Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1889
Revised: April 30, 2024
Accepted: August 1, 2024
Published online: September 15, 2024
Processing time: 203 Days and 17.8 Hours
Diabetes is a chronic metabolic syndrome that has become a global public health problem with significant morbidity and mortality. It is a pro-inflammatory and pro-thrombotic condition characterized by increased platelet activation and alterations in platelet indices. However, the use of platelet indices as predictors of poor glucoregulation has not been fully evaluated in this context, and evidence for their role as predictors of poor glycemic status in diabetic patients is limited.
To evaluate platelet indices and determine their prognostic significance in relation to inadequate glucoregulation among individuals diagnosed with type 2 diabetes at Bishoftu General Hospital in Ethiopia, from June 15 to August 12, 2022.
A comparative cross-sectional study was conducted in 261 participants including 174 individuals with type 2 diabetes mellitus (T2DM) and 87 non-diabetic controls. The systematic random sampling technique was used to select par-ticipants. Data were collected using structured questionnaires, physical measurements, checklists, and laboratory tests. Platelet parameters and fasting blood glucose levels were determined from blood samples using Sysmex-XN550 and CobasC311 analyzers, respectively. The hematology analyzer output was checked and participants were also screened for malaria parasites using a prepared blood smear. Collected data were entered into Epi-data version 3.1 and exported to SPSS version 25 for analysis. The χ2 test, Mann-Whitney U test, Kruskal-Wallis test, post hoc test, Spearman correlation, and receiver operating characteristic curve were used for analysis. A P value < 0.05 was considered statistically significant.
The results of our study indicate that diabetic patients have significantly higher levels of platelet distribution width (PDW), mean platelet volume (MPV), platelet large cell ratio (PLCR), and plateletcrit (PCT) compared to healthy individuals (P < 0.001). Furthermore, these indices were found to be significantly elevated in individuals with poor glycemic control in T2DM compared to those with good glycemic control and healthy controls. We also observed significant correlations between these indices and various anthropometric and clinical variables. Our findings suggest that PDW, with a cut-off value of 15.75 fL and an area under the curve (AUC) of 0.803, MPV, with a cut-off value of 12.25 fL and an AUC of 0.774, PLCR, with a cut-off value of 36.3% and an AUC of 0.775, and PCT, with a cut-off value of 0.24% and an AUC of 0.761, can serve as predictors of poor glycemic control in patients with diabetes mellitus.
The observed correlation between diabetic patients and a significant increase in platelet indices has highlighted their potential as predictors of poor glycemic control in diabetes. Therefore, regular screening and profiling of platelet indices is recommended as part of the follow-up process for individuals with diabetes mellitus.
Core Tip: The main aim of this study was to evaluate platelet indices and their ability to predict poor glucoregulation in patients with type 2 diabetes mellitus (T2DM). Platelet indices such as platelet distribution width, mean platelet volume, platelet large cell ratio, and plateletcrit showed varying levels of specificity, sensitivity, area under the curve, and cutoff value, and were identified as potential markers of poor glucoregulation in patients with type 2 diabetes. Therefore, these platelet indices could be utilized in the monitoring of patients with T2DM, especially in developing nations like Ethiopia.
- Citation: Regassa DA, Berihun GA, Habtu BF, Haile WB, Nagaash RS, Kiya GT. Platelet indices as predictors of poor glucoregulation in type 2 diabetes mellitus adults at Bishoftu General Hospital, Ethiopia. World J Diabetes 2024; 15(9): 1889-1902
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1889.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1889
Diabetes is a chronic metabolic condition that results from various factors and is characterized by consistently high levels of glucose due to impaired carbohydrate, lipid, and protein metabolism caused by irregular insulin secretion, function, or both[1-4].
The global incidence of diabetes mellitus (DM) has significantly increased, with cases rising from 108 million in 1980 to a staggering 537 million in 2021, representing a five-fold increase[3,4]. DM affects approximately 73 million individuals in the Middle East and North Africa, with an additional 24 million people in Sub-Saharan Africa also impacted by the condition[4]. By 2030, the prevalence of diabetes is expected to reach 643 million individuals, and this number is projected to increase to 783 million by 2045[4]. Type 2 diabetes is the most common form of diabetes worldwide, accounting for over 90% of cases, and it is particularly prevalent in low and middle-income countries[1,2]. Ethiopia has a high pre-valence of diabetes, with an overall estimated prevalence of 6.5%, affecting approximately 3.3% of the country’s adult population[4,5].
Approximately 541 million individuals within the working-age population face the potential threat of developing type 2 diabetes. It is one of the world's major public health challenges, affecting individuals, families, and threatening the well-being of entire communities[4]. Urban areas exhibit a higher prevalence of diabetes compared to rural areas, with middle-income countries projected to have a greater burden of the disease in the period spanning from 2021 to 2045 when contrasted with high- and low-income countries[4].
Expenditures on a global scale for the management of diabetes experienced a substantial increase, climbing from $232 billion in 2007 to $760 billion in 2019, and then further escalating to $966 billion in 2021. This surge represents a remarkable 316% growth over 15 years[4]. In sub-Saharan Africa, annual costs are projected at $67.03 billion, translating to $8836 per individual with diabetes. Moreover, it is anticipated that diabetes-related expenses will reach $7 trillion by the year 2025[6].
Diabetes is a pro-inflammatory and prothrombotic disorder characterized by altered platelet indices and increased platelet reactivity[7]. Activated platelets predispose patients to macrovascular complications such as cardiovascular diseases, stroke, and arterial disease, as well as microvascular complications like neuropathy, nephropathy, and retinopathy, making diabetic patients two to three times more likely to have a stroke or heart attack[3,8]. These phe-nomena increase diabetes morbidity and mortality[8]. Large platelets are enzymatically and metabolically active, rich in prothrombotic molecules such as platelet factor 4, P-selectin, serotonin, thromboxane A2, and plateletderived growth protein[9-11]. Increased formation of larger platelets leads to atherothrombotic complications in diabetes. Atherothrombosis is the leading cause of vascular disease in patients with type 2 diabetes and increases the risk of coronary artery disease, stroke, and peripheral artery disease[10,11].
Approximately 80% of individuals with DM die due to thrombosis. Of those, 75% of deaths are due to cardiovascular complications, while the remaining are due to cerebrovascular events and peripheral vascular complications. Endothelial abnormalities play a role in the increased activation of platelets and clotting factors seen in diabetes[12].
According to the World Health Organization (WHO) mortality database collected from 108 countries from 2000 to 2016, approximately 7.1 million deaths were recorded due to diabetes complications[13]. A review of diabetes in sub-Saharan Africa reported that the proportion of patients with diabetic complications ranged from 7% to 63% for retinopathy, 27% to 66% for neuropathy, and 10% to 83% for nephropathy[6].
Timely identification of inadequate blood sugar control in individuals with diabetes is crucial for effectively managing the disease, as well as for preventing cardiovascular complications, slowing progression of the disease, and reducing both mortality and morbidity rates[3,4]. There is some evidence to support the recent views on the effects of oral hypoglycemic agents on platelet counts (PLT) and indices[14,15].
However, there are no data from Ethiopia on the glycemic status and complications status in diabetic patients. To diagnose the glycemic status and complications status in diabetic patients, it requires the measurement of various parameters at a time and the availability of these tests is limited[3]. There are methods to determine if platelets are activated, but most of these methods are timeconsuming, expensive, and require specific training[16]. Almost all of these methods are unavailable in our setting. Studies have been conducted on the determination of platelet indices in complicated DM patients in Ethiopia, but most of them did not include a control group.
Therefore, the identification of accessible, dependable, and cost-effective indicators from laboratory findings is crucial for the early detection and management of diabetes-related complications, particularly in resourcelimited settings such as Ethiopia. Evidence has shown an association between platelet indices and DM[9,17-21].
Studies conducted on platelet indices in DM have yielded controversial conclusions. A significant increase in platelet indices and their potential for predicting poor glycemic control, and vascular complications in diabetic patients have been identified[7,15,22-32].
However, the use of platelet indices as an indicator of inadequate glucoregulation remains relatively unfamiliar in Ethiopia. Therefore, the primary objective of this study was to evaluate platelet indices among adult patients with type 2 diabetes compared to a group of healthy individuals. Additionally, we aimed to determine the prognostic significance of platelet indices in predicting suboptimal glucoregulation in DM at Bishoftu Hospital, located in the central region of Ethiopia in 2022.
From June 15 to August 12, 2022, a comparative cross-sectional study was carried out at Bishoftu General Hospital, located in Bishoftu town, Oromia Regional State, Ethiopia.
This study included all adult patients previously diagnosed with type 2 DM (T2DM) receiving care at the chronic care clinic of Bishoftu General Hospital. Additionally, individuals who were healthy, non-diabetic, and age and sex matched to the patient group, as well as patient attendants at Bishoftu General Hospital, were included as the control group for this study.
The study excluded type 2 diabetic patients with existing chronic diseases such as renal failure, liver disease, and hematological malignancy. Patients with a history of infectious diseases such as human immunodeficiency virus/Acquired Immune Deficiency Syndrome, hepatitis B, hepatitis C, and malaria were also excluded. Additionally, patients with asthma, rheumatoid arthritis, severe illness, those on anticoagulant or antiplatelet therapy were excluded. Patients experiencing bleeding or thrombosis, pregnant women, smokers, alcoholics, anemic patients, and individuals under 15 or over 65 years of age were not included in the study. Furthermore, the health status of the control group was evaluated, and individuals with a history of malarial infection in the past two months, a positive C-reactive protein (CRP) test, smokers, and alcoholics were also excluded from the study.
The sample size was determined using the two-population mean formula with the help of G-power software version 3.1. The process involved considering a 95%CI (2-tailed, α = 0.05), 80% power, a control-to-case ratio of 1:1, an effect size (d) of 0.45, and accounting for a non-respondents rate of 10%. Mean and standard deviation values for mean platelet volume (MPV) in diabetic patients and the control group were extracted from a previous study[33]. For type 2 diabetes, the mean was 10.4 ± 1.1, and for the control group, the mean was 9.9 ± 1.1. To enhance precision, the researchers applied the rule of thumb proposed by Morgan et al[34].
The intervention group had twice as many instances as the control group, resulting in a sample size of 174 for T2DM (approximately 87 participants in the poor glucoregulation group, and 87 in the good glucoregulation group). Ad-ditionally, around 87 participants were assigned to the control group, making a total of 261 participants in the study.
Participants were selected using a systematic random sampling technique, based on the sequence of follow-up visits attended. The sampling interval, represented by the kth value, was determined by dividing the estimated total number of individuals in the source population by the sample size, resulting in a value of around 7. The hospital's quarterly report indicated 1260 individuals with type 2 diabetes attending follow-up visits, while the required sample size was 174. Therefore, the kth value was calculated as 1260 divided by 174, resulting in 7. The first participant was chosen using a lottery method, where the medical record numbers of the initial seven participants were written on separate pieces of paper, and the individual corresponding to the second number drawn was selected as the first participant. Subsequently, patients with type 2 diabetes were interviewed face-to-face at every seventh interval of the follow-up visit order.
This study included the recruitment of 261 participants from two distinct groups. The first group included 174 in-dividuals diagnosed with T2DM, while the second group consisted of 87 non-diabetic healthy controls. The classification of these groups was based on the American Diabetes Association-2017 guidelines[35]. Within the 174 T2DM participants, two subgroups were formed. One subgroup included 87 individuals with poor glycemic control, identified by a fasting blood glucose (FBG) level exceeding 130 mg/dL, and a history of more severe clinical symptoms. The other subgroup comprised the remaining 87 participants who displayed good glycemic control, with FBG levels between 80 and 130 mg/dL, and a history of milder clinical manifestations.
Clinical features, including the duration of DM, the presence of microvascular complications, the specific oral hypoglycemic medications taken, and FBG levels over the previous two months, were extracted from the patient's medical records using checklists. The average blood glucose level was calculated based on FBG measurements from diabetic patients for at least three months, which included the most recent reading.
After a rest period of over five minutes, the participants underwent blood pressure (BP) assessment on the upper arm of the left hand at heart level using a sphygmomanometer and stethoscope. This process was carried out twice simultaneously with a two-minute interval, and the results were recorded in millimeters of mercury (mmHg). If the BP difference exceeded 5 mmHg, the measurement was repeated. Additionally, height (measured to the nearest 0.1 cm without shoes); and weight (measured to the nearest 0.1 kg without shoes and wearing light clothing) were determined using a stadiometer and weighing scale, respectively. Subsequently, the body mass index (BMI) was calculated by dividing the weight in kg by the height in meters squared.
The waist circumference (WC) was measured using a non-stretched tape at the midpoint between the least palpable inferior margin of the rib and the iliac crest, while the participant was in a normal exhaled state. Similarly, hip circumference (HC) was measured using the same tape around the widest part of the buttocks. The waist-to-hip ratio (WHR) was calculated by dividing the WC in centimeters by the HC. All anthropometric measurements followed the protocol established by the WHO and were conducted by clinical nurses. Both anthropometric and BP measurements were taken twice, and the average values were used for analysis.
After completing an interview, reviewing records, and measuring anthropometric and BP values by a clinical nurse, study participants were directed to a central laboratory. The vein in the antecubital fossa of the forearm was sterilized with 70% alcohol solution. A tourniquet was applied as needed, and 5 mL of venous blood was collected from each patient diagnosed with T2DM using the vacutainer method. Trained laboratory technologists divided the blood sample into two tubes. Blood (3 mL) was placed in a K2-EDTA test tube for platelet parameters and a blood smear, while 2 mL was collected in a fluoride test tube for glucose analysis.
A serum separator tube was used to collect a blood sample, which was left at room temperature for 15 min, then centrifuged at 3600 rpm for 3 min using a Sorvall-ST-16 centrifuge to separate the serum for glucose level determination. Following the same procedure, 3 mL of venous blood was collected from each participant in the control group in a K2-EDTA test tube for PLT parameters, blood smears, and the CRP Ab test, which assesses inflammatory status. The CRP test was used as a criterion for inclusion or exclusion providing a positive or negative result through latex agglutination.
The CobasC311 system from Roche, Germany, was used to analyze FBG levels. The serum sample from a serum separator test tube was underwent photometric analysis to detect glucose level spectrophotometrically at a wavelength of 340 nm[36]. The Sysmex XN550, an automated hematological analyzer developed by Sysmex in Germany, utilized hydro-dynamically focused impedance measurement for red blood cells and PLT, a flow cytometer method for white blood cell differential count, and photometric principle for hemoglobin determination[37].
A thin blood film was created, marked, dried, and stained with Wright stain, to verify PLT parameters from the hematology machine and assess morphological arrangement, as well as screen for malaria. FBG levels, PLT, and platelet indices [MPV, platelet distribution width (PDW), plateletcrit (PCT), and platelet-large cell ratio] were examined at the central laboratory of Bishoftu General Hospital. The outcome for each study participant was printed, attached to the request paper, and recorded using the laboratory result registration format.
To ensure the accuracy and reliability of the data, questionnaires and informed consent forms originally written in English were translated into the local languages of Afaan Oromo and Amharic. These translations were then back-translated into English to ensure consistency and precision. Prior to the actual data collection process, a pretest was conducted on a small portion of the total sample size, specifically 5% (consisting of 13 subjects who met the eligibility criteria), at Mojo General Hospital in East Shewa, Mojo town. To minimize potential technical or observation bias, a half-day training session was provided to the four data collectors involved in the study, including two clinical nurses and two laboratory technologists. The training covered the study objectives, data collection procedures, and the importance of maintaining confidentiality when handling the collected information.
Throughout the study period, the quality of sociodemographic, anthropometric, and clinical data was ensured through daily checks for completeness and consistency by on-site supervision of data collectors. To uphold confidentiality, a code was utilized to safeguard the test results of study participants, with records securely stored in an undisclosed location. Feedback and necessary corrections were provided throughout the daily data collection process.
The laboratory ensured data integrity by following manufacturer guidelines and adhering to standard operating procedures during specimen collection, analysis of complete blood count, FBG determination, and blood film preparation and examination. To prevent hemolysis after collection, the blood sample was carefully dispensed into a K2-EDTA test tube, and gently mixed by inverting it 8-10 times. PLTs and indices were subsequently calculated, and a Wright-stained thin blood film was examined under a microscope to evaluate PLTs falling below, above, or within the reference range.
Samples collected were evaluated to determine compliance with specific acceptance criteria, such as the absence of hemolysis, absence of clotting, and sufficient sample quantity. To avoid confusion, both the container holding the sample and the corresponding request form were marked with identical identification codes following the collection process. The standard operating procedure of the hospital laboratory mandated the utilization of control materials with varying levels (low, normal, and high) for the hematology analyzer, as well as normal and pathological control materials for the Cobasc311 analyzer during glucose level measurements. Routine background measurements were conducted daily to reduce the risk of background carryover effects, and the expiration dates of reagents were confirmed before analyzing patient samples. All laboratory tests were carried out within a 2-h window from sample collection, with the outcomes documented, reported, and samples managed appropriately.
The collected data underwent a thorough verification process to ensure completeness and consistency. Subsequently, the verified data were entered into Epi-Data version 3.1, software developed by the Epi-Data Association in Denmark, for further analysis. For the analysis, the Statistical Package for Social Sciences (SPSS) software version 25, developed by IBM SPSS Statistics in the United States, was utilized. Histograms and the Kolmogorov-Smirnov test were employed to assesses the normality of the data distribution. The results of categorical variables were presented in terms of frequency and percentage. The χ2 test was employed to determine any statistical differences among these categorical variables.
The goodness-of-fit model test revealed that the continuous parameters displayed a non-normal distribution. Consequently, continuous parameters were presented in the form of median [interquartile range (IQR)]. The Mann-Whitney U test and Kruskal-Wallis analyses were employed to compare platelet indices between different groups. Bonferroni's test was utilized as a post-hoc analysis to further assess platelet indices between different groups. Bivariate Spearman's rank correlation coefficients were used to assess the correlations between platelet indices and independent variables, such as anthropometrics and clinical variables. Receiver operating characteristic (ROC) curves were constructed to determine the sensitivity, specificity, cutoff value, area under the curve, positive predictive values (PPV), and negative predictive values (NPV) for distinguishing poor glucoregulation from good glycemic control in diabetic patients. A P value of less than 0.05 was considered statistically significant.
The sociodemographic data indicated that there were no significant variations in age and gender among the cohorts of individuals with type 2 diabetes and those without the condition (P > 0.05). The study primarily included male participants, with 139 (79.9%) diagnosed with type 2 diabetes and 66 (75.9%) classified as healthy controls (Table 1).
| Variables | Categories | T2DM patients | Healthy controls | P value |
| Age (years) | Median (IQR) | 33 (28-39) | 31 (27-38) | 0.322 |
| Sex | M | 139 (79.9) | 66 (75.9) | 0.46 |
| F | 35 (20.1) | 21 (24.1) | ||
| Residence | Urban | 147 (84.5) | 68 (78.2) | 0.21 |
| Rural | 27 (15.5) | 19 (21.8) | ||
| Educational level | Unable to write & read | 8 (4.6) | 2 (2.3) | 0.04 |
| Can write and read | 11 (6.3) | 2 (2.3) | ||
| Primary school | 25 (14.4) | 9 (10.3) | ||
| Secondary school | 66 (37.9) | 50 (57.5) | ||
| Higher education | 64 (36.8) | 24 (27.6) | ||
| Occupation status | Unemployed | 37 (21.3) | 37 (42.5) | 0.004 |
| Merchant | 47 (27.0) | 20 (23) | ||
| Farmer | 25 (14.4) | 7 (8) | ||
| Gov’t employee | 65 (37.4) | 23 (26.4) | ||
| Marital status | Single | 59 (33.9) | 33 (37.9) | 0.94 |
| Married | 75 (43.1) | 35 (40.2) | ||
| Divorced | 29 (16.7) | 14 (16.1) | ||
| Widowed | 11 (6.3) | 5 (5.7) |
Substantial differences across the groups in terms of BMI, WHR, systolic BP (SBP), and diastolic BP (DBP) at a significance threshold of P < 0.001 were observed, whereas for WC the significance threshold was P = 0.017. Among individuals with type 2 diabetes and poorly managed glycemic levels, around 45 (51.7%) had experienced at least one type of mic-rovascular complication (Table 2).
| Variables | Categories | T2DM patients | Healthy control | P value | |
| BMI (kg/m2) | Median (IQR) | 23.2 (20.6-25.3) | 20.3(19.1-22.03) | < 0.001 | |
| WC (cm) | Median (IQR) | 90 (87-97) | 89 (86-92) | 0.017 | |
| WHR | Median (IQR) | 0.91 (0.86-0.98) | 0.87 (0.83-0.92) | < 0.001 | |
| SBP (mmHg) | Median (IQR) | 141 (133-150) | 131 (126-135) | < 0.001 | |
| DBP (mmHg) | Median (IQR) | 101 (94.0-109.0) | 90 (87.0-92.0) | < 0.001 | |
| FBG level (mg/dL) | Median (IQR) | 131.2 (114.7-150) | - | - | |
| Duration of DM illness (years) | Median (IQR) | 6.0 (3.0-10.0) | - | - | |
| Presence of microvascular complications at least one type | Poor glycemic DM (87) | Yes | 45 (51.7) | - | - |
| No | 42 (48.3) | ||||
| Good glycemic DM (87) | Yes | 0 (0) | - | ||
| No | 87 (100) | ||||
| Current oral hypoglycemic therapy used by DM patients | Glibenclamide | 41 (23.60) | - | - | |
| Metformin | 89 (51.1) | - | |||
| Metformin + Glibenclamide | 44 (25.3) | - | |||
Morphological analysis report: Microscopic examination of the peripheral blood film showed that approximately 102 (58.6%) T2DM patients had giant platelets, while 61 (35.1%) T2DM patients had large platelets.
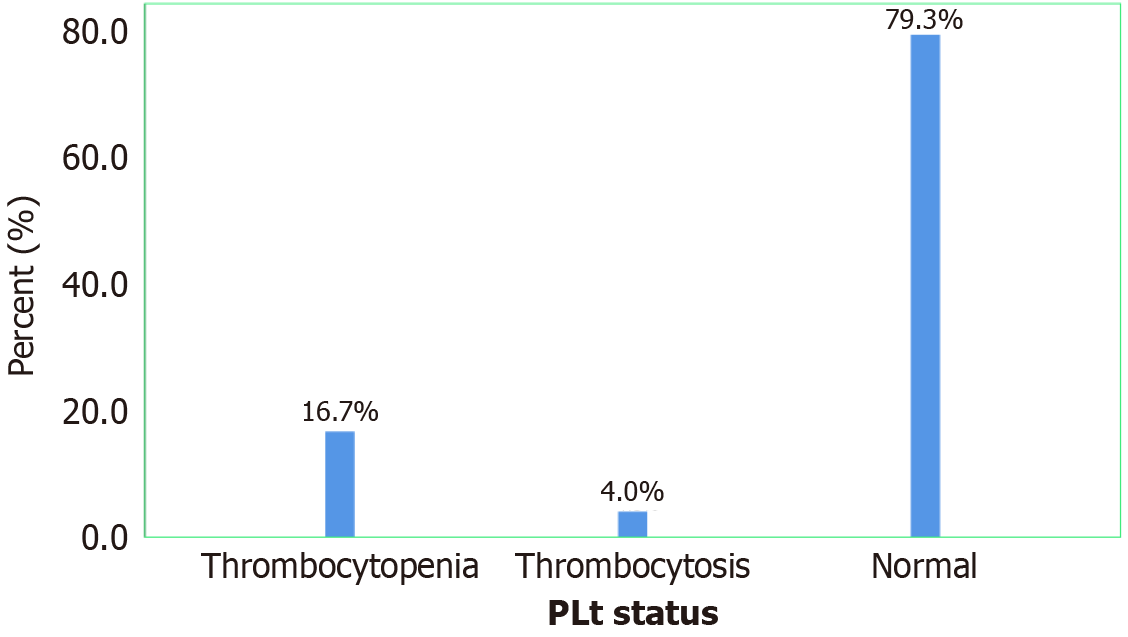
Frequency of quantitative platelet abnormalities among diabetic patients: According to this study, twenty-nine of our study participants experienced thrombocytopenia, accounting for 16.7%, while seven of our study participants had thrombocytosis, accounting for 4.0% (Figure 1).
Comparisons of platelet indices between diabetic patients and healthy controls: Diabetic patients exhibited significantly higher values of PDW, MPV, and platelet large cell ratio (PLCR) compared to healthy controls (P < 0.001). Additionally, the PCT showed a significant difference between the two groups (P = 0.001; Table 3).
| Platelet parameters | Diabetes mellitus group, media (IQR) | Healthy control group, media (IQR) | P value |
| Platelet count (103/µL) | 198 (160-291) | 208 (170-232) | 0.269 |
| PDW (fL) | 13.7 (11.8-20.1) | 11.1 (10.1-13.1) | < 0.001 |
| MPV (fL) | 11.1 (10.3-13.2) | 9.9 (9.6-10.9) | < 0.001 |
| PLCR (%) | 33.2 (27.3-46.2) | 24.5 (21.2-31.8) | < 0.001 |
| PCT (%) | 0.24 (0.2-0.32) | 0.23 (0.19-0.26) | 0.001 |
The platelet indices were compared among individuals with poorly controlled T2DM, well-controlled T2DM, and healthy controls using the Kruskal-Wallis test and subsequent post hoc analysis: Except for PLT count, all values of platelet indices were significantly different between the three groups (P < 0.001; Table 4).
| Platelet indices | Poorly controlled T2DM median (IQR) | Good controlled T2DM median (IQR) | Healthy controls median (IQR) | Kruskal Wallis test | Post hoc test (Bonferroni test) | ||
| P value | aP value | bP value | cP value | ||||
| PLT count (103/µL) | 188 (159-350) | 210 (162-251) | 208 (170-232) | 0.512 | - | - | - |
| PDW (fL) | 19.1 (13.3-24.2) | 12.4 (11.6-13.7) | 11.1 (10.1-13.1) | < 0.001 | 0.009 | < 0.001 | < 0.001 |
| MPV (fL) | 12.8 (10.8-14.8) | 10.7 (10-11.2) | 9.9 (9.6-10.9) | < 0.001 | 0.01 | < 0.001 | < 0.001 |
| PLCR (%) | 44.4 (31.6-55.2) | 30 (25.8-34) | 24.5 (21.2-31.8) | < 0.001 | 0.009 | < 0.001 | < 0.001 |
| PCT (%) | 0.27 (0.22-0.38) | 0.23 (0.2-0.28) | 0.23 (0.19-0.26) | < 0.001 | 1.000 | 0.001 | < 0.001 |
Comparisons of platelet indices across microvascular complicated and uncomplicated T2DM: Type 2 diabetic individuals with microvascular complications exhibited notably higher median (IQR) levels of PDW, MPV, and PLCR compared to type 2 diabetic patients without complications (P < 0.001; Table 5).
| Platelet indices | Complicated diabetic patients, median (IQR) (n = 45) | Uncomplicated diabetic patients, median (IQR) (n = 42) | P value |
| Platelet count (103/µL) | 169 (142-187) | 334 (193-422) | < 0.001 |
| PDW (fL) | 23.1 (19.1-24.8) | 14.7 (12-18.1) | < 0.001 |
| MPV (fL) | 13.9 (12.9-15.1) | 11.4 (10.1-12.7) | < 0.001 |
| PLCR (%) | 52.8 (47.0-57.0) | 33.3 (26.2-42.8) | < 0.001 |
| PCT (%) | 0.24 (0.20-0.28) | 0.38 (0.27-0.42) | < 0.001 |
Correlational analysis of the platelet indices with BMI, WC, and WHR among study participants: In type 2 diabetic patients, PDW (P = 0.033), PLCR (P = 0.018), and PCT (P = 0.009) values were positively correlated with BMI. On the other hand, in T2DM, the PLT count (P = 0.011) was positively correlated with WHR, and the PCT value was also significantly positively correlated with WC (P = 0.006) and WHR (P < 0.001; Table 6).
| PLT indices | T2DM patient group | Healthy control group | ||||
| BMI rho (P) | WC rho (P) | WHR rho (P) | BMI rho (P) | WC rho (P) | WHR rho (P) | |
| PLT | 0.092 (0.227) | 0.117 (0.124) | 0.192 (0.011)a | -0.209 (0.052) | 0.079 (0.467) | 0.075 (0.49) |
| PDW | 0.162 (0.033)a | 0.063 (0.408) | 0.072 (0.348) | 0.126 (0.246) | -0.117 (0.282) | -0.129 (0.234) |
| MPV | 0.132 (0.081) | 0.058 (0.445) | 0.033 (0.667) | 0.142 (0.188) | -0.043 (0.692) | -0.049 (0.652) |
| PLCR | 0.179 (0.018)a | 0.044 (0.568) | 0.016 (0.833) | 0.135 (0.212) | -0.082 (0.448) | -0.076 (0.483) |
| PCT | 0.199 (0.009)b | 0.208 (0.006)b | 0.298 (0.001)b | -0.088 (0.417) | 0.083 (0.444) | 0.159 (0.142) |
Correlational analysis of the platelet indices with clinical variables and BP among study participants: It was shown that PDW, MPV, and PLCR values had positive and significant correlations with SBP at P ≤ 0.001. On the other hand, PDW, MPV, PLCR and PCT showed a significant positive correlation with DBP. The data also showed that PDW, MPV, PLCR, and PCT were significantly positively correlated with FBS. Additionally, PDW, MPV, and PLCR showed a significant positive correlation with DM duration (P < 0.001) in type 2 diabetic patients (Table 7).
| Platelet indices | Diabetic patient group | Healthy control group | ||||
| SBP rho (P) | DBP rho (P) | FBS rho (P) | Duration rho (P) | SBP rho (P) | DBP rho (P) | |
| PLT | 07 (0.358) | 0.067 (0.378) | 0.048 (0.529) | 0.133 (0.137) | -0.024 (0.826) | -0.071 (0.511) |
| PDW | 0.416 (0.001)b | 0.44 (0.001)b | 0.525 (0.001)b | 0.351 (0.001)b | -0.12 (0.27) | 0.031 (0.779) |
| MPV | 0.355 (0.001)b | 0.398 (0.001)b | 0.474 (0.001)b | 0.298 (0.001)b | -0.061 (0.575) | -0.005 (0.964) |
| PLCR | 0.421 (0.001)b | 0.394 (0.001)a | 0.509 (0.001)b | 0.372 (0.001)b | -0.059 (0.586) | -0.005 (0.965) |
| PCT | 0.12 (0.113) | 0.206 (0.006)b | 0.164 (0.03)a | 0.078 (0.304) | -0.036 (0.738) | 0.139 (0.198) |
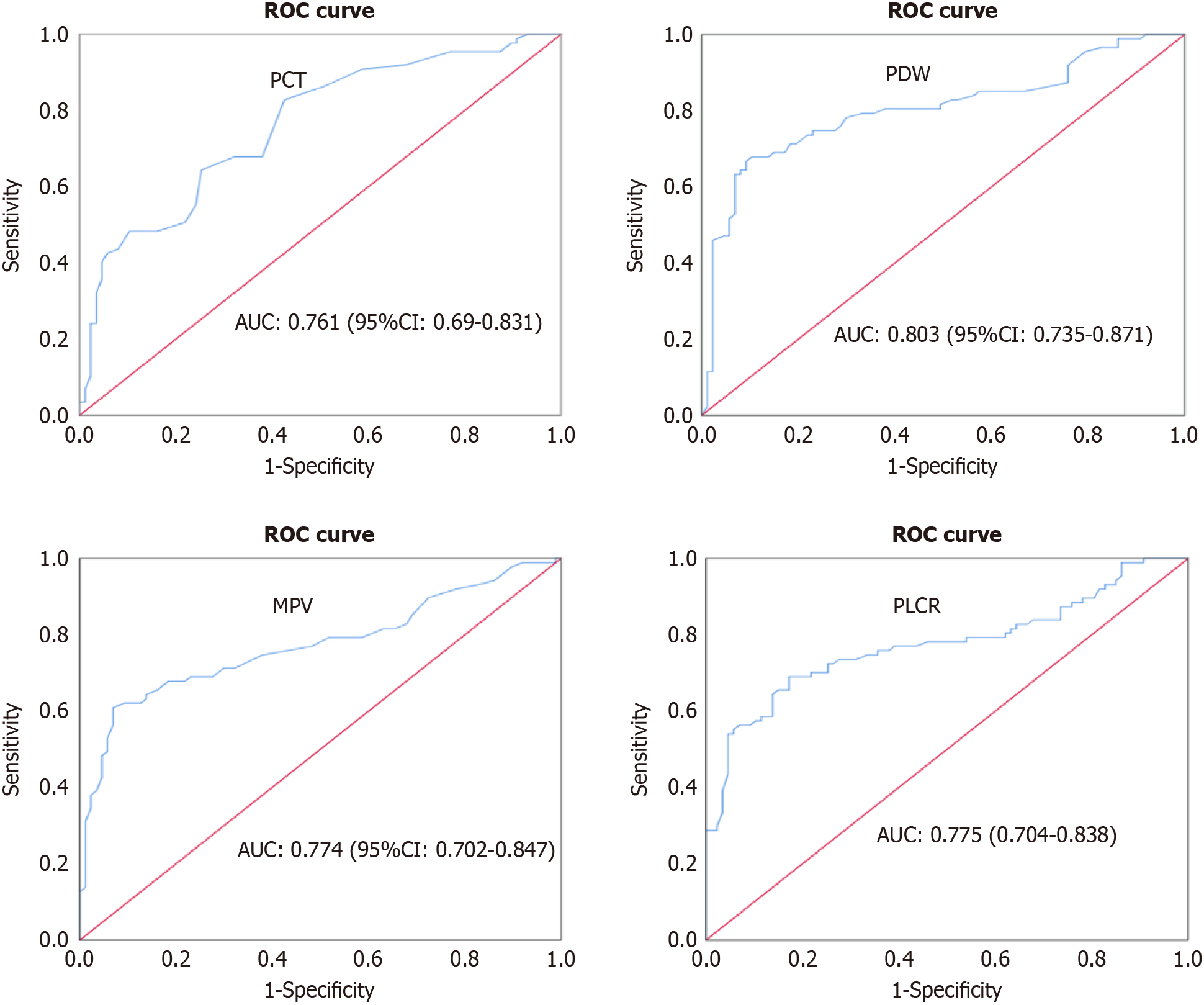
ROC analysis was employed to ascertain the predictive values of platelet indices in assessing inadequate glucoregulation in individuals with diabetes: A ROC analysis was conducted to assess the predictive capability of platelet indices in identifying poor glycemic control among diabetic patients. By employing various cut-off values, the sensitivity, specificity, PPV, and NPV were determined, along with the AUC of the platelet indices. These findings establish platelet indices as a valuable tool for predicting poor glycemic control in diabetic patients (Table 8, Figure 2).
| Platelet indices | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off value | AUC | 95%CI | P value |
| PDW (fL) | 68 | 90 | 87.2 | 73.8 | ≥ 15.75 | 0.803 | 0.735-0.871 | < 0.001 |
| MPV (fL) | 61 | 93 | 89.7 | 70.5 | ≥ 12.25 | 0.774 | 0.702-0.847 | < 0.001 |
| PLCR (%) | 69 | 83 | 80.2 | 72.3 | ≥ 36.3 | 0.775 | 0.704-0.838 | < 0.001 |
| PCT (%) | 83 | 57 | 65.9 | 87.7 | ≥ 0.24 | 0.761 | 0.69-0.831 | < 0.001 |
The results of the current study revealed elevated PCT levels in diabetic patients compared to the control group. PCT is a parameter calculated from PLT and MPV, and is considered a guide to prevent vascular complications due to glycemic dysregulation. Platelets play a central role in the inflammatory process, but, PCT plays a better role than PLT in assessing the inflammatory status in diabetic patients. A high PCT value is indicative of inflammatory status in diabetic patients[8,38]. Our findings were consistent with studies conducted in India[25] and Brazil[22].
However, our findings contrasted with those in a study conducted in Egypt[24]. The discrepancy might be due to our study’s result of relatively extreme increases in MPV values in cases compared to the control group. As PCT value is determined by analyzer calculation using the formula (PCT = PLT × MPV/10000), an increase in MPV without a significant difference in PLT between the case and control groups may result in an increment of PCT in the case group compared to the control group.
Furthermore, the present investigation revealed that diabetic individuals with inadequate glycemic control exhibited elevated PCT levels compared to those with well-managed diabetes and healthy individuals. Comparable findings were observed in studies conducted in India[23,25]. According to the findings of this study, PCT was identified as a discriminator between poorly glycemic-controlled type 2 diabetes and well glycemic-controlled type 2 diabetes. Based on its AUC value, it is a good predictor of poor glucoregulation in type 2 diabetic patients[39]. To the best of our knowledge, we could not find any studies that determined the predictive ability of PCT for poor glucoregulation which limited us to comparing and contrasting our findings.
In our study, PDW was found to be higher in individuals with type 2 diabetes compared to healthy controls. This increase in PDW may be attributed to in vivo activation of protein kinase C and non-enzymatic glycation of platelet surface proteins, leading to decreased platelet membrane fluidity and increased activation[18]. In patients with type 2 diabetes, activated platelets differ in size from inactive platelets, forming pseudopodia structures that cause shape changes from biconcave discs to spheres, ultimately affecting PDW. This results in an increased base of the histogram plot of platelet distribution[22].
Studies conducted in Egypt[24], Turkey[29], India[30], and Brazil[22], have reported similar findings to those in our study. Additionally, PDW levels were significantly higher in diabetic individuals with poor glycemic control compared to those with satisfactory control and individuals without diabetes. This consistent pattern was also observed in studies from India[23,25,26,30], Greece[28], Brazil[22], and Egypt[24].
In our study, the results of ROC curve analysis showed that PDW could distinguish between poor glycemic control status and good control in T2DM. This indicates that PDW is an excellent predictor of poor glycemic control in diabetic patients. Our study results showed a slightly higher cut-off value, as well as lower sensitivity, PPV, and specificity values of PDW compared to a study conducted in India[30]. A study conducted in Bangladesh[31] reported lower values for all parameters of the ROC curve output compared to ours.
The current study found that diabetic patients had increased MPV values compared to nondiabetic controls. The background reason for platelet activation in DM is, that chronic hyperglycemia can lead to nonenzymatic glycation of platelet surface proteins. Elevated blood glucose and some glucose metabolites also cause platelet osmotic swelling, increasing platelet reactivity and shortening platelet lifespan, reflecting the recruitment of young, large platelets[26]. Reports from the studies conducted in Egypt[24] and Brazil[22] showed consistent results to ours. However, studies conducted in Ethiopia[40], Iraq[41], and Sudan[42], reached different conclusions. A possible reason for this discrepancy might be; that in our study, the majority of diabetic patients did not utilize antiplatelet medications such as Clopidogrel and Vasoprin during their follow-up. Clopidogrel’s mechanism involves the irreversible restriction of the P2Y12 ADP receptor subtype on the platelet cell membrane, effectively hindering the initiation and cross-linking of platelets by fibrin[43]. Activated platelets exhibit an increased size, and the anticipation of platelet activation could potentially hinder the surge in average platelet size and MPV. However, we cannot hypothesize that clopidogrel is the only explanation for this effect.
Furthermore, the findings of this investigation revealed a significant increase in MPV among individuals diagnosed with diabetes and exhibiting suboptimal glycemic control, compared to those with well-managed glycemic levels and non-diabetic individuals. This finding was consistent with studies conducted in India[23,25], Egypt[24], Brazil[22], and Greece[28]. However, a report from a study in Turkey[44], contradicts our findings. The discrepancy between our results, and the study from Turkey may be due to our study participants not strictly following the prescribe diet and medication regimen. When diabetes patients carefully maintained optimal glycemic control by adhering to the recommended diet and medication plan provided by health care providers, the MPV significantly decreased at the three-month follow-up as compared to the baseline value of MPV[45].
The current study found that MPV is a good predictor of poor glycemic control in type 2 diabetes. Our study revealed that MPV had elevated cut-off, specificity, and PPV values compared to a study conducted in India[30], and a study conducted in Bangladesh showed a lower ROC curve output than ours[31]. On the other hand, a study conducted in Bosnia and Herzegovina[32], showed a lower cut-off value, specificity, higher sensitivity values, and AUC value comparable to ours.
The results of this study indicate that PLCR was significantly higher in diabetic patients compared to controls. A possible explanation for the higher PLCR in diabetic patients is that when platelets are activated in DM, there is a release of the largest platelet fraction which is typically measured as PLCR. PLCR indirectly correlates with platelet counts, and directly with MPV and PDW, but is more sensitive to increased platelet size[8].
Our findings are similar to those in studies conducted in Brazil[22], India[23], and Egypt[24]. Conversely, a study conducted in Ethiopia[40] reported different conclusions. This discrepancy may be due to the inclusion of newly diagnosed diabetic patients in the previous study. In newly diagnosed diabetes, inflammation and hyperglycemia are typically at near-normal levels, which do not result in changes in platelet activation or an increase in platelet indices. Additionally, a significant increase in PLCR was observed among diabetic individuals with inadequate glycemic control compared to those with well-managed glycemic levels and healthy individuals. Similar results have been reported in studies conducted in India[23,26].
In our study, PLCR was identified as a predictor of poor glycemic control in diabetic patients. Following the general rule of thumb for interpreting AUC to evaluate the diagnostic ability of a test in discriminating the real aliment status of a patient, PLCR was shown to be a good parameter for predicting poor glycemic control based on its AUC value[39]. The PLCR is not often quoted in the literature, probably because it is a relatively new PLT index, mostly generated by a few machines, including Sysmex analyzer generations[27].
In our study, PDW, MPV, and PLCR were significantly elevated in complicated T2DM compared to uncomplicated T2DM. Our study results were consistent with studies conducted in Brazil[23], India[24], and Belgium[27]. DM is a multi-systemic disease that affects the eyes, kidneys, and peripheral nerves leading to micro- and macroangiopathy through chronic hyperglycemia, which enhances the alteration of platelet indices in diabetic patients with complications[8]. A study from Pakistan[46] reached a conflicting conclusion compared to our findings, possibly due to differences in the onset of complications in the current and previous study populations, as complications can exacerbate platelet activation over time.
Regarding the correlation between platelet indices and anthropometric measurements, the results of the current study showed significant positive correlations between PDW, PLCR, and PCT with BMI, PLT counts with WHR, and PCT with WC and WHR showing a significant positive correlation. A possible explanation for the correlation between platelet indices and anthropometric measurements, is that studies have shown that BMI, WC, and WHR are indicators of metabolic disorders such as obesity. Obesity is characterized by an abnormal accumulation of adipose tissue caused due to metabolic disorders such as dyslipidemia, hyperglycemia, and hypertension. These disorders result in changes in various cytokines that promote platelet activation and alteration of platelet indices[47,48].
Contrary to the findings of this study, a study conducted in Ethiopia[33] found that PDW, MPV, and PLT were negatively correlated with BMI and WHR (P > 0.05). This inconsistency may be due to variations in procedural measurements of BMI, WC, and WHR between study populations and differences in the study period. Differences in the study period could be considered as the reason for this discrepancy as seasonal variations can result in differences in BMI, WC, and WHR values due to the seasonal effects on body physiology.
In our findings, the Spearman correlation regarding BP showed that PDW, MPV, and PLCR were significantly positively correlated with SBP. While PDW, MPV, PLCR, and PCT showed a significant positive correlation with DBP. The possible reason for this correlation is that hyperglycemia in DM causes larger platelets to be released into circulation. Increased formation of reticulated platelets leads to complications of atherothrombosis in diabetic patients[10,11]. These factors contribute to increased vasoconstriction variability and changes in blood vessel properties, ultimately leading to platelet activation and changes in PLT indices. Consistent with our finding, a study conducted in Turkey[7] also found that PDW was significantly and positively correlated with DBP, and a study conducted in Ethiopia[34] found that PLT count and MPV were positively correlated with SBP.
Our study results showed a significant positive correlation between PDW, MPV, PLCR, PCT, and FBG levels. This finding supports evidence for a strong relationship between glycemic status and platelet hyper-activation suggesting a possible etiology of vascular complications in diabetes[18,19]. Our results align with a study conducted in India which showed that FBG levels were positively correlated with PDW, MPV, and PLCR, while PLT count and PCT did not correlate with FBG[26]. Another study from India[30] found that PDW was positively correlated with FBG. A study conducted in Brazil[22], also showed positive correlations between MPV, PDW, PCT, and FBG, and a study from Ethiopia[33] showed a positive correlation between MPV and FBG. The negative correlation obtained in the study carried out in India[26] may be due to significantly decreased values of the PLT count that cannot compensate for the MPV increment resulting in lower PCT.
In our study, PDW, MPV, and PLCR were significantly positively correlated with duration of DM disease. This correlation may be explained by the fact that vascular complications increase with longer disease duration, leading to endothelial dysfunction and increased platelet activation in adult patients with type 2 diabetes[49]. Similar findings were reported in studies conducted in India[23] where it was found that PDW and MPV were significantly and positively correlated with duration of DM disease, and a study conducted in Ethiopia[34] found that PLT and MPV were significantly and positively associated with duration of DM disease. Another study from Ethiopia[40] reported that PLT correlated with diabetes duration.
This research is a comprehensive investigation aimed at evaluating a variety of platelet indices in various scenarios involving individuals with type 2 diabetes. The results of this study may offer valuable insights into the role of platelet indices in predicting poor blood sugar among diabetic patients. Morphological analysis was also conducted to characterize the platelets and assess the hematological automation output of PLT as well as the arrangement of platelets.
The research utilized a cross-sectional study design, which limits the ability to establish causal relationships. Evaluation of the coagulation profile and immature platelet fraction was not conducted. Instead, serum glucose levels were used in place of HgbA1c due to limitations associated with the HgbA1c test at the hospital.
In summary, our investigation revealed notable increases in PDW, MPV, PLCR, and PCT levels among individuals with diabetes compared to the control group. Additionally, we observed significantly higher PDW, MPV, PLCR, and PCT values in T2DM patients with poor glycemic control compared to those with well-controlled T2DM and healthy controls. PDW, MPV, and PLCR were significantly raised in complicated T2DM patients compared to uncomplicated T2DM. At various levels, PLT, PDW, MPV, PLCR, and PCT were significantly correlated with anthropometric measurements. Furthermore, PDW, MPV, PLCR, and PCT showed strong and significant correlations with FBG, BP, and duration of DM illness. These factors have been identified as potential predictors of poor glucoregulation in diabetic patients. Therefore, paying close attention to abnormalities in platelet indices during follow-up and monitoring of DM can help mitigate diabetes-related disorders. It may be beneficial to use PLT indices in combination to diagnose DM, as they complement each other’s limitations and could provide a more accurate diagnosis.
All authors would like to thank Jimma University, Bishoftu General Hospital staff members, all data collectors, supervisors, study participants, and questionnaire translators for their assistance in conducting this study.
| 1. | World Health Organization. Classification of Diabetes Mellitus. Geneva: World Health Organization, 2019: 4-28. |
| 2. | American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 1964] [Article Influence: 327.3] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Diabetes key facts Overview. Geneva: World Health Organization, 2021: 1-5. |
| 4. | International Diabetes Federation. IDF Diabetes Atlas, 10th ed. Brussels, Belgium: International Diabetes Federation, 2021: 9-65. |
| 5. | Zeru MA, Tesfa E, Mitiku AA, Seyoum A, Bokoro TA. Prevalence and risk factors of type-2 diabetes mellitus in Ethiopia: systematic review and meta-analysis. Sci Rep. 2021;11:21733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 7. | Kizilgul M, Sencar E, Ucan B, Beysel S, Ozcelik O, Ozbek M, Cakal E. Components of the Complete Blood Count in Type 2 Diabetes Mellitus with Inadequate Glycemic Control. Dicle Med J. 2018;45:113-120. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Yilmaz T, Yilmaz A. Relationship between Altered Platelet Morphological Parameters and Retinopathy in Patients with Type 2 Diabetes Mellitus. J Ophthalmol. 2016;2016:9213623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Kraakman MJ, Lee MK, Al-Sharea A, Dragoljevic D, Barrett TJ, Montenont E, Basu D, Heywood S, Kammoun HL, Flynn M, Whillas A, Hanssen NM, Febbraio MA, Westein E, Fisher EA, Chin-Dusting J, Cooper ME, Berger JS, Goldberg IJ, Nagareddy PR, Murphy AJ. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest. 2017;127:2133-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 11. | Suslova TE, Sitozhevskii AV, Ogurkova ON, Kravchenko ES, Kologrivova IV, Anfinogenova Y, Karpov RS. Platelet hemostasis in patients with metabolic syndrome and type 2 diabetes mellitus: cGMP- and NO-dependent mechanisms in the insulin-mediated platelet aggregation. Front Physiol. 2014;5:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 496] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Ling W, Huang Y, Huang YM, Fan RR, Sui Y, Zhao HL. Global trend of diabetes mortality attributed to vascular complications, 2000-2016. Cardiovasc Diabetol. 2020;19:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Papazafiropoulou A, Papanas N, Pappas S, Maltezos E, Mikhailidis DP. Effects of oral hypoglycemic agents on platelet function. J Diabetes Complications. 2015;29:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Hussein HA, Hussein FEA, Babikir DM, Samaan MA. The Effect of Metformin and Glimepiride on Platelet Count and Indices Among Diabetic Patients Were Attending Jaber Abu Aliz Diabetic Center in Khartoum State. J Biomed Pharm Res. 2021;10:93-98. [DOI] [Full Text] |
| 16. | Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103:20A-26A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Vaidya AR, Wolska N, Vara D, Mailer RK, Schröder K, Pula G. Diabetes and Thrombosis: A Central Role for Vascular Oxidative Stress. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Li X, Weber NC, Cohn DM, Hollmann MW, DeVries JH, Hermanides J, Preckel B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Randriamboavonjy V, Fleming I. Insulin, insulin resistance, and platelet signaling in diabetes. Diabetes Care. 2009;32:528-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Matsuno H, Tokuda H, Ishisaki A, Zhou Y, Kitajima Y, Kozawa O. P2Y12 receptors play a significant role in the development of platelet microaggregation in patients with diabetes. J Clin Endocrinol Metab. 2005;90:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Alhadas K, Santos S, Freitas M, Viana SA, Ribeiro L, Costa M. Are platelet indices useful in the evaluation of type 2 diabetic patients? J Bras Patol Med Lab. 2016;52:96-102. |
| 23. | Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, Singh S. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | DerisBesadaa MS, Elsayeda AA, Alia AE, Hashem AA. Platelet indicies as an indicators of diabetic nephropathy. SVU Int J Med Sci. 2020;4:41-48. [DOI] [Full Text] |
| 25. | Mukta P, Himani G, Charu A, Varsha C, Kanika S, Shveta L. Can they serve as biomarkers of glycemic control or development of complications in evaluation of type 2 diabetes mellitus? Iraqi J Hematol. 2018;7:72-78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Rajagopal L, Arunachalam S, Abdullah SM, Ganesan V, Kathamuthu K, Ramraj B. Can Mean Platelet Volume and Platelet Distribution Width be used as Predictive Markers for Impending Diabetic Vascular Complications ? J Clin Diagnostic Res. 2018;12:1-5. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Dwivedi T, Davangeri R. Variation of Platelet Indices among Patients with Diabetes Mellitus Attending Tertiary Care Hospital. J Clin Diagnostic Res. 2018;12:EC22-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Dalamaga M, Karmaniolas K, Lekka A, Antonakos G, Thrasyvoulides A, Papadavid E, Spanos N, Dionyssiou-Asteriou A. Platelet markers correlate with glycemic indices in diabetic, but not diabetic-myelodysplastic patients with normal platelet count. Dis Markers. 2010;29:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Atak BM, Duman TT, Aktas G, Kocak MZ, Savli H. Platelet Distribution Width is Associated with Type 2 Diabetes Mellitus and Diabetic Nephropathy and Neuropathy. Natl J Heal Sci. 2018;3:95-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Joshi AA, Jaison J. Platelet Parameters- Mean Platelet Volume (MPV) and Platelet Distribution Width (PDW) in Type 2 Diabetes Mellitus. J Ann Pathol Lab Med. 2019;8:407-413. [DOI] [Full Text] |
| 31. | Jaman S, Rezwan S, Alam S, Islam R, Husna AU, Sayeed MA. Association of Mean Platelet Volume and Platelet Distribution Width with Hba1c. J Endocrinol Diabetes. 4:1-6. [DOI] [Full Text] |
| 32. | Kadić D, Hasić S, Spahić E. Mean platelet volume predicts the glycemic control deterioration in diabetes mellitus type 2 patients. Med Glas (Zenica). 2016;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Biadgo B, Melku M, Abebe SM, Abebe M. Hematological indices and their correlation with fasting blood glucose level and anthropometric measurements in type 2 diabetes mellitus patients in Gondar, Northwest Ethiopia. Diabetes Metab Syndr Obes. 2016;9:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Morgan C, Voorhis WV, Betsy L. Understanding Power and Rules of Thumb for Determining Sample Sizes. Tutor Quant Methods Psychol. 2007;3:43-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 783] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 35. | Ivanov TD, Ivanov M. American Diabetes Association. Standards of Medical Care in Diabetes - 2017. Kidneys. 2021;6:47-63. [DOI] [Full Text] |
| 36. | Little R. Laboratory Procedure Manual for Fasting Glucose by Roche Cobas C311. 2017. [cited 23 May 2024]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/GLU_I_MET_C311.pdf. |
| 37. | Europe GA. Sysmex XN-550 Complete Blood Count and Parameters Whole Blood-standard Operating Procedure. 2019. [cited 23 May 2024]. Available from: https://www.sysmexeurope.com/products/products-detail/xn-550/. |
| 38. | Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb). 2016;26:178-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Yang S, Berdine G. The receiver operating characteristic (ROC) curve. Southwest Respir Crit Care Chronicles. 2017;5:34-36. [DOI] [Full Text] |
| 40. | Adane T, Asrie F, Getaneh Z, Getawa S. White blood cells and platelet profiles of diabetic patients at University of Gondar specialized referral hospital: A comparative cross-sectional study. J Clin Lab Anal. 2021;35:e23808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Ali M, Hassan A. Assessment of the Alteration of Blood Indices in Patients with Type 2 Diabetic Mellitus : A Cross - Sectional Study. J Mustansiriya Med. 2019;18:24-29. [DOI] [Full Text] |
| 42. | Adam NKA, Abderahman NAM, Ahmed MAI, Eisa IMA, Wardi HM, Abdrabo AEA. Hematological Parameters in Sudanese Type-2 Diabetes Mellitus. SAR J Med Biochem. 2021;2:46-49. [DOI] [Full Text] |
| 43. | Maegdefessel L, Azuma J, Tsao PS. Modern role for clopidogrel in management of atrial fibrillation and stroke reduction. Vasc Health Risk Manag. 2010;6:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Ünübol M, Ayhan M, Güney E. The relationship between mean platelet volume with microalbuminuria and glycemic control in patients with type II diabetes mellitus. Platelets. 2012;23:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2019;23:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Jiskani SA, Singh D. Platelets Indices as Biomarkers of Glycemic Control and Progression of Complications in Patients of Diabetes Mellitus Type II. J Haematol Stem Cell Res. 2021;1:21-24. |
| 47. | World Health Organisation (WHO). Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation. Geneva: World Health Organization, 2008: 1-47. |
| 48. | Anfossi G, Russo I, Trovati M. Platelet dysfunction in central obesity. Nutr Metab Cardiovasc Dis. 2019;19:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, Marre M, Patel A, Poulter N, Williams B, Chalmers J; ADVANCE Collaborative group. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57:2465-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |