Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.104665
Revised: March 24, 2025
Accepted: May 7, 2025
Published online: June 15, 2025
Processing time: 168 Days and 9.8 Hours
Ferroptosis is a new type of programmed cell death caused by the accumulation of iron-dependent lipid peroxides, and it plays a role in the occurrence and progression of diverse diseases. Diabetic cardiomyopathy (DCM), a serious cardiovascular complication in patients with diabetes, eventually progresses to refractory heart failure (HF), which increases the risk of hospitalization for HF and cardiovascular death in patients with diabetes. Despite glycemic control, effective strategies to prevent DCM onset are currently lacking. Accumulating evidence suggests that ferroptosis is involved in oxidative stress, inflammation, and abnormal autophagy in diabetic myocardium, which plays an important role in myocardial apoptosis, hypertrophy, and cardiac fibrosis. The inhibition of ferroptosis can relieve DCM. Presently, ferroptosis inhibitors have been broadly suggested for the treatment of iron overload-related cardiomyopathy. This article reviewed relevant studies to offer a new therapeutic target for DCM.
Core Tip: Ferroptosis, a form of iron-dependent programmed cell death, plays a key role in the development of diabetic cardiomyopathy (DCM), contributing to oxidative stress, inflammation, and myocardial damage. Inhibiting ferroptosis can potentially alleviate DCM and prevent its progression to heart failure. This emerging pathway offers a promising therapeutic target for DCM, providing new hope for the management of DCM.
- Citation: Li GZ, Liu JY, Zhou H. Ferroptosis: A novel therapeutic target for diabetic cardiomyopathy. World J Diabetes 2025; 16(6): 104665
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/104665.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.104665
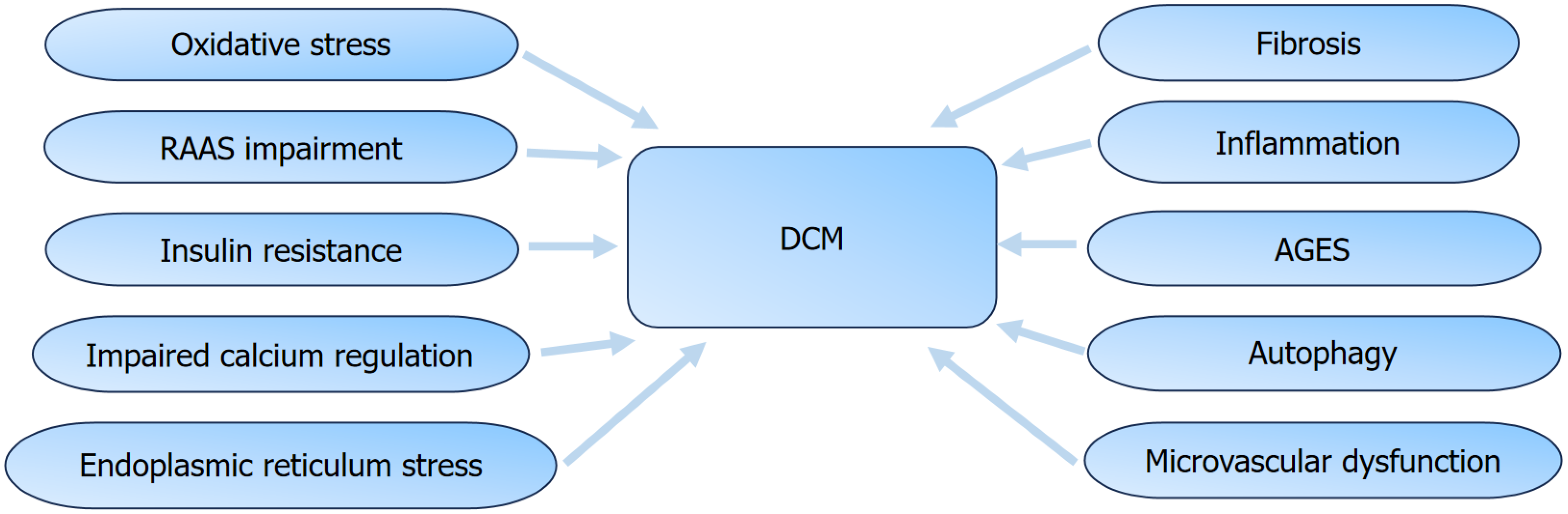
It was projected that by 2030, the global occurrence of diabetes will rise to 9%-10.2%[1]. Diabetes poses serious health risks, particularly due to its cardiovascular complications, which account for 50% to 80% of deaths among patients with diabetes[2]. Diabetic cardiomyopathy (DCM) was proposed by Rubler et al[3] in 1972 as a clinical condition due to the disturbances in glycolipid metabolism, resulting in coronary heart disease, hypertension, or valvular heart disease, ultimately progressing to heart failure[3-5]. DCM includes an early subclinical stage, characterized by diastolic dysfunction, which gradually progresses to systolic dysfunction, eventually progressing to refractory heart failure with reduced ejection fraction[6-8]. The pathogenesis of DCM is complex, including impaired cellular Ca2+ regulation, endoplasmic reticulum stress (ERS)[9], activation of the renin-angiotensin-aldosterone system, mitochondrial dysfunction[10], oxidative stress, inflammation, and dysfunction of myocardial mitochondria and microvasculature. These factors contribute to myocardial cell apoptosis, hypertrophy, and fibrosis[11,12].
Ferroptosis, first named in 2012, is a recently recognized form of non-apoptotic cell death characterized by intracellular iron overload, accumulation of free radicals, and glutathione (GSH) depletion, leading to increased lipid peroxidation[13-15]. It differs from autophagy, necrosis, and apoptosis in terms of morphology, genetics, and biochemistry. Elevated iron-dependent reactive oxygen species (ROS) production and redox imbalance, promote ferroptosis by causing peroxidation of polyunsaturated fatty acids (PUFAs) in cell membranes. Free iron within cells accelerates the production of lipid peroxides through the Fenton reaction, while decreased GSH synthesis and increased consumption of GSH peroxidase 4 (GPX4) further promote lipid radical generation and cell death. Therefore, the main regulatory mechanisms of ferroptosis are iron overload, lipid peroxidation, and GSH synthesis and metabolism[16]. Currently, researchers are investigating the pathological roles of ferroptosis in different diseases, including brain ischemia, gastrointestinal diseases, tumors, cancers, Parkinson’s syndrome, and neurodegenerative diseases[17,18]. Recent studies have demonstrated that ferroptosis is closely linked to the pathogenesis of cardiovascular diseases (CVDs)[16]. Diabetes mellitus (DM) is a slowly progressive inflammatory metabolic disease characterized by pancreatic β-cell dysfunction. Ferroptosis promotes the occurrence of diabetes and its complications by activating inflammation and increasing lipid peroxidation[19]. Li et al[20] have confirmed that ferroptosis in pancreatic β-cells of patients with type 2 DM (T2DM) can be inhibited by quercetin, with the mechanism involving the inhibition of iron overload, GSH depletion, and lipid peroxidation. Diabetic kidney disease, occurring in approximately 30%-40% of patients with diabetes, is among the serious complications of diabetes[20]. Zhao et al[21] have suggested that cadmium-induced iron depletion in renal tubular epithelial cells may be considerably associated with autophagy mediated by ERS. Ferroptosis also worsens diabetes-related hepatic metabolic and functional disorders, contributing to liver fibrosis and the progression of nonalcoholic fatty liver disease[22].
Growing evidence from a number of studies have shown that ferroptosis plays a critical role in the development of DCM[23-25]. Numerous studies have indicated that inhibiting ferroptosis is beneficial for alleviating myocardial injury in DCM and improving cardiac dysfunction[26,27]. The application of ferroptosis inhibitors can delay the progression of DCM by reducing oxidative stress, inflammation, and myocardial remodeling in patients with diabetes[28,29]. Thus, suppressing ferroptosis has provided new insights for the clinical treatment of DCM. Therefore, the present review aimed to discuss the mechanisms of ferroptosis in the development of DCM and summarize the current knowledge on the prevention and treatment of DCM.
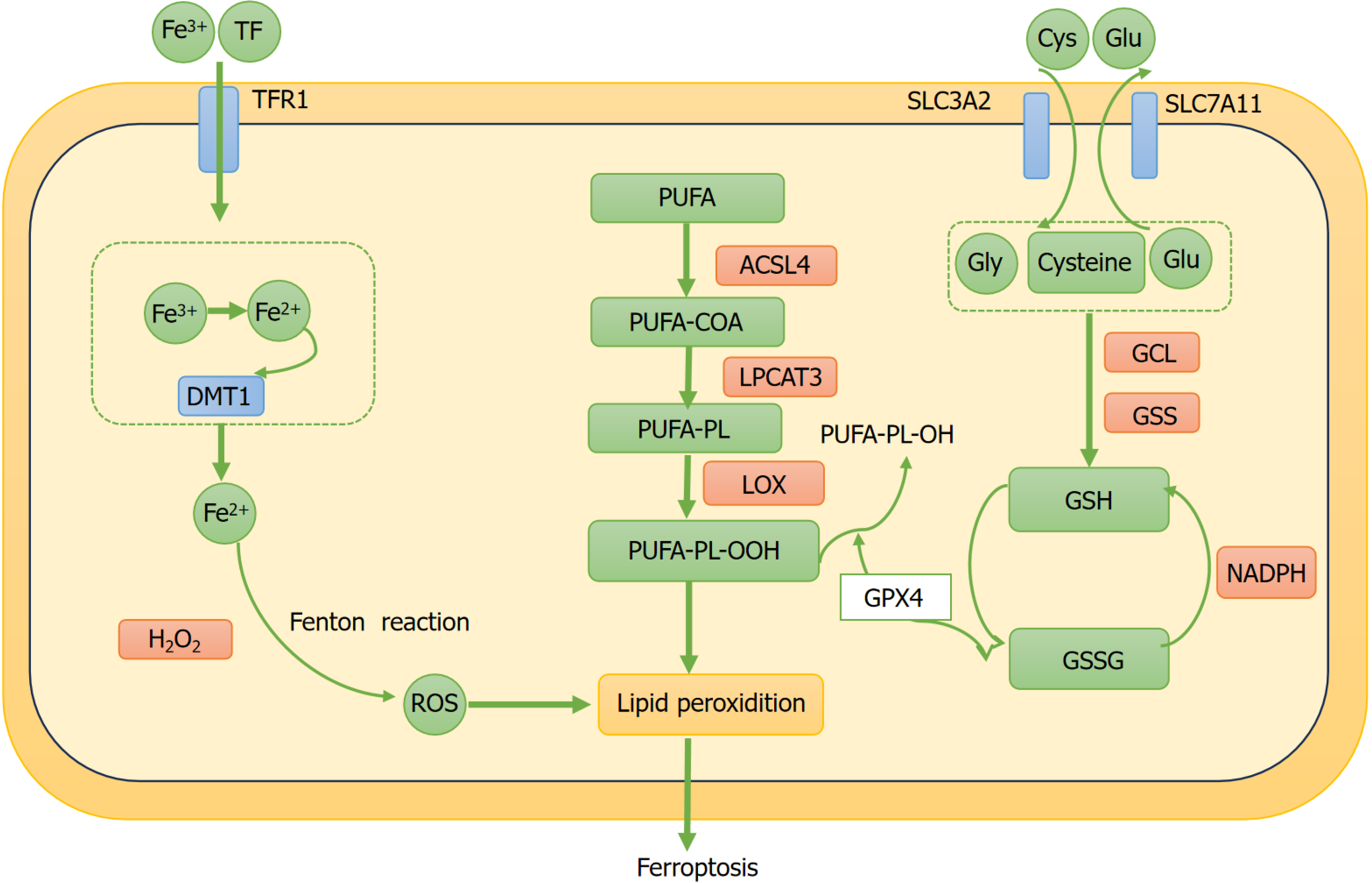
Ferroptosis is a newly discovered form of programmed cell death that differs from apoptosis and is closely related to the depletion of GSH synthesis and inactivation of antioxidant enzymes. It is characterized by lipid peroxidation reactions induced by ROS associated with iron overload[30]. Ferroptosis cannot be inhibited by inhibitors of apoptosis, autophagy, or necrosis but can be suppressed by iron chelators. Cellular iron homeostasis is crucial for the human body because ferric (Fe3+) and ferrous (Fe2+) ions are involved in numerous physiological and biochemical processes and are essential trace elements[31]. From a biochemical perspective, ferroptosis is mainly induced by excess iron and lipid peroxidation. Intracellular iron overload can increase the ROS levels through the Fenton reaction, promoting the production of lipid peroxides and facilitating ferroptosis. Moreover, the reduction of intracellular GPX4 activity and depletion of GSH results in the accumulation of lipid peroxides, further aggravating ferroptosis[32-35]. Morphologically, mitochondrial atrophy, reduced cristae numbers, and mitochondrial membrane rupture are considered characteristics of ferroptosis[13]. At the genetic level, ferroptotic cells show changes in the expression of genes regulating iron homeostasis and lipid peroxidation[36]. Notably, biomarkers of ferroptosis include overexpression of genes, such as acyl-CoA synthetase long-chain family member 4 (ACSL4)[37]. Therefore, the regulation of ferroptosis mainly involves iron overload, increased lipid pero
The body precisely regulates iron metabolism to maintain a balance between cellular uptake, storage, and utilization. Circulating iron primarily exists in the Fe3+ form, which is delivered into the cells via the receptor-mediated endocytosis of transferrin-bound Fe3+. Within the lysosome, Fe3+ is reduced to Fe2+. Subsequently, divalent metal transporter 1 transfers Fe2+ into the labile iron pool. Ferroptosis occurs owing to the overloaded Fe2+ generating ROS through the Fenton reaction, resulting in increased synthesis of lipid peroxides[40].
The increase in the peroxidation of PUFA phospholipids (PUFA-PLs) leads to ferroptosis[35,41]. The production of PUFA-PLs depends on the main enzyme ACSL4. ACSL4 activates free PUFAs by CoA, forming PUFA-CoA, which is subsequently transformed into PUFA-PLs by lysophosphatidylcholine acyltransferase 3 (LPCAT3). Consequently, ACSL4 and LPCAT3have a significant impact on increasing the levels of polyunsaturated phospholipids and triggering ferroptosis. PUFAs are converted to phospholipids under the action of ACSL4, leading to ferroptosis. PUFAs, which contain dienyl groups, are highly susceptible to peroxidation, with their abundance being a critical factor in susceptibility to lipid oxidation. Failure to convert lipid peroxides to lipid hydroperoxides and further to non-toxic alcohols enables the continuation of free radical-mediated reactions, resulting in substantial secondary product formation, membrane damage, and ferroptosis[42,43]. Importantly, the pro-ferroptotic role of ACSL4 is inversely correlated with the anti-ferroptotic function of GPX4. Reduced GPX4 expression or activity is associated with elevated ACSL4 levels and increased ferroptosis, whereas higher GPX4 expression or activity results in decreased ACSL4 levels and diminished ferroptosis[44].
GSH comprises cysteine, glutamate, and glycine. GSH primarily exists in the following forms: reduced GSH, oxidized GSH, and conjugated forms. These forms are important in maintaining the antioxidant status of cells and overall metabolism. Among these forms, GSH is the most common, possessing antioxidant functions that can neutralize free radicals and other oxidants. GSH is an essential cofactor for GPX4. GPX4 can inhibit the lipoxygenase (LOX) and eliminate lipid peroxides generated from iron accumulation, thereby reducing the production of ROS and suppressing ferroptosis. GPX4 is an essential regulatory factor in ferroptosis. The reduction of intracellular GSH considerably lowers the GPX4 activity and increases the lipid ROS levels within the cells, leading to reduced antioxidant ability, lipid ROS accumulation, and ferroptosis[45,46].
System Xc- is an amino acid antiporter located on the cell membrane, which is composed of solute carrier family 3 member 2 (SLC3A2) and solute carrier family 7 member 11 (SLC7A11). The production of GSH requires system Xc- to import extracellular cystine into the cell, where it consumes nicotinamide adenine dinucleotide phosphate (NADPH) to reduce cystine to cysteine[47]. Cysteine and glutamate combine through γ-glutamylcysteine ligase to form γ-glutamylcysteine, which is then transformed to GSH by GSH synthase (GSS)[48]. GSH, as an important cofactor for GPX4, reduces lipid peroxidation, thereby inhibiting ferroptosis[49]. A substantial number of studies have demonstrated that the suppression of the system Xc- pathway induces ferroptosis in cells, whereas its activation can counteract ferroptosis[50]. The small molecule erastin-an inhibitor of system Xc--binds to SLC7A11 and subsequently diminishes intracellular GSH synthesis. As GSH is an essential cofactor for GPX4, its depletion compromises GPX4’s capacity to effectively attenuate lipid peroxidation, thereby inducing ferroptosis[51]. Notably, this ferroptotic process can be counteracted either through cysteine supplementation or administration of ferroptosis inhibitors such as ferrostatin-1, which substantiates the pivotal role of System Xc- in this pathway[52]. It is essential to highlight that the GSH metabolism pathway is a key metabolic route for ferroptosis (Figure 1).
Growing evidence suggests that ferroptosis plays a significant role in T2DM and its complications[53]. Intracellular iron homeostasis is crucial for maintaining endocrine system stability, as iron is involved in numerous glucose metabolic pathways, including insulin secretion and hepatic, renal, and lipid metabolism[54]. Excess Fe2+ generates a large amount of ROS through the Fenton reaction. Pancreatic β-cells have a weak antioxidant capacity and are damaged by the effects of ROS. It is characterized by the downregulation of GPX4 expression and increased inactivation[55]. Ferroptosis in pancreatic β-cells reduces insulin synthesis and secretion, ultimately leading to diabetes[56].
Chronic hyperglycemia can lead to vascular complications, including macrovascular and microvascular complications[57]. The macrovascular complications are associated with vascular endothelial cell (VEC) dysfunction and vascular smooth muscle cell (VSMC) impairment[58]. Diabetes significantly increases the risk of atherosclerosis and related diseases, including ischemic cerebrovascular, coronary artery, and peripheral artery diseases. Patients with diabetes face a significantly increased risk of developing atherosclerotic CVD as compared with individuals with diabetes, making it one of the leading causes of death in those with T2DM[59]. In VSMCs and VECs, certain markers that promote iron dysregulation exhibit a positive relationship with the progression of atherosclerosis-related plaques. Fe-1, a ferroptosis inhibitor, can suppress atherosclerosis progression, while ferroptosis inducers exacerbate it[60]. A considerable amount of evidence points to the fact that ferroptosis is involved in the development of CVDs[61]. A common comorbidity in patients with CVDs is diabetes, which can exacerbate the heart’s susceptibility to ischemia–reperfusion injury (IRI)[62]. In streptozotocin (STZ)-induced diabetic mouse models, ferroptosis mediates myocardial IRI[63]. In addition, ERS induced by hyperglycemia seems to be linked to myocardial cell damage mediated by ferroptosis[61]. Li et al[64] found that Ferrostatin-1 and iron chelators improved the symptoms of HF induced by acute and chronic IRI. This implies that targeting ferroptosis is a strategy for preventing and treating CVDs.
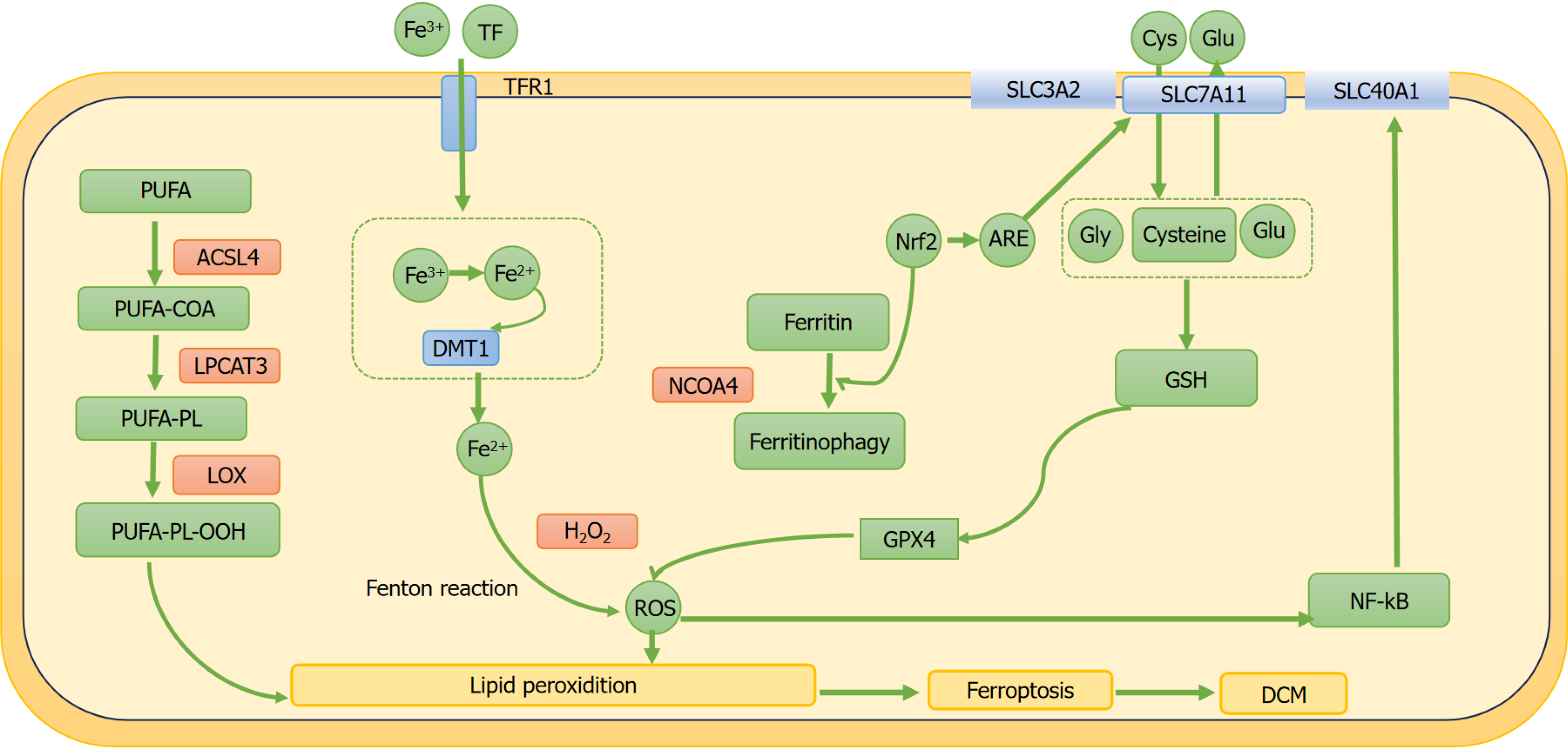
DCM is among the most common complications of diabetes and a major cause of HF and mortality[5-7]. DCM is characterized by myocardial damage induced by DM, featuring interstitial fibrosis and diastolic dysfunction[8,9]. The pathological manifestations of DCM include myocardial cell hypertrophy, apoptosis, and fibrosis, with complex underlying mechanisms involving lipid deposition, oxidative stress, hyperglycemia, inflammation, insulin resistance, ERS, autophagy, and accumulation of advanced glycation end products. Despite extensive research over the past few decades, knowledge on the pathogenesis and diagnostic criteria of DCM remains limited. Excessive iron accumulation beyond normal physiological levels can cause structural myocardial damage and cardiac dysfunction[60] (Figure 2). Recent research has shown that ferroptosis may play a role in the pathogenesis of DCM[13,14]. Numerous studies have shown that lowering oxidative stress or improving the endogenous antioxidant defense system can effectively treat DCM[65-67]. Therefore, suppressing lipid peroxidation caused by iron overload may be vital for enhancing the body’s antioxidant capacity and alleviating the pathological damage of DCM[65]. Studies have shown that exogenous spermidine has a cardioprotective effect on myocardial cells in STZ-induced diabetic rats. Exogenous spermidine can improve cardiac impairment in STZ-induced diabetic rats by inhibiting the nuclear factor erythroid 2-related factor 2 (Nrf2)-ROS-p53-MuRF1 axis, effectively clearing ROS and boosting the calcium-sensing receptor levels, thereby decreasing ERS and oxidative stress. Global knockout of Nrf2 inhibited cardiac remodeling, oxidative stress, and cell apoptosis, which are associated with the occurrence and progression of cardiac dysfunction in patients with T1DM[68,69]. Thus, ferroptosis plays a crucial role in DCM pathogenesis (Figure 3).
One of the primary factors influencing the occurrence and progression of DCM is oxidative stress[70]. Increased generation of ROS inside the cells triggers oxidative stress, leading to cardiomyocyte damage[65,71]. Fe2+ increases the production of ROS within the cells through the Fenton reaction[72]. There is evidence that the imbalance in iron regulation among patients with diabetes increases ROS production, triggering oxidative stress in the myocardium and damaging the cardiomyocytes[26,73]. Erastin, an inducer of ferroptosis, can promote the accumulation of intracellular free radicals, thereby triggering ferroptosis[27,74]. Patients with DCM treated with erastin showed rapidly increasing ROS levels in the myocardial cells. In vitro studies have found that the use of ferroptosis inhibitors considerably inhibits apoptosis in H9C2 cells[62,74,75]. In STZ-induced diabetic rats, the oxidative stress in the myocardial tissue increased, whereas the left ventricular ejection fraction decreased. However, supplementation with the ferroptosis inhibitor vitamin E reduced myocardial oxidative stress and improved hemodynamics, further confirming the protective role of ferroptosis inhibitors in DCM[76]. Beyond vitamin E, other broad-spectrum antioxidants also show potential in regulating ferroptosis. Vitamin K, through its role in redox cycling, attenuates lipid peroxidation by regenerating reduced forms of lipid-soluble antioxidants[77]. N-acetylcysteine, a precursor of GSH, enhances cellular GSH pools, supporting GPX4 activity to neutralize lipid peroxides[78]. Coenzyme Q10 (CoQ10), a critical component of the mitochondrial electron transport chain, acts as a lipid-soluble antioxidant, scavenging free radicals and inhibiting ferroptosis by maintaining membrane integrity[77]. Statins significantly reduce cardiovascular risk through their antioxidant, lipid-lowering, and anti-inflammatory mechanisms[79]. However, their inhibition of the mevalonate pathway leads to the depletion of CoQ10 and GPX4, thereby promoting ferroptosis[80]. Studies have shown that atorvastatin induces ferroptosis in a dose-dependent manner, resulting in cardiomyocyte damage[81]. As statins exhibit dual effects, their use should be carefully tailored to individual patients to balance their cardiovascular protective benefits with the potential risk of ferroptosis.
An important antioxidant mechanism for preventing ferroptosis is the GSH metabolism pathway[82]. As mentioned earlier, the biosynthesis of endogenous GSH is based on the Xc- system, which is composed of the SLC7A11 and SLC3A2[38]. When oxidative stress occurs within the cells, the Xc- system can regulate the ferroptosis mechanism by swapping glutamate and cystine in a 1:1 ratio. Cystine is converted to cysteine within the cells and then transformed into GSH by GSS. With the aid of GSH, GPX4 changes PUFA-PL-OH into a non-toxic form, thereby mitigating iron dysregulation[30,83]. Wu et al[84] found that ferroptosis is increased in mice with high-fat diet and STZ-induced diabetes, which promotes the development of DCM. This effect is primarily due to the reduction of SLC7A11 expression, leading to decreased GSH levels, increased lipid peroxidation, and enhanced ferroptosis, resulting in cell damage, which contributes to the onset of DCM. Additionally, the researchers also validated these findings using H9C2 and primary neonatal mouse cardiomyocytes treated with palmitic acid and HG in vitro[84]. Similarly, Ghosh et al[85] proved that, in the STZ-induced diabetic rat model, the GSH levels in the cardiomyocytes were reduced, whereas the ROS levels were elevated, leading to increased apoptosis of cardiomyocytes. Sulforaphane (SFN) mitigates ferroptosis by activating AMPK and stimulating ferritin and SLC7A11 downstream expressions. In addition, SFN enhances the role of AMPK in age-induced endothelial cell dysfunction and diabetic cardiac remodeling[86]. These studies suggest that lipotoxicity-induced ferroptosis plays a vital role in the pathogenesis of DCM[87].
Nrf2 is engaged in regulating cellular lipid peroxidation, antioxidant response, and ferroptosis[88]. Nrf2 regulates the expression of different genes, including those that participate in transport proteins, antioxidant defense, scavenger receptors, and autophagic degradation[89]. It also plays a key role in cellular antioxidant response, as it facilitates the transcription of downstream genes by interacting with the antioxidant response elements (AREs) of those genes[90]. The Nrf2/ARE transcriptional pathway is an antioxidant pathway in cells involved in preventing ferroptosis[91]. A previous study first reported the presence of ferroptosis in the hearts of mice with diabetes, and the activation of Nrf2 may inhibit ferroptosis by elevating the ferritin and SLC7A11 levels[23]. The Nrf2/ARE pathway is crucial in the cellular system defense responses, as it regulates and ensures sufficient redox processes and oxidative signaling molecules[92]. In the presence of Nrf2, the SLC40A1 expression is increased, promoting the export of intracellular Fe2+ and reducing ROS generation to prevent ferroptosis[93]. Curcumin improves fibrosis in high-glucose-treated H9C2 cells. When curcumin is activated, the levels of HO-1 and GPX4 increases, resulting in the inhibition of iron-dependent ROS production, thereby suppressing ferroptosis[27]. Wu et al[94] found that 6-gingerol alleviated myocardial fibrosis in diabetic mice induced by STZ combined with a high-fat diet, which was primarily achieved through 6-G’s enhancement of the Nrf2/HO-1 pathway, resulting in reduced ferroptosis and inflammation. In vitro, similar results were observed in the HG and palmitic acid-induced H9c2 cells treated with 6-gingerol. Li et al[95] have found that dexmedetomidine inhibits ferroptosis by activating the Nrf2/GPX4 pathway, thereby reducing the HG-induced apoptosis in the H9C2 cells. Wang et al[23] found that SFN reduced Fe2+ accumulation and increased the intracellular cysteine levels by activating the AMPK/Nrf2/ARE pathway, thereby suppressing ferroptosis and alleviating the progression of DCM. In an in vivo model of STZ-induced diabetic mice and H9C2 cells with HG in vitro, some studies[26,75] confirmed that canagliflozin inhibited ferroptosis in cardiac cells by triggering the AMPK/Nrf2/ARE pathway, which suppressed myocardial fibrosis and slowed the progression of DCM.
In the context of diabetes, hyperglycemia induces an increase in pro-inflammatory cytokines, including interleukins (ILs), tumor necrosis factor-alpha, and transforming growth factor-beta1, which leads to sustained myocardial inflammation[96]. The nucleotide-binding oligomerization domain-like receptor 3 (NLRP3) inflammasome is activated by inflammation, contributing to DCM[97]. The occurrence and progression of DCM are linked to the activation of the NLRP3 inflammasome[98]. A prominent early response to diabetes is myocardial inflammation, which is plays a critical role in the development of DCM. The NLRP3 inflammasome affects the progression of DCM. A previous study has found that silencing the NLRP3 gene in STZ-induced diabetic rats reduced cardiac inflammation and fibrosis and improved cardiac function[98]. The activation of NLRP3 can accelerate the secretion of IL-1β, which promotes increased collagen expression, leading to myocardial cell dysfunction and apoptosis. These factors collectively play a part in the progression of DCM[99-101].
Recent research has indicated that NF-κB expression is upregulated in hyperglycemic conditions. Inhibiting NF-κB signaling pathway can effectively alleviate cardiac oxidative stress and myocardial fibrosis, thereby reversing the deterioration of cardiac function. NF-κB can activate the NLRP3 inflammatory complex and enhance the expression of pro-inflammatory cytokines, such as IL-β and IL-18, exacerbating the occurrence and progression of cardiac inflammation[102]. Research has shown that suppressing the NF-κB pathway within zebrafish hepatocytes enhanced the SLC40A1 activity, reduced the iron overload, and alleviated ferroptosis[103]. The activation of NLRP3 is attributed to ROS produced by iron overload and activation of the NF-κB signaling pathway, thereby exacerbating the progression of DCM[84,103]. Iron chelators and ferroptosis inhibitors have been found to lower the expression of NF-κB in diabetic hearts and reduced myocardial fibrosis associated with DCM[28]. In summary, inflammation plays a key role in the association between ferroptosis and DCM, promoting the onset and progression of DCM.
Autophagy is a mechanism by which cells utilize the constituents of the cell to maintain intracellular homeostasis through the breakdown and reuse of organelles and proteins, which is crucial for maintaining normal cardiac cell function[104]. However, excessive autophagy can result in programmed cell death of cardiomyocytes, commonly called autophagy-dependent cell death[105]. The inhibition of autophagy may disrupt the metabolic processes of the heart and blood vessels, causing cardiac fibrosis and exacerbation of cardiomyocyte injury[106]. Autophagy is intricately associated with ferroptosis, with excessive autophagy potentially promoting ferroptosis[107,108]. Recent research has highlighted that ferritinophagy, mediated by nuclear receptor coactivator 4 (NCOA4), signifies a unique form of targeted autophagy[109]. Ferritinophagy leads to iron overload through increased NCOA4 expression, thereby facilitating ferroptosis[110]. The autophagic response involving NCOA4 converts ferritin into free Fe2+, which in turn promotes lipid peroxidation and intracellular ROS production, thereby activating autophagy[111]. Autophagy activation promotes the production of free Fe2+ within the cells[112-114]. The use of autophagy inhibitors has been shown to help alleviate ferroptosis[115,116]. A previous study has reported that several autophagic vesicles have been observed in the cardiomyocytes of patients with diabetes[117]. Research has confirmed that autophagy can be activated by diabetes, leading to the generation of increased amount of ROS within the cardiomyocytes[51,118]. Inhibiting autophagy can upregulate NRF2 expression, which reduces lipid peroxidation and iron deposition, thereby inhibiting ferroptosis in cardiomyocytes and delaying the progression of DCM[119].
Ferroptosis inhibitors have been extensively used for treating iron overload-related cardiomyopathy[75,120,121]. Resveratrol can inhibit oxidative stress in cardiomyocytes of mice with STZ-induced diabetes and reduces cardiomyocyte apoptosis, which slows down DCM progression[122]. In mice with STZ-induced diabetes, sulforaphane-activated NRF2 inhibits ferroptosis in DCM by increasing ferritin and SLC7A11 levels, thereby alleviating DCM-induced cardiac dysfunction[23]. In an in vivo model of mice with STZ-induced diabetes and an in vitro model of H9C2 cells, canagliflozin promoted the Xc-/GSH/GPX4-axis system, inhibited cardiomyocyte ferroptosis, and slowed the progression of DCM[26]. In STZ-induced and HFD-fed diabetic mice, the ferroptosis inhibitor 6-gingerol mitigated DCM-associated cardiomyocyte hypertrophy and interstitial fibrosis by activating the NRF2/HO-1 signaling pathway to enhance antioxidant defenses[94]. Moreover, by inhibiting myocardial lipid peroxidation, liproxstatin-1 (a ferroptosis inhibitor) can delay DCM progression in mice with STZ-induced diabetes[123]. DPP-4 inhibitors are currently widely used as hypoglycemic agents in clinical practice. DPP-4 inhibitors (such as vildagliptin, alogliptin, and linagliptin) have been demonstrated to inhibit ferroptosis in tumor models by blocking NADPH oxidase-mediated ROS generation and lipid peroxidation[124]. Further studies have revealed that DPP-4 inhibitors reduce oxidative stress and lipid peroxidation, thereby mitigating ferroptosis, through the stabilization of Golgi function[125]. Pham et al[126] demonstrated that evogliptin significantly alleviates cardiac lipotoxicity, mitochondrial damage, and fibrosis in db/db mice by inhibiting DPP-4 activity, thereby delaying the progression of DCM (Table 1). Although clinical studies have not yet conclusively demonstrated cardiovascular benefits, the in-depth exploration of the multifaceted mechanisms of DPP-4 inhibitors suggests their emerging potential in cardiovascular disease prevention, highlighting the need for further investigation into their therapeutic applications. Future studies should leverage innovative approaches such as PROTAC probe technology and proteomics to identify key molecular targets and elucidate the interplay between ferroptosis and DCM[127]. By applying the PROTAC probe technology, we may successfully precisely identify and manipulate the proteins involved in ferroptosis within the context of DCM, while proteomics can provide a comprehensive view of the protein alterations associated with the disease process. This would potentially open up new avenues for the development of more effective therapeutics. Hopefully, with the utilization of these advanced tools, we can decipher the complex interplay between ferroptosis and DCM, ultimately translating these findings into improved clinical outcomes for patients.
| Drug name | Mechanism of action | Experimental model | Key effects |
| Ferrostatin-1[64] | Inhibits lipid peroxidation, scavenges free radicals | Mouse DCM model | Anti-oxidative stress, improves myocardial fibrosis |
| Liproxstatin-1[123] | Stabilizes mitochondrial membrane potential, inhibits lipid ROS generation | Diabetic rat model | Improves cardiac function, inhibits PTGS2 expression |
| Vitamin E[76] | Lipid-soluble antioxidant, inhibits lipid peroxidation chain reaction | STZ-induced diabetic rats | Reduces myocardial oxidative damage, improves mitochondrial function |
| CoQ10[77] | Maintains mitochondrial electron transport chain, reduces ROS generation | Diabetic rat model | Enhances myocardial energy metabolism, reduces lipid peroxidation |
| Resveratrol[122] | Activates Nrf2 pathway, upregulates antioxidant enzymes (e.g., GPX4) | Diabetic mice | Inhibits ferroptosis, reduces apoptosis |
| Canagliflozin[26] | Promotes the Xc-/GSH/GPX4 axis system | Diabetic mice and high glucose-induced H9C2 cells | Improves mitochondrial dysfunction and alleviates myocardial fibrosis |
| Sulforaphane[23] | Activates Nrf2 pathway, enhances cellular antioxidant capacity | Diabetic mouse model | Reduces myocardial ferroptosis, improves cardiac remodeling |
| Curcumin[27] | Enhances the Nrf2/HO-1 pathway | STZ-induced and HFD-fed diabetic mice | Anti-oxidative stress, alleviates and improves myocardial fibrosis |
| Dexmedetomidine[95] | Activates the Nrf2/GPX4 pathway | HG-induced H9C2 cells | Reduces cardiomyocyte apoptosis |
| DPP-4 inhibitors (evogliptin)[126] | Blocks NADPH oxidase-mediated ROS generation and lipid peroxidation | db/db mice | Alleviates cardiac lipotoxicity, mitochondrial damage, and fibrosis |
Currently, despite the presence of glycemic control, there are still no effective ways for preventing the progression of DCM. Accumulating evidence suggests that HG and/or high-fat-induced oxidative stress, inflammation, and autophagy participate in ferroptosis in the diabetic myocardium, which play significant roles in the pathological process of DCM. These studies offer a new insight into the pathogenic mechanism of DCM. Ferroptosis inhibitors such as SFN demonstrate the potential to delay DCM in experimental models by inhibiting myocardial lipid peroxidation, alleviating oxidative stress, and reducing cardiomyocyte apoptosis. These findings suggest that ferroptosis is a promising therapeutic target for DCM, and the inhibition of ferroptosis may alleviate the progression of DCM. However, the pathophysiological roles of ferroptosis in DCM are not yet fully understood; hence, more in-depth research is needed in the future.
We would like to express our sincere gratitude to Na Li and Meng-Nan Zhao for their expert advice and invaluable guidance during the preparation of this manuscript.
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5938] [Article Influence: 989.7] [Reference Citation Analysis (8)] |
| 2. | Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 808] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 3. | Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1267] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 4. | Liu D, Xing R, Zhang Q, Tian X, Qi Y, Song H, Liu Y, Yu H, Zhang X, Jing Q, Yan C, Han Y. The CREG1-FBXO27-LAMP2 axis alleviates diabetic cardiomyopathy by promoting autophagy in cardiomyocytes. Exp Mol Med. 2023;55:2025-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Picano E. Diabetic cardiomyopathy. the importance of being earliest. J Am Coll Cardiol. 2003;42:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Liu X, Yang ZG, Gao Y, Xie LJ, Jiang L, Hu BY, Diao KY, Shi K, Xu HY, Shen MT, Ren Y, Guo YK. Left ventricular subclinical myocardial dysfunction in uncomplicated type 2 diabetes mellitus is associated with impaired myocardial perfusion: a contrast-enhanced cardiovascular magnetic resonance study. Cardiovasc Diabetol. 2018;17:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Park JJ. Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes. Diabetes Metab J. 2021;45:146-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 8. | Nakamura M, Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol. 2020;598:2977-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Dhalla NS, Shah AK, Tappia PS. Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy. Int J Mol Sci. 2020;21:2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Qiu J, Liu D, Li P, Zhou L, Zhou L, Liu X, Zhang Y, Yuan M, Tse G, Li G, Liu T. NADPH Oxidase Mediates Oxidative Stress and Ventricular Remodeling through SIRT3/FOXO3a Pathway in Diabetic Mice. Antioxidants (Basel). 2022;11:1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Peng C, Zhang Y, Lang X, Zhang Y. Role of mitochondrial metabolic disorder and immune infiltration in diabetic cardiomyopathy: new insights from bioinformatics analysis. J Transl Med. 2023;21:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 12. | Wang T, Li N, Yuan L, Zhao M, Li G, Chen Y, Zhou H. MALAT1/miR-185-5p mediated high glucose-induced oxidative stress, mitochondrial injury and cardiomyocyte apoptosis via the RhoA/ROCK pathway. J Cell Mol Med. 2023;27:2495-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 13. | Zhao WK, Zhou Y, Xu TT, Wu Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021;2021:9929687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 14. | Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25:424-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 367] [Article Influence: 367.0] [Reference Citation Analysis (0)] |
| 15. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11762] [Article Influence: 904.8] [Reference Citation Analysis (1)] |
| 16. | Liu G, Xie X, Liao W, Chen S, Zhong R, Qin J, He P, Xie J. Ferroptosis in cardiovascular disease. Biomed Pharmacother. 2024;170:116057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 17. | Sun S, Shen J, Jiang J, Wang F, Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct Target Ther. 2023;8:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 239] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Lv MN, Zhao WJ. Research on ferroptosis as a therapeutic target for the treatment of neurodegenerative diseases. Ageing Res Rev. 2023;91:102035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 19. | Liu P, Zhang Z, Cai Y, Li Z, Zhou Q, Chen Q. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 52] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 20. | Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12:2954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 21. | Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, Liu Z, Hu X, Zhang N, Wang T, Fu Y. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 22. | Guo T, Yan W, Cui X, Liu N, Wei X, Sun Y, Fan K, Liu J, Zhu Y, Wang Z, Zhang Y, Chen L. Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol Med. 2023;29:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 23. | Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, Wang Q, Tan Y, Keller BB, Tong Q, Zheng Y, Cai L. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12:708-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 321] [Article Influence: 107.0] [Reference Citation Analysis (37)] |
| 24. | Chen Z, Li S, Liu M, Yin M, Chen J, Li Y, Li Q, Zhou Y, Xia Y, Chen A, Lu D, Li C, Chen Y, Qian J, Ge J. Nicorandil alleviates cardiac microvascular ferroptosis in diabetic cardiomyopathy: Role of the mitochondria-localized AMPK-Parkin-ACSL4 signaling pathway. Pharmacol Res. 2024;200:107057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 25. | Lou X, Zhang Y, Guo J, Gao L, Ding Y, Zhuo X, Lei Q, Bian J, Lei R, Gong W, Zhang X, Jiao Q. What is the impact of ferroptosis on diabetic cardiomyopathy: a systematic review. Heart Fail Rev. 2024;29:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Du S, Shi H, Xiong L, Wang P, Shi Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front Endocrinol (Lausanne). 2022;13:1011669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (3)] |
| 27. | Wei Z, Shaohuan Q, Pinfang K, Chao S. Curcumin Attenuates Ferroptosis-Induced Myocardial Injury in Diabetic Cardiomyopathy through the Nrf2 Pathway. Cardiovasc Ther. 2022;2022:3159717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 28. | Zou C, Liu X, Xie R, Bao Y, Jin Q, Jia X, Li L, Liu R. Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochem Biophys Res Commun. 2017;486:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Zheng Y, Li XK, Wang Y, Cai L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. Hemoglobin. 2008;32:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Sun H, Chen D, Xin W, Ren L, Li Q, Han X. Targeting ferroptosis as a promising therapeutic strategy to treat cardiomyopathy. Front Pharmacol. 2023;14:1146651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Nemeth E, Ganz T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int J Mol Sci. 2021;22:6493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 32. | Wang H, Liu C, Zhao Y, Gao G. Mitochondria regulation in ferroptosis. Eur J Cell Biol. 2020;99:151058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 33. | Jiang Y, Glandorff C, Sun M. GSH and Ferroptosis: Side-by-Side Partners in the Fight against Tumors. Antioxidants (Basel). 2024;13:697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 34. | Zhang H, Pan J, Huang S, Chen X, Chang ACY, Wang C, Zhang J, Zhang H. Hydrogen sulfide protects cardiomyocytes from doxorubicin-induced ferroptosis through the SLC7A11/GSH/GPx4 pathway by Keap1 S-sulfhydration and Nrf2 activation. Redox Biol. 2024;70:103066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 78] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 35. | Xu X, Xu XD, Ma MQ, Liang Y, Cai YB, Zhu ZX, Xu T, Zhu L, Ren K. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed Pharmacother. 2024;171:116112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 36. | Shen C, Liu J, Liu H, Li G, Wang H, Tian H, Mao Y, Hua D. Timosaponin AIII induces lipid peroxidation and ferroptosis by enhancing Rab7-mediated lipophagy in colorectal cancer cells. Phytomedicine. 2024;122:155079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Ding K, Liu C, Li L, Yang M, Jiang N, Luo S, Sun L. Acyl-CoA synthase ACSL4: an essential target in ferroptosis and fatty acid metabolism. Chin Med J (Engl). 2023;136:2521-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 38. | Hu X, Bao Y, Li M, Zhang W, Chen C. The role of ferroptosis and its mechanism in ischemic stroke. Exp Neurol. 2024;372:114630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Pope LE, Dixon SJ. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023;33:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 240] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 40. | Dai E, Chen X, Linkermann A, Jiang X, Kang R, Kagan VE, Bayir H, Yang WS, Garcia-Saez AJ, Ioannou MS, Janowitz T, Ran Q, Gu W, Gan B, Krysko DV, Zhu X, Wang J, Krautwald S, Toyokuni S, Xie Y, Greten FR, Yi Q, Schick J, Liu J, Gabrilovich DI, Liu J, Zeh HJ, Zhang DD, Yang M, Iovanna J, Kopf M, Adolph TE, Chi JT, Li C, Ichijo H, Karin M, Sankaran VG, Zou W, Galluzzi L, Bush AI, Li B, Melino G, Baehrecke EH, Lotze MT, Klionsky DJ, Stockwell BR, Kroemer G, Tang D. A guideline on the molecular ecosystem regulating ferroptosis. Nat Cell Biol. 2024;26:1447-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 98] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 41. | Morgan PK, Pernes G, Huynh K, Giles C, Paul S, Smith AAT, Mellett NA, Liang A, van Buuren-Milne T, Veiga CB, Collins TJC, Xu Y, Lee MKS, De Silva TM, Meikle PJ, Lancaster GI, Murphy AJ. A lipid atlas of human and mouse immune cells provides insights into ferroptosis susceptibility. Nat Cell Biol. 2024;26:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 42. | Cui J, Wang Y, Tian X, Miao Y, Ma L, Zhang C, Xu X, Wang J, Fang W, Zhang X. LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates with ACSL4 and YAP to Determine Ferroptosis Sensitivity. Antioxid Redox Signal. 2023;39:491-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 54] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 43. | Xue Q, Kang R, Klionsky DJ, Tang D, Liu J, Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;19:2175-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 298] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 44. | Niu C, Jiang D, Guo Y, Wang Z, Sun Q, Wang X, Ling W, An X, Ji C, Li S, Zhao H, Kang B. Spermidine suppresses oxidative stress and ferroptosis by Nrf2/HO-1/GPX4 and Akt/FHC/ACSL4 pathway to alleviate ovarian damage. Life Sci. 2023;332:122109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 45. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4260] [Article Influence: 1065.0] [Reference Citation Analysis (0)] |
| 46. | Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, Liu Y, Zhao X, Qian L, Liu P, Xiong Y. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 447] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 47. | Wu Y, Jia Q, Tang Q, Deng H, He Y, Tang F. Berberine-mediated Ferroptosis through System Xc(-)/GSH/GPX4 Axis Inhibits Metastasis of Nasopharyngeal Carcinoma. J Cancer. 2024;15:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 48. | Zheng J, Fang Y, Zhang M, Gao Q, Li J, Yuan H, Jin W, Lin Z, Lin W. Mechanisms of ferroptosis in hypoxic-ischemic brain damage in neonatal rats. Exp Neurol. 2024;372:114641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 49. | Zhang XD, Liu ZY, Wang MS, Guo YX, Wang XK, Luo K, Huang S, Li RF. Mechanisms and regulations of ferroptosis. Front Immunol. 2023;14:1269451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 50. | Xie Y, Kang R, Klionsky DJ, Tang D. GPX4 in cell death, autophagy, and disease. Autophagy. 2023;19:2621-2638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 255] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 51. | Sha W, Hu F, Xi Y, Chu Y, Bu S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J Diabetes Res. 2021;2021:9999612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 52. | Scarpellini C, Klejborowska G, Lanthier C, Hassannia B, Vanden Berghe T, Augustyns K. Beyond ferrostatin-1: a comprehensive review of ferroptosis inhibitors. Trends Pharmacol Sci. 2023;44:902-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 53. | Deng Q, Zhu Y, Zhang M, Fei A, Liang J, Zheng J, Zhang Q, Cheng T, Ge X. Ferroptosis as a potential new therapeutic target for diabetes and its complications. Endocr Connect. 2023;12:e220419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Gao H, Jin Z, Bandyopadhyay G, Wang G, Zhang D, Rocha KCE, Liu X, Zhao H, Kisseleva T, Brenner DA, Karin M, Ying W. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab. 2022;34:1201-1213.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 55. | Wang J, Wang H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid Med Cell Longev. 2017;2017:1930261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 56. | Miao R, Fang X, Zhang Y, Wei J, Zhang Y, Tian J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: mechanisms and therapeutic opportunities. Cell Death Dis. 2023;14:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 57. | Lu Y, Wang W, Liu J, Xie M, Liu Q, Li S. Vascular complications of diabetes: A narrative review. Medicine (Baltimore). 2023;102:e35285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (2)] |
| 58. | Naderi-Meshkin H, Cornelius VA, Eleftheriadou M, Potel KN, Setyaningsih WAW, Margariti A. Vascular organoids: unveiling advantages, applications, challenges, and disease modelling strategies. Stem Cell Res Ther. 2023;14:292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Townsend N, Kazakiewicz D, Lucy Wright F, Timmis A, Huculeci R, Torbica A, Gale CP, Achenbach S, Weidinger F, Vardas P. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 385] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 60. | Zhang M, Li J, Hu W. The complex interplay between ferroptosis and atherosclerosis. Biomed Pharmacother. 2024;178:117183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 61. | Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20:7-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 602] [Article Influence: 301.0] [Reference Citation Analysis (0)] |
| 62. | Huang Q, Tian H, Tian L, Zhao X, Li L, Zhang Y, Qiu Z, Lei S, Xia Z. Inhibiting Rev-erbα-mediated ferroptosis alleviates susceptibility to myocardial ischemia-reperfusion injury in type 2 diabetes. Free Radic Biol Med. 2023;209:135-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Li W, Li W, Leng Y, Xiong Y, Xia Z. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020;39:210-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 64. | Li X, Ma N, Xu J, Zhang Y, Yang P, Su X, Xing Y, An N, Yang F, Zhang G, Zhang L, Xing Y. Targeting Ferroptosis: Pathological Mechanism and Treatment of Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021;2021:1587922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 65. | Byrne NJ, Rajasekaran NS, Abel ED, Bugger H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med. 2021;169:317-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 66. | Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, Ikeda S, Shirakabe A, Sadoshima J. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ Res. 2019;124:1360-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 364] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 67. | Lu S, Liao Z, Lu X, Katschinski DM, Mercola M, Chen J, Heller Brown J, Molkentin JD, Bossuyt J, Bers DM. Hyperglycemia Acutely Increases Cytosolic Reactive Oxygen Species via O-linked GlcNAcylation and CaMKII Activation in Mouse Ventricular Myocytes. Circ Res. 2020;126:e80-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 68. | Wang Y, Chen J, Li S, Zhang X, Guo Z, Hu J, Shao X, Song N, Zhao Y, Li H, Yang G, Xu C, Wei C. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox Biol. 2020;32:101514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Fang W, Xie S, Deng W. Ferroptosis mechanisms and regulations in cardiovascular diseases in the past, present, and future. Cell Biol Toxicol. 2024;40:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 70. | Cai W, Chong K, Huang Y, Huang C, Yin L. Empagliflozin improves mitochondrial dysfunction in diabetic cardiomyopathy by modulating ketone body metabolism and oxidative stress. Redox Biol. 2024;69:103010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 71. | Xu N, Liu S, Zhang Y, Chen Y, Zuo Y, Tan X, Liao B, Li P, Feng J. Oxidative stress signaling in the pathogenesis of diabetic cardiomyopathy and the potential therapeutic role of antioxidant naringenin. Redox Rep. 2023;28:2246720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Zhang K, Tian XM, Li W, Hao LY. Ferroptosis in cardiac hypertrophy and heart failure. Biomed Pharmacother. 2023;168:115765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 73. | Zhao P, Lv X, Zhou Z, Yang X, Huang Y, Liu J. Indexes of ferroptosis and iron metabolism were associated with the severity of diabetic nephropathy in patients with type 2 diabetes mellitus: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1297166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 74. | Zhou Y, Jia Z, Wang J, Huang S, Yang S, Xiao S, Xia D, Zhou Y. Curcumin reverses erastin-induced chondrocyte ferroptosis by upregulating Nrf2. Heliyon. 2023;9:e20163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 75. | Zhang W, Lu J, Wang Y, Sun P, Gao T, Xu N, Zhang Y, Xie W. Canagliflozin Attenuates Lipotoxicity in Cardiomyocytes by Inhibiting Inflammation and Ferroptosis through Activating AMPK Pathway. Int J Mol Sci. 2023;24:858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 76. | Hamblin M, Smith HM, Hill MF. Dietary supplementation with vitamin E ameliorates cardiac failure in type I diabetic cardiomyopathy by suppressing myocardial generation of 8-iso-prostaglandin F2alpha and oxidized glutathione. J Card Fail. 2007;13:884-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Zhang M, Chen X, Zhang Y. Mechanisms of Vitamins Inhibiting Ferroptosis. Antioxidants (Basel). 2024;13:1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 78. | Raghu G, Berk M, Campochiaro PA, Jaeschke H, Marenzi G, Richeldi L, Wen FQ, Nicoletti F, Calverley PMA. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr Neuropharmacol. 2021;19:1202-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 79. | Sahebkar A, Foroutan Z, Katsiki N, Jamialahmadi T, Mantzoros CS. Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: Is there a role for statin therapy? Metabolism. 2023;146:155659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 80. | Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 787] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 81. | Zhang Q, Qu H, Chen Y, Luo X, Chen C, Xiao B, Ding X, Zhao P, Lu Y, Chen AF, Yu Y. Atorvastatin Induces Mitochondria-Dependent Ferroptosis via the Modulation of Nrf2-xCT/GPx4 Axis. Front Cell Dev Biol. 2022;10:806081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 82. | Zeng L, Liu X, Geng C, Gao X, Liu L. Ferroptosis in cancer (Review). Oncol Lett. 2024;28:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 83. | Yan X, Xie Y, Liu H, Huang M, Yang Z, An D, Jiang G. Iron accumulation and lipid peroxidation: implication of ferroptosis in diabetic cardiomyopathy. Diabetol Metab Syndr. 2023;15:161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 84. | Wu S, Zhou Y, Liang J, Ying P, Situ Q, Tan X, Zhu J. Upregulation of NF-κB by USP24 aggravates ferroptosis in diabetic cardiomyopathy. Free Radic Biol Med. 2024;210:352-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 85. | Ghosh S, Ting S, Lau H, Pulinilkunnil T, An D, Qi D, Abrahani MA, Rodrigues B. Increased efflux of glutathione conjugate in acutely diabetic cardiomyocytes. Can J Physiol Pharmacol. 2004;82:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Strassburger M, Bloch W, Sulyok S, Schüller J, Keist AF, Schmidt A, Wenk J, Peters T, Wlaschek M, Lenart J, Krieg T, Hafner M, Kümin A, Werner S, Müller W, Scharffetter-Kochanek K. Heterozygous deficiency of manganese superoxide dismutase results in severe lipid peroxidation and spontaneous apoptosis in murine myocardium in vivo. Free Radic Biol Med. 2005;38:1458-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Beharier O, Tyurin VA, Goff JP, Guerrero-Santoro J, Kajiwara K, Chu T, Tyurina YY, St Croix CM, Wallace CT, Parry S, Parks WT, Kagan VE, Sadovsky Y. PLA2G6 guards placental trophoblasts against ferroptotic injury. Proc Natl Acad Sci U S A. 2020;117:27319-27328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 88. | Galy B, Conrad M, Muckenthaler M. Mechanisms controlling cellular and systemic iron homeostasis. Nat Rev Mol Cell Biol. 2024;25:133-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 279] [Article Influence: 279.0] [Reference Citation Analysis (0)] |
| 89. | Zang H, Mathew RO, Cui T. The Dark Side of Nrf2 in the Heart. Front Physiol. 2020;11:722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 90. | Sun R, Liu M, Xu K, Pu Y, Huang J, Liu J, Zhang J, Yin L, Pu Y. Ferroptosis is involved in the benzene-induced hematotoxicity in mice via iron metabolism, oxidative stress and NRF2 signaling pathway. Chem Biol Interact. 2022;362:110004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 91. | Chen C, Chen W, Zhou X, Li Y, Pan X, Chen X. Hyperbaric oxygen protects HT22 cells and PC12 cells from damage caused by oxygen-glucose deprivation/reperfusion via the inhibition of Nrf2/System Xc-/GPX4 axis-mediated ferroptosis. PLoS One. 2022;17:e0276083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Ghanim BY, Qinna NA. Nrf2/ARE axis signalling in hepatocyte cellular death. Mol Biol Rep. 2022;49:4039-4053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Ma DY, Liu JX, Wang LD, Zhi XY, Luo L, Zhao JY, Qin Y. GSK-3β-dependent Nrf2 antioxidant response modulates ferroptosis of lens epithelial cells in age-related cataract. Free Radic Biol Med. 2023;204:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 94. | Wu S, Zhu J, Wu G, Hu Z, Ying P, Bao Z, Ding Z, Tan X. 6-Gingerol Alleviates Ferroptosis and Inflammation of Diabetic Cardiomyopathy via the Nrf2/HO-1 Pathway. Oxid Med Cell Longev. 2022;2022:3027514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 95. | Li F, Hu Z, Huang Y, Zhan H. Dexmedetomidine ameliorates diabetic cardiomyopathy by inhibiting ferroptosis through the Nrf2/GPX4 pathway. J Cardiothorac Surg. 2023;18:223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 96. | Wen C, Liu C, Li Y, Xia T, Zhang X, Xue S, Olatunji OJ. Ameliorative potentials of the ethanolic extract from Lycium chinense leaf extract against diabetic cardiomyopathy. Insight into oxido-inflammatory and apoptosis modulation. Biomed Pharmacother. 2022;154:113583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 97. | Hu Y, Zhang S, Lou H, Mikaye MS, Xu R, Meng Z, Du M, Tang P, Chen Z, Chen Y, Liu X, Du Z, Zhang Y. Aloe-Emodin Derivative, an Anthraquinone Compound, Attenuates Pyroptosis by Targeting NLRP3 Inflammasome in Diabetic Cardiomyopathy. Pharmaceuticals (Basel). 2023;16:1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 98. | Sun Y, Ding S. NLRP3 Inflammasome in Diabetic Cardiomyopathy and Exercise Intervention. Int J Mol Sci. 2021;22:13228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 99. | Gao R, Shi H, Chang S, Gao Y, Li X, Lv C, Yang H, Xiang H, Yang J, Xu L, Tang Y. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol. 2019;74:105575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 100. | Wei C, Xu J, Liu Y, Qadir J, Zhang S, Yuan H. Exogenous Spermidine Alleviates Diabetic Myocardial Fibrosis Via Suppressing Inflammation and Pyroptosis in db/db Mice. Balkan Med J. 2023;40:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 101. | Cai L, Tan Y, Islam MS, Horowitz M, Wintergerst KA. Diabetic cardiomyopathy: Importance of direct evidence to support the roles of NOD-like receptor protein 3 inflammasome and pyroptosis. World J Diabetes. 2024;15:1659-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (3)] |
| 102. | Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, An F. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 2014;9:e104771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 103. | Cheng K, Huang Y, Wang C. 1,25(OH)(2)D(3) Inhibited Ferroptosis in Zebrafish Liver Cells (ZFL) by Regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin Axis. Int J Mol Sci. 2021;22:11334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 104. | Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023;14:648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 381] [Reference Citation Analysis (0)] |
| 105. | Mellor KM, Reichelt ME, Delbridge LM. Autophagy anomalies in the diabetic myocardium. Autophagy. 2011;7:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 2021;68:101338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 107. | Liu J, Liu Y, Wang Y, Li C, Xie Y, Klionsky DJ, Kang R, Tang D. TMEM164 is a new determinant of autophagy-dependent ferroptosis. Autophagy. 2023;19:945-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 109] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 108. | Lee S, Hwang N, Seok BG, Lee S, Lee SJ, Chung SW. Autophagy mediates an amplification loop during ferroptosis. Cell Death Dis. 2023;14:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 114] [Reference Citation Analysis (0)] |
| 109. | Xie Y, Zhou Y, Wang J, Du L, Ren Y, Liu F. Ferroptosis, autophagy, tumor and immunity. Heliyon. 2023;9:e19799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 110. | Jia D, Zhang M, Li M, Gong W, Huang W, Wang R, Chen Y, Yin Q, Wu J, Jin Z, Wang J, Liu Y, Liang C, Ji Y. NCOA4-mediated ferritinophagy participates in cadmium-triggered ferroptosis in spermatogonia. Toxicology. 2024;505:153831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 111. | Zhao H, Lu Y, Zhang J, Sun Z, Cheng C, Liu Y, Wu L, Zhang M, He W, Hao S, Li K. NCOA4 requires a [3Fe-4S] to sense and maintain the iron homeostasis. J Biol Chem. 2024;300:105612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 112. | Kuno S, Fujita H, Tanaka YK, Ogra Y, Iwai K. Iron-induced NCOA4 condensation regulates ferritin fate and iron homeostasis. EMBO Rep. 2022;23:e54278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 113. | Sheng SY, Li JM, Hu XY, Wang Y. Regulated cell death pathways in cardiomyopathy. Acta Pharmacol Sin. 2023;44:1521-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 28.5] [Reference Citation Analysis (35)] |
| 114. | Chen Y, Zhang J, Tian Y, Xu X, Wang B, Huang Z, Lou S, Kang J, Zhang N, Weng J, Liang Y, Ma W. Iron accumulation in ovarian microenvironment damages the local redox balance and oocyte quality in aging mice. Redox Biol. 2024;73:103195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 115. | Li H, Yang H, Lu S, Wang X, Shi X, Mao P. Autophagy-dependent ferroptosis is involved in the development of endometriosis. Gynecol Endocrinol. 2023;39:2242962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Chen HY, Xiao ZZ, Ling X, Xu RN, Zhu P, Zheng SY. ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med. 2021;27:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 117. | Xiao C, Chen MY, Han YP, Liu LJ, Yan JL, Qian LB. The protection of luteolin against diabetic cardiomyopathy in rats is related to reversing JNK-suppressed autophagy. Food Funct. 2023;14:2740-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 118. | Guan Y, Zhou L, Zhang Y, Tian H, Li A, Han X. Effects of PP2A/Nrf2 on experimental diabetes mellitus-related cardiomyopathy by regulation of autophagy and apoptosis through ROS dependent pathway. Cell Signal. 2019;62:109339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 119. | Fang Q, Liu X, Ding J, Zhang Z, Chen G, Du T, Wang Y, Xu R. Soluble Epoxide Hydrolase Inhibition Protected against Diabetic Cardiomyopathy through Inducing Autophagy and Reducing Apoptosis Relying on Nrf2 Upregulation and Transcription Activation. Oxid Med Cell Longev. 2022;2022:3773415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 120. | Chen H, Zhu J, Le Y, Pan J, Liu Y, Liu Z, Wang C, Dou X, Lu D. Salidroside inhibits doxorubicin-induced cardiomyopathy by modulating a ferroptosis-dependent pathway. Phytomedicine. 2022;99:153964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 121. | Yan J, Li Z, Liang Y, Yang C, Ou W, Mo H, Tang M, Chen D, Zhong C, Que D, Feng L, Xiao H, Song X, Yang P. Fucoxanthin alleviated myocardial ischemia and reperfusion injury through inhibition of ferroptosis via the NRF2 signaling pathway. Food Funct. 2023;14:10052-10068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 122. | Yang L, Gao Z, Zhao H, Zhang Z, Song G. Resveratrol Delays Diabetic Cardiomyopathy Fibrosis by Regulating Mitochondrial Autophagy. Altern Ther Health Med. 2025;31:143-149. [PubMed] |
| 123. | Zhao Y, Pan B, Lv X, Chen C, Li K, Wang Y, Liu J. Ferroptosis: roles and molecular mechanisms in diabetic cardiomyopathy. Front Endocrinol (Lausanne). 2023;14:1140644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 124. | Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, Lotze MT, Zeh HJ 3rd, Kang R, Kroemer G, Tang D. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017;20:1692-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 688] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 125. | Alborzinia H, Ignashkova TI, Dejure FR, Gendarme M, Theobald J, Wölfl S, Lindemann RK, Reiling JH. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun Biol. 2018;1:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 126. | Pham TK, Nguyen THT, Yi JM, Kim GS, Yun HR, Kim HK, Won JC. Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice. Exp Mol Med. 2023;55:767-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 127. | Yan S, Zhang G, Luo W, Xu M, Peng R, Du Z, Liu Y, Bai Z, Xiao X, Qin S. PROTAC technology: From drug development to probe technology for target deconvolution. Eur J Med Chem. 2024;276:116725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |