. 2008;19:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Proctor SD, Mamo JC. Intimal retention of cholesterol derived from apolipoprotein B100- and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2003;23:1595-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Karpe F, de Faire U, Mercuri M, Bond MG, Hellénius ML, Hamsten A. Magnitude of alimentary lipemia is related to intima-media thickness of the common carotid artery in middle-aged men. Atherosclerosis. 1998;141:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Groot PH, van Stiphout WA, Krauss XH, Jansen H, van Tol A, van Ramshorst E, Chin-On S, Hofman A, Cresswell SR, Havekes L. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 61. | Patsch JR, Miesenböck G, Hopferwieser T, Mühlberger V, Knapp E, Dunn JK, Gotto AM, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 774] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 62. | Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98:458-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 63. | Reyes-Soffer G, Holleran S, Karmally W, Ngai CI, Chen NT, Torres M, Ramakrishnan R, Blaner WS, Berglund L, Ginsberg HN. Measures of postprandial lipoproteins are not associated with coronary artery disease in patients with type 2 diabetes mellitus. J Lipid Res. 2009;50:1901-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Werner C, Filmer A, Fritshc M, Groenewald S, Gräber S, Böhm M, Laufs U. Prospective evaluation of post-prandial triglycerides and cardiovascular events in patients with coronary artery disease. 2011;. |
| 65. | Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1153] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 66. | Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1548] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 67. | Eberly LE, Stamler J, Neaton JD. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 466] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 69. | Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1059] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 70. | Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1866] [Cited by in RCA: 2107] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 71. | Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, Shepherd MD, Seibel JA. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012;18 Suppl 1:1-78. [PubMed] |
| 72. | Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 73. | Langsted A, Nordestgaard BG. Nonfasting lipids, lipoproteins, and apolipoproteins in individuals with and without diabetes: 58 434 individuals from the Copenhagen General Population Study. Clin Chem. 2011;57:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Mihas C, Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K. Diagnostic value of postprandial triglyceride testing in healthy subjects: a meta-analysis. Curr Vasc Pharmacol. 2011;9:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Lupattelli G, Pasqualini L, Siepi D, Marchesi S, Pirro M, Vaudo G, Ciuffetti G, Mannarino E. Increased postprandial lipemia in patients with normolipemic peripheral arterial disease. Am Heart J. 2002;143:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Valdivielso P, Hidalgo A, Rioja J, Aguilar I, Ariza MJ, González-Alegre T, González-Santos P. Smoking and postprandial triglycerides are associated with vascular disease in patients with type 2 diabetes. Atherosclerosis. 2007;194:391-396. [PubMed] |
| 77. | Valdivielso P, Puerta S, Rioja J, Alonso I, Ariza MJ, Sánchez-Chaparro MA, Palacios R, González-Santos P. Postprandial apolipoprotein B48 is associated with asymptomatic peripheral arterial disease: a study in patients with type 2 diabetes and controls. Clin Chim Acta. 2010;411:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Mancera-Romero J, Sánchez-Chaparro MA, Rioja J, Ariza MJ, Olivecrona G, González-Santos P, Valdivielso P. Fasting apolipoprotein B48 is a marker for peripheral arterial disease in type 2 diabetes. Acta Diabetol. 2013;50:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Lapice E, Cipriano P, Patti L, Romano G, Vaccaro O, Rivellese AA. Fasting apolipoprotein B48 is associated with asymptomatic peripheral arterial disease in type 2 diabetic subjects: a case-control study. Atherosclerosis. 2012;223:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 81. | Horton TJ, Commerford SR, Pagliassotti MJ, Bessesen DH. Postprandial leg uptake of triglyceride is greater in women than in men. Am J Physiol Endocrinol Metab. 2002;283:E1192-E1202. [PubMed] |
| 82. | Ariza MJ, Sánchez-Chaparro MA, Barón FJ, Hornos AM, Calvo-Bonacho E, Rioja J, Valdivielso P, Gelpi JA, González-Santos P. Additive effects of LPL, APOA5 and APOE variant combinations on triglyceride levels and hypertriglyceridemia: results of the ICARIA genetic sub-study. BMC Med Genet. 2010;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Coca-Prieto I, Kroupa O, Gonzalez-Santos P, Magne J, Olivecrona G, Ehrenborg E, Valdivielso P. Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass: identification of a novel GPIHBP1 mutation. J Intern Med. 2011;270:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis. 2008;197:1-11. [PubMed] |
| 85. | Martorell L, Gentile M, Rius J, Rodríguez C, Crespo J, Badimon L, Martínez-González J. The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol Cell Biol. 2009;29:5828-5842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 871] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 87. | Lichtenstein L, Kersten S. Modulation of plasma TG lipolysis by Angiopoietin-like proteins and GPIHBP1. Biochim Biophys Acta. 2010;1801:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O’Byrne SM, Kroupa O. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 89. | Ena J, Argente CR, Molina M, Gonzalez-Sanchez V, Alvarez CE, Lozano T. Infradiagnóstico de enfermedad arterial periférica en pacientes con diabetes mellitus atendidos en consultas de segundo nivel. Avances en Diabetología. 2013;29:175-181. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |