Published online Aug 15, 2024. doi: 10.4239/wjd.v15.i8.1811
Revised: May 24, 2024
Accepted: June 18, 2024
Published online: August 15, 2024
Processing time: 125 Days and 16.8 Hours
Transient neonatal diabetes mellitus (TNDM) is a rare form of diabetes mellitus that usually presents within the first 6 mo of life. Patients often enter remission within several months, although relapse can occur later in life. Mutations in the ABCC8 gene, which encodes the sulfonylurea receptor 1 of the ATP-sensitive potassium channel in pancreatic beta cells, are associated with TNDM and permanent neonatal diabetes. This study describes a novel de novo c.3880C>T heterozygous ABCC8 variant that causes TNDM and can be treated with sulf-onylurea therapy.
We retrospectively analyzed 2 Chinese patients with TNDM who were diagnosed, treated, or referred for follow-up between September 2017 and September 2023. The patients were tested for mutations using targeted next-generation sequencing. Patients with neonatal diabetes mellitus caused by a c.3880C>T heterozygous missense variant in the ABCC8 gene have not been reported before. Both children had an onset of post-infectious diabetic ketoacidosis, which is worth noting. At a follow-up visit after discontinuing insulin injection, oral glyburide was found to be effective with no adverse reactions.
Early genetic testing of neonatal diabetes mellitus aids in accurate diagnosis and treatment and helps avoid daily insulin injections that may cause pain.
Core Tip: To date there have been no reports of the c.3880C>T heterozygous variant of the ABCC8 gene in patients with diabetes mellitus. After finding that this heterozygous variant can cause transient neonatal diabetes mellitus, we determined that oral sulfonylurea a was safe and effective therapy that successfully replaced insulin in 2 patients with the c.3880C>T heterozygous variant of the ABCC8 gene. This genetic diagnosis will inform clinicians about the probable course and optimal management of diabetes in such patients.
- Citation: Shen LH, Cui Y, Fu DX, Yang W, Wu SN, Wang HZ, Yang HH, Chen YX, Wei HY. Transient diabetes mellitus with ABCC8 variant successfully treated with sulfonylurea: Two case reports and review of literature. World J Diabetes 2024; 15(8): 1811-1819
- URL: https://www.wjgnet.com/1948-9358/full/v15/i8/1811.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i8.1811
Neonatal diabetes mellitus (NDM) is characterized by the onset of diabetes within the first 6 mo of life, but some cases are diagnosed between 6 mo and 12 mo of age. NDM is a type of monogenic diabetes that is very rare and difficult to treat, as reported by Kitsell in 1851[7]. The incidence of NDM is estimated to be approximately 1/89000-1:500000 live births[8], but it is not known in China. There are two clinical NDM subtypes depending on the permanence of hyperglycemia, transient (TNDM) and permanent (PNDM)[9]. The latter is a lifelong disease without remission; but TNDM, which accounts for 50%-60% of the NDM cases, usually enters remission after 6-12 mo. However, such patients may relapse in adolescence or early adulthood[10]. There are over 20 known genetic causes of NDM[11]. Approximately two-thirds of TNDM cases are related to abnormalities in an imprinted region on chromosome 6q24. Activating mutations in either of the genes encoding the two subunits of the ATP-sensitive potassium (KATP) channel of the pancreatic β-cell membrane [potassium inwardly rectifying channel subfamily j member 11 (KCNJ11) or ABCC8] are responsible in most of the remaining cases (KATP-NDM)[12,13]. Patients with KATP-NDM respond to sulfonylurea (SU) therapy, and approximately 90%-95% may be successfully transitioned to SU therapy, with complete discontinuation of insulin and a significant decrease in glycated hemoglobin levels[14,15]. In China, reports of the ABCC8 gene variant causing TNDM are rare. Here we describe a clinical phenotype of TNDM caused by a novel de novo ABCC8 gene c.3880C>T heterozygous variant and report effective SU treatment in 2 Chinese infants.
Case 1: A male infant 2 mo and 14 d of age was admitted to our hospital following a fever of 4 d, dyspnea of 3 d, and high blood glucose of 1 d duration.
Case 2: A male infant 7 mo and 20 d of age was admitted to our hospital following polydipsia and polyuria of 13 d, intermittent fever 6 d, and poor spirits of 5 d duration.
Case 1: Four days before admission, the parents complained of fever and increased water intake; however, the specific urine volume was not known. They reported that no chills, convulsions, nasal congestion, runny nose, cough, vomiting, abdominal distension, diarrhea, abnormal crying, or other symptoms had occurred. The patient had been given oral medication for a respiratory infection at a local clinic for 1 d. However, recurrent fever persisted. 3 d before admission he had dyspnea and was treated at a local hospital. But the response to atomization and oral medication was poor. One day earlier, he had an occasional cough and sputum in his throat accompanied by dyspnea, poor milk intake, poor spirits, and drowsiness. He was transferred to a superior local hospital for treatment. His blood glucose was high, and blood gas analysis revealed a pH of 7.06, a bicarbonate( HCO3-) level of 3.4 mmol/L, and a base excess(BE) of −24.8 mmol/L. The patient was treated with antibiotics, fluid replacement, and insulin therapy for 1 d. His breathing improved, and his body temperature was normal, but his blood glucose level was not well controlled. Therefore, he was transferred to our hospital for further treatment.
Case 2: Thirteen days before admission, the patient developed polydipsia and polyuria without evident cause, and the water intake and urine volumes were not known. Increased nocturia, evident overeating, or weight loss were not noted, but 6 d before admission, he had a fever with a peak temperature of 38 °C and occasional coughing. Oral ibuprofen effectively normalized the body temperature but was accompanied by frequent episodes of crying and agitation. He was taken to a local hospital and was treated with oral drugs for bronchitis. Furthermore, 5 d before admission, he manifested a poor spirit, mouth breathing, and tachypnea, with no nasal congestion, runny nose, cough, expectoration, rash, or convulsions. On a hospital revisit, he was considered to have severe pneumonia or respiratory failure. After receiving intravenous diazepam and treatment with cardiotonic drugs, his heart rate increased to 180-190 bpm, and he was transferred from the local facility to the intensive care unit of a tertiary hospital. His blood glucose was high, and blood gas analysis revealed a pH of 7.12, a HCO3- of 2.1 mmol/L, and a BE of −27 mmol/L. In addition, ventilator-assisted breathing, oxygen inhalation, sedation, anti-infectives, rehydration therapy, and other symptomatic treatments were administered. Insulin was given for 3 h but blood glucose levels remained at > 30 mmol/L. After the patient’s condition stabilized, an insulin pump was implanted for continuous subcutaneous insulin infusion. During hospitalization, the patient had an intermittent fever with a peak of 38.5 °C, accompanied by a cough and expectoration. 2 d before admission, the treatment was switched to regular subcutaneous insulin injection of 1.2 IU every 6 h. The blood glucose level fluctuated between 4-19 mmol/L, and the patient’s mental state and respiration were significantly improved. Subsequently, the patient was transferred to our hospital for further treatment.
Cases 1 and 2: The patients had no specific medical histories.
Case 1: The patient was born at term by cesarean section, with a birth weight of 3.85 kg. The parents denied a history of asphyxia rescue. He was breastfed after birth, and his parents were not consanguineous. No family history of diabetes was reported.
Case 2: The patient was the second live birth of the mother (gravida 2, para 2), was delivered uneventfully at 38 wk, with a birth weight of 3 kg. The parents denied a history of asphyxia rescue. He was breastfed after birth and was not given complementary food. His parents were not consanguineous. No family history of diabetes was reported.
Case 1: Physical examination at admission showed a body temperature of 36.4 °C, pulse rate of 168 bpm, a respiratory rate of 42 breaths/min, blood pressure of 75/40 mmHg, height of 52 cm, and a weight of 7 kg. There was a poor spirit, no jaundice, no bleeding spots, normal skin elasticity, soft neck, normal fontanelle, equal pupils, sensitivity to light reflex, ruddy lips, smooth oral mucosa, rapid and regular breathing with thick breath sounds in both lungs, strong heart sounds, normal abdomen, normal spine and limbs, normal muscle strength, but poor peripheral circulation.
Case 2: Physical examination at admission showed a body temperature of 36.5 °C, pulse rate of 108 bpm, respiratory rate of 25 breaths/min, blood pressure of 80/45 mmHg, height of 66.8 cm (3rd-10th percentile), and weight of 10 kg. Clear consciousness, moderate nutrition, anemic appearance, pale complexion, no jaundice, no bleeding spots, normal skin elasticity, equal and round pupils, sensitivity to light reflex, soft neck, smooth breathing, thick breath sounds, and phlegm rales in both lungs, strong heart sounds, normal abdomen, normal spine and limbs, normal muscle strength, normal reflexes, slightly pale nail beds.
Case 1: Laboratory examinations were performed at admission. The venous blood glucose level was 11.15 mmol/L after admission and multiple random peripheral blood glucose levels were > 11.1 mmol/L. Blood gas analysis revealed a pH of 7.356, a HCO3- of 19.9 mmol/L, and a BE of 4.5 mmol/L. Urine glucose was markedly elevated (++++) and there was a weak positive result for urine ketone bodies. Blood ammonia and lactic acid levels were 67.8 μmol/L and 3.4 mmol/L, respectively. Routine blood tests revealed a white blood cell (WBC) count of 10.14 × 109/L, red blood cell (RBC) count of 3.3 × 1012/L, Hb level of 99 g/L, and a platelet (PLT) count of 101 × 109/L. Blood biochemistry results were total cholesterol 6.27 mmol/L (reference range 0.36-6.2 mmol/L), triglycerides 2.46 mmol/L (reference range, 0.38-2.25 mmol/L), and normal liver function and renal function, myocardial enzyme levels, electrolyte levels, and thyroid function results were normal. The C-peptide level was 0.88 ng/mL (reference range, 1.1-4.4 ng/mL), and glycosylated Hb (HbA1c) was 9.52%. Insulinulin autoantibodies (glutamic acid decarboxylase, islet cell, and insulin antibodies) were negative.
Case 2: Laboratory examination was performed at admission. The venous blood glucose level was 13.26 mmol/L, and multiple random peripheral blood glucose samples were > 11.1 mmol/L. Blood gas analysis revealed a PH of 7.411, a HCO3- of −24.5 mmol/L, and a BE of 0.4 mmol/L.Urine glucose and urine ketone bodies were weakly positive, respectively. The blood ammonia level was 15.5 μmol/L and the lactic acid level was 2.4 mmol/L. Routine blood tests showed a WBC count of 11.57 × 109/L, RBC count of 4.14 × 1012/L, Hb level of 66 g/L, and PLT count of 317 × 109/L. Blood biochemistry results were triglycerides 3.61 mmol/L (reference range, 0.38-2.25 mmol/L) and normal liver function, renal function, myocardial enzymes, electrolytes, and thyroid function. The C-peptide level was 1.44 ng/mL (reference range, 1.1-4.4 ng/mL), HbA1c was 12.63%; and five insulin antibodies (glutamic acid decarboxylase autoantibodies, islet cell antibodies, insulin autoantibody, zinc transporter-8 antibody, and tyrosine phosphatase antibody) were negative.
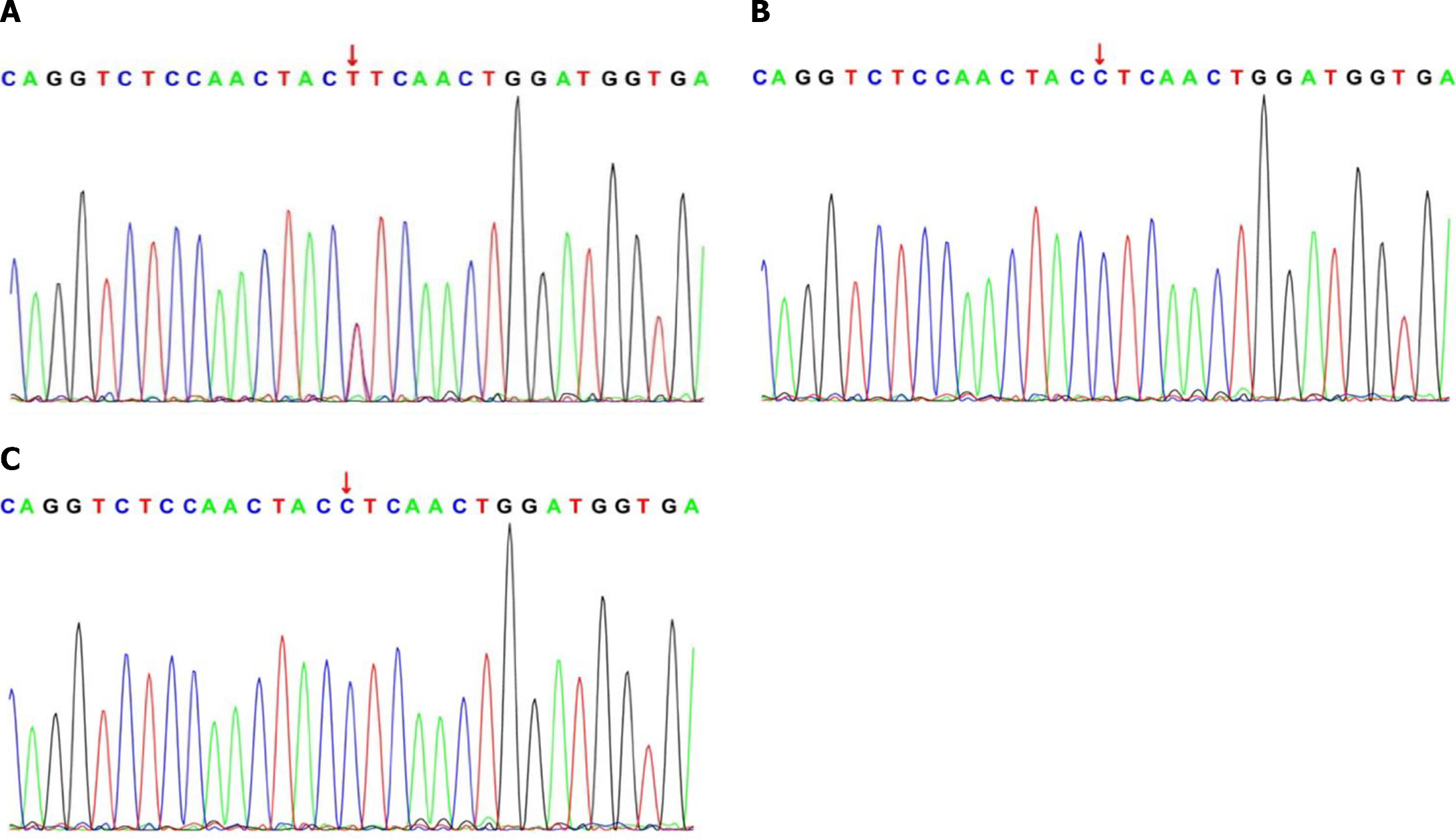
Genetic testing and mutation analysis: The Ethics Committee of the Children’s Hospital Affiliated with Zhengzhou University approved this study (Approval No. 2023-K-123). Informed consent was obtained from the patients’ guardians. DNA samples were sent to the hospital's Institute of Pediatrics for next-generation sequencing. The procedure was performed following the standard protocol. Sanger sequencing was used to verify the variant sites in the patients, and sequence analysis was performed on samples from the parents to determine the source of variation. A heterozygous missense variant in ABCC8 was found in both patients, resulting in a C>T variant at position 3880 and the substitution of leucine (L) by phenylalanine (F) at amino acid position 1294 of the encoded protein. No corresponding gene variants were detected in the parents of either infant (Figure 1).
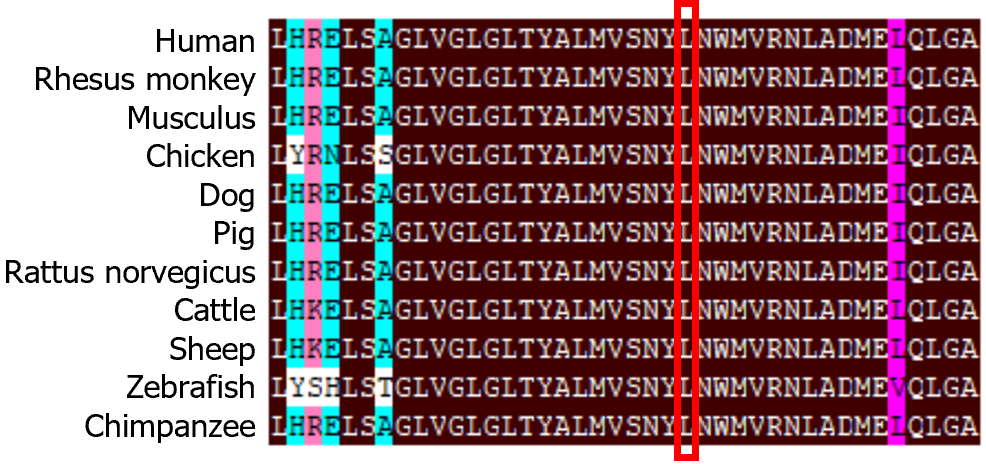
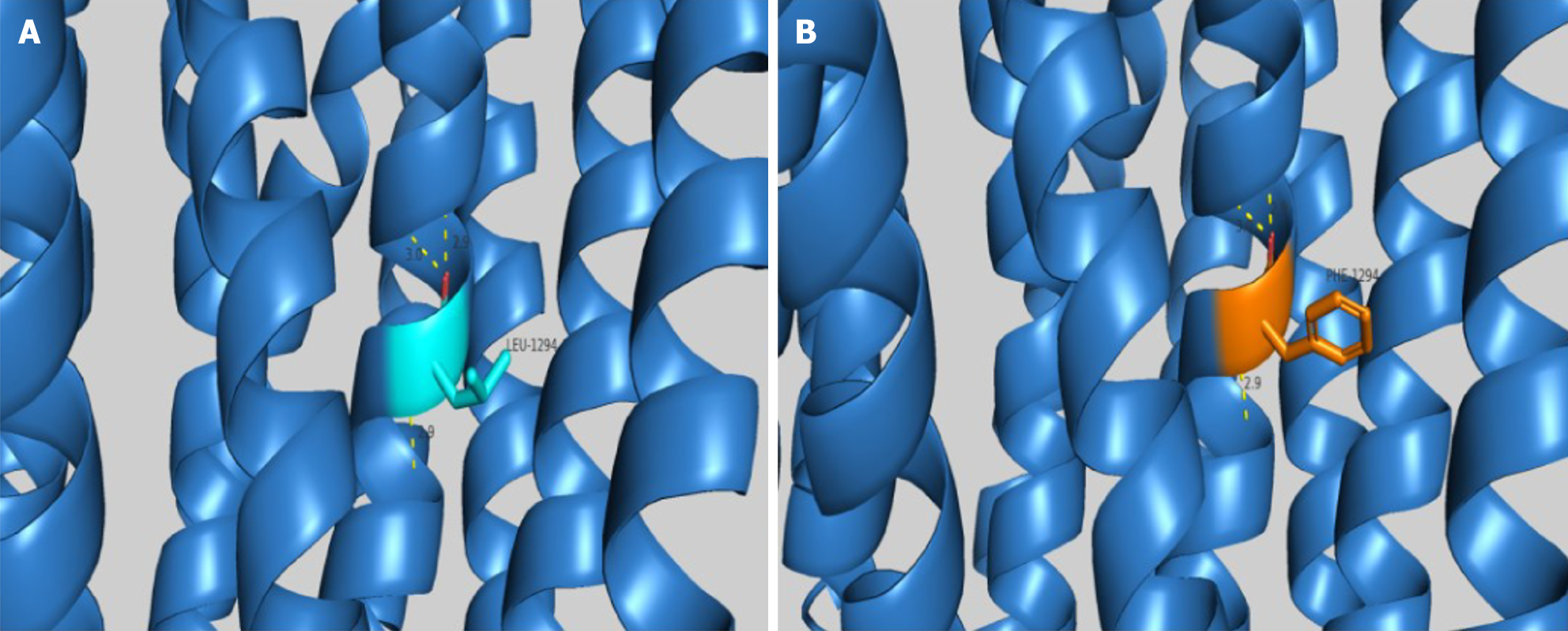
The ABCC8 c.3880C>T variant has not been reported in PubMed or other databases such as the Human Gene Mutation Database, ClinVar, and the Database for Single Nucleotide Polymorphisms (dbSNP). Homologous sequence comparison using DNAMAN software (Lynnon Biosoft Bioinformatics Solutions, San Ramon, CA, United States) showed that amino acid L at position 1294 of the ABCC8 protein was highly conserved (Figure 2). Bioinformatics software, such as Mutation Taster (https://www.mutationtaster.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), and SIFT (http://sift.bii.a-star.edu.sg/), predicted that the c.3880C>T variant was pathogenic or likely pathogenic. This variant changed the nonpolar hydrophobic aliphatic L to a nonpolar hydrophobic aromatic F at position 1294, altering the side chain structure with insignificant changes in hydrogen bonding (Figure 3). The c.3880C>T variant is classified as pathogenic (PM1 + PM2 + PP3 + PP4) by the American College of Medical Genetics and Genomics (ACMG) Classification of Genetic Variations[16].
The final diagnosis was TNDM.
Both infants were treated with low-dose insulin after diabetic ketoacidosis. The initial dose was as follows: after ketoacidosis was corrected, neutral protamine Hagedorn insulin was administered three times, and glyburide was used as an experimental treatment when the blood glucose level was relatively stable. The initial dose of glyburide was 0.1-0.2 mg/kg/day. The glyburide dose was gradually adjusted by the results of blood glucose monitoring and insulin was gradually discontinued.
In both cases, blood glucose levels were in the normal range during outpatient follow-up. Glyburide was effective and was discontinued after 1 mo of oral administration in case 1 and after 1 year in case 2. No major side effects of glyburide were found. The growth and development of the 2 children were similar to that of their peers, and no neurological abnormalities were detected.
TNDM is a genetically heterogeneous form of NDM characterized by hyperglycemia and is usually diagnosed before 6 mo of age. It remits during infancy but recurs in later life in most patients[17,18]. Subcutaneous insulin was routinely used to treat NDM previously. However, establishing an effective long-term insulin therapy for NDM is a great challenge for pediatricians and parents because of the irregular feeding habits of infants. Consequently, oral SU therapy has been found to improve glycemic control and to be a more effective in improving the quality of life[19,20].
KATP channels are hetero-octameric complexes formed by four pore-forming Kir6.2 subunits and four SU receptor-1 (SUR1) regulatory subunits and encoded by the KCNJ11 and ABCC8 genes, respectively. In normal pancreatic beta cells, increased glucose across glucose transporter 2 is metabolized by the enzyme glucokinase, resulting in increased production of ATP. This closes the KATP channel, which in turn depolarizes the cell membrane and activates an influx of calcium through voltage-gated calcium channels that subsequently permit exocytosis of insulin granules. ABCC8 gene mutations cause the KATP channels to remain inappropriately open, even in the presence of hyperglycemia. Without channel closure, the cell membrane depolarizes and blocks insulin release from the beta cells, resulting in the clinical manifestations of diabetes mellitus. SU closes the KATP channel through an ATP-independent route, leading to increased insulin secretion[14,21]. Patients with NDM carrying ABCC8 variations have been successfully switched from insulin to oral SU treatment[20,22,23]. However, the appropriate treatment may differ owing to different types of variants and variable clinical phenotypes. In addition, the sensitivity to SU varied among patients with ABCC8 variants. Notably, most patients, but not all, were successful in transitioning from insulin therapy to SU[22]. The ABCC8 gene, encoding a 1582 amino acid protein, is located on the short arm of chromosome 11 (11p15.1) and comprises 39 exons. Over 700 pathogenic or likely pathogenic ABCC8 mutations have been identified in the ABCC gene, and these include point, missense, nonsense, frame, splicing, and deletion variations[24-26].
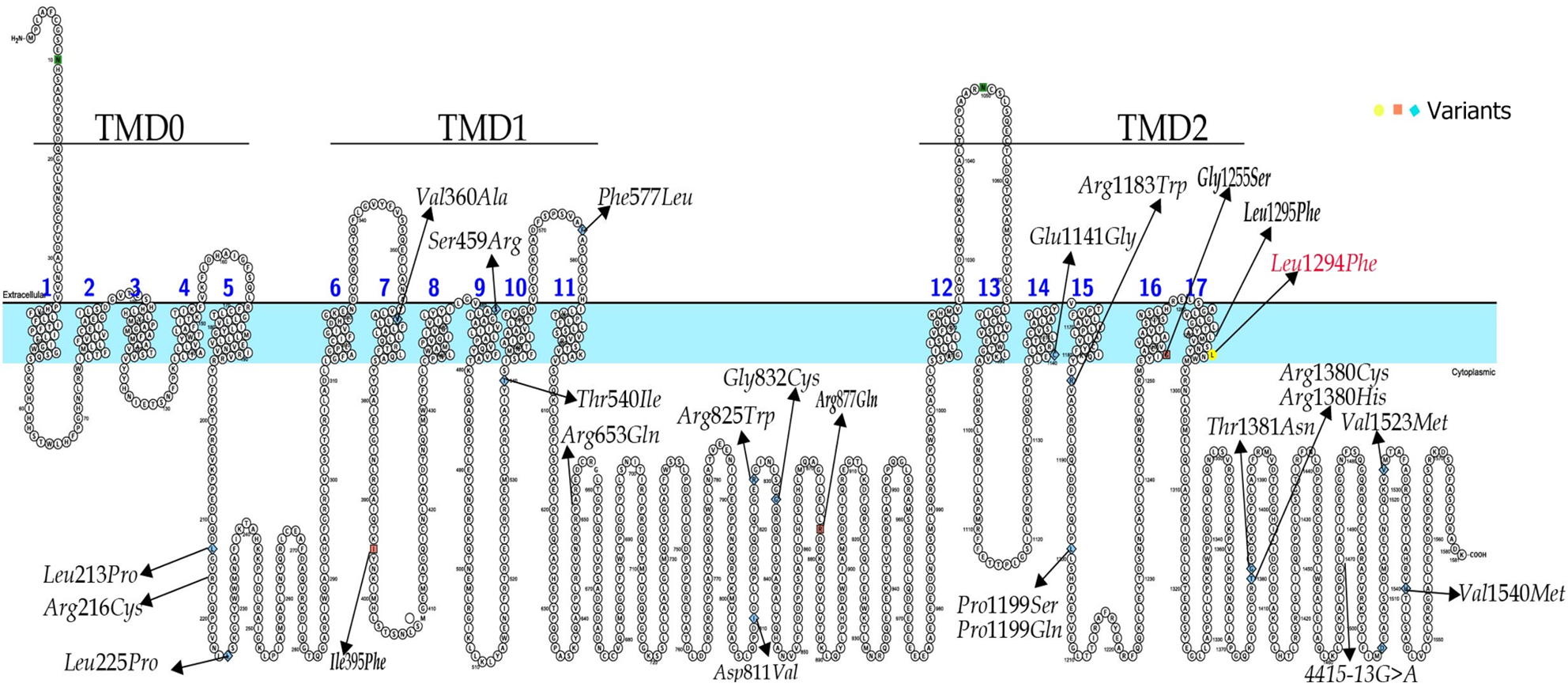
A large prospective cohort study by Busiah et al[27] of neonatal diabetes diagnosed before 1 year of age in 68 French centers reported that of 31 patients with NDM caused by an ABCC8 mutation, 11 (13%) were < 1 mo, 19 (27%) were between 1 mo and 6 mo, and 1 (6%) was between 6 mo and 1 year of age. At the end of follow-up, 24 cases were identified as TNDM (78%) and 5 as PNDM (16%)[27]. In 2020, De Franco et al[24] described a total of 748 ABCC8 pathogenic and likely pathogenic variants associated with NDM and congenital hyperinsulinism that had been identified in various countries. To date, the only patients with ABCC8 variants associated with TNDM in China were reported by Li et al[28]. We searched the literature in PubMed and Chinese literature databases, such as China National Knowledge In-frastructure and Wanfang. The ABCC8 gene variants associated with TNDM were reviewed and are summarized in Figure 4, with the majority of variants located in the coding region of the gene. A total of 12 cases with detailed clinical information and the outcomes of SU therapy transfer are summarized in Table 1. The c.3880C>T variation is in the TMD2 domain of the SUR1 subunit in exon 32 of ABCC8 and results in a change from amino acid L to amino acid F at the 1294 position in the encoded protein. According to the ACMG guidelines for classifying genetic variants, this variant is likely to be pathogenic (PM1 + PM2 + PP3 + PP4). So far, no reports of the ABCC8 c.3880C>T variation have been reported, and further functional studies are needed. The age at onset in case 1 was 2 mo, and that in case 2 was 7 mo and 20 d. Both patients had ketoacidosis and no family history of diabetes mellitus. During the follow-up period, glyburide proved to be effective and was discontinued after 1 mo and 1 year of oral administration, respectively. No significant side effects of glyburide were observed, suggesting that the age at onset and duration of glyburide treatment differ in patients with the same mutation site. Owing to the ABCC8 variations, patients with TNDM have a high possibility of recurrence in the future, and we will continue to follow-up the children for an extended period.
| Case | Age | DKA | PH | HCO3- | BE | HbA1c | C-peptide | Neurological symptoms | Mutation | Zygosity | Inheritance | Treatment | Ref. |
| 1 | 36 d | Severe | 7.08 | 3.3 | −26 | 7.6 | 0.17 | No | Arg1183Trp | Het | Paternal | Insulin → SU remission in 24 mo | Ngoc et al[29], 2021 |
| 2 | 50 d | Moderate | 7.19 | 8 | −19 | 7.58 | 0.41 | Convultion | Glu1141Gly | Het | Paternal | Insulin → SU remission in 6 mo | Ngoc et al[29], 2021 |
| 3 | 35 d | Yes | NA | NA | NA | NA | NA | No | Arg216Cys | Hom | Both parents | Insulin → SU remission in 14 mo | Nayak et al[30], 2021 |
| 4 | 270 d | No | Normal | NA | NA | NA | NA | No | Leu1295Phe | Het | Paternal | Insulin remission in 48 mo | Nayak et al[30], 2021 |
| 5 | 4 d | No | Normal | NA | NA | NA | 0.06 | NA | Arg1380Cys | NA | NA | Insulin remission in 4 mo | Torbjörnsdotter et al[31], 2020 |
| 6 | 96 d | NA | NA | NA | NA | 7.2 | NA | Developmental delay | Arg653Gln | Het | NA | NA | Balamurugan et al[32], 2019 |
| 7 | 12 d | NA | NA | NA | NA | 4 | 0.33 | NA | Ile395Phe | NA | NA | Insulin → SU unresponsive remission in 5.5 mo | Li et al[28], 2018 |
| 8 | 60 d | NA | NA | NA | NA | 7.2 | < 0.5 | NA | Arg877Gln | NA | NA | Insulin remission in 4 mo | Li et al[28], 2018 |
| 9 | 105 d | NA | NA | NA | NA | 7.9 | 0.42 | NA | Gly1255Ser | NA | NA | Insulin remission in 3.5 mo relapse in 8 yr | Li et al[28], 2018 |
| 10 | 35 d | No | 7.459 | 22.5 | −0.4 | 4.3 | 0.6 | No | Gly832Cys | Het | De novo | Insulin → SU remission in 1 yr and 10 mo | Yamazaki M et al[33], 2017 |
| 11 | 74 d | Severe | 7.06 | 3.4 | −24.8 | 0.88 | 9.52 | No | Leu1294Phe | Het | De novo | Insulin → SU remission in 1 mo | This study |
| 12 | 230 d | Severe | 7.12 | 2.1 | −27 | 1.44 | 12.63 | No | Leu1294Phe | Het | De novo | Insulin → SU remission in 12 mo | This study |
NDM is rarely encountered in clinical practice. In this study, we retrospectively analyzed the clinical phenotypes and ABCC8 c.3880C>T mutations in 2 infants with TNDM. SU glyburide treatment was effective, and this novel de novo variation expands the pathogenic gene mutation spectrum of NDM. All patients diagnosed with diabetes before the 1 year of age should be referred for genetic testing, regardless of their current age, to identify those cases likely to benefit from SU treatment. However, the sample size was limited; therefore, additional evidence and experience should be accumulated over long follow-up periods.
We express our gratitude to the children, parents, and caregivers who willingly participated in this study.
| 1. | Kanakatti Shankar R, Pihoker C, Dolan LM, Standiford D, Badaru A, Dabelea D, Rodriguez B, Black MH, Imperatore G, Hattersley A, Ellard S, Gilliam LK; SEARCH for Diabetes in Youth Study Group. Permanent neonatal diabetes mellitus: prevalence and genetic diagnosis in the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2013;14:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Rubio-Cabezas O, Flanagan SE, Damhuis A, Hattersley AT, Ellard S. KATP channel mutations in infants with permanent diabetes diagnosed after 6 months of life. Pediatr Diabetes. 2012;13:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Mohamadi A, Clark LM, Lipkin PH, Mahone EM, Wodka EL, Plotnick LP. Medical and developmental impact of transition from subcutaneous insulin to oral glyburide in a 15-yr-old boy with neonatal diabetes mellitus and intermediate DEND syndrome: extending the age of KCNJ11 mutation testing in neonatal DM. Pediatr Diabetes. 2010;11:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Thomas ER, Brackenridge A, Kidd J, Kariyawasam D, Carroll P, Colclough K, Ellard S. Diagnosis of monogenic diabetes: 10-Year experience in a large multi-ethnic diabetes center. J Diabetes Investig. 2016;7:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1299] [Article Influence: 649.5] [Reference Citation Analysis (70)] |
| 6. | Greeley SAW, Polak M, Njølstad PR, Barbetti F, Williams R, Castano L, Raile K, Chi DV, Habeb A, Hattersley AT, Codner E. ISPAD Clinical Practice Consensus Guidelines 2022: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2022;23:1188-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 7. | Shield JP. Neonatal diabetes. Horm Res. 2007;68 Suppl 5:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Grulich-Henn J, Wagner V, Thon A, Schober E, Marg W, Kapellen TM, Haberland H, Raile K, Ellard S, Flanagan SE, Hattersley AT, Holl RW. Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPV). Diabet Med. 2010;27:709-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Rearson MA, McKnight-Menci H, Steinkrauss L. Neonatal diabetes: current trends in diagnosis and management. MCN Am J Matern Child Nurs. 2011;36:17-22; quiz 23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Bowman P, Mathews F, Barbetti F, Shepherd MH, Sanchez J, Piccini B, Beltrand J, Letourneau-Freiberg LR, Polak M, Greeley SAW, Rawlins E, Babiker T, Thomas NJ, De Franco E, Ellard S, Flanagan SE, Hattersley AT; Neonatal Diabetes International Collaborative Group. Long-term Follow-up of Glycemic and Neurological Outcomes in an International Series of Patients With Sulfonylurea-Treated ABCC8 Permanent Neonatal Diabetes. Diabetes Care. 2021;44:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Groop L. Genetics and neonatal diabetes: towards precision medicine. Lancet. 2015;386:934-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI; Neonatal Diabetes International Collaborative Group. Insulinulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040-15044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 411] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 13. | De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, Ellard S, Hattersley AT. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386:957-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 14. | Lemelman MB, Letourneau L, Greeley SAW. Neonatal Diabetes Mellitus: An Update on Diagnosis and Management. Clin Perinatol. 2018;45:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT; Neonatal Diabetes International Collaborative Group. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 669] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 16. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22423] [Article Influence: 2242.3] [Reference Citation Analysis (0)] |
| 17. | Hattersley AT, Greeley SAW, Polak M, Rubio-Cabezas O, Njølstad PR, Mlynarski W, Castano L, Carlsson A, Raile K, Chi DV, Ellard S, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 18. | Barbetti F, D'Annunzio G. Genetic causes and treatment of neonatal diabetes and early childhood diabetes. Best Pract Res Clin Endocrinol Metab. 2018;32:575-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Carmody D, Beca FA, Bell CD, Hwang JL, Dickens JT, Devine NA, Mackay DJ, Temple IK, Hays LR, Naylor RN, Philipson LH, Greeley SA. Role of noninsulin therapies alone or in combination in chromosome 6q24-related transient neonatal diabetes: sulfonylurea improves but does not always normalize insulin secretion. Diabetes Care. 2015;38:e86-e87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Zhang M, Chen X, Shen S, Li T, Chen L, Hu M, Cao L, Cheng R, Zhao Z, Luo F. Sulfonylurea in the treatment of neonatal diabetes mellitus children with heterogeneous genetic backgrounds. J Pediatr Endocrinol Metab. 2015;28:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Inagaki N, Gonoi T, Clement JP 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1235] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 22. | Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT; Neonatal Diabetes International Collaborative Group. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Cao B, Gong C, Wu D, Lu C, Liu F, Liu X, Zhang Y, Gu Y, Qi Z, Li X, Liu M, Li W, Su C, Liang X, Feng M. Genetic Analysis and Follow-Up of 25 Neonatal Diabetes Mellitus Patients in China. J Diabetes Res. 2016;2016:6314368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | De Franco E, Saint-Martin C, Brusgaard K, Knight Johnson AE, Aguilar-Bryan L, Bowman P, Arnoux JB, Larsen AR, Sanyoura M, Greeley SAW, Calzada-León R, Harman B, Houghton JAL, Nishimura-Meguro E, Laver TW, Ellard S, Del Gaudio D, Christesen HT, Bellanné-Chantelot C, Flanagan SE. Update of variants identified in the pancreatic β-cell K(ATP) channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum Mutat. 2020;41:884-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev Endocr Metab Disord. 2010;11:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Busiah K, Drunat S, Vaivre-Douret L, Bonnefond A, Simon A, Flechtner I, Gérard B, Pouvreau N, Elie C, Nimri R, De Vries L, Tubiana-Rufi N, Metz C, Bertrand AM, Nivot-Adamiak S, de Kerdanet M, Stuckens C, Jennane F, Souchon PF, Le Tallec C, Désirée C, Pereira S, Dechaume A, Robert JJ, Phillip M, Scharfmann R, Czernichow P, Froguel P, Vaxillaire M, Polak M, Cavé H; French NDM study group. Neuropsychological dysfunction and developmental defects associated with genetic changes in infants with neonatal diabetes mellitus: a prospective cohort study [corrected]. Lancet Diabetes Endocrinol. 2013;1:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Li X, Xu A, Sheng H, Ting TH, Mao X, Huang X, Jiang M, Cheng J, Liu L. Early transition from insulin to sulfonylureas in neonatal diabetes and follow-up: Experience from China. Pediatr Diabetes. 2018;19:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Ngoc CTB, Dien TM, De Franco E, Ellard S, Houghton JAL, Lan NN, Thao BP, Khanh NN, Flanagan SE, Craig ME, Dung VC. Molecular Genetics, Clinical Characteristics, and Treatment Outcomes of K(ATP)-Channel Neonatal Diabetes Mellitus in Vietnam National Children's Hospital. Front Endocrinol (Lausanne). 2021;12:727083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Nayak S, Sarangi AN, Sahoo SK, Mangla P, Tripathy M, Rao S, Gupta S, Paliwal VK, Sudhanshu S, Ravi C, Joshi K, Bhatia V, Bhatia E. Neonatal Diabetes Mellitus: Novel Mutations. Indian J Pediatr. 2021;88:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Torbjörnsdotter T, Marosvari-Barna E, Henckel E, Corrias M, Norgren S, Janson A. Successful treatment of a cohort of infants with neonatal diabetes using insulin pumps including data on genetics and estimated incidence. Acta Paediatr. 2020;109:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Balamurugan K, Kavitha B, Yang Z, Mohan V, Radha V, Shyng SL. Functional characterization of activating mutations in the sulfonylurea receptor 1 (ABCC8) causing neonatal diabetes mellitus in Asian Indian children. Pediatr Diabetes. 2019;20:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Yamazaki M, Sugie H, Oguma M, Yorifuji T, Tajima T, Yamagata T. Sulfonylurea treatment in an infant with transient neonatal diabetes mellitus caused by an adenosine triphosphate binding cassette subfamily C member 8 gene mutation. Clin Pediatr Endocrinol. 2017;26:165-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |