Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.103244
Revised: February 12, 2025
Accepted: February 25, 2025
Published online: May 15, 2025
Processing time: 163 Days and 12.7 Hours
Beinaglutide, a short-acting glucagon-like polypeptide-1 receptor agonist, has shown variable efficacy in weight reduction and metabolic control in randomized controlled trials (RCTs).
To summarize the therapeutic effects of beinaglutide in patients with over
RCTs involving patients receiving beinaglutide in the intervention arm and placebo or active comparator in the control arm were searched through multiple electronic databases. The change from baseline in body weight was the primary outcome; secondary outcomes included changes in body mass index (BMI), waist circumference (WC), blood pressure, glycemic parameters, lipids, and adverse events (AEs). RevMan web was used to conduct meta-analysis using random-effects models. Outcomes were presented as mean differences (MDs), odds ratios (ORs), or risk ratios (RRs) with 95% confidence intervals (95%CIs).
Six RCTs (n = 800) with mostly some concerns about the risk of bias were included. Over 12-24 weeks, beinaglutide 0.1-0.2 mg thrice daily was superior to the control group in reducing total (MD = -3.25 kg, 95%CI: -4.52 to -1.98, I2 = 84%, P < 0.00001) and percent (MD = -4.13%, 95%CI: -4.87 to -3.39, I2 = 54%, P < 0.00001) body weight reduction. Beinaglutide also outperformed the control group in achieving weight loss by 5% (OR 4.61) and 10% (OR = 5.34). The superiority of beinaglutide vs the control group was also found in reducing BMI (MD = -1.22 kg/m2, 95%CI:
Short-term data from RCTs suggested that beinaglutide causes modest benefits in reducing body weight, BMI, and WC, with no significant difference in glycemic and other metabolic endpoints compared to the control arm. Safety data were consistent with those of the other drugs in the glucagon-like polypeptide-1 receptor agonist class. Larger RCTs are warranted to prove the longer-term metabolic benefits of beinaglutide.
Core Tip: This systematic review assessed available randomized controlled trials involving patients receiving beinaglutide, a short-acting glucagon-like polypeptide-1 receptor agonist. Beinaglutide at doses of 0.1-0.2 mg three times daily for 12-24 weeks was more effective than the control group at reducing body weight. Beinaglutide also demonstrated superiority over the control group in reducing body mass index and waist circumference. Beinaglutide posed greater risks of treatment discontinuation due to adverse events, including nausea, vomiting, palpitations, headaches, and dizziness, compared to the control group. However, the two groups had identical risks of total and serious adverse events, diarrhea, fatigue, and hypoglycemia.
- Citation: Kamrul-Hasan ABM, Ganakumar V, Nagendra L, Dutta D, Islam MR, Pappachan JM. Effect of beinaglutide, a thrice-daily GLP-1 receptor agonist, on body weight and metabolic parameters: A systematic review and meta-analysis. World J Diabetes 2025; 16(5): 103244
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/103244.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.103244
Around 2.5 billion people are overweight worldwide, translating to as many as one in four individuals being overweight and one in eight individuals having obesity[1]. Obesity has significant implications for cardiometabolic health, including an increased risk of type 2 diabetes (T2D), hypertension, dyslipidemia, metabolic dysfunction-associated steatotic liver disease, obstructive sleep apnea, chronic kidney disease, and cardiovascular (CV) disease. Additionally, it is associated with musculoskeletal complications and an increased risk of dementia and several malignancies[2]. T2D remains the most clinically significant metabolic complication of obesity due to a strong shared pathophysiology[3].
Until recently, treating obesity has been quite challenging, as older anti-obesity drugs frequently provided inadequate weight loss benefits and raised safety concerns. Over the past 50 years significant strides have been achieved in addressing obesity and the related health issues linked to excess weight. Newer therapeutic agents like glucagon-like polypeptide-1 (GLP-1) receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors have been the breakthroughs in advancing our armamentarium against obesity and T2D[4]. The ubiquitous nature of the two conditions in the modern era, along with elevated CV risk, has mandated a more integrated approach to managing diabetes, extending beyond targeting normoglycemia. The advent of these new therapeutic agents has prompted a substantial shift from the glucocentric approach towards a more holistic approach to managing T2D that prioritizes not only glycemic and metabolic control but also CV and renal protection as vital components of patient care[5].
GLP-1RAs amplify glucose-dependent insulin secretion and inhibit glucagon secretion from the pancreas. They also promote beta cell function and mass expansion, suggesting a potentially disease-modifying effect in T2D. Additionally, they have a potent anorexigenic effect by central action via modulation of appetite-regulating neural centers and by delaying gastric emptying peripherally, leading to significant weight loss[6]. In clinical trials, GLP-1RAs demonstrated substantial reductions in body weight, body mass index (BMI), and waist circumference (WC), regardless of diabetes status, the specific GLP-1RA used, or the route of administration. Subcutaneous liraglutide, semaglutide, and tirzepatide are approved GLP-1RA-based chronic weight management therapies, while many are in the pipeline[7].
Exenatide, the first GLP-1RA approved for clinical use, was launched in 2005 as a twice-daily injection for T2D treatment. Exenatide twice-daily was followed by once-daily liraglutide, once-weekly exenatide, once-daily lixisenatide, once-daily oral semaglutide, and several once-weekly medicines, including albiglutide, dulaglutide, semaglutide, and tirzepatide. The superiority of GLP-1RAs over other antidiabetic drugs in reducing glycated hemoglobin (HbA1c) and facilitating weight loss without the risk of hypoglycemic episodes has been well-established. Semaglutide and tirzepatide showed the highest efficacy in lowering HbA1c by 1.70% and 2.37%, respectively[8]. GLP-1RAs also have anti-inflammatory and anti-atherogenic effects and address several other cardiometabolic risk factors, including hypertension, dyslipidemia, and renal disease progression, potentially contributing to CV risk reduction[6]. Consequently, GLP-1RAs signify a pivotal advancement in managing obesity and diabetes, enhancing the tenets of holistic care and CV risk mitigation[7].
Beinaglutide (formerly known as benaglutide) is a novel recombinant GLP-1RA with nearly 100% structural homology to human GLP-1 (7-36)[9]. With a half-life of 15 min and a duration of action of 2 h, it is a subcutaneously administered short-acting GLP-1RA typically administered preprandially three times a day (TID). It more effectively stimulates the postprandial glucose-dependent insulin secretion and controls postprandial plasma glucose[10]. It demonstrated effectiveness in lowering fasting plasma glucose (FPG), postprandial plasma glucose, and HbA1c in several randomized controlled trials (RCTs) in China, after which it received the approval of the Chinese Food and Drug Administration for treating T2D in 2016[10]. It is only the second molecule to have also received approval from China’s National Medical Products Association for managing obesity following significant weight loss benefits noted in the studies in individuals with or without T2D[10]. It has proven to be an important addition to the GLP-1RA class of agents, featuring a unique structure and pharmacokinetics, and is the only medication in this class that is administered TID. Hence, its role in the contemporary management of obesity and diabetes needs to be evaluated.
With this systematic review and meta-analysis (SRM), we shed light on this molecule by summarizing the available evidence for beinaglutide and its effects on body weight and other metabolic parameters.
This SRM followed the recommended reporting items for Systematic Reviews and Meta-Analyses checklists and the procedures described in the Cochrane Handbook for Systematic Reviews of Interventions[11,12]. The SRM was registered with PROSPERO (CRD42024600540), and the protocol summary is accessible online.
Several databases and registers were systematically searched, including MEDLINE (via PubMed), Scopus, Cochrane Central Register, Google Scholar, ClinicalTrials.gov, and the Chinese Clinical Trial Register. The search covered these sources from their commencement to September 30, 2024. The search terms were applied to titles and abstracts; the search technique followed a Boolean approach using the terms “Beinaglutide” OR “Benaglutide” AND “type 2 diabetes” OR “type 2 diabetes mellitus” OR “T2D” OR “T2DM” OR “obesity” OR “overweight”. Every recently published or unpublished clinical study in English was searched exhaustively and carefully. This search involved reviewing pertinent publications and references in the clinical trials included in the present work.
Population, Intervention, Comparison, Outcomes, and Study (PICOS) design was used as a framework to formulate eligibility criteria for the clinical trials in this SRM. The patient population (P) consisted of adults with overweight/obesity with/without T2D, the intervention (I) was beinaglutide subcutaneous injections, the comparison or control (C) included individuals receiving either a placebo or lifestyle interventions or other glucose-lowering drugs (GLDs), the outcomes (O) included change from baseline in body weight, and only RCTs were considered as the study type (S) for inclusion. This study comprised RCTs spanning a minimum duration of 12 weeks with study individuals aged at least 18 years. The trials had at least two treatment arms/groups: One group receiving beinaglutide 0.1-0.2 mg subcutaneous TID with or without lifestyle interventions or other GLDs and the other group receiving either a placebo or lifestyle interventions or GLDs. Excluded from consideration were nonrandomized trials, retrospective studies, pooled analyses of clinical trials, conference proceedings, letters to editors, case reports, and articles that did not provide data on outcomes of interest. Clinical trials involving animals or healthy humans and RCTs with less than 12 weeks were also excluded.
Four review authors independently extracted data using standardized forms, with details provided elsewhere[13]. The handling of missing data has also been elaborated upon in the same source[13].
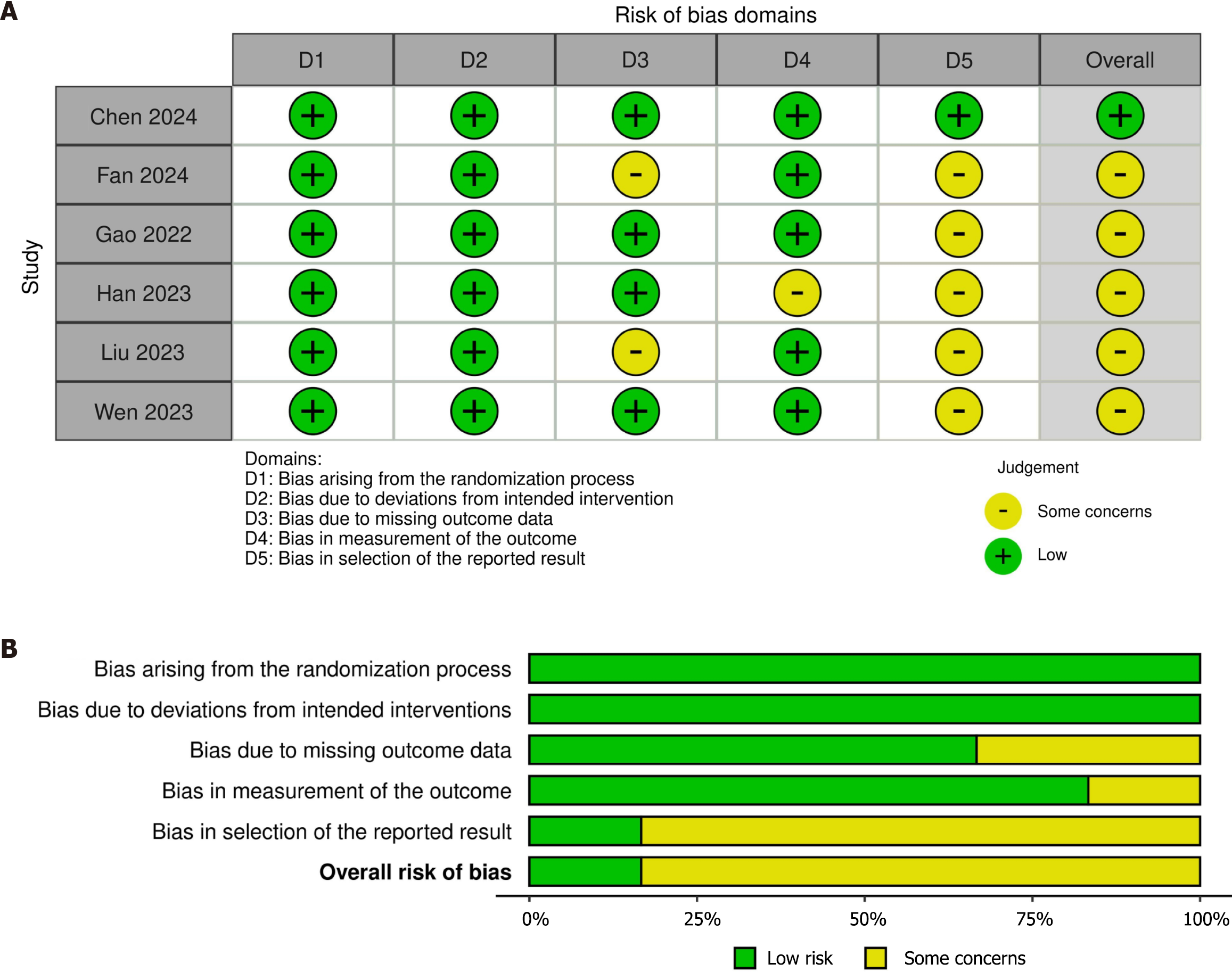
Four authors independently performed the risk of bias (RoB) assessment using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB2) in the Review Manager (RevMan) computer program, version 7.2.0.[14,15]. The domains included in RoB2 cover all types of bias currently understood to affect the results of RCTs, e.g., bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. The risk-of-bias judgment assigned one of three levels to each domain: low RoB; some concerns; or high RoB. The least favorable assessment across the domains of bias was considered the overall RoB for the result[14]. The Risk-of-bias VISualization (robvis) web app was used to create risk-of-bias plots[16].
The primary outcome of interest was the change from baseline body weight (absolute and percentage) after the trials ended. Additional efficacy endpoints included subjects achieving weight reduction of 5% and 10%, changes in BMI, WC, blood pressure (BP), FPG, HbA1c, fasting insulin, homeostatic model assessment for insulin resistance (HOMA-IR), and lipid levels. Safety variables consisted of adverse events (AEs), serious AEs, gastrointestinal AEs, neurological AEs, and hypoglycemic episodes.
The results of the outcomes were expressed as mean differences (MDs) for continuous variables and as odds ratios (ORs) or risk ratios (RRs) for dichotomous variables with 95% confidence intervals (95%CIs). The RevMan-generated forest plots portrayed the standardized MD or OR or RR for the outcomes; the left side of the forest plot favored beinaglutide, and the right side favored the control group(s)[15]. Random effects analysis models were chosen to address the anticipated heterogeneity resulting from population characteristics and trial length variations. The inverse variance statistical method was applied for all instances. The SRM encompassed forest plots that integrated data from at least two trials. A significance level of P < 0.05 was used.
The assessment of heterogeneity was initially conducted by studying forest plots. Subsequently, a χ2 test was performed using N-1 degrees of freedom and a significance level of 0.05 to determine the statistical significance. The I2 test was also employed in the subsequent analysis[17]. The specifics of understanding I2 values have already been explained in depth elsewhere[13].
An overall assessment of the evidence quality related to the primary and major secondary outcomes of the meta-analysis was conducted using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach[18]. The process of creating the summary of findings table and evaluating the quality of evidence as “high”, “moderate”, “low”, or “very low” has been previously described in depth elsewhere[13].
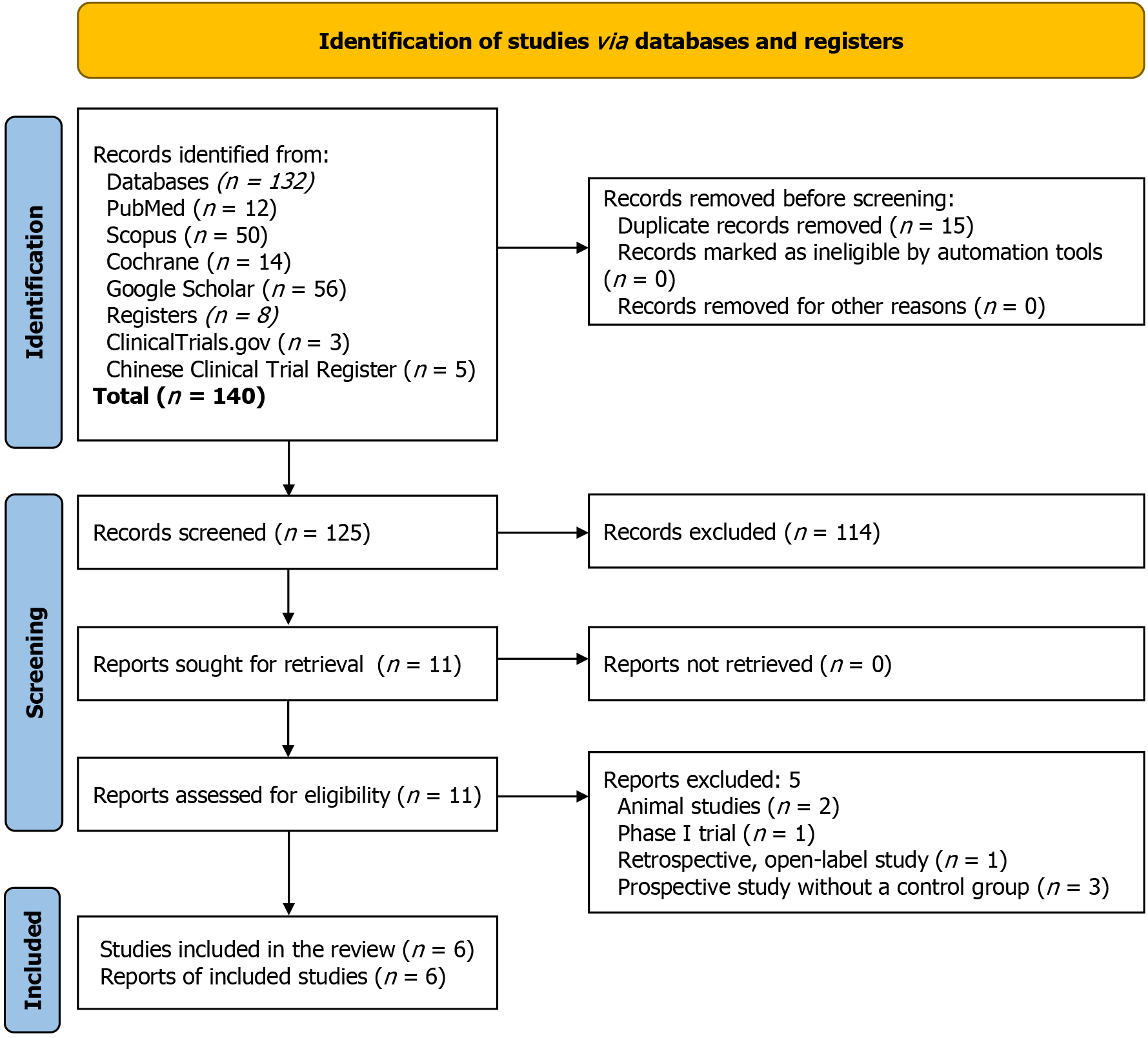
The steps for selecting studies are depicted in Figure 1. The initial search identified 140 articles; the number was narrowed to 11 after screening titles, abstracts, and subsequent full-text reviews. Finally, six RCTs involving 800 subjects meeting all the prespecified criteria were included in this SRM[19-24]. Five studies were excluded[25-29]; among them, two were animal studies[25,28], one was a phase I study[26], one was a prospective open-label study without a control group[27], and the other was a retrospective open-label study[29].
All six RCTs in this SRM were conducted in China; two were multicenter[19,23], and the other four were single-center studies[20-22,24]. Three trials included adults with T2D[20,22,23], and one of them included subjects with nonalcoholic fatty liver disease[20]. Three included trials were conducted among subjects with overweight/obesity having no diabetes[19,21,24]; one of these trials investigated adult females with polycystic ovary syndrome[24].
Study subjects in the beinaglutide arm of all included RCTs received the drug as preprandial subcutaneous injections TID, starting at a low dose with gradual up-titration of the dose to a maximum of 0.1-0.2 mg. In the control arm, Chen et al[19] used a placebo in 2024, Fan et al[20] used lifestyle intervention in 2024, Gao et al[21] used metformin 0.5 gm TID in 2022, Han et al[22] used insulin aspart subcutaneous injections TID in 2023, Liu et al[23] used insulin glargine subcutaneous injections before bed in 2023, and Wen et al[24] used metformin 850 mg two times a day in 2023. Both the beinaglutide and control arm of Han et al[22] received metformin in 2023. Wen et al[24] used metformin 850 mg two times a day in addition to beinaglutide 0.1-0.2 mg TID in the beinaglutide arm in 2023.
Gao et al[21] and Wen et al[24] had trial durations of 12 weeks, Chen et al[19] had a duration of 16 weeks with a 12-week post-treatment observation period in 2024, Liu et al[23] conducte a 16-week duration with an 8-week randomized period in 2023, Fan et al[20] had a duration of 24 weeks in 2024, and the study by Han et al[22] spanned over 6 months in 2023. The baseline characteristics of the included RCTs were matched throughout the trial arms. The specifics of the included and excluded studies are shown in Table 1 and Supplementary Table 1, respectively.
| Registration no. and study place | Major baseline characteristics of the study subjects | Study arms | n | Age (years, mean ± SD) | Female (%) | Baseline body weight (kg, mean ± SD) | Baseline BMI | Duration of RCT |
| ChiCTR1900023428, Multicenter in China[19] | Adults with BMI ≥ 28 kg/m2 or BMI ≥ 24-27.9 kg/m2 with weight-related complication; No DM | Beinaglutide 0.2 mg s.c. TID | 282 | 35.3 ± 9.1 | 51.4 | 89.35 ± 18.30 | 31.74 ± 4.60 | 16 weeks with a 12-week post-treatment observation |
| Placebo | 138 | 36.9 ± 8.7 | 52.9 | 88.04 ± 18.10 | 31.5 ± 4.70 | |||
| ChiCTR1900023611, Single center in China[20] | Adults with T2D with HbA1c ≤ 9.5%; NAFLD, MRS showing IHTG ≥ 15% | Beinaglutide 0.1 mg s.c. TID | 25 | 47.2 ± 13.0 | 48 | 85.2 ± 14.9 | 30.5 ± 4.0 | 24 weeks |
| Lifestyle intervention | 25 | 52.8 ± 15.2 | 40 | 87.4 ± 20.2 | 30.1 ± 4.7 | |||
| NCT03593668, Single center in China[21] | Adults with BMI ≥ 28-37.5 kg/m2 or BMI ≥ 24-27.9 kg/m2 with weight-related complication; No DM | Beinaglutide 0.2 mg s.c. TID | 32 | 32.5 ± 1 .6 | 50.0 | 94.0 ± 2.5 | 32.3 ± 0.4 | 12 weeks |
| Metformin 0.5 gm orally TID | 32 | 32.3 ± 1.4 | 56.3 | 88.0 ± 2.5 | 31.2 ± 0.6 | |||
| ChiCTR2200061003, Single center in China[22] | Adults with T2D; BMI 22-40 kg/m2; At least 8 weeks of stable treatment with metformin alone (daily dose ≥ 1 g) prior to screening | Beinaglutide 0.1-0.2 mg s.c. TID + Metformin | 67 | 52.1 ± 9.2 | NA | 84.34 ± 13.86 | 27.08 ± 3.64 | 6 months |
| Insulin Aspart + Metformin | 67 | 51.8 ± 8.3 | NA | 83.12 ± 16.09 | 26.87 ± 2.94 | |||
| NCT03829891, Multicenter in China[23] | Adults with T2D for ≥ 6 months; HbA1c ≥ 7.5 - ≤ 11.0%; BMI 21-35 kg/m2; Stable OHA (alone or in combination therapy, excluding glinides, incretin-based therapies, and insulin) for ≥ 1 month | Beinaglutide 0.1-0.2 mg s.c. TID | 35 | 52.6 ± 11.5 | 34.3 | 69.73 ± 14.45 | 24.71 ± 3.44 | 16 weeks, 8 weeks randomized period |
| Insulin Glargine before bed | 33 | 53.3 ± 8.13 | 21.2 | 72.36 ± 10.57 | 24.87 ± 2.48 | |||
| ChiCTR2000033741, Single center in China[24] | Females with PCOS; Age 18-40 years; BMI ≥ 24 kg/m2 | Beinaglutide 0.1-0.2 mg s.c. TID + Metformin 850 mg BID | 32 | 26.75 ± 4.40 | 100 | 73.95 ± 6.71 | 28.65 ± 1.93 | 12 weeks |
| Metformin 850 mg BID | 32 | 25.43 ± 3.10 | 100 | 72.68 ± 6.23 | 28.79 ± 2.12 |
Figure 2 illustrates the specific and overall RoB in the six RCTs. One (16.7%) trial [19] had low overall RoB, and the other five (83.3%) had some concerns. The trials with some concerns had biases due to missing outcome data[20,23], measurement of the outcome [22], and the selection of the reported result [20-24]]. Publication bias was not assessed due to the inadequate number of RCTs (at least 10) in forest plots[30].
The grades for the certainty of evidence of the results are given in the summary of findings table (Supplementary Table 2).
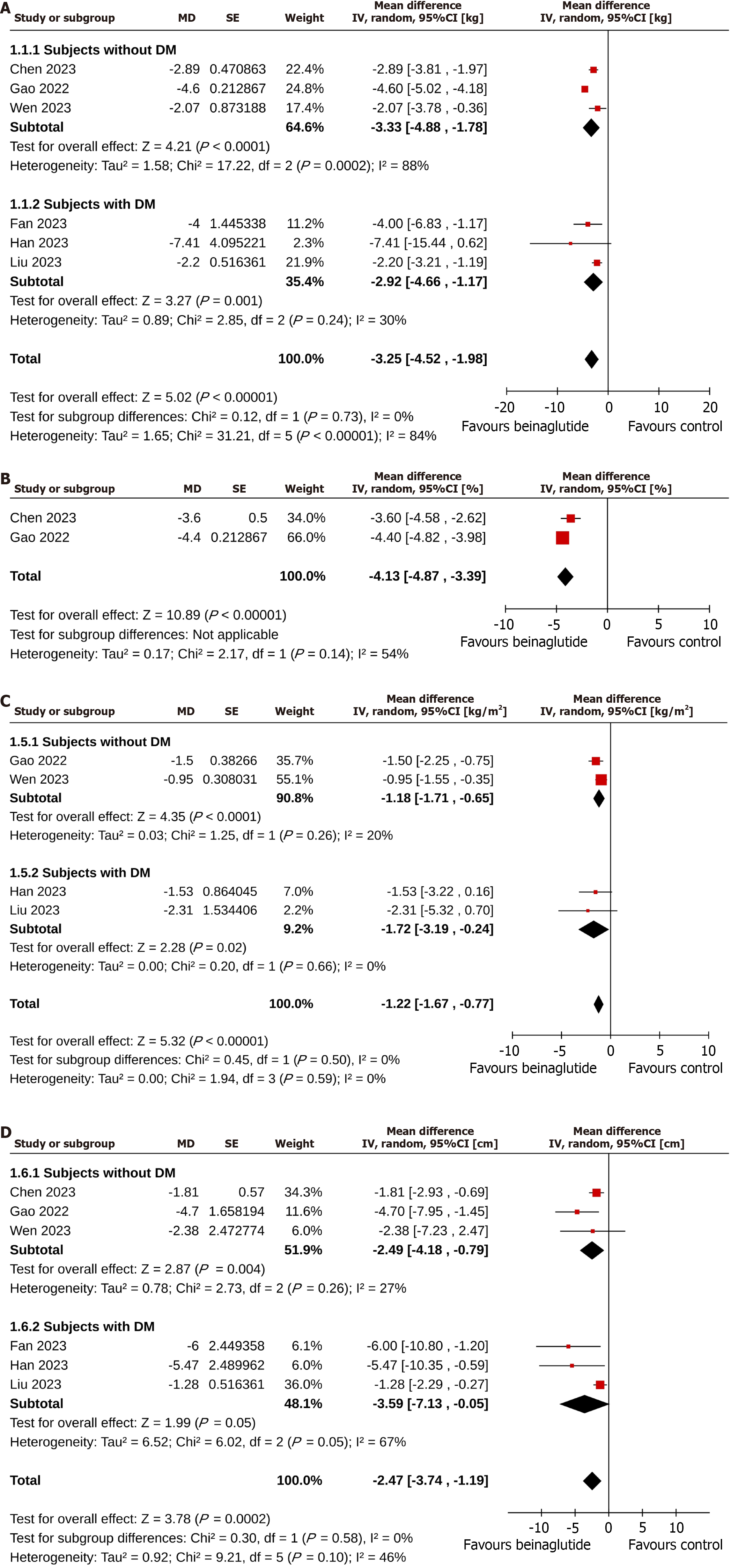
Subjects in the beinaglutide group achieved greater body weight reduction than those in the control group (MD = -3.25 kg, 95%CI: -4.52 to -1.98), I2 = 84%, P < 0.00001, very low certainty of the evidence). The superiority of beinaglutide over the control group in weight reduction was found in subjects with T2D (MD = -2.92 kg, 95%CI: -4.66 to -1.17, I2 = 30%, P = 0.001) and without T2D (MD = -3.33 kg, 95%CI: -4.88 to -1.78, I2 = 88%, P < 0.0001). The extent of weight reduction in subjects with and without T2D was similar (P for subgroup differences = 0.73; Figure 3A). The percent reduction in weight was also greater in the beinaglutide group than in the control group (MD = -4.13%, 95%CI: -4.87 to -3.39, I2 = 54%, P < 0.00001, low certainty of the evidence; Figure 3B). More study subjects in the beinaglutide group than in the control group achieved 5% (OR = 4.61, 95%CI: 3.07 to 6.93, I2 = 0%, P < 0.00001, moderate certainty of the evidence) and 10% (OR = 5.34, 95%CI: 2.78 to 10.25, I2 = 0%, P < 0.00001, moderate certainty of the evidence) weight reductions (Table 2).
| Outcome variables | No. of RCT included | No. of participants with outcome/participants analyzed | Pooled effect size, OR (95%CI) | I2 (%) | P value | |
| Beinaglutide arm | Control arm | |||||
| Categorical | ||||||
| Subjects achieving BW reduction 5% | 3 | 206/335 | 55/193 | 4.61 (3.07 to 6.93) | 0 | < 0.00001 |
| Subjects achieving BW reduction 10% | 3 | 80/335 | 12/193 | 5.34 (2.78 to 10.25) | 0 | < 0.00001 |
| Continuous | ||||||
| SBP (mmHg) | 3 | 349 | 203 | -1.11 (-2.74 to 0.53) | 0 | 0.19 |
| DBP (mmHg) | 3 | 349 | 203 | -0.57 (-1.87 to 0.72) | 0 | 0.38 |
| FPG (mmol/L) in subjects without T2D | 3 | 339 | 195 | -0.11 (-0.26 to 0.04) | 43 | 0.15 |
| FPG (mmol/L) in subjects with T2D | 3 | 107 | 108 | 0.32 (-0.50 to 1.15) | 63 | 0.44 |
| HbA1c (%) in subjects with T2D | 3 | 107 | 108 | 0.05 (-0.27 to 0.38) | 22 | 0.75 |
| Fasting insulin (mIU/L) in subjects without T2D | 2 | 309 | 165 | -2.28 (-10.97 to 6.41) | 69 | 0.61 |
| HOMA-IR in subjects without T2D | 3 | 339 | 195 | -0.11 (-1.76 to 1.55) | 70 | 0.9 |
| ALT (U/L) | 3 | 99 | 102 | -4.31 (-11.87 to 3.24) | 0 | 0.26 |
| AST (U/L) | 3 | 99 | 102 | -2.65 (-8.11 to 2.81) | 41 | 0.34 |
| TC (mmol/L) | 6 | 446 | 303 | 0.18 (-0.41 to 0.76) | 95 | 0.55 |
| LDL-C (mmol/L) | 6 | 446 | 303 | 0.17 (-0.41 to 0.75) | 99 | 0.56 |
| HDL-C (mmol/L) | 6 | 446 | 303 | 0.18 (-0.36 to 0.71) | 99 | 0.52 |
| TG (mmol/L) | 6 | 446 | 303 | 0.16 (-0.43 to 0.75) | 94 | 0.6 |
| FFA (mmol/L) | 2 | 331 | 190 | 0.54 (-0.54 to 1.61) | 99 | 0.33 |
Leave-one-out sensitivity analyses were performed for body weight change to detect the changes in the statistical significance levels and heterogenicity (at least 2-step change; Supplementary Table 3). There were no changes in the statistical significance levels of body weight change of beinaglutide vs control. The heterogenicity among the studies was reduced (I2 = 84% to I2 = 0%) after removing the study Gao et al[21] (2022).
Reduction in BMI was greater in the beinaglutide group than in the control group (MD = -1.22 kg/m2, 95%CI: -1.67 to
Table 2 summarizes the findings of the meta-analysis on other metabolic outcomes. Beinaglutide and control arms achieved identical changes in systolic BP (MD = -1.11 mmHg, 95%CI: -2.74 to 0.53, I2 = 0%, P = 0.19), diastolic BP (MD =
The safety outcome findings in the meta-analysis are summarized in Table 3. Similar proportions of study subjects in the beinaglutide and the control arms experienced at least one AE (RR = 2.90, 95%CI: 0.28 to 30.29, I2 = 83%, P = 0.37) and serious AEs (RR = 1.80, 95%CI: 0.08 to 42.54, I2 = 55%, P = 0.71). Although the risks of treatment discontinuation for any reason were identical in the two groups (RR = 1.22, 95%CI: 0.79 to 1.90, I2 = 0%, P = 0.37), the risk of treatment discontinuation due to AEs was higher with beinaglutide (RR = 3.15, 95%CI: 1.32 to 7.54, I2 = 0%, P = 0.010, moderate certainty of the evidence). The risks with beinaglutide vs the control were higher for nausea (RR = 4.51, 95%CI: 1.16 to 17.53, I2 = 87%, P = 0.03, very low certainty of the evidence) and vomiting (RR = 8.19, 95%CI: 2.32 to 28.90, I2 = 15%, P = 0.001, moderate certainty of the evidence) but not for diarrhea (RR = 0.27, 95%CI: 0.04 to 1.67, I2 = 63%, P = 0.16). The two groups had identical risks of fatigue (RR = 2.53, 95%CI: 0.91 to 7.04, I2 = 0%, P = 0.08), but beinaglutide increased the risks for palpitation (RR = 3.95, 95%CI: 1.06 to 14.80, I2 = 0%, P = 0.04, moderate certainty of the evidence), headache (RR = 2.87, 95%CI: 1.31 to 6.32, I2 = 0%, P = 0.009, moderate certainty of the evidence), and dizziness (RR = 6.07, 95%CI: 2.92 to 12.61,
| Outcome variables | No. of RCT included | No. of participants with outcome/participants analyzed | Pooled effect size, RR (95%CI) | I2 (%) | P value | |
| Beinaglutide arm | Control arm | |||||
| At least one AE | 2 | 252/311 | 113/166 | 2.90 (0.28 to 30.29) | 83 | 0.37 |
| Serious AEs | 2 | 8/350 | 1/205 | 1.80 (0.08 to 42.54) | 55 | 0.71 |
| Treatment discontinuation for any reason | 5 | 36/198 | 29/196 | 1.22 (0.79 to 1.90) | 0 | 0.37 |
| Treatment discontinuation due to AEs | 5 | 31/452 | 6/305 | 3.15 (1.32 to 7.54) | 0 | 0.010 |
| Nausea | 5 | 191/417 | 27/270 | 4.51 (1.16 to 17.53) | 87 | 0.03 |
| Vomiting | 4 | 77/382 | 2/237 | 8.19 (2.32 to 28.90) | 15 | 0.001 |
| Diarrhea | 4 | 17/382 | 37 /237 | 0.27 (0.04 to 1.67) | 63 | 0.16 |
| Palpitation | 2 | 18/325 | 2/180 | 3.95 (1.06 to 14.80) | 0 | 0.04 |
| Fatigue | 3 | 19/357 | 4/212 | 2.53 (0.91 to 7.04) | 0 | 0.08 |
| Headache | 4 | 36/382 | 6/237 | 2.87 (1.31 to 6.32) | 0 | 0.009 |
| Dizziness | 3 | 76/350 | 7/205 | 6.07 (2.92 to 12.61) | 0 | < 0.00001 |
| Upper RTI | 3 | 28/350 | 14/205 | 0.99 (0.54 to 1.81) | 0 | 0.98 |
| Hypoglycemia | 3 | 6/99 | 9/97 | 0.64 (0.2 to 1.92) | 7 | 0.43 |
This SRM was based on six RCTs with 800 participants with overweight/obesity with/without T2D treated with subcutaneous beinaglutide in the intervention arm (0.1-0.2 mg preprandial thrice a day) vs placebo/lifestyle inter
Subjects in the beinaglutide arm had a significantly higher weight loss (MD = -3.25 kg) and percent reduction in body weight (MD = -4.13%) than the control group. Additionally, beinaglutide use was associated with significantly greater odds of achieving 5% (OR = 4.61) and 10% (OR = 5.34) weight reduction and a significant reduction in BMI (MD = -1.22 kg/m2) and WC (MD = -2.47 cm). Subgroup comparison revealed that the extent of reduction in all these parameters was similar in individuals with and without T2D.
The GLP-1RAs in use have demonstrated variable efficacy in diabetes and obesity outcomes. Initial GLP-1RAs include short-acting ones like exenatide and lixisenatide, whose predominant mode of action is delaying gastric emptying and controlling postprandial blood glucose excursions. These excursions are short-lived and small in magnitude compared to the 24-h glycemic burden. This, along with the relative lack of effect on nocturnal/FPG and central nervous system satiety centers, is likely responsible for only a modest lowering of HbA1c and weight loss with these agents[31]. On the other hand, long-acting GLP-1RAs are designed by molecular alterations of exendin-4 (once weekly exenatide) or mammalian GLP-1 (daily liraglutide, weekly injectable semaglutide, albiglutide, and dulaglutide) and exhibit slower elimination and prolonged half-lives, enabling a decrease in the dosing frequency[32]. They also lead to a constant and more substantial GLP-1R stimulation, leading to a more pronounced action on the central nervous system satiety centers (leading to higher weight reduction) and effective lowering of FPG and consequently HbA1c[31].
The newer dual and triple agonist molecules like tirzepatide, CagriSema, mazdutide, and retatrutide have demonstrated more encouraging results concerning metabolic outcomes by harvesting the multiple incretin pathways and the intricate ways of their interaction. A recent meta-analysis by Yao et al[33], which compiled data from 76 trials in 39246 participants with T2D, confirmed this pattern. The highest body weight reduction (kg) was noted with newer molecules like CagriSema (MD = -14.03 kg), tirzepatide (MD = -8.47 kg), retatrutide (MD = -7.87 kg), and orforglipron (MD = -4.88 kg) followed by semaglutide (MD = -3.13 kg) and liraglutide (MD = -1.33 kg). However, mazdutide, dulaglutide, PEGylated exenatide, and short-acting GLP-1RAs (exenatide, lixisenatide) were not associated with significantly reducing body weight. Similar results were also noted in the meta-analyses by Huthmacher et al[31] and Ma et al[34], who stated that long-acting GLP-1RAs were associated with better FPG, HbA1c, and body weight control as compared with short-acting GLP-1RAs.
Our meta-analysis revealed that beinaglutide use was associated with a significantly higher body weight reduction as compared with the control arm, occurring to a similar degree in individuals with and without diabetes. Compared with other agents, the treatment effect with beinaglutide was encouraging, faring better than other short-acting GLP-1RAs and almost comparable to some long-acting GLP-1RAs. The nearly five times increased odds of achieving 5% and 10% weight loss noted with beinaglutide use in our study can potentially result in significant reductions in the burden of obesity-related comorbidities with long-term use. The nearly 100% structural homology to human GLP-1 might account for better efficacy than other short-acting GLP-1RAs. The delineation of the areas targeted by beinaglutide could aid in further understanding its weight-loss-promoting effects. However, the fact that the RCTs for beinaglutide were exclusively conducted in China, along with the variable comorbidity profile and doses used (antihyperglycemic doses vs the higher anti-obesity doses) in other GLP-1RA trials, preclude direct comparison of results. The possibility of the role of higher doses of beinaglutide in non-diabetic overweight/obesity should be explored in trials for possible augmentation of weight-loss benefits while carefully weighing against tolerability concerns.
On the other hand, beinaglutide use was not associated with significant differences in glycemic endpoints (FPG, HbA1c), in addition to other metabolic endpoints like lipid parameters (total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglyceride, free fatty acid), liver enzymes (aspartate aminotransferase, alanine aminotransferase), insulin resistance indices (fasting insulin, HOMA-IR), and BP endpoints (systolic BP, diastolic BP) in our SRM.
Yao et al[33] reported the highest HbA1c reduction with newer coformulations of tirzepatide (-2.17%), mazdutide
Among the safety parameters, there was no significant difference in AEs and serious AEs in the beinaglutide group and the control group. However, beinaglutide use was associated with a three times higher rate of treatment discontinuation due to AEs compared to the control arm and a significantly increased incidence of nausea, vomiting, palpitations, dizziness, and headache as compared with the control group. Conversely, the risk of diarrhea, fatigue, upper respiratory tract infection, and hypoglycemia were similar in the two groups.
These findings are identical to the safety outcomes reported with other GLP-1RAs. Ma et al[34] reported the highest rates of discontinuation due to AEs with the short-acting GLP-1RA exenatide (OR = 2.96) or with higher doses of long-acting GLP-1RAs- liraglutide 3.0 mg (OR = 2.88) and semaglutide 2.4 mg (OR = 1.88). Similarly, Yao et al[33] reported 2-3 times increased odds of discontinuation due to AEs with lixisenatide, exenatide, liraglutide, and tirzepatide. Hence, the risk of discontinuation of therapy seemed to be related to the use of either higher doses of medications or the use of short-acting GLP-1RAs. The increased discontinuation rates with beinaglutide and other short-acting GLP-1RAs may be due to abrupt fluctuations in appetite due to a shorter half-life and the burden of multiple daily injections. These issues could harness more research towards developing a long-acting drug preparation with better efficacy and tolerability in the future, as in the case of weekly exenatide.
One of the major issues with beinaglutide use was the higher dosing frequency that can result in drug adherence issues. Disease management plans and the likelihood of treatment adherence primarily depend on patient choices, especially for chronic diseases. Higher injection burden with the currently available formulation could potentially impact therapeutic compliance, as patients often opt for a GLP-1RA molecule with a lower dosing frequency. This issue demands the development of an extended release preparation as in the case of exenatide extended release that could mitigate adherence issues and better acceptability of the drug molecule in future.
Our SRM provided an up-to-date evidence base for beinaglutide, a distinct GLP-1RA, for body weight and other metabolic parameters in individuals with overweight/obese with/without T2D. The molecule has been studied in China in trials with variable comorbidities. Our SRM has allowed the compilation of the data encompassing six RCTs and 800 patients to provide objective evidence on its efficacy and safety.
We acknowledge several limitations of our study. The six eligible RCTs in the meta-analysis were all conducted in China, four of which were single-center studies. The clinical responsiveness to GLP-1RAs is majorly affected by ethnicity; hence, studies in a single ethnic population limit the generalizability of the study findings globally[35]. Secondly, most studies (83.3%) had bias concerns due to risks in different domains. Publication bias could not be assessed because of a limited number of RCTs. Thirdly, the treatment received by the control group in the included RCTs was highly variable (placebo/lifestyle interventions/GLDs), which may have impacted the study results. Fourthly, the relatively short duration of the trials in the SRM is not ideal for concluding a chronic condition like obesity and associated comorbidities, and longer trials, including CV outcome trials, are needed to estimate the true potential of the novel molecule accurately. Last but not least, the certainty of the evidence generated for the primary outcomes was very low (for body weight) to low (for percent body weight). Despite these limitations, our results should empower clinicians with a better knowledge base and provide the impetus to plan longer-term studies with more participants to generate a more robust evidence base.
Beinaglutide is a novel GLP-1RA with a distinct structure (near 100% homology to human GLP-1) and pharmacokinetics compared to other GLP-1RAs. Our SRM revealed that beinaglutide use at 0.1-0.2 mg subcutaneously TID for 12-24 weeks was associated with modest benefits concerning body weight, BMI, and WC reduction, with no significant difference in glycemic and other metabolic end-points as compared to the control arm. Safety data was consistent with the other drugs in the GLP-1RA class, with a higher incidence of gastrointestinal adverse effects and treatment discontinuation due to AEs in the treatment arm, possibly due to the shorter half-life and multiple daily injections. The lack of longer duration trials, availability, and cost-effective analyses for beinaglutide vs other available GLP-1RAs limits its usability in the current scenario. However, the benefits concerning obesity/overweight outcomes, including measures of central adiposity, are encouraging despite the shorter half-life of the drug and duration of trials. Hence, longer-duration trials with multi-ethnic representation are needed to estimate the true potential of the molecule and find its place in the contemporary management of obesity and diabetes.
| 1. | World Health Organization. Obesity and overweight. Mar 1 2024. [cited 5 October 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. |
| 2. | Kivimäki M, Strandberg T, Pentti J, Nyberg ST, Frank P, Jokela M, Ervasti J, Suominen SB, Vahtera J, Sipilä PN, Lindbohm JV, Ferrie JE. Body-mass index and risk of obesity-related complex multimorbidity: an observational multicohort study. Lancet Diabetes Endocrinol. 2022;10:253-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 285] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 3. | Chandrasekaran P, Weiskirchen R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 113] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 4. | Gourdy P, Darmon P, Dievart F, Halimi JM, Guerci B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc Diabetol. 2023;22:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 5. | American Diabetes Association Professional Practice Committee. Erratum. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024;47(Suppl. 1):S158-S178. Diabetes Care. 2024;47:1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1106] [Article Influence: 158.0] [Reference Citation Analysis (1)] |
| 7. | Popoviciu MS, Păduraru L, Yahya G, Metwally K, Cavalu S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 113] [Reference Citation Analysis (0)] |
| 8. | Petrova L, Andreevska K, Parvova I, Petkova V. Systematic review of the efficacy and safety of GLP-1 receptor agonists in the treatment of patients with type 2 diabetes mellitus. PHAR. 2024;71:1-17. [DOI] [Full Text] |
| 9. | Ding B, Hu Y, Yuan L, Yan RN, Ma JH. Effectiveness of beinaglutide in a patient with late dumping syndrome after gastrectomy: A case report. Medicine (Baltimore). 2021;100:e26086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Chinese Diabetes Society; National Office for Primary Diabetes Care. [National guidelines for the prevention and control of diabetes in primary care (2022)]. Zhonghua Nei Ke Za Zhi. 2022;61:249-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40616] [Article Influence: 10154.0] [Reference Citation Analysis (2)] |
| 12. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. August 22 2024. [cited 1 October 2024]. Available from: https://www.training.cochrane.org/handbook. |
| 13. | Kamrul-Hasan ABM, Alam MS, Talukder SK, Dutta D, Selim S. Efficacy and Safety of Omarigliptin, a Novel Once-Weekly Dipeptidyl Peptidase-4 Inhibitor, in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocrinol Metab (Seoul). 2024;39:109-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Higgin JPT; Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. [cited 1 October 2024]. Available from: https://training.cochrane.org/handbook/current/chapter-08. |
| 15. | Review Manager (RevMan) [Computer program]. Version 7.2.0. The Cochrane Collaboration, 2024. [cited 1 October 2024]. Available from: https://revman.cochrane.org. |
| 16. | McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 2562] [Article Influence: 512.4] [Reference Citation Analysis (0)] |
| 17. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46550] [Article Influence: 2115.9] [Reference Citation Analysis (3)] |
| 18. | Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4813] [Cited by in RCA: 7117] [Article Influence: 474.5] [Reference Citation Analysis (0)] |
| 19. | Chen K, Chen L, Shan Z, Wang G, Qu S, Qin G, Yu X, Xin W, Hsieh TH, Mu Y. Beinaglutide for weight management in Chinese individuals with overweight or obesity: A phase 3 randomized controlled clinical study. Diabetes Obes Metab. 2024;26:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Fan Y, Xia M, Yan H, Li X, Chang X. Efficacy of beinaglutide in the treatment of hepatic steatosis in type 2 diabetes patients with nonalcoholic fatty liver disease: A randomized, open-label, controlled trial. Diabetes Obes Metab. 2024;26:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 21. | Gao L, Huang H, Zhang L, Zhang N, Fu Y, Zhu D, Bi Y, Feng W. Comparison of Beinaglutide Versus Metformin for Weight Loss in Overweight and Obese Non-diabetic Patients. Exp Clin Endocrinol Diabetes. 2022;130:358-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Han CY, Lu JP, Ye XM, Jin HY, Xu WW, Wang P, Zhang M. Effect of beinaglutide combined with metformin versus aspart 30 with metformin on metabolic profiles and antidrug antibodies in patients with type 2 diabetes: a randomized clinical trial. Front Endocrinol (Lausanne). 2023;14:1267503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Liu X, Yang W, Liu J, Huang X, Fang Y, Ming J, Lai J, Fu J, Ji Q, Wang L. The efficacy and safety of beinaglutide alone or in combination with insulin glargine in Chinese patients with type 2 diabetes mellitus who are inadequately controlled with oral antihyperglycemic therapy: A multicenter, open-label, randomized trial. J Diabetes. 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Wen Q, Fang S, Liang Y, Tian Y, Chen Y, Yuan J, Chen Q. Short-term effect of beinaglutide combined with metformin versus metformin alone on weight loss and metabolic profiles in obese patients with polycystic ovary syndrome: a pilot randomized trial. Front Endocrinol (Lausanne). 2023;14:1156521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Fang X, Du Z, Duan C, Zhan S, Wang T, Zhu M, Shi J, Meng J, Zhang X, Yang M, Zuo Y. Beinaglutide shows significantly beneficial effects in diabetes/obesity-induced nonalcoholic steatohepatitis in ob/ob mouse model. Life Sci. 2021;270:118966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Lin P, Li C, Liu Y, Sun F, Hsieh TH, Lin Zhang, Ma Y, Gao X, Yu Q, Cao Y. Pharmacokinetics and safety profiles of beinaglutide injection, a recombinant human GLP-1, in adults with overweight/obesity: results from a phase I clinical trial. Front Pharmacol. 2024;15:1433587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Wang G, Wu P, Qiu Y, Dong X, Wang Y, Chi Y, Zhang F, Li Y, Zhang J, Huang Z, Du X, Du Z. Effect of beinaglutide treatment on weight loss in Chinese patients with type 2 diabetes mellitus and overweight/obesity. Arch Endocrinol Metab. 2021;65:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Zhang F, Chen Z, Wu D, Tian L, Chen Q, Ye Y, Chen W, Wu X, Wu P, Yuan W, Qiu Y, Zhou Z, Du Z, Hu F. Recombinant human GLP-1 beinaglutide regulates lipid metabolism of adipose tissues in diet-induced obese mice. iScience. 2021;24:103382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Zhang YL, Zhou C, Li XF, Yang MN, Tao L, Zheng XY, Jia YS. Beinaglutide showed significant weight-loss benefit and effective glycaemic control for the treatment of type 2 diabetes in a real-world setting: a 3-month, multicentre, observational, retrospective, open-label study. Obes Sci Pract. 2019;5:366-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Debray TPA, Moons KGM, Riley RD. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res Synth Methods. 2018;9:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Huthmacher JA, Meier JJ, Nauck MA. Efficacy and Safety of Short- and Long-Acting Glucagon-Like Peptide 1 Receptor Agonists on a Background of Basal Insulin in Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2020;43:2303-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 865] [Article Influence: 216.3] [Reference Citation Analysis (0)] |
| 33. | Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (12)] |
| 34. | Ma H, Lin YH, Dai LZ, Lin CS, Huang Y, Liu SY. Efficacy and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in overweight/obese patients with or without diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2023;13:e061807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 35. | Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |