Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.100371
Revised: October 24, 2024
Accepted: November 26, 2024
Published online: February 15, 2025
Processing time: 137 Days and 21.9 Hours
The risk factors for type 2 diabetes mellitus (T2DM) have been increasingly researched, but the lack of systematic identification and categorization makes it difficult for clinicians to quickly and accurately access and understand all the risk factors, which are categorized in this paper into five categories: Social determinants, lifestyle, checkable/testable risk factors, history of illness and medication, and other factors, which are discussed in a narrative review. Meanwhile, this paper points out the problems of the current research, helps to improve the systematic categorisation and practicality of T2DM risk factors, and provides a professional research basis for clinical practice and industry decision-making.
Core Tip: Type 2 diabetes mellitus (T2DM), as a worldwide public health problem, poses an enormous burden on public health and socioeconomics. This article sum
- Citation: Tang SS, Zhao XF, An XD, Sun WJ, Kang XM, Sun YT, Jiang LL, Gao Q, Li ZH, Ji HY, Lian FM. Classification and identification of risk factors for type 2 diabetes. World J Diabetes 2025; 16(2): 100371
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/100371.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.100371
The number of diabetics aged 20-79 years worldwide totalled 529 million in 2021, and the global prevalence of diabetes is projected to reach 1.31 billion in 2050[1], with type 2 diabetes mellitus (T2DM) being the most predominant component of the diabetic population[2], accounting for 90% of the diabetes prevalence[3] and posing a huge economic burden to the world[4]. Currently, most preventive measures for T2DM focus on lifestyle interventions such as weight management, dietary adjustments, and addressing abdominal obesity. Early screening is recommended for individuals with a family history of T2DM to lower the risk of developing the condition. T2DM is a chronic disease that results from the interplay of various risk factors and their long-term effects on the body. More and more studies are proposing new risk factors for T2DM, but the lack of uniform identification and classification makes it difficult for clinicians to quickly and accurately access and understand all relevant risk factors so that they cannot carry out disease risk prevention and control in a timely manner, and provide personalized and targeted preventive and therapeutic advice to patients, while the complex and fragmented information also makes patients' self-management risk increase, which affects disease clinical outcomes.
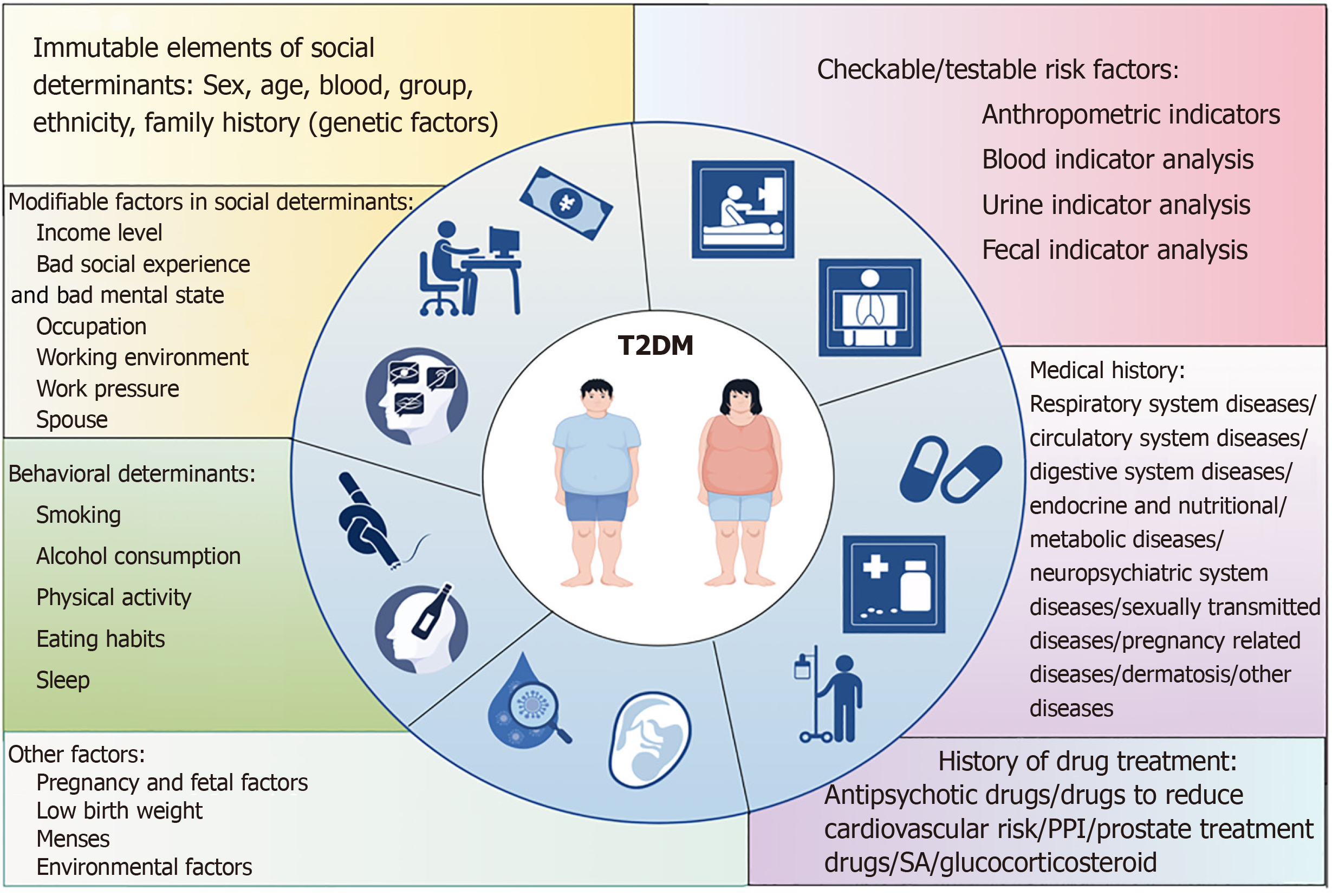
This study was conducted by searching PubMed, EMBASE, and Cochrane databases, and the clinical studies retrieved spanned the years 2006-2024, mostly focusing on the last 5 years. Through a systematic and comprehensive reading of more than 200 high-quality articles, the risk factors for T2DM were categorized and identified into five categories: Social determinants, lifestyle, checkable/testable risk factors, medical and medication history, and other factors to develop a narrative review (Figure 1), in order to identify the high-risk groups of T2DM, to carry out risk management for such groups, to interrupt the development of T2DM, to provide clinicians with practical strategies for disease prevention, to further standardize medical behaviour, to scientifically allocate medical resources, to protect the rights and interests of patients, and to maximize the benefits for the risk groups of T2DM.
Immutable elements of social determinants: Biological sex, age, blood group, and ethnicity are considered risk factors for T2DM. Research has shown that gender plays a significant role in the development of T2DM[5,6], with men being more susceptible to the condition compared to women in many regions worldwide[7-9]. Men are more predisposed to obesity, insulin resistance (IR), and hyperglycemia compared to women[10]. This disparity can be attributed to the presence of estrogen in women, which has been shown to decrease IR and the incidence of T2DM[11,12]. T2DM is most commonly diagnosed in adults aged 40 years and older[13]. Research indicates that individuals with blood type A or B have a higher risk of developing T2DM compared to those with blood type O[14], with a 76% higher prevalence of diabetes in individuals with blood type A[15]. Studies focusing on specific ethnicities have shown that native Americans, Blacks, and Hispanics/Latinos are at a higher risk for T2DM[16], with Blacks being more predisposed to diabetes compared to Whites[17].
The development of T2DM is closely linked to genetics. Sixty-nine percent of the risk of developing T2DM in individuals aged 35-60 years is attributable to genetic factors[18]. When the father, mother, or both have T2DM, the risk of a T2DM diagnosis in the offspring is approximately 2-4 times greater[19]. The hundreds of gene loci associated with T2DM can be divided into functionally specific groups of genes that work together to regulate the development of T2DM, and within this vast network of gene regulation is the central hub RFX6, whose reduced expression is associated with defective β-cell function[20]. The SLC30A8 gene polymorphism rs13266634 may be an important genetic factor in the risk of developing T2DM in Asian and European populations[21].
Modifiable factors in social determinants: Income level affects the incidence of T2DM. More than 80% of people with T2DM live in low- and middle-income countries[4]. There is an increased overall risk of T2DM in low socioeconomic status populations[22]. Children and adolescents in low-income families have a significantly higher risk of T2DM than those in high-income families[23]. In the United States, living in poor neighbourhoods increases the risk of diabetes among Blacks and poor Whites[17].
Adverse social experiences, as well as poor psychological states, increase the risk of developing T2DM. A better social environment is linked to better health[24]. Individuals who experience childhood adversity are at higher risk of developing T2DM in early adulthood, according to results from a 1.2 million-person Danish cohort study[25]. Individuals with adverse childhood experiences are at increased risk of developing diabetes in adulthood[26,27]. Experiences of violence and marginalized societies increase the risk of T2DM worldwide[28]. Clinicians should pay more attention to groups affected by crisis, displacement, and food insecurity by organizing diabetes screening programmes for such vulnerable groups[29]. Gene-environment interactions due to early famine and the consequent increased intergenerational risk of T2DM are the main causes of the current T2DM epidemic in China[30]. Famine exposure may increase the risk of developing T2DM, which may vary depending on the duration of the famine, the definition of diabetes, gender, and the duration of famine exposure[31]. Lower psychological well-being scores (poorer overall mental health) were found to be associated with an increased risk of developing T2DM in a dose-response relationship, independent of traditional risk factors and unrelated to genetic predisposition[32]. Two large prospective cohort studies from Europe and East Asia have shown that both social isolation and loneliness are associated with an increased risk of T2DM[33]. Individuals who feel lonely have a 2-fold increased risk of developing T2DM[34].
Occupation, work environment, and work stress can be risk factors for T2DM. The non-skilled occupation was found to be an independent risk factor for diabetes in a developed Asian setting[35]. A study screening all employees in Sweden found that among the 30 most common occupations, motor vehicle drivers had the highest prevalence of T2DM among men (8.8%), and manufacturing workers had the highest prevalence of T2DM among women (6.4%)[36]. Two large prospective cohort studies in the United States, conducted over a span of 22-24 years and involving a sample of over 140000 female nurses, revealed that nurses working night shifts faced an elevated risk of developing T2DM. The risk increased by 11%, 28%, and 46% for those working night shifts for 1-5, 5-9, and 10 years or more, respectively. Upon adjusting for various factors, the researchers observed that for every additional five years of night shift work, the likelihood of developing T2DM rose by 31%. Furthermore, when coupled with unhealthy lifestyle habits, the risk of developing the disease surged to 2.83 times higher[37]. Work stress is a significant risk factor for T2DM, particularly in women[38]. Research has shown that high work stress and heavy domestic responsibilities are linked to a higher risk of T2DM in women over the age of 60[39]. Conversely, a positive working environment, good relationships with coworkers, and lower levels of work stress have been found to decrease the risk of T2DM among employees[40,41].
The age and physical condition of the spouses in a marriage, as well as the quality of the marriage, can be risk factors for T2DM. The age of a man in a marriage is associated with his partner's risk of gestational diabetes mellitus (GDM), with men aged ≥ 45 years having a 28% increased risk of GDM in their partners compared with men aged 25-34 years; this risk increases to 34% when the man is over 55 years of age[42]. One spouse with T2DM has a 26% increased risk of the other spouse developing T2DM[43]. Among older British men, having an obese spouse increases their own risk of T2DM[44]. Additionally, poorer marital quality may be a unique risk factor for diabetes[45].
Dietary habits: Dietary habits, dietary structure, and eating speed have a significant impact on the development of T2DM. An analysis of epidemiological data from 184 countries worldwide reveals that 70% of new cases of T2DM can be linked to 11 specific poor dietary habits. The most notable contributors include inadequate consumption of whole grains (26.1%), excessive intake of refined rice and pasta (24.6%), and high consumption of processed meat (20.3%) and unprocessed red meat (20.1%)[46]. Results from the China Health and Nutrition Examination Survey (1997-2018) showed that the risk of T2DM was negatively associated with meat intake below 75 g/day and positively associated with intake above 165 g/day[47]. Diets high in glycaemic index (GI) and glycaemic load have been linked to an increased incidence of T2DM[48]. Specifically, a higher consumption of white rice has been associated with a significantly elevated risk of T2DM, particularly in Asian populations[49,50]. Higher intake of dietary heme iron has been linked to a notable rise in the risk of developing T2DM[51,52]. Similarly, a higher consumption of sugar-sweetened beverages has been found to be positively correlated with an increased occurrence of T2DM[53,54]. Notably, the consumption of potatoes, particularly in the form of French fries, has also been associated with an elevated risk of T2DM. Specifically, a 10% increase in the risk of developing T2DM is linked to a 100 g/day increase in consumption of French fries[55]. Women consuming temperate fruits such as apples have a lower risk of developing T2DM, whereas men consuming high GI fruits such as bananas have a higher risk of developing T2DM, and the effect of fruit intake on the risk of diabetes varies according to fruit type[56]. Furthermore, studies indicate that a higher consumption of highly processed foods is associated with an increased risk of developing T2DM[57]. The study suggests that red, processed meat consumption is a risk factor for T2DM[53,58,59]. In a large prospective cohort of French adults, a direct association was found between the risk of T2DM and exposure to a variety of food additive emulsifiers widely used in industrial foods, with the greatest increase in risk being for tripotassium phosphate, with an increase of 500 mg/day associated with a 15% increase in risk[60]. In the dietary habits of new-onset diabetes, eating too fast was the only predisposing factor[61], and there is experimental data to support this idea[62]. This may be due to the positive correlation between rapid eating and body mass index (BMI) and weight gain[63]. A plant-based dietary pattern consisting of healthy plant foods and a small amount of animal foods may reduce the risk of T2DM by improving liver and kidney function and reducing underlying inflammation[64,65]. Some studies have found that intermittent fasting plus early time-restricted eating with calorie restriction improves postprandial glucose metabolism to a large extent in people at risk of T2DM[66].
Physical activity: Physical activity is a first-line strategy for the prevention and management of T2DM[67,68]. Accumulation of daily physical activity is a major determinant of insulin sensitivity[69]. Pedometer data were obtained from 7118 participants, with a 5.5% reduction in the risk of progression to diabetes for every 2000-step increase in the average daily step count (up to 10000), and an adjusted relative risk reduction of > 6%[70]. In multifactorial analyses, an increase of 2 hour/day in television viewing was associated with a 14% increase in the risk of diabetes, an increase of 2 hour/day in sitting at work was associated with a 7% increase in the risk of diabetes, and a 12% reduction in diabetes was associated with standing or walking at home (2 hours/day). A brisk walk every hour of the day is associated with a 34% reduction in diabetes[71]. Compared to those who did little or no exercise per day, moderate-to-vigorous exercise of 5.3-25.9 minutes per day was associated with a 37% reduction in the risk of T2DM; for 26-68.4 minutes, the risk was reduced by 59%, and for more than 68.4 minutes, the risk was reduced by 74%[72].
Sleep: A U-shaped dose-response relationship was observed between sleep duration and the risk of developing T2DM in a study involving 482502 participants followed for 2.5 to 16 years. The group with 7 to 8 hours of sleep per day had the lowest risk, while both shorter and longer sleep durations were linked to a significant increase in the risk of developing T2DM[73]. Habitual short sleep duration is associated with an increased risk of developing T2DM[74]. Individuals with poor sleep quality are at a significantly higher risk of developing T2DM compared to those with good sleep quality[75]. Moreover, people with insomnia have a 28% higher risk of developing T2DM compared to those without insomnia[76]. Research has indicated that the risk of developing insomnia rises as the follow-up period extends. This heightened risk is especially noticeable in individuals under the age of 40, with a 1.14-fold, 1.38-fold, and 1.51-fold increase in the risk of developing T2DM for insomnia durations of less than 4 years, 4-8 years, and over 8 years, respectively[77].
Smoking: Smoking is linked to an increased risk of diabetes[78-80]. The risk of developing T2DM is higher with greater amounts and longer durations of smoking. Quitting smoking at a young age may help lower the chances of developing T2DM[81]. Both active and passive smoking significantly increases the risk of T2DM, and new quitters have an increased risk of diabetes, but the risk of diabetes decreases substantially with longer periods of smoking cessation[82]. High consumption of snuff is a risk factor for T2DM, and this risk is similar to that of smokers, which means that smokers cannot reduce their risk of developing T2DM by changing their smoking patterns, and the findings similarly support the idea that nicotine increases the risk of T2DM[83].
Alcohol consumption: Alcohol consumption is associated with T2DM through effects on IR, changes in alcohol metabolite levels, and anti-inflammatory effects[84]. The relative risk of T2DM in men is most protective at 22 g/day of alcohol intake and becomes harmful at 60 g/day of alcohol or more, and in women, 24 g/day of alcohol intake is the most protective and becomes harmful at around 50 g/day of alcohol intake[85]. In women with a history of GDM, alcohol consumption of 5.0-14.9 g/day was negatively associated with the risk of T2DM[86].
Anthropometric and obesity indicators: Anthropometric and obesity indicators of T2DM risk factors include visceral adiposity index (VAI), BMI, hip circumference, body size index (ABSI, A Body Shape Index), age-related weight change, C-index, and relative fat mass. Studies have shown that higher VAI and body shape index scores are independently associated with the risk of diabetes[87] and can be utilized to predict diabetes progression[88]. Additionally, VAI has been found to be positively correlated with the risk of developing T2DM in Chinese populations[89]. Each 1-unit increase in VAI is correlated with a 42% higher risk of developing T2DM[90]. In obese adults, both excess visceral fat and IR are independently linked to the development of pre-DM and T2DM[91]. BMI is considered an independent risk factor for T2DM. Research has shown that the risk of developing T2DM significantly rises by 5.03% when BMI reaches 31 kg/m2[92]. Additionally, for every 5-unit increase in BMI, the risk of developing T2DM increases by 72%[90]. Hip circumference is negatively associated with an increased risk of developing T2DM[93]. ABSI and BMI are significantly associated with diabetes, with some studies suggesting that ABSI is a better predictor of diabetes risk than BMI[94]. Long-term weight gain from early adulthood to middle age increases the risk of developing T2DM, with this risk further escalating in women's later life as a result of weight gain[95]. It is noteworthy that moderate-to-heavy weight gain in early life presents a greater risk for the onset of diabetes compared to weight gain occurring after the age of 40[96]. The study revealed that certain calculated metrics, specifically the C-index and relative fat mass (RFM), were strongly linked to the development of new-onset T2DM. These metrics could potentially serve as valuable tools for assessing the risk of diabetes in extensive epidemiological research[97].
Indicators of atherosclerosis analysis: Brachial ankle pulse wave velocity (baPWV), an indicator of atherosclerosis analysis, is associated with the risk of developing T2DM. Low ABI was found to be mildly but independently associated with an increased risk of developing diabetes in the general population, and clinical attention should be paid to the glycaemic trajectory in people with low ABI but no diabetes[98]. A significant association was found between elevated baPWV and an increased risk of developing T2DM in middle-aged and older populations, and an elevated total white blood cell (WBC) count may partially mediate this association[99].
Resting heart rate: It has been suggested that high resting heart rate (RHR) is associated with an increased risk of metabolic syndrome (MetS), three MetS components [elevated blood pressure (BP), elevated triglycerides (TG), and elevated fasting plasma glucose (FPG)], and aggregated metabolic risk[100]. RHR was independently associated with an increased risk of T2DM, with a 19% increase in the risk of T2DM for every ten bpm increase in RHR[101]. A faster RHR is associated with a higher risk of impaired fasting glucose (IFG) and diabetes in the Chinese population, and this correlation is stronger in younger adults[102]. Moreover, there is a suggestion that the connection between elevated RHR and the development of T2DM is more noticeable in middle-aged individuals with significant vascular lesions[103].
BP: People with pre-hypertension are at increased risk of diabetes, a risk mediated by IR[104]. It has been found that people with elevated BP have an increased risk of diabetes, with increases in systolic BP (SBP) of 20 mmHg and diastolic BP of 10 mmHg increasing the risk of new-onset diabetes by 58% and 52%, respectively[105]. Patients with a mean SBP of 130-140 and/or a diastolic BP (DBP) of 80-90 mmHg at the time of treatment were found to have a 24% increased risk of diabetes compared to those with a mean SBP < 130 and a DBP of < 80 mmHg in the China Primary Stroke Prevention trial[106]. In a large sample of treated nondiabetic hypertensive patients, uncontrolled BP was associated with a 2-fold increased risk of developing diabetes mellitus (DM) and was not associated with age, BMI, baseline BP, or FPG[107]. Genetically elevated SBP is associated with an increased risk of developing type 2 diabetes[108]. There is an increased risk of T2DM in offspring of mothers with maternal hypertensive disorders of pregnancy (HDP) compared to offspring of mothers without[109]. BP reduction has a preventive effect on the risk of T2DM, with an 11% reduction in the risk of new-onset T2DM for every 5 mmHg reduction in SBP[110].
Blood glucose: The risk of diabetes was found to increase with increasing FPG even within the currently accepted normal range, with a 6% increase in the risk of diabetes for every 1 mg/dL increase in FPG, and subjects with blood glucose levels of 95-99 mg/dL were 2.33 times more likely to develop diabetes compared to those with FPG levels of less than 85 mg/dL, and subjects with blood glucose levels of 90-94 mg/dL were 49% more likely to progress to diabetes[111]. According to the Korean national health data, the risk of T2DM was found to increase progressively with increasing IFG exposure scores, with a 3.75-9.77-fold increase in the hazard ratio (HR) of developing diabetes in subjects with IFG exposure scores of 2, 3, or 4, and cumulative IFG exposure was associated with a higher risk of T2DM in a dose-response manner[112]. Random blood glucose ≥ 100 mg/dL (5.6 mmol/L) was the strongest predictor of undiagnosed diabetes[113]. Glycosylated haemoglobin (A1C) values between 5.5%-6.5% were associated with a significantly increased risk of developing diabetes[114].
Lipids: TG, high-density lipoprotein (HDL), and TG/HDL ratio in blood lipids are strongly associated with the risk of developing T2DM. Increases in TG levels within the normal range were found to lead to a sustained increase in the incidence of T2DM, even in healthy subjects[115]. For every one mmol/L increase in TG, the risk of T2DM increased by 81%[116]. The study from the Netherlands analysed the relationship between the levels of seven different HDL subtypes from H1P-H7P and the risk of T2DM and showed that H2P increased the risk of T2DM, with a corresponding 15% increase in risk for every one standard deviation increase in its level[117]. Low mean and high variability of HDL-C are independent predictors of diabetes with additive effects and elevating and stabilising HDL-C may be an important target for reducing diabetes risk[43]. Non-traditional lipid parameters, especially TG/HDL-C ratio, were associated with the risk of pre-DM and T2DM, and high TG/HDL-C, defined by sex-specific TG/HDL-C cut-off points, was a risk factor for pre-DM and T2DM[118], and an elevated TG/HDL-C ratio was significantly associated with an increased risk of new-onset DM (NOD)[119].
Uric acid: In a follow-up of 4536 subjects without diabetes at baseline for an average of 10.1 years, subjects with higher blood uric acid levels were found to be more susceptible to T2DM, with 1/4 cases of diabetes attributable to high blood uric acid levels, suggesting that blood uric acid serves as a strong and independent risk factor for diabetes[120]. It has been suggested that the association between hyperuricaemia and diabetes is mediated in part by the MetS, with participants in the highest uric acid quintile with the MetS having 3.3 times the risk of developing diabetes than those in the lowest uric acid quintile without the MetS[121].
Vitamin D levels: Blood vitamin D levels were negatively associated with diabetes risk, with a greater reduction in diabetes risk in participants with blood 25-hydroxyvitamin D levels above 125 nmol/L[122]. Vitamin D deficiency at baseline may be associated with a 50% increased risk of developing DM and a 62% increased risk of developing pre-DM after 4 years of follow-up[123]. The multivariable-adjusted HR per 10 nmol/L increase was 0.88[124]. At the same time, however, some studies have argued against it, suggesting that increasing 25 hydroxyvitamin D [25-(OH)D] concentrations may not reduce the risk of T2DM as expected from observational evidence[125,126].
Thyroid function: Adolescents with thyroid disease have an approximately 2-fold increased risk of developing T2DM compared to those without, and this association is confirmed in normal goitre and hypothyroidism and is apparent by the age of 30 years[127]. Abnormal thyroid hormone levels were found to be associated with an increased risk of T2DM by meta-analysis, with a J-shaped relationship with thyroid-stimulating hormone and an inverted J-shaped relationship with free triiodothyronine and free thyroxine[128]. Subclinical hypothyroidism increases IR only in the normoglycemic population, with an increased risk of developing diabetes as central thyroid sensitivity decreases[129].
Serum magnesium levels: Serum magnesium concentration is negatively correlated with DM, BMI, blood glucose, insulin, HbA1c, and homeostasis model assessment-IR (HOMA-IR)[130]. Lower serum magnesium concentrations are associated with a higher risk of IR and diabetes; when serum magnesium levels are below 0.82 mmol/L, the risk ratio for IR increases incrementally with increasing serum magnesium levels; when serum magnesium levels reach about 0.82 mmol/L, the risk of IR decreases with increasing serum magnesium levels, however, when serum magnesium is greater than about 0.93 mmol/L the risk of IR increased rapidly[131]. Mechanisms of serum magnesium effects on pre-DM and diabetes risk may be mediated on the one hand through IR and on the other hand through genetic variants in the magnesium-regulated genes TRPM6, CLDN19, SLC41A2, CNNM2, and FXYD2[132].
Inflammatory markers: A meta-analysis included ten prospective studies that detected a significant dose-response relationship between interleukin 6 (IL-6) levels, and the risk of T2DM, and elevated C-reactive protein levels were significantly associated with an increased risk of T2DM without publication bias[133]. Platelet count was independently associated with an increased risk of developing T2DM only in women[134]. Shorter WBC telomere relative length (rLTL) (a biomarker of biological ageing) was found to be associated with a higher risk of glycaemic progression, with an average 1.69-fold increase in the risk of diabetes progression for each 1-base decrease in absolute LTL value[135].
Liver function: In cross-sectional analyses, alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were strongly associated with obesity, IR, and MetS, and GGT and ALT were significant predictors of T2DM[136]. Meta-analyses have also shown that ALT levels have a dose-response effect on the risk of T2DM, with the risk of T2DM increasing by approximately 20% for every 5 IU/L increase in ALT levels[137]. The association of alanine, phenylalanine, and tyrosine with future T2DM risk was confirmed in two prospective cohorts of Chinese adults, and palmitoylcarnitine was further identified as a new metabolic marker for new-onset T2DM[138].
Sex hormones: Sex steroid hormones play a role in the metabolism, accumulation, and distribution of adipose tissue[139]. Low serum levels of sex hormone-binding globulin (SHBG) are linked to IR, compensated hyperinsulinemia, and abnormal glucose-lipid metabolism in individuals with polycystic ovary syndrome (PCOS)[140]. SHBG levels are a strong predictor of the risk of developing T2DM[141]. High testosterone levels are associated with a higher risk of developing T2DM in women but a lower risk in men; the inverse association of SHBG with risk is stronger in women than in men[12]. Higher endogenous plasma estradiol (TE) and testosterone levels are strongly associated with an increased risk of T2DM[142]. SHBG and TE are independent risk factors for the development of T2DM in women[143]. Maternal hype
Triglyceride glucose-BMI: Elevated triglyceride glucose (TyG) index is associated with impaired β-cell function[145]. A longitudinal cohort study in Japan demonstrated that baseline TyG-BMI was positively associated with the risk of developing T2DM in a normoglycaemic population, that this risk was significantly higher in young people (18-44 years), women, non-hypertensive people and non-drinkers, and that TyG-BMI could be used as an independent predictor of T2DM development[146].
Branched-chain amino acids: Research findings have shown a negative correlation between peripheral insulin sensitivity and plasma branched-chain amino acid (BCAA) levels[147-149]. In three large prospective cohorts of men and women in the United States, consistent associations were observed between long-term intake of BCAAs, including leucine, isoleucine, and valine, either individually or in total, and increased risk of developing T2DM, and these associations were independent of traditional diabetes risk factors, including BMI[150].
Proteinuria: The presence of proteinuria was significantly associated with an increased risk of T2DM, with studies finding that test-paper proteinuria was an independent risk factor for the development of new-onset T2DM, and that the risk of T2DM increased proportionally with the severity of proteinuria[151].
Microbe-associated metabolites: Several bacteria (e.g., B. burgdorferi and E. faecalis) and enzymes (e.g., xylanase EC 3.2.1.156) involved in fibre degradation have been found to be positively correlated with fibre intake, inversely correlated with the incidence of T2DM, and positively correlated with metabolic profiles associated with T2DM[152]. Alterations in the gut microbiota have been associated with the growing prevalence of metabolic disorders, including obesity, IR, and T2DM[153]. The gut flora contributes to the disease development process as a trigger for metabolic inflammation in obesity and T2DM[154]. Additionally, T2DM has been linked to a deficiency in short-chain fatty acids[155]. Elevated levels of trimethylamine N-oxide, a choline metabolite produced by intestinal bacteria, have been associated with an increased risk of DM, with an odds ratio (OR) of 1.89[156]. Furthermore, an increased relative abundance of the family Oxalobacteraceae (OR = 1.0704), the genus Oxalobacter (OR = 1.0874), and the species faecis (OR = 0.9460) have been respectively associated with an increased or decreased risk of developing T2DM. Other microbial taxa such as β-Ascomycetes, Lactobacteriaceae, Sclerobacterium, and Proboscidea have also shown significant associations with T2DM[157]. The abundance of Blautia wexlerae was found to be negatively associated with obesity and T2DM in a cross-sectional study of Japanese adults[158].
Genomics: A chromatin map of pancreatic islet cells was created through single-cell nucleus ATAC-seq analysis. Among 15298 cells, a positive correlation was identified between fasting blood glucose levels and the enrichment of T2DM and β-cell transcription factor motifs, including PDX. In relation to fasting blood glucose, the strongest enrichment was observed for state-specific transcription factor motifs related to high insulin levels, with RFX and NEUROD being the most notable[159]. Mutations in the GIGYF1 gene, found in approximately 1 in 3000 individuals, significantly raise the likelihood of Y chromosome deletions and increase the risk of developing T2DM to 30%, as opposed to the 5% risk in the general population. This suggests that mutations in the GIGYF1 gene can elevate the risk of T2DM by up to 6-fold[160]. Additionally, simultaneous exposure to NPC1 L1 and other genetically reduced LDL-C loci is linked to a heightened risk of T2DM[161].
Medical history: (1) Respiratory system diseases. Snoring and obstructive sleep apnea-hypopnea syndrome (OSAHS): In the Chinese adult population, habitual snoring has been found to be independently linked to a higher risk of developing T2DM[162]. Habitual snoring is associated with an increased incidence of DM over a 10-year period in 30-69-year-old men in Uppsala[163]. Research indicates that snoring is connected to impaired glucose metabolism. Even in metabolically normal adults, higher snoring intensity and frequency are positively correlated with fasting glucose and HbA1c levels[164]. The main features of OSAHS include transient hypoxemia and sleep fragmentation, which are believed to be the primary factors leading to metabolic dysfunction[165]. The Apnea-Hypopnea Index shows a moderate positive corre
Coronavirus disease 2019 (COVID-19): A large cohort study involving over 47.1 million participants demonstrated a notable link between COVID-19 and the development of new-onset diabetes[169]. Research indicates that individuals infected with severe acute respiratory syndrome coronavirus 2 have a higher likelihood of developing T2DM, with a roughly 60% increased risk of new-onset diabetes compared to non-infected individuals[170];
(2) Circulatory system diseases. Heart failure (HF) is a state of IR in which chronically increased sympathetic nervous system activity can lead to reduced insulin responsiveness, glucose utilisation, and cellular insulin secretion by affecting vasodilatory tone, free fatty acid levels, and oxidative stress. By reducing insulin sensitivity, HF may select patients with a genetic predisposition to develop cellular hyposecretion and diabetes[171]. Additionally, a Danish nationwide cohort study revealed that the severity of HF was linked to an increasing risk of diabetes[172];
(3) Digestive system diseases: Metabolic dysfunction-associated fatty liver disease (MAFLD). MAFLD, a metabolic disorder characterized by excessive accumulation of liver fat[173], increases the risk of developing T2DM[174,175]. Three hundred and ninety-five studies from 40 countries or territories covering 8051205 patients with MAFLD found that the prevalence of comorbid T2DM was 28.3% (95% confidence interval: 25.2%-31.6%) in patients with MAFLD, and 26.2% (23.9%-28.6%) globally[176]. Young people with MAFLD have a 6.1 times higher risk of developing diabetes compared to their peers without MAFLD[177]. About 35% of subjects with metabolically healthy abdominal obesity (MHAO) with MAFLD showed an excess risk of pre-DM plus diabetes[178].
Inflammatory bowel disease (IBD): Ulcerative colitis (UC) and Crohn's disease (CD), collectively referred to as IBD, are idiopathic, chronic, and recurrent inflammatory diseases of the intestine[179]. A nationwide Danish cohort study involving 6028844 individuals compared data from those diagnosed with IBD (CD or UC) to the general population, revealing a significantly increased risk of developing T2DM[180]. The link between T2DM and IBD/UC may be attributed to changes in various metabolic pathways and immune responses mediated by cytotoxic T-lymphocyte antigen 4[181].
Pancreas-related diseases: Fatty pancreas and pancreatitis have been identified as risk factors for developing T2DM. A 10-year prospective cohort study revealed that individuals with a pancreatic fat content exceeding 10.4% had a 1.81-fold higher risk of developing diabetes[182]. Additionally, it was observed that individuals who experienced acute pancreatitis had a 2-fold increased risk of developing diabetes, potentially attributed to pancreatic necrosis and reduction in β-cell area[183].
Gallbladder-related diseases: Both gallstone disease and cholecystectomy increase the risk of developing T2DM. Cholelithiasis was independently associated with an increased risk of developing T2DM[184]. Chinese community-dwelling individuals who have undergone cholecystectomy have an increased risk of developing abnormal blood glucose[185]. Cholecystectomy is an independent risk factor for the development of T2DM, and cholecystectomy increases the risk of developing T2DM by 20%[186];
(4) Endocrine and nutritional metabolic diseases: Obesity and MetS. Obesity is associated with an increased risk of developing IR and T2DM, and in obese individuals, adipose tissue releases large amounts of factors involved in the development of IR, and abnormal pancreatic β-cell function is a key determinant of the development of T2DM in obese patients[187]. Increased abdominal and intra-abdominal fat distribution and increased intrahepatic and intramuscular triglyceride content are associated with T2DM, causing both IR and β-cell dysfunction[188]. Individuals who switch from a metabolically healthy state to an unhealthy phenotype may be at increased risk for diabetes[189]. Central obesity was independently associated with an increased risk of developing diabetes, and after adjusting for confounding covariates, centrally obese individuals had a 72% higher risk of diabetes than non-centrally obese individuals in the propensity score matching cohort[190]. MetS is a cluster of metabolism-related symptoms, and IR and hyperinsulinemia are consistent features of MetS that significantly increase the risk of developing T2DM[191].
Rheumatoid arthritis (RA): There is increasing evidence suggesting that patients with RA are at a higher risk of developing DM and that RA can worsen the metabolic imbalances associated with DM. This is due to various factors present in RA, such as pro-inflammatory cytokines (e.g., tumor necrosis factor-α, IL-6, and IL-1β), RA-specific autoantibodies (e.g., antibodies to rheumatoid factor and cyclic citrullinated peptide), and elevated levels of adipokines associated with RA (e.g., leptin), all of which contribute to IR and the onset of DM[192].
PCOS: The primary causes of PCOS are IR and elevated levels of androgens[193]. Normal PCOS (NA PCOS) is an independent risk factor for T2DM; the incidence of type 2 diabetes in patients with NA PCOS is twice as high as in non-PCOS patients, and women with the hyperandrogenic PCOS phenotype face a higher risk of T2DM than women with the NA PCOS phenotype[194];
(5) Neuropsychiatric system diseases: Depression. Depression shares similar environmental and lifestyle factors with T2DM, such as socioeconomic deprivation, social adversity, smoking, and reduced physical activity[195]. The presence of depressive symptoms is associated with a modestly increased risk of T2DM[196]. Depressed adults have a 37% increased risk of developing T2DM[197]. Furthermore, studies suggest that in younger adults aged 20-50, depression may increase the risk of diabetes by 23%[198];
(6) Sexually transmitted diseases: Human immunodeficiency virus (HIV) infection. Studies have shown that among HIV-infected individuals, the risk of T2DM is higher. The older the age, the higher the BMI, the higher the TG, the lower the total cholesterol, the longer the duration of HIV infection, and the lower the lowest value of CD4. The prevalence of T2DM in HIV-infected individuals is almost twice as high as that in the healthy population, which is related to the typical risk factors in the general population, as well as to the duration of HIV infection and low minimum CD4 Levels[199];
(7) Pregnancy related diseases: Abnormalities of blood glucose during pregnancy. In a representative national cohort study of GDM screening, abnormal glucose tolerance that did not meet the GDM threshold was associated with an increased risk of T2DM that could be as high as ninefold[200]. Women with GDM are at an increased risk of being diagnosed with T2DM[201-203]. Women with a history of GDM have a nearly 10-fold higher risk of developing T2DM than women with normal blood sugar[201]. It was found that diabetes before pregnancy leaves a metabolic imprint in oocytes - a significant decrease in levels of the DNA demethylase Tet3 - and offspring are more likely to be insulin-deficient[204];
(8) Dermatosis (psoriasis): One study found more IR in patients with psoriasis compared to healthy controls, which supports the idea that psoriasis may be a pre-DM state[205]. In patients with psoriasis, psoriasis is associated with a 59% increased prevalence of diabetes and a 27% increased risk of diabetes, and the risk of diabetes may be higher in patients with severe psoriasis, especially in younger patients[206]. Furthermore, research indicated that pre-pregnancy diabetes can leave a metabolic imprint on oocytes, leading to reduced levels of the DNA demethylase Tet3, potentially resulting in offspring with insulin deficiency[207];
And (9) Other diseases: Cancer. A longitudinal study conducted over an 11-year period in Denmark revealed that the presence of lung, pancreatic, breast, brain, urethral, or uterine cancer is associated with an elevated risk of T2DM, with pancreatic cancer showing the highest risk[208]. This increased risk of developing diabetes is noticeable shortly after the diagnosis of cancer and is most pronounced within the initial two years following the cancer diagnosis[209]. Similarly, findings from a nationwide cohort study in Korea indicated that patients with thyroid cancer who underwent thyroidectomy faced an increased risk of T2DM, irrespective of age. Moreover, there was an observed U-shaped relationship between the dosage of postoperative levothyroxine and the risk of developing T2DM[210].
Solid organ transplantation (SOT): T2DM occurs in 10% to 15% of renal transplant recipients, and the pathogenesis of this DM is characterized by β-cell dysfunction and associated with reduced insulin sensitivity in the liver, muscle, and adipose tissues[211]. Recipients of SOTs are exposed to high doses of methylprednisolone intravenously at the time of surgery, followed by oral tapering glucocorticosteroid therapy, and, in the clinical setting, glucocorticoids induce increased transcription of gluconeogenic enzymes leading to hepatic IR, which results in abnormally elevated blood glucose[212].
History of drug treatment: (1) Antipsychotic drugs: A cohort study conducted on a Danish population revealed a significantly increased risk of developing diabetes after starting antipsychotic medication compared to schizophrenic patients who were not taking antipsychotic medication[213]. Another cohort study focusing on children aged 6 to 17 years found that the risk of T2DM was over three times higher in individuals using antipsychotic medication. Furthermore, there was a notable rise in risk with higher cumulative doses, and the risk persisted for up to 1 year post-discontinuation of the antipsychotic medication[214]. A study indicated a significant increase in the risk of developing T2DM in children and adolescents with psychiatric disorders who were exposed to atypical antipsychotics compared to those who were not[215]. Additionally, treatment with antiseizure medications (ASMs) was linked to a higher risk of developing diabetes compared to ASMs without enzymatic interactions. These effects are related to the cytochrome P450 family (CYPs), body weight, and IR. The risk of T2DM development tends to increase with the duration of ASM treat
(2) Drugs to reduce cardiovascular risk: Four classes of drugs commonly used to reduce cardiovascular risk, namely, statins, niacin, thiazide diuretics, and beta-blockers, have been shown in meta-analyses or large-scale clinical trials to increase the risk of NOD by 9%-43%[223]. Statins can impair insulin sensitivity and secretory function of pancreatic β-cells and increase IR in peripheral tissues[224]. In examining the effects of the major antihypertensive drug classes, it was found that beta-blockers and thiazide diuretics increased the risk of disease compared with placebo[110];
(3) Proton pump inhibitors (PPIs): There is a significant association between PPIs and the risk of developing T2DM[225,226]. During the follow-up of 2127471 people, 10105 incident cases of diabetes were recorded, and the risk of developing diabetes was 24% higher in those who regularly used PPIs than in those who did not, with the risk of developing diabetes increasing with the duration of PPI use[227];
(4) Prostate treatment drugs: Androgen deprivation therapy (ADT) reduces testosterone levels and creates a state of IR that worsens glycaemic control, and ADT is associated with worsened diabetes control and increased HbA1c levels[228]. Additionally, men with an enlarged prostate are commonly prescribed 5-alpha-reductase inhibitors to decrease androgen production, with research indicating a heightened risk of developing T2DM in men using these medications[229];
(5) Somatostatin analogues (SAs): A phase III trial found that the SA drug paregoric acid increased new-onset diabetes threefold and hyperglycaemic adverse events in up to 30% of patients and that SAs inhibit insulin and glucagon secretion, which can lead to diabetes[230];
And (6) Glucocorticosteroids: Studies have shown that glucocorticoid oral drug use is associated with 2% of new-onset diabetes[231]. Glucocorticoid-induced hyperglycaemia is caused by stimulation of hepatic glucose production and increased lipolysis in adipose tissue, resulting in systemic IR and impaired insulin production and secretion by pancreatic beta cells[232].
Pregnancy and foetal factors: (1) Premature and multiple births. Multiple and premature births are associated with the development of diabetes[233]. Preterm birth is thought to play an important role in the development of diabetes. Studies have shown that preterm birth is an important and independent risk factor for both T1DM and T2DM[234]. Preterm birth before 35 weeks of gestation is associated with an increased risk of developing T2DM in adulthood, which is independent of the risk associated with slow fetal growth[235]. Multiple births increase the risk of diabetes by impairing the proliferative capacity of pancreatic beta cells[236];
And (2) Low birth weight: Low birth weight is a known risk factor for T2DM[237,238]. Being thin in childhood increases the risk of developing T2DM in individuals who are obese in adulthood[239]. As birth weight increases (< 5000 g), the risk of developing T2DM decreases significantly, and the association between birth weight and T2DM is curvilinear and L-shaped[240]. It has been suggested that birth weight significantly alters circulating insulin-like growth factor-1 levels in adulthood, thereby affecting the risk of developing T2DM[241]. Fasting and 2-hour insulin concentrations and HOMA-IR were negatively correlated with birth weight[242]. Low birth weight and overweight in early adulthood are major determinants of the risk of developing T2DM in adult men, and they increase the risk of developing T2DM in an additive manner[243].
Menses: Women with prolonged or highly irregular menstrual cycles are at a significantly increased risk of developing T2DM[244]. After adjusting for potential confounders, those women who reported irregular menstrual cycles between the ages of 14-17, 18-22, and 29-46 had a 32%, 41%, and 66% increased risk of T2DM, respectively, compared with women of the same age with very regular menstrual cycles[245]. Early age at natural menopause is an independent risk factor for the development of T2DM, and subjects with significantly earlier menopause (before 40 years of age) have a nearly fourfold increased risk of developing T2DM compared with those with late-onset menopause, which may be due to disruption of the hypothalamic-pituitary-ovarian axis, resulting in an increased pituitary release of gonadotropins and follicle-stimulating hormone[246]. Scarcity of menstruation predicts MetS and IFG + T2DM, and the pattern of delayed menstruation during puberty should be considered an important risk factor for IFG + T2DM, MetS, menstrual scarcity, and future development of PCOS in young adults[247].
Environmental factors: Traffic noise, environmental pollution, and exposure to outdoor light all increase the risk of developing T2DM. The study found that for every 10 dB increase in traffic noise exposure, there was an 8% increase in the risk of developing T2DM among people aged 35-100 years living in Toronto[248]. A large number of recent studies have confirmed that long-term exposure to particulate matter with an aerodynamic diameter ≤ 2.5 mm (PM2.5) is a newly identified risk factor for diabetes[249-251]. Organic matter may be the most important factor in the PM2.5-diabetes relationship[252]. Arsenic is considered to be a toxic metalloid, mainly from drinking water and food (e.g., rice and cereals)[253]. Arsenic exposure was found to be positively associated with T2DM risk, with arsenic exposure levels measured by arsenic in drinking water or urine[254,255]. Genetic susceptibility to arsenic metabolism correlates with arsenic metabolism efficiency and may modify the correlation between inorganic arsenic and risk of T2DM[256]. As levels of organochlorine pesticides (OCPs) in groundwater increase, blood OCP levels tend to increase the risk of T2DM[257]. A prospective study originating in the United Kingdom with a 14-year follow-up of 283374 individuals found that middle-aged and older adults living in areas with high outdoor nighttime exposure to outdoor light levels may be at higher risk for T2DM and low sleep quality[258].
Current research has revealed a large number of new multifaceted risk factors for T2DM; however, these findings face an important challenge in their practical application: The lack of systematic classification and organization. This leads to difficulties for clinicians in fully understanding and utilizing these findings to guide patient management. Physicians in the clinic need to consider a variety of factors to assess a patient's risk of disease and develop individualized prevention and treatment plans. Due to the lack of effective classification and organization tools, it is often difficult for physicians to quickly and accurately extract useful information from numerous research findings. In order to better translate research results into effective tools in clinical practice, this paper provides a systematic categorization and identification of T2DM risk factors and a systematic and comprehensive overview of known risk factors according to different dimensions (social determinants, lifestyle, examinable/examined risk factors, medical and medication history, and other factors), which can help clinicians to improve the rate of early diagnosis of the disease, and help public health policymakers to better understand the risk characteristics of different groups and take more targeted preventive measures. At the same time, it can help establish risk prediction models. Currently, diabetes prediction models are usually based on a series of risk factors, such as age, weight, BP, and family history, and the relationship between these factors and the occurrence of diabetes is determined through statistical analysis. This paper adds more possibilities to help construct a more accurate and comprehensive risk prediction model through a more comprehensive comb, and this approach, from the careful consideration of multiple factors, also better reflects the complexity of the aetiology of diabetes, which is not caused by a single factor, but is the result of the combined effect of multiple factors.
At the same time, the review of risk factor-related research found that there are some shortcomings in the current research; the following will be discussed: The clinical significance of the current risk factor research and the shortcomings of the current risk factor research, in order to improve the systematic categorisation and practicality of T2DM risk factors by improving the shortcomings of current studies, thus providing a more scientific basis for clinicians and improving the effect of disease prevention and control.
During the review, we found that some of the risk factors are the easiest and least costly to change; such individual modifiable risk factors include dietary preferences, behavioural activities, mental health, and disease medication choices. Individuals can reduce the risk of developing T2DM by eating right, exercising scientifically, losing weight, adjusting emotions, regular screening, and making sensible drug choices. Studies have found that at least 75% of T2DM can be prevented through a healthy lifestyle[259]. HbA1c can be reduced by 2% in newly diagnosed T2DM patients guided by medical nutrition therapy[260]. In addition, the following is helpful to prevent T2DM: Choosing a low-fat, low-sugar, high-fibre diet; avoiding excessive alcohol consumption; strengthening physical exercise and active sports, with at least 150 minutes of moderate-intensity aerobic exercise per week; paying attention to emotional regulation; maintaining a positive state of mind, and overcoming negative thinking and irritability and other negative emotions; undergoing regular screening to help determine the progression of the disease; and timely adjusting the therapeutic use of medication to reduce the risk of the onset of T2DM or delay its occurrence. The risk of T2DM can be reduced by timely adjusting the treatment and medication plan, truncating or delaying the occurrence of T2DM, and providing health benefits in the later stage through the improvement of personal lifestyle and emotions.
T2DM, as a chronic disease, is a gradual process due to the accumulation, superimposition, and synergistic effect of health risk factors on the body over time. This gradual process will not stop as long as these risk factors persist. Clinicians often advocate a good three-level prevention of T2DM[261], in which "primary prevention" is mainly to identify T2DM risk factors and adopt a healthy lifestyle to prevent the occurrence of T2DM; "secondary prevention" is to delay the disease progression through drug treatment and prevent the occurrence of T2DM complications; and "tertiary prevention" is to delay the progression of T2DM complications, reduce disability and mortality, and improve the quality of life for patients through standardised treatment. By reviewing the risk factors of T2DM, we propose the possibility to move the intervention forward, front-loading health regulation, and avoiding the occurrence of T2DM earlier, i.e., to achieve the "level 0 prevention" of T2DM so that the prevention of T2DM starts from the risk control before the occurrence of T2DM, and further move the gate of T2DM prevention forward, which is a more proactive health promotion approach. This is a more proactive health promotion management model, which is also a missing part of the current research. A review of risk factors for T2DM found that immutable social determinants include gender, age, blood group, ethnicity, and family history (genetic factors). With regard to age of onset, we can reduce the incidence of T2DM through early attention and intervention at the age when T2DM is most prevalent. Also the review found that family history (genetic factors) plays an important role in the development of T2DM. Our review of the literature found that multiple behaviours of the mother (including those during pregnancy) affect the health of the offspring[262-264], which has also been verified in several animal studies[265-268]. One article clearly suggested that mothers can inherit glucose intolerance to their offspring through oocyte TET3 deficiency[204]. Also foetal undernutrition has been suggested to be a predisposing factor for IR in individuals[269]. We propose that risk screening and intervention for the mother's offspring, including genetic testing of the mother's offspring to predict the individual's risk of developing the disease, motivating high-risk individuals to change their lifestyles before a clinical phenotype emerges, helping the offspring to stay healthy by changing the mother's health status, and focusing on the mother's health management during pregnancy to reduce the risk of the offspring's adult T2DM disease, could transform this immutable risk factor into a modifiable risk factor that can be effectively intervened upon. Fully recognising the possibility and importance of starting intervention at an early stage of life fundamentally changes the thinking of disease prevention and control, providing new insights into the prevention and treatment of T2DM. The above methods block T2DM from the source of development, prevent and control chronic diseases from the source of life, promote the health and development of human beings throughout the life cycle, and provide new perspectives and strategies to ensure the health and safety of the world.
There is a lack of research to categorize T2DM risk factors according to the different disease periods of T2DM. The prevention of T2DM can be divided into three main parts: Prevention of occurrence, prevention of progression (without complications), and reduction of disability and mortality (with complications). The risk factors for the development of T2DM reviewed in the paper also have an impact on the T2DM disease process. We further categorised the risk factors for T2DM to include risk factors associated with three phases: Pre-T2DM [The American Diabetes Association defines a patient with prediabetes as the presence of IFG and/or IGT and/or an A1C of 5.7-6.4 percent (270)] as well as during the period of healthy individuals, T2DM without complications, and T2DM with complications. Regarding risk factors that are closely associated with pre-T2DM as well as in healthy individuals, they can include body mass-related index, family history (risk genes), mental health status, lifestyle (diet, physical activity, smoking, alcohol consumption, and sleep), and blood glucose-related index. This is due to the fact that T2DM is prevalent in obese patients, and weight control is the first and foremost method to prevent T2DM. Intervention and control of the mother's generation of risk genes is an early-life intervention to prevent T2DM by improving the mother's generation, while adjusting the mood, quitting smoking and drinking, regular work and rest, and optimising the dietary structure are to increase the health index of the individual through optimising the individual's lifestyle and reduce the incidence of T2DM. At the same time, attention should be paid to screening blood glucose indicators, so as to keep abreast of the body's blood glucose situation and facilitate the adoption of therapeutic measures as soon as possible. Risk factors that are closely associated with the uncomplicated stage of T2DM include lifestyle and checkable/testable risk factors. At this stage, patients are affected by the "three more and one less" symptoms of diabetes and gradually lose weight, or even become thin, and the risk of disease caused by body weight is weakened. In this stage, diet control and exercise therapy become the most important, supplemented by drugs to bring good glucose-lowering effect, and attention should be paid to regular monitoring of blood glucose to avoid acute and chronic complications caused by fluctuations in blood glucose. Risk factors closely associated with T2DM complication stage may include: Lifestyle, medical and medication history, and examination/tests. Lifestyle intervention in this stage is equally important, while patients should pay more attention to medication, and screening and selection of drugs, to avoid other drugs and diseases that aggravate the burden of blood glucose. In the period of complications, attention should be paid to the examination, inspection, and blood glucose monitoring to understand the status of blood glucose control to achieve blood glucose individualisation and timely adjustment of glucose-lowering programmes. The relevant complications should be examined every 3 to 6 months to reduce the rate of disability and mortality, improve quality of life, and prolong the life. We can see that there is a different focus on risk factor interventions during the development of the disease, but the importance of lifestyle interventions is emphasised by the fact that they are carried out by the individual throughout the development of the disease. At the same time, some articles have suggested the importance of prioritising risk factors that contribute significantly to diabetes in different age groups for effective prevention and management of diabetes[271]. This is a direction for future research to help slow disease progression by establishing a map of diabetes risk factors that can be tested through a large number of clinical studies to develop personalised interventions for patients at different ages and disease stages.
In this article, we found that a number of examination and laboratory indicators can be used as risk factors for diabetes, and through a series of examinations and laboratory tests, the health status and physiological function of various groups of people can be assessed and at the same time, the diagnosis, assessment, treatment, and follow-up of the disease can be carried out. Among these risk factors that can be obtained through examinations/tests, in China, some of the tests are easy to obtain, which are mostly simple, inexpensive, fast, and sensitive, with high acceptance by patients, and can be done in hospitals or central laboratories. On the other hand, some of the tests are not easily available in the clinic due to their high price, high operator requirements, poor reproducibility, and high requirements for the testing environment. For example, gene sequencing of the human body is more expensive and less accepted by patients. The intestinal flora, with its high professional requirements for sample collection, preservation, and transportation conditions, is therefore not very generalizable and cannot be used as a routine test for large samples. Moreover, ethnicity, region, lifestyle, and customs[272-274] contribute more to the differences in individual flora, and factors such as race and geography should not be ignored so that only the study of intestinal flora for specific populations can provide guidance for precision nutrition and precision medicine. In order to solve the above problems and improve the universality and reproducibility of the test results of clinical trials, it is necessary to formulate standard operating procedures, strictly control the quality of instrumentation and reagents, converge the sample processing and storage conditions of different laboratories, provide professional skills training for laboratory personnel, and join the external quality control program. Through the above measures, the generalizability and reproducibility of laboratory results can be maximized to ensure that reliable data are obtained to guide clinical practice.
Scientific inquiry is a step forward through various contradictions, verified (or disproved) step by step by various studies. In the article, we have included the findings of many large clinical trials, but some of the results of clinical trials have some contradictions, such as the effect of vitamin D levels and antidepressant drugs on the risk of T2DM. Some previous studies have concluded that vitamin D supplementation can prevent T2DM, and the use of antidepressants increases the risk of T2DM, but there are also some studies that do not support this conclusion, which is contradictory. In the later stage, we should pay more attention to the contradictory viewpoints in the previous clinical trials, carry out more in-depth explorations, and carry out more high-quality clinical studies through a complete protocol design so as to provide more high-level evidence-based basis for guiding the clinical practice, and extend the study from whether there is a risk association to the direction of the association (positive correlation/negative correlation), the number of associations (single correlation/complex correlation/biased correlation), and the strength of the association (significant correlation/highly correlated correlation). We should continue to think about and improve the results in order to provide more scientific support for disease prevention.
Among the immutable social factors, we found that there are relatively few studies on ethnicity, and it is very important to consider the ethnic background when establishing risk stratification for clinical management in the era of precision medicine. Currently, there is a lack of high-quality, large-sample ethnicity studies to clarify the risk groups for T2DM in different ethnic populations, and most are single-country studies concentrated in Western countries, which results in a lack of ethnic diversity. In order to prevent further deterioration of existing health disparities, subsequent studies need to incorporate multi-country, multi-ethnic studies in order to obtain more generalizable and impactful results. There is a lack of uniformity and accuracy in the impact of blood glucose, blood lipids, blood uric acid, and other testing indicators on the risk of T2DM in the current study, and it is not possible to clarify what levels of blood glucose, blood lipids, and other testing indicators will form a qualitative change that will increase the risk of T2DM, and there is a lack of global consensus, which prevents clinicians from providing clear guidance, and does not help them to set up strict and clear blood glucose and blood lipid targets for their patients to manage disease risk. More high-level evidence-based studies are needed to help us form worldwide consensus guidelines to help clinicians make prevention and treatment decisions.
This article is a narrative review of T2DM risk factors, aiming to provide clinicians with a reference for T2DM prevention strategies, and timely identification and early warning of high-risk groups, thus delaying the onset of the disease. By optimizing treatment regimens and identifying potential drug targets, the preventive effect can be effectively improved. In the future, these risk factors can be translated into scales or disease prediction models to predict and estimate individual risk of disease. Although some of the findings can be directly applied to the clinic, not all of them need to be evaluated in the context of the actual clinical situation. Predictive models based on the risk factor identification and categorization in this review can help in the design of community-based multilevel screening strategies and can provide strong support for the development of public health policies and the improvement of related industry standards, especially in the discipline of endocrinology, metabolism, and other related medical fields. Overall, this paper provides important reference information on the prevention and treatment of T2DM, promotes multidisciplinary collaboration, improves risk assessment, and provides a professional research basis for clinical practice and industry decision-making.
| 1. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1726] [Article Influence: 863.0] [Reference Citation Analysis (18)] |
| 2. | The Lancet. Diabetes: a defining disease of the 21st century. Lancet. 2023;401:2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 3. | Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400:1803-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 501] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 4. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4744] [Article Influence: 1581.3] [Reference Citation Analysis (36)] |
| 5. | Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab. 2018;15:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (1)] |
| 6. | Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37:278-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1214] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 7. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2522] [Article Influence: 280.2] [Reference Citation Analysis (0)] |
| 8. | Peters SAE, Muntner P, Woodward M. Sex Differences in the Prevalence of, and Trends in, Cardiovascular Risk Factors, Treatment, and Control in the United States, 2001 to 2016. Circulation. 2019;139:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 9. | Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2451] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 10. | Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 530] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 11. | Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 360] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 12. | Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1001] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 13. | Vaiserman A, Lushchak O. Developmental origins of type 2 diabetes: Focus on epigenetics. Ageing Res Rev. 2019;55:100957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Fagherazzi G, Gusto G, Clavel-Chapelon F, Balkau B, Bonnet F. ABO and Rhesus blood groups and risk of type 2 diabetes: evidence from the large E3N cohort study. Diabetologia. 2015;58:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Navabi J, Navabi SM, Hemmati N, Shaahmadi Z, Aghaei A. Higher Odds of Type 2 Diabetes for Some Blood Groups. Public Health Genomics. 2020;23:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Tuchman AM. Diabetes and race. A historical perspective. Am J Public Health. 2011;101:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Gaskin DJ, Thorpe RJ Jr, McGinty EE, Bower K, Rohde C, Young JH, LaVeist TA, Dubay L. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health. 2014;104:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 18. | Almgren P, Lehtovirta M, Isomaa B, Sarelin L, Taskinen MR, Lyssenko V, Tuomi T, Groop L; Botnia Study Group. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia. 2011;54:2811-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Papazafiropoulou AK, Papanas N, Melidonis A, Maltezos E. Family History of Type 2 Diabetes: Does Having a Diabetic Parent Increase the Risk? Curr Diabetes Rev. 2017;13:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Walker JT, Saunders DC, Rai V, Chen HH, Orchard P, Dai C, Pettway YD, Hopkirk AL, Reihsmann CV, Tao Y, Fan S, Shrestha S, Varshney A, Petty LE, Wright JJ, Ventresca C, Agarwala S, Aramandla R, Poffenberger G, Jenkins R, Mei S, Hart NJ, Phillips S, Kang H, Greiner DL, Shultz LD, Bottino R, Liu J, Below JE; HPAP Consortium, Parker SCJ, Powers AC, Brissova M. Genetic risk converges on regulatory networks mediating early type 2 diabetes. Nature. 2023;624:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Cheng L, Zhang D, Zhou L, Zhao J, Chen B. Association between SLC30A8 rs13266634 Polymorphism and Type 2 Diabetes Risk: A Meta-Analysis. Med Sci Monit. 2015;21:2178-2189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:804-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 646] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 23. | Yen FS, Wei JCC, Liu JS, Hwu CM, Hsu CC. Parental Income Level and Risk of Developing Type 2 Diabetes in Youth. JAMA Netw Open. 2023;6:e2345812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 24. | Marmot M, Bell R, Goldblatt P. Action on the social determinants of health. Rev Epidemiol Sante Publique. 2013;61 Suppl 3:S127-S132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Elsenburg LK, Bengtsson J, Rieckmann A, Rod NH. Childhood adversity and risk of type 2 diabetes in early adulthood: results from a population-wide cohort study of 1.2 million individuals. Diabetologia. 2023;66:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Zhu S, Shan S, Liu W, Li S, Hou L, Huang X, Liu Y, Yi Q, Sun W, Tang K, Adeloye D, Rudan I, Song P; Global Health Epidemiology Research Group (GHERG). Adverse childhood experiences and risk of diabetes: A systematic review and meta-analysis. J Glob Health. 2022;12:04082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Kivimäki M, Bartolomucci A, Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. 2023;19:10-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 28. | Mendenhall E, Kohrt BA, Norris SA, Ndetei D, Prabhakaran D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet. 2017;389:951-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 29. | Carruth L, Mendenhall E. Social aetiologies of type 2 diabetes. BMJ. 2018;361:k1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Zimmet P, Shi Z, El-Osta A, Ji L. Epidemic T2DM, early development and epigenetics: implications of the Chinese Famine. Nat Rev Endocrinol. 2018;14:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Liu H, Chen X, Shi T, Qu G, Zhao T, Xuan K, Sun Y. Association of famine exposure with the risk of type 2 diabetes: A meta-analysis. Clin Nutr. 2020;39:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Sun Y, Yu Y, Zhang H, Wang B, Chen C, Wang Y, Tan X, Zhang J, Chen Y, Xia F, Lu Y, Wang N. Joint Exposure to Positive Affect, Life Satisfaction, Depressive Symptoms, and Neuroticism and Incident Type 2 Diabetes. J Clin Endocrinol Metab. 2022;107:e3186-e3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Song Y, Zhu C, Shi B, Song C, Cui K, Chang Z, Gao G, Jia L, Fu R, Dong Q, Feng L, Zhu C, Yin D, Manson JE, Dou K. Social isolation, loneliness, and incident type 2 diabetes mellitus: results from two large prospective cohorts in Europe and East Asia and Mendelian randomization. EClinicalMedicine. 2023;64:102236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 34. | Henriksen RE, Nilsen RM, Strandberg RB. Loneliness increases the risk of type 2 diabetes: a 20 year follow-up - results from the HUNT study. Diabetologia. 2023;66:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 35. | Hung HHY, Chan EYY, Chow EYK, Chung GKK, Lai FTT, Yeoh EK. Non-skilled occupation as a risk factor of diabetes among working population: A population-based study of community-dwelling adults in Hong Kong. Health Soc Care Community. 2022;30:e86-e94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Carlsson S, Andersson T, Talbäck M, Feychting M. Incidence and prevalence of type 2 diabetes by occupation: results from all Swedish employees. Diabetologia. 2020;63:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Shan Z, Li Y, Zong G, Guo Y, Li J, Manson JE, Hu FB, Willett WC, Schernhammer ES, Bhupathiraju SN. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363:k4641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 38. | Li W, Yi G, Chen Z, Dai X, Wu J, Peng Y, Ruan W, Lu Z, Wang D. Is job strain associated with a higher risk of type 2 diabetes mellitus? A systematic review and meta-analysis of prospective cohort studies. Scand J Work Environ Health. 2021;47:249-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Pan KY, Xu W, Mangialasche F, Fratiglioni L, Wang HX. Work-related psychosocial stress and the risk of type 2 diabetes in later life. J Intern Med. 2017;281:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Lian Y, Sun Q, Guan S, Ge H, Tao N, Jiang Y, Zhang Y, Ning L, Xiao J, Liu J. Effect of Changing Work Stressors and Coping Resources on the Risk of Type 2 Diabetes: The OHSPIW Cohort Study. Diabetes Care. 2018;41:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Xu T, Clark AJ, Pentti J, Rugulies R, Lange T, Vahtera J, Magnusson Hanson LL, Westerlund H, Kivimäki M, Rod NH. Characteristics of Workplace Psychosocial Resources and Risk of Diabetes: A Prospective Cohort Study. Diabetes Care. 2022;45:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Khandwala YS, Baker VL, Shaw GM, Stevenson DK, Lu Y, Eisenberg ML. Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: population based cohort study. BMJ. 2018;363:k4372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: a systematic review and meta-analysis. BMC Med. 2014;12:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Nielsen J, Hulman A, Witte DR. Spousal cardiometabolic risk factors and incidence of type 2 diabetes: a prospective analysis from the English Longitudinal Study of Ageing. Diabetologia. 2018;61:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Whisman MA, Li A, Sbarra DA, Raison CL. Marital quality and diabetes: results from the Health and Retirement Study. Health Psychol. 2014;33:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | O'Hearn M, Lara-Castor L, Cudhea F, Miller V, Reedy J, Shi P, Zhang J, Wong JB, Economos CD, Micha R, Mozaffarian D; Global Dietary Database. Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat Med. 2023;29:982-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 47. | Liu M, Wang H, Du S, Jiao Y, Wang Q, Su C, Zhang B, Ding G. Trajectories of Meat Intake and Risk of Type 2 Diabetes: Findings from the China Health and Nutrition Survey (1997-2018). Nutrients. 2023;15:3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 48. | Miller V, Jenkins DA, Dehghan M, Srichaikul K, Rangarajan S, Mente A, Mohan V, Swaminathan S, Ismail R, Luz Diaz M, Ravindran RM, Zatonska K, Bahonar A, Altuntas Y, Khatib R, Lopez-Jaramillo P, Yusufali A, Yeates K, Chifamba J, Iqbal R, Yusuf R, Catherina Swart E, Bo H, Han G, Li X, Alhabib KF, Rosengren A, Avezum A, Lanas F, Yusuf S; Prospective Urban and Rural Epidemiology (PURE) study investigators. Associations of the glycaemic index and the glycaemic load with risk of type 2 diabetes in 127 594 people from 20 countries (PURE): a prospective cohort study. Lancet Diabetes Endocrinol. 2024;12:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 49. | Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344:e1454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 50. | Bhavadharini B, Mohan V, Dehghan M, Rangarajan S, Swaminathan S, Rosengren A, Wielgosz A, Avezum A, Lopez-Jaramillo P, Lanas F, Dans AL, Yeates K, Poirier P, Chifamba J, Alhabib KF, Mohammadifard N, Zatońska K, Khatib R, Vural Keskinler M, Wei L, Wang C, Liu X, Iqbal R, Yusuf R, Wentzel-Viljoen E, Yusufali A, Diaz R, Keat NK, Lakshmi PVM, Ismail N, Gupta R, Palileo-Villanueva LM, Sheridan P, Mente A, Yusuf S. White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care. 2020;43:2643-2650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 51. | Rajpathak S, Ma J, Manson J, Willett WC, Hu FB. Iron intake and the risk of type 2 diabetes in women: a prospective cohort study. Diabetes Care. 2006;29:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Bao W, Rong Y, Rong S, Liu L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012;10:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 53. | Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:363-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 527] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 54. | InterAct Consortium; Romaguera D, Norat T, Wark PA, Vergnaud AC, Schulze MB, van Woudenbergh GJ, Drogan D, Amiano P, Molina-Montes E, Sánchez MJ, Balkau B, Barricarte A, Beulens JW, Clavel-Chapelon F, Crispim SP, Fagherazzi G, Franks PW, Grote VA, Huybrechts I, Kaaks R, Key TJ, Khaw KT, Nilsson P, Overvad K, Palli D, Panico S, Quirós JR, Rolandsson O, Sacerdote C, Sieri S, Slimani N, Spijkerman AM, Tjonneland A, Tormo MJ, Tumino R, van den Berg SW, Wermeling PR, Zamara-Ros R, Feskens EJ, Langenberg C, Sharp SJ, Forouhi NG, Riboli E, Wareham NJ. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56:1520-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 55. | Quan W, Jiao Y, Xue C, Li Y, Wang Z, Zeng M, Qin F, He Z, Chen J. Processed potatoes intake and risk of type 2 diabetes: a systematic review and meta-analysis of nine prospective cohort studies. Crit Rev Food Sci Nutr. 2022;62:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Alperet DJ, Butler LM, Koh WP, Yuan JM, van Dam RM. Influence of temperate, subtropical, and tropical fruit consumption on risk of type 2 diabetes in an Asian population. Am J Clin Nutr. 2017;105:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Debras C, Druesne-Pecollo N, Chazelas E, Deschasaux M, Hercberg S, Galan P, Monteiro CA, Julia C, Touvier M. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern Med. 2020;180:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 58. | Tian S, Xu Q, Jiang R, Han T, Sun C, Na L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients. 2017;9:982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 59. | Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. 2018;361:k2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 60. | Sellem L, Srour B, Javaux G, Chazelas E, Chassaing B, Viennois E, Debras C, Salamé C, Druesne-Pecollo N, Esseddik Y, de Edelenyi FS, Agaësse C, De Sa A, Lutchia R, Louveau E, Huybrechts I, Pierre F, Coumoul X, Fezeu LK, Julia C, Kesse-Guyot E, Allès B, Galan P, Hercberg S, Deschasaux-Tanguy M, Touvier M. Food additive emulsifiers and risk of cardiovascular disease in the NutriNet-Santé cohort: prospective cohort study. BMJ. 2023;382:e076058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 61. | Kudo A, Asahi K, Satoh H, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Fujimoto S, Narita I, Konta T, Kondo M, Shibagaki Y, Kasahara M, Watanabe T, Shimabukuro M. Fast eating is a strong risk factor for new-onset diabetes among the Japanese general population. Sci Rep. 2019;9:8210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Radzevičienė L, Ostrauskas R. Fast eating and the risk of type 2 diabetes mellitus: a case-control study. Clin Nutr. 2013;32:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Maruyama K, Sato S, Ohira T, Maeda K, Noda H, Kubota Y, Nishimura S, Kitamura A, Kiyama M, Okada T, Imano H, Nakamura M, Ishikawa Y, Kurokawa M, Sasaki S, Iso H. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: cross sectional survey. BMJ. 2008;337:a2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 64. | Thompson AS, Candussi CJ, Tresserra-Rimbau A, Jennings A, Bondonno NP, Hill C, Sowah SA, Cassidy A, Kühn T. A healthful plant-based diet is associated with lower type 2 diabetes risk via improved metabolic state and organ function: A prospective cohort study. Diabetes Metab. 2024;50:101499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 65. | Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 66. | Teong XT, Liu K, Vincent AD, Bensalem J, Liu B, Hattersley KJ, Zhao L, Feinle-Bisset C, Sargeant TJ, Wittert GA, Hutchison AT, Heilbronn LK. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nat Med. 2023;29:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 67. | Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527-2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 68. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1614] [Article Influence: 179.3] [Reference Citation Analysis (1)] |
| 69. | Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E; EGIR-RISC Study Group. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57:2613-2618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 70. | Kraus WE, Yates T, Tuomilehto J, Sun JL, Thomas L, McMurray JJV, Bethel MA, Holman RR. Relationship between baseline physical activity assessed by pedometer count and new-onset diabetes in the NAVIGATOR trial. BMJ Open Diabetes Res Care. 2018;6:e000523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1089] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 72. | Luo M, Yu C, Del Pozo Cruz B, Chen L, Ding D. Accelerometer-measured intensity-specific physical activity, genetic risk and incident type 2 diabetes: a prospective cohort study. Br J Sports Med. 2023;57:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 73. | Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 621] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 74. | Nôga DA, Meth EMES, Pacheco AP, Tan X, Cedernaes J, van Egmond LT, Xue P, Benedict C. Habitual Short Sleep Duration, Diet, and Development of Type 2 Diabetes in Adults. JAMA Netw Open. 2024;7:e241147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 75. | Song Y, Chang Z, Song C, Cui K, Yuan S, Qiao Z, Bian X, Gao Y, Dou K. Association of sleep quality, its change and sleep duration with the risk of type 2 diabetes mellitus: Findings from the English longitudinal study of ageing. Diabetes Metab Res Rev. 2023;39:e3669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 76. | LeBlanc ES, Smith NX, Nichols GA, Allison MJ, Clarke GN. Insomnia is associated with an increased risk of type 2 diabetes in the clinical setting. BMJ Open Diabetes Res Care. 2018;6:e000604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Lin CL, Chien WC, Chung CH, Wu FL. Risk of type 2 diabetes in patients with insomnia: A population-based historical cohort study. Diabetes Metab Res Rev. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Liu X, Bragg F, Yang L, Kartsonaki C, Guo Y, Du H, Bian Z, Chen Y, Yu C, Lv J, Wang K, Zhang H, Chen J, Clarke R, Collins R, Peto R, Li L, Chen Z; China Kadoorie Biobank Collaborative Group. Smoking and smoking cessation in relation to risk of diabetes in Chinese men and women: a 9-year prospective study of 0·5 million people. Lancet Public Health. 2018;3:e167-e176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 79. | Durlach V, Vergès B, Al-Salameh A, Bahougne T, Benzerouk F, Berlin I, Clair C, Mansourati J, Rouland A, Thomas D, Thuillier P, Tramunt B, Le Faou AL. Smoking and diabetes interplay: A comprehensive review and joint statement. Diabetes Metab. 2022;48:101370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 80. | Rimm EB, Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Hennekens CH, Speizer FE. Cigarette smoking and the risk of diabetes in women. Am J Public Health. 1993;83:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Huh Y, Han K, Choi MJ, Kim JH, Kim SM, Nam GE. Association of Smoking Status With the Risk of Type 2 Diabetes Among Young Adults: A Nationwide Cohort Study in South Korea. Nicotine Tob Res. 2022;24:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 377] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 83. | Carlsson S, Andersson T, Araghi M, Galanti R, Lager A, Lundberg M, Nilsson P, Norberg M, Pedersen NL, Trolle-Lagerros Y, Magnusson C. Smokeless tobacco (snus) is associated with an increased risk of type 2 diabetes: results from five pooled cohorts. J Intern Med. 2017;281:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Bonnet F, Disse E, Laville M, Mari A, Hojlund K, Anderwald CH, Piatti P, Balkau B; RISC Study Group. Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia. 2012;55:3228-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2009;32:2123-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 477] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 86. | Hinkle SN, Bao W, Wu J, Sun Y, Ley SH, Tobias DK, Qian F, Rawal S, Zhu Y, Chavarro JE, Hu FB, Zhang C. Association of Habitual Alcohol Consumption With Long-term Risk of Type 2 Diabetes Among Women With a History of Gestational Diabetes. JAMA Netw Open. 2021;4:e2124669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of Visceral Adiposity Index, Body Shape Index, Body Mass Index and Waist Circumference and Their Associations with Diabetes Mellitus in Adults. Nutrients. 2019;11:1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 88. | Koloverou E, Panagiotakos DB, Kyrou I, Stefanadis C, Chrysohoou C, Georgousopoulou EN, Skoumas I, Tousoulis D, Pitsavos C; ATTICA Study group. Visceral adiposity index outperforms common anthropometric indices in predicting 10-year diabetes risk: Results from the ATTICA study. Diabetes Metab Res Rev. 2019;35:e3161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y, Liu D, Zhang D, Guo C, Zhou Q, Tian G, Huang S, Wu X, Li Y, Yang X, Zhao Y, Feng Y, Liu Y, Li H, Sun X, Chen Q, Wang T, Chen X, Hu D, Zhang M. Chinese visceral adiposity index: A reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev. 2021;37:e3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 90. | Jayedi A, Soltani S, Motlagh SZ, Emadi A, Shahinfar H, Moosavi H, Shab-Bidar S. Anthropometric and adiposity indicators and risk of type 2 diabetes: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2022;376:e067516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 91. | Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 92. | Guo Z, Liu L, Yu F, Cai Y, Wang J, Gao Y, Ping Z. The causal association between body mass index and type 2 diabetes mellitus-evidence based on regression discontinuity design. Diabetes Metab Res Rev. 2021;37:e3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Janghorbani M, Momeni F, Dehghani M. Hip circumference, height and risk of type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2012;13:1172-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Bawadi H, Abouwatfa M, Alsaeed S, Kerkadi A, Shi Z. Body Shape Index Is a Stronger Predictor of Diabetes. Nutrients. 2019;11:1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 95. | Nanri A, Mizoue T, Takahashi Y, Matsushita Y, Noda M, Inoue M, Tsugane S; Japan Public Health Center-based Prospective Study Group. Association of weight change in different periods of adulthood with risk of type 2 diabetes in Japanese men and women: the Japan Public Health Center-Based Prospective Study. J Epidemiol Community Health. 2011;65:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 97. | Wang D, Chen Z, Wu Y, Ren J, Shen D, Hu G, Mao C. Association between two novel anthropometric measures and type 2 diabetes in a Chinese population. Diabetes Obes Metab. 2024;26:3238-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 98. | Hua S, Loehr LR, Tanaka H, Heiss G, Coresh J, Selvin E, Matsushita K. Ankle-brachial index and incident diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Cardiovasc Diabetol. 2016;15:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Liu Y, Lai X, Guo W, Ma L, Li W, Fang Q, Yang H, Cai Y, Liu M, Zhang X, Yang L. Total White Blood Cell Count Mediated the Association Between Increased Arterial Stiffness and Risk of Type 2 Diabetes Mellitus in Chinese Adults. Arterioscler Thromb Vasc Biol. 2020;40:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Zhang X, Hong F, Qin Z, Liu L, Yang J, Tang X, Li X, Zhang J, Luo P. Resting heart rate is associated with the risk of metabolic syndrome and its components among Dong adults in southwest China: Cross-sectional findings of the China Multi-Ethnic Cohort Study. Diabetes Metab Res Rev. 2022;38:e3475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Lee DH, de Rezende LFM, Hu FB, Jeon JY, Giovannucci EL. Resting heart rate and risk of type 2 diabetes: A prospective cohort study and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, Zhu Y, Li D, Hu FB, Wu S, Gao X. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. Int J Epidemiol. 2015;44:689-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 103. | Bemelmans RH, Wassink AM, van der Graaf Y, Nathoe HM, Vernooij JW, Spiering W, Visseren FL; SMART Study Group. Risk of elevated resting heart rate on the development of type 2 diabetes in patients with clinically manifest vascular diseases. Eur J Endocrinol. 2012;166:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Mullican DR, Lorenzo C, Haffner SM. Is prehypertension a risk factor for the development of type 2 diabetes? Diabetes Care. 2009;32:1870-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual Blood Pressure and Risk of New-Onset Diabetes: Evidence From 4.1 Million Adults and a Meta-Analysis of Prospective Studies. J Am Coll Cardiol. 2015;66:1552-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 106. | Zhang Y, Nie J, Zhang Y, Li J, Liang M, Wang G, Tian J, Liu C, Wang B, Cui Y, Wang X, Huo Y, Xu X, Hou FF, Qin X. Degree of Blood Pressure Control and Incident Diabetes Mellitus in Chinese Adults With Hypertension. J Am Heart Assoc. 2020;9:e017015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 107. | Izzo R, de Simone G, Chinali M, Iaccarino G, Trimarco V, Rozza F, Giudice R, Trimarco B, De Luca N. Insufficient control of blood pressure and incident diabetes. Diabetes Care. 2009;32:845-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Aikens RC, Zhao W, Saleheen D, Reilly MP, Epstein SE, Tikkanen E, Salomaa V, Voight BF. Systolic Blood Pressure and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes. 2017;66:543-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 109. | Yang L, Huang C, Zhao M, Lee PMY, Zhang C, Yu Y, Xi B, Li J. Maternal hypertensive disorders during pregnancy and the risk of offspring diabetes mellitus in childhood, adolescence, and early adulthood: a nationwide population-based cohort study. BMC Med. 2023;21:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 110. | Nazarzadeh M, Bidel Z, Canoy D, Copland E, Wamil M, Majert J, Smith Byrne K, Sundström J, Teo K, Davis BR, Chalmers J, Pepine CJ, Dehghan A, Bennett DA, Smith GD, Rahimi K; Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure lowering and risk of new-onset type 2 diabetes: an individual participant data meta-analysis. Lancet. 2021;398:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 111. | Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 112. | Kim MK, Han K, Koh ES, Hong OK, Baek KH, Song KH, Kwon HS. Cumulative exposure to impaired fasting glucose and future risk of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2021;175:108799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 113. | Bowen ME, Xuan L, Lingvay I, Halm EA. Random blood glucose: a robust risk factor for type 2 diabetes. J Clin Endocrinol Metab. 2015;100:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, Imperatore G, Williams DE, Albright AL. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 115. | Szili-Torok T, Bakker SJL, Tietge UJF. Normal fasting triglyceride levels and incident type 2 diabetes in the general population. Cardiovasc Diabetol. 2022;21:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 116. | Guo R, Wei L, Cao Y, Zhao W. Normal triglyceride concentration and the risk of diabetes mellitus type 2 in the general population of China. Front Endocrinol (Lausanne). 2024;15:1330650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 117. | Sokooti S, Flores-Guerrero JL, Kieneker LM, Heerspink HJL, Connelly MA, Bakker SJL, Dullaart RPF. HDL Particle Subspecies and Their Association With Incident Type 2 Diabetes: The PREVEND Study. J Clin Endocrinol Metab. 2021;106:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 118. | Yang T, Liu Y, Li L, Zheng Y, Wang Y, Su J, Yang R, Luo M, Yu C. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and Type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 119. | Kim J, Shin SJ, Kim YS, Kang HT. Positive association between the ratio of triglycerides to high-density lipoprotein cholesterol and diabetes incidence in Korean adults. Cardiovasc Diabetol. 2021;20:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 120. | Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 121. | Chien KL, Chen MF, Hsu HC, Chang WT, Su TC, Lee YT, Hu FB. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem. 2008;54:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 122. | Pittas AG, Kawahara T, Jorde R, Dawson-Hughes B, Vickery EM, Angellotti E, Nelson J, Trikalinos TA, Balk EM. Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes : A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Ann Intern Med. 2023;176:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 123. | McCarthy K, Laird E, O'Halloran AM, Walsh C, Healy M, Fitzpatrick AL, Walsh JB, Hernández B, Fallon P, Molloy AM, Kenny RA. Association between vitamin D deficiency and the risk of prevalent type 2 diabetes and incident prediabetes: A prospective cohort study using data from The Irish Longitudinal Study on Ageing (TILDA). EClinicalMedicine. 2022;53:101654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 124. | Wang M, Zhou T, Li X, Ma H, Liang Z, Fonseca VA, Heianza Y, Qi L. Baseline Vitamin D Status, Sleep Patterns, and the Risk of Incident Type 2 Diabetes in Data From the UK Biobank Study. Diabetes Care. 2020;43:2776-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 125. | Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F; InterAct Consortium, Langenberg C, Wareham NJ, Forouhi NG. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 126. | Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. J Clin Endocrinol Metab. 2016;101:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 127. | Bardugo A, Derazne E, Zucker I, Bendor CD, Puris G, Lutski M, Pinhas-Hamiel O, Cukierman-Yaffe T, Mosenzon O, Schechter M, Tzur D, Afek A, Tirosh A, Gerstein HC, Raz I, Twig G. Adolescent Thyroid Disorders and Risk for Type 2 Diabetes in Young Adulthood. J Clin Endocrinol Metab. 2021;106:e3426-e3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Rong F, Dai H, Wu Y, Li J, Liu G, Chen H, Zhang X. Association between thyroid dysfunction and type 2 diabetes: a meta-analysis of prospective observational studies. BMC Med. 2021;19:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 129. | Yang W, Jin C, Wang H, Lai Y, Li J, Shan Z. Subclinical hypothyroidism increases insulin resistance in normoglycemic people. Front Endocrinol (Lausanne). 2023;14:1106968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 130. | Bertinato J, Wang KC, Hayward S. Serum Magnesium Concentrations in the Canadian Population and Associations with Diabetes, Glycemic Regulation, and Insulin Resistance. Nutrients. 2017;9:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 131. | Li W, Jiao Y, Wang L, Wang S, Hao L, Wang Z, Wang H, Zhang B, Ding G, Jiang H. Association of Serum Magnesium with Insulin Resistance and Type 2 Diabetes among Adults in China. Nutrients. 2022;14:1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 132. | Kieboom BCT, Ligthart S, Dehghan A, Kurstjens S, de Baaij JHF, Franco OH, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Serum magnesium and the risk of prediabetes: a population-based cohort study. Diabetologia. 2017;60:843-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 133. | Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 621] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 134. | Yun EK, Seo IH, Lee HS, Seol SY, Lee YJ. Sex differences in the relationship between platelet count and type 2 diabetes risk in community-dwelling adults: Longitudinal findings over 14 years. Diabetes Metab Res Rev. 2023;39:e3641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 135. | Cheng F, Luk AO, Shi M, Huang C, Jiang G, Yang A, Wu H, Lim CKP, Tam CHT, Fan B, Lau ESH, Ng ACW, Wong KK, Carroll L, Lee HM, Kong AP, Keech AC, Chow E, Joglekar MV, Tsui SKW, So WY, So HC, Hardikar AA, Jenkins AJ, Chan JCN, Ma RCW. Shortened Leukocyte Telomere Length Is Associated With Glycemic Progression in Type 2 Diabetes: A Prospective and Mendelian Randomization Analysis. Diabetes Care. 2022;45:701-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 136. | Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care. 2005;28:2913-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 137. | Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol. 2013;178:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 138. | Qiu G, Zheng Y, Wang H, Sun J, Ma H, Xiao Y, Li Y, Yuan Y, Yang H, Li X, Min X, Zhang C, Xu C, Jiang Y, Zhang X, He M, Yang M, Hu Z, Tang H, Shen H, Hu FB, Pan A, Wu T. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int J Epidemiol. 2016;45:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 139. | Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 341] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 140. | Zhu JL, Chen Z, Feng WJ, Long SL, Mo ZC. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 141. | Zhu T, Cui J, Goodarzi MO. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes. 2021;70:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 142. | Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 143. | Muka T, Nano J, Jaspers L, Meun C, Bramer WM, Hofman A, Dehghan A, Kavousi M, Laven JS, Franco OH. Associations of Steroid Sex Hormones and Sex Hormone-Binding Globulin With the Risk of Type 2 Diabetes in Women: A Population-Based Cohort Study and Meta-analysis. Diabetes. 2017;66:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 144. | Noroozzadeh M, Rahmati M, Behboudi-Gandevani S, Ramezani Tehrani F. Maternal hyperandrogenism is associated with a higher risk of type 2 diabetes mellitus and overweight in adolescent and adult female offspring: a long-term population-based follow-up study. J Endocrinol Invest. 2022;45:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 145. | Chen Z, Wen J. Elevated triglyceride-glucose (TyG) index predicts impaired islet β-cell function: A hospital-based cross-sectional study. Front Endocrinol (Lausanne). 2022;13:973655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 146. | Song B, Zhao X, Yao T, Lu W, Zhang H, Liu T, Liu C, Wang K. Triglyceride Glucose-Body Mass Index and Risk of Incident Type 2 Diabetes Mellitus in Japanese People With Normal Glycemic Level: A Population-Based Longitudinal Cohort Study. Front Endocrinol (Lausanne). 2022;13:907973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 147. | Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, Viikari JS, Raitakari OT, Ala-Korpela M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 425] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 148. | Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB, Svetkey LP. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 149. | Vanweert F, de Ligt M, Hoeks J, Hesselink MKC, Schrauwen P, Phielix E. Elevated Plasma Branched-Chain Amino Acid Levels Correlate With Type 2 Diabetes-Related Metabolic Disturbances. J Clin Endocrinol Metab. 2021;106:e1827-e1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 150. | Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, Wolpin BM, Hu FB, Qi L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45:1482-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 151. | Jeon J, Kim J. Dipstick proteinuria and risk of type 2 diabetes mellitus: a nationwide population-based cohort study. J Transl Med. 2021;19:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 152. | Wang Z, Peters BA, Yu B, Grove ML, Wang T, Xue X, Thyagarajan B, Daviglus ML, Boerwinkle E, Hu G, Mossavar-Rahmani Y, Isasi CR, Knight R, Burk RD, Kaplan RC, Qi Q. Gut Microbiota and Blood Metabolites Related to Fiber Intake and Type 2 Diabetes. Circ Res. 2024;134:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 153. | Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172:R167-R177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 154. | Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, Herrema H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol. 2020;11:571731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 155. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1536] [Article Influence: 219.4] [Reference Citation Analysis (68)] |
| 156. | Zhuang R, Ge X, Han L, Yu P, Gong X, Meng Q, Zhang Y, Fan H, Zheng L, Liu Z, Zhou X. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes Rev. 2019;20:883-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 157. | Zhang H, Ma L, Peng W, Wang B, Sun Y. Association between gut microbiota and onset of type 2 diabetes mellitus: a two-sample Mendelian randomization study. Front Cell Infect Microbiol. 2024;14:1327032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 158. | Hosomi K, Saito M, Park J, Murakami H, Shibata N, Ando M, Nagatake T, Konishi K, Ohno H, Tanisawa K, Mohsen A, Chen YA, Kawashima H, Natsume-Kitatani Y, Oka Y, Shimizu H, Furuta M, Tojima Y, Sawane K, Saika A, Kondo S, Yonejima Y, Takeyama H, Matsutani A, Mizuguchi K, Miyachi M, Kunisawa J. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat Commun. 2022;13:4477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 209] [Reference Citation Analysis (0)] |
| 159. | Chiou J, Zeng C, Cheng Z, Han JY, Schlichting M, Miller M, Mendez R, Huang S, Wang J, Sui Y, Deogaygay A, Okino ML, Qiu Y, Sun Y, Kudtarkar P, Fang R, Preissl S, Sander M, Gorkin DU, Gaulton KJ. Single-cell chromatin accessibility identifies pancreatic islet cell type- and state-specific regulatory programs of diabetes risk. Nat Genet. 2021;53:455-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 160. | Zhao Y, Stankovic S, Koprulu M, Wheeler E, Day FR, Lango Allen H, Kerrison ND, Pietzner M, Loh PR, Wareham NJ, Langenberg C, Ong KK, Perry JRB. GIGYF1 loss of function is associated with clonal mosaicism and adverse metabolic health. Nat Commun. 2021;12:4178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 161. | Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, Luan J, Ardanaz E, Arriola L, Balkau B, Boeing H, Deloukas P, Forouhi NG, Franks PW, Grioni S, Kaaks R, Key TJ, Navarro C, Nilsson PM, Overvad K, Palli D, Panico S, Quirós JR, Riboli E, Rolandsson O, Sacerdote C, Salamanca EC, Slimani N, Spijkerman AM, Tjonneland A, Tumino R, van der A DL, van der Schouw YT, McCarthy MI, Barroso I, O'Rahilly S, Savage DB, Sattar N, Langenberg C, Scott RA, Wareham NJ. Association Between Low-Density Lipoprotein Cholesterol-Lowering Genetic Variants and Risk of Type 2 Diabetes: A Meta-analysis. JAMA. 2016;316:1383-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 304] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 162. | Wei Y, Zheng B, Fan J, Lv J, Guo Y, Bian Z, Du H, Yang L, Chen Y, Chen J, Zhong X, Chen J, Chen Z, Yu C, Li L; China Kadoorie Biobank Collaborative Group. Habitual snoring, adiposity measures and risk of type 2 diabetes in 0.5 million Chinese adults: a 10-year cohort. BMJ Open Diabetes Res Care. 2020;8:e001015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 163. | Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med. 2000;248:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 164. | Cho SMJ, Lee H, Shim JS, Kim HC. Association of Snoring with Prediabetes and Type 2 Diabetes Mellitus: The Cardiovascular and Metabolic Diseases Etiology Research Center Cohort. Diabetes Metab J. 2020;44:687-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 165. | Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest. 2017;152:1070-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 422] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 166. | Khaire SS, Gada JV, Utpat KV, Shah N, Varthakavi PK, Bhagwat NM. A study of glycemic variability in patients with type 2 diabetes mellitus with obstructive sleep apnea syndrome using a continuous glucose monitoring system. Clin Diabetes Endocrinol. 2020;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 167. | Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31 Suppl 2:S303-S309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 168. | Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev. 2016;30:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 414] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 169. | Lai H, Yang M, Sun M, Pan B, Wang Q, Wang J, Tian J, Ding G, Yang K, Song X, Ge L. Risk of incident diabetes after COVID-19 infection: A systematic review and meta-analysis. Metabolism. 2022;137:155330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 170. | Wong R, Lam E, Bramante CT, Johnson SG, Reusch J, Wilkins KJ, Yeh HC. Does COVID-19 Infection Increase the Risk of Diabetes? Current Evidence. Curr Diab Rep. 2023;23:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 171. | Kostis JB, Sanders M. The association of heart failure with insulin resistance and the development of type 2 diabetes. Am J Hypertens. 2005;18:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 172. | Demant MN, Gislason GH, Køber L, Vaag A, Torp-Pedersen C, Andersson C. Association of heart failure severity with risk of diabetes: a Danish nationwide cohort study. Diabetologia. 2014;57:1595-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 173. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1672] [Article Influence: 418.0] [Reference Citation Analysis (33)] |
| 174. | Chen C, Zhang Y, Fan Y, Ying Z, Su Q, Li X, Qin L. The change of non-alcoholic fatty liver disease is associated with risk of incident diabetes. Front Endocrinol (Lausanne). 2023;14:1108442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 175. | Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 176. | Cao L, An Y, Liu H, Jiang J, Liu W, Zhou Y, Shi M, Dai W, Lv Y, Zhao Y, Lu Y, Chen L, Xia Y. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta-analysis. BMC Med. 2024;22:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 177. | Ha J, Hong OK, Han K, Kwon HS. Metabolic dysfunction-associated fatty liver disease increases the risk of type 2 diabetes mellitus in young Korean adults. Diabetes Res Clin Pract. 2024;212:111584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 178. | Zhang J, Xu Q, Lai F, Chen N, Lin M, Liu Y, Zhang W, Liu C, Wang S, Li Z. Joint associations of metabolically healthy abdominal obesity and non-alcoholic fatty liver disease with prediabetes and diabetes in Chinese adults. BMJ Open Diabetes Res Care. 2021;9:e002362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 179. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1560] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 180. | Jess T, Jensen BW, Andersson M, Villumsen M, Allin KH. Inflammatory Bowel Diseases Increase Risk of Type 2 Diabetes in a Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2020;18:881-888.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 181. | Xiao X, Wu X, Yi L, You F, Li X, Xiao C. Causal linkage between type 2 diabetes mellitus and inflammatory bowel disease: an integrated Mendelian randomization study and bioinformatics analysis. Front Endocrinol (Lausanne). 2024;15:1275699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 182. | Chan TT, Tse YK, Lui RN, Wong GL, Chim AM, Kong AP, Woo J, Yeung DK, Abrigo JM, Chu WC, Wong VW, Tang RS. Fatty Pancreas Is Independently Associated With Subsequent Diabetes Mellitus Development: A 10-Year Prospective Cohort Study. Clin Gastroenterol Hepatol. 2022;20:2014-2022.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 183. | Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol. 2015;110:1698-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 184. | Wang F, Wang J, Li Y, Yuan J, Yao P, Wei S, Guo H, Zhang X, Yang H, Wu T, He M. Gallstone Disease and Type 2 Diabetes Risk: A Mendelian Randomization Study. Hepatology. 2019;70:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 185. | Sang M, Xie C, Qiu S, Wang X, Horowitz M, Jones KL, Rayner CK, Sun Z, Wu T. Cholecystectomy is associated with dysglycaemia: Cross-sectional and prospective analyses. Diabetes Obes Metab. 2022;24:1656-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 186. | Huh JH, Lee KJ, Cho YK, Moon S, Kim YJ, Roh E, Han KD, Koh DH, Kang JG, Lee SJ, Ihm SH. Cholecystectomy Increases the Risk of Type 2 Diabetes in the Korean Population: Data From the National Health Insurance Cooperation Health Checkup 2010-2017. Ann Surg. 2023;278:e264-e271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 187. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3638] [Article Influence: 202.1] [Reference Citation Analysis (0)] |
| 188. | Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab. 2022;34:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 434] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 189. | Song Z, Gao M, Lv J, Yu C, Guo Y, Bian Z, Wei Y, Yang L, Du H, Chen Y, Zhang J, Yao J, Chen J, Chen Z, Huang T, Li L; China Kadoorie Biobank (CKB) Collaborative Group. Metabolically healthy obesity, transition to unhealthy phenotypes, and type 2 diabetes in 0.5 million Chinese adults: the China Kadoorie Biobank. Eur J Endocrinol. 2022;186:233-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 190. | Cao C, Hu H, Zheng X, Zhang X, Wang Y, He Y. Association between central obesity and incident diabetes mellitus among Japanese: a retrospective cohort study using propensity score matching. Sci Rep. 2022;12:13445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 191. | Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129:4001-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 192. | Li J, Chen Y, Liu Q, Tian Z, Zhang Y. Mechanistic and therapeutic links between rheumatoid arthritis and diabetes mellitus. Clin Exp Med. 2023;23:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 193. | Ding H, Zhang J, Zhang F, Zhang S, Chen X, Liang W, Xie Q. Resistance to the Insulin and Elevated Level of Androgen: A Major Cause of Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2021;12:741764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 194. | Persson S, Elenis E, Turkmen S, Kramer MS, Yong EL, Poromaa IS. Higher risk of type 2 diabetes in women with hyperandrogenic polycystic ovary syndrome. Fertil Steril. 2021;116:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 195. | Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 2015;3:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 424] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 196. | Arroyo C, Hu FB, Ryan LM, Kawachi I, Colditz GA, Speizer FE, Manson J. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care. 2004;27:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 197. | Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 617] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 198. | Brown LC, Majumdar SR, Newman SC, Johnson JA. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care. 2005;28:1063-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 199. | Galli L, Salpietro S, Pellicciotta G, Galliani A, Piatti P, Hasson H, Guffanti M, Gianotti N, Bigoloni A, Lazzarin A, Castagna A. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol. 2012;27:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 200. | Bardugo A, Bendor CD, Rotem RS, Tsur AM, Derazne E, Gerstein HC, Tzur D, Pinhas-Hamiel O, Cukierman-Yaffe T, Raz I, Hod M, Tirosh A, Lebenthal Y, Afek A, Chodick G, Twig G. Glucose intolerance in pregnancy and risk of early-onset type 2 diabetes: a population-based cohort study. Lancet Diabetes Endocrinol. 2023;11:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 201. | Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 615] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 202. | Diaz-Santana MV, O'Brien KM, Park YM, Sandler DP, Weinberg CR. Persistence of Risk for Type 2 Diabetes After Gestational Diabetes Mellitus. Diabetes Care. 2022;45:864-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 203. | You H, Hu J, Liu Y, Luo B, Lei A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian J Med Res. 2021;154:62-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 204. | Chen B, Du YR, Zhu H, Sun ML, Wang C, Cheng Y, Pang H, Ding G, Gao J, Tan Y, Tong X, Lv P, Zhou F, Zhan Q, Xu ZM, Wang L, Luo D, Ye Y, Jin L, Zhang S, Zhu Y, Lin X, Wu Y, Jin L, Zhou Y, Yan C, Sheng J, Flatt PR, Xu GL, Huang H. Maternal inheritance of glucose intolerance via oocyte TET3 insufficiency. Nature. 2022;605:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 205. | Gyldenløve M, Storgaard H, Holst JJ, Vilsbøll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol. 2015;72:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 206. | Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 207. | Evans EA, Sayers SR, Kodji X, Xia Y, Shaikh M, Rizvi A, Frame J, Brain SD, Philpott MP, Hannen RF, Caton PW. Psoriatic skin inflammation induces a pre-diabetic phenotype via the endocrine actions of skin secretome. Mol Metab. 2020;41:101047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 208. | Sylow L, Grand MK, von Heymann A, Persson F, Siersma V, Kriegbaum M, Lykkegaard Andersen C, Johansen C. Incidence of New-Onset Type 2 Diabetes After Cancer: A Danish Cohort Study. Diabetes Care. 2022;45:e105-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 209. | Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, Chang YJ, Choi KS, Jung SY, Woo SM, Ahn JS, Sim SH, Hong YS, Pastor-Barriuso R, Guallar E, Lee ES, Kong SY, Cho J. Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol. 2018;4:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 210. | Roh E, Noh E, Hwang SY, Kim JA, Song E, Park M, Choi KM, Baik SH, Cho GJ, Yoo HJ. Increased Risk of Type 2 Diabetes in Patients With Thyroid Cancer After Thyroidectomy: A Nationwide Cohort Study. J Clin Endocrinol Metab. 2022;107:e1047-e1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 211. | Jenssen T, Hartmann A. Emerging treatments for post-transplantation diabetes mellitus. Nat Rev Nephrol. 2015;11:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 212. | Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019;15:172-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 213. | Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Børglum AD, Gasse C. Endogenous and Antipsychotic-Related Risks for Diabetes Mellitus in Young People With Schizophrenia: A Danish Population-Based Cohort Study. Am J Psychiatry. 2017;174:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 214. | Bobo WV, Cooper WO, Stein CM, Olfson M, Graham D, Daugherty J, Fuchs DC, Ray WA. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 215. | Lee H, Song DH, Kwon JW, Han E, Chang MJ, Kang HY. Assessing the risk of type 2 diabetes mellitus among children and adolescents with psychiatric disorders treated with atypical antipsychotics: a population-based nested case-control study. Eur Child Adolesc Psychiatry. 2018;27:1321-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 216. | Tseng WJ, Chang CW, Hwang JS, Ko PC, Liu CJ, Lim SN. Association of Long-term Antiseizure Medication Use and Incident Type 2 Diabetes Mellitus. Neurology. 2023;100:e2071-e2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 217. | Blumenthal SR, Castro VM, Clements CC, Rosenfield HR, Murphy SN, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Smoller JW, Perlis RH. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry. 2014;71:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 218. | van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand. 2010;122:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 219. | Kivimäki M, Hamer M, Batty GD, Geddes JR, Tabak AG, Pentti J, Virtanen M, Vahtera J. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010;33:2611-2616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 220. | Sun JW, Hernández-Díaz S, Haneuse S, Bourgeois FT, Vine SM, Olfson M, Bateman BT, Huybrechts KF. Association of Selective Serotonin Reuptake Inhibitors With the Risk of Type 2 Diabetes in Children and Adolescents. JAMA Psychiatry. 2021;78:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 221. | Kivimäki M, Batty GD, Jokela M, Ebmeier KP, Vahtera J, Virtanen M, Brunner EJ, Tabak AG, Witte DR, Kumari M, Singh-Manoux A, Hamer M. Antidepressant medication use and risk of hyperglycemia and diabetes mellitus: a noncausal association? Biol Psychiatry. 2011;70:978-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 222. | Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 2013;36:3337-3345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 223. | Ong KL, Barter PJ, Waters DD. Cardiovascular drugs that increase the risk of new-onset diabetes. Am Heart J. 2014;167:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 224. | Galicia-Garcia U, Jebari S, Larrea-Sebal A, Uribe KB, Siddiqi H, Ostolaza H, Benito-Vicente A, Martín C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int J Mol Sci. 2020;21:4725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 225. | Xia B, He Q, Smith FG, Gkoutos VG, Nirantharakumar K, Kuo ZC, Wang D, Feng Q, Cheung EC, Dai L, Huang J, Yu Y, Meng W, Qin X, Yuan J. Individualized prevention of proton pump inhibitor related adverse events by risk stratification. Nat Commun. 2024;15:3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 226. | Loosen SH, Kostev K, Luedde M, Qvartskhava N, Luedde T, Roderburg C. Long-term use of proton pump inhibitors (PPIs) is associated with an increased risk of type 2 diabetes. Gut. 2022;71:1687-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 227. | Yuan J, He Q, Nguyen LH, Wong MCS, Huang J, Yu Y, Xia B, Tang Y, He Y, Zhang C. Regular use of proton pump inhibitors and risk of type 2 diabetes: results from three prospective cohort studies. Gut. 2021;70:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 228. | Keating NL, Liu PH, O'Malley AJ, Freedland SJ, Smith MR. Androgen-deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol. 2014;65:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 229. | Wei L, Lai EC, Kao-Yang YH, Walker BR, MacDonald TM, Andrew R. Incidence of type 2 diabetes mellitus in men receiving steroid 5α-reductase inhibitors: population based cohort study. BMJ. 2019;365:l1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 230. | Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, Pronin V, Raverot G, Shimon I, Lievre KK, Fleck J, Aout M, Pedroncelli AM, Colao A; Pasireotide C2402 Study Group. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 231. | Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care. 2006;29:2728-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 232. | Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022;18:540-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 233. | Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 234. | Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2014;15:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 235. | Kajantie E, Osmond C, Barker DJ, Eriksson JG. Preterm birth--a risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care. 2010;33:2623-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 236. | Moon JH, Lee J, Kim KH, Kim HJ, Kim H, Cha HN, Park J, Lee H, Park SY, Jang HC, Kim H. Multiparity increases the risk of diabetes by impairing the proliferative capacity of pancreatic β cells. Exp Mol Med. 2023;55:2269-2280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 237. | Tyrrell JS, Yaghootkar H, Freathy RM, Hattersley AT, Frayling TM. Parental diabetes and birthweight in 236 030 individuals in the UK biobank study. Int J Epidemiol. 2013;42:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 238. | Wibaek R, Andersen GS, Linneberg A, Hansen T, Grarup N, Thuesen ACB, Jensen RT, Wells JCK, Pilgaard KA, Brøns C, Vistisen D, Vaag AA. Low birthweight is associated with a higher incidence of type 2 diabetes over two decades independent of adult BMI and genetic predisposition. Diabetologia. 2023;66:1669-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 239. | Carrasquilla GD, Ängquist L, Sørensen TIA, Kilpeläinen TO, Loos RJF. Child-to-adult body size change and risk of type 2 diabetes and cardiovascular disease. Diabetologia. 2024;67:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 240. | Tian G, Guo C, Li Q, Liu Y, Sun X, Yin Z, Li H, Chen X, Liu X, Zhang D, Cheng C, Liu L, Liu F, Zhou Q, Wang C, Li L, Wang B, Zhao Y, Liu D, Zhang M, Hu D. Birth weight and risk of type 2 diabetes: A dose-response meta-analysis of cohort studies. Diabetes Metab Res Rev. 2019;35:e3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 241. | Geng T, Wang M, Li X, Zhou T, Ma H, Fonseca VA, Koh WP, Huang T, Heianza Y, Qi L. Birth weight modifies the relation between adulthood levels of insulin-like growth factor-1 and type 2 diabetes: a prospective cohort study. BMJ Open Diabetes Res Care. 2021;9:e001885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 242. | Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 243. | Célind J, Bygdell M, Bramsved R, Martikainen J, Ohlsson C, Kindblom JM. Low birthweight and overweight during childhood and young adulthood and the risk of type 2 diabetes in men: a population-based cohort study. Diabetologia. 2024;67:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 244. | Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, Colditz GA, Speizer FE, Manson JE. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286:2421-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 245. | Wang YX, Shan Z, Arvizu M, Pan A, Manson JE, Missmer SA, Sun Q, Chavarro JE. Associations of Menstrual Cycle Characteristics Across the Reproductive Life Span and Lifestyle Factors With Risk of Type 2 Diabetes. JAMA Netw Open. 2020;3:e2027928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 246. | Muka T, Asllanaj E, Avazverdi N, Jaspers L, Stringa N, Milic J, Ligthart S, Ikram MA, Laven JSE, Kavousi M, Dehghan A, Franco OH. Age at natural menopause and risk of type 2 diabetes: a prospective cohort study. Diabetologia. 2017;60:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 247. | Glueck CJ, Woo JG, Khoury PR, Morrison JA, Daniels SR, Wang P. Adolescent oligomenorrhea (age 14-19) tracks into the third decade of life (age 20-28) and predicts increased cardiovascular risk factors and metabolic syndrome. Metabolism. 2015;64:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 248. | Shin S, Bai L, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Goldberg MS, Copes R, Kopp A, Chen H. Association Between Road Traffic Noise and Incidence of Diabetes Mellitus and Hypertension in Toronto, Canada: A Population-Based Cohort Study. J Am Heart Assoc. 2020;9:e013021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 249. | Yang BY, Qian ZM, Li S, Chen G, Bloom MS, Elliott M, Syberg KW, Heinrich J, Markevych I, Wang SQ, Chen D, Ma H, Chen DH, Liu Y, Komppula M, Leskinen A, Liu KK, Zeng XW, Hu LW, Guo Y, Dong GH. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in China: a cross-sectional study with findings from the 33 Communities Chinese Health Study. Lancet Planet Health. 2018;2:e64-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 250. | Sørensen M, Poulsen AH, Hvidtfeldt UA, Frohn LM, Ketzel M, Christensen JH, Brandt J, Geels C, Raaschou-Nielsen O. Exposure to source-specific air pollution and risk for type 2 diabetes: a nationwide study covering Denmark. Int J Epidemiol. 2022;51:1219-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 251. | Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. The 2016 global and national burden of diabetes mellitus attributable to PM(2·5) air pollution. Lancet Planet Health. 2018;2:e301-e312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 252. | Li S, Guo B, Jiang Y, Wang X, Chen L, Wang X, Chen T, Yang L, Silang Y, Hong F, Yin J, Lin H, Zhao X. Long-term Exposure to Ambient PM2.5 and Its Components Associated With Diabetes: Evidence From a Large Population-Based Cohort From China. Diabetes Care. 2023;46:111-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 253. | Singh R, Singh S, Parihar P, Singh VP, Prasad SM. Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf. 2015;112:247-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 254. | Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 255. | Islam R, Khan I, Hassan SN, McEvoy M, D'Este C, Attia J, Peel R, Sultana M, Akter S, Milton AH. Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health. 2012;11:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 256. | Li W, Li Z, Yan Y, Zhang J, Zhou Q, Jia C, Xu Y, Cui H, Xie S, Liu Q, Guan Y, Liu Y, He M. Urinary arsenic metabolism, genetic susceptibility, and their interaction on type 2 diabetes. Chemosphere. 2023;345:140536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 257. | Tyagi S, Siddarth M, Mishra BK, Banerjee BD, Urfi AJ, Madhu SV. High levels of organochlorine pesticides in drinking water as a risk factor for type 2 diabetes: A study in north India. Environ Pollut. 2021;271:116287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 258. | Xu Z, Jin J, Yang T, Wang Y, Huang J, Pan X, Frank K, Li G. Outdoor light at night, genetic predisposition and type 2 diabetes mellitus: A prospective cohort study. Environ Res. 2023;219:115157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 259. | Zhang Y, Pan XF, Chen J, Xia L, Cao A, Zhang Y, Wang J, Li H, Yang K, Guo K, He M, Pan A. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020;63:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 260. | Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 261. | Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, Zou D, Guo L, Ji Q, Chen L, Chen L, Dou J, Guo X, Kuang H, Li L, Li Q, Li X, Liu J, Ran X, Shi L, Song G, Xiao X, Yang L, Zhao Z; Chinese Diabetes Society. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35:e3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 262. | Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB, Raubenheimer D, Collins CE. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr. 2012;96:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 263. | Tirumalaraju V, Suchting R, Evans J, Goetzl L, Refuerzo J, Neumann A, Anand D, Ravikumar R, Green CE, Cowen PJ, Selvaraj S. Risk of Depression in the Adolescent and Adult Offspring of Mothers With Perinatal Depression: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e208783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 264. | Wang Y, Wang K, Du M, Khandpur N, Rossato SL, Lo CH, VanEvery H, Kim DY, Zhang FF, Chavarro JE, Sun Q, Huttenhower C, Song M, Nguyen LH, Chan AT. Maternal consumption of ultra-processed foods and subsequent risk of offspring overweight or obesity: results from three prospective cohort studies. BMJ. 2022;379:e071767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 265. | Kusuyama J, Makarewicz NS, Albertson BG, Alves-Wagner AB, Conlin RH, Prince NB, Alves CRR, Ramachandran K, Kozuka C, Xiudong Y, Xia Y, Hirshman MF, Hatta T, Nagatomi R, Nozik ES, Goodyear LJ. Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High-Fat Diet on Offspring Metabolism Through Stabilization of H3K4me3 and Protection Against WDR82 Carbonylation. Diabetes. 2022;71:1170-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 266. | Kim E, Paik D, Ramirez RN, Biggs DG, Park Y, Kwon HK, Choi GB, Huh JR. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4(+) T cells. Immunity. 2022;55:145-158.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 267. | Aiken CE, Tarry-Adkins JL, Penfold NC, Dearden L, Ozanne SE. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 2016;30:1548-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 268. | Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 863] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 269. | Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 270. | American Diabetes Association Professional Practice Committee. 3. Prevention or Delay of Type 2 Diabetes and Associated Comorbidities: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S39-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 271. | Wang T, Zhao Z, Wang G, Li Q, Xu Y, Li M, Hu R, Chen G, Su Q, Mu Y, Tang X, Yan L, Qin G, Wan Q, Gao Z, Yu X, Shen F, Luo Z, Qin Y, Chen L, Huo Y, Zeng T, Chen L, Ye Z, Zhang Y, Liu C, Wang Y, Wu S, Yang T, Deng H, Zhao J, Shi L, Xu Y, Xu M, Chen Y, Wang S, Lu J, Bi Y, Ning G, Wang W. Age-related disparities in diabetes risk attributable to modifiable risk factor profiles in Chinese adults: a nationwide, population-based, cohort study. Lancet Healthy Longev. 2021;2:e618-e628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 272. | Borrello K, Lim U, Park SY, Monroe KR, Maskarinec G, Boushey CJ, Wilkens LR, Randolph TW, Le Marchand L, Hullar MA, Lampe JW. Dietary Intake Mediates Ethnic Differences in Gut Microbial Composition. Nutrients. 2022;14:660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 273. | Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients. 2019;11:2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 274. | Wegierska AE, Charitos IA, Topi S, Potenza MA, Montagnani M, Santacroce L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022;52:2355-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |