Published online Jul 15, 2024. doi: 10.4239/wjd.v15.i7.1509
Revised: April 7, 2024
Accepted: April 26, 2024
Published online: July 15, 2024
Processing time: 133 Days and 6.8 Hours
Magnetic resonance imaging (MRI) combined with serum endothelin and galactagoglobin-3 (Gal-3) can improve the clinical diagnosis of diabetes mellitus complicated with cerebral infarction.
To analyze the clinical value of MRI combined with serum endolipin and Gal-3 for the diagnosis of cerebral infarction in the elderly with diabetes mellitus.
One hundred and fifty patients with acute cerebral infarction hospitalized between January 2021 and December 2023 were divided into two groups accor
Serum endolipin and Gal-3 expression were higher in the diabetic cerebral infarction group (P < 0.05). The arterial wall area, vessel area, normalized wall index, and lumen stenosis rate were higher in the diabetic cerebral infarction group, while the rate of arterial lumen moderate and severe stenosis was 48.39% higher (36.36%, P < 0.05). The percentage of large (29.03%) and multiple infarcts (33.87%) in the diabetic cerebral infarction group was higher (13.64% and 20.45%), and the incidence rate of lacunar infarcts was lower (37.10% vs 65.91%) (P < 0.05). The total incidence of arterial plaque in patients in the diabetic cerebral infarction group was 96.77% higher (69.32%), while the incidence of necrotic lipid core plaque was 58.06% higher (26.14%) (P < 0.05). Receiver operating characteristic curve analysis was performed to assess the diagnosis utility of these techniques. MRI in combination with serum endoglin and Gal-3 had the highest area under the curve, the Yoden index, sensitivity and specificity (P < 0.05).
The expression of serum endolipin and Gal-3 in elderly patients with diabetes mellitus with cerebral infarction showed an elevated trend, and the degree of luminal stenosis was severe. MRI predominantly revealed large and multiple infarct foci. This combined index examination can improve the clinical diagnosis of diabetes mellitus combined with cerebral infarction.
Core Tip: Magnetic resonance imaging (MRI) and serum markers aid diagnosis of cerebral infarction in elderly diabetics. Elevated serum endolipin and galactagoglobin-3 levels were observed in diabetic patients. MRI revealed severe luminal stenosis and multiple infarct foci. Combined with serum markers, MRI showed the highest diagnostic utility. This study highlights the clinical value of these techniques for diagnosing cerebral infarction in elderly diabetics.
- Citation: Zhang YH, Liang D. Magnetic resonance imaging combined with serum endolipin and galactagoglobin-3 to diagnose cerebral infarction in the elderly with diabetes mellitus. World J Diabetes 2024; 15(7): 1509-1517
- URL: https://www.wjgnet.com/1948-9358/full/v15/i7/1509.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i7.1509
Cerebral infarction is a common disease that jeopardizes the physical and mental health of patients, with mortality and disability rates exceeding those of cardiovascular diseases, making it a focus of clinical attention. Diabetes mellitus has been confirmed as an independent risk factor for cerebral infarction, as it causes intracranial macrovascular and microvascular lesions through long-term hyperglycemia which induces sustained and direct damage to patients' central nervous function; furthermore, it affects patients' synaptic plasticity, and increases the degree of neuronal damage[1]. With the deepening of clinical understanding of diabetes mellitus and cerebrovascular disease, most scholars and physicians recognize the importance of early disease prevention and early detection to achieve the goal of "early diagnosis and early intervention", reduce the risk of cerebrovascular disease and improve the prognosis of patients. Serological indicators are commonly used as clinical diagnostic items. Examples include endolipin, an adipocytokine, which functions to regulate glucose metabolism, lipid metabolism, insulin resistance, and vascular endothelial function[2]; and galactagoglobin-3 (Gal-3), a chimeric galactoglucan lectin, which promotes the activity of situational cells, monocyte chemotaxis, and release of inflammatory mediators, and participates in atherosclerosis occurrence and development process[3]. However, these serological indicators are affected by detection time, medication use, diet and other factors, which decreases their diagnostic sensitivity and specificity. Magnetic resonance imaging (MRI) is a non-invasive, high image resolution examination, which in the multi-parameter, multi-sequence mode can quantitatively assess the degree of stenosis of the carotid artery, and the location and morphology of plaque, etc., and can clearly display the specific morphology and structure of the lumen of intracranial arteries and the wall of the arteries[4]. Currently, cranial MRI is the primary means of clinical diagnosis of acute cerebral infarction, and its diagnostic value has been widely clinically recognized. However, the diagnosis of cerebral infarction combined with diabetes mellitus in the elderly using differences, and the specific imaging differences, have rarely been reported in clinical practice. The present study was therefore conducted to analyze the clinical value of MRI combined with serum endolipin and Gal-3 in the diagnosis of cerebral infarction combined with diabetes mellitus in the elderly.
In total, 150 cases of elderly patients with acute cerebral infarction admitted to the hospital between January 2021 and December 2023 were selected and divided into two groups according to the presence of comorbid diabetes mellitus, including 62 cases in the diabetic cerebral infarction group and 88 cases in the nondiabetic cerebral infarction group. A comparison of the clinical data of the patients in the two groups (P > 0.05) revealed no significant differences (Table 1).
| Indicators | Diabetic cerebral infarction group (n = 62) | Non-diabetic cerebral infarction group (n = 88) | t/χ2 | P value |
| Gender (cases) | ||||
| Male | 36 | 50 | 0.023 | 0.879 |
| Female | 26 | 38 | ||
| Smoking history (cases) | ||||
| Yes | 21 | 30 | 0.001 | 0.978 |
| No | 41 | 58 | ||
| Drinking history (cases) | ||||
| Yes | 15 | 21 | 0.002 | 0.963 |
| No | 47 | 67 | ||
| Infarction location (cases) | ||||
| Left side | 32 | 45 | 0.003 | 0.954 |
| Right side | 30 | 43 | ||
| Age (yr) | 68.85 ± 4.18 | 69.10 ± 4.24 | 0.128 | 0.721 |
| Diastolic blood pressure (mmHg) | 76.53 ± 3.34 | 76.10 ± 3.40 | 0.59 | 0.444 |
| Systolic blood pressure (mmHg) | 121.23 ± 8.53 | 120.76 ± 8.64 | 0.109 | 0.742 |
| BMI index (kg/m2) | 22.04 ± 4.16 | 21.89 ± 4.20 | 0.047 | 0.829 |
| Lesion volume (cm3) | 8.80 ± 1.16 | 8.74 ± 1.21 | 0.093 | 0.761 |
| Time from onset to admission (h) | 2.50 ± 1.03 | 2.53 ± 1.05 | 0.03 | 0.862 |
| Waist circumference (cm) | 82.05 ± 8.56 | 81.78 ± 8.62 | 0.036 | 0.85 |
The conditions for the enrollment of cases were as follows: (1) Patients enrolled in the group met the criteria for acute cerebral infarction after physical examination, cranial MRI, computed tomography, and other comprehensive examinations[5]; (2) age ≥ 60 years; (3) diabetic group: Previous examination by glucose tolerance and other tests, with results meeting the criteria for diabetes mellitus[6]; (4) complete examination data; and (5) able to understand the specific details of the study, and signed the consent form. The case exclusion criteria were: (1) Comorbid cerebral hemorrhage or cardiogenic cerebral infarction; (2) hepatic and renal insufficiency, malignant tumors; (3) coronary artery disease, hypertension, blood diseases and immune disorders; (4) secondary diabetes mellitus, diabetic ketoacidosis, hyperosmolar coma and other conditions; and (5) history of use of immunosuppressants, steroids, or other drugs in the last 4 wk.
Serological indicators: 5 mL of elbow venous blood was collected from all patients at the time of admission. Samples were centrifuged for 10 min at 3000 RPM, and the upper layer of serum samples was stored at -80 ℃. The concentrations of of endolipin and Gal-3 were detected by enzyme immunoassay using the kit from Shanghai Xitang Biotechnology Co. Before beginning the experiment, all blank, standard and sample wells were set up, and the absorbance value (OD) at 450 nm was measured on the enzyme labeling instrument to calculate the expression.
MRI examination: Patients in both groups underwent examination by cranial MRI after admission to the hospital using a Philips Achieva 3. 0 T MR diagnostic machine with 8-channel head coil. The scanning sequence was as follows: Diffusion-weighted imaging sequence: Repetition time (TR) was 2194 ms, echo time (TE) was 84 ms, field of view: 230 mm × 230 mm; matrix was 152 × 121; scanning time was 46 s; three-dimensional time-of-flight method (3D-TOF MRA): TR was 25 ms, TE was 3.5 ms, field of view was 200 mm × 200 mm; matrix was 572 × 1290 The scan time was at 548 s; T2-weighted imaging: TR of 3000 ms, TE of 53 ms, field of view of 180 mm × 200 mm; matrix of 360 × 398; scan time was at 11 min; T1WI sequence: TR of 600 ms, TE of 10 ms, field of view of 230 mm × 183 mm; matrix of 256 × 163; scan time was at 298 s; the scan range was from the carotid root to the carotid artery. The range of scanning was from the root of the carotid artery to above the corpus callosum. The scanned images were uploaded to the post-processing workstation for processing and analysis, and the lumen area (LA), wall area (WA), vessel area (VA), normalized wall index (NWI), NWI = WA/(WA + LA) × 100%, and luminal stenosis rate of the carotid vessels of the patients were measured. The patients' lesion morphology was defined as follows: Lacunar cerebral infarction, diameter ≤ 1.5 cm; large infarction, diameter > 1.5 cm; multifocal infarction, infarct lesions ≥ 2. Arterial luminal plaque formation was also assessed, with a plaque defined as a region in the localized wall of the artery that was eccentrically thickened, where the thickness of the thinnest position of the wall was less than 1/2 of the thickest part of the wall.
The observation indices for comparison between the two groups were as follows: (1) Expression of serum endolipin and Gal-3; (2) parameters of carotid artery MRI examination, including LA, WA, VA and the degree of stenosis; (3) infarction morphology, including: Luminal infarction, large infarction, multiple infarcts; (4) the nature of plaques, e.g. necrotic liponucleated plaques, plaque hemorrhage, plaque ulceration and plaque calcification incidence; and (5) arterial lumen stenosis: Mild, moderate, and severe stenosis: Stenosis < 50%, 50%-69%, and > 69%.
All statistical analyses were performed using SPSS26.0 statistical software. Measured data are shown as the mean ± SD, and comparisons were performed using independent samples t-test is used when it meets the normal distribution; continuous variable data, if non-normally distributed. Non-normally distributed data are expressed as M (P25, P75), and were compared using the Mann-Whitney U-test. Counting data are expressed as a rate (%), and were compared using a χ2 -test. Rank data were tested by the rank-sum Z-test. Analysis of the diagnostic value of MRI in combination with serological indicators of the disease was calculated using the receiver operating characteristic curve (ROC), and the difference was statistically significant when P < 0.05.
Serum endolipin and Gal-3 expression in the diabetic cerebral infarction group were higher than those in the nondiabetic cerebral infarction group (P < 0.05; Table 2).
| Group | Visfatin (µg/L) | Gal-3 (ng/mL) |
| Diabetic cerebral infarction group (n = 62) | 36.58 ± 8.12 | 10.58 ± 3.48 |
| Non-diabetic cerebral infarction group (n = 88) | 26.13 ± 6.82 | 6.28 ± 2.39 |
| t | 8.536 | 8.975 |
| P value | < 0.001 | < 0.001 |
Arterial VA, WA, NWI, and lumen stenosis rates were higher in the diabetic cerebral infarction group than in the nondiabetic cerebral infarction group (P < 0.05), while LA showed no difference between the two groups (P > 0.05), as shown in Table 3.
| Group | VA (mm2) | LA (mm2) | WA (mm2) | NWI | Stenosis rate (%) |
| Diabetic cerebral infarction group (n = 62) | 131.28 ± 24.23 | 67.14 ± 18.53 | 58.15 ± 6.64 | 0.48 ± 0.10 | 48.52 ± 12.08 |
| Non-diabetic cerebral infarction group (n = 88) | 118.82 ± 21.02 | 65.82 ± 16.20 | 48.28 ± 6.35 | 0.42 ± 0.08 | 36.12 ± 8.83 |
| t | 3.555 | 0.463 | 9.199 | 4.075 | 7.264 |
| P value | 0.001 | 0.644 | < 0.001 | < 0.001 | < 0.001 |
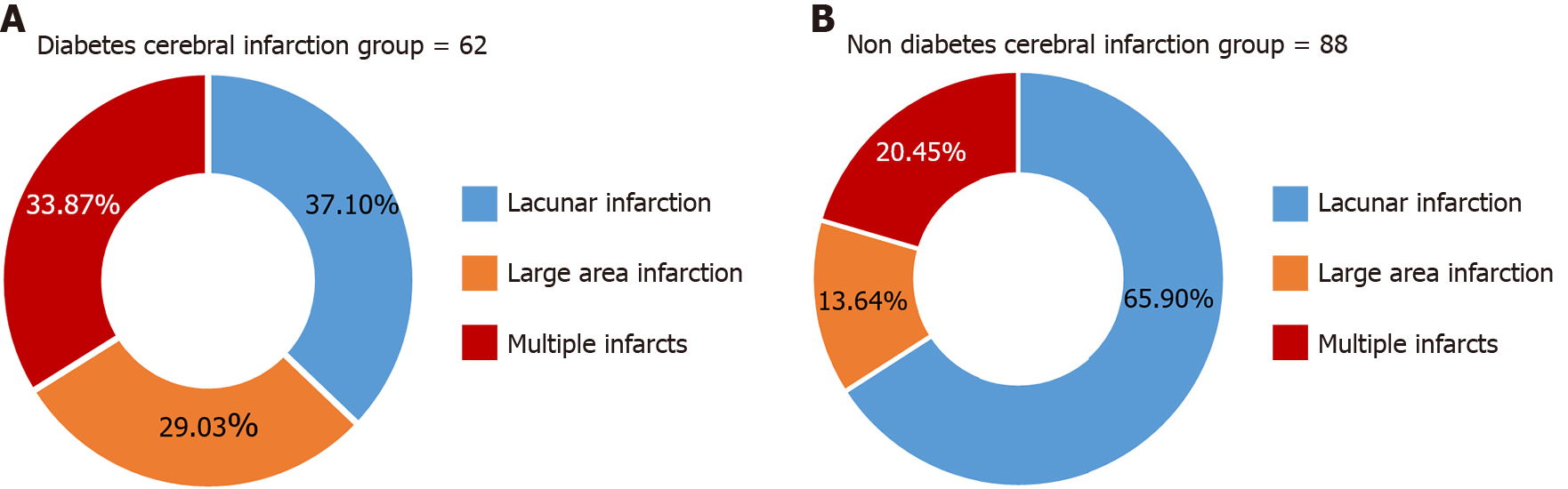
The percentage of large infarcts (29.03%) and multiple infarcts (33.87%) in the diabetic cerebral infarction group was higher than that of the non-diabetic cerebral infarction group (13.64% and 20.45%, respectively) and the incidence rate of lacunar infarcts was lower than that of the non-diabetic cerebral infarction group (65.91%), and the difference was statistically significant (Z = 5.339; P = 0.020), as shown in Figure 1.
The total incidence of arterial plaques in the diabetic cerebral infarction group was 96.77% higher than that in the nondiabetic cerebral infarction group (69.32%), while the incidence of necrotic liponuclear plaques was 58.06% higher than that in the nondiabetic cerebral infarction group (26.14%) (P < 0.05). However, there was no difference in the incidence of intra-plaque hemorrhage, plaque ulceration, and plaque calcification between the two groups (P > 0.05), as shown in Table 4.
| Group | Necrotic lipid nuclear plaque | Plaque intraplaque hemorrhage | Plaque ulceration | Plaque calcification | Total |
| Diabetic cerebral infarction group (n = 62) | 36 (58.06) | 8 (12.90) | 3 (4.84) | 13 (20.97) | 60 (96.77) |
| Non-diabetic cerebral infarction group (n = 88) | 23 (26.14) | 15 (17.05) | 10 (11.36) | 13 (14.77) | 61 (69.32) |
| Z | 15.539 | 0.481 | 1.956 | 0.974 | 17.582 |
| P value | < 0.001 | 0.488 | 0.162 | 0.324 | < 0.001 |
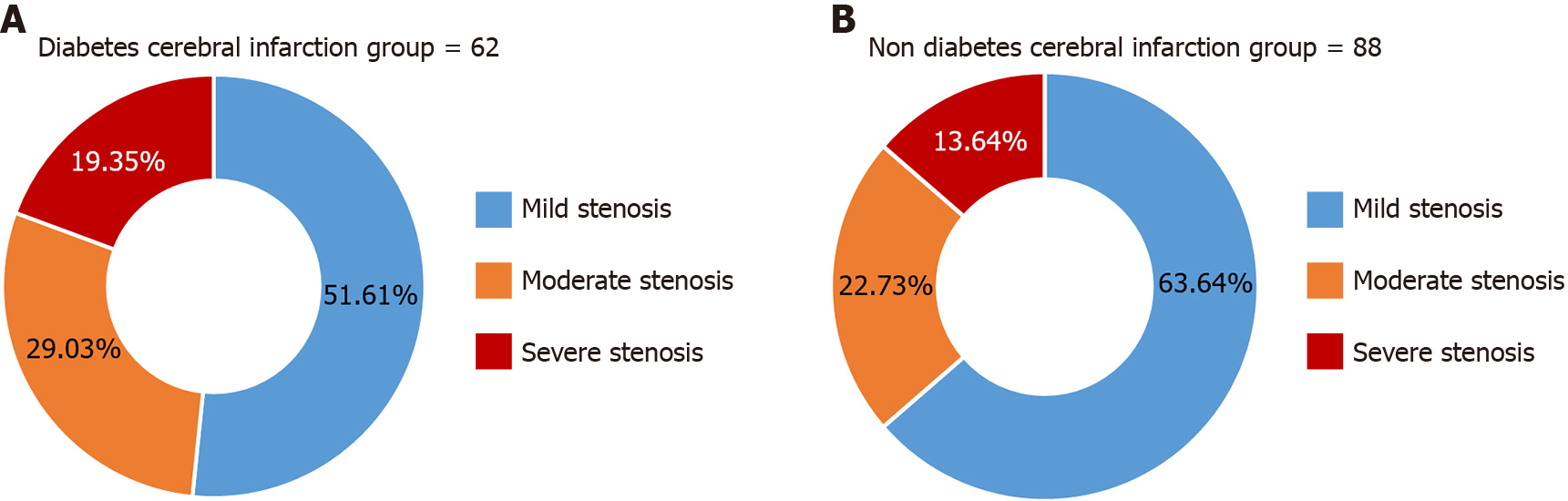
The rate of moderate and severe stenosis of the arterial lumen of patients in the diabetic cerebral infarction group was 48.39% higher than that of the non-diabetic cerebral infarction group, which was 36.36%, and the difference was statistically significant (Z = 10.777, P = 0.001), as shown in Figure 2.
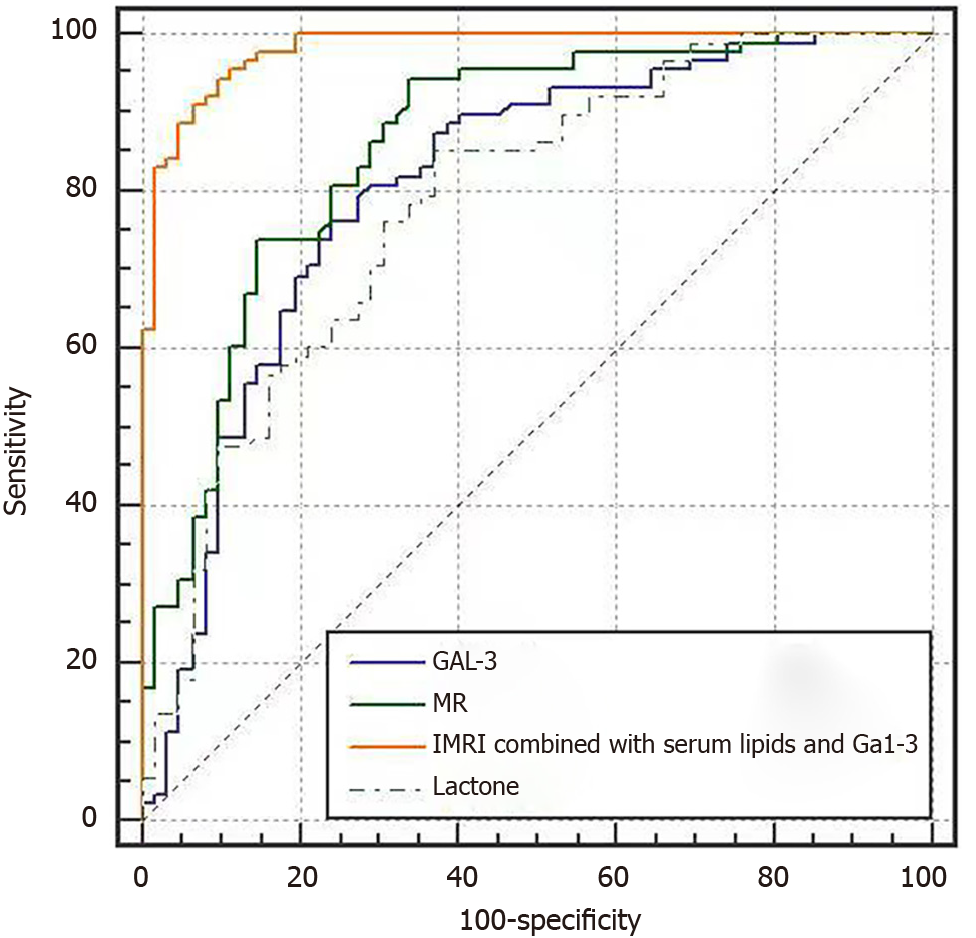
ROC curve analysis showed that MRI combined with serum endolipin and Gal-3 had the highest area under the curve (AUC), Jordon's index, sensitivity, and specificity for the diagnosis of diabetic cerebral infarction (P < 0.05), as shown in Figure 3 and Table 5.
| Indicators | AUC | Youden index | Sensitivity (%) | Specificity (%) | SE | 95%CI |
| MRI | 0.858 | 0.605 | 94.30 | 66.10 | 0.0317 | 0.792-0.910 |
| Visfatin | 0.784 | 0.481 | 85.20 | 62.90 | 0.0383 | 0.710-0.847 |
| Gal-3 | 0.807 | 0.521 | 79.50 | 72.60 | 0.0378 | 0.735-0.867 |
| MRI combined with serum Visfatin and Gal-3 | 0.981 | 0.846 | 94.30 | 90.30 | 0.00865 | 0.944-0.996 |
In recent years, the incidence of diabetes mellitus and acute cerebral infarction has increased. Diabetes mellitus is an independent risk factor for acute cerebral infarction, with one study showing that diabetic patients are 3.6 times more likely to suffer from acute cerebral infarction than non-diabetic individuals[7]. This is because long-term hyperglycemia results in damage to the endothelial cells of the blood vessels, reducing the elasticity of the blood vessel wall and increasing the activity of platelets and lipid metabolism, resulting in hypercoagulability of the blood and the continuous release of oxygen radicals. This can affect the patient’s microcirculation, inducing atherosclerosis, which is the pathological mechanism underlying cerebrovascular diseases[8]. Diabetes mellitus has also been indicated as a poor prognostic marker of acute cerebral infarction. Indeed, one study[9] showed that the total effective rate of treatment of acute cerebral infarction was 60% lower in patients with diabetes mellitus than in those without (80%). Shou et al[10] reported that patients with a glycated hemoglobin > 8.2% have a worse short-term prognosis, and the AUC of predicting the patient's short-term prognosis of adverse events is 0.842. Therefore, among elderly patients with diabetes mellitus combined with cerebral infarction, early diagnosis and treatment is essential to prevent the disease from progressing, which in turn will provide a reference basis for the clinical treatment.
Serological indicators are commonly used for clinical diagnosis. Endolipin is a serological marker which is widely distributed in visceral adipocytes, while Gal-3 is mainly distributed in neutrophils, macrophages, etc., and is often used as an inflammatory marker. Both of these markers have been extensively studies in cardiovascular and cerebrovascular diseases and research. In a prior study, Ruan et al[11] showed that endolipin is an independent risk factor for the occurrence of stroke in hypertensive patients. Susairaj et al[12] showed that serum endostatin predicted type 2 diabetes mellitus with an AUC of 0.77 and a sensitivity and specificity of 75%. Kumar et al[13] stated that Gal-3 expression is closely related to the risk of microvascular complications in patients with type 2 diabetes mellitus, and could be used as a valid factor in the diagnosis and evaluation of microvascular and macrovascular complications in patients with type 2 diabetes mellitus. Tan et al[14] showed that when serum Gal-3 expression was elevated, the risk of carotid atherosclerosis and cardiovascular events and all-cause mortality was significantly higher. The present study showed that serum endolipin and Gal-3 expression were higher in diabetic cerebral infarction group than in non-diabetic cerebral infarction group (P < 0.05). These results suggest that serum endolipin and Gal-3 expression are significantly elevated in elderly patients with acute cerebral infarction combined with diabetes mellitus, with levels higher than those with acute cerebral infarction alone. In elderly patients with combined cerebral infarction and diabetes mellitus, long-term hypertension, abnormal lipid metabolism, insulin resistance, and endothelial function abnormality all cause atherosclerosis, increase the thickness of capillary basement membrane, and subsequently increase the risk of cerebral infarction, of which lipocalin and Gal-3 are closely related to their pathological mechanisms. Endostatin can promote the expression of triacylglycerol, induce the expression of fatty acid synthase, lipocalin and other adipocyte differentiation factors, promote the generation of foam cells, and induce the formation of atherosclerotic plaques. Further, endostatin is mainly distributed in macrophages, which function to regulate the body's inflammatory response; as such, we speculate that endostatin functions as a pro-inflammatory factor, inducing atherosclerosis, increasing the risk of acute cerebral infarction.
In regards to acute cerebral infarction risk, insulin resistance leads to endothelial dysfunction through lipotoxicity and glucotoxicity, inducing atherosclerosis. When diabetic patients lack or resist insulin secretion, long-term hyperglycemic stimulation promotes the expression of endolipoxins, and the subsequent sustained expression of endolipoxins damages insulin receptors, exacerbating insulin resistance[15]. Therefore, endostatin may be involved in the processes underlying diabetes and atherosclerosis by affecting glucose and lipid metabolism, vascular inflammatory response, insulin resistance, vascular endothelial function, etc. We found that endostatin it is highly expressed in diabetic patients with cerebral infarction, where it may exacerbate the risk of disease pathology. Gal-3 is primarily produced after macrophage activity, which can activate macrophage activity, promote the release of inflammatory factors, and inhibit the proliferation of smooth muscle cells and promote the formation of atherosclerotic plaques[16]. Therefore, Gal-3 is involved in the process of diabetes and atherogenesis by regulating the body's inflammatory response, and the two influence each other, which results in abnormally elevated Gal-3 expression, which is significantly higher than that in patients with acute cerebral infarction. However, when diagnosing diabetic cerebral infarction only based on serological indexes, the lack of intuitive and objective results presented makes its clinical diagnostic value low.
MRI is an important method for the clinical diagnosis of cerebrovascular diseases, as it can clearly show the degree of stenosis of vascular lumen and the location of infarction foci, and thus provide a reference basis for the diagnosis of cerebral infarction. The present study showed that the rate of arterial LA, WA, NWI and luminal stenosis in the diabetic cerebral infarction group was higher than that in the nondiabetic cerebral infarction group, as were the rates of large and multiple infarcts (29.03% vs 13.64% and 33.87% vs 20.45%, respectively,). Furthermore, the total incidence of arterial plaques was higher in the diabetic than the non-diabetic group (96.77% vs 69.32%). Among them, the incidence of necrotic lipid core plaque was higher in the diabetic group (58.06% vs 26.14%) (P < 0.05). The results showed that patients with diabetic cerebral infarction had a higher risk of carotid plaque formation, and the infarct area was large and more serious. Liu et al[17] found that the percentage of multiple cerebral infarcts in patients with diabetic cerebral infarction group was 59.26% higher than that in the simple cerebral infarction group (35.19%), and the degree of cerebral vascular stenosis was significantly higher (P < 0.05). Another study has shown that in patients with acute cerebral infarction combined with diabetes mellitus, long-term hyperglycemic stimulation will aggravate vascular hypoxia and vascular endothelial dysfunction, resulting in atherosclerosis and plaque formation, and predominantly unstable plaques, causing extensive and small vascular lesions[18]. Simultaneously, hyperglycemia promotes apoptosis of the ischemic hemidiaphragm zone, thus increasing the area of infarcted area, and the risk of cerebral ischemia. In addition, hyperglycemia will can affect lipid metabolism, impairing the function of vascular endothelium, promoting platelet adhesion and aggregation, and affecting the patients' compensatory function of the collateral circulation, thereby increasing the infarcted foci area and number of the patients, and aggravating arterial stenosis degree[19].
In this study, ROC curve analysis showed that MRI combined with serum endolipin and Gal-3 had the highest AUC, Jordon's index, sensitivity, and specificity for diagnosing diabetic cerebral infarction (P < 0.05). These results indicate that combining MRI with serum endolipin and Gal-3 could allow effective diagnosis of diabetes mellitus combined with cerebral infarction. Diabetes mellitus is an important risk factor for cerebral infarction. Under long-term hyperglycemia, it promotes an increase in metabolites, causing endothelial damage and vascular inflammation. Diabetes mellitus is often accompanied by obesity, an increased waist-to-hip ratio, dyslipidemia and other risk factors, which will aggravate arterial endothelial damage, intima-media thickening, aggravating arterial stenosis and occlusion, and inducing cerebral infarction. The series of pathological processes induced by diabetes mellitus are related to serum endostatin and Gal-3, which are highly expressed in patients with diabetic cerebral infarction. Due to the small, tortuous and complex distribution structure of intracranial arterial vessels, it is impossible to accurately evaluate the degree of lesions in patients based on lumen stenosis alone. In cranial MRI diagnosis, the application and development of various functional imaging modes can effectively inhibit blood flow and cerebrospinal fluid signals, reduce the impact of volume effect on image diagnosis, so as to clearly display the specific structure and morphology of the vascular lumen, as well as show the signal changes and nature of the arterial lumen plaques. In addition, MRI can clearly reflect the extent and scope of atherosclerosis, to achieve the quantitative evaluation of the arterial plaques, and effectively evaluate their nature, thereby providing a reference for the diagnosis of the disease[19]. As such, MRI combined with serum endolipin and Gal-3 is of great significance in disease diagnosis. This modality can not only clearly and objectively show the structure and extent of lesions, but can also predict the risk of acute cerebral infarction in diabetic patients in advance.
In summary, the present study showed that serum endolipin and Gal-3 expression were abnormally elevated in elderly patients with diabetes mellitus combined with cerebral infarction, and MRI examination showed that patients with diabetes mellitus combined with cerebral infarction had severe stenosis of arterial lumen, which was dominated by large infarcts and multiple infarcts, and the combination of the indexes can improve the diagnostic value of diabetes mellitus combined with cerebral infarction and provide a reference for the clinical diagnosis.
| 1. | Kudo I, Sasaki M, Suzuki A, Ishikawa T. Functional outcomes and associated factors of cerebral infarction and intracerebral hemorrhage in an area with aging populations in change over time: Evidence from the Akita Stroke Registry. Geriatr Gerontol Int. 2023;23:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 2. | Jianjun L, Xiaona L, Hong L. The effect of visfatin genotype on insulin pump therapy on quality of life in patients with type I diabetes. Cell Mol Biol (Noisy-le-grand). 2022;67:195-202. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Nishikawa H, Nakatsuka Y, Shiba M, Kawakita F, Fujimoto M, Suzuki H; pSEED group. Increased Plasma Galectin-3 Preceding the Development of Delayed Cerebral Infarction and Eventual Poor Outcome in Non-Severe Aneurysmal Subarachnoid Hemorrhage. Transl Stroke Res. 2018;9:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Zheng D, Li X, Fu Y. Risk factors of acute cerebral infarction in patients with primary hypertension. Ir J Med Sci. 2023;192:2441-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Sveinsson ÓÁ, Kjartansson Ó, Valdimarsson EM. [Cerebral ischemia/infarction - diagnosis and treatment]. Laeknabladid. 2014;100:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Im K, Ju H, Lee M, Joo BE, Kwon KY, Roh H, Ahn MY, Hwang HW, Lee KB. Recent glycemic control can predict the progressive motor deficits of acute subcortical infarction with diabetes or prediabetes. Neurol Sci. 2021;42:285-291. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Cho KH, Kwon SU, Lee JS, Yu S, Cho AH. Newly diagnosed diabetes has high risk for cardiovascular outcome in ischemic stroke patients. Sci Rep. 2021;11:12929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Georgakis MK, Harshfield EL, Malik R, Franceschini N, Langenberg C, Wareham NJ, Markus HS, Dichgans M. Diabetes Mellitus, Glycemic Traits, and Cerebrovascular Disease: A Mendelian Randomization Study. Neurology. 2021;96:e1732-e1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Shou GL, Luo S, Zhu FF, Xu YY, Li YZ, Zhao H. Correlation between glycated hemoglobin and early neurological function and short-term prognosis in patients with type 2 diabetes mellitus complicated with acute cerebral infarction. Jiangsu Daxue xuebao (Yixueban). 2022;32:172-175. [DOI] [Full Text] |
| 11. | Ruan W, Zhai X, Guan L, Wei X, Zhang S. Expressions of serum adiponectin and visfatin in patients with hypertension in cerebrovascular accidents and analysis of risk factors. Am J Transl Res. 2022;14:7852-7859. [PubMed] |
| 12. | Susairaj P, Snehalatha C, Nanditha A, Satheesh K, Raghavan A, Vinitha R, Ramachandran A. Analysis of an Indian diabetes prevention programme on association of adipokines and a hepatokine with incident diabetes. Sci Rep. 2021;11:20327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kumar S, Ranawat CS, Bhandiwad C, Arya H, Mali M, Singh CP, Sharma N, Lathwal N, Wasim S. Galectin-3 as a Potential Biomarker of Microvascular Complications in Patients with Type 2 Diabetes. Indian J Endocrinol Metab. 2022;26:490-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Tan KCB, Cheung CL, Lee ACH, Lam JKY, Wong Y, Shiu SWM. Galectin-3 and risk of cardiovascular events and all-cause mortality in type 2 diabetes. Diabetes Metab Res Rev. 2019;35:e3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases. 2022;10:10840-10851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Manouchehrian O, Andersson E, Eriksson-Hallberg B, Deierborg T. Galectin-3 ablation does not affect infarct size or inflammatory cytokines after experimental stroke in 24-month-old female mice. Neuroreport. 2022;33:266-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Liu B, Tong C, Wu XQ. Differential diagnostic value of head and neck CTA on diabetic cerebral infarction. Yixue Yingxiangxue Zazhi. 2022;7:1230-1233. |
| 18. | Maida CD, Daidone M, Pacinella G, Norrito RL, Pinto A, Tuttolomondo A. Diabetes and Ischemic Stroke: An Old and New Relationship an Overview of the Close Interaction between These Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 19. | Sawamura T, Sawada K, Ohmori A. Fresh Cerebral Infarction-Like MRI Findings Mimicking Hyperosmolar Hyperglycemic Syndrome With Seizures. Cureus. 2022;14:e25675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |