Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.108245
Revised: June 4, 2025
Accepted: July 14, 2025
Published online: August 15, 2025
Processing time: 118 Days and 2.2 Hours
The specific mechanism of diabetic nephropathy (DN) has not been fully elu
To investigate the correlation between intestinal microbiota dysbiosis, low-grade inflammatory status, renal function impairment, and disease severity in older patients with DN, in order to provide a basis for the prevention and therapeutic intervention of DN.
We enrolled 167 older patients with DN, diagnosed in the Department of Neph
In the DN group, the Chao, Ace, and Shannon indices were significantly lower, while the Simpson index was significantly higher than the control group. The relative abundances of Bacteroides and Bifidobacterium were significantly lower, whereas the relative abundances of Clostridium, Butyricimonas, Klebsiella, Enterococcus, Veillonella, and Megamonas were significantly higher than those in the control group (P < 0.05). Estimated glomerular filtration rate was positively correlated with the Chao, Ace, and Shannon diversity indices of the gut microbiota, as well as with the relative abundances of Bacteroides, Bifidobacterium, and Akkermansia, and was negatively correlated with the relative abundances of Clostridium, Klebsiella, and Enterococcus (P < 0.05). Logistic regression analysis indicated that lower Chao, Ace, and Shannon indices and higher Simpson index were associated with an increased risk of developing DN. After one year of follow-up, patients in the progression group exhibited a significantly greater decrease in Chao, Ace, and Shannon indices and a greater increase in Simpson index than the stable group. The reduction in the relative abundances of Bacteroides, Clostridium, Bifidobacterium, and Butyricimonas, as well as the increase in Klebsiella, Enterococcus, Veillonella, and Megamonas, were significantly more pronounced in the progression group than in the stable group (P < 0.05). Regression analysis indicated that greater declines in Chao, Ace, and Shannon indices and Bacteroides relative abundance, along with greater increases in Simpson index and Enterococcus relative abundance, were associated with a more rapid decline in renal function.
The onset and progression of DN in older patients with diabetes are closely associated with gut microbiota composition. The more severe the dysbiosis, the lower the abundance of beneficial bacteria and the higher the abundance of harmful bacteria, leading to an increased risk of both DN occurrence and disease progression.
Core Tip: At present, there are many studies on the relationship between intestinal microbiota and inflammation level and the development of diabetes, but the relationship between intestinal microbiota and the development of diabetic nephropathy (DN) is still unclear. This study investigated elderly patients with DN and revealed that they have dysbiosis of the gut microbiota and inflammatory response, which worsen with disease progression. We expect that these results will provide reference for the prevention and treatment of secondary DN in diabetic patients.
- Citation: Shi YP, Pan ZL, Zhang J, Xue LY, Li MQ. Gut dysbiosis, low-grade inflammation, and renal impairment severity in elderly diabetic nephropathy. World J Diabetes 2025; 16(8): 108245
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/108245.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.108245
Diabetic nephropathy (DN) is the most common microvascular complication in patients with diabetes and is generally considered to be a long-term hyperglycemia-related condition. Hyperglycemia causes increased renal vascular pressure in the microvasculature, ultimately leading to altered renal structure and function[1]. Metabolic disorders, unhealthy dietary patterns, oxidative stress, and inflammatory cytokines have all been confirmed to perpetuate the onset and progression of DN[2]. However, the exact mechanisms underlying DN remain incompletely understood, and further research is needed to provide a basis for the prevention and development of targeted therapeutic strategies in patients with diabetes[3]. Multiple studies have shown that gut microbiota is involved not only in the digestion and absorption of food but also closely related to the host’s metabolic function and immune system regulation. Gut microbiota dysbiosis has been found to be associated with the progression of diabetes, as well as the development of cardiovascular and cerebrovascular diseases and immune-mediated disorders[4,5]. Low-grade inflammation is characterized by a persistent, systemic, low-intensity inflammatory state, primarily mediated by continuous activation of the monocyte-macrophage system. This condition is commonly observed in patients with diabetes, and recent evidence indicates that the onset and progression of DN are closely associated with this chronic inflammatory process[6]. Although numerous studies have explored the associations among gut microbiota composition, low-grade inflammation, and DN pathogenesis, the precise mechanisms through which these factors contribute to DN progression remain incompletely understood. To address this knowledge gap, the present study systematically investigates the role of gut microbial communities and the dynamics of low-grade inflammation in the development and progression of DN, aiming to provide a theoretical basis for the prevention and therapeutic intervention of diabetes-related kidney disease.
This study included 252 patients with type 2 diabetes diagnosed in our hospital between June 2020 and June 2023. DN was diagnosed according to the National Guidelines for the Prevention and Treatment of Diabetes at the Grassroots Level (2018)[7], with an estimated glomerular filtration rate (eGFR) ≤ 90 mL/minute considered as the diagnostic criteria. A total of 85 patients with eGFR ≥ 90 mL/minute were assigned to the control group, while 167 patients with eGFR < 90 mL/minute were assigned to the DN group. All enrolled patients received standard treatment, including blood glucose control, blood pressure management, dietary adjustment, lipid regulation, and management of proteinuria. During the one-year follow-up, the difference in eGFR between the enrollment date and the one-year mark was calculated. A decline in eGFR of ≥ 5 mL/minute/year was defined as disease progression. Based on this criterion, patients were divided into the progression (n = 118) and the stable group (n = 134).
The inclusion criteria were as follows: (1) Age ≥ 60 years; (2) The eGFR > 30 mL/minute; (3) Meet the diagnostic criteria for type 2 diabetes mellitus as per the “Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes Mellitus (2020)”[8]; (4) Duration of diabetes ≥ 5 years; (5) No history of mental illness or cognitive dysfunction; and (6) A comprehensive understanding of the research program and voluntary participation in this study, accepting the relevant clinical and laboratory evaluation.
We established the following exclusion criteria: (1) Patients with primary renal disease; (2) Patients with a history of renal surgery, renal trauma, or nephrotoxic drug administration; (3) Patients with severe dysfunction of important organs such as the heart, liver, and lungs; (4) Those with immune system disorder, hematological and severe infectious diseases, and malignant tumors; (5) Patients who had taken medications affecting the gut microbiota within 3 months prior to the start of the study; (6) Patients with a history of gastrointestinal surgery or severe gastrointestinal diseases; and (7) Patients who had taken medications affecting renal function within two weeks prior to the start of the study.
General data: We collected general patient data on the day of enrollment, including age, body mass index, sex, duration of diabetes, history of smoking/drinking, and blood pressure.
Intestinal flora detection: Fresh fecal samples (7-10 g) were collected from the central portion of the stool using sterile fecal collection containers, avoiding surface contamination, on the day of enrollment and again after 1 year (365 ± 5 days). Samples were labeled and stored at -80 °C until analysis. The intestinal microbiota composition was assessed using real-time fluorescence quantitative polymerase chain reaction (PCR). Total DNA was extracted using the QIAamp DNA Stool Mini Kit, with proteinase digestion performed in a 56 °C water bath for 1 hour. Quality control criteria included an A260/280 ratio of 1.8-2.0 as measured by a Nanodrop spectrophotometer, and intact DNA bands without degradation as verified by agarose gel electrophoresis. PCR Amplification and Library Construction: The 16S ribosomal RNA gene was amplified using PCR, targeting the V3-V4 hypervariable regions with universal primers. The forward primer was 5’-CCTACGGGNGGCWGCAG-3’, and the reverse primer was 5’-GACTACHVGGGTATCTAATCC-3’. First-round PCR amplification was performed under the following thermal cycling conditions: Initial denaturation at 95 °C for 3 minutes; followed by 40 cycles of denaturation at 94 °C for 15 seconds, annealing at 50 °C for 30 seconds, and extension at 70 °C for 30 seconds. Second-round PCR amplification was conducted to add Illumina adapter and index sequences, with eight additional cycles to minimize over-amplification. Sequencing was performed on the Illumina NovaSeq platform using paired-end 300 bp reads. Low-quality sequences were filtered using QIIME2, and taxonomic annotation of microbial communities was conducted by aligning reads against the Silva reference database. QIIME2 was also used to calculate α-diversity, including microbial richness (Chao1 and Ace) and diversity indices (Shannon and Simpson). β-diversity was assessed based on Bray-Curtis distance. Using the formula: Relative abundance = number of sequences assigned to a given taxon/total number of sequences. Quality control criteria included: A minimum of 50000 high-quality reads per sample, sequence alignment similarity ≥ 95% for taxonomic annotation, and a Pearson correlation coefficient (R²) ≥ 0.95 between technical replicates. Using the formula: Change value = value at enrollment-value at one-year follow-up. To calculate the changes in gut microbiota indicators from baseline to one-year follow-up in patients.
Detection of glucose and lipid metabolism indicators: On the day of enrollment and at the one-year follow-up (365 ± 5 days), fasting blood glucose (FBG) and 2-hour postprandial blood glucose (PBG) were measured using the glucose oxidase method. Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography, and fasting insulin (FINS) was measured by chemiluminescent immunoassay. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the formula: HOMA-IR = (FBG × FINS)/22.5. Lipid metabolism indicators, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and free fatty acids (FFA), were measured using a fully automatic biochFemical analyzer (Mindray BS-390).
Microinflammatory indicator detection: The patients were followed up for 1 year (365 ± 5 days). We collected 5 mL of peripheral venous blood from the patients and separated the serum by centrifugation at 3500 rpm for 15 minutes with a radius of 12 cm. We assessed the concentrations of serum C-reactive protein (CRP), tumor necrosis factor α (TNF-α), and interleukin (IL)-6 by enzyme-linked immunosorbent assay using the CRP (Zhongsheng Beikong Biotechnology Co., Ltd., Jingji Zhun 20162400692), TNF-α (Suzhou Changguanghua Doctor Medical Engineering Co., Ltd., Guoji Zhun 20153401473), and IL-6 (Ruilai Biotechnology Jiangsu Co., Ltd., Suji Zhun 20202400651) test kits, respectively. We investigated IL-1β using an immunoturbidimetric assay kit purchased from Jidan Biotechnology Co., Ltd., Suzu Injection 20232401747.
Renal function index detection: At enrollment and at one-year follow-up (365 ± 5 days), we measured cystatin C (Cys-C), serum creatinine (Scr), and blood urea nitrogen (BUN) levels using an automatic biochemistry analyzer (Mindray, BS-390). The assays were performed using Cys-C (Wuhan Zhongtai Biotechnology Co., Ltd., registration number 20132401915), Scr (Hebei Aichi Biotechnology Co., Ltd., registration number 20172400112), and BUN (Lanyi Technology Group Co., Ltd., registration number 20142400123) reagent kits.
At enrollment and at one-year follow-up (365 ± 5 days), we collected 5 mL of morning urine and measured urinary microalbumin and creatinine levels using an immunoturbidimetric method. We calculated the urinary albumin-to-creatinine ratio (ACR) using urinary microalbumin (Shanghai Kaichuang Biotechnology Co., Ltd., registration number 2014240034) and creatinine (Suzhou Boyuan Medical Technology Co., Ltd., registration number 20172400351) reagent kits.
We analyzed the data using the statistical software SPSS 27.0. All the measured parameters followed a normal distribution. We used the mean ± SD description as well as t-test for comparisons between two groups. One-way analysis of variance was used to compare the three groups. The least-significant difference test was used for comparisons among the three groups. We described the count data as n (%) and used the χ2 test for comparisons between groups. Analysis of covariance was used to adjust for baseline differences in age, sex, and disease duration. Repeated measures analysis of variance was used to evaluate time effects and interaction effects between groups. Logistic regression analysis was employed to identify factors associated with the occurrence and progression of DN. A P value < 0.05 was considered statistically significant.
The average age and diabetes duration were significantly higher in the DN group than that in the control group (P < 0.05). However, we observed no significant differences between the two groups concerning sex distribution, body mass index, smoking and drinking proportions, and systolic and diastolic blood pressures (P > 0.05) (Table 1).
| Variables | DN group (n = 167) | Control group (n = 85) | t/χ2 | P value |
| Age (year), mean ± SD | 73.34 ± 6.10 | 69.81 ± 5.92 | 2.901 | 0.008 |
| Gender | 0.757 | 0.354 | ||
| Male (example) | 98 (58.68) | 45 (52.94) | ||
| Female (example) | 69 (41.32) | 40 (47.06) | ||
| BMI (kg/m2), mean ± SD | 24.20 ± 1.96 | 23.81 ± 2.10 | 1.458 | 0.146 |
| Course of diabetes (years), mean ± SD | 10.72 ± 3.18 | 8.95 ± 2.64 | 2.741 | 0.015 |
| Smoking (example) | 59 (35.33) | 38 (44.71) | 2.092 | 0.148 |
| Drinking alcohol (example) | 55 (32.93) | 36 (42.35) | 2.166 | 0.141 |
| Systolic pressure (mmHg), mean ± SD | 128.34 ± 7.31 | 126.51 ± 7.44 | 1.868 | 0.063 |
| Diastolic pressure (mmHg), mean ± SD | 78.54 ± 6.60 | 76.80 ± 6.92 | 1.946 | 0.053 |
Patients in the DN group showed lower Chao, Ace, and Shannon indices of gut microbiota compared to the control group, while the Simpson index was higher than that of the control group (P < 0.05) (Table 2). In the DN group, the relative abundances of Bacteroides and Bifidobacterium were significantly lower than those in the control group, while the relative abundances of Clostridium, Butyricimonas, Klebsiella, Enterococcus, Veillonella, and Megamonas were significantly higher (P < 0.05). There were no statistically significant differences between the two groups in the relative abundances of Blautia, Prevotella, Rareactinobacteria, Trichococcus, Roseburia, or Escherichia-Shigella (P > 0.05) (Table 3).
| Variables | DN group (n = 167) | Control group (n = 85) | t | P value |
| Chao | 55.10 ± 8.56 | 68.85 ± 11.31 | -10.789 | 0.000 |
| Ace | 57.26 ± 12.61 | 72.97 ± 16.26 | -8.457 | 0.000 |
| Simpson | 0.34 ± 0.09 | 0.29 ± 0.07 | 4.402 | 0.000 |
| Shannon | 1.38 ± 0.18 | 1.48 ± 0.23 | -3.745 | 0.000 |
| Variables | DN group (n = 167) | Control group (n = 85) | t | P value |
| Bacteroides (%) | 18.44 ± 3.64 | 21.76 ± 3.69 | -6.811 | 0.000 |
| Fusobacterium (%) | 7.88 ± 2.13 | 4.00 ± 1.58 | 14.883 | 0.000 |
| Blautia (%) | 8.37 ± 1.12 | 8.59 ± 1.10 | -1.487 | 0.138 |
| Bifidobacterium (%) | 2.09 ± 0.55 | 4.30 ± 0.88 | -24.326 | 0.000 |
| Prevotella (%) | 3.42 ± 0.39 | 3.46 ± 0.46 | -0.725 | 0.469 |
| Alloprevotella (%) | 2.59 ± 0.39 | 2.60 ± 0.42 | -0.124 | 0.901 |
| Lachnospira (%) | 2.35 ± 0.49 | 2.52 ± 0.48 | -2.697 | 0.007 |
| Roseburia (%) | 2.37 ± 0.44 | 2.29 ± 0.35 | 1.378 | 0.170 |
| Butyricimonas (%) | 5.46 ± 0.84 | 5.87 ± 0.39 | -1.500 | 0.135 |
| Escherichia-Shigella (%) | 1.40 ± 0.30 | 1.44 ± 0.29 | -1.085 | 0.279 |
| Klebsiella (%) | 4.22 ± 0.30 | 2.51 ± 0.33 | 42.544 | 0.000 |
| Enterococcus (%) | 3.91 ± 0.42 | 2.39 ± 0.19 | 31.516 | 0.000 |
| Veillonella (%) | 2.87 ± 0.57 | 2.23 ± 0.39 | 9.321 | 0.000 |
| Megamonas (%) | 2.33 ± 0.51 | 1.68 ± 0.29 | 10.766 | 0.000 |
Patients in the DN group had higher levels of FBG, PBG, HbA1c, FINS, HOMA-IR, TC, TG, LDL-C, and FFA compared to the control group, while HDL-C levels were lower (P < 0.05) (Table 4).
| Variables | DN group (n = 167) | Control group (n = 85) | t | P value |
| FBG (mmol/L) | 8.14 ± 0.60 | 7.38 ± 0.61 | 9.290 | 0.000 |
| PBG (mmol/L) | 11.58 ± 1.39 | 10.39 ± 0.96 | 7.067 | 0.000 |
| HbA1c (%) | 10.68 ± 1.47 | 8.53 ± 0.86 | 12.435 | 0.000 |
| FINS (pmol/mL) | 36.96 ± 8.84 | 27.52 ± 4.99 | 9.136 | 0.000 |
| HOMA-IR | 13.39 ± 3.41 | 9.03 ± 1.79 | 11.019 | 0.000 |
| TC (mmol/L) | 6.58 ± 2.19 | 6.08 ± 1.33 | 1.889 | 0.060 |
| TG (mmol/L) | 2.47 ± 0.68 | 1.90 ± 0.41 | 7.182 | 0.000 |
| LDL-C (mmol/L) | 3.66 ± 0.75 | 3.11 ± 0.52 | 6.112 | 0.000 |
| HDL-C (mmol/L) | 0.61 ± 0.12 | 0.80 ± 0.14 | -11.578 | 0.000 |
| FFA (mmol/L) | 0.85 ± 0.39 | 0.68 ± 0.31 | 3.620 | 0.000 |
Patients in the DN group exhibited higher levels of CRP, TNF-α, IL-6, and IL-1β compared to the control group (P < 0.05) (Table 5).
| Variables | DN group (n = 167) | Control group (n = 85) | t | P value |
| CRP (mg/L) | 7.83 ± 2.39 | 3.75 ± 1.28 | 14.662 | 0.000 |
| TNF-α (μg/L) | 44.64 ± 8.96 | 27.52 ± 5.37 | 16.186 | 0.000 |
| IL-6 (μg/L) | 154.42 ± 31.61 | 83.29 ± 16.14 | 19.480 | 0.000 |
| IL-1β (μg/L) | 69.67 ± 19.14 | 27.06 ± 8.18 | 19.617 | 0.000 |
Patients in the DN group had lower eGFR levels and higher levels of Cys-C, Scr, BUN, and ACR compared to the control group (P < 0.05) (Table 6).
| Variables | DN group (n = 167) | Control group (n = 85) | t | P value |
| eGFR (mL/minute) | 79.67 ± 8.77 | 115.22 ± 10.17 | -27.163 | 0.000 |
| Cys-C (mg/L) | 5.98 ± 2.57 | 3.80 ± 0.98 | 7.554 | 0.000 |
| Scr (μmol/L) | 95.48 ± 16.27 | 66.81 ± 11.51 | 14.494 | 0.000 |
| BUN (mmol/L) | 8.60 ± 2.36 | 6.56 ± 1.71 | 7.108 | 0.000 |
| ACR (mg/g) | 289.93 ± 105.69 | 160.73 ± 33.62 | 10.982 | 0.000 |
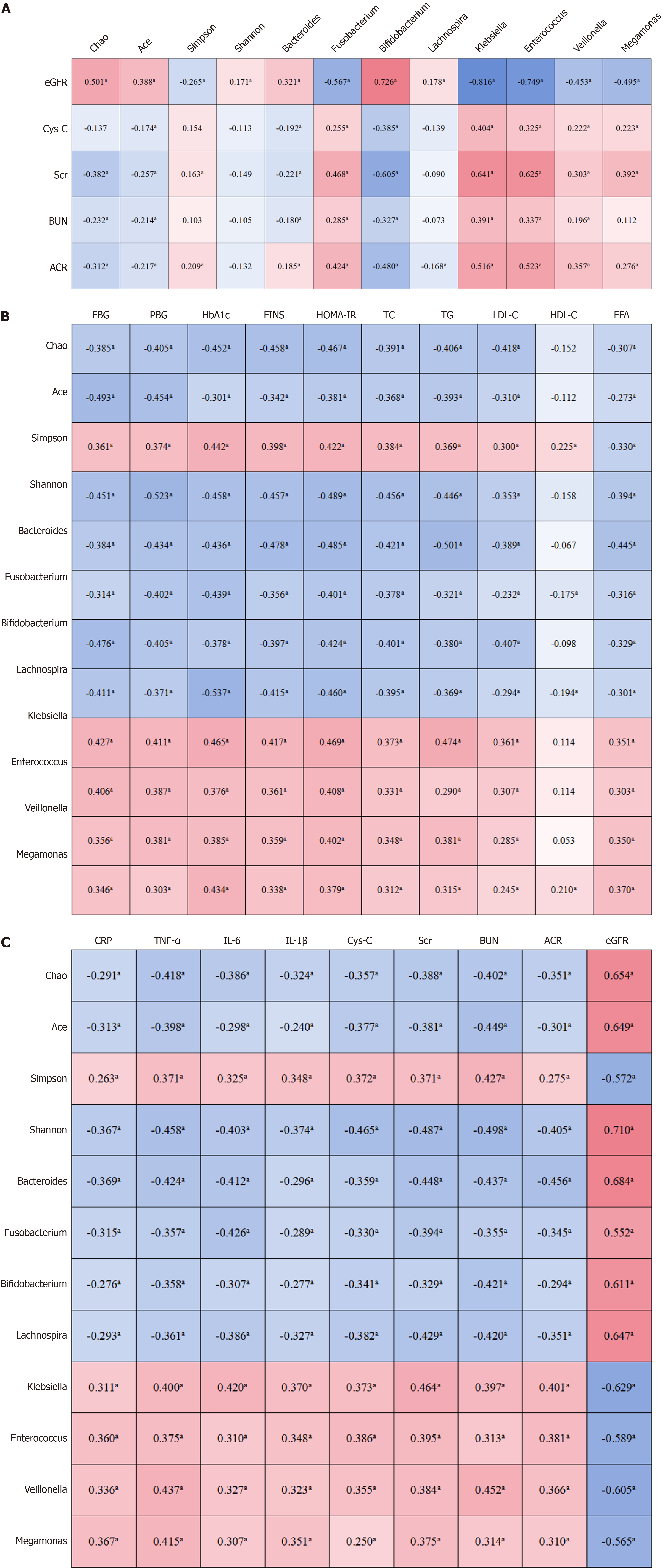
The Chao, Ace, and Shannon indices of gut microbiota were positively correlated with eGFR levels. The relative abundances of Bacteroides, Bifidobacterium, and Lachnospira were also positively correlated with eGFR, whereas the relative abundances of Fusobacterium, Klebsiella, Enterococcus, Veillonella, and Megamonas were negatively correlated with eGFR (P < 0.05) (Figure 1A).
The presence of DN was coded as 1, and absence as 0. Logistic regression analysis showed that lower Chao, Ace, and Shannon indices, higher Simpson index, lower relative abundances of Bacteroides, Bifidobacterium, and Helicobacter, and higher relative abundances of Clostridium, Klebsiella, Enterococcus, Veillonella, and Megamonas were associated with an increased risk of developing DN in patients with diabetes (P < 0.05) (Table 7).
| Variables | β | SE | Z | P | OR (95%CI) |
| Chao | -0.143 | 0.019 | -7.440 | 0.000 | 0.866 (0.834-0.900) |
| Ace | -0.079 | 0.012 | -6.668 | 0.000 | 0.927 (0.903-0.946) |
| Simpson | 7.090 | 1.728 | 4.103 | 0.000 | 9.623 (4.570-17.138) |
| Shannon | -2.516 | 0.714 | -3.522 | 0.000 | 0.081 (0.020-0.328) |
| Bacteroides | -0.295 | 0.049 | -6.034 | 0.000 | 0.745 (0.677-0.819) |
| Fusobacterium | 1.086 | 0.140 | 7.765 | 0.000 | 2.964 (2.253-3.899) |
| Bifidobacterium | -4.255 | 0.662 | -6.427 | 0.000 | 0.014 (0.004-0.052) |
| Lachnospira | -3.789 | 0.508 | -7.457 | 0.000 | 0.234 (0.038-0.763) |
| Klebsiella | 9.569 | 3.291 | 3.211 | 0.000 | 14.404 (3.428-28.438) |
| Enterococcus | 5.807 | 5.611 | 2.016 | 0.000 | 9.622 (2.273-16.725) |
| Veillonella | 2.403 | 0.353 | 7.014 | 0.000 | 5.859 (2.942-9.669) |
| Megamonas | 3.663 | 0.496 | 7.383 | 0.000 | 7.975 (4.739-13.062) |
The progression group exhibited a greater decrease in Chao, Ace, and Shannon indices, and a greater increase in the Simpson index compared to the stable group (P < 0.05) (Table 8). The progression group showed a greater decrease in the relative abundances of Bacteroides, Clostridium, Bifidobacterium, and Butyricimonas, and a greater increase in the relative abundances of Klebsiella, Enterococcus, Veillonella, and Megamonas compared to the stable group (P < 0.05) (Table 9).
| Variables | Progress group (n = 118) | Stable group (n = 134) | t | P value |
| Chao | 4.21 ± 2.12 | 1.44 ± 1.89 | 11.006 | 0.000 |
| Ace | 4.51 ± 2.60 | 1.46 ± 2.16 | 10.146 | 0.000 |
| Simpson | -0.21 ± 0.01 | -0.01 ± 0.01 | -9.722 | 0.000 |
| Shannon | 0.10 ± 0.04 | 0.03 ± 0.05 | 12.177 | 0.000 |
| Variables | Progress group (n = 118) | Stable group (n = 134) | t | P value |
| Bacteroides (%) | 1.42 ± 0.67 | 0.39 ± 0.63 | 12.375 | 0.000 |
| Fusobacterium (%) | 0.45 ± 0.31 | 0.15 ± 0.24 | 8.417 | 0.000 |
| Bifidobacterium (%) | 0.19 ± 0.12 | 0.05 ± 0.11 | 9.923 | 0.000 |
| Butyricimonas (%) | 0.32 ± 0.16 | 0.11 ± 0.17 | 10.183 | 0.000 |
| Klebsiella (%) | -0.23 ± 0.12 | -0.06 ± 0.07 | -9.859 | 0.000 |
| Enterococcus (%) | -0.21 ± 0.12 | -0.08 ± 0.11 | -8.403 | 0.000 |
| Veillonella (%) | -0.16 ± 0.09 | -0.06 ± 0.07 | -9.503 | 0.000 |
| Megamonas (%) | -0.13 ± 0.08 | -0.05 ± 0.07 | -7.965 | 0.000 |
Patients in the progression group showed greater increases in FBG, PBG, HbA1c, FINS, HOMA-IR, TC, TG, LDL-C, and FFA levels compared to the stable group, while the decrease in HDL-C was smaller than that observed in the stable group (P < 0.05) (Table 10).
| Variables | Progress group (n = 118) | Stable group (n = 134) | t | P value |
| FBG (mmol/L) | -0.51 ± 0.22 | -0.16 ± 0.24 | -11.635 | 0.000 |
| PBG (mmol/L) | -0.72 ± 0.35 | -0.21 ± 0.38 | -11.253 | 0.000 |
| HbA1c (%) | -0.64 ± 0.30 | -0.19 ± 0.35 | -10.798 | 0.000 |
| FINS (pmol/mL) | -4.62 ± 3.98 | 1.55 ± 3.87 | -12.523 | 0.000 |
| HOMA-IR | -2.44 ± 1.61 | 0.29 ± 1.51 | -13.897 | 0.000 |
| TC (mmol/L) | -0.73 ± 0.60 | 0.34 ± 0.71 | -12.609 | 0.000 |
| TG (mmol/L) | -0.34 ± 0.27 | 0.14 ± 0.24 | -13.969 | 0.000 |
| LDL-C (mmol/L) | -0.43 ± 0.32 | 0.07 ± 0.37 | -11.596 | 0.000 |
| HDL-C (mmol/L) | 0.01 ± 0.07 | 0.06 ± 0.08 | -6.113 | 0.000 |
| FFA (mmol/L) | -0.12 ± 0.12 | 0.03 ± 0.11 | -10.743 | 0.000 |
Patients in the progression group exhibited greater increases in CRP, TNF-α, IL-6, and IL-1β levels compared to the stable group (P < 0.05) (Table 11).
| Variables | Progress group (n = 118) | Stable group (n = 134) | t | P value |
| CRP (mg/L) | -1.15 ± 1.07 | 0.39 ± 1.02 | -11.420 | 0.000 |
| TNF-α (μg/L) | -6.30 ± 4.08 | 6.45 ± 6.44 | -16.091 | 0.000 |
| IL-6 (μg/L) | -28.69 ± 23.63 | 17.64 ± 25.37 | -14.936 | 0.000 |
| IL-1β (μg/L) | -7.74 ± 9.80 | 6.39 ± 5.84 | -11.399 | 0.000 |
Patients in the progression group showed a greater decrease in eGFR and greater increases in Cys-C, Scr, BUN, and ACR compared to the stable group (P < 0.05) (Table 12).
| Variables | Progress group (n = 118) | Stable group (n = 134) | t | P value |
| eGFR (mL/minute) | 7.02 ± 1.10 | 2.65 ± 1.43 | -13.235 | 0.000 |
| Cys-C (mg/L) | -0.98 ± 0.86 | 0.44 ± 0.81 | -14.554 | 0.000 |
| Scr (μmol/L) | -13.13 ± 10.27 | 5.17 ± 9.65 | -16.448 | 0.000 |
| BUN (mmol/L) | -1.62 ± 1.19 | 0.83 ± 1.16 | -13.621 | 0.000 |
| ACR (mg/g) | -61.04 ± 53.67 | 24.66 ± 46.82 | 27.248 | 0.000 |
From enrollment to the one-year follow-up, the changes in FBG, PBG, HbA1c, FINS, HOMA-IR, TC, TG, LDL-C, and FFA levels were negatively correlated with changes in the Chao, ACE, and Shannon indices, as well as with changes in the relative abundances of Bacteroides, Fusobacterium, Bifidobacterium, and Lachnospira; and positively correlated with changes in the Simpson index and the relative abundances of Klebsiella, Enterococcus, Veillonella, and Megamonas (P < 0.05). Changes in HDL-C levels were positively correlated with changes in the Simpson index and the relative abundance of Megamonas, and negatively correlated with changes in the relative abundances of Fusobacterium and Lachnospira (p < 0.05) (Figure 1B).
From enrollment to the one-year follow-up, the changes in CRP, TNF-α, IL-6, IL-1β, Cys-C, Scr, BUN, and ACR were negatively correlated with changes in the Chao, Ace, and Shannon indices, as well as with changes in the relative abundances of Bacteroides, Fusobacterium, Bifidobacterium, and Lachnospira; and positively correlated with changes in the Simpson index and the relative abundances of Klebsiella, Enterococcus, Veillonella, and Megamonas (P < 0.05). Changes in eGFR were also negatively correlated with changes in the Chao, Ace, and Shannon indices, and with the relative abundances of Bacteroides, Fusobacterium, Bifidobacterium, and Lachnospira, while positively correlated with changes in the Simpson index and the relative abundances of Klebsiella, Enterococcus, Veillonella, and Megamonas (P < 0.05) (Figure 1C).
Progression within one year was coded as 1, and no progression as 0. Logistic regression analysis showed that greater decreases in the Chao, Ace, and Shannon indices, as well as in the relative abundance of Bacteroides, and greater increases in the Simpson index and the relative abundance of Enterococcus over one year were associated with a higher risk of disease progression (P < 0.05) (Table 13).
| Variables | β | SE | Z | P value | OR (95%CI) |
| Chao | 0.308 | 0.147 | 4.393 | 0.036 | 1.361 (1.020-1.815) |
| Ace | 0.285 | 0.133 | 4.622 | 0.032 | 1.330 (1.026-1.725) |
| Simpson | -0.768 | 2.231 | 5.145 | 0.023 | 1.495 (1.042-1.898) |
| Shannon | 0.553 | 7.167 | 1.111 | 0.292 | 1.201 (0.872-1.405) |
| Bacteroides | 1.831 | 0.503 | 13.245 | 0.000 | 6.240 (2.328-16.729) |
| Fusobacterium | 1.098 | 1.222 | 0.807 | 0.369 | 2.998 (0.273-32.898) |
| Bifidobacterium | 2.095 | 2.505 | 0.699 | 0.403 | 8.122 (0.006-11.422) |
| Lachnospira | 3.383 | 1.969 | 2.952 | 0.086 | 29.449 (0.621-95.825) |
| Klebsiella | -4.635 | 2.492 | 3.458 | 0.063 | 0.001 (0.000-1.284) |
| Enterococcus | -7.111 | 2.794 | 6.478 | 0.011 | 0.001 (0.000-0.195) |
| Veillonella | -6.936 | 3.562 | 3.792 | 0.051 | 0.001 (0.000-1.046) |
| Megamonas | -4.320 | 3.937 | 1.204 | 0.273 | 0.013 (0.000-9.843) |
To date, the mechanisms underlying the onset and progression of DN are not yet fully understood. In most cases, DN develops secondary to diabetes and is typically asymptomatic in its early stages, with no obvious clinical signs or symptoms[9]. Previous studies have shown that older age, longer duration of diabetes, and female sex are associated with a higher risk of developing DN[10,11]. The findings of the present study are consistent with previous results, as patients in the DN group had a higher average age and longer diabetes duration compared to those without DN. This may be attributed to the fact that prolonged disease duration increases the impact of elevated blood lipids and lipid deposition, which leads to sustained stimulation of the glomerular basement membrane and alterations in microvascular tension, thereby increasing the risk of renal damage[12]. Additionally, advanced age is associated with a higher risk of DN, partly due to age-related physiological decline, and partly because older diabetic patients tend to have a longer overall disease duration[13]. However, this study did not observe a significant difference in sex distribution between patients with and without DN. This may be attributed to the fact that all enrolled patients had a diabetes duration of ≥ 5 years, and the relatively long disease course may have attenuated sex-related differences.
Human gut harbor a vast and diverse microbial community, and numerous recent studies have highlighted the bidirectional interactions between the gut and the kidneys. Chen et al[14] reported that kidney disease can lead to gut microbiota dysbiosis and impaired intestinal barrier function. Conversely, disruption of gut homeostasis promotes the production of gut-derived toxins, which may trigger systemic inflammatory responses and further exacerbate renal impairment. Multiple studies have demonstrated that patients with DN exhibit lower Chao, Ace, and Shannon indices compared to diabetic patients without DN, whereas the Simpson index is significantly higher in DN patients[15,16]. The findings of this study are consistent with the above-mentioned research, confirming that patients with DN have reduced gut microbial richness, diversity, and stability, along with a greater degree of imbalance in dominant bacterial species. Furthermore, this study revealed that the relative abundances of beneficial bacteria such as Bacteroides and Bifidobacterium were lower in DN patients, while the relative abundances of potentially harmful bacteria, including Clostridium, Klebsiella, and Enterococcus, were higher compared to diabetic patients without DN. Similar findings were reported in the study by Sun et al[17], where an imbalance in gut microbial homeostasis was observed in DN patients, characterized by a decrease in beneficial bacteria and an increase in harmful bacteria. This result suggested that the increase in pathogenic bacteria and the reduction of beneficial bacteria in the gut microbiota of diabetic patients may be important factors contributing to the development of DN. The underlying mechanism may involve a decline in host immune and metabolic function due to the loss of beneficial bacteria, while the proliferation of pathogenic bacteria can trigger inflammatory responses through the release of endotoxins. Persistent elevation of inflammatory cytokines may further activate systems such as the renin-angiotensin system, leading to endothelial injury and promoting the onset of DN[17,18].
Numerous studies have established that patients with DN exhibit impaired glucose and lipid metabolism, reduced renal function, and a more pronounced inflammatory response compared to diabetic patients without DN. Kawamura et al[19] reported a mutually reinforcing relationship among gut microbiota, low-grade inflammation, diabetes, and DN. Their study found that the more severe the DN, the more significant the gut microbiota dysbiosis and inflammatory response. The findings of the present study are consistent with these results. Compared to diabetic patients without DN, those with DN demonstrated concurrent deterioration in glucose and lipid metabolism indicators, inflammatory markers, and renal function parameters. Correlation analysis further revealed that eGFR levels were positively associated with the Chao, Ace, and Shannon indices of gut microbiota. Correlation analysis between eGFR levels and the relative abundances of gut microbiota genera revealed that eGFR was positively associated with the relative abundances of Bacteroides, Bifidobacterium, and Helicobacter, and negatively associated with the relative abundances of Clostridium, Klebsiella, Enterococcus, Veillonella, and Megamonas. These findings suggest that Bacteroides, Bifidobacterium, and Helicobacter may have a protective effect on renal function in patients. The underlying mechanisms may be as follows: Bacteroides can degrade complex polysaccharides, thereby reducing the generation of uremic toxins from protein fermentation. This contributes to the improvement of intestinal barrier function, limits the translocation of endotoxins into the bloodstream, and reduces renal tubular epithelial cell injury and systemic inflammation that continuously impairs renal function[18]. Bifidobacterium may activate the toll-like receptor 2 signaling pathway in dendritic cells, thereby decreasing the levels of proinflammatory cytokines. It can also inhibit reactive oxygen species production, delay glomerular injury, and synthesize folate precursors to improve abnormal homocysteine metabolism[19]. Lachnospira produces butyrate as its major metabolite, which can suppress the nuclear factor kappa B signaling pathway, reduce the expression of renal interstitial fibrosis-related factors, and enhance the expression of Klotho protein to help maintain renal homeostasis. Moreover, it competitively consumes aromatic amino acid substrates in the gut, thereby reducing tubular damage[20,21]. After adjusting for confounding factors such as age, sex, and disease duration using a regression model, it was further confirmed that reduced relative abundances of Bacteroides, Bifidobacterium, and Lachnospira were independent risk factors for the development of DN in patients with diabetes.
Current research in the field primarily focuses on comparing gut microbiota characteristics between patients with DN and those with diabetes but without DN. In order to evaluate the association between gut microbiota and the onset and progression of DN. Other studies have explored molecular mechanisms and the gut-kidney axis theory to investigate how gut dysbiosis contributes to the development of DN. Collectively, these studies indicate that the occurrence and progression of DN are closely associated with alterations in the gut microbiota[22,23]. However, most existing studies lack longitudinal follow-up data. In this study, we conducted a one-year prospective follow-up of enrolled patients and found that both DN and non-DN diabetic patients exhibited an overall decline in gut microbial homeostasis, glucose and lipid metabolism, and renal function over time. Notably, patients with a decline in eGFR ≥ 5 mL/minute/year showed more pronounced changes. Specifically, they had greater reductions in Chao, Ace, and Shannon indices, as well as in the relative abundances of Bacteroides and Bifidobacterium. Concurrently, they exhibited greater increases in FBG, HbA1c, HOMA-IR, TC, and FFA levels, along with elevated levels of CRP, TNF-α, IL-6, IL-1β, Cys-C, Scr, BUN, and ACR. Correlation analysis yielded consistent results, suggesting that a reduction in beneficial gut bacteria and an increase in harmful bacteria is associated with worsening glucose and lipid metabolism, declining renal function, and enhanced inflammatory responses in patients.
After adjusting for confounding factors such as age, sex, and disease duration using logistic regression analysis, the results showed that greater decreases in the Chao, Ace, and Shannon indices, as well as greater increases in the Simpson index, were associated with a higher risk of eGFR decline ≥ 5 mL/minute/year. Regarding changes in the relative abundance of bacterial genera, only a decrease in Bacteroides and an increase in Enterococcus were significantly associated with an increased risk of rapid eGFR decline (≥ 5 mL/minute/year), while no statistically significant associations were found for other genera. These findings suggest that Bacteroides and Enterococcus may play more critical roles in the onset and progression of DN. One possible explanation is that Bacteroides is a dominant genus within the gut microbiota and exerts multiple beneficial functions, including the clearance of uremic toxins, regulation of nitrogen metabolism, modulation of endotoxin levels, interference with key fibrotic signaling pathways, regulation of metabolic signaling, and suppression of pro-inflammatory cytokine release[24]. Although Enterococcus constitutes a relatively small proportion of the gut microbiota, it can contribute to renal injury through retrograde urinary tract infection, promotion of toxin production, and disruption of nitrogen metabolism. It also plays a role in immune suppression and inflammatory cascade activation, and may aggravate renal oxidative stress and fibrosis by competing with beneficial bacteria[25]. The combined effect of decreased Bacteroides abundance and increased Enterococcus abundance may synergistically exacerbate renal impairment.
This study has certain limitations. Due to the low incidence of new-onset DN among non-DN patients during the follow-up period, the collected data could not directly verify the causal relationship between gut microbiota alterations and the onset of DN. The study could only observe the influence of gut microbiota changes on renal function, such as accelerating the decline in eGFR and worsening glucose and lipid metabolism. Moreover, during the follow-up period, treatment strategies varied among patients due to differences in clinical conditions, making it difficult to completely eliminate the potential confounding effects of treatment regimens on the study outcomes. Moreover, as all samples were collected from a single provincial hospital in China, region-specific dietary habits and climatic conditions may introduce sampling bias, potentially limiting the generalizability of the findings. Future research should involve larger sample sizes and improved study designs to better control for confounding variables and strengthen the validity of the findings.
In summary, reduced gut microbiota diversity, a decrease in beneficial bacteria, an increase in pathogenic bacteria, and heightened inflammatory responses are important contributors to the development of DN in patients with diabetes. Dysbiosis of the gut microbiota and the intensification of low-grade inflammation may promote the progression of DN and exacerbate renal injury in affected patients. Therefore, for the prevention of DN in diabetic patients and in strategies aimed at halting its progression, greater attention should be given to the regulation of gut microbiota and the control of inflammatory responses.
The onset and progression of DN in older patients with diabetes are closely associated with gut microbiota composition. The more severe the dysbiosis, the lower the abundance of beneficial bacteria and the higher the abundance of harmful bacteria, leading to an increased risk of both DN occurrence and disease progression. Future studies with longer follow-up periods including different ethnic groups are needed to improve our understanding on how gut dysbiosis affects DN onset and progression among patients with diabetes.
| 1. | Zelniker TA, Raz I, Mosenzon O, Dwyer JP, Heerspink HHJL, Cahn A, Goodrich EL, Im K, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Gause-Nilsson I, Langkilde AM, Sabatine MS, Wiviott SD. Effect of Dapagliflozin on Cardiovascular Outcomes According to Baseline Kidney Function and Albuminuria Status in Patients With Type 2 Diabetes: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2021;6:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, Baeres FMM, Idorn T, Bosch-Traberg H, Lausvig NL, Pratley R; FLOW Trial Committees and Investigators. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. 2024;391:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 547] [Article Influence: 547.0] [Reference Citation Analysis (1)] |
| 3. | Singh K, Kondal D, Jagannathan R, Ali MK, Prabhakaran D, Narayan KMV, Anand S, Tandon N; CARRS Trial Investigators. Rate and risk factors of kidney function decline among South Asians with type 2 diabetes: analysis of the CARRS Trial. BMJ Open Diabetes Res Care. 2024;12:e004218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | McEwan P, Gabb PD, Davis JA, Garcia Sanchez JJ, Sjöström CD, Barone S, Kashioulis P, Ouwens M, Cassimaty S, Correa-Rotter R, Rossing P, Wheeler DC, Heerspink HJL. The long-term effects of dapagliflozin in chronic kidney disease: a time-to-event analysis. Nephrol Dial Transplant. 2024;39:2040-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 1074] [Article Influence: 214.8] [Reference Citation Analysis (0)] |
| 6. | Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Toto RD, Sjöström CD, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 341] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 7. | Cai C. [Interpretation of the management requirements of national guidelines for the prevention and control of diabetes in primary care (2018)]. Zhonghua Nei Ke Za Zhi. 2019;58:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Chinese Diabetes Society. [Guidelines for the prevention and treatment of type 2 diabetes in China (2020 edition)]. Zhongguo Shiyong Neike Zazhi. 41:668-695. [DOI] [Full Text] |
| 9. | Sulaj A, Kopf S, von Rauchhaupt E, Kliemank E, Brune M, Kender Z, Bartl H, Cortizo FG, Klepac K, Han Z, Kumar V, Longo V, Teleman A, Okun JG, Morgenstern J, Fleming T, Szendroedi J, Herzig S, Nawroth PP. Six-Month Periodic Fasting in Patients With Type 2 Diabetes and Diabetic Nephropathy: A Proof-of-Concept Study. J Clin Endocrinol Metab. 2022;107:2167-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Zhang W, Hu FJ, Yao CX, Li BP, Zhang M, Yang XM. [Visualization analysis of research hotspots in pathogenesis of diabetic nephropathy in China]. Zhonghua Yu Fang Yi Xue Za Zhi. 2023;57:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Liu S, Gui Y, Wang MS, Zhang L, Xu T, Pan Y, Zhang K, Yu Y, Xiao L, Qiao Y, Bonin C, Hargis G, Huan T, Yu Y, Tao J, Zhang R, Kreutzer DL, Zhou Y, Tian XJ, Wang Y, Fu H, An X, Liu S, Zhou D. Serum integrative omics reveals the landscape of human diabetic kidney disease. Mol Metab. 2021;54:101367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Ueki K, Sasako T, Okazaki Y, Miyake K, Nangaku M, Ohashi Y, Noda M, Kadowaki T; J-DOIT3 Study Group. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Qian F, Zhao L, Zhang D, Yu M, Zhou W, Jin J. Serum metabolomics detected by LDI-TOF-MS can be used to distinguish between diabetic patients with and without diabetic kidney disease. FEBS Open Bio. 2023;13:1844-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Chen L, Liu B, Ren L, Du H, Fei C, Qian C, Li B, Zhang R, Liu H, Li Z, Ma Z. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front Cell Infect Microbiol. 2023;13:1069954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Ren H, Zhao C, Shi Z, Qiu L, Yang F, Zhou X, Han X, Wu K, Zhong H, Li Y, Li J, Ji L. Metagenomic analysis reveals crosstalk between gut microbiota and glucose-lowering drugs targeting the gastrointestinal tract in Chinese patients with type 2 diabetes: a 6 month, two-arm randomised trial. Diabetologia. 2022;65:1613-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | McFarlane C, Krishnasamy R, Stanton T, Savill E, Snelson M, Mihala G, Kelly JT, Morrison M, Johnson DW, Campbell KL. Synbiotics Easing Renal Failure by Improving Gut Microbiology II (SYNERGY II): A Feasibility Randomized Controlled Trial. Nutrients. 2021;13:4481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Sun K, Gao Y, Wu H, Huang X. The causal relationship between gut microbiota and type 2 diabetes: a two-sample Mendelian randomized study. Front Public Health. 2023;11:1255059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 18. | Yang J, Yang X, Wu G, Huang F, Shi X, Wei W, Zhang Y, Zhang H, Cheng L, Yu L, Shang J, Lv Y, Wang X, Zhai R, Li P, Cui B, Fang Y, Deng X, Tang S, Wang L, Yuan Q, Zhao L, Zhang F, Zhang C, Yuan H. Gut microbiota modulate distal symmetric polyneuropathy in patients with diabetes. Cell Metab. 2023;35:1548-1562.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 19. | Kawamura T, Umemura T, Umegaki H, Imamine R, Kawano N, Tanaka C, Kawai M, Minatoguchi M, Kusama M, Kouchi Y, Watarai A, Kanai A, Nakashima E, Hotta N. Effect of renal impairment on cognitive function during a 3-year follow up in elderly patients with type 2 diabetes: Association with microinflammation. J Diabetes Investig. 2014;5:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Chen L, Zhao C, Chen Z, Xiao S, Yin X, Wu N, Yang L, Xu J, Zhou H, Wu Q, Shao R, Xu W. Corrigendum to "Polysaccharides from Cynanchum auriculatum Royle ex Wight ameliorate symptoms of hyperglycemia by regulating gut microbiota in type 2 diabetes mellitus mice" [Int. J. Biol. Macromol. 299 (2025) 139878]. Int J Biol Macromol. 2025;303:140624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Dulai AS, Min M, Sivamani RK. The Gut Microbiome's Influence on Incretins and Impact on Blood Glucose Control. Biomedicines. 2024;12:2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Razavi S, Amirmozafari N, Zahedi Bialvaei A, Navab-Moghadam F, Khamseh ME, Alaei-Shahmiri F, Sedighi M. Gut microbiota composition and type 2 diabetes: Are these subjects linked Together? Heliyon. 2024;10:e39464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Gao LW, Yang XY, Yu YF, Yin S, Tong KK, Hu G, Jian WX, Tian Z. Bibliometric analysis of intestinal microbiota in diabetic nephropathy. Eur Rev Med Pharmacol Sci. 2023;27:8812-8828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Tsai HJ, Tsai WC, Hung WC, Hung WW, Chang CC, Dai CY, Tsai YC. Gut Microbiota and Subclinical Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Nutrients. 2021;13:2679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Hung WC, Hung WW, Tsai HJ, Chang CC, Chiu YW, Hwang SJ, Kuo MC, Chen SC, Dai CY, Tsai YC. The Association of Targeted Gut Microbiota with Body Composition in Type 2 Diabetes Mellitus. Int J Med Sci. 2021;18:511-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |