Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108209
Revised: April 24, 2025
Accepted: May 16, 2025
Published online: July 15, 2025
Processing time: 98 Days and 23.4 Hours
This editorial delves into the potential of systemic immune indicators (SIIs) as early predictors of renal damage in children with newly diagnosed type 1 diabetes mellitus. By exploring the recent study published by Cao et al, this article aims to highlight the importance of early detection and intervention. This study comprehensively analyzes various SIIs, examining their correlation with renal compli
Core Tip: Taking into consideration the growing concern of diabetic nephropathy in children with type 1 diabetes mellitus and examining the promise of systemic immune indicators as predictive tools, healthcare providers have the opportunity to reshape the narrative of diabetes management, offering hope for a healthier future for pediatric patients. It is also imperative that healthcare providers and researchers continue to explore the role of systemic immune indicators in diabetes management to further enhance the understanding and treatment of this chronic condition.
- Citation: Nagoba BS, Gavkare AM, Nanaware N, Mumbre SS, Bhavthankar S. Systemic immune indicators: Early predictors of renal damage in children with newly diagnosed type 1 diabetes mellitus. World J Diabetes 2025; 16(7): 108209
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/108209.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.108209
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease that typically manifests in childhood, imposing significant challenges for patients and their families. Its prevalence has been increasing steadily, bringing a host of complications that can significantly impact quality of life. While advancements in medical science have greatly improved the management of this condition, the emergence of complications such as diabetic nephropathy remains a daunting hurdle. Diabetic nephropathy, being a serious renal complication, often develops gradually, making early detection critical for effective intervention[1]. Predicting renal damage in T1DM patients, especially children, poses a challenge for healthcare providers. Traditional diagnostic tools often identify complications at a stage when interventions can only mitigate damage rather than prevent it. The search for innovative, non-invasive, and accurate markers to predict complications early has led researchers to explore systemic immune indicators (SIIs), a promising frontier in diabetes care[2].
The recently published study[3] in the World Journal of Diabetes entitled “Systemic immune indicators for predicting renal damage in newly diagnosed type 1 diabetic children” marks a significant breakthrough in this endeavor. The study introduces the potential of SIIs, including metrics such as the SII, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio, as reliable predictors of renal damage in newly diagnosed T1DM pediatric patients[3]. These markers, derived from routine blood tests, could revolutionize diabetes care by enabling proactive management and improving long-term outcomes. This editorial seeks to delve into the findings of this study, providing an overview of T1DM in children, exploring the growing concern of diabetic nephropathy, and examining the promise of SIIs as predictive tools. By integrating these insights into clinical practice, healthcare providers have the opportunity to reshape the narrative of diabetes management, offering hope for a healthier future for pediatric patients.
T1DM is an autoimmune disease predominantly diagnosed during childhood or adolescence. Unlike type 2 diabetes, which is often influenced by lifestyle factors, T1DM is the result of destruction of the insulin-producing beta cells of the pancreas by auto-antibodies. This destruction leads to an absolute deficiency of insulin, a hormone crucial for regulating blood glucose levels. T1DM is characterized by its abrupt onset and the severity of its symptoms in children. Common symptoms include increased thirst (polydipsia), frequent urination (polyuria), unintended weight loss, fatigue, and blurred vision. Diagnosing T1DM in children typically involves measuring blood glucose levels, detecting autoantibodies associated with the autoimmune process, and assessing C-peptide levels to evaluate residual beta-cell function[1].
Managing T1DM in children presents unique challenges due to the dynamic nature of their growth and development. Insulin therapy remains the cornerstone of treatment, with a combination of long-acting and rapid-acting insulin tailored to each child’s needs. Blood glucose monitoring, whether through traditional methods or continuous glucose monitoring systems, plays a vital role in ensuring optimal glycemic control. Moreover, children and their families require education on carbohydrate counting, physical activity adjustments, and recognizing the signs of hypoglycemia and hyperglycemia.
Beyond the physical symptoms, T1DM can have a profound psychosocial impact on children and their caregivers. The constant vigilance required to manage the disease, combined with the fear of complications, can lead to emotional stress. Schools and peer interactions can also pose challenges, underscoring the importance of a supportive environment[4]. T1DM in children is not merely a medical condition but a multifaceted challenge requiring a holistic approach to care. Through advancements in technology, improved understanding of its pathophysiology, and supportive healthcare systems, children with T1DM can lead fulfilling lives while minimizing the risks associated with this chronic illness.
Diabetic nephropathy, also known as diabetic kidney disease, is a serious complication of diabetes that affects the kidneys. It is characterized by damage to the small blood vessels in the kidneys due to prolonged hyperglycemia, leading to impaired kidney function. This condition progresses through stages, starting with increased albuminuria, eventually leading to end-stage renal disease if untreated. Recent study highlights the role of inflammation, oxidative stress, and podocyte injury in the progression of diabetic nephropathy. Sodium-glucose cotransporter type 2 inhibitors have emerged as promising treatments, offering renoprotective effects by reducing glucose levels and mitigating kidney damage[5]. Understanding the molecular mechanisms and biomarkers associated with diabetic nephropathy is crucial for early diagnosis and effective management.
Studies indicate that diabetic nephropathy develops in approximately 25%-40% of individuals with T1DM, with early signs such as microalbuminuria appearing soon after disease onset[6]. In a study conducted in India, the prevalence of diabetic nephropathy among children and young people with T1DM was found to be 13.4%, with hypertension being a significant predictor[7]. Another study highlighted that childhood-onset T1DM is associated with a higher risk of diabetic nephropathy compared to later-onset cases. Early screening and timely intervention are crucial in preventing disease progression and reducing the risk of end-stage renal disease[8].
Diabetic nephropathy in pediatric patients can lead to significant long-term health challenges. Persistent albuminuria and declining kidney function are common outcomes, which may progress to end-stage renal disease requiring dialysis or kidney transplantation. Additionally, children with diabetic nephropathy face an increased risk of cardiovascular complications, including hypertension and heart disease, due to the systemic effects of kidney dysfunction. The condition also impacts growth and development, as chronic kidney disease can interfere with normal physical and hormonal growth processes. Furthermore, the psychological burden of managing a chronic illness from a young age can affect mental health and quality of life. Early intervention, including strict glycemic control, blood pressure management, and regular monitoring of kidney function, is crucial to mitigate these long-term impacts[9].
Early detection of complications in children with T1DM is a cornerstone of effective disease management. Complications such as diabetic nephropathy, retinopathy, and neuropathy are often insidious, developing silently before symptoms become apparent. By the time clinical signs manifest, the damage may already be substantial, limiting the effectiveness of interventions. This highlights the critical need for predictive tools and markers that can identify these complications at a nascent stage.
The kidneys, for instance, have remarkable compensatory mechanisms, which often delay the clinical detection of diabetic nephropathy until significant damage has occurred. Early identification of microalbuminuria, an early marker of kidney dysfunction, can enable healthcare providers to intervene promptly, potentially halting or slowing disease progression before it leads to chronic kidney disease or end-stage renal failure.
Complications due to T1DM affect the quality of life and increase the long-term health burden. For example, a child with diabetic nephropathy faces a heightened risk of cardiovascular disease in adulthood. Early detection allows for personalized interventions, including optimized blood glucose control, lifestyle modifications, and pharmacological therapies, to mitigate these risks and improve overall health outcomes.
From a public health perspective, early detection is cost-effective. Managing advanced complications involves prolonged hospital stays, invasive procedures, and specialized care, which can be financially and emotionally taxing for families. Conversely, preventive measures based on early identification of at-risk patients can significantly reduce healthcare costs while improving patient outcomes.
Beyond clinical benefits, early detection empowers families and patients by fostering greater awareness of potential risks. It enables caregivers to adopt proactive strategies to safeguard their child’s health, including adherence to treatment regimens and regular follow-ups. This proactive approach helps build confidence and resilience in managing the disease.
The study on SIIs underscores the transformative potential of emerging diagnostic markers. SIIs such as the SII and systemic inflammation response index (SIRI) are promising because they are derived from routine blood tests and are cost-effective. Their ability to predict renal damage before clinical symptoms emerge could revolutionize the paradigm of diabetes care, shifting the focus from reactive to proactive management[10].
A large-scale observational study analyzing data from the National Health and Nutrition Examination Survey (1999-2018) assessed the association between log2-transformed SII and SIRI levels with chronic kidney disease prevalence using weighted logistic regression models. Additionally, Cox regression models were utilized to estimate mortality risk, with subgroup and sensitivity analyses confirming the robustness of findings[11]. Another prospective study examined the predictive accuracy of various systemic inflammatory indicators, including the SIIs, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio, using Kaplan-Meier survival curves, restricted cubic spline modeling, and receiver operating characteristic analysis[12]. These methodological approaches have demonstrated that elevated SII and SIRI levels are significantly associated with increased chronic kidney disease incidence and mortality risk, reinforcing their potential as early biomarkers for renal damage. Thus, early detection of complications in T1DM is not merely a clinical necessity, it is a holistic strategy that ensures better physical, emotional, and financial outcomes for pediatric patients and their families. By embracing innovative approaches like SIIs, the medical community can move closer to achieving comprehensive and compassionate diabetes care.
SIIs have emerged as a promising tool in medical diagnostics, particularly in identifying complications associated with chronic diseases like T1DM. These indicators, derived from routine blood tests, leverage easily measurable immune system components, such as neutrophils, lymphocytes, and platelets, to assess systemic inflammatory responses. The simplicity and cost-effectiveness of SIIs make them highly accessible, even in resource-limited settings, offering great potential for widespread clinical use[13].
SIIs are quantitative measures that reflect the balance and interplay of different immune cells in the body. For example, a SII is calculated using the formula: Platelet count × neutrophil count/lymphocyte count. Similarly, the SIRI considers neutrophils, monocytes, and lymphocytes to provide a nuanced view of the inflammatory state. These indicators are particularly valuable because they are based on standard complete blood count parameters routinely assessed in clinical practice[14].
T1DM is characterized by an autoimmune destruction of pancreatic beta cells, which leads to systemic inflammatory responses. Over time, this chronic inflammation can contribute to complications such as diabetic nephropathy. Traditional markers for predicting complications often require specialized and costly diagnostic techniques, limiting their utility for regular monitoring. In contrast, SIIs provide a readily available and economical alternative, offering a non-invasive way to monitor the systemic inflammation associated with T1DM and its complications.
The ability of SIIs to predict complications like diabetic nephropathy lies in their sensitivity to inflammatory changes. For example, elevated neutrophil and monocyte counts, as reflected in SIIs and SIRI, indicate heightened inflammation, a known precursor to organ damage and a low lymphocyte count, another component of these indices, may signal an impaired immune response, further exacerbating the risk of complications. This underscores the potential of SIIs as reliable tools for risk stratification and early intervention.
Integrating SIIs into diabetes care protocols has the potential to bridge significant gaps in early detection. By providing actionable insights into a patient’s inflammatory status, SIIs can help clinicians to identify high-risk patients before clinical symptoms of complications, such as microalbuminuria, become apparent. This early warning system can facilitate timely therapeutic interventions, thereby preventing or delaying the progression of complications such as diabetic nephropathy[15]. This emphasizes that SIIs represent a paradigm shift in managing T1DM and its complications. Their accessibility, affordability, and predictive capabilities position them as indispensable tools in modern healthcare, particularly for pediatric patients who stand to benefit immensely from early detection and intervention.
A study published in the Egyptian Pediatric Association Gazette investigated the association between systemic immune markers and renal function in children with T1DM. The researchers found that elevated levels of inflammatory markers, such as C-reactive protein and interleukin-6, were associated with lower estimated glomerular filtration rate and increased risk of renal damage in these children. This suggests that SIIs can be early predictors of renal complications in this population[16]. Another study explored the role of immune cells in the development of diabetic nephropathy in children with T1DM. This study reported that higher levels of circulating T cells and pro-inflammatory cytokines were associated with the progression of renal damage in these children. This highlights the importance of immune dysregulation in the pathogenesis of diabetic nephropathy and the potential of immune markers as predictive tools[17]. Recent technological advancements have also allowed for the development of novel immune-based biomarkers for predicting renal damage in type 1 diabetic children. A study by Zhu et al[18] utilized a machine learning approach to identify a panel of immune markers that could accurately predict the risk of diabetic nephropathy in this population. This personalized approach holds promise for early detection and intervention in children at high risk of renal complications. In conclusion, the use of SIIs for predicting renal damage in newly diagnosed T1DM children is a rapidly evolving field with significant implications for clinical practice. Current research suggests that inflammatory markers, immune cells, and novel biomarkers can be valuable tools for identifying children at risk of diabetic nephropathy.
A study by Elmeazawy et al[16] investigated the role of SIIs and SIRI in predicting early renal impairment in children with T1DM. It found that these indices, calculated using blood parameters like neutrophils, lymphocytes, and platelets, were significantly associated with diabetic nephropathy. These markers are affordable and accessible, making them promising tools for early detection.
A research study highlighted the prognostic value of SIIs and SIRI in patients with diabetic nephropathy, as elevated levels of these indices were linked to increased risks of all-cause, cardiovascular, and kidney disease mortality, emphasizing their utility in clinical assessments[19].
A study evaluated the prognostic value of SIIs in patients undergoing immune-based therapy for hepatocellular carcinoma. While not specific to diabetes, this research demonstrates the broader applicability of SIIs in predicting treatment outcomes and survival, which could inspire similar approaches in diabetic care[20].
A systematic review by Kou et al[21] analyzed the relationship between SIIs and survival outcomes in cancer patients receiving immunotherapy. The findings suggest that high SII levels correlate with poorer treatment responses and prognosis, reinforcing the importance of SIIs in personalized medicine.
A study provided direct evidence on the relationship between SIIs, SIRI, and cumulative glycemic exposure in children with T1DM[22]. This study involved 159 pediatric patients and found that elevated SII and SIRI levels correlated with increased glycemic burden, suggesting a potential link between systemic inflammation and long-term renal complications.
While SIIs, such as the SII and SIRI, have shown promise as early predictors of renal damage, it is acknowledged that their interpretation must consider several confounding factors. One of the primary limitations of SIIs is their non-specific nature, as they reflect generalized immune activation rather than disease-specific inflammatory pathways. This means that elevated SII and SIRI levels may not exclusively indicate renal impairment but could also be influenced by acute infections, autoimmune conditions, or systemic inflammatory responses unrelated to diabetic nephropathy. Additionally, medications such as corticosteroids, immunosuppressants, and certain antihyperglycemic agents can significantly alter immune parameters, leading to fluctuations in SII and SIRI values. A study examined the impact of immunomodulatory therapies on systemic inflammation markers and found that SII and SIRI levels varied significantly in patients receiving corticosteroids or biologic agents[23]. This highlights the need for careful interpretation of these indices in clinical settings. Furthermore, another study demonstrated that SII values can be influenced by age-related inflammatory changes, making it essential to establish population-specific reference ranges[24]. In pediatric T1DM populations, additional studies are required to determine whether these markers maintain predictive accuracy independent of transient inflammatory fluctuations.
Recent studies have identified lumican, an extracellular matrix-related biomarker, as a potential predictor of diabetic nephropathy progression. Lumican plays a crucial role in renal fibrosis and extracellular matrix remodeling, which are hallmarks of diabetic nephropathy[25]. Additionally, urinary epidermal growth factor has been recognized as a non-invasive biomarker for early renal decline, with studies demonstrating its correlation with glomerular filtration rate and renal tubular function[26]. Furthermore, circulating microRNAs, particularly microRNA-21 and microRNA-192, have been implicated in diabetic nephropathy pathogenesis, offering insights into inflammatory and fibrotic pathways[27].
Some promising areas for future research in SIIs and T1DM: (1) Refining SII metrics: Investigate ways to enhance the accuracy and specificity of SII calculations, accounting for factors like age, gender, and comorbidities; (2) Predictive models for disease progression: Develop advanced algorithms integrating SIIs with genetic, environmental, and lifestyle factors to predict the onset and progression of T1DM; (3) Role of SIIs in early diagnosis: Explore the potential of SIIs as biomarkers for detecting preclinical stages of T1DM, enabling earlier interventions; (4) Impact of immunotherapy: Study how SIIs can guide and monitor the effectiveness of immunotherapy in preserving pancreatic beta-cell function; (5) Longitudinal studies: Long-term studies should be conducted to assess the correlation between SII levels and renal damage progression in T1DM patients; (6) Integration with digital health tools: Investigate the use of wearable devices and mobile apps to track SIIs in real time, providing actionable insights for personalized care; (7) Global and multicentric research: Validate the utility of SIIs across diverse populations and healthcare settings to ensure widespread applicability; and (8) Combination biomarker approaches: Explore the synergistic use of SIIs with other biomarkers to improve diagnostic and prognostic accuracy. These areas could significantly advance the understanding and application of SIIs in managing T1DM.
Future research should focus on establishing robust methodological frameworks to explore the early immune indicators of renal damage in children with newly diagnosed T1DM. Longitudinal cohort studies tracking immune responses over time could provide deeper insights into disease progression. Additionally, multi-center clinical trials examining standardized immune biomarker panels could enhance diagnostic precision. A significant knowledge gap exists in understanding the interplay between systemic inflammation and renal dysfunction at a molecular level, warranting further exploration through advanced immunogenomic and proteomic approaches. Prioritizing research in these areas is critical for early intervention strategies, potentially mitigating long-term renal complications. By integrating these specific directions, future investigations can offer more targeted and impactful contributions to clinical practice.
A robust sample size is crucial to ensure the statistical power and generalizability of findings related to SIIs in newly diagnosed T1DM. Future studies should employ appropriate sample size estimation methods, such as power analysis, to ensure adequate representation of diverse patient populations. Additionally, potential biases, including selection bias, measurement error, and confounding variables, must be rigorously assessed to enhance the reliability of findings. Strategies such as randomization, standardized diagnostic criteria, and multivariate adjustments should be incorporated to mitigate these biases. Before SIIs can be implemented in routine clinical practice, comprehensive validation studies are necessary, utilizing external cohorts and multi-center trials to assess reproducibility and diagnostic accuracy. Establishing standardized protocols and threshold values for immune biomarkers will further refine their clinical applicability, paving the way for their integration into precision medicine strategies.
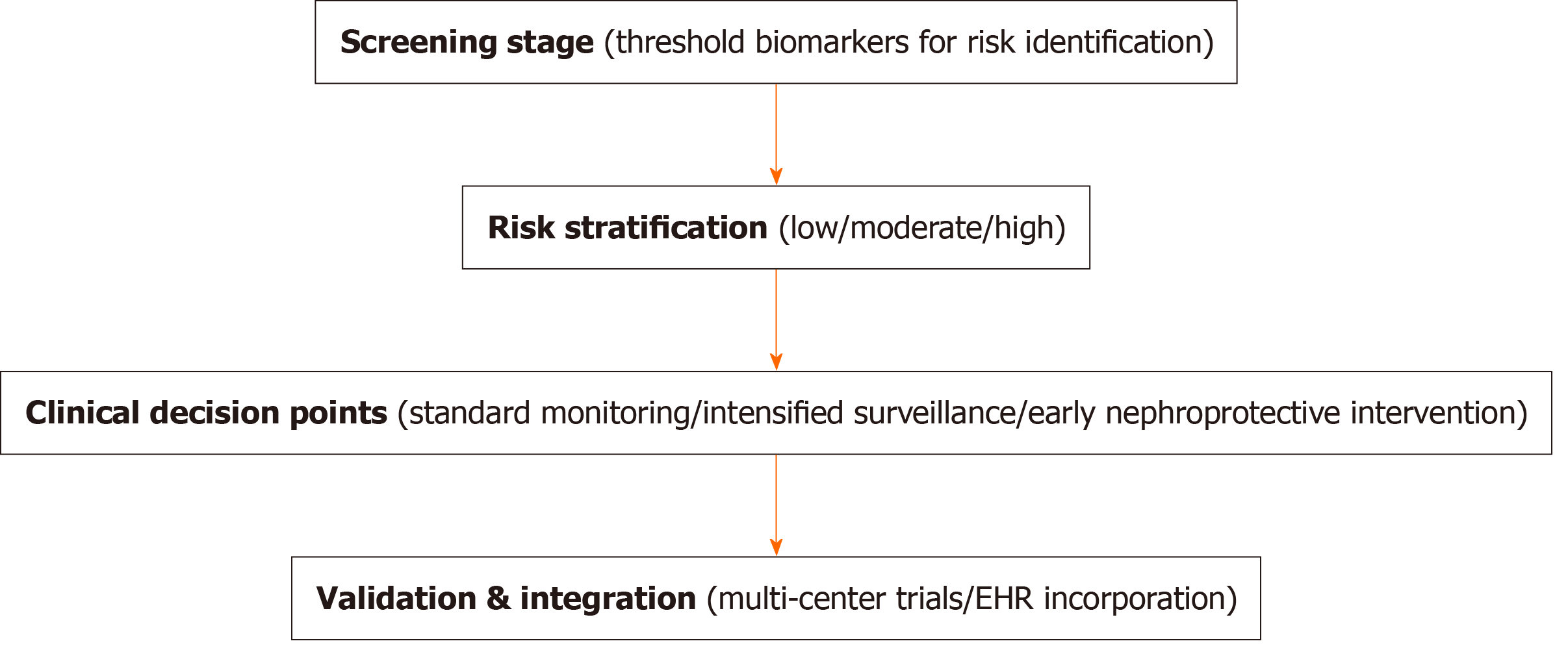
To enhance the clinical applicability of SIIs in pediatric diabetes care, a structured decision framework for early identification and risk stratification of renal complications was proposed. This includes: (1) Early screening stage: Defining thresholds for immune biomarkers that indicate potential renal risk in newly diagnosed T1DM; (2) Risk stratification: Categorizing patients based on biomarker levels into low-, moderate-, and high-risk groups; (3) Clinical decision points: Specifying intervention strategies for each risk category, such as intensified monitoring, early nephroprotective treatment, or additional diagnostics; and (4) Validation and integration: Ensuring reproducibility of the algorithm through multi-center validation and integrating it into electronic health records for streamlined clinical use. A conceptual visual schematic is shown in Figure 1.
The study highlights the significance of SIIs in predicting renal damage in newly diagnosed T1DM children. By integrating these markers into clinical practice, healthcare providers can improve outcomes and pave the way for a brighter future in diabetes management. Continued research and collaboration are essential to unlock the full potential of these promising tools.
| 1. | Popoviciu MS, Kaka N, Sethi Y, Patel N, Chopra H, Cavalu S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J Pers Med. 2023;13:422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 2. | Rivetti G, Hursh BE, Miraglia Del Giudice E, Marzuillo P. Acute and chronic kidney complications in children with type 1 diabetes mellitus. Pediatr Nephrol. 2023;38:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Cao LF, Xu QB, Yang L. Systemic immune indicators for predicting renal damage in newly diagnosed type 1 diabetic children. World J Diabetes. 2025;16:104482. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Bahal M, Pande V, Dua J, Mane S. Advances in Type 1 Diabetes Mellitus Management in Children. Cureus. 2024;16:e67377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Lim AKh. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 6. | Lin YB; Taiwan Diabetes Registry Study Group, Chang TJ. Age at onset of type 1 diabetes between puberty and 30 years old is associated with increased diabetic nephropathy risk. Sci Rep. 2024;14:3611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Karguppikar M, Oza C, Shah N, Khadilkar V, Gondhalekar K, Khadilkar A. Prevalence of nephropathy in Indian children and youth with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2022;35:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Muntean C, Starcea IM, Banescu C. Diabetic kidney disease in pediatric patients: A current review. World J Diabetes. 2022;13:587-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 9. | White NH. Long-term Outcomes in Youths with Diabetes Mellitus. Pediatr Clin North Am. 2015;62:889-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Sochett E, Daneman D. Early diabetes-related complications in children and adolescents with type 1 diabetes. Implications for screening and intervention. Endocrinol Metab Clin North Am. 1999;28:865-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Huang P, Mai Y, Zhao J, Yi Y, Wen Y. Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: observational study of 40,937 adults. Inflamm Res. 2024;73:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Nie Y, Wu J, Li C, Zheng L, Zhu B, Min Y, Ling T, Liu X. Association between systemic inflammatory indicators with the survival of chronic kidney disease: a prospective study based on NHANES. Front Immunol. 2024;15:1365591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 13. | Tian BW, Yang YF, Yang CC, Yan LJ, Ding ZN, Liu H, Xue JS, Dong ZR, Chen ZQ, Hong JG, Wang DX, Han CL, Mao XC, Li T. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy. 2022;14:1481-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Wijeratne T, Murphy MJ, Wijeratne C, Martelletti P, Karimi L, Apostolopoulos V, Sales C, Riddell N, Crewther SG. Serial systemic immune inflammation indices: markers of acute migraine events or indicators of persistent inflammatory status? J Headache Pain. 2025;26:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Aljuraiban GS, Alharbi FJ, Aljohi AO, Almeshari AZ, Alotaibi MN, AlShammari SS, Al-Musharaf S, Aldhwayan MM, Abudawood M. Systemic Immune-Inflammation Index in Relation to Diabetes Markers in Saudi Adults: A Retrospective Study. Medicina (Kaunas). 2024;60:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Elmeazawy R, El Shall S, Abdelsamea MZ, Emara MH. Systemic immune-inflammatory index and systemic inflammation response index in predicting renal impairment in children with type 1 diabetes mellitus. Egypt Pediatric Association Gaz. 2024;72:49. [DOI] [Full Text] |
| 17. | Peng QY, An Y, Jiang ZZ, Xu Y. The Role of Immune Cells in DKD: Mechanisms and Targeted Therapies. J Inflamm Res. 2024;17:2103-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Zhu Y, Zhang Y, Yang M, Tang N, Liu L, Wu J, Yang Y. Machine Learning-Based Predictive Modeling of Diabetic Nephropathy in Type 2 Diabetes Using Integrated Biomarkers: A Single-Center Retrospective Study. Diabetes Metab Syndr Obes. 2024;17:1987-1997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Zhang F, Han Y, Mao Y, Li W. The systemic immune-inflammation index and systemic inflammation response index are useful for predicting mortality in patients with diabetic nephropathy. Diabetol Metab Syndr. 2024;16:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | He T, Xu B, Wang LN, Wang ZY, Shi HC, Zhong CJ, Zhu XD, Shen YH, Zhou J, Fan J, Sun HC, Hu B, Huang C. The prognostic value of systemic immune-inflammation index in patients with unresectable hepatocellular carcinoma treated with immune-based therapy. Biomark Res. 2025;13:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Kou J, Huang J, Li J, Wu Z, Ni L. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: a systematic review and metaanalysis. Clin Exp Med. 2023;23:3895-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Ozde S, Akture G, Ozel MA, Yavuzyilmaz F, Arslanoglu I, Ozde C, Kayapinar O, Coskun G. Evaluation of the systemic-immune inflammation index (SII) and systemic immune-inflammation response index (SIRI) in children with type 1 diabetes mellitus and its relationship with cumulative glycemic exposure. J Pediatr Endocrinol Metab. 2024;37:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Yang CH, Wang XY, Zhang YH, Ding N. SIRI and SII as potential biomarkers of disease activity and lupus nephritis in systemic lupus erythematosus. Front Immunol. 2025;16:1530534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Cataltepe E, Ceker E, Fadiloglu A, Gungor F, Karakurt N, Ulger Z, Varan HD. Association between the systemic immune-inflammation index and sarcopenia in older adults: a cross-sectional study. BMC Geriatr. 2025;25:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Tao Y, Liu Y, Wang Z, Tang L, Zhang Y, Zheng S, Wang R, Wei K, Liu S. Lumican as a potential biomarker for diabetic nephropathy. Ren Fail. 2025;47:2480245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Shetty S, Suvarna R, Awasthi A, Bhojaraja MV, Pappachan JM. Emerging Biomarkers and Innovative Therapeutic Strategies in Diabetic Kidney Disease: A Pathway to Precision Medicine. Diagnostics (Basel). 2025;15:973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Li B, Zhao X, Xie W, Hong Z, Zhang Y. Integrative analyses of biomarkers and pathways for diabetic nephropathy. Front Genet. 2023;14:1128136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |