Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.104350
Revised: January 27, 2025
Accepted: March 3, 2025
Published online: May 15, 2025
Processing time: 128 Days and 17.4 Hours
Negative pressure wound therapy (NPWT) is a potential treatment for diabetic foot ulcers (DFUs), although the mechanisms underlying its effectiveness remain unclear. This study posits that NPWT may improve wound healing by promoting angiogenesis and activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like epichlorohydrin-associated protein 1 (Keap1) signaling path
To study the mechanism of NPWT in DFUs.
This study included a total of 40 hospitalized patients with DFUs from Xuzhou Central Hospital, who were divided into Control group (n = 21) and NPWT group (n = 19). The levels of Nrf2 and Keap1 were analyzed in the granulation tissue 7 days after treatment. The wound condition, erythrocyte sedimentation rate (ESR), procalcitonin (PCT), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b-FGF), cluster of differentiation 31 (CD31), and levels of oxidative stress [malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (T-AOC)] were analyzed before and 7 days after treatment by the Mann-Whitney U test.
The NPWT group demonstrated significant improvements in wound healing compared to the control group after 7 days of treatment. The levels of ESR, PCT, IL-6, and TNF-α were significantly reduced in the NPWT group compared to the control group (P < 0.05), while the levels of CD31, VEGF, and b-FGF showed significant increases
NPWT may contribute to the healing of DFUs by potentially reducing levels of oxidative stress. Its effects could possibly be enhanced through the action of Nrf2.
Core Tip: This study investigated the potential of negative pressure wound therapy (NPWT) in diabetic foot ulcers (DFUs) by assessing wound healing, inflammatory markers, cytokines, growth factors, and oxidative stress. NPWT was associated with improved wound conditions and reduced inflammation, while also increasing angiogenesis markers and antioxidant defenses that may be mediated by nuclear factor erythroid 2-related factor 2 (Nrf2). The study suggests that Nrf2 could be involved in the therapeutic effects of NPWT on DFUs.
- Citation: Sun HJ, Si SW, Ma YM, Liu XK, Geng HF, Liang J. Role of nuclear factor erythroid 2-related factor 2 in negative pressure wound therapy for diabetic foot ulcers. World J Diabetes 2025; 16(5): 104350
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/104350.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.104350
Diabetic foot ulcers (DFUs) are a severe complication of diabetes, often leading to significant disability and increased mortality rates. The incidence of DFUs is estimated at approximately 6.3%, with a lifetime risk between 19% and 34%[1]. Among patients with DFUs, the overall amputation rate is 31%[2]. Additionally, the annual mortality rate linked to DFUs is reported at 13.1%[3]. The high prevalence of diabetic foot, along with its amputation and mortality rates, poses a significant challenge to global public health. Moreover, the healing process of DFUs is slow and challenging, resulting in a heavy burden for both patients and society. Delayed healing is linked to abnormalities in several molecular signaling pathways including chronic inflammation, angiogenesis, the accumulation of advanced glycation end products, impaired neuropeptide signaling, and oxidative stress[4]. DFUs often involve excessive accumulation of reactive oxygen species, heightened oxidative stress, reduced antioxidant capacity[5], and impaired wound healing, and can eventually develop into chronic wounds that are notoriously challenging to treat. Consequently, a crucial focus in the treatment of DFUs lies in promoting wound healing by actively addressing and mitigating the wound oxidative stress response.
Negative pressure wound therapy (NPWT) is increasingly being utilized for the treatment of both acute and chronic wounds. Previous studies have demonstrated its efficacy in managing DFUs by significantly shortening healing times and accelerating the formation of granulation tissue[6]. NPWT achieves these beneficial effects through a variety of mechanisms. It stimulates the production of essential factors that support blood vessel formation, including transforming growth factor beta, vascular endothelial growth factor (VEGF), platelet-derived growth factor, and fibroblast growth factor 2 (FGF2). Furthermore, NPWT helps to decrease the expression of inflammatory factors such as interleukin 1 beta (IL-1b) and tumor necrosis factor alpha (TNF-α)[7]. NPWT aids in reducing bacterial load in the wound, thereby pro
The nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like epichlorohydrin-associated protein 1 (Keap1) signaling pathway is the body’s main defense against oxidative stress. It protects against damage to various organs, nerves, and blood vessels. Nrf2 is crucial for maintaining redox balance and helps counteract oxidative stress by increasing antioxidant proteins and detoxification enzymes. Normally, Nrf2 forms a complex with its inhibitor Keap1. However, when exposed to external stimuli, this complex breaks apart, allowing Nrf2 to move into the nucleus. Inside the nucleus, Nrf2 activates the transcription of several antioxidant factors, such as heme oxygenase 1 (HO-1), nicotinamide adenine dinucleotide phosphate: Quinone oxidoreductase 1 (NQO-1), catalase (CAT), superoxide dismutase (SOD), and gluta
This study recruited 40 hospitalized patients with DFUs. The patients were divided into two groups: Control group (standard wound care, n = 21) and NPWT group (standard wound care with NPWT, n = 19), both receiving treatment for 7 days. Inclusion criteria included: (1) Type 2 diabetes mellitus; (2) Ages 40 to 75 years; (3) Wagner grades 2 to 4; (4) An ankle-brachial index of 0.7 to 1.2; and (5) A wound area greater than 2.0 cm². Exclusion criteria included malignant wounds, coagulation dysfunction, and recent treatment (within the past month) with growth factors, immunosuppressive drugs, or hyperbaric oxygen therapy. The study was conducted under the supervision of a specialized foot care team. The Ethics Committee of Xuzhou Central Hospital (No. XZXY-LK-20211223-053; Xuzhou, China) approved the study protocol, and all patients provided informed consent to participate. The study adhered to the principles outlined in the Helsinki Declaration.
All patients received systematic treatment according to standard wound care protocols. This comprehensive approach included managing blood glucose levels, enhancing circulation, supplying nerve nourishment, and addressing lipid metabolism disorders. Initially, empirical antibiotics were administered. Adjustments were then made based on the results of antibiotic susceptibility tests and the effectiveness of the clinical treatment. Besides standard wound care, the NPWT group benefited from using NPWT.
This process included removing necrotic tissue from the wound, opening sinus tracts, extracting dead bone, and performing amputations when indicated.
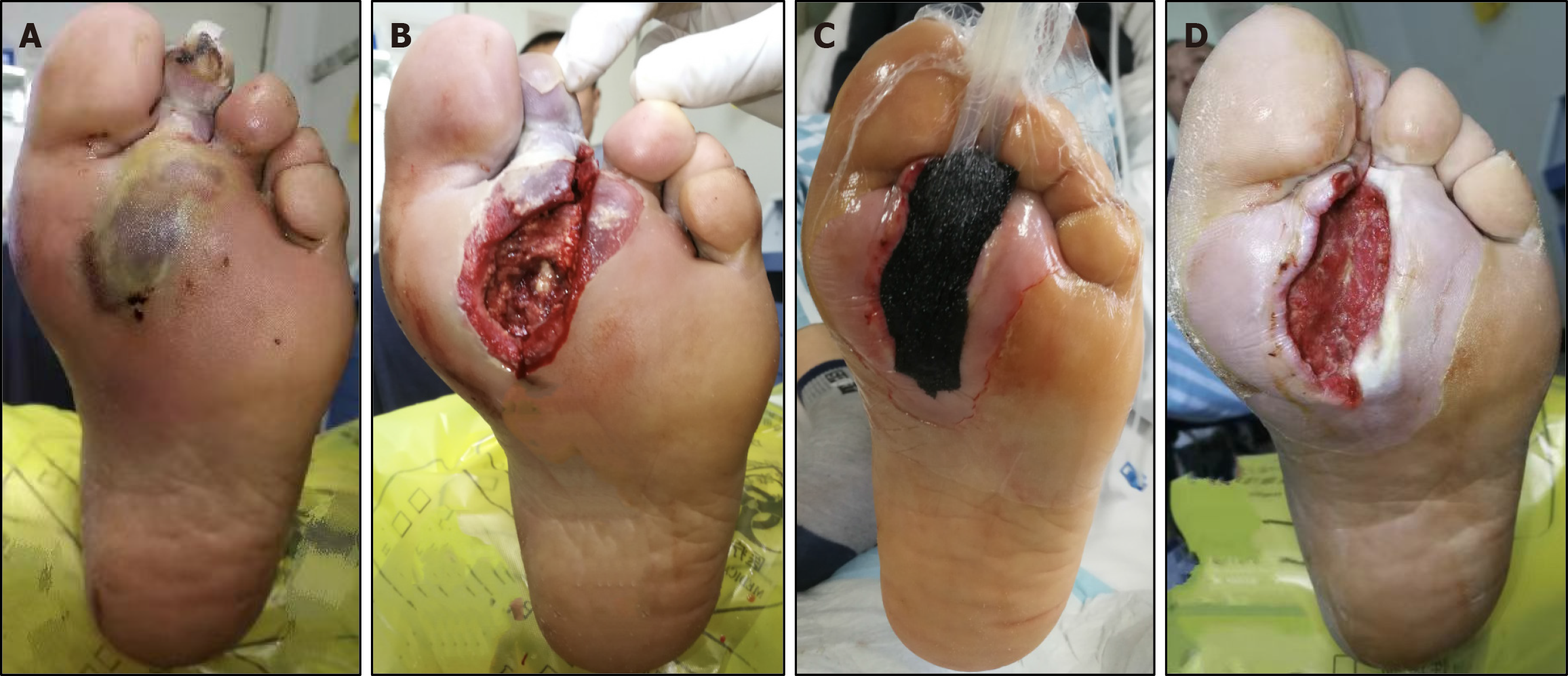
In the NPWT group, patients underwent treatment using the negative pressure wound treatment system (Wuhan VSD Medical Technology, Wuhan, China). NPWT was administered following wound bed preparation. A polyurethane foam dressing, tailored to match the size of the wound, was applied and connected to a wall suction device with a continuous negative pressure of -125 mmHg (Figure 1). A polyurethane foam dressing should be changed based on the clinical appearance of the wound. The control group received conventional wound dressing treatment using Vaseline gauze. The frequency of dressing changes was based on the amount of wound exudate. During dressing changes, necrotic tissue was removed and the wound was filled with Vaseline gauze.
The Bates-Jensen wound assessment tool is an effective tool for evaluating different wound characteristics[15]. This assessment tool includes 13 distinct aspects (wound size, edges, depth, type of necrotic tissue, undermining, granulation and epithelialization tissue, necrotic tissue volume, exudate volume and type, edema, surrounding skin color, and induration), with each aspect consisting of five individual characteristics. The total score is obtained by adding the scores of all individual items. A higher total score is indicative of a more severe wound condition. Wound scores were calculated to ascertain and compare the impacts of two treatment schemes on the DFU healing.
Blood specimens were collected from two groups both before treatment and 7 days after treatment. The blood specimens were centrifuged at 3000 rpm for 10 minutes to isolate the serum layer. Granulation tissue specimens in the edge of wounds were carefully collected from wound after 7 days of treatment, with dimensions approximately measuring 5 mm × 5 mm × 2 mm (length × width × depth). During the operation, local infiltration anesthesia was used. The tissues were divided into two portions. One portion was frozen at -80 °C for Western blot analysis, while the other portion was fixed in a 4% paraformaldehyde solution at room temperature to prepare paraffin-embedded specimens. These paraffin-embedded specimens served for immunohistochemical staining.
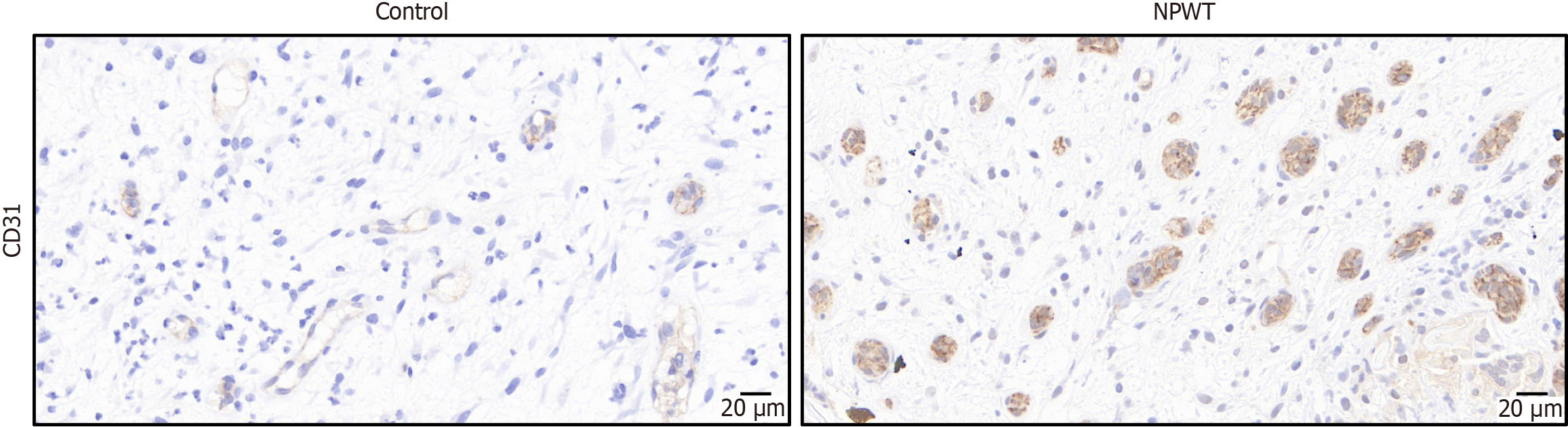
Tissue samples were fixed in a 4% paraformaldehyde solution, dehydrated through a graded series of alcohol, and subsequently embedded in paraffin. The prepared tissue sections had a standardized thickness of 3 microns. For immunohistochemical staining, antigens were extracted under heat conditions, and the sections were allowed to cool to room temperature before incubating with the primary antibody. cluster of differentiation 31 (CD31), a marker indicative of endothelial cells expressed during the early stages of vascular development, was employed to detect and identify newly formed blood vessels. The results of the immunohistochemical staining were recorded as the average positive areas, appearing as brownish yellow or tan regions, through the use of the Medical Image Analysis System (Case Viewer Image Analysis System; 3DHISTECH Ltd., Budapest, Hungary). To quantify the obtained data, the average microvascular density was calculated by examining a minimum of three randomly selected fields of view (FOV) per tissue section at 40 × magnification, allowing for the determination of the number of new blood vessels present per unit of FOV.
IL-6 and TNF-α in serum were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific Co., Ltd., Shanghai, China). VEGF and basic FGF (b-FGF) levels in serum were determined using an ELISA kit (Jianglai Biotechnology Co., Ltd., Shanghai, China). The measurements were carried out according to the instructions provided by the respective manufacturers.
Using relevant kits (Jiancheng Institute of Biological Engineering, Nanjing, Jiangsu Province, China), the levels of SOD, CAT, malondialdehyde (MDA), and total antioxidant capacity (T-AOC) were precisely measured. SOD was assessed using a convenient water-soluble tetrazole assay, while CAT was determined employing a visible light method. MDA content was measured through the thiobarbituric acid assay. T-AOC was determined using a colorimetric method.
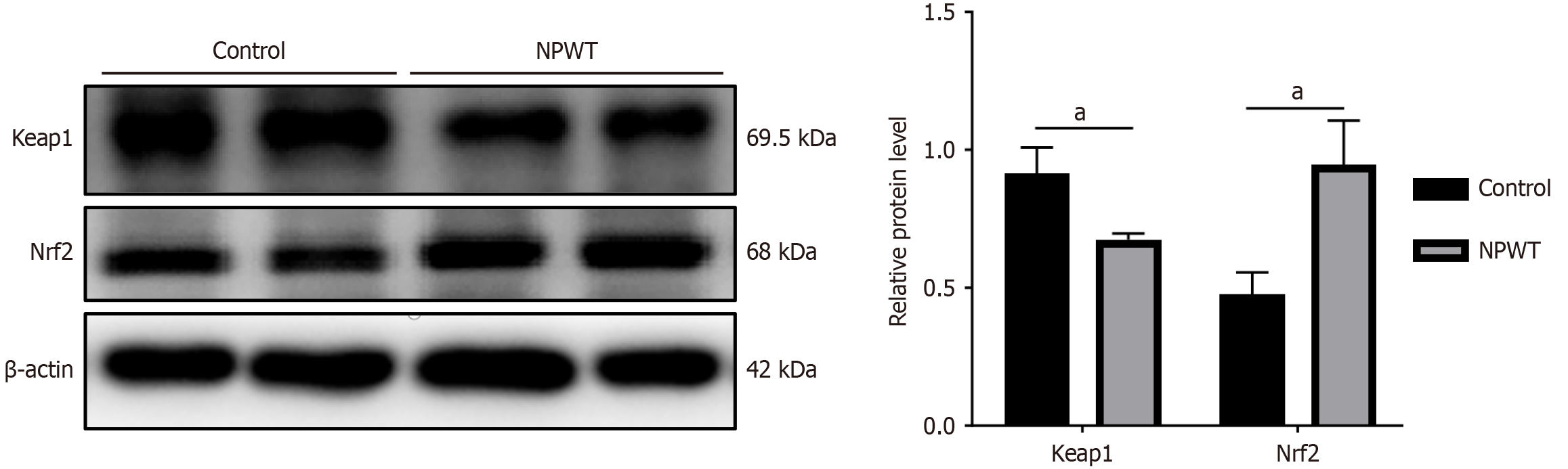
The wound tissue was lysed on ice for at least 15 minutes. The lysates were collected in 1.5 mL Eppendorf tubes, and the protein concentration was determined using the bicinchoninic acid assay method. Equal amounts of protein were loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 5 × loading buffer. The separated proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane from EMD Millipore (Burlington, MA, United States). The PVDF membrane was blocked in either 5% skim milk for 2 hours at room temperature. Antibodies specific to Nrf2 (1:1000; Abcam, Cambridge, MA, United States) and Keap1 (1:1000; Abcam) were incubated with the proteins overnight in a shaker set at 4 °C. Next, the membranes were incubated with corresponding secondary antibodies for 2 hours at room temperature. After incubation, the membranes were washed three times with tris buffered saline with tween for 5 minutes each, incubated with enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Stevenage, United Kingdom) and developed. Quantity one software (Bio-Rad, Hercules, CA, United States) was used to analyze the collected images.
Statistical Package for Social Sciences 26.0 (Statistical Product and Service Solutions, IBM Corp., Armonk, NY, United States) software and GraphPad Prism 10.0 (GraphPad Software, San Diego, CA, United States) software were used for statistical analysis. R 4.3.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) was used for plotting. Descriptive statistics such as mean, standard deviation, median (interquartile range), frequency, and percentage were employed to analyze the study data. The Mann-Whitney U test analyzed continuous variable comparisons, while the χ2 test and Fisher’s exact test were used for categorical data comparisons. The Δ value indicates the difference observed before and after treatment. P < 0.05 was considered statistically significant.
Table 1 provides an overview of the demographic characteristics and personal attributes of all patients. The results suggested that there were no significant differences between the two groups, except for the average dressing frequency, which was significantly different (P < 0.001).
| Variables | Control group (n = 21) | NPWT group (n = 19) | P value |
| Sex (male/female) | 15/6 | 13/6 | 0.836 |
| Smoking | 9 (42.9) | 8 (42.1) | 0.962 |
| Drinking | 7 (33.3) | 7 (36.8) | 0.816 |
| Wagner grade | 1.000 | ||
| 2 | 4 (19.0) | 3 (15.8) | |
| 3 | 11 (52.4) | 10 (52.6) | |
| 4 | 6 (28.6) | 6 (31.6) | |
| Duration of diabetes (year) | 12 (3, 18) | 15 (12, 18) | 0.615 |
| Duration of diabetic foot (day) | 14 (10, 15) | 22 (7, 83) | 0.486 |
| Age (year) | 61.19 ± 9.35 | 56.89 ± 9.40 | 0.107 |
| FPG (mmol/L) | 9.76 ± 3.65 | 9.95 ± 3.95 | 0.989 |
| HbA1c (%) | 10.52 ± 2.83 | 9.62 ± 3.11 | 0.316 |
| Estimated glomerular filtration rate (mL/minute/1.73 m2) | 111.72 ± 18.73 | 105.49 ± 16.94 | 0.261 |
| Wound area (cm2) | 13.12 ± 3.16 | 13.38 ± 3.16 | 0.978 |
| Ankle brachial index | 0.85 ± 0.08 | 0.84 ± 0.07 | 0.654 |
| Average dressing frequency | 8.67 ± 1.65 | 1.74 ± 0.87 | 0.000 |
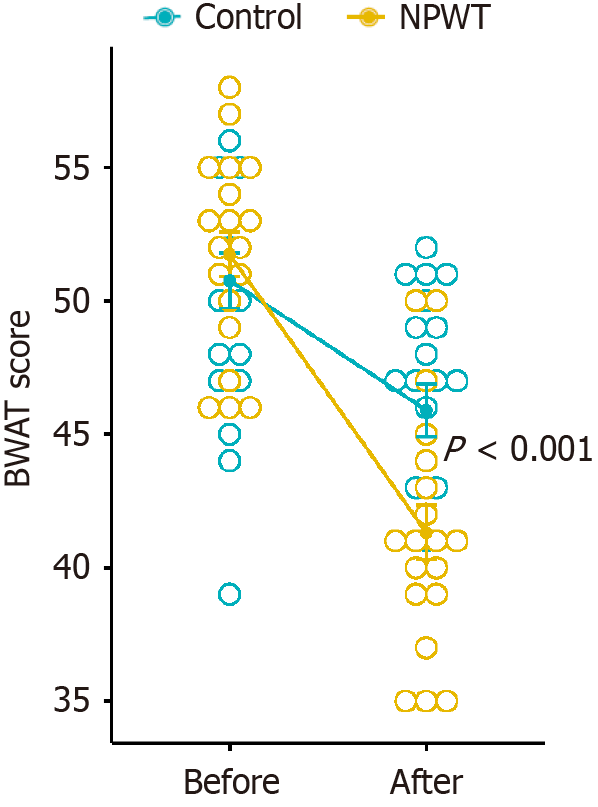
The Bates-Jensen wound assessment tool was used to assess the wounds. Before treatment, no statistically significant differences in wound scores were observed between the two groups (50.76 ± 4.71 vs 51.74 ± 3.66, P = 0.615). However, after 7 days of treatment, the NPWT group showed a significantly greater reduction in wound scores than the control group (41.32 ± 4.47 vs 45.90 ± 4.50, P = 0.003; Δ -10.42 ± 3.12 vs -4.86 ± 1.42, P < 0.001) (Figure 2). There were no adverse events during the treatment.
Prior to treatment, there were no statistically significant differences observed in the levels of erythrocyte sedimentation rate (ESR) (74.26 ± 39.37 vs 71.90 ± 33.79 mm/hour, P = 0.755) and procalcitonin (PCT) (0.37 ± 0.37 vs 0.24 ± 0.31 ng/mL, P = 0.176) between the two groups. However, after 7 days of treatment, the NPWT group had significantly lower levels of ESR (29.79 ± 17.55 vs 58.57 ± 29.36 mm/hour, P = 0.002; Δ -44.47 ± 37.28 vs -13.33 ± 19.28 mm/hour, P = 0.014) and PCT (0.03 ± 0.40 vs 0.15 ± 0.20 ng/mL, P = 0.043; Δ -0.34 ± 0.35 vs -0.09 ± 0.11 ng/mL, P = 0.023) than the control group (Supplementary Figure 1).
Before treatment, there were no differences in IL-6 (19.00 ± 2.12 vs 18.79 ± 2.38 pg/mL, P = 0.635) and TNF-α (33.51 ± 6.01 vs 33.31 ± 4.98 pg/mL, P = 0.989) levels between the NPWT and the control group. Following 7 days of treatment, there were significant differences in IL-6 (8.38 ± 2.72 vs 9.90 ± 1.95 pg/mL, P = 0.047; Δ -10.62 ± 1.82 vs -8.88 ± 1.93 pg/mL, P = 0.010) and TNF-α (19.11 ± 3.49 vs 22.22 ± 3.68 pg/mL, P = 0.025; Δ -14.40 ± 4.89 vs -11.09 ± 4.12 pg/mL, P = 0.047) levels (Supplementary Figure 2).
Immunohistochemical staining of CD31 was conducted at 40 × magnification, revealing positively stained cells that appeared brownish-yellow or brown. After 7 days of treatment, the NPWT group showed a notable increase in both newly formed blood vessels. The mean microvessel density value in the NPWT group was significantly higher than that of the control group (25.84 ± 6.51 vs 16.10 ± 5.97; P < 0.001) (Figure 3).
Before treatment, no significant differences in VEGF (76.38 ± 6.26 vs 75.73 ± 9.38 pg/mL, P = 0.989) and b-FGF (40.67 ± 6.70 vs 41.97 ± 7.44 pg/mL, P = 0.542) levels were found between the NPWT and control group. However, following 7 days of treatment, VEGF (106.23 ± 12.03 vs 91.24 ± 6.60 pg/mL, P < 0.001; Δ 29.85 ± 10.43 vs 15.51 ± 4.90 pg/mL, P < 0.001) and b-FGF (96.48 ± 8.63 vs 62.87 ± 8.24 pg/mL, P < 0.001; Δ 55.82 ± 8.42 vs 20.90 ± 5.16 pg/mL, P < 0.001) levels in the NPWT group were significantly higher than those in the control group (Supplementary Figure 3).
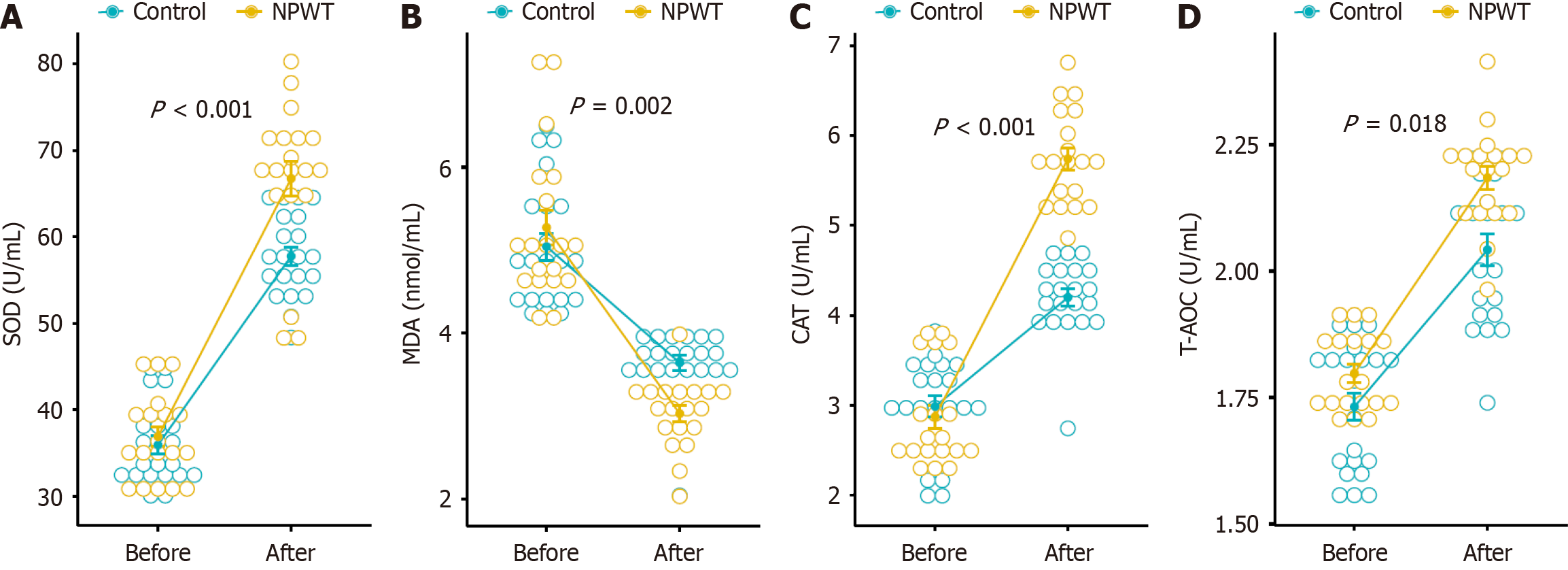
Before treatment, there were no statistically significant differences in the expression of SOD (35.94 ± 4.79 vs 36.90 ± 4.98 U/mL, P = 0.448), T-AOC (1.73 ± 0.12 vs 1.80 ± 0.08 U/mL, P = 0.093), CAT (2.99 ± 0.53 vs 2.87 ± 0.56 U/mL, P = 0.343), and MDA (5.04 ± 0.75 vs 5.27 ± 0.92 nmol/mL, P = 0.432) between the control group and NPWT group. After 7 days of treatment, compared to the control group, there was a significant increase in the expression of SOD (57.7 ± 4.84 vs 66.74 ± 8.89 U/mL, P < 0.001; Δ 21.82 ± 1.50 vs 29.83 ± 5.14 U/mL, P < 0.001), T-AOC (2.04 ± 0.14 vs 2.18 ± 0.10 U/mL, P = 0.002; Δ 0.31 ± 0.14 vs 0.39 ± 0.11 U/mL, P = 0.018), and CAT (4.20 ± 0.43 vs 5.74 ± 0.53 U/mL, P < 0.001; Δ 1.21 ± 0.64 vs 2.87 ± 0.94 U/mL, P < 0.001). Conversely, there was a significant decrease in the expression of MDA (3.64 ± 0.41 vs 3.03 ± 0.43 nmol/mL, P < 0.001; Δ -1.40 ± 0.90 vs -2.24 ± 1.08 nmol/mL, P = 0.020) (Figure 4).
The Western blot analysis revealed a statistically significant difference in the expression of Nrf2 and Keap1 after treatment (P < 0.05). Following 7 days of treatment, the level of Nrf2 in the NPWT group significantly increased, indicating up-regulation. Conversely, the level of Keap1 in the NPWT group significantly decreased after 7 days of treatment, indicating down-regulation (Figure 5).
NPWT is an additional treatment option for patients with chronic non-healing DFUs. Several research studies have indicated that NPWT is more effective in managing chronic DFUs compared to standard treatment[16,17]. A meta-analysis highlighted the limitations of current evidence from randomized controlled trials. This suggests that more research is needed to clarify the decision to use NPWT as a treatment option for DFUs[18]. Therefore, further con
NPWT has been found to accelerate wound healing through several mechanisms. The application of negative pressure creates mechanical tension on the wound edge, leading to minor changes in the wound’s shape. This mechanical pull stimulates the proliferation of fibroblasts and upregulates the expression of FGF2, thereby facilitating wound healing. Additionally, negative pressure establishes a low-oxygen environment that inhibits bacterial growth and reproduction, effectively reducing infections and preventing the development of drug-resistant bacteria caused by excessive antibiotic use. Furthermore, NPWT aids in pus drainage, mitigates tissue edema, decreases levels of inflammatory cytokines, improves microcirculation, and promotes the formation of granulation tissue[7,19]. A meta-analysis showed that NPWT is more effective than traditional dressing changes in reducing wound area and promoting DFUs healing[20]. This study highlights the superior efficacy of NPWT, compared to standard treatment. The wound status of patients undergoing NPWT, as evaluated by the Bates-Jensen wound scoring tool, dramatically improved by the end of treatment, while the degree of DFUs wound change in the routine dressing group was significantly less than that in the NPWT group. Furthermore, NPWT was found to alleviate inflammatory biomarkers in patients with DFUs. In this study, the levels of inflammatory biomarkers such as PCT and ESR, significantly decreased in patients treated with NPWT. Inflammation has a profound impact on the prognosis of DFUs[21]. Effective management of inflammation plays a crucial role in promoting wound healing.
While NPWT has been shown to enhance wound healing through various mechanisms, the exact ways it works in the treatment of DFUs are still under investigation. DFUs possess distinct characteristics compared to other chronic wounds. Hyperglycemia, along with excessive oxidative stress and inflammation, can significantly delay the healing process of DFUs. Hence, it becomes apparent that modifying the oxidative stress status of the wound significantly impacts the process of wound healing. The Nrf2 signaling pathway is a crucial component of the body’s antioxidant defense system. By inducing downstream targets, Nrf2 protects tissues from damage caused by oxidative stress[22]. Normally, Nrf2 is primarily located in the cytoplasm where it binds to Keap1. However, under conditions of oxidative stress, Keap1 dissociates from Nrf2, allowing Nrf2 to translocate to the nucleus and form a heterodimer with the Maf transcription factor. These heterodimers identify antioxidant response elements and trigger the expression of key antioxidant genes, such as HO-1, NQO-1, CAT, SOD, and GSH. They play vital roles in maintaining cellular redox homeostasis, supporting detoxification processes, and responding to stress. Decreased levels of circulating and tissue Nrf2 contribute to heightened oxidative stress, further aggravating endothelial dysfunction and abnormal angiogenesis, both of which are common manifestations in diabetes[23]. Previous studies highlighted Nrf2 as a key therapeutic target for promoting healing in DFUs[13]. While NPWT has been found to facilitate wound healing through various pathways like mitogen-activated protein kinase/c-Jun N-terminal kinase[24], there haven’t been any studies investigating the activation of the Nrf2/Keap-1 pathway by NPWT to promote wound healing. Our study showed that NPWT treatment significantly raises Nrf2 levels while lowering Keap1 levels in DFUs, providing fresh evidence for the effectiveness of NPWT in managing these ulcers.
In the NPWT group, levels of SOD, CAT, and T-AOC increased, while MDA levels decreased. This suggests that NPWT may enhance the antioxidant capacity of the wound and improve oxidative stress. We speculate that NPWT promotes the healing of DFUs by activating the Nrf2/Keap1 pathway. Nrf2 plays a vital role in reducing oxidative stress by regulating the synthesis of endogenous antioxidants and the expression of enzymes that scavenge reactive oxygen species. Additionally, it helps maintain mitochondrial homeostasis and prevents excessive cell damage caused by autophagy. Nrf2 downregulates the transcription of pro-inflammatory cytokine genes mediated by nuclear factor kappa-B, leading to a decrease in inflammatory cytokines and reducing the inflammatory response[25]. Both CAT and SOD are crucial enzymes that effectively remove reactive oxygen species. SOD converts superoxide into hydrogen peroxide, while CAT catalyzes the conversion of hydrogen peroxide into water and oxygen, thus improving the oxygen supply to local tissues while also removing free radicals. MDA, which is widely accepted as the biomarker of oxidative stress, serves as an important indicator for evaluating the generation of oxygen free radicals and tissue damage. Excessive accumulation of MDA leads to endothelial dysfunction, which can impact blood vessel formation and impede wound healing[26]. Thus, the reduction in MDA levels after NPWT treatment suggests an improvement in oxidative stress in DFUs.
This study found that NPWT treatment increased serum levels of VEGF and b-FGF in patients. These markers play a crucial role in angiogenesis. VEGF is predominantly expressed in endothelial cells, macrophages, smooth muscle cells, platelets, and neutrophils. It not only promotes the formation of new blood vessels but also facilitates the deposition of collagen and epithelial cell growth, thereby promoting wound healing[27]. These findings align with a study conducted by Karam et al[28]. In addition, b-FGF stimulates fibroblast proliferation and contributes to blood vessel formation during the intermediate and late stages, working alongside VEGF. The expression of VEGF and b-FGF increases after NPWT is applied to the wound[29,30]. Their observations revealed that applying moderate pressure to the wounds promoted wound healing by accelerating granulation tissue growth, increasing the production of angiogenic factors, and improving the deposition of collagen fibers. Moreover, Valcarcel's study found that disrupting the Nrf2 signaling pathway impacts various essential steps of angiogenesis, such as adhesion, proliferation, migration, and the formation of capillary-like structures[31]. Nrf2 regulates cancer angiogenesis by targeting VEGF[32]. Knockdown of Nrf2 under hypoxic conditions downregulates VEGF expression through the phosphatidylinositol 3-kinase/protein kinase B signaling pathway in cerebral microvascular endothelial cells, leading to the inhibition of angiogenesis[33]. The current study results suggest a potential association between VEGF and Nrf2 in regulating angiogenesis. These findings provide insights into the molecular mechanisms underlying NPWT therapy, particularly in the context of wound healing. Additionally, it has been proposed that Nrf2-regulated HO-1 expression contributes to a positive feedback loop, where VEGF activates Nrf2 in an extracellular regulated protein kinases 2/1-dependent manner. Furthermore, the upregulation of HO-1 may increase VEGF expression through the production of carbon monoxide and the stabilization of HIF-1α[34]. Studies on diabetic retinopathy and oxidative stress have suggested that the activation and nuclear translocation of Nrf2 mediate the expression of VEGF induced by oxidative stress[35]. Therefore, we speculate that NPWT can promote the healing of DFUs by activating the Nrf2 antioxidant pathway, potentially through the alleviation of oxidative stress and the regulation of angiogenesis.
This study had several limitations. First, the study was performed in a small group of patients without adjusting confounding variables, which increased the likelihood of biased results. Second, the present results were examined by non-parametric methods. The results may not be generalizable to other study populations. Third, this study provides initial insights suggesting that NPWT may facilitate the healing of DFUs via the Nrf2 pathway. Moving forward, we plan to conduct further research using animal models to explore additional protein molecules and strengthen the robustness of our findings.
This study provides preliminary insights into the potential role of Nrf2 in the wound healing process of DFUs treated with NPWT. Our findings indicate that NPWT may enhance wound healing, possibly through the upregulation of Nrf2. This upregulation may activate antioxidant defense mechanisms, promote angiogenesis, and reduce inflammation, although further research is needed to confirm these effects.
We would like to express gratitude to the colleagues for their invaluable guidance and assistance in data analysis.
| 1. | Lim QH, Loy LC, Abdul Hadi H, Faheem NAN, Shaharuddin IS, Sri La Ponnampalavanar S, Lim LL. Diabetic foot ulcer in the Western Pacific Region: Current data on ulceration rates and microbial profiles, gaps and charting strategies. Prim Care Diabetes. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Luo Y, Liu C, Li C, Jin M, Pi L, Jin Z. The incidence of lower extremity amputation and its associated risk factors in patients with diabetic foot ulcers: A meta-analysis. Int Wound J. 2024;21:e14931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: A systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2023;25:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 93] [Article Influence: 46.5] [Reference Citation Analysis (1)] |
| 4. | Song J, Liu A, Liu B, Huang W, Jiang Z, Bai X, Hu L, Zheng S, Guo S, Wu J, Chen Q. Natural Biologics Accelerate Healing of Diabetic Foot Ulcers by Regulating Oxidative Stress. Front Biosci (Landmark Ed). 2022;27:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Liu Z, Hu L, Zhang T, Xu H, Li H, Yang Z, Zhou M, Smith HS, Li J, Ran J, Deng Z. PKCβ increases ROS levels leading to vascular endothelial injury in diabetic foot ulcers. Am J Transl Res. 2020;12:6409-6421. [PubMed] |
| 6. | James SMD, Sureshkumar S, Elamurugan TP, Debasis N, Vijayakumar C, Palanivel C. Comparison of Vacuum-Assisted Closure Therapy and Conventional Dressing on Wound Healing in Patients with Diabetic Foot Ulcer: A Randomized Controlled Trial. Niger J Surg. 2019;25:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Borys S, Hohendorff J, Frankfurter C, Kiec-Wilk B, Malecki MT. Negative pressure wound therapy use in diabetic foot syndrome-from mechanisms of action to clinical practice. Eur J Clin Invest. 2019;49:e13067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Bobkiewicz A, Francuzik W, Martinkosky A, Borejsza-Wysocki M, Ledwosinski W, Szmyt K, Banasiewicz T, Krokowicz L. Negative Pressure Level and Effects on Bacterial Growth Kinetics in an in vitro Wound Model. Pol J Microbiol. 2024;73:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Silverman RP. Negative Pressure Wound Therapy With Instillation and Dwell Time: Mechanisms of Action Literature Review. Eplasty. 2023;23:e54. [PubMed] |
| 10. | Normandin S, Safran T, Winocour S, Chu CK, Vorstenbosch J, Murphy AM, Davison PG. Negative Pressure Wound Therapy: Mechanism of Action and Clinical Applications. Semin Plast Surg. 2021;35:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Liu X, Zhao P, Wu X, Zhao Y, Zhou F, Luo Y, Jia X, Zhong W, Xing M, Lyu G. Negative Pressure Smart Patch to Sense and Heal the Wound. Adv Sci (Weinh). 2025;12:e2408077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, Zheng H, Zhang DD. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes. 2016;65:780-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Teena R, Dhamodharan U, Jayasuriya R, Ali D, Kesavan R, Ramkumar KM. Analysis of the Exonic Single Nucleotide Polymorphism rs182428269 of the NRF2 Gene in Patients with Diabetic Foot Ulcer. Arch Med Res. 2021;52:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Jayasuriya R, Dhamodharan U, Karan AN, Anandharaj A, Rajesh K, Ramkumar KM. Role of Nrf2 in MALAT1/ HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic Biol Med. 2020;156:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates-Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Wu Y, Shen G, Hao C. Negative pressure wound therapy (NPWT) is superior to conventional moist dressings in wound bed preparation for diabetic foot ulcers: A randomized controlled trial. Saudi Med J. 2023;44:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Seidel D, Lefering R; DiaFu study group. NPWT resource use compared with standard moist wound care in diabetic foot wounds: DiaFu randomized clinical trial results. J Foot Ankle Res. 2022;15:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Liu Z, Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, Peinemann F. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2018;10:CD010318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Schlosser KA, Otero J, Lincourt A, Augenstein VA. Management of Surgical Incisions Using Incisional Negative-Pressure Therapy. Plast Reconstr Surg. 2019;143:15S-20S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Huang Q, Wang JT, Gu HC, Cao G, Cao JC. Comparison of Vacuum Sealing Drainage and Traditional Therapy for Treatment of Diabetic Foot Ulcers: A Meta-Analysis. J Foot Ankle Surg. 2019;58:954-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Aydın MS, Eren MA, Uyar N, Kankılıç N, Karaaslan H, Sabuncu T, Çelik H. Relationship between systemic immune inflammation index and amputation in patients with diabetic foot ulcer. J Orthop Sci. 2024;29:1060-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | Xu WD, Yang C, Huang AF. The role of Nrf2 in immune cells and inflammatory autoimmune diseases: a comprehensive review. Expert Opin Ther Targets. 2024;28:789-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Wang T, Li X, Fan L, Chen B, Liu J, Tao Y, Wang X. Negative pressure wound therapy promoted wound healing by suppressing inflammation via down-regulating MAPK-JNK signaling pathway in diabetic foot patients. Diabetes Res Clin Pract. 2019;150:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 827] [Cited by in RCA: 1336] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 26. | Huang X, Liang P, Jiang B, Zhang P, Yu W, Duan M, Guo L, Cui X, Huang M, Huang X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020;259:118246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 27. | Shams F, Moravvej H, Hosseinzadeh S, Mostafavi E, Bayat H, Kazemi B, Bandehpour M, Rostami E, Rahimpour A, Moosavian H. Overexpression of VEGF in dermal fibroblast cells accelerates the angiogenesis and wound healing function: in vitro and in vivo studies. Sci Rep. 2022;12:18529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Karam RA, Rezk NA, Abdel Rahman TM, Al Saeed M. Effect of negative pressure wound therapy on molecular markers in diabetic foot ulcers. Gene. 2018;667:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Guo H, Xue Z, Mei S, Li T, Yu H, Ning T, Fu Y. Clinical efficacy of antibiotic-loaded bone cement and negative pressure wound therapy in multidrug-resistant organisms diabetic foot ulcers: a retrospective analysis. Front Cell Infect Microbiol. 2024;14:1521199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Fujii M, Bessho R, Miyagi Y, Nitta T. Negative-pressure sternal wound closure with interrupted subcuticular suturing and a subcutaneous drain tube reduces the incidence of poststernotomy wound infection after coronary artery bypass grafting surgery. Surg Today. 2020;50:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Huang Y, Yang Y, Xu Y, Ma Q, Guo F, Zhao Y, Tao Y, Li M, Guo J. Nrf2/HO-1 Axis Regulates the Angiogenesis of Gastric Cancer via Targeting VEGF. Cancer Manag Res. 2021;13:3155-3169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Huang Y, Mao Y, Li H, Shen G, Nan G. Knockdown of Nrf2 inhibits angiogenesis by downregulating VEGF expression through PI3K/Akt signaling pathway in cerebral microvascular endothelial cells under hypoxic conditions. Biochem Cell Biol. 2018;96:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Li L, Pan H, Wang H, Li X, Bu X, Wang Q, Gao Y, Wen G, Zhou Y, Cong Z, Yang Y, Tang C, Liu Z. Interplay between VEGF and Nrf2 regulates angiogenesis due to intracranial venous hypertension. Sci Rep. 2016;6:37338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Rossino MG, Lulli M, Amato R, Cammalleri M, Monte MD, Casini G. Oxidative Stress Induces a VEGF Autocrine Loop in the Retina: Relevance for Diabetic Retinopathy. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |