Published online Aug 15, 2013. doi: 10.4239/wjd.v4.i4.145
Revised: April 24, 2013
Accepted: June 18, 2013
Published online: August 15, 2013
Processing time: 162 Days and 11.2 Hours
AIM: To evaluate properties of bone quantity/quality using young non-obese Type 1 (T1D)-diabetic (NOD) prone and syngenic non-diabetic (NOD.scid) mice.
METHODS: Quantitative bone assessment of tibia was conducted using dual-energy X-ray absorptiometry (DXA) for the evaluation of body mass, bone mineral content, body fat mass and lean mass. Qualitative assessment was accomplished by three-point breakage for assessment of force to failure and micro-computed tomography for evaluation of trabecular and cortical properties of bone. In addition, fasting blood was evaluated prior to sacrifice at week eleven and fifteen to evaluate and compare glucose homeostasis between the strains of mice.
RESULTS: Our findings support a perturbation in the relationship between bone quantity, quality, and subsequently, the association between structure and strength. There were no differences in DXA-assessed body composition (body fat, % fat mass and lean mass) and bone composition (bone mineral content and bone mineral density) between strains. However, relative to NOD.scid, NOD mice had lower trabecular bone volume, relative trabecular bone volume, trabecular number and trabecular total material density (P < 0.05). Conversely, NOD mice had greater cortical total mean volume (P < 0.05). General linear models analysis adjusted for body weight revealed a significant contribution of T1D to bone health as early as 5 wk.
CONCLUSION: It is well-established that diabetes is a significant risk factor for increased fractures, although the underlying mechanisms are not fully understood. Investigation of bone parameters encompassing strength and structure early in the life course will facilitate the elucidation of the pathogenesis of impaired bone integrity.
Core tip: Diabetes-related impairment in bone microarchitectural properties and parameters of quality was apparent as early as 5 wk.
- Citation: Casazza K, Hanks LJ, Clines GA, Tse HM, Eberhardt AW. Diabetes-related impairment in bone strength is established early in the life course. World J Diabetes 2013; 4(4): 145-150
- URL: https://www.wjgnet.com/1948-9358/full/v4/i4/145.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i4.145
Apart from the classical complications of diabetes, adverse effects associated with bone health are becoming increasingly apparent. Individuals with diabetes have higher incidence of fracture and greater prevalence of osteoporosis. Many[1,2], but not all[3-7] investigations report low bone mineral content (BMC) and density (BMD). Fracture healing is also compromised in diabetics with as high as 87% recovery delay relative to “healthy” counterparts[8-10]. While the skeletal manifestations of dysregulated glucose metabolism have been primarily considered in terms of bone quantity (i.e., low bone mass), bone strength, the most obvious characteristic of bone structure/health is dependent upon various qualitative aspects.
A variety of animal models have been developed and used to examine the mechanisms of diabetes-related complications. Autoimmune-prone non-obese diabetic (NOD) mice are a widely studied model of spontaneous type 1 diabetes (T1D)[11-13]. In contrast to the pharmacologic streptozotocin (STZ)-induced T1D model, NOD mice become spontaneously diabetic secondary to a progressive diminished capacity of insulin-producing pancreatic beta islet cell function due to autoimmune destruction of the islet beta-cells. The earliest signs of autoimmune pathology in the NOD mouse occur at approximately 4 to 5 wk of age with leukocytes beginning to accumulate around the pancreatic islets, progressively intensifying and eventually leading to destruction of the insulin-producing beta cells at about 12 wk of age[14]. Whereas some studies have investigated bone phenotypes in adult NOD mice, the skeletal effects at disease initiation, to our knowledge have not been investigated. As a comparator strain, syngenic autoimmune-deficient NOD.scid mice lack functional lymphocytes, precluding the autoimmune destruction of beta cells and unlike NOD mice, do not develop T1D[11,13-16].
While insulin stimulates not only osteoblastic cell differentiation, but also osteoblastogenesis and thus plays a pivotal role in bone metabolism[17,18], an impairment in insulin regulation compromises bone processes giving rise to an altered phenotype. Accordingly, disease states related to insulin homeostasis/glucose handling might be expected to elicit profound physiologic alterations, particularly during growth[19]. The objective of the study was to evaluate properties of bone quantity (via DXA) and quality (via microCT, three point breakage) using young NOD vs NOD.scid mice, two different models in terms of insulin response to glucose. We hypothesized that as diabetes progressed in the NOD mice, the strength-structure impairments would manifest as illustrated by decreased force required to break, as well as trabecular and cortical bone measures.
Five-to-seven week-old female NOD (n = 24) and NOD.scid mice (n = 23) were bred and housed at the Research Support Building of the University of Alabama at Birmingham, under pathogen-free conditions and observing IACUC approved mouse protocols. Mice were kept under a normal diurnal cycle in a temperature-controlled room and were fed with standard chow (NIH316 formula, Purina item #5K52) from age of weaning.
For assessment of bone quantity by dual-energy X-ray absorptiometry (DXA) analysis, animals were briefly anesthetized with isoflurane (2%) and placed in a prostrate position on the imaging plate. BMD, bone mineral content (BMC) was assessed in vivo (GE-Lunar PIXImus, software version 1.45, GE-Lunar). Additionally, lean mass and fat mass as well as animal area was obtained at 5, 8, 11 and 15 wk.
For qualitative assessment, three point breakage and micro-computed tomography was used. Tibia strength was assessed by three-point breakage analysis using an MTS 858 MiniBionix (MTS Systems, Eden Prairie, MN) with a 100 N load cell. The span was 9 mm and the bones were loaded at a rate of 0.1 mm/s. For the determination of the 3D architecture of the trabecular and cortical bone, mouse femurs were scanned using the Scanco μCT40 desktop cone-beam micro-CT scanner (Scanco Medical AG, Brüttisellen, Switzerland). Femurs were placed vertically, but inverted (distal femur at the top) in 12 μm diameter scanning holders and scanned twice: one for cortical and one for trabecular bone. Scans were performed at the following settings: 12 mm resolution, 55 kvp, 145 μA with an integration time of 200 ms. Scans were automatically reconstructed into 2-D slices, and the region of interest was outlined in each slice using the micro-computed tomography (μCT) Evaluation Program (v5.0A, Scanco Medical). Cortical bone was determined at the mid-shaft of the femur with a scan of 25 slices. The region of interest (ROI) tool was used to outline the outside edge of the cortical bone. Cortical bone was identified and separated from the marrow by using a threshold value of 294. A 3D reconstruction was performed on the ROI consisting of everything within the outer cortical surface. Data was obtained for bone volume, (BV), bone density, total volume (TV) (bone plus marrow), bone volume fraction (BV/TV), trabecular thickness (Tb. Tk.), number (Tb. N) and cortical thickness. The scan of the trabecular bone was performed at the distal femur below the growth plate (on the inverted bone). Each scan consisted of 209 slices of which 100 were used for the analysis. ROI’s were drawn on each of the 100 slices just inside the cortical bone, to include only the trabecular bone and marrow. Trabecular bone threshold was set at 226 HU, to distinguish it from the marrow. The 3D reconstruction was performed on the ROI which only contained trabecular bone; no cortical bone was present in these ROI’s.
Analysis of variance (ANOVA) was used to determine differences in bone properties between strains. If an aging effect was apparent for a given parameter, a post hoc comparison was performed. Statistical significance was set at α < 0.05. In order to determine whether the strength-structure relationship was different, general linear models in which group, weight and body area were the covariates were conducted. All analyses were conducted using SAS (Institute Inc., Cary NC).
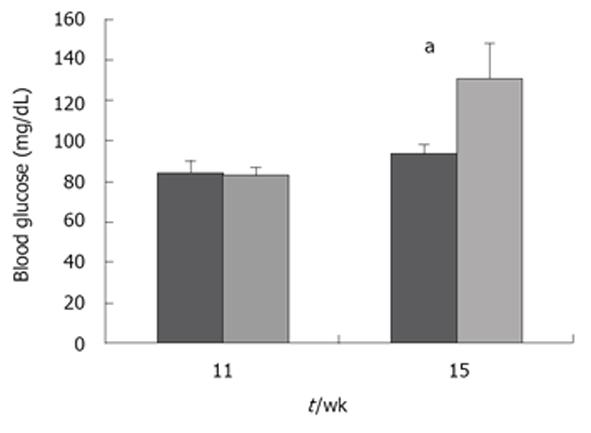
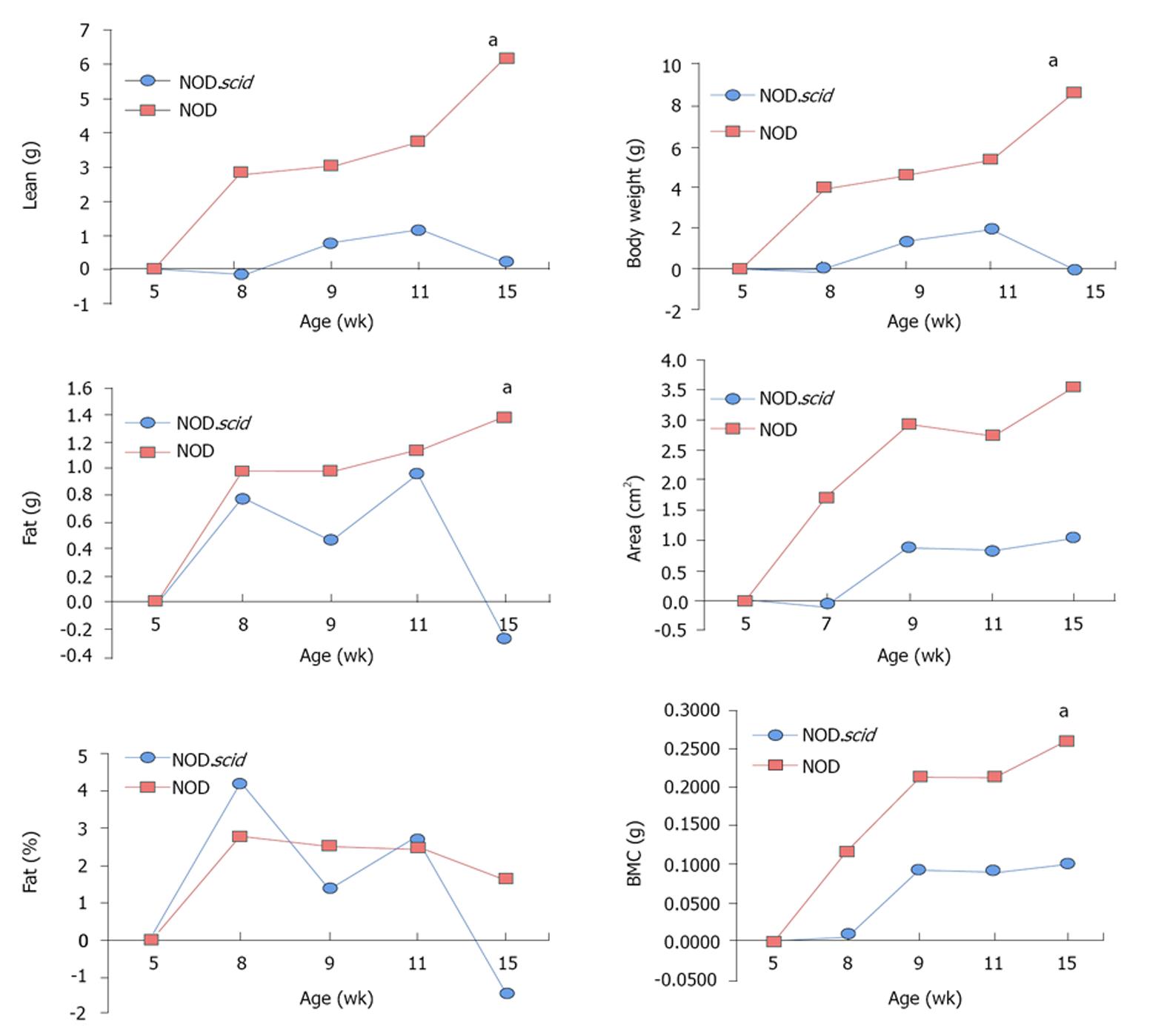
Serum glucose was collected from 11 and 15 wk old NOD and NOD.scid mice. As expected, NOD mice displayed increased glucose beginning at 15 wk (Figure 1) and is consistent with the phenotype of this autoimmune T1D mouse model[11-13]. The growth characteristics were then determined between NOD and NOD.scid mice. At 5 wk, NOD mice were smaller than NOD.scid as represented by significantly less total mass, lean mass, body weight. However, by 8 wk size and weight did not differ and the similarity in body dimensions. At 15 wk NOD mice were significantly heavier (Figure 2).
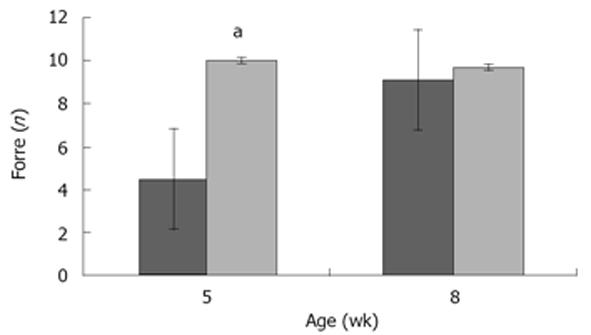
We next analyzed the bone mechanical properties of tibiae harvested from 5 and 8 wk old NOD and NOD.scid mice. In a three-point bending test, NOD mice demonstrated less mechanical strength than NOD.scid mice at 5 wk but not at 8 wk of age (Figure 3).
Body composition and bone parameters of 5 and 8 wk old NOD and NOD.scid mice were next examined (Table 1). The weight of the NOD and NOD.scid mice were similar (Table 1). There were no differences in body composition (body fat, fat mass and lean mass) and bone composition (bone mineral content and bone mineral density) were detected properties in NOD compared to NOD.scid mice (Table 1).
| Parameter | mean ± SEMNOD | NOD.scid |
| Body composition properties (DXA) | ||
| Body weight | 19.7 ± 0.6 | 19.9 ± 0.4 |
| BMC | 0.37 ± 0.40 | 0.34 ± 0.30 |
| BMD | 0.04 ± 0.04 | 0.040 ± 0.001 |
| Body fat | 2.8 ± 0.1 | 2.9 ± 0.2 |
| Lean mass | 14.7 ± 0.4 | 14.8 ± 0.2 |
| % Fat | 15.8 ± 0.7 | 16.2 ± 0.7 |
| Trabecular bone properties (microCT) | ||
| TV | 1.5 ± 0.1 | 1.5 ± 0.1 |
| BV | 0.10 ± 0.01 | 0.15 ± 0.011 |
| BV/TV | 0.06 ± 0.01 | 0.10 ± 0.011 |
| Tb. Th | 0.050 ± 0.002 | 0.050 ± 0.002 |
| Tb. N | 2.3 ± 0.2 | 2.80 ± 0.11 |
| Tb. MBV | 931.9 ± 20.1 | 922.9 ± 20.7 |
| Tb. TMD | 92.2 ± 8.8 | 124.5 ± 9.71 |
| Cortical bone properties (microCT) | ||
| TV | 0.19 ± 0.10 | 0.21 ± 0.01 |
| BV | 0.12 ± 0.02 | 0.120 ± 0.004 |
| BV/TV | 0.60 ± 0.01 | 0.60 ± 0.01 |
| Ct. TMV | 801.8 ± 22.5 | 770.4 ± 17.51 |
| Ct. TMD | 1288.5 ± 15.9 | 1291.6 ± 16.3 |
Because DXA can be an insensitive bone research technique, we performed microCT analyses to dissect the differences in trabecular and cortical bone. Tibiae were harvested from 5 and 8 wk old NOD and NOD.scid mice. Including all mice, there was not a significant difference in bending strength between NOD and NOD.scid mice. However, relative to NOD.scid, NOD mice had lower trabecular bone volume, relative trabecular bone volume, Tb.N, and trabecular total material density (P < 0.05) (Table 1). Conversely, NOD mice had greater cortical total mean volume (P < 0.05). We next performed a general linear models analysis that adjusted for body weight that revealed a significant contribution of age (Table 2). Accordingly, analysis was conducted by age. Table 2 presents the bone quality measures using microCT assessed at 5 and 8 wk. Diabetes-related impairment in bone microarchitectural properties and parameters of quality was apparent as early as 5 wk.
| 5 wk | 8 wk | |||
| NOD(n = 4) | NOD.scid(n = 4) | NOD(n = 6) | NOD.scid(n = 4) | |
| Body weight | 14.9 ± 0.2 | 19.2 ± 0.41 | 19.2 ± 0.5 | 19.9 ± 0.6 |
| BMC | 0.19 ± 0.01 | 0.30 ± 0.011 | 0.30 ± 0.02 | 0.30 ± 0.01 |
| BMD | 0.030 ± 0.001 | 0.040 ± 0 .002 | 0.040 ± 0.001 | 0.040 ± 0.001 |
| Tibia strength | 4.5 ± 0.2 | 10.0 ± 0.8 | 8.4 ± 0.7 | 8.2 ± 0.8 |
| Trabecular bone properties | ||||
| TV | 1.4 ± 0.2 | 1.6 ± 0.1 | 1.50 ± 0.06 | 1.40 ± 0.04 |
| BV | 0.08 ± 0.02 | 0.14 ± 0.01 | 0.11 ± 0.02 | 0.16 ± 0.05 |
| BV/TV | 0.05 ± 0.021 | 0.09 ± 0.00 | 0.07 ± 0.01 | 0.11 ± 0.041 |
| Tb. Tk | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.01 |
| Tb. N | 2.0 ± 0.31 | 2.8 ± 0.3 | 2.40 ± 0.09 | 2.90 ± 0.58 |
| Tb. TMV | 63.8 ± 4.9 | 108.9 ± 7.41 | 104.3 ± 8.9 | 137.0 ± 14.6 |
| Tb. TMD | 872.7 ± 42.5 | 930.5 ± 17.6 | 891.8 ± 21.3 | 948.8 ± 65.2 |
| Cortical bone properties | ||||
| TV | 0.18 ± 0.021 | 0.23 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.02 |
| BV | 0.10 ± 0.02 | 0.14 ± 0.02 | 0.12 ± 0.00 | 0.11 ± 0.01 |
| BV/TV | 0.60 ± 0.03 | 0.60 ± 0.01 | 0.63 ± 0.011 | 0.58 ± 0.01 |
| Ct. TMV | 743.5 ± 56.9 | 787.4 ± 21.5 | 831.0 ± 13.2 | 753.0 ± 16.21 |
| Ct. TMD | 1262.9 ± 18.9 | 1324.1 ± 20.8 | 1304.3 ± 11.0 | 1258.6 ± 13.4 |
There is a paucity of mechanistic information on how disease initiation and progression affect bone. While low bone mass in diabetes is often reported, material properties of bone, specifically those addressing initiation of impairment in material properties is lacking. The present study provides support of impaired bone structure/architecture with diabetes on bone via impairment of bone structure/architecture early in the life course.
Pre-diabetic NOD mice had lower trabecular properties, but greater cortical volume, suggesting compositional differences exist in tissue properties prior to disease progression. Nyman and colleagues recently reported a time-dependent alteration in matrix organization beginning approximately ten weeks after STZ injection, in line with consistent reports[20]. The mice in the study were 11 wk old when injected. While the authors noted a decrement in mineralization, they subsequently observed an increase in non-enzymatic collagen cross-linking[21]. It is possible that injections in close proximity or prior to rapid skeletal growth may lead to accelerated change in the strength-structure relationship. Further, a later-induced diabetic phenotype provokes skeletal phenotypes via different pathways and several conditions in both models may have indirect effects on the reported properties of bone. Notably, beyond those initiated with diabetic onset further changes in strength-structure relationship were not observed by Nyman and colleagues[20]. The increased Ct.MTV and Ct.TMD in NOD mice at 8 wk which did not translate into increased mechanical strength was surprising. Speculatively, a compensatory increase in insulin early in T1D prior to insulinitis may enhance anabolic properties at the outer surface. However, assessment of the strength-structure relationship requires evaluation of both outer and inner surfaces as well as the intrinsic properties within the bone (Ego Seeman, personal communication).
Particularly relevant during rapid skeletal growth, insulin has direct anabolic effects on periosteal apposition[17,18,21]. This would explain why diabetes did not affect the BMC or BMD, despite lower trabecular microarchitecture. In the context of humans, while it was recently reported that as adolescents with T1D attained reproductive maturation, had “normal” cortical cross sectional area[19], fracture risk remains greater among this population. It is important to note that it was recently reported that while mechanical properties of bone in humans with diabetes were impaired relative to non-diabetic controls, strength was not different between middle-aged and older adults with diabetes[22], supporting our findings of the deleterious effects on bone integrity initiated early in disease progression.
While NOD mice are used to examine spontaneous T1D progression, immune-deficient NOD.scid mice may incur indirect effects on bone properties, which may explain the reported differences in the literature. Lacking mature B and T cells, NOD.scid mice are both insulitis- and diabetes-free throughout life. However, because of a high incidence of thymic lymphomas, the mean lifespan is relatively short[13,23,24]. Accordingly, while the unique immune defects provide an excellent in vivo environment for hematopoietic investigation extending to effects within the marrow compartment, this model may not be suitable for assessing bone material properties. The long-term tumorigenic effects provoked an unanticipated effect on growth in NOD.scid mice that likely affected strength-structure relationship. Further investigation in a comparable strain with optimal growth conditions [e.g., non-obese diabetes resistant (NOR)] are needed to confirm our findings.
In conclusion, the T1D mouse model revealed complex changes early in the developmental process. There was diminished trabecular microarchitecture which manifested into weakened bone strength relative to non-diabetic mice, independent of bone mass. Our findings support a perturbation in the relationship between bone structure and strength and a need for intervention efforts to promote bone parameters during growth and development.
The authors wish to thank Sasanka Ramanadham, Jake Fletcher, William Hancock and Maria Johnson for their invaluable contribution and assistance in bone parameter and body composition assessment.
While the skeletal manifestations of dysregulated glucose metabolism have been primarily considered in terms of bone quantity (i.e., low bone mass), bone strength, the most obvious characteristic of bone structure/health is dependent upon various qualitative aspects.
Diabetes-related impairments in insulin/glucose handling alter growth processes including those associated with bone development. However, the precise mechanisms accounting for perturbed bone accretion processes are not known and may differ, at least in part, by degree of glucose control.
Maintenance of glucose in circulation within a “normal” range, as well as the standard of “normality” throughout dynamic growth processes, varies, and has particular relevance to body composition trajectories during critical periods of development.
Peak bone mass, a major determinant of adult bone health is largely achieved by the end of sexual and skeletal maturity. Thus, an emerging area of investigation is the contribution of insulin/glucose homeostasis to bone (re)modeling, with considerable interest in understanding influential factors serving to maximize bone mass accrual in childhood, and therefore optimize bone phenotype throughout life.
A potential explanation for observed differences in bone parameters in adults may be related to impairment in the remodeling-associated bone-resorption/formation coupling, which is maximally operational during the rapid skeletal development phase in childhood. Although the process of bone remodeling is complex, accumulating evidence supports glucose homeostasis as an integral part of bone formation (quantity) and micro-architecture (quality). Consistent with this, individuals exhibiting impaired glucose handling are at risk for compromised bone mass and integrity. In particular, type 1 diabetes mellitus is associated with increased bone mass loss (e.g., osteopenia, osteoporosis), increased risk of fragility fracture, and poor bone healing following injury. Investigation early in the life course is highly relevant.
P- Reviewers Robinson MK, Yin YW S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr. 2013;79:68-74. [PubMed] |
| 2. | Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complications. 2012;26:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab. 2013;98:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 325] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 4. | Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone--osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102:1343-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Merlotti D, Gennari L, Dotta F, Lauro D, Nuti R. Mechanisms of impaired bone strength in type 1 and 2 diabetes. Nutr Metab Cardiovasc Dis. 2010;20:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Yaturu S. Diabetes and skeletal health. J Diabetes. 2009;1:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Adami S. Bone health in diabetes: considerations for clinical management. Curr Med Res Opin. 2009;25:1057-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kawai M, de Paula FJ, Rosen CJ. New insights into osteoporosis: the bone-fat connection. J Intern Med. 2012;272:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Kachapati K, Adams D, Bednar K, Ridgway WM. The non-obese diabetic (NOD) mouse as a model of human type 1 diabetes. Methods Mol Biol. 2012;933:3-16. [PubMed] |
| 12. | Thayer TC, Wilson SB, Mathews CE. Use of nonobese diabetic mice to understand human type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:541-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Mouse Models for Type 1 Diabetes. Drug Discov Today Dis Models. 2009;6:41-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Wu J, Kakoola DN, Lenchik NI, Desiderio DM, Marshall DR, Gerling IC. Molecular phenotyping of immune cells from young NOD mice reveals abnormal metabolic pathways in the early induction phase of autoimmune diabetes. PLoS One. 2012;7:e46941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Hanson MS, Park EE, Sears ML, Greenwood KK, Danobeitia JS, Hullett DA, Fernandez LA. A simplified approach to human islet quality assessment. Transplantation. 2010;89:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Mellanby RJ, Koonce CH, Monti A, Phillips JM, Cooke A, Bikoff EK. Loss of invariant chain protects nonobese diabetic mice against type 1 diabetes. J Immunol. 2006;177:7588-7598. [PubMed] |
| 17. | Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 910] [Cited by in RCA: 824] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 18. | Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 574] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 19. | Bechtold S, Putzker S, Bonfig W, Fuchs O, Dirlenbach I, Schwarz HP. Bone size normalizes with age in children and adolescents with type 1 diabetes. Diabetes Care. 2007;30:2046-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR, Cockrell GE, Wahl EC, Bunn RC, Lumpkin CK, Fowlkes JL. Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone. 2011;48:733-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Nyman JS, Makowski AJ. The contribution of the extracellular matrix to the fracture resistance of bone. Curr Osteoporos Rep. 2012;10:169-1=77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Fleischli JG, Laughlin TJ, Athanasiou K, Lanctot DR, Lavery L, Wang X, Agrawal CM. Effect of diabetes mellitus on the material properties of the distal tibia. J Am Podiatr Med Assoc. 2006;96:91-95. [PubMed] |
| 23. | Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. 2008;Chapter 15:Unit 15.21. [PubMed] |
| 24. | Shultz LD, Banuelos S, Lyons B, Samuels R, Burzenski L, Gott B, Lang P, Leif J, Appel M, Rossini A. NOD/LtSz-Rag1nullPfpnull mice: a new model system with increased levels of human peripheral leukocyte and hematopoietic stem-cell engraftment. Transplantation. 2003;76:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |