Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1853
Revised: May 11, 2024
Accepted: May 31, 2024
Published online: September 15, 2024
Processing time: 146 Days and 1.8 Hours
Inflammatory markers and mediators that affect the development of car-diovascular diseases have been the focus of recent scientific work. Thus, the purpose of this editorial is to promote a critical debate about the article titled “Nε-carboxymethyl-lysine and inflammatory cytokines, markers, and mediators of coronary artery disease progression in diabetes”, published in the World Journal of Diabetes in 2024. This work directs us to reflect on the role of advanced glycation end products, which are pro-inflammatory products arising from the metabolism of fatty acids and sugars whose main marker in tissues is Nε-carboxymethyl-lysine (NML). Recent studies have linked high levels of pro-inflammatory agents with the development of coronary artery disease (CAD), especially tumor necrosis factor alpha, interleukins, and C-reactive protein. These inflammatory agents increase the production of reactive oxygen species (ROS), of which people with diabetes are known to have an increased production. The increase in ROS promotes lipid peroxidation, which causes damage to myocytes, promoting myocardial damage. Furthermore, oxidative stress induces the binding of NML to its receptor RAGE, which in turn activates the nuclear factor-kB, and conse-quently, inflammatory cytokines. These inflammatory cytokines induce endo-thelial dysfunction, with increased expression of adhesion molecules, changes in endothelial permeability and changes in the expression of nitric oxide. In this sense, the therapeutic use of monoclonal antibodies (inflammatory reducers such as statins and sodium-glucose transport inhibitors) has demonstrated positive results in the regression of atherogenic plaques and consequently CAD. On the other hand, many studies have demonstrated a relationship between mito-chondrial dynamics, diabetes, and cardiovascular diseases. This link occurs since ROS have their origin in the imbalance in glucose metabolism that occurs in the mitochondrial matrix, and this imbalance can have its origin in inadequate diet as well as some pathologies. Photobiomodulation (PBM) has recently been considered a possible therapeutic agent for cardiovascular diseases due to its effects on mitochondrial dynamics and oxidative stress. In this sense, therapies such as PBM that act on pro-inflammatory mediators and mitochondrial modulation could benefit those with cardiovascular diseases.
Core Tip: Increased oxidative stress promoted by pro-inflammatory mediators such as Nε-carboxymethyl-lysine causes changes in mitochondrial dynamics and has been associated with insulin resistance and cardiovascular diseases. Therapies that promote the health of mitochondria by balancing the mechanisms of mitochondrial fusion and fission may be a path forward in the context of coronary artery disease.
- Citation: Tatmatsu-Rocha JC, Mendes-Costa LS. Inflammatory markers, oxidative stress, and mitochondrial dynamics: Repercussions on coronary artery disease in diabetes. World J Diabetes 2024; 15(9): 1853-1857
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1853.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1853
The accumulation of lipids and hypertension associated with hyperglycemia for decades has been the main risk factor for reducing cholesterol and developing coronary artery disease (CAD) in patients with diabetes mellitus (DM). However, pro-inflammatory agents are now considered the major orchestrators of CAD development and atherosclerotic implications[1]. Among these agents, Nε-(carboxymethyl) lysine (NML) stands out as a stimulant of macrophage uptake via receptors for advanced glycation end products (AGEs). AGEs result from the reaction between reducing sugars and the amino group of proteins, leading to dysfunctional glycated proteins[2,3], which play a significant role in diabetes pathophysiology. Additionally, NML is associated with atherogenic precursors by stimulating foam cell formation[4]. Another mechanism by which these pro-inflammatory mediators cause endothelial dysfunction is through decreased nitric oxide (NO) release, increased adhesion molecules, and increased permeability[5]. One of these pro-inflammatory protagonists is Pyrin Receptor 3 (NLRP3), which induces the release of pro-inflammatory cytokines. Moreover, the thioredoxin (TXNIP)-NLRP3 complex promotes pro-caspase-1 activation and apoptosis. In pathological situations, the myocardium increases reactive oxygen species (ROS) production, leading to increased K+ efflux and mitochondrial damage. Bisht et al[6] suggested that NLRP3 could be an interesting target in myocardial injury treatment. Recent studies have linked high levels of pro-inflammatory agents with CAD development, particularly tumor necrosis factor-alpha, interleukins, and C-reactive protein.
The hyperglycemic state increases oxidative stress, which in turn induces the dissociation of the thioredoxin complex that interacts with TXNIP[7]. Concomitantly, NO acts on certain adhesion and pro-inflammatory molecules, decreasing their release through an inhibition mechanism of nuclear factor-kappa B (NF-κB)[8]. NF-κB induces the transcription of pro-inflammatory cytokines and activates NLRP3, thereby increasing the inflammatory response[9]. In conditions where the inflammatory process is exacerbated (as in DM), there is an increase in the production of peroxynitrite anion and ROS that end up inhibiting the activities of NO[10]. In turn, increased ROS promotes the degradation of tetrahydrobiopterin, an important cofactor of NO synthesis. Therefore, there is a decrease in NO production, and endothelial dysfunction progresses, progressively affecting the muscle tone of the endothelium[10]. Some authors have argued that the therapeutic target in this clinical setting should be the pursuit of mitochondrial health[11,12], as mitochondria mediate the conversion of substrates into adenosine triphosphate (ATP), delivering energy to maintain cellular functions. Fur-thermore, they regulate signaling pathways in the cell and buffer intracellular calcium and apoptosis. These organelles have a significant capacity for self-regulation through cycles of fusion and fission, known as mitochondrial dynamics[12]. It is important to highlight that insulin resistance (IR) is associated with dysfunctional mitochondria, characterized by reduced bioenergetic responses to insulin stimulation and decreased mitochondrial biogenesis[13]. Changes in the transcription of mitochondrial genes, lipotoxicity, and glucotoxicity appear to be some of the mechanisms involved in IR[11]. Additionally, mitochondrial dysfunction promotes a reduction in energy expenditure, overproduction of ROS, as well as altered oxidation of fatty acids, thereby aggravating IR. The balance between mitochondrial fusion and fission is fundamental for cardiometabolic homeostasis. Mitofusins (MFN1) and (MFN2) are proteins that act in mitochondrial fusion, together with dynamin-related protein 1 (DRP1) and fission protein 1 (FIS1)[12,13]. Due to this crucial role of mitochondrial dynamics in metabolism, therapeutic approaches aimed at mitochondrial homeostasis could strongly impact the treatment of these pathologies. In this sense, some antihyperglycemic drugs have played a modulatory role in mitochondrial homeostasis, acting on the cardiovascular system by reducing DRP1 levels and increasing OPA1 and MFN2 protein levels in cardiomyocytes[14]. Among these drugs, we can highlight glucagon-like peptide 1 receptor agonists and sodium-glucose cotransporter 2 inhibitors (SGLT2i), which act on glycemic control and body weight[15]. Shao et al[16] demonstrated that SGLT2i increased the protein expression of OPA1, DRP1, and MFN1, as well as mitochondrial respiration, suggesting that SGLT2i could attenuate defects in mitochondrial function in diabetic cardiomyopathy by modulating mitochondrial dynamics[16]. According to these authors, several studies[17-19] have observed that SGLT2 inhibitors minimize the risks of non-fatal myocardial infarction and non-fatal death or stroke related to heart failure. Some theories about the possible mechanisms of cardioprotection by SGLT-2 inhibitors, including apoptosis, antioxidant effects, prevention of cardiac inflammation, mitochondrial dysfunction, and ionic homeostasis[20-22], suggest repolarization through the improvement of mitochondrial health and oxidative stress, as well as prolonged ventricular pressure suppression. Other authors, such as Lee et al[23], highlight a late Na+ and Na+/H+ exchange current and changes in Ca2+ regulation.
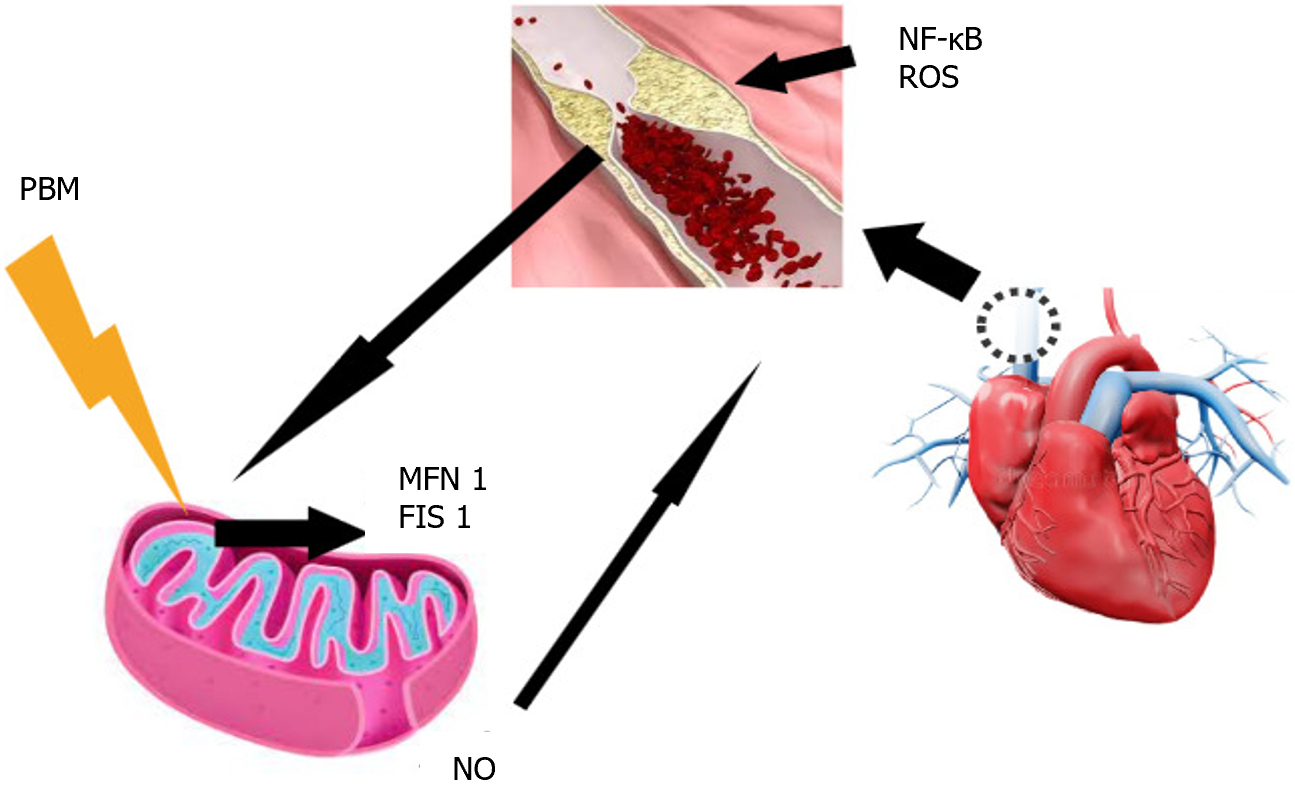
Photobiomodulation (PBM) is a therapy based on electromagnetic irradiation using a light emitter such as a laser or light-emitting diode[24]. The effects of this electromagnetic light irradiation primarily target the mitochondrial membrane, where it acts on cytochrome c oxidase of the respiratory chain, facilitating electron transport and resulting in increased ATP production and transmembrane proton gradient[25]. Previous studies have shown that PBM initially increases ROS production, followed by an adaptive decrease in oxidative stress[26,27], and that PBM acts on mitochondrial dynamics[21], modulating the expressions of MFN2 and FIS1 in diabetic-induced rat models[27]. Furthermore, it activates the redox-sensitive transcription factor NF-κB through brief generation of ROS. This modulation of NF-κB attenuates inflammatory responses in vascular smooth muscle cells, endothelial cells, and macrophages[28], as well as the production of inflammatory cytokines tumor necrosis factor-alpha and NO[29] (Figure 1).
The interaction between inflammatory mediators, oxidative stress, and mitochondrial dynamics substantially influences the pathogenesis of cardiovascular diseases, especially in the context of DM. Pro-inflammatory agents play a fundamental role in the development of CAD and atherosclerosis, contributing to endothelial dysfunction and subsequent cardiovascular complications. Mitochondrial dysfunction, exacerbated by hyperglycemia and IR, further intensifies oxidative stress and inflammation, leading to compromised cellular metabolism and impaired energy production. Therapeutic strategies targeting mitochondrial homeostasis, such as certain antidiabetic medications like SGLT2 inhibitors, have the potential to mitigate cardiovascular risks by modulating mitochondrial dynamics and oxidative stress. Additionally, PBM emerges as a potential therapeutic approach, exerting anti-inflammatory effects and influencing mitochondrial function. In light of this, innovative therapeutic interventions can be developed to effectively manage cardiovascular diseases associated with DM.
| 1. | Attiq A, Afzal S, Ahmad W, Kandeel M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. 2024;966:176338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 60] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 2. | Aragno M, Mastrocola R. Dietary Sugars and Endogenous Formation of Advanced Glycation Endproducts: Emerging Mechanisms of Disease. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Eiras S. Nε-carboxymethyl-lysine and inflammatory cytokines, markers and mediators of coronary artery disease progression in diabetes. World J Diabetes. 2024;15:575-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Wang ZQ, Yao HP, Sun Z. N(ε)-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products. World J Diabetes. 2023;14:222-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Macvanin MT, Stanimirovic J, Isenovic ER. Methods for Measurements of Oxidized LDL, Homocysteine and Nitric Oxide as Clinical Parameters of Oxidative Stress and Endothelial Dysfunction. CAC. 2022;18:1040-1056. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Bisht K, Verma VK, Abdullah Z, Prajapati V, Rajiv N, Bhatia J, Ray R, Nag TC, Arya DS. Arglabin: A mediator of inflammasome modulated and independent myocardial injury (PARA-AMI study). Eur J Pharmacol. 2024;970:176465. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Tian L, Li N, Li K, Tan Y, Han J, Lin B, Lai W, Liu H, Shi Y, Xi Z, Liu X. Ambient ozone exposure induces ROS related-mitophagy and pyroptosis via NLRP3 inflammasome activation in rat lung cells. Ecotoxicol Environ Saf. 2022;240:113663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Attiq A, Yao LJ, Afzal S, Khan MA. The triumvirate of NF-κB, inflammation and cytokine storm in COVID-19. Int Immunopharmacol. 2021;101:108255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Hendawy N, Salaheldin TH, Abuelezz SA. PCSK9 Inhibition Reduces Depressive like Behavior in CUMS-Exposed Rats: Highlights on HMGB1/RAGE/TLR4 Pathway, NLRP3 Inflammasome Complex and IDO-1. J Neuroimmune Pharmacol. 2023;18:195-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, Brandner S, Daniels JD, Schmitt-Kopplin P, Hauck SM, Stockwell BR, Hadian K, Schick JA. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 825] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 11. | García-Peña LM, Abel ED, Pereira RO. Mitochondrial Dynamics, Diabetes, and Cardiovascular Disease. Diabetes. 2024;73:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 12. | Tatmatsu-Rocha JC, Tim CR, Avo L, Bernardes-Filho R, Brassolatti P, Kido HW, Hamblin MR, Parizotto NA. Mitochondrial dynamics (fission and fusion) and collagen production in a rat model of diabetic wound healing treated by photobiomodulation: comparison of 904 nm laser and 850 nm light-emitting diode (LED). J Photochem Photobiol B. 2018;187:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31:25-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Atef MM, Hafez YM, El-Deeb OS, Basha EH, Ismail R, Alshenawy H, El-Esawy RO, Eltokhy AK. The cardioprotective effect of human glucagon-like peptide-1 receptor agonist (semaglutide) on cisplatin-induced cardiotoxicity in rats: Targeting mitochondrial functions, dynamics, biogenesis, and redox status pathways. Cell Biochem Funct. 2023;41:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Turan B, Durak A, Olgar Y, Tuncay E. Comparisons of pleiotropic effects of SGLT2 inhibition and GLP-1 agonism on cardiac glucose intolerance in heart dysfunction. Mol Cell Biochem. 2022;477:2609-2625. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 17. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8230] [Article Influence: 823.0] [Reference Citation Analysis (1)] |
| 18. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5359] [Article Influence: 669.9] [Reference Citation Analysis (0)] |
| 19. | Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, Shaw W, Law G, Walton MK, Rosenthal N, de Zeeuw D, Matthews DR; CANVAS Program collaborative group. Optimizing the analysis strategy for the CANVAS Program: A prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017;19:926-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ. Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients. Front Physiol. 2018;9:1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Lahnwong C, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Durak A, Olgar Y, Degirmenci S, Akkus E, Tuncay E, Turan B. A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc Diabetol. 2018;17:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 23. | Lee TI, Chen YC, Lin YK, Chung CC, Lu YY, Kao YH, Chen YJ. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Dos Santos Mendes-Costa L, de Lima VG, Barbosa MPR, Dos Santos LE, de Siqueira Rodrigues Fleury Rosa S, Tatmatsu-Rocha JC. Photobiomodulation: systematic review and meta-analysis of the most used parameters in the resolution diabetic foot ulcers. Lasers Med Sci. 2021;36:1129-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4:337-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 603] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 26. | Bensadoun RJ, Nair RG. Low-Level Laser Therapy in the Management of Mucositis and Dermatitis Induced by Cancer Therapy. Photomed Laser Surg. 2015;33:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6:e22453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 532] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 28. | Sena C, Pereira A, Fernandes R, Santos-silva D, Faustino A, Ceica R. P759Novel therapeutic approach to target endothelial dysfunction in type 2 diabetes. Cardiovasc Res. 2014;103:S139.2-S139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Kashiwagi S, Morita A, Yokomizo S, Ogawa E, Komai E, Huang PL, Bragin DE, Atochin DN. Photobiomodulation and nitric oxide signaling. Nitric Oxide. 2023;130:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |