Published online Jul 15, 2024. doi: 10.4239/wjd.v15.i7.1531
Revised: April 25, 2024
Accepted: May 23, 2024
Published online: July 15, 2024
Processing time: 199 Days and 0.9 Hours
Glycated hemoglobin A1c (HbA1c) is considered the most suitable for diabetes mellitus diagnosis due to its accuracy and convenience. However, the effect of HbA1c on diabetic retinopathy (DR) in the Han and Korean populations in Jilin, China, remains inconclusive.
To determine the best cut-off of HbA1c for diagnosing DR among the Chinese.
This cross-sectional study included 1933 participants from the Yanbian area of Jilin Province, China. Trained investigators employed a questionnaire-based survey, physical examination, laboratory tests, and fundus photography for the investigation. The best cut-off value for HbA1c was established via the receiver operating characteristic curve. The factors associated with HbA1c-associated risk factors were determined via linear regression.
The analysis included 887 eligible Chinese Han and Korean participants, 591 of whom were assigned randomly to the training set and 296 to the validation set. The prevalence of DR was 3.27% in the total population. HbA1c of 6.2% was the best cut-off value in the training set, while it was 5.9% in the validation set. In both Chinese Han and Korean populations, an HbA1c level of 6.2% was the best cut-off value. The optimal cut-off values of fasting blood glucose (FBG) ≥ 7 mmol/L and < 7 mmol/L were 8.1% and 6.2% respectively in Han populations, while those in Korean populations were 6.9% and 5.3%, respectively. Age, body mass index, and FBG were determined as the risk factors impacting HbA1c levels.
HbA1c may serve as a useful diagnostic indicator for DR. An HbA1c level of 6.2% may be an appropriate cut-off value for DR detection in the Chinese population.
Core Tip: This cross-sectional analysis of data from 887 participants in Yanbian area of China from March to April 2017 determined the best glycated hemoglobin A1c (HbA1c) cut-off point for the diagnosis of diabetic retinopathy (DR) among the Chinese population. This study implies that HbA1c is a practical diagnostic marker for DR, and an HbA1c level of 6.2% may be an appropriate cut-off value for DR detection in the Chinese population.
- Citation: Wen Y, Wang Q. Cut-off value of glycated hemoglobin A1c for detecting diabetic retinopathy in the Chinese population. World J Diabetes 2024; 15(7): 1531-1536
- URL: https://www.wjgnet.com/1948-9358/full/v15/i7/1531.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i7.1531
Diabetes mellitus has significantly impacted global health and medical expenses in recent decades, reaching pandemic proportions[1]. By 2030, projections indicate that it will impact 643 million people[2]. Diabetic retinopathy (DR), the most common microvascular consequence of diabetes, is the primary lead to blindness and is expected to influence 191 million people globally by 2030[3]. DR is usually asymptomatic in its initial stages, progresses gradually over years, and sym-ptoms may not manifest until it reaches an advanced stage, which might hinder timely treatment and lead to poor therapeutic outcomes[4]. Hence, early detection of DR is imperative to promptly implement therapeutic interventions and prevent visual loss.
Diabetes duration, blood pressure control, and glucose control have a significant correlation with DR and are the primary risk factors affecting its severity[5]. Glucose control plays a key role in the progression of DR, accounting for about 11% of DR risk. The relationship between glucose control and DR is established through glycated hemoglobin A1c (HbA1c), fasting blood glucose (FBG), and 2-h plasma glucose (2 h-PG). HbA1c is the gold standard for evaluating glycaemic control. HbA1c is considered the most suitable for DR diagnosis due to its accuracy and convenience[6-8]. However, the effect of HbA1c on DR in the Chinese population remains inconclusive. This study aimed to explore the diagnostic significance of HbA1c in DR and measure the best HbA1c threshold for detecting DR in a Chinese population.
This cross-sectional study was conducted in Jilin Province, China, from March to April 2017. Two neighborhood committees in the Yanbian area were randomly selected to enlist the residents aged 18-85 years. Three days before the study, investigators visited the house to schedule a field investigation and obtained written informed consent with the assistance of the local staff. The field investigation conducted by the trained investigators included a questionnaire-based survey (including name, sex, age, nationality, medical history, medication history, occupation, and income), physical examination (including weight, height, waist circumference, pulse rate, blood pressure), laboratory measurement program (including FBG, 2 h-PG, and HbA1c), blood lipids (including total cholesterol, low- and high-density lipoprotein, triglyceride), renal function (including creatinine, urea nitrogen, and uric acid), routine blood examination parameters (including white and red blood cell count, hemoglobin, and blood platelet count), and fundus photography. Investigators conducted face-to-face questionnaire-based surveys and recorded them. Physical examinations were performed using standard methods. Fasting blood was collected in the morning fasting for at least 10 h. Laboratory tests, except for HbA1c, were conducted using the AU58000 automatic biochemistry analyzer (Beckman Coulter Inc., CA, United States). HbA1c was measured using the D-10TM Hemoglobin Testing System (Bio-Rad Laboratories Inc., CA, United States). Two digital fundus photographs were captured for each eye using a Canon CR-2 AF nonmydriatic digital fundus camera (Canon, Tokyo, Japan). The images were graded by two professional ophthalmologists in a double-blind manner. A total of 1933 participants were enrolled. Participants who were older adults, frail, had restricted mobility, suffered from severe liver and kidney diseases, or had incomplete data were excluded. Finally, 887 participants were involved in the data analysis and were grouped into a training set and a validation set according to a ratio of 2:1 at random.
The Early Treatment Diabetic Retinopathy Study (ETDRS) scale was utilized for DR diagnosis[9]. The presence of DR in the participant and its grading were determined based on the eye with the higher score. Herein, the specific level of DR was defined by an ETDRS letter score of ≥ 31 (mild nonproliferative DR or more severe DR).
The R software (version 4.1.2) was applied to statistical analysis. Counting data was described as frequency number and percentage, and experimental data was presented as mean ± SD. Data corresponding to a normal distribution was compared using variance analysis, while data fitting a non-normal distribution was compared by Mann-Whitney U test. The best HbA1c cut-off value was determined by the receiver operating characteristic (ROC) curve. Factors related to HbA1c were determined via linear regression. A P value < 0.05 was deemed statistically significant.
A total of 887 eligible participants representing the Chinese Han and Korean populations were involved in the analysis, of which 591 were grouped randomly into the training set and 296 were allocated to the validation set. The proportion of the population representing Chinese Korean nationality accounted for 67.68% of the population in the training set and 63.85% in the validation set, respectively. In the total population, the prevalence of DR was 3.27%. The baseline characteristics of the participants are displayed in Table 1. No significant differences regarding demographic information, physical examination, or lab test results were found between the two sets.
| Training set (n = 591) | Validation set (n = 296) | P value | |
| Chinese Korean population, n (%) | 400 (67.68) | 189 (63.85) | 0.2832 |
| Female, n (%) | 387 (65.48) | 186 (62.84) | 0.4759 |
| Age (yr) | 55.82 ± 11.41 | 54.81 ± 11.24 | 0.1913 |
| BMI (kg/m2) | 25.38 ± 3.84 | 25.66 ± 4.03 | 0.5643 |
| Waist circumference (cm) | 80.17 ± 9.77 | 80.88 ± 9.84 | 0.4620 |
| Systolic blood pressure (mmHg) | 136.67 ± 21.83 | 137.62 ± 20.48 | 0.4574 |
| Diastolic blood pressure (mmHg) | 80.56 ± 12.59 | 80.57 ± 12.26 | 0.4661 |
| Beats per minute | 80.58 ± 11.85 | 80.54 ± 13.04 | 0.9233 |
| FBG (mmol/L) | 6.20 ± 1.62 | 6.14 ± 1.65 | 0.1461 |
| 2 h-PG (mmol/L) | 7.59 ± 3.02 | 7.58 ± 3.62 | 0.7261 |
| HbA1c (%) | 5.83 ± 1.06 | 5.81 ± 1.07 | 0.7282 |
| White blood cell count (109/L) | 5.73 ± 1.47 | 5.89 ± 1.69 | 0.2423 |
| Red blood cell count (1012/L) | 4.71 ± 0.45 | 4.74 ± 0.48 | 0.7093 |
| Blood platelet count (109/L) | 225.47 ± 57.29 | 227.50 ± 57.49 | 0.6463 |
| Hemoglobin (g/L) | 144.99 ± 16.27 | 145.81 ± 15.45 | 0.7393 |
| Total cholesterol (mmol/L) | 5.28 ± 1.05 | 5.29 ± 1.17 | 0.9639 |
| Triglyceride (mmol/L) | 1.76 ± 1.41 | 1.79 ± 1.44 | 0.8220 |
| Low-density lipoprotein (mmol/L) | 3.02 ± 0.83 | 3.04 ± 0.88 | 0.8941 |
| High-density lipoprotein (mmol/L) | 1.49 ± 0.37 | 1.49 ± 0.32 | 0.8240 |
| Creatinine (μmol/L) | 57.88 ± 15.08 | 57.87 ± 15.02 | 0.8231 |
| Urea nitrogen (mmol/L) | 5.03 ± 1.42 | 4.96 ± 1.33 | 0.6231 |
| Uric acid (μmol/L) | 280.60 ± 82.06 | 284.96 ± 81.33 | 0.6276 |
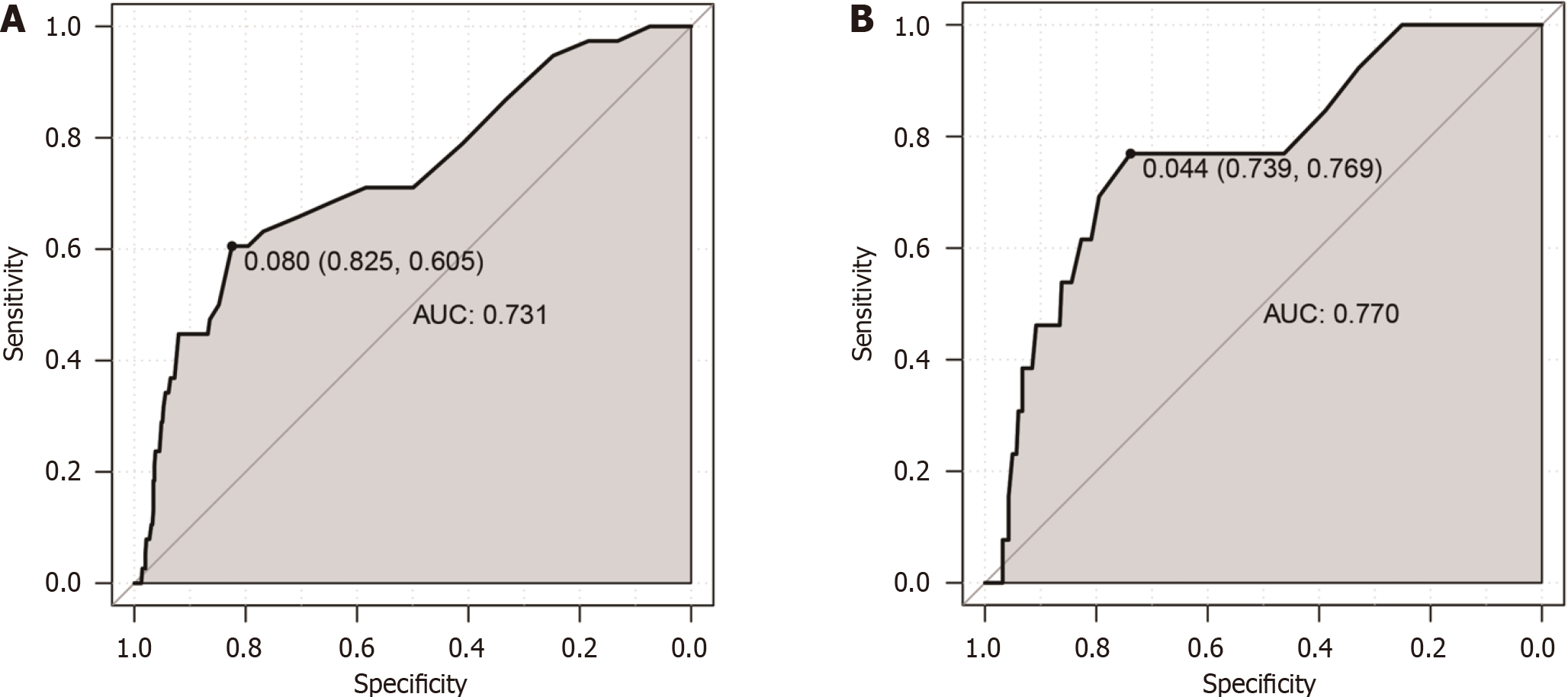
The ROC curve was utilized to measure the optimal HbA1c cut-off value for diagnosing DR, and the area under the ROC curve (AUC) was applied to assess its diagnostic efficacy. In the training set, the AUC was 0.731 [95% confidence interval (CI): 0.682-0.773], and an HbA1c of 6.2% was the optimal cut-off value with a specificity of 82.5% and a sensitivity of 60.5% (Figure 1A). The AUC for the validation set was 0.770 (95%CI: 0.679-0.861), and an optimal HbA1c cut-off value of 5.9% was determined, corresponding to a specificity of 73.9% and a sensitivity of 76.9% (Figure 1B). The best cut-off value for both the Chinese Han and Korean populations was HbA1c of 6.2%, which had a high specificity (81.2% and 84.9%, respectively) and a relatively low sensitivity (60.0% and 50.0%, respectively). The corresponding AUC was 0.721 and 0.685, respectively (Supplementary Figure 1).
Age, body mass index (BMI), and FBG were determined to be significant risk factors associated with HbA1c (Table 2). An HbA1c level of 6.3% was the best cut-off value in the older adults (Supplementary Table 1, AUC: 0.694, sensitivity: 62.4%, specificity: 78.9%), whereas in the young people, it was 5.9% (AUC: 0.752, sensitivity: 66.7%, specificity: 82.4%). An HbA1c level of 6.2% was the best cut-off value in the overweight and obese individuals (AUC: 0.702, sensitivity: 68.5%, specificity: 79.7%), while it was 6.0% in the healthy participants (AUC: 0.738, sensitivity: 72.6%, specificity: 88.6%).
| Coefficient | SE | P value | |
| Age (yr) | 0.350 | 0.0017 | 0.0042 |
| BMI (kg/m2) | 0.283 | 0.0643 | < 0.0001 |
| FBG (mmol/L) | 0.547 | 0.0121 | < 0.0001 |
Diabetes and its complications present a serious global health problem, and China is its primary epicenter[2]. The present study proposes using an HbA1c cut-off value of 6.2% to detect DR in the Chinese population. HbA1c is an internationally utilized indicator to assess glycemic control over extended periods, reflecting the average blood glucose level over the previous three months. The association between the glucose level and diabetic complications, especially DR, is fun-damental to diabetes diagnosis. The American Diabetes Association recommends using HbA1c for diabetes diagnosis[10]. As HbA1c levels rise, the occurrence of DR significantly increases[11]. Conversely, lowering HbA1c levels is cor
Ethnicity is correlated with variations in HbA1c diagnostic performance and thresholds, suggesting that the best HbA1c cut-off value should be modified to account for local conditions[13]. For Asians, the HbA1c cut-off value for diag
Age, BMI, and FBG were identified as the risk factors influencing HbA1c cut-off values. An increased BMI and advancing age were associated with a higher HbA1c cut-off value. Aging is associated with an elevation in HbA1c levels. Furthermore, age is correlated with the rising prevalence of overweight and obesity[18-20], a correlation that could potentially be attributed to the increased HbA1c levels. These findings indicated that age may be a factor in establishing the HbA1c cut-off value among different groups of people.
The study has several limitations. Because this experiment was a cross-sectional design, the causal link between HbA1c and DR could not be established. The sample size was limited, making it extremely crucial to exercise caution when extrapolating the results to other populations. In the future, large-scale, multicenter prospective studies are recom-mended.
HbA1c is a useful diagnostic marker for DR, and an HbA1c level of 6.2% may be an appropriate cut-off value for DR detection in the Chinese population.
| 1. | Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The Growing Epidemic of Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 3. | Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Zhang B, Zhang B, Zhou Z, Guo Y, Wang D. The value of glycosylated hemoglobin in the diagnosis of diabetic retinopathy: a systematic review and Meta-analysis. BMC Endocr Disord. 2021;21:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3524] [Cited by in RCA: 3107] [Article Influence: 239.0] [Reference Citation Analysis (3)] |
| 6. | Kim HU, Park SP, Kim YK. Long-term HbA1c variability and the development and progression of diabetic retinopathy in subjects with type 2 diabetes. Sci Rep. 2021;11:473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Martínez-Vizcaíno V, Cavero-Redondo I, Álvarez-Bueno C, Rodríguez-Artalejo F. The Accuracy of Diagnostic Methods for Diabetic Retinopathy: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0154411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Gomez-Peralta F, Choudhary P, Cosson E, Irace C, Rami-Merhar B, Seibold A. Understanding the clinical implications of differences between glucose management indicator and glycated haemoglobin. Diabetes Obes Metab. 2022;24:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786-806. [PubMed] |
| 10. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1310] [Article Influence: 655.0] [Reference Citation Analysis (70)] |
| 11. | Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12:1322-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 12. | Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia. 2006;49:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, Narayan KM, Koch DD, Phillips LS. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Park YM, Ko SH, Lee JM, Kim DJ, Han K, Bower JK, Ahn YB; Committee of Clinical Practice Guideline, Korean Diabetes Association. Glycaemic and haemoglobin A1c thresholds for detecting diabetic retinopathy: the fifth Korea National Health and Nutrition Examination Survey (2011). Diabetes Res Clin Pract. 2014;104:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Mukai N, Yasuda M, Ninomiya T, Hata J, Hirakawa Y, Ikeda F, Fukuhara M, Hotta T, Koga M, Nakamura U, Kang D, Kitazono T, Kiyohara Y. Thresholds of various glycemic measures for diagnosing diabetes based on prevalence of retinopathy in community-dwelling Japanese subjects: the Hisayama Study. Cardiovasc Diabetol. 2014;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Xin Z, Yuan MX, Li HX, Hua L, Feng JP, Shi J, Zhu XR, Cao X, Yang JK. Evaluation for fasting and 2-hour glucose and HbA1c for diagnosing diabetes based on prevalence of retinopathy in a Chinese population. PLoS One. 2012;7:e40610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Wang B, Liu MC, Li XY, Liu XH, Feng QX, Lu L, Zhu Z, Liu YS, Zhao W, Gao ZN. Cutoff Point of HbA1c for Diagnosis of Diabetes Mellitus in Chinese Individuals. PLoS One. 2016;11:e0166597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Hu J, Gao J, Li J. Sex and age discrepancy of HbA1c and fetal hemoglobin determined by HPLC in a large Chinese Han population. J Diabetes. 2018;10:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care. 2008;31:1991-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1738] [Article Influence: 289.7] [Reference Citation Analysis (0)] |