Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.106833
Revised: April 30, 2025
Accepted: July 7, 2025
Published online: August 15, 2025
Processing time: 156 Days and 2.8 Hours
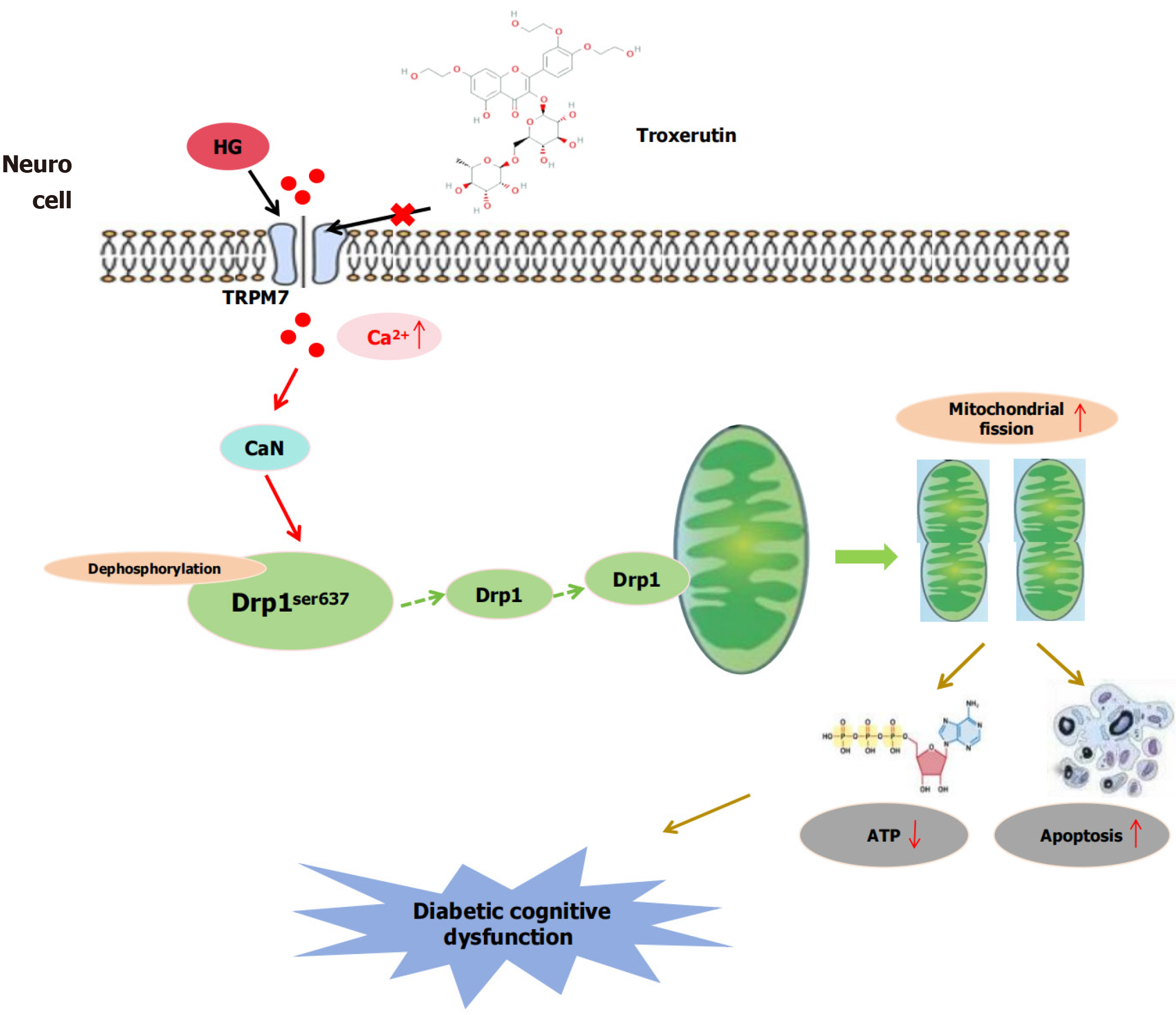
Diabetic cognitive dysfunction (DCD) is one of the chronic complications of diabetes, but its mechanism is currently unknown. Studies have shown that mitochondrial fission mediated by calcium overload is an important mechanism of DCD. Blocking calcium overload and restoring calcium homeostasis are key steps in treatment. Transient receptor potential melastatin 7 (TRPM7) is a novel player in causing calcium overload. Our previous studies have shown that genetic silencing of TRPM7 in type 1 diabetic rats leads to significant improvements in cognitive function, but the specific mechanism remains unclear. Troxerutin, extracted from the flowers of Sophora japonica, is one of the derivatives of rutin and has been shown to have neuroprotective effects. However, its association with TRPM7 remains unclear.
To use animal and cellular models, we investigated whether TRPM7 mediated mitochondrial fission by upregulation of calcineurin (CaN)/dynamin-related protein 1 (Drp1)ser637 in DCD, and whether Troxerutin improved DCD by inhibiting TRPM7-mediated mitoch
In this study, we used db/db mice and hippocampal neuronal cell lines (HT22) treated with high-concentration glucose as our study subjects. We evaluated cognitive function using Morris water maze, novel object recognition tasks, and Nesting tests. We observed mitochondrial morphology using transmission electron microscopy and measured mitochondrial energy metabolism indicators using a spectrophotometer. We also detected mRNA and protein expression of TRPM7, CaN, p-Drp1ser637, caspase-3, B-cell lymphoma 2 associated X protein, and B-cell lymphoma 2 using quantitative real-time polymerase chain reaction, western blotting, and immunofluorescence.
In the db/db diabetic mice with cognitive dysfunction, as well as in hippocampal neurons exposed to high-concentration glucose, TRPM7 and CaN expression were upregulated, phosphorylated Drp1ser637 expression was downregulated, and mitochondrial fission was increased. By modulating (inhibiting or overexpressing) TRPM7, it was further validated that TRPM7 activates the CaN/Drp1ser637 pathway, resulting in an increase in mitochondrial fission and neuronal cell apoptosis. Troxerutin downregulated TRPM7/CaN/Drp1ser637, reduced mitochondrial fission, and improved DCD.
TRPM7 promotes mitochondrial fission via the CaN/Drp1ser637 pathway. Troxerutin improves mitochondrial function and reduces neuronal damage by inhibiting this pathway, suggesting TRPM7 as a potential therapeutic target for DCD.
Core Tip: The expression of transient receptor potential melastatin 7 (TRPM7) was increased in diabetic cognitive dysfunction (DCD) mice and hippocampal neurons treated with high-concentration glucose. TRPM7 promoted mi
- Citation: Li J, Gao M, Wang JX, Li HY, Wang P, Yuan F, Liu AJ, Zhang SY. Troxerutin improves diabetic cognitive dysfunction by inhibiting mitochondrial fission mediated by transient receptor potential melastatin 7/calcineurin/dynamin-related protein 1ser637. World J Diabetes 2025; 16(8): 106833
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/106833.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.106833
Type 2 diabetes mellitus is a global health issue, with diabetic cognitive dysfunction (DCD) being a significant complication. Patients with mild DCD may experience reduced learning and memory, attentional issues, and cognitive sluggishness[1,2], whereas severe cases can even progress to dementia at a young age, creating a financial burden on families and the wider community. Current treatments for diabetes-related cognitive dysfunction have limited efficacy. Conventional measures such as controlling blood sugar, blood pressure, and nourishing the nerves can only delay the progression of the dementia, but cannot prevent it. Therefore, identifying key pathogenic mechanisms of diabetes-related cognitive dysfunction and potential therapeutic targets is essential.
Neurons are especially vulnerable to mitochondrial damage as their survival and synaptic plasticity rely heavily on mitochondrial energy. The balance between mitochondrial fission and fusion is the foundation for maintaining normal mitochondrial structure and function. The hippocampus is crucial for memory, learning, and spatial discrimination. When the fission of mitochondria is increased, causing an imbalance between fission and fusion, it will lead to mitochondrial dysfunction, a decrease in adenosine triphosphate (ATP) production, harm to hippocampal neurons, and is an important mechanism of DCD[3,4]. Dynamin-related protein 1 (Drp1), is a key mitochondrial fission protein, that is activated in the cytoplasm and then translocated to the mitochondrial outer membrane to initiate fission. The localization of Drp1 is contingent upon calcium/calcineurin (CaN)[5-9]. In studies of ischemic stroke and Alzheimer’s disease, cytoplasmic calcium overload activates CaN, which dephosphorylates Drp1ser637, leading to Drp1 translocation to the mitochondrial outer membrane and accelerating mitochondrial fission[10-14]. However, the potential mechanism of mitochondrial fission enhancement in DCD is still unclear.
Transient receptor potential melastatin 7 (TRPM7), a calcium ion channel protein[15], is closely associated with Alzheimer’s disease and age-related cognitive decline[16,17]. In studies of diabetes complications, Huang et al[18] have shown that TRPM7 mediates high-glucose-induced endoplasmic reticulum stress and neuronal cell apoptosis. TRPM7 is also involved in the initiation of neuropathic pain caused by calcium overload in type 2 diabetic mice[19]. Our previous research has shown that the expression of TRPM7 is increased in the hippocampus of type 1 DCD rats, and silencing TRPM7 can improve cognitive function[20]. Accordingly, we speculate that TRPM7 plays a certain role in the mechanism of DCD occurrence in type 2 diabetic mice.
Cytoplasmic calcium overload induces CaN activation, which in turn mediates Drp1ser637 dephosphorylation, thereby promoting mitochondrial fission. Moreover, TRPM7 activation potentially causes an excess of calcium, which in turn activates CaN[21-23]. However, it remains unclear whether TRPM7 promotes mitochondrial fission by mediating CaN activation. In this study, we hypothesize that TRPM7 plays a critical role in mitochondrial fission and investigate whether it induces DCD by upregulating the CaN/Drp1ser637-mediated mitochondrial fission pathway.
Additionally, troxerutin is a flavonoid compound with antioxidant and anti-inflammatory effects that is derived from food (Sophora japonica flowers) and has low toxicity. Troxerutin can improve oxidative stress levels and cognitive function in type 1 diabetic rats[24,25]. Given that regulating the generation of reactive oxygen species is a fundamental mitochondrial function, and Troxerutin can increase ATP production in cardiomyocytes[26]. We reasonably speculate that the neuroprotective effect of Troxerutin is related to improved mitochondrial function. If TRPM7 mediates mitochondrial division by up-regulating CaN/Drp1ser637 in DCD, the cognitive improvement observed with Troxerutin may be owing to inhibition of TRPM7-mediated mitochondrial division. In this study, we established a type 2 DCD mouse model and a high-concentration glucose (HG)-induced mouse hippocampal neuronal cell model to explore whether TRPM7 mediates mitochondrial fission through upregulating CaN/Drp1ser637 in DCD, and whether Troxerutin can improve DCD by inhibiting TRPM7-mediated mitochondrial fission (Figure 1).
In this study, we used db/db mice and hippocampal neuronal cell lines (HT22) treated with HG as our study subjects. We conducted this study from 2021 to 2024 at the Hebei Key Laboratory of Rare Disease, Shijiazhuang, 050000, Hebei Province, China.
A total of 16 specific pathogen free-grade healthy male db/m mice (8-weeks old, body weight 181 g) and 26 db/db mice (8 weeks old, body weight 412 g) were purchased from Changzhou Cavens Experimental Animal Co., Ltd. No. 202140985). The animal care and experimental procedures were strictly in accordance with the approval of the Animal Care and Management Committee of the Second Hospital of Hebei Medical University.
After one week of adaptive feeding, the mice were randomly divided into five groups: (1) Control group (NC) (dm, n = 8); (2) Control+ troxerutin group (dm-T, n = 8); (3) Diabetes group (db, n = 8); (4) Diabetes + troxerutin group (db-T, n = 8); and (5) Diabetes + troxerutin + bradykinin group (db-T-B, n = 10). The dm-T, db-T, and db-T-B groups were given intraperitoneal injections of troxerutin dissolved in physiological saline (60 mg/kg/day, MedChemExpress, China) for 8 weeks. The db-T-B group was given an additional intraperitoneal injection of Bradykinin (10 mg/kg/day, ApexBio, United States), an agonist of TRPM7, after 6 weeks of Troxerutin treatment. At the 17th week, the mice were fasted and sacrificed by an intraperitoneal injection of sodium pentobarbital (100 mg/kg, Sinopharm, Shanghai, China). The hippocampal tissue was then collected.
The HT22 cells (JennioBiotech, Guangzhou, Guangdong Province, China) were cultured in 25 cm2 culture flask with Dulbecco’s essential medium (GIBCO, Shanghai, China), supplemented with 10% fetal bovine serum (EXCELL, Nanjing, Jiangsu Province, China) and 1% penicillin-streptomycin (Solarbio Life Sciences, Beijing, China).
The cells were passaged using trypsin digestion and were inoculated in six-well plates at a density of 5 × 104 cells/mL for the first-stage experiment. The experimental groups were divided as follows: NC (25 mmol/L glucose), hypertonic group (25 mmol/L glucose + 75 mmol/L mannitol), HG (100 mmol/L glucose), NC + small interfering RNA (siRNA) empty vector group (NC + siRNA), NC +si-TRPM7 group (NC + si-TRPM7), NC + pcDNA4 empty vector group (NC + pcDNA4), NC + pcDNA4-TRPM7 group (NC + pcDNA4-TRPM7), NC + dimethyl sulfoxide group, NC + pcDNA4-TRPM7 + FK506 group (NC + pcDNA4-TRPM7 + FK506, 50 mmol/L/10 μM). FK506 is a CaN inhibitor and was purchased from AG Scientific (San Diego, California, United States). The second-stage grouping: NC, control + troxerutin group (NC + T, 10 μM), HG, and high-glucose + troxerutin group (HG + T), as well as high glucose + troxerutin + pcDNA4-TRPM7 group (HG +T + pcDNA4-TRPM7).
Morris water maze test: The Morris water maze test was constructed with a black circular pool (160 cm diameter and 60 cm height) filled with water (30 cm depth) with a temperature of 24 °C ± 2 °C. The Morris water maze test was divided into four equal quadrants. A circular platform (10 cm × 10 cm) was hidden 1 cm below the water surface and fixed in the center of the northwest quadrant. The testing process remains the same as the previous research[24].
Novel object recognition: The Novel object recognition test was performed in a plexiglass white box of dimensions 40 cm × 40 cm × 30 cm. The experiment process was divided into three stages, namely adaptation, familiarization, and testing. The relative identification index was used to assess the learning and memory of mice. It was calculated as new/(new + familiar) × 100%, where new refers to the time to explore the novel object and familiar refers to the time to explore familiar objects.
Nesting test: Before beginning the nesting experiment, keep the mice in a single cage for at least 24 hours. Place 5 cm × 5 cm tasteless tissue paper with uniform cutting size in the cage, with two piles of eight sheets each. After a period of 24 hours, take pictures and use a blind scoring system based on the scoring criteria to determine the nesting score of the mice. 0 points: No contact with the tissues; 1 point: Tissues scattered throughout the cage, but without any visible tearing; 2 points: Tissues clustered on one side of the cage, but without any tearing; 3 points: Tissues clustered in one corner or one side, with a small part torn but no visible, formed nest; 4 points: Most of the tissues are torn and gathered into a nest.
To assess the cell cytotoxicity of HG and troxerutin, the cell counting kit-8 assay was used to detect cell viability (according to the manual).
ATP content in hippocampal tissues and HT22 cells of each group was determined using mitochondrial ATP detection kit (Grace Biotechnology, Jiangsu Province, China), according to the operation steps of the kit. JC-1 detection kit (KeyGen Biotech, Jiangsu Province, China) was used to detect mitochondrial membrane potential in HT22 cells, which was displayed under a fluorescence microscope.
Analyze apoptosis through morphological methods. Stain the neurons of each group with Hoechst 33258 staining solution (KeyGen Biotech, Jiangsu Province, China) for cell apoptosis analysis, and observe the apoptotic cells of each group under a fluorescence microscope. Characteristic morphology was used to identify apoptotic cells.
Total RNA was extracted from the mouse hippocampal tissue and HT22 neuronal cells using the Total RNA Rapid Extraction Kit (GeneCopoeia), and cDNA was synthesized with All-in-One First-Strand cDNA Synthesis Kit (Gene
| Primers | Forward (5’-3’) | Reverse (5’-3’) |
| TRPM7 | TCCTGTGACATGGTTTTGG | CAGGGGTGTAGTCATTCCTCT |
| CaN | CTCCCAGTTCAGCGTCAA | ATCGCCATCCTTATCCAG |
| Caspase-3 | AGAACTGGACTGTGGCATTG | TAACCAGGTGCTGTGGAGTA |
| Bax | TTTCCGAGTGGCAGCTG | CAAAGTAGAAAAGGGCGACAAC |
| Bcl-2 | GGATGCCTTTGTGGAACTGT | CACTTGTGGCTCAGATAGGC |
| GAPDH | GCTGAAGGTCGGTGTGAAGG | CTCGCTCCTGGAAGATGGTG |
Total protein from tissues or cells was extracted using RIPA lysis buffer (Solarbio Life Sciences, Beijing, China) containing protease inhibitors. Protein quantification was carried out using the BCA protein assay kit (Solarbio Life Sciences, Beijing, China). Membranes were transferred at different currents according to the molecular weight, blocked with 5% non-fat milk at room temperature for 2 h, and then incubated with TRPM7 (1:500, affinity), CaN (1:1000, 13433-1-AP, Santa), Drp1 (1:1000, Affinity, China), Phospho-Drp1ser637 (1:1000, Affinity, China), caspase -3 (1:1000, Cell Signaling), B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) (1:1000, Cell Signaling), Bcl-2 (1:1000, Cell Signaling), glyceraldehyde-3-phosphate dehydrogenase (1:1000, Bioworld Technology), and β-Tubulin (1:1000, Bioworld Technology) specific antibodies overnight at 4 °C. The next day, the membrane was washed with TBST three times and incubated with the secondary antibody (1:10000, Rockland) at room temperature for 1 h. The blot was then scanned using an infrared scanner (LICOR Biosciences, United States). The results were quantified using ImageJ software.
After anesthesia, mice were perfused with pre-cooled normal saline for 2 minutes, followed by perfusion with 4% paraformaldehyde. After tissue fixation, cryostat (Thermo Fisher Scientific, United States) was used to slice the hippocampal tissue with a thickness of 15 μm. The slices were then blocked with 10% donkey serum at 37 °C for 1 hour. The slices were incubated with TRPM7 antibody (1:150, DF7513, Affinity, China), CaN antibody (1:200, 13433-1-AP, Santa), and Phospho-Drp1ser637 antibody (1:200, DF2980, Affinity, China) at 4 °C overnight. On the second day, the slices were warmed up at room temperature for 30 minutes and then incubated with the corresponding secondary antibody at 37 °C in the dark for 1 h. The nuclei were stained with 4’,6-diamidino-2-phenylindole (Southern Biotech, United States). Observation and photography were performed under a confocal laser scanning microscope.
Tissue blocks of mouse hippocampus (size < 1 mm3) from each group were fixed overnight in 2.5% glutaraldehyde, dehydrated with ethanol, and then resin-embedded. Ultra-thin sections (60-80 nm) were cut using an ultramicrotome (UC7, Leica). Observations were made using a transmission electron microscope (TEM) (HT7700, Hitachi). Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, United States) was used for the analysis with a 12.0k × 500.0 nm and 6.0k × 1 μm scale. Mitochondrial morphology was recorded for each sample and mitochondrial length and width were measured in high-power fields.
The siRNA-TRPM7 of mouse (target sequence: CCACAAACAGACAGAGGAA) was synthesized by RiboBio Biotechnology (Guangzhou, Guangdong Province, China). A non-targeting siRNA (RiboBio Biotechnology, Guangzhou, Guangdong Province, China) was used as a negative control. Cells were transfected with 100 nM siRNA or control siRNA using the transfection reagent RiboFECTTMCP (RiboBio Biotechnology) according to the manufacturer’s instructions. pcDNA4/TO encoding TRPM7 (NM_021450.2) was also synthesized by RiboBio Biotechnology. Cells were transfected with 2.5 μg of plasmid using transfection reagent Lipofectamine® 3000 (Invitrogen) according to the manufacturer’s instructions. Cells were used two days later for experiments.
Data were processed and analyzed using the SPSS 17.0 statistical software package, and the results were expressed as mean ± SD. The data were tested for normality and homogeneity of variance before differential analysis. Escape latency was analyzed using repeated-measures analysis of variance, whereas the remaining data were analyzed by one-way analysis of variance, followed by a post hoc Tukey’s honest significant difference test for multiple comparisons among the groups. Two-sided tests were used to assess statistical significance, with P < 0.05 considered significant.
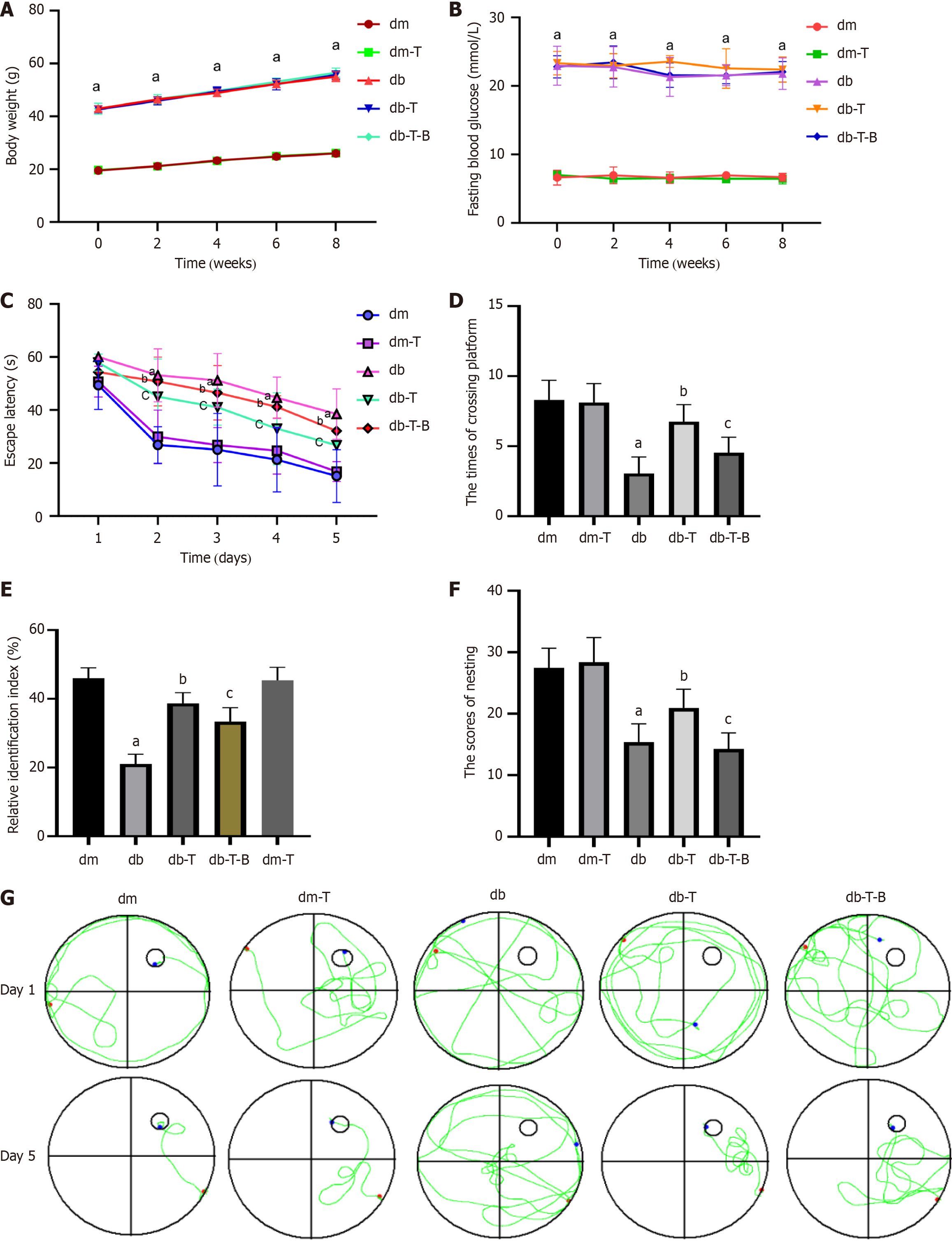
Compared with the dm group, the db group had significantly higher body weight and blood glucose levels, whereas there were no significant differences in body weight and blood glucose levels among the db group mice (Figure 2A and B). After 8 weeks of feeding (17 weeks of age), cognitive function was evaluated using water maze, novel object recognition, and nesting tests. Compared with the dm group, the db group mice had increased escape latencies starting on day 2 (P < 0.0001) (Figure 2C), reduced platform crossings (P < 0.0001) (Figure 2D), lower novel object relative recognition indices (P < 0.0001) (Figure 2E), and lower nesting scores (P < 0.0001) (Figure 2F). This suggests that 17-week-old type 2 diabetic mice exhibit cognitive dysfunction. Additionally, swimming trajectory analysis revealed that the swimming path of dm group mice was directional, whereas the swimming path of db group mice was disordered (Figure 2G).
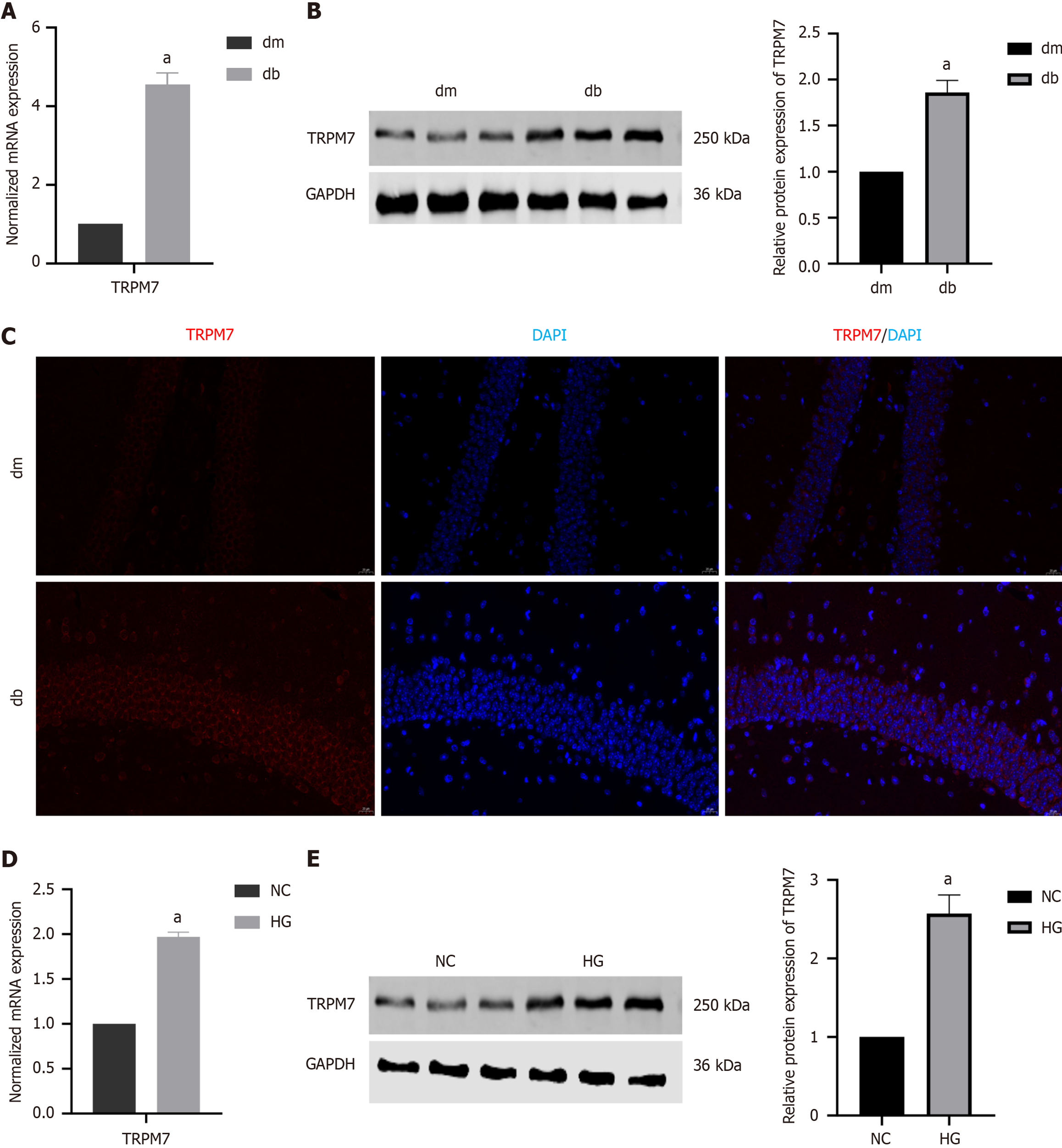
Compared with the dm group, the db group had higher mRNA and protein expression levels of TRPM7 in the hippocampal tissue (4.54 ± 0.16 vs 1.00; 1.86 ± 0.09 vs 1.00 ± 0.06, P < 0.0001, P = 0.0016) (Figure 3A and B), which was also observed through immunofluorescence staining (Figure 3C). Similarly, we also found increased TRPM7 expression levels in HG-induced HT22 cells (1.97 ± 0.03 vs 1.00; 2.57 ± 0.20 vs 1.00 ± 0.10, P < 0.0001, P = 0.0011) (Figure 3D and E). These results suggest that TRPM7 may be an important target for diabetic neuronal injury.
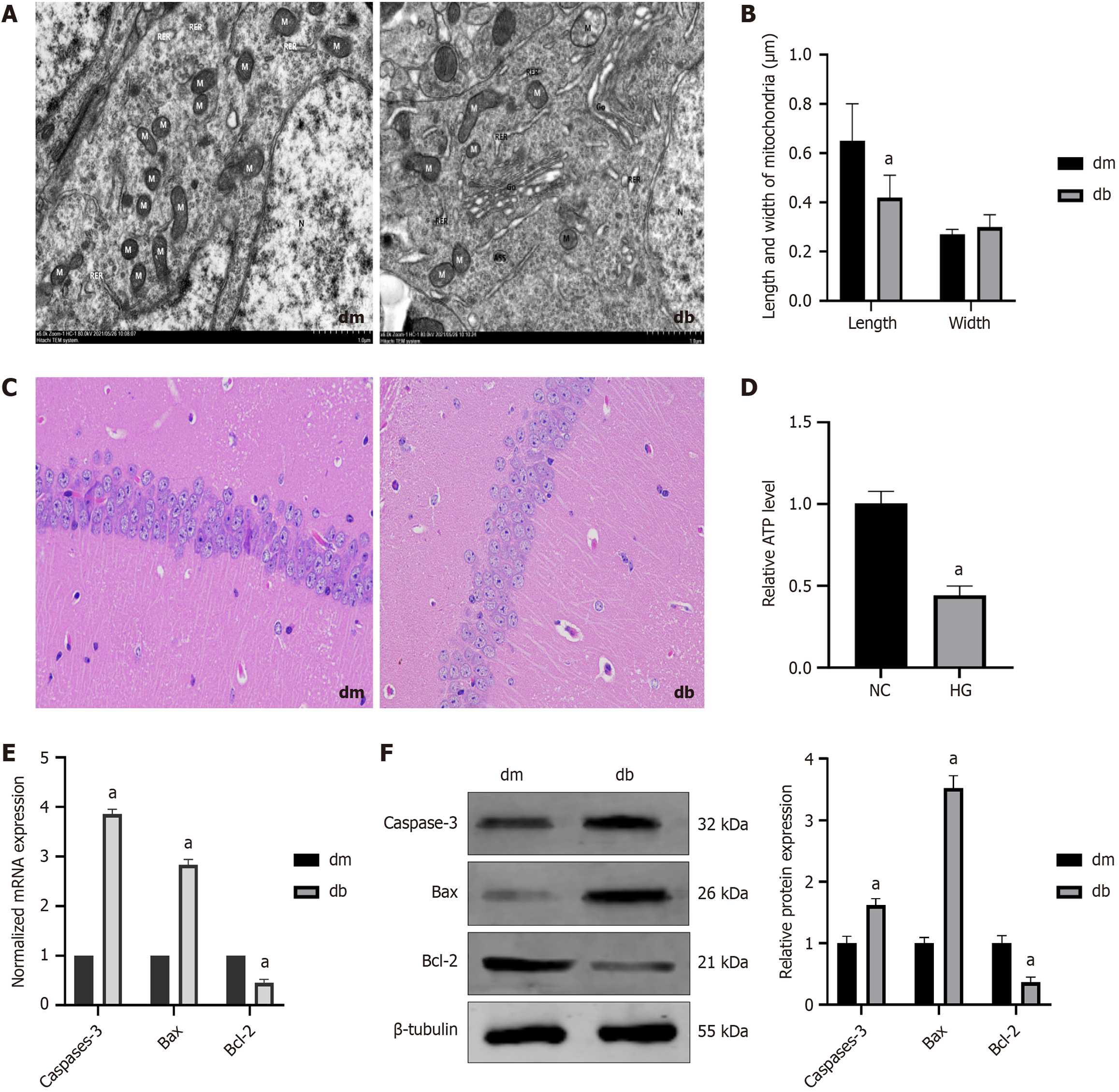
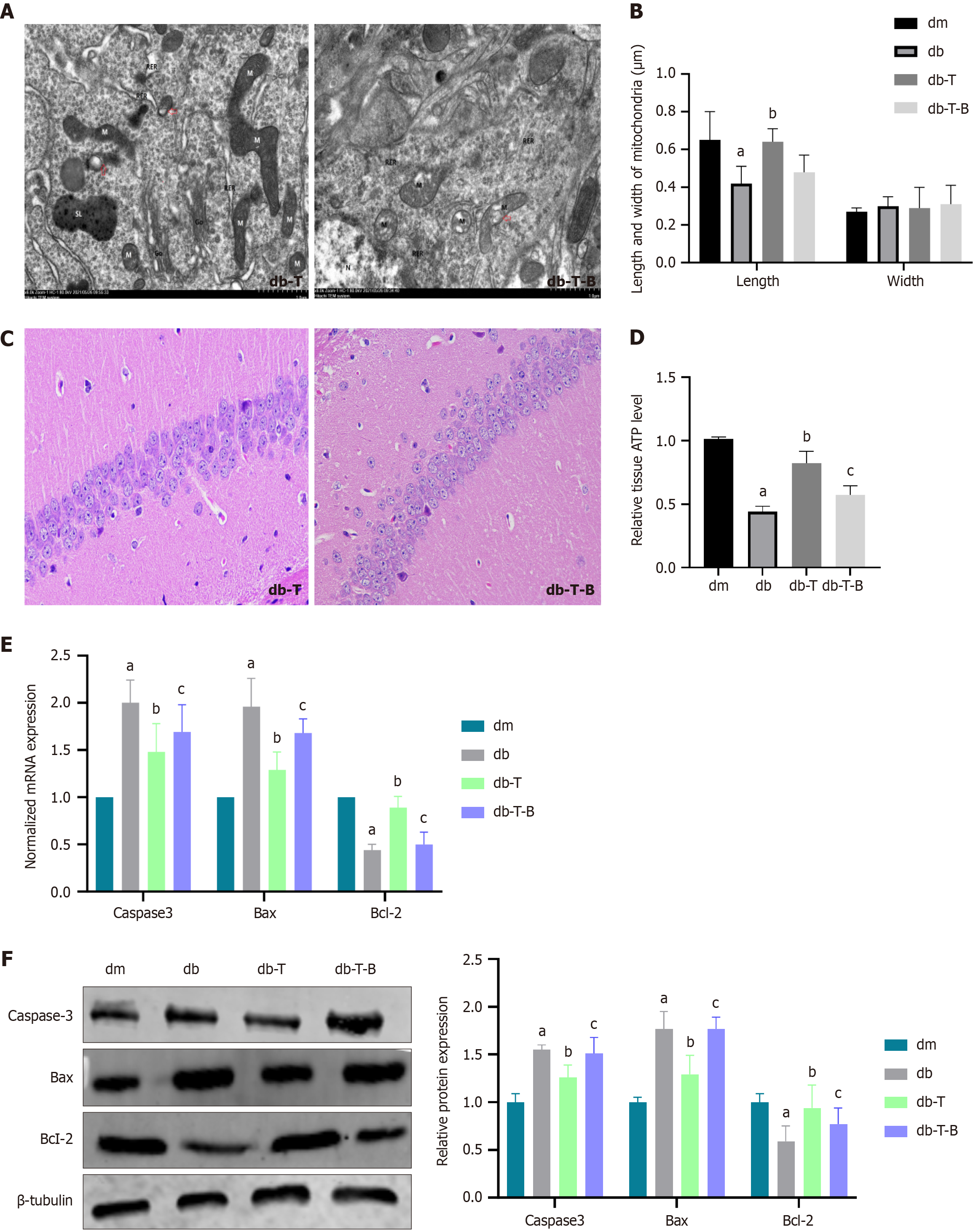
TEM images revealed that the mitochondria in the hippocampus of db group were swollen, with cristae broken and reduced, as well as increased mitochondrial fission and mitochondrial microautophagy structure (The damaged mitochondria are surrounded by membranous structures) (Figure 4A). Furthermore, at high magnification, the length of mitochondria in the db was found to be decreased in comparison to the dm group (0.65 ± 0.15 vs 0.42 ± 0.09, P < 0.0001), whereas the width of the mitochondria showed no significant difference (Figure 4B).
Hematoxylin and eosin staining revealed that the hippocampal pyramidal neurons in the dm group were arranged neatly with intact morphology, whereas the db group showed a decrease in cell number, unclear cell membranes and nuclei, nuclear pyknosis and fragmentation, reduced cytoplasm, and vacuolar degeneration (Figure 4C).
ATP levels can reflect mitochondrial function. We found that ATP content was decreased in the db group compared to the dm group (0.44 ± 0.16 vs 1.01 ± 0.09, P < 0.0001) (Figure 4D). Additionally, we detected apoptotic proteins in the hippocampal tissue, including increased expression of caspase -3 (P = 0.0001, P < 0.0001) and Bax (P < 0.0001, P < 0.0001) and decreased expression of Bcl-2 (P = 0.0001, P < 0.0001) (Figure 4E and F). These results suggest that mitochondrial fission increases, ATP synthesis decreases, and neuronal cell damage occurs in DCD mice.
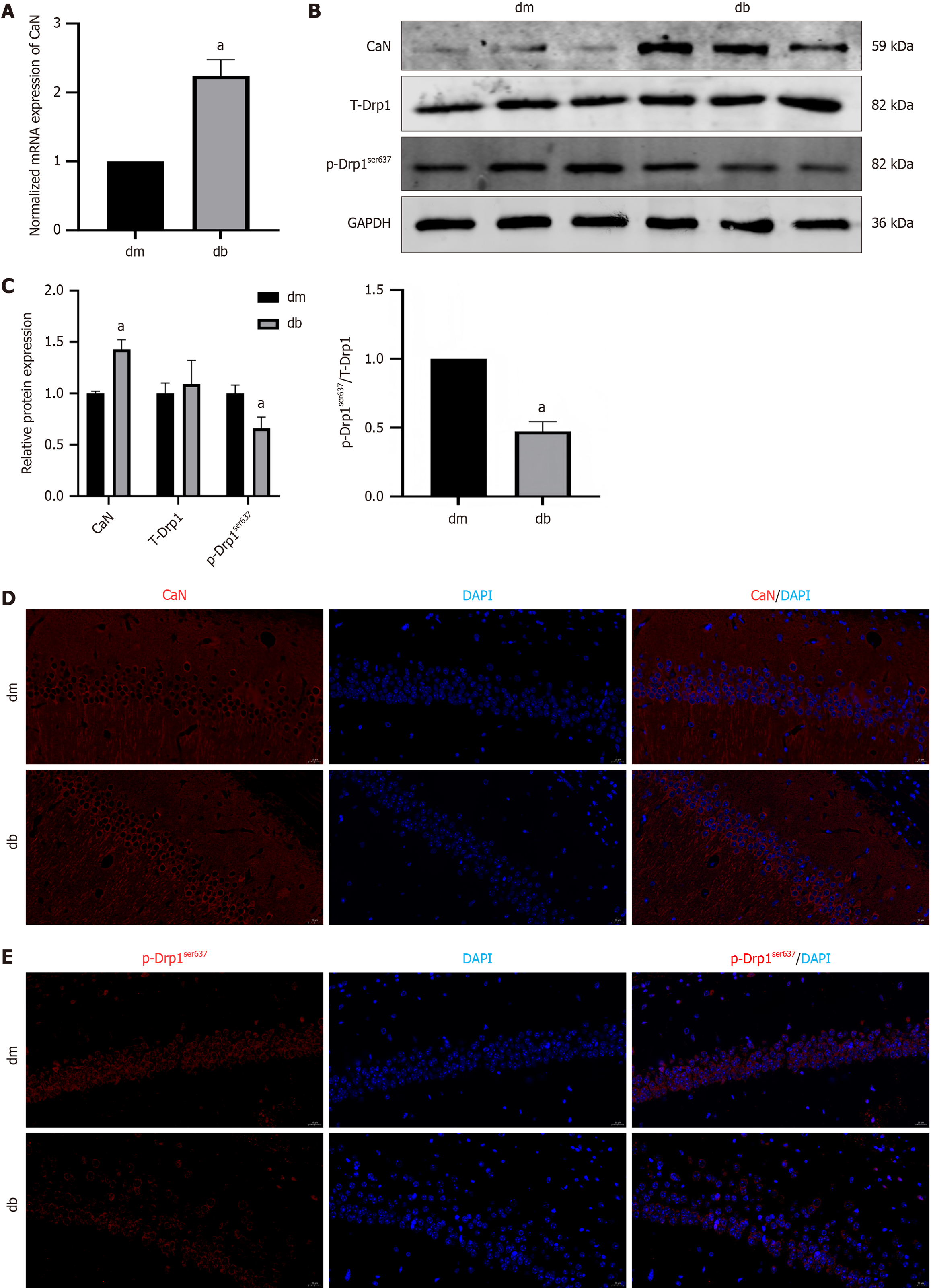
TRPM7, a calcium ion channel, is associated with calcium overload, activation of CaN, and initiation of signal transduction. We also observed an increase in CaN mRNA (P < 0.0001) and protein (P = 0.0031) expression in the hippocampal tissue of the db group (2.24 ± 0.11 vs 1.00; 1.43 ± 0.09 vs 1.00 ± 0.02) (Figure 5A-C). Drp1 mitochondrial translocation is a key process in mitochondrial fission. This translocation is mainly regulated by phosphorylation. We analyzed phosphorylation of Drp1ser637, which is associated with Drp1 activation-induced apoptosis. Compared to the dm group, there was no significant difference in total Drp1 protein expression in the hippocampal tissue of the db group, whereas p-Drp1ser637 protein expression was reduced (0.66 ± 0.11 vs 1.00 ± 0.08, P < 0.0001) (Figure 5B and C), and the same changes were observed by immunofluorescence (Figure 5D and E).
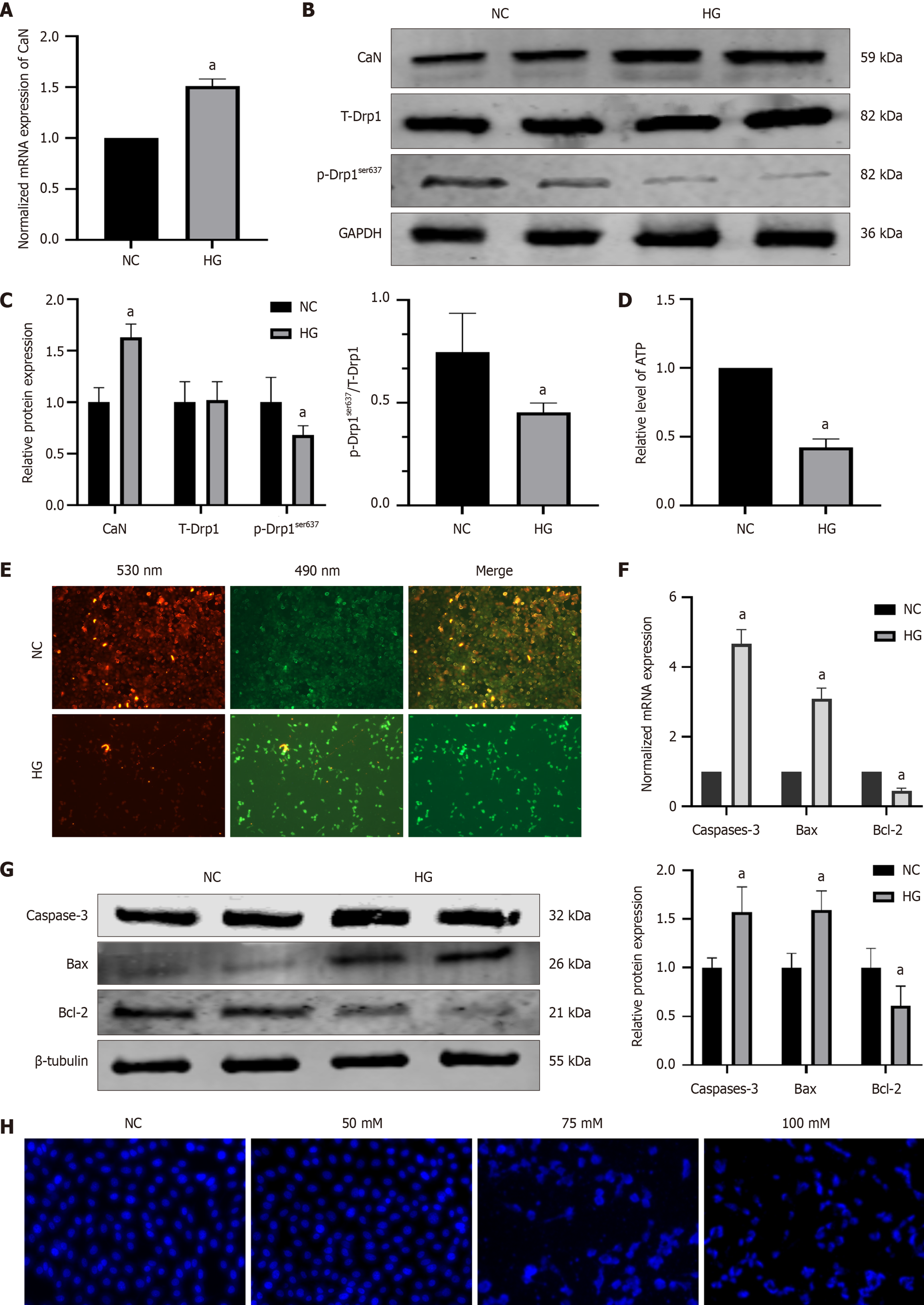
However, in HG-induced HT22 cells, we observed that compared to the NC group, the HG group also had increased mRNA (P = 0.0002) and protein (P = 0.0021) expression of CaN (1.49 ± 0.10 vs 1.00; 1.63 ± 0.13 vs 1.00 ± 0.14,*) (Figure 6A-C), with no difference in total Drp1 protein levels but reduced p-Drp1ser637 protein expression (0.68 ± 0.09 vs 1.00 ± 0.14, P = 0.0042 ) (Figure 6B and C). There was a decrease in ATP synthesis and mitochondrial membrane potential (P < 0.0001) (Figure 6D and E). Furthermore, the expression of caspase 3 (P < 0.0001, P = 0.0100) and Bax increased (P < 0.0001, P = 0.0080), and the expression of Bcl-2 (P < 0.0001, P = 0.0034) decreased (Figure 6F and G). Further observation of cell apoptosis was conducted using Hoechst 33258 fluorescence staining and fluorescence microscopy. The NC group cells had a uniform faint blue nuclear coloration and a round or oval shape, whereas most cells in the HG group presented typical features of apoptotic cells, with bright blue nuclear staining owing to chromatin condensation, or nuclei that were lobulated or fragmented (Figure 6H).
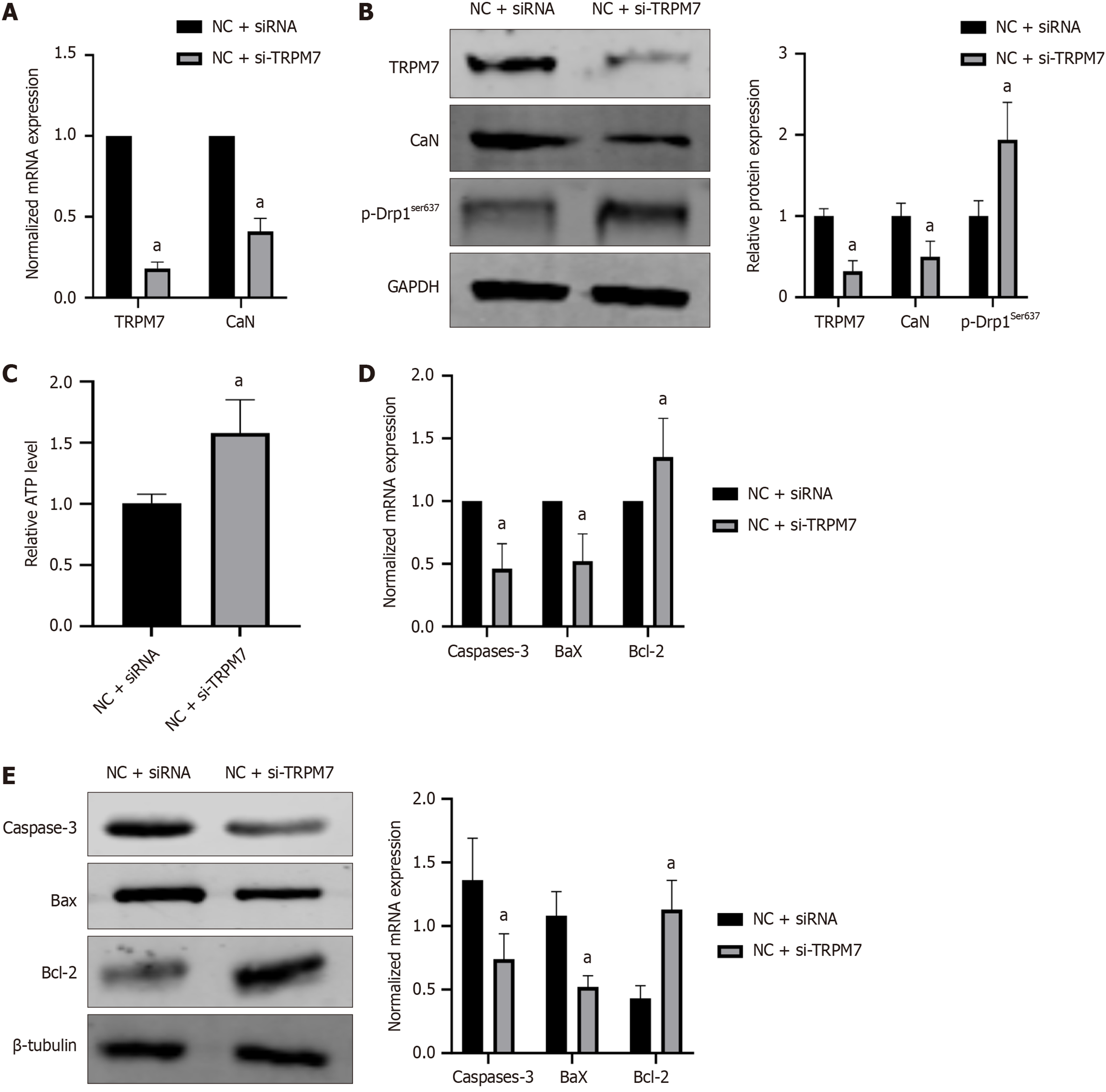
To better understand the specific mechanism of TRPM7 and how it affects neuronal cell damage, we employed the use of specific siRNA to silence TRPM7. Compared to the NC + siRNA group, the NC + si-TRPM7 group had a significant reduction in TRPM7 mRNA relative expression down to 0.18 ± 0.04 (P < 0.0001) (Figure 7A), and TRPM7 protein (P = 0.0124) relative expression down to 0.32 ± 0.13 (Figure 7B). Downstream CaN mRNA (P < 0.0001) and protein levels (P = 0.024) also decreased to 0.41 ± 0.08 and 0.50 ± 0.19 respectively (Figure 7A and B). Simultaneously, p-Drp1ser637 (P = 0.0011) increased to 1.94 ± 0.46 (Figure 7A and B), and ATP level rose to 1.58 ± 0.11 (compared to 1.01 ± 0.08, P = 0.0006) (Figure 7C). Additionally, caspase 3 (P = 0.0080, P = 0.0095) and Bax (P = 0.0172, P = 0.0182) expression decreased, whereas Bcl-2 expression increased (P = 0.035, P = 0.004) (Figure 7D and E).
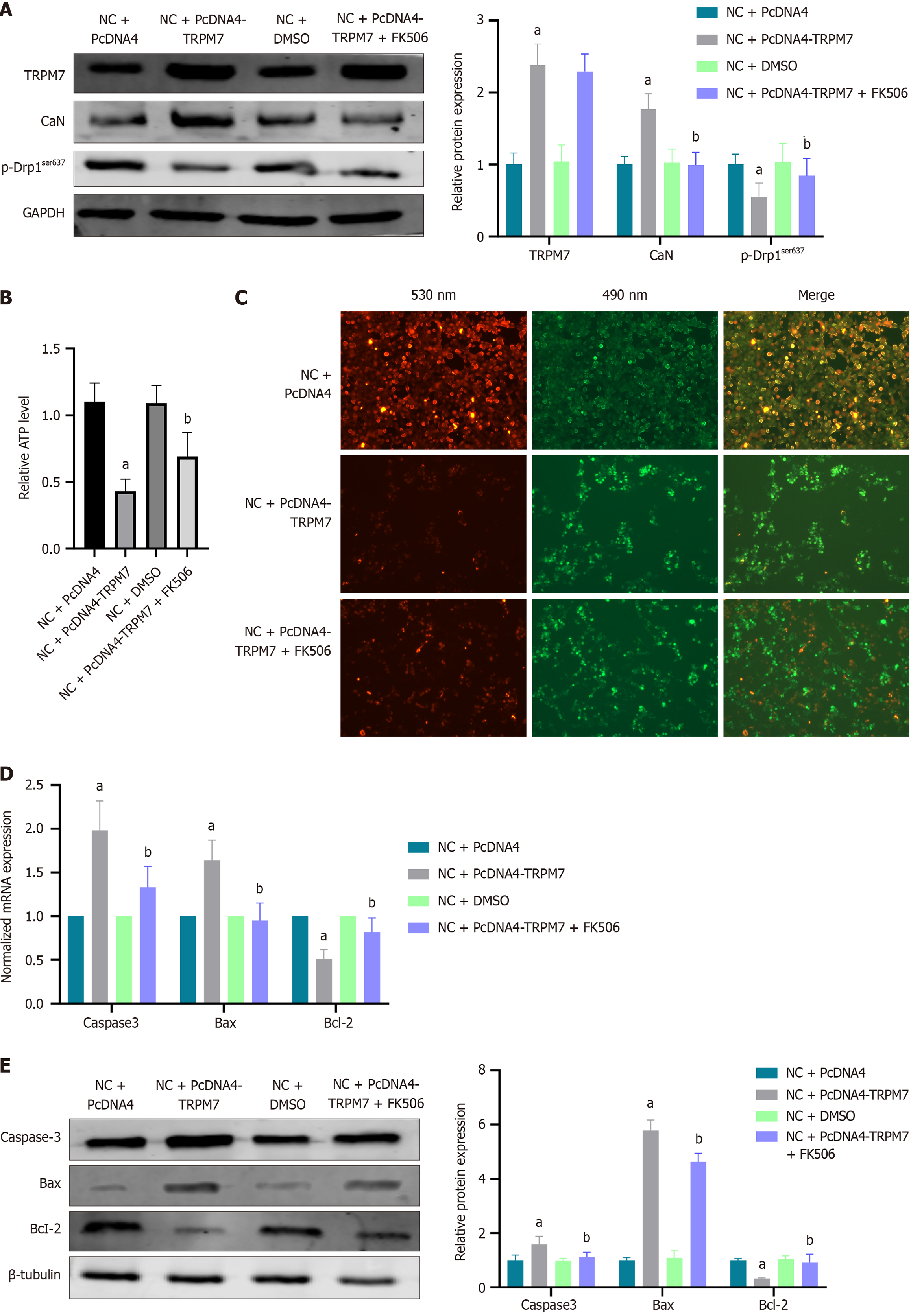
After 48 hours of transient transfection of HT22 cells with pcDNA4-TRPM7, the protein levels of TRPM7 (P < 0.0001) and CaN (P = 0.0004) in the NC + pcDNA4-TRPM7 group were significantly higher than the NC + pcDNA4 group (2.38 ± 0.29 vs 1.00 ± 0.16; 1.77 ± 0.21 vs 1.00 ± 0.11). Additionally, the p-Drp1ser637 protein levels were significantly decreased (0.55 ± 0.19 vs 1.00 ± 0.14, P = 0.0381) (Figure 8A). Furthermore, the cellular ATP levels were reduced (P < 0.0001) (Figure 8B) and the mitochondrial membrane potential levels were decreased (Figure 8C). The expressions of caspase-3 (P < 0.0001, P = 0.0120) and Bax (P = 0.001, P < 0.0001) were increased, whereas the expression of Bcl-2 was decreased (P = 0.0026, P = 0.0032) (Figure 8D and E).
To further clarify the relationship between TRPM7 and downstream CaN/Drp1ser637, FK506, a CaN inhibitor, was added during transient transfection with pcDNA4-TRPM7. The results showed that the protein level of TRPM7 remained unchanged, indicating that TRPM7 is upstream of CaN. The protein level of CaN significantly decreased (0.99 ± 0.18 vs 1.77 ± 0.21, bP = 0.0004), followed by a significant increase in p-Drp1ser637 protein level (0.84 ± 0.24 vs 0.55 ± 0.19, bP = 0.026) (Figure 8A). ATP levels (bP = 0.0059) and mitochondrial membrane potential increased (Figure 8B and C), whereas caspase-3 (bP = 0.0001, bP = 0.0316) and Bax (bP < 0.0001, bP < 0.0001) expression decreased and Bcl-2 (bP = 0.0344, bP = 0.0093) expression increased (Figure 8D and E). These results support the hypothesis that TRPM7 upregulates CaN/Drp1ser637, increases mitochondrial fission in hippocampal neurons, causes ATP synthesis disorders, and promotes neuronal apoptosis.
After preventive Troxerutin treatment, compared to the db group, the db-T group had a decreased escape latency (P = 0.0327) (Figure 2C), increased number of platform crossings (P < 0.0001) (Figure 2D), increased relative recognition index (P = 0.0048) (Figure 2E), and increased nesting score (P = 0.0053) (Figure 2F), suggesting Troxerutin improved learning and memory functions in mice, consistent with our previous research. There were no significant cognitive differences between the dm and dm-T groups. TEM showed that, compared to the db group, the db-T group had reduced mitochondrial fission (Figure 9A) and increased mitochondrial length (P < 0.0001) (Figure 9B). Hematoxylin and eosin staining showed that hippocampal pyramidal cells were more regularly arranged in the db-T group compared to the db group, with reduced nuclear condensation and mitochondrial fission (Figure 9C). ATP levels increased (P < 0.0001) (Figure 9D), caspase-3(P = 0.0061, P = 0.00245) and Bax (P = 0.0005, P = 0.0017) expression decreased, Bcl-2(P = 0.0182, P = 0.0220) expression increased in db-T group compared to db group (Figure 9E and F).
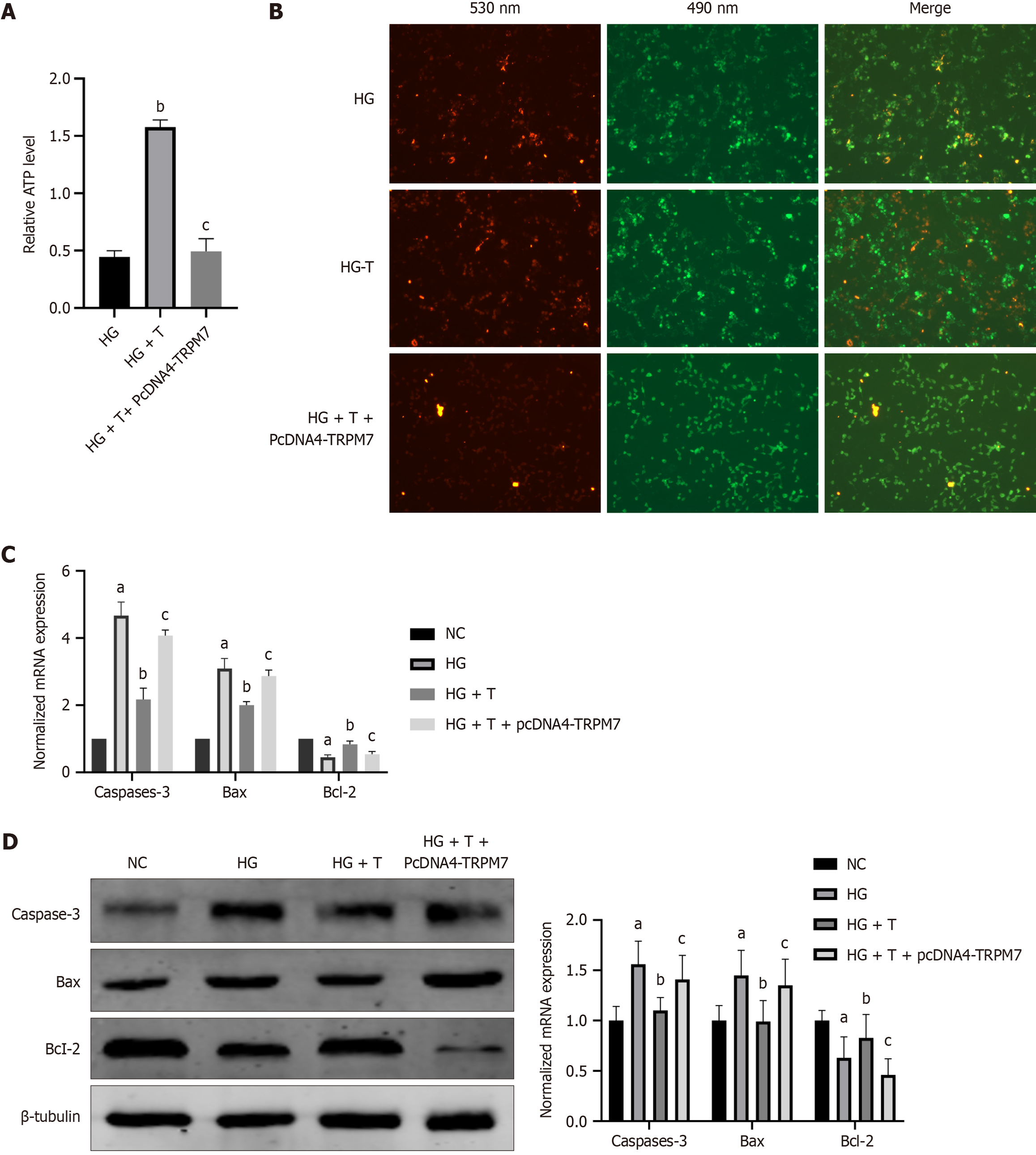
First, we added different concentrations (5 μM, 10 μM, 15 μM, 20 μM, and 25 μM) of quercetin to cells for 48 hours. Based on the survival rate and ATP level of each group of cells, we ultimately selected 10 μM as the experimental concentration. In the HG group, quercetin (10 μM, 48 hours) was added, and the ATP level in the HG + T group was significantly higher than that in the HG group (P < 0.0001) (Figure 10A). Similarly, the mitochondrial membrane potential in the HG + T group was significantly higher than that in the HG group (Figure 10B). Moreover, the mRNA and protein levels of caspase-3 (P < 0.0001, bP = 0.00246) and Bax (P < 0.0001, P = 0.00246) in the HG + T group decreased, whereas the expression of Bcl-2 (P = 0.0022, bP = 0.036) increased (Figure 10C and D).
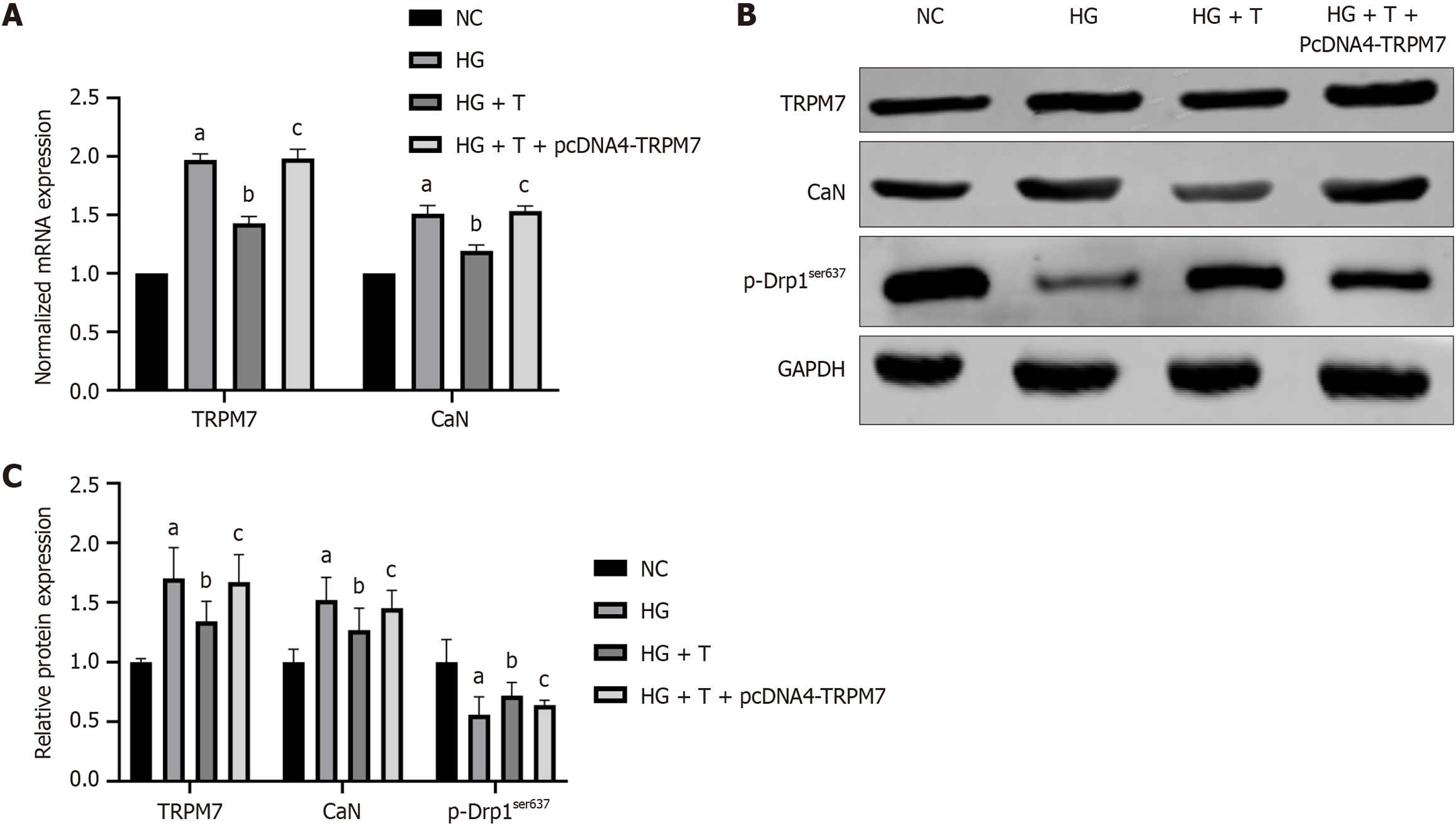
Compared with the HG group, the HG + T group had reduced protein and mRNA levels of TRPM7(P < 0.0001, P = 0.0346), CaN (P < 0.0001, P = 0.0295), and p-Drp1ser637 (P = 0.0255) (Figure 11A and B). After TRPM7 overexpression was induced in the HG + T group, the HG + T + pcDNA4-TRPM7 group had increased protein and mRNA levels of TRPM7(P < 0.0001, P = 0.0360), CaN (P < 0.0001, P = 0.0243), and decreased p-Drp1ser637 (P = 0.0219) protein expression (Figure 11A and B), reduced ATP levels (P < 0.0001) (Figure 10A), and decreased mitochondrial membrane potential (Figure 10C). These results indicate that Troxerutin has neuroprotective effects by inhibiting the TRPM7/CaN/Drp1ser637 pathway.
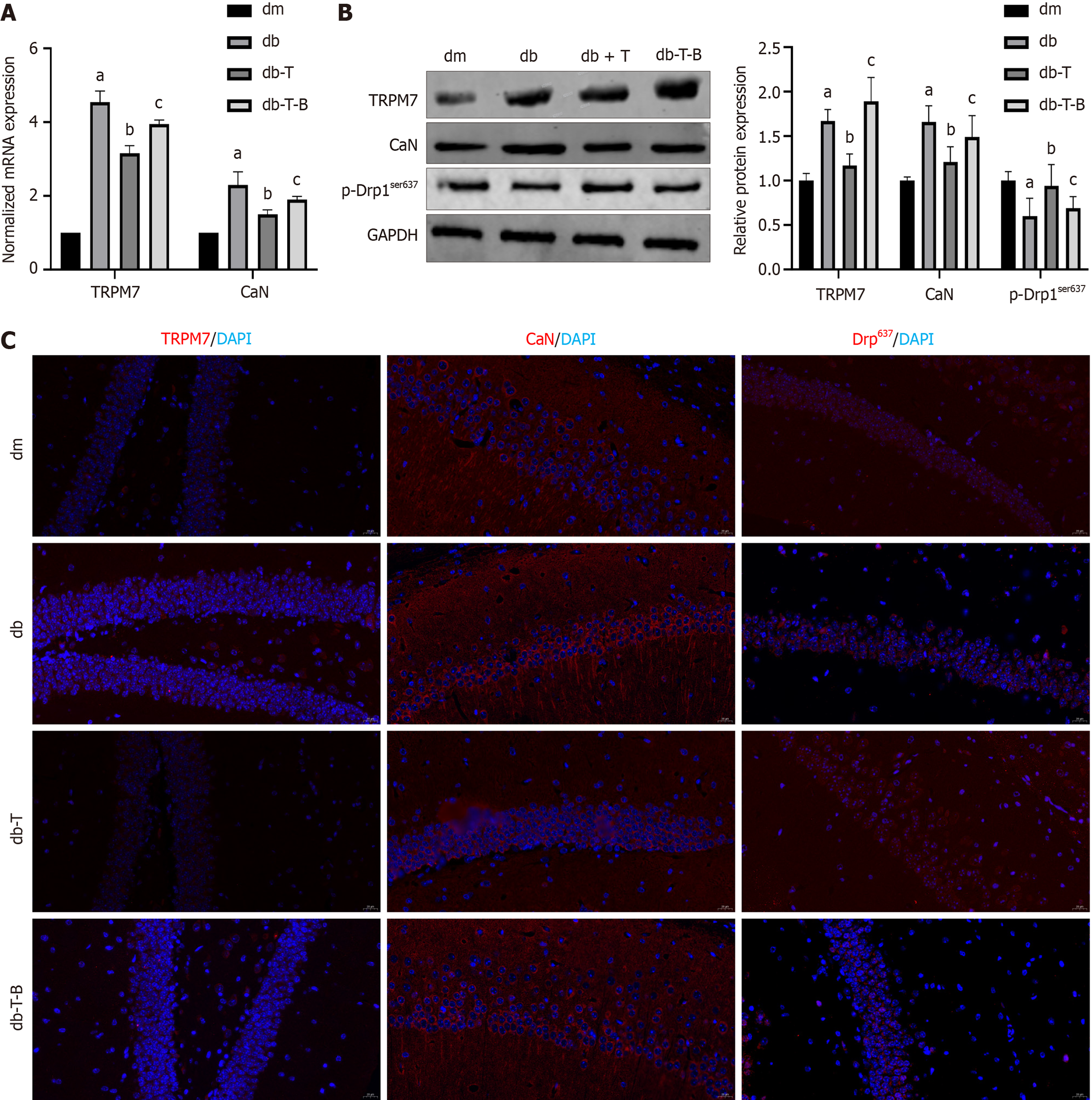
To gain a better comprehension of the mechanism of troxerutin protecting neuronal cells, we re-examined it in an animal model. After 6 weeks of treatment with Troxerutin, the db-T-B group was additionally given bradykinin (TRPM7 agonist) for 2 weeks. Compared to the db-T group, the db-T-B group mice showed an increase in escape latency (P = 0.0392) (Figure 2C), a decrease in crossing platforms (P = 0.0041) (Figure 2D), a decrease in relative recognition index of new objects (P = 0.0127) (Figure 2E), and a decrease in nesting score (P = 0.0008) (Figure 2F). The expression of TRPM7 (P < 0.0001), CaN (P = 0.0189, P = 0.0324) mRNA and protein in hippocampal tissue was increased, whereas the expression level of p-Drp1ser637 protein decreased (P = 0.0289) (Figure 12A and B); The immunofluorescence results also showed a similar pattern (Figure 12C). These results suggest that bradykinin weakens the effect of Troxerutin, further demon
DCD is a complication of diabetes that affects the central nervous system and is more widespread than AD, affecting people of all ages. Studies show that Ca2+ overload-induced mitochondrial fission plays a critical role in DCD, calcium overload induces pathological mitochondrial fission, establishing a crucial disease mechanism. In this research, HT22 cell and db/db mice models were employed to look into the effects of HG on TRPM7, CaN, and p-Drp1ser637, as well as the potential mechanism of troxerutin to ameliorate DCD. We found that: (1) TRPM7 expression was increased in DCD mice and HG-induced hippocampal neurons; (2) TRPM7 mediates mitochondrial fission by upregulating CaN/Drp1ser637; (3) The preventive application of Troxerutin was able to improve cognitive dysfunction in type 2 diabetic mice, as well as increase ATP production and reduce cell apoptosis in HG-induced hippocampal neurons; and (4) Using TRPM7 overexpression and agonists, we showed that Troxerutin reduces mitochondrial fission by downregulating TRPM7/CaN/Drp1ser637. Our findings suggest that TRPM7 is a potential target for the treatment of DCD, and Troxerutin improves mitochondrial fission and DCD by downregulating TRPM7/CaN/Drp1ser637.
TRPM7, a novel calcium ion channel, has been revealed to be significant in ischemic stroke and other neurodegenerative diseases by causing calcium overload[8,25,26]. Our previous study found that si-TRPM7 can improve DCD in type 1 diabetic rats. In this study, we further explored the role of TRPM7 in cognitive dysfunction in type 2 diabetes and delved into the specific mechanisms by which it affects cognitive impairment.
First, we found that TRPM7 expression is increased in hippocampal neurons treated with HG and in type 2 DCD mice. DCD is a complex pathological process involving multiple mechanisms, of which mitochondrial fission and fusion imbalance is an important pathogenesis. Studies have demonstrated that DCD mice have an augmented mitochondrial fission[6], however, the exact cause is still unknown. The activity of Drp1, a protein that regulates mitochondrial fission, is triggered by post-translational modifications, with phosphorylation being the most studied[7]. Dephosphorylation of ser637 can foster mitochondrial fission.
Our study revealed a decrease in the p-drp1ser637 protein level and an increase in mitochondrial fission in the db group of mice. Dephosphorylation of p-drp1ser637 is CaN dependent[5], and we also observed an increase in CaN mRNA and protein levels in the db group. TRPM7 activation leads to calcium influx and subsequent activation of CaN[27,28]. Therefore, we speculate that TRPM7 causes calcium overload, activating CaN/Drp1ser637, increasing mitochondrial fission, reducing ATP synthesis, and resulting in neuronal damage.
Next, we used the HT22 cells to investigate the effects of TRPM7 by suppression and overexpression. The results showed that in the NC + si-TRPM7 group, TRPM7 expression was decreased, CaN expression was decreased, p-Drp1ser637 expression was increased, ATP synthesis was increased, and cell apoptosis was reduced. Conversely, in the NC + pcDNA4-TRPM7 group, TRPM7 expression was increased, CaN expression was increased, p-Drp1ser637 expression was decreased, ATP synthesis was reduced, and cell apoptosis was enhanced. Furthermore, when the CaN inhibitor FK506 was added, p-Drp1ser637 expression was increased, ATP synthesis was increased, and cell apoptosis was reduced. These results suggest that the TRPM7/CaN/Drp1ser637 pathway plays a pivotal role in the increase of mitochondrial division mediated by HG.
Troxerutin, a flavonoid drug, has been observed to have beneficial effects on the brain. Our prior research[21,22] showed that Troxerutin can improve DCD in type 1 diabetic rats, yet the underlying mechanism is still unclear. Through in vitro experiments, it was observed that Troxerutin was able to reduce ATP loss and cell apoptosis in neurons caused by HG, while also decreasing TRPM7 and CaN expression and increasing p-drp1ser637 expression. However, when TRPM7 was overexpressed, the effects of Troxerutin were weakened. These results suggest that Troxerutin improves mito
Furthermore, our in vivo studies have also confirmed the neuroprotective effect of Troxerutin in DCD mice. Con
Overall, current research indicates that TRPM7 is an important target for the prevention of DCD, and that Troxerutin can improve DCD. Specifically, Troxerutin acts by inhibiting TRPM7, reducing calcium influx and CaN activation, thereby upregulating the phosphorylation of Drp1ser637, inhibiting mitochondrial fission, increasing ATP synthesis, and improving neuronal cell injury. Although we have demonstrated troxerutin’s influence on the TRPM7/CaN/Drp1ser637 pathway, the specific mechanistic basis for this modulation requires systematic investigation through additional fundamental studies.
We conclusively show that TRPM7 mediates mitochondrial fission by upregulating CaN/Drp1ser637, and provides evidence that Troxerutin improves mitochondrial function and neuronal cell damage by inhibiting the TRPM7/CaN/Drp1ser637 pathway. Inhibitors of TRPM7 may provide a novel approach for the management of DCD. However, it should be noted that the precise molecular mechanisms underlying Troxerutin's modulation of this pathway require further in-depth investigation.
| 1. | Luo A, Xie Z, Wang Y, Wang X, Li S, Yan J, Zhan G, Zhou Z, Zhao Y, Li S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci Biobehav Rev. 2022;137:104642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Yang Y, Zhao JJ, Yu XF. Expert Consensus on Cognitive Dysfunction in Diabetes. Curr Med Sci. 2022;42:286-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Hu Y, Zhou Y, Yang Y, Tang H, Si Y, Chen Z, Shi Y, Fang H. Metformin Protects Against Diabetes-Induced Cognitive Dysfunction by Inhibiting Mitochondrial Fission Protein DRP1. Front Pharmacol. 2022;13:832707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Maneechote C, Chunchai T, Apaijai N, Chattipakorn N, Chattipakorn SC. Pharmacological Targeting of Mitochondrial Fission and Fusion Alleviates Cognitive Impairment and Brain Pathologies in Pre-diabetic Rats. Mol Neurobiol. 2022;59:3690-3702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 5. | Song SB, Park JS, Jang SY, Hwang ES. Nicotinamide Treatment Facilitates Mitochondrial Fission through Drp1 Activation Mediated by SIRT1-Induced Changes in Cellular Levels of cAMP and Ca(2). Cells. 2021;10:612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Rosdah AA, Smiles WJ, Oakhill JS, Scott JW, Langendorf CG, Delbridge LMD, Holien JK, Lim SY. New perspectives on the role of Drp1 isoforms in regulating mitochondrial pathophysiology. Pharmacol Ther. 2020;213:107594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Cao Y, Sun W, Liu C, Zhou Z, Deng Z, Zhang M, Yan M, Yin X, Zhu X. Resveratrol ameliorates diabetic encephalopathy through PDE4D/PKA/Drp1 signaling. Brain Res Bull. 2023;203:110763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Lai YS, Chang CC, Chen YY, Nguyen TMH, Xu J, Chen YC, Chang YF, Wang CY, Chen PS, Lin SC, Peng IC, Tsai SJ, Chiu WT. Optogenetically engineered Ca2+ oscillation-mediated DRP1 activation promotes mitochondrial fission and cell death. J Cell Sci. 2023;136:jcs260819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Lin L, Xu H, Yao Z, Zeng X, Kang L, Li Y, Zhou G, Wang S, Zhang Y, Cheng D, Chen Q, Zhao X, Li R. Jin-Xin-Kang alleviates heart failure by mitigating mitochondrial dysfunction through the Calcineurin/Dynamin-Related Protein 1 signaling pathway. J Ethnopharmacol. 2024;335:118685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Ciocci Pardo A, González Arbeláez LF, Fantinelli JC, Aiello EA, Mosca SM. Calcineurin/P38MAPK/HSP27-dependent pathways are involved in the attenuation of postischemic mitochondrial injury afforded by sodium bicarbonate co-transporter (NBCe1) inhibition. Biochem Pharmacol. 2019;161:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Wong H, Levenga J, Cain P, Rothermel B, Klann E, Hoeffer C. RCAN1 overexpression promotes age-dependent mitochondrial dysregulation related to neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2015;130:829-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Yu X, Jia L, Yu W, Du H. Dephosphorylation by calcineurin regulates translocation of dynamin-related protein 1 to mitochondria in hepatic ischemia reperfusion induced hippocampus injury in young mice. Brain Res. 2019;1711:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Lee DG, Min JS, Lee HS, Lee DS. Isoliquiritigenin attenuates glutamate-induced mitochondrial fission via calcineurin-mediated Drp1 dephosphorylation in HT22 hippocampal neuron cells. Neurotoxicology. 2018;68:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Park J, Lee DG, Kim B, Park SJ, Kim JH, Lee SR, Chang KT, Lee HS, Lee DS. Iron overload triggers mitochondrial fragmentation via calcineurin-sensitive signals in HT-22 hippocampal neuron cells. Toxicology. 2015;337:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Nadezhdin KD, Correia L, Narangoda C, Patel DS, Neuberger A, Gudermann T, Kurnikova MG, Chubanov V, Sobolevsky AI. Structural mechanisms of TRPM7 activation and inhibition. Nat Commun. 2023;14:2639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Zhang S, Cao F, Li W, Abumaria N. TRPM7 kinase activity induces amyloid-β degradation to reverse synaptic and cognitive deficits in mouse models of Alzheimer's disease. Sci Signal. 2023;16:eade6325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Zhao Y, Kiss T, DelFavero J, Li L, Li X, Zheng L, Wang J, Jiang C, Shi J, Ungvari Z, Csiszar A, Zhang XA. CD82-TRPM7-Numb signaling mediates age-related cognitive impairment. Geroscience. 2020;42:595-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Huang Y, Leng TD, Inoue K, Yang T, Liu M, Horgen FD, Fleig A, Li J, Xiong ZG. TRPM7 channels play a role in high glucose-induced endoplasmic reticulum stress and neuronal cell apoptosis. J Biol Chem. 2018;293:14393-14406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Aydın B, Nazıroğlu M. Involvement of TRPM7 Channel on the Induction of Diabetic Neuropathic Pain in Mice: Protective Role of Selenium and Curcumin. Biol Trace Elem Res. 2023;201:2377-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Zhang QJ, Li J, Zhang SY. Effects of TRPM7/miR-34a Gene Silencing on Spatial Cognitive Function and Hippocampal Neurogenesis in Mice with Type 1 Diabetes Mellitus. Mol Neurobiol. 2018;55:1568-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Turlova E, Wong R, Xu B, Li F, Du L, Habbous S, Horgen FD, Fleig A, Feng ZP, Sun HS. TRPM7 Mediates Neuronal Cell Death Upstream of Calcium/Calmodulin-Dependent Protein Kinase II and Calcineurin Mechanism in Neonatal Hypoxic-Ischemic Brain Injury. Transl Stroke Res. 2021;12:164-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Wu W, Wang X, Liao L, Chen J, Wang Y, Yao M, Zhu L, Li J, Wang X, Chen AF, Zhang G, Zhang Z, Bai Y. The TRPM7 channel reprograms cellular glycolysis to drive tumorigenesis and angiogenesis. Cell Death Dis. 2023;14:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 23. | Hotka M, Cagalinec M, Hilber K, Hool L, Boehm S, Kubista H. L-type Ca(2+) channel-mediated Ca(2+) influx adjusts neuronal mitochondrial function to physiological and pathophysiological conditions. Sci Signal. 2020;13:eaaw6923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Gao M, Kang Y, Zhang L, Li H, Qu C, Luan X, Liu L, Zhang S. Troxerutin attenuates cognitive decline in the hippocampus of male diabetic rats by inhibiting NADPH oxidase and activating the Nrf2/ARE signaling pathway. Int J Mol Med. 2020;46:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Zhang S, Li H, Zhang L, Li J, Wang R, Wang M. Effects of troxerutin on cognitive deficits and glutamate cysteine ligase subunits in the hippocampus of streptozotocin-induced type 1 diabetes mellitus rats. Brain Res. 2017;1657:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Babaei-Kouchaki S, Babapour V, Panahi N, Badalzadeh R. Effect of troxerutin on oxidative stress and expression of genes regulating mitochondrial biogenesis in doxorubicin-induced myocardial injury in rats. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Li J, Ren H, Wang Y, Hoang DM, Li Y, Yao X. Mechanism of Stat1 in the neuronal Ca(2+) overload after intracerebral hemorrhage via the H3K27ac/Trpm7 axis. J Neurophysiol. 2022;128:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Schappe MS, Stremska ME, Busey GW, Downs TK, Seegren PV, Mendu SK, Flegal Z, Doyle CA, Stipes EJ, Desai BN. Efferocytosis requires periphagosomal Ca(2+)-signaling and TRPM7-mediated electrical activity. Nat Commun. 2022;13:3230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |