Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1340
Revised: March 12, 2024
Accepted: April 15, 2024
Published online: June 15, 2024
Processing time: 113 Days and 6.7 Hours
The mechanism of improvement of type 2 diabetes after duodenal-jejunal bypass (DJB) surgery is not clear.
To study the morphological and functional changes in adipose tissue after DJB and explore the potential mechanisms contributing to postoperative insulin sensitivity improvement of adipose tissue in a diabetic male rat model.
DJB and sham surgery was performed in a-high-fat-diet/streptozotocin-induced diabetic rat model. All adipose tissue was weighed and observed under mi-croscope. Use inguinal fat to represent subcutaneous adipose tissue (SAT) and mesangial fat to represent visceral adipose tissue. RNA-sequencing was utilized to evaluate gene expression alterations adipocytes. The hematoxylin and eosin staining, reverse transcription-quantitative polymerase chain reaction, western blot, and enzyme-linked immunosorbent assay were used to study the changes. Insulin resistance was evaluated by immunofluorescence.
After DJB, whole body blood glucose metabolism and insulin sensitivity in adipose tissue improved. Fat cell volume in both visceral adipose tissue (VAT) and SAT increased. Compared to SAT, VAT showed more significantly functional alterations after DJB and KEGG analysis indicated growth hormone (GH) pathway and downstream adiponectin secretion were involved in metabolic regulation. The circulating GH and adiponectin levels and GH receptor and adiponectin levels in VAT increased. Cytological experiment showed that GH stimulated adiponectin secretion and improve insulin sensitivity.
GH improves insulin resistance in VAT in male diabetic rats after receiving DJB, possibly by increasing adiponectin secretion.
Core Tip: Our results provide focused insight into the effects of duodenal-jejunal bypass (DJB) on adipose tissue function and insulin resistance in diabetic male rats. Unlike previous studies that often focused on broader metabolic improvements after DJB, this study focused on adipocytes and their molecular changes. Establishing a link between growth hormone (GH), adiponectin, and insulin sensitivity by identifying alterations in the GH pathway.
- Citation: Liu ZT, Yang GW, Zhao X, Dong SH, Jiao Y, Ge Z, Yu A, Zhang XQ, Xu XZ, Cheng ZQ, Zhang X, Wang KX. Growth hormone improves insulin resistance in visceral adipose tissue after duodenal-jejunal bypass by regulating adiponectin secretion. World J Diabetes 2024; 15(6): 1340-1352
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1340.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1340
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by elevated blood glucose levels, frequently resulting in detrimental health complications. The prevalence of T2DM is notably augmented by obesity, and weight loss can effectively improve metabolism and ameliorate insulin resistance[1,2]. Both human and animal studies have demonstrated the efficacy of metabolic surgery in maintaining weight loss and improving metabolism. Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) are most widely performed worldwide at the moment[3-6]. Duodenal-jejunal bypass (DJB), a modified operation of RYGB, involving duodenal-jejunal anastomosis without gastric resection, has the capacity to induce rapid and durable improvement in glucose tolerance in both rodent models and humans[7-9]. However, unlike SG and RYGB, DJB does not reduce body weight, and therefore, DJB is more used as an experimental model for weight-loss independent mechanism investigation.
Obesity primarily presents as the accumulation of fat cells, yet not all instances of fat accumulation result in metabolic abnormalities. Research has indicated that adipose tissue and adipokines play a significant role in metabolic regulation and amelioration of insulin resistance[10,11]. Adipose tissue could be roughly classified as visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), which exhibit distinct functions. Previous research has regarded SAT as advantageous adipose tissue due to its heightened insulin sensitivity and pronounced affinity for circulating free fatty acids and triglycerides, thereby facilitating metabolic processes. Conversely, VAT has been identified as a significant contributor to metabolic disorders, with increased amounts of VAT being associated with aggravated insulin resistance[12-15]. Following DJB, the body exhibited metabolically healthy obesity and we found that insulin resistance in adipose tissue improved, suggesting that DJB has significant effect on adipose tissue alterations. However, the specific changes, either morphological or functional, of adipose tissues after DJB have not been investigated. Therefore, the present study aims to evaluate every specific adipose tissue change and to explore the potential mechanisms contributing to postoperative metabolic improvement based on RNA sequencing.
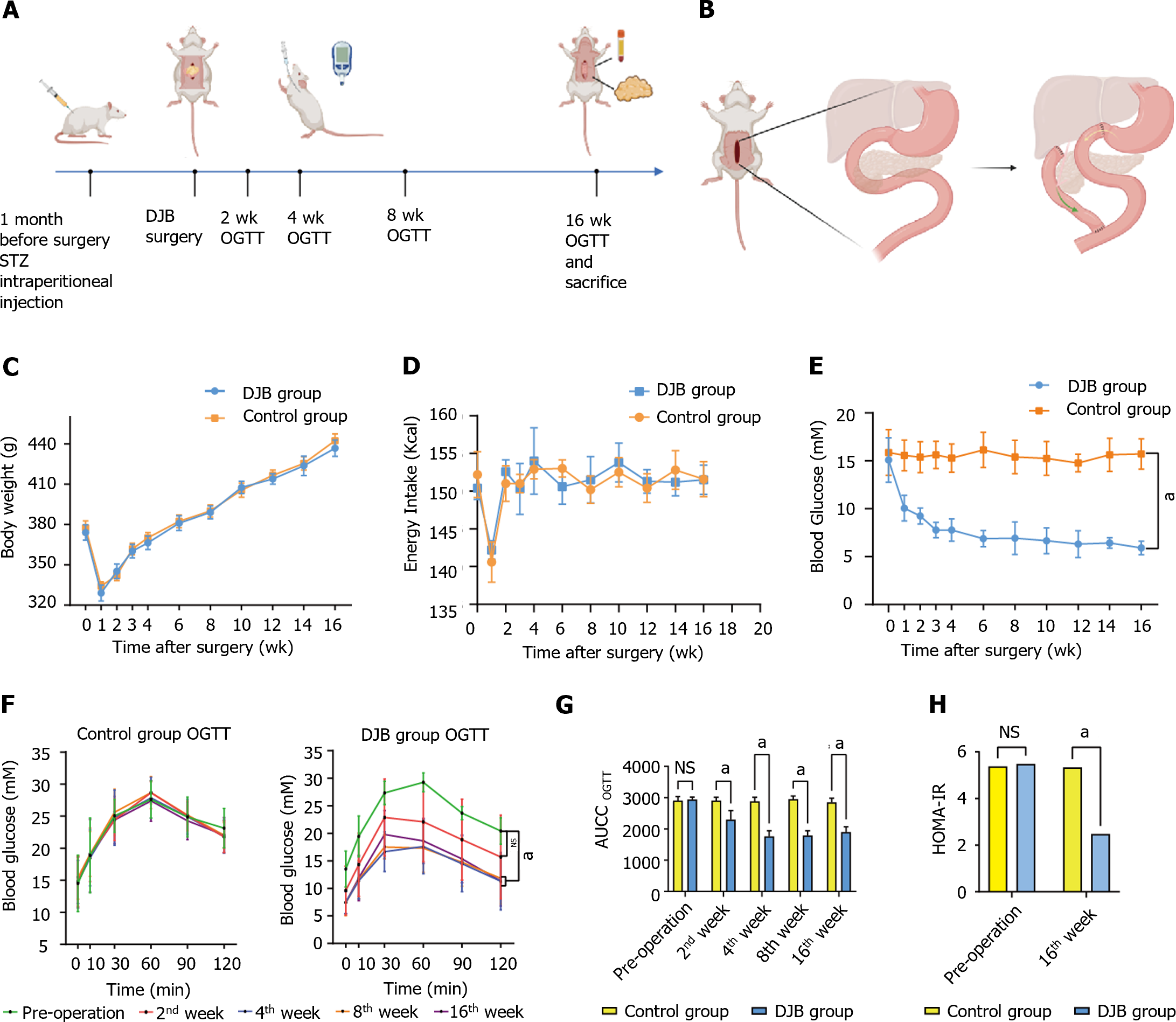
Six-week-old male Wistar rats (200 g on average, Huafukang, China) were individually housed in independent ventilated cages with 12-h light/dark cycle under constant temperature (24–26°C) and humidity (50%–70%). After 1 wk of adaptive feeding, the rats were administered a high-fat diet (HFD, 45% Calories from Fat, Xietong Pharmaceutical Bio-engineering Co, China), for 4 wk to induce insulin resistance. After 12-h fasting, 35 mg/kg streptozotocin (STZ; Sigma Aldrich, United States) dissolved in sodium citrate buffer was injected intraperitoneally to induce a diabetic state. Random blood glucose levels were measured after 72 h. The rats were then continued to be fed with a HFD. After 2 wk, an oral glucose tolerance test (OGTT) was conducted, and 20 rats with random blood glucose over 16.7 mmol/L were randomly allocated to DJB group and control group (n = 10 each). All animal experimental procedures in this study were approved by the Animal Care and Utilization Committee of Qilu Hospital of Shandong University in Jinan, China.
One month after STZ injection, all diabetic rats were subjected to a residue-free diet (Enteral Nutritional Powder, 3%, 13.5 Kcal, Abbott Laboratories, China) for 3 d. After a 12-h fasting, rats underwent DJB or SHAM operation (n = 10 each). OGTT was conducted at specific intervals of 2, 4, 8, and 16 wk after operation. Following an 8-h fasting, all rats were administered 20% glucose (5 mL/kg) via oral gavage. Subsequently, blood glucose levels were measured at 5 distinct intervals (0, 10, 30, 60, and 120 minutes). Finally 20 rats all survived until the end of the study. All rats were sacrificed 4 months after operation. Blood and tissue samples were collected for later tests (Figure 1A).
DJB was conducted in 10 diabetic rats. The rats were weighed under inhalational anesthesia using isoflurane at a concentration of 3% and a flow rate of 0.25 L/min. Pentobarbital was injected intraperitoneally at a concentration of 0.3% and 30 mg/kg dosage. The duodenum was transected 1 cm distal to the pylorus, and the stump was closed by 7–0 nylon suture (Ningbo medical, China). Then the jejunum was transected 10 cm distal to the ligament of Treitz, and the distal end was anastomosed to the proximal end of the duodenum (duodenojejunal anastomosis). At last, the biliopancreatic limb was anastomosed to the alimentary limb 15 cm distal to the duodenojejunal anastomosis in a Roux-en-Y fashion[16] (Figure 1B).
The rats in the control group underwent sham surgery. The bowels were transected at the sites where enterotomies were performed in DJB, and reanastomosis was made in situ. The operation time was prolonged similar to that of DJB to acquire similar surgical and anesthetic stress.
The adipose tissue was categorized into two distinct types: VAT and SAT. VAT encompassed various intra-abdominal fat deposits, including mesangial, perirenal, epididymal, retro-abdominal and round ligament fat of the liver. SAT consisted of inguinal fat, waist and back fat, hip fat, armpit fat and neck fat. The collected specimens were subsequently weighed and subjected to comparative analysis. Specifically, we chose mesangial fat and inguinal fat as representatives to analyze the functional difference between VAT and SAT.
The adipose tissue samples from the DJB and control groups were preserved in RNA later (Beyotime, China). Total RNA was extracted by Trizol reagent (Thermofisher, 15596018). The high-quality RNA samples obtained were utilized to construct a sequencing library. The mRNA was fragmented into shorter fragments and subjected to 2 × 150 bp paired-end sequencing (PE150) on an Illumina Novaseq™ 6000 (LC-Bio Technology CO. Ltd, China). The study involved comparing the results between the DJB and control groups, while also conducting an intra-group analysis to examine the distinctions between VAT and SAT.
The tissues from DJB and control groups were embedded in paraffin and subsequently transformed into paraffin sections. Hematoxylin-eosin staining was conducted on the sections to examine the morphological alterations of adipocytes.
Serum samples were subjected to analysis of growth hormone (GH), adiponectin, leptin, and adipsin concentrations using ELISA Kit (Keyybio, China), following the manufacturer's protocols. Absorbance at a wavelength of 450 nm was measured using a microplate reader. The concentrations of adiponectin and GH in serum samples were determined using the standard curve interpolation method.
The C3H10T1/2 cells were cultured using Dulbecco's modified Eagle's medium (DMEM, BasalMedia, China) supplemented with 10% fetal bovine serum (FBS, Gibco, United States) and penicillin-streptomycin (NCMbio, China) at a temperature of 37°C in a 5% CO2 incubator. The mature adipocyte model was induced through adipocyte cocktail induction protocol. High-fat and high-glucose environment simulation was performed through sodium palmitate (PA, Meilunbio, China)[17,18]. This medium comprised 0.3 μmol/L sodium palmitate (Meilunbio) in DMEM, with the glucose concentration adjusted to 16.7 mmol/L.Mature adipocytes were raised in HG (high glucose) and PA (palmitic acid) medium for 48 h, then stimulated by 25 ug/L GH (MCE) for 48 h.
Primary VAT cells were isolated using collagenase-type I (Sigma, United States) digestion, followed by density separation. In brief, VAT tissue was minced and digested in 1 mg/mL collagenase at 37°C for 60 min. The digestion process was halted using DMEM/F12 (BasalMedia, China) containing 20% FBS (Gibco, United States) and 1% penicillin-streptomycin (Biosharp, China). Subsequently, the digested mixture was centrifuged for 10 min at room temperature and 1700 × g. The resulting sediment was re-suspended in DMEM/F12 supplemented with 20% FBS and diluted to a final concentration of 106 cells/mL. These cells were then cultured in a humidified atmosphere at 37°C containing 5% CO2.
Following successful differentiation, adipocytes were treated with a newly prepared Oil red O dyeing solution (Solarbio, China) and were immersed. The water was washed two to five times until all excess dye was removed. Mayer hematoxylin staining solution (Solarbio, China) was added to counterstain the nucleus. Finally, the cells were covered with distilled water and were observed under a microscope.
The cells and adipose tissue were added to the lysis buffer. After lysis, the lysates were separated by centrifugation at 13000 g for 5 min at 4°C. The lysates were then supplemented with 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample loading buffer and heated at 95°C for 5 min. Immunoblotting was conducted using 10% Bis-Tris gels (Epizyme, China) for 2 h at 80 V. Following gel electrophoresis, the proteins were transferred onto polyvinylidene fluoride membranes (0.2 μm, Millipore, United States). The membranes were blocked and then incubated with primary antibodies at 4°C or an extended duration, and were then incubated with secondary antibody for 1 h. Protein expression was visualized using anti-rabbit horseradish peroxidase and detected through chemiluminescence with the ECL luminescence reagent (Millipore, United States).
The total RNA was extracted using the RNA fast 200 test kit (Fastagen, China) and cDNA transcription using the Rever Tra qPCR reaction RT Kit (TOYOBO, Japan). The qPCR was carried out using the SYBR Green Realtime polymerase chain reaction Master Mix (TOYOBO, Japan), and the reaction was conducted using the LightCycler 480 Instrument II. Gene expression levels were determined using the 2–∆∆Ct method. The primers used are shown in Table 1.
| Genes | Forward | Reverse |
| β-tublin (rat) | 5′-CGTCCACCTTCATCGGCAACAG-3′ | 5′-TCGGCCTCGGTGAACTCCATC-3′ |
| Adiponectin (rat) | 5’-TCTTCATTCCTGTCTGTACGAGTG-3’ | 5’-CCTTCATGACTGGGCAGGATTAA-3’ |
| GHR (rat) | 5’-GATCTTTGGCGGGTGTTCTTAAC-3’ | 5’-ACTTTCCCTCAGGCTTGGATTAA-3’ |
| Adipsin (rat) | 5’-CCTGTCCAGTCCTGAACCCTAC-3’ | 5’-AGTGAGGCATTGTGGGAGAGC-3’ |
| Leptin (rat) | 5’-TCCTGTGGCTTTGGTCCTATCTG-3’ | 5’-CCTGGTGACAATGGTCTTGATGAG-3’ |
| β-actin (mouse) | 5’-TGACCCAGGACTCTCTCTTCTATGA-3’ | 5’-GAGCGCGTAACCCTCATAGAT-3’ |
| Adiponectin (mouse) | 5’-ACTAATGAGACCTGGCCACTTTC-3’ | 5’-AACAGGAGAGCTTGCAACAGTAG-3’ |
| GHR (mouse) | 5’-CATGCTACTGGACAGAAGGAGAT-3’ | 5’-CTGGGTCCATTCATGAGCAATTC-3’ |
| Adipsin (mouse) | 5’-GATGCAGTCGAAGGTGTGGTTAC-3’ | 5’-CGGTAGGATGACACTCGGGTATAG-3’ |
| Leptin (mouse) | 5’-TCCTGTGGCTTTGGTCCTATCTG-3’ | 5’-CCTGGTGACAATGGTCTTGATGAG-3’ |
For lentivirus constructs, the short hairpin RNA for Adiponectin was cloned into a hU6-MCS-CBh-gcGFP-IRES-puromycin (GV493, Jikai Genechem, China) vector. After lentiviral-mediated transfection and puromycin selection, transfection efficiency was verified by reverse transcription-qPCR and WB.
Mature adipocytes were fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.25% Triton X-100 for 10 min, and then blocked with 10% goat serum for 1 h at room temperature while protected from light. The cell coverslips were incubated with antibodies against GLUT4 (Proteintech, China) or INSR (Proteintech, China) overnight at 4 ℃. The next day, the bound primary antibodies were detected using fluorescence-conjugated goat anti-rabbit IgG (H + L; Proteintech, China) for 1 h at room temperature. The coverslips were then mounted on slides with mounting medium containing DAPI (Abcam), and the fluorescence signal was imaged using a Zeiss Axio Vest.A1 fluorescence microscopy.
The statistical analyses were conducted using IBM SPSS Statistics version 24.0 (IBM, United States), employing the two-tailed Student's t-test, one-way ANOVA, or two-way ANOVA. The data are presented as mean ± SD. Statistical significance is denoted by P < 0.05, P < 0.01, and P < 0.001, and ns indicates no significance. The statistical details are included in the respective figure legends.
There were ten rats in DJB group and ten rats in control group, and all survived until the end of the study.
Baseline body weight did not differ between DJB and control groups. There was no difference in body weight or energy intake between DJB and control groups postoperatively (Figure 1C and D). Consistent with our previous report[16], DJB group showed much better fasting blood glucose and glucose tolerance than control group (Figure 1E-G). At week 16 postoperatively, insulin resistance was greatly improved after DJB compared to control group based on the homeostasis model of assessment for insulin resistance index (2.474 ± 0.043 vs 5.339 ± 0.169, P < 0.001, Figure 1H).
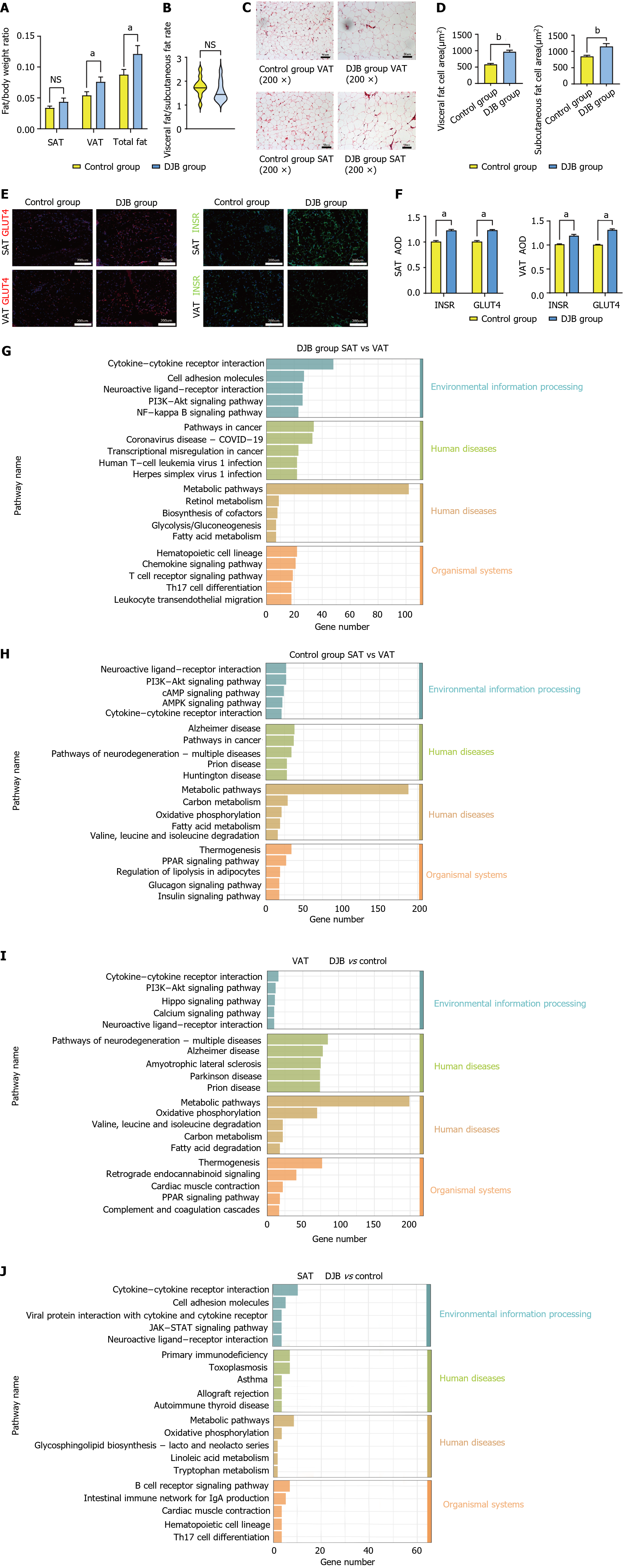
At week 16 postoperatively, the weight proportion of both total body adipose tissue and VAT in DJB group was significantly greater than that in control group (0.121 ± 0.013 vs 0.087 ± 0.008, P < 0.05; 0.076 ± 0.08 vs 0.054 ± 0.06, P < 0.05, Figure 2A). In contrast, no significant differences were detected between two groups regarding the weight proportion of SAT (Figure 2A) or VAT/SAT ratio (Figure 2B).
The size of adipocytes was significantly greater in DJB group than that in control group in both SAT and VAT, as evidenced by the average SAT area measurements of 1230.89 ± 96.09 µm² compared to 1049.10 ± 86.34 µm² (P < 0.001), and the average VAT area measurements of 987.56 ± 58.23 µm²compared to 675.66 ± 61.62 µm² (P < 0.001; Figure 2C and D). Furthermore, immunofluorescence staining revealed an elevation in the expression of GLUT4 and IR within both VAT and SAT after DJB, suggesting an amelioration of insulin resistance in whole body adipose tissue (Figure 2E and F).
Based on comparative analysis of the transcriptional variances between VAT and SAT, utilizing the KEGG analysis, it was observed that within each group, the most differentially expressed genes lied in metabolic pathways, suggesting different functions of VAT and SAT in metabolism regulation (Figure 2G and H). The intergroup differences also resided within metabolic pathways, but only significantly in VAT (Figure 2I), suggesting that VAT, instead of SAT, might play a major role in metabolic improvement after DJB (Figure 2J).
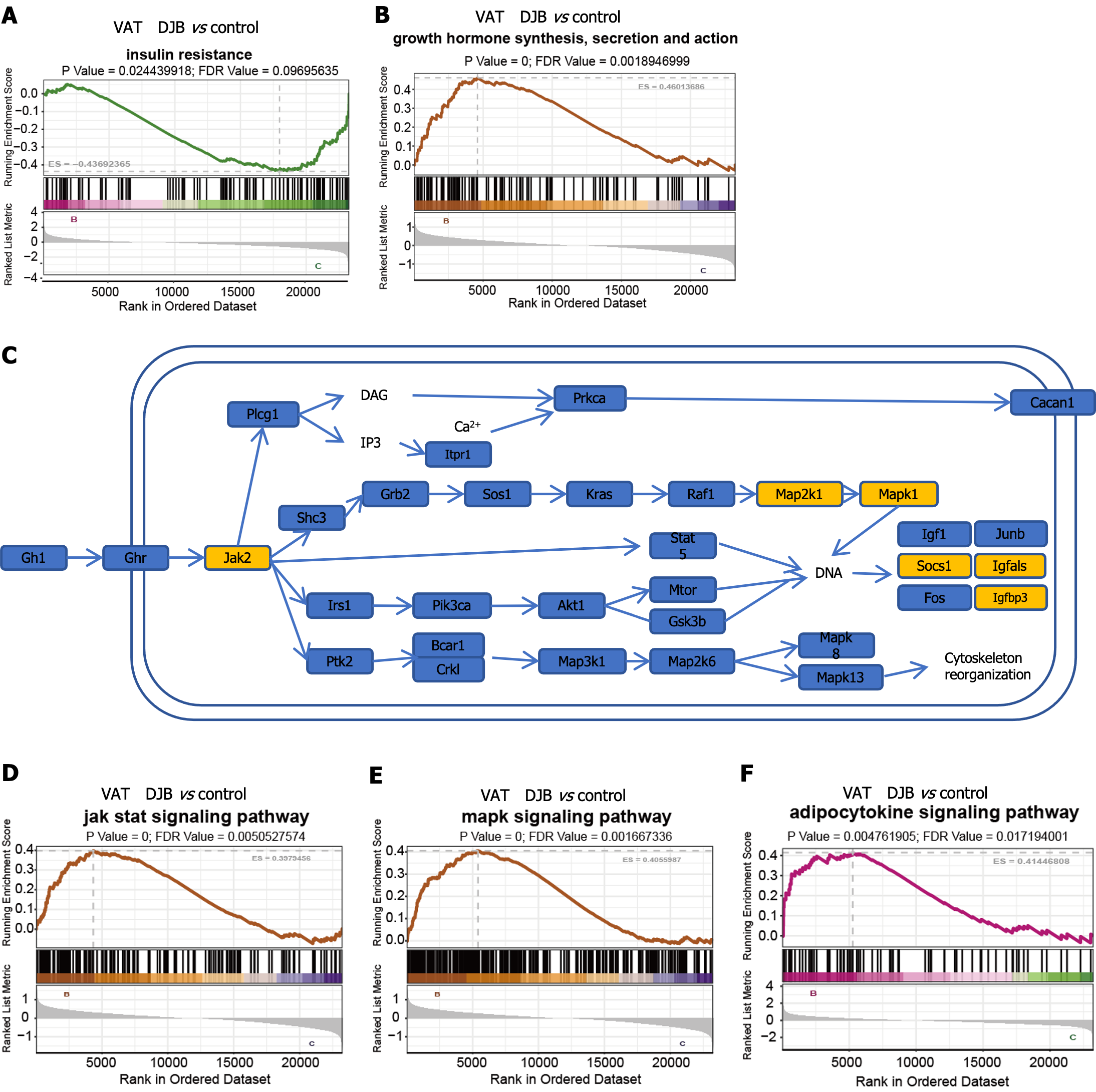
Hence, our attention was directed towards examining the disparities in VAT between control group and DJB group. Consistent with the results from Immunofluorescence of GLUT4 and INSR mentioned above, the findings of the gene set enrichment analysis (GSEA) also revealed a significant improvement in insulin sensitivity within VAT after DJB (Figure 3A). Additionally, the metabolic pathway analysis revealed a substantial enhancement in functional pathways of GH within VAT in all metabolic pathway analyses (Figure 3B). To further investigate the significant distinctions, a GH functional pathway map was constructed utilizing the KEGG database (Figure 3C), and subsequent GSEA analysis revealed notable differences in the downstream MAPK and JAK pathways (Figure 3D and E). Furthermore, a couple of downstream factors of the GH pathway were also recognized (Figure 3C); some of the factors (such as Socs1, Igf, etc.) were found to be involved in adipocytokine signaling pathway in adipocytes, and GSEA analysis revealed significant differences in the adipocytokine signaling pathway between control group and DJB group (Figure 3F).
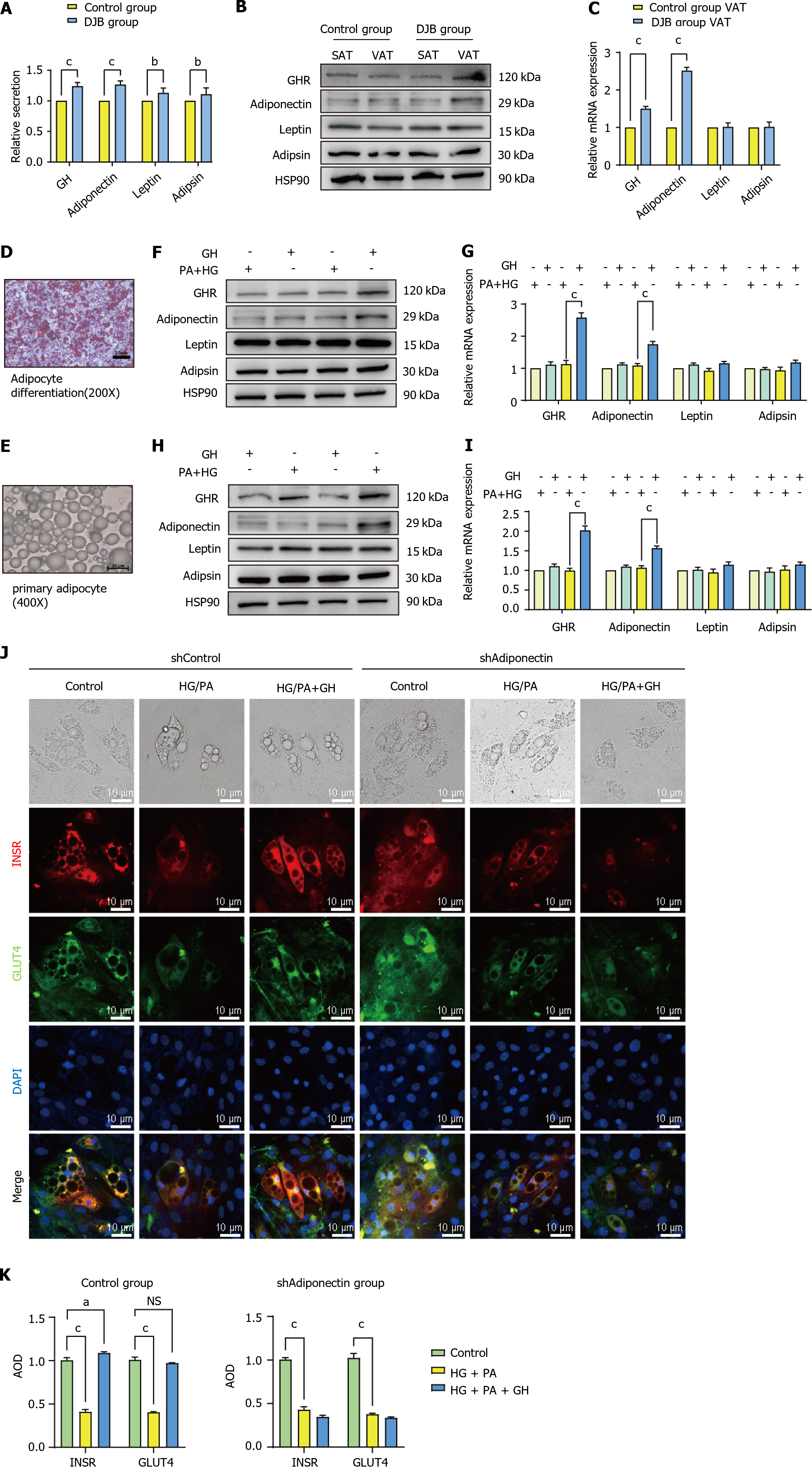
Both serum GH and adipocytokine (adiponectin, leptin, and adipsin) concentrations increased significantly after DJB compared to control group (P < 0.001; Figure 4A). In comparison to control group, the expressions of GH receptor (GHR) and adiponectin in VAT, but not in SAT, were found to be significantly greater in DJB group (P < 0.001). No significant difference was observed in the expression of leptin or adipsin in either VAT or SAT (Figure 4B and C).
Observed the degree of differentiation of adipocytes and observe the status of primary adipocytes under microscope before conducting experiments (Figure 4D and E). Results from both adipocyte cell line culture and primary adipocyte extraction showed that GH stimulation had no effect on adipocytokine secretion in the ordinary culture condition, but significantly increased adiponectin secretion in HG + PA conditions, without any effect on leptin or adipsin secretion. Additionally, it was discovered that the expression of GHR, when stimulated by GH, exhibited a significantly increased expression (P < 0.001, Figure 4F-I).
The regulatory impact of GH on insulin resistance is mediated through adiponectin was be demonstrated. Through utilization of fluorescent staining on mature adipocytes, it was observed that the expression of GLUT4 and INSR in adipocytes decreased in a HG + PA environment. After GH stimulation, the expression of both GLUT4 and INSR had returned to their original levels. However, GH was unable to reverse the expression of GLUT4 and INSR in adipocytes when Adiponectin had been knocked down in a HG + PA environment (Figure 4J and K).
The role of adipose tissue in regulating endocrine function is significant, and we observed a tendency to increase adipose tissue mass after DJB, which was accompanied by an improvement in insulin resistance. However, the specific mechanism behind this phenomenon remains unclear. To address this knowledge gap, we conducted a study and established a DJB surgical model. This allows us to examine morphological and functional changes in SAT and VAT. The results of this study showed that after DJB surgery, an increase in adipose tissue mass and cell volume was observed, and notably, the changes in VAT were highly significant compared with SAT. To ensure the reliability of our findings, we used transcriptome analysis and experimental validation to validate our results, and the results showed that VAT was the adipose tissue with the greatest difference after DJB surgery. Since VAT is rich in blood supply, we believe that there are factors in plasma that affect the structure and function of VAT. Combining fat volume growth and transcriptome analysis results, we found that GH plays a crucial role in the changes in VAT after DJB. This is supported by increased circulating GH levels and upregulation of GH receptor expression in adipose tissue. Furthermore, GH administration stimulates adipocytes to secrete adiponectin, thereby ameliorating insulin resistance in HG + PA environments. Adiponectin, as an important adipokine, plays a key role in promoting metabolism. In the presence of elevated glucose and fat levels, adipocytes exhibit increased adiponectin expression following GH stimulation. In addition, GLUT4 and INSR levels within adipocytes were significantly increased, indicating significantly enhanced insulin resistance. Therefore, our findings indicate that GH improves insulin resistance in VAT in male diabetic rats after receiving DJB, possibly by increasing adiponectin secretion.
Adipose tissue, being extensively distributed throughout the body, serves as a crucial reservoir for energy storage. It is classified into two distinct categories, namely VAT and SAT, based on their respective anatomical placements. Research findings have consistently demonstrated notable variations in metabolic functionality between VAT and SAT. Notably, VAT exhibits enhanced capabilities in free fatty acid production and glucose uptake. Conversely, SAT demonstrates a greater affinity for circulating free fatty acids and triglycerides[14,19]. Furthermore, adipocytes possess a distinctive function in metabolic regulation. Notably, the adipokine adipsin, secreted by SAT, has been substantiated to play a crucial role in safeguarding islet β cells. Adipsin effectively impedes the differentiation and apoptosis of islet β cells, thereby facilitating the restoration of insulin secretion[20]. Leptin has the capacity to diminish appetite and enhance energy expenditure[21]. Adiponectin, predominantly secreted by VAT, has been scientifically demonstrated to enhance insulin sensitivity in peripheral tissues and potentially contribute to weight loss by regulating hypothalamic function. Nevertheless, VAT is commonly linked to metabolic disorders[22,23]. Weight loss surgery facilitates the redistribution of fat and substantially decreases fat mass by altering fat metabolism in various regions, particularly through SG and RYGB procedures. However, the alteration in fat weight subsequent to DJB was not readily discernible and exhibited a progressive increase over time[7,8]. The weight augmentation following DJB was concomitant with modifications in fat functionality, and certain scholarly articles have posited an elevation in circulating adiponectin levels subsequent to DJB[24]. In our investigation, we scrutinized the morphological and functional transformations of adipocytes in the context of DJB, wherein SAT displayed analogous attributes and no statistically significant disparities compared to the control group. However, it is noteworthy that the visceral adipocytes underwent substantial alterations subsequent to DJB, as evidenced by an increase in volume, active adiponectin secretion, and amelioration of insulin resistance. These findings imply that VAT primarily governs the modifications in adipose tissue following DJB, and the modifications in its structure and secretory function play a role in the metabolic regulation of the organism. Therefore, the role of adipose tissue in different parts of the body under different metabolic environments should be analyzed separately.
The importance of GH as a neuroendocrine hormone is emphasized by its ability to affect a variety of target cells. However, conflicting findings have emerged regarding its role in regulating adipocyte secretion. Some studies have shown that elevated GH levels can hinder adipocyte mass growth and reduce adiponectin secretion, but multiple studies have shown that long-term GH stimulation can enhance adipocyte secretion of adiponectin, thereby improving insulin resistance[25-27]. However, it needs to be emphasized that there are limited studies on the effects of GH on adipocytes in HG + PA environments. Our results suggest that, under normal conditions, GH does not effectively stimulate adiponectin secretion. However, after HG + PA stimulation, enhanced sensitivity of VAT to GH was observed, accompanied by a significant increase in adipocyte adiponectin secretion. Our immunofluorescence experiments demonstrated that GH has the ability to improve adipose tissue insulin resistance in high-glucose and high-fat environments. In the rat model after DJB, an increase in serum GH levels was observed, and an increase in immunofluorescence of insulin sensitivity-related indicators in adipose tissue was observed, indicating an improvement in insulin resistance. These findings confirm the effect of GH on VAT after DJB and reveal a new mechanism by which DJB improves metabolism and insulin resistance.
The present study has identified several limitations. Specifically, our research focused on discerning the functional disparities between VAT and SAT, utilizing mesentery fat and inguinal fat as representative samples. In order to enhance our understanding, it is imperative to undertake a more refined classification of VAT and SAT in subsequent research endeavors, while also broadening the range of sample types to comprehensively analyze the functional distinctions among different types of adipose tissue. Additionally, this study elucidated the impact of GH on VAT subsequent to DJB, yet failed to elucidate its precise molecular mechanism of action. The utilization of rats as the animal model, which diverges from humans, necessitates the collection of human specimens to thoroughly analyze the influence of GH on adipose tissue.
In male rats with diabetes and DJB surgery, GH has the potential to regulate body metabolism and increase insulin resistance by promoting the secretion of adiponectin, primarily mediated by VAT.
We express our gratitude to Biorender for their provision of drawing materials and assistance in creating presentation diagrams and flow charts. Additionally, we acknowledge the support from GO and KEGG databases in facilitating data analysis. Furthermore, we extend our appreciation to Lianchuan Company for their contribution of RNA-Seq data analysis tools.
| 1. | Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 950] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 2. | 2 Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, Ling Lim GP, Rong Tay NW, Annan NC, Bhattamisra SK, Candasamy M. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. 2019;13:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 3. | Batterham RL, Cummings DE. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care. 2016;39:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 4. | Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and Endocrine Consequences of Bariatric Surgery. Front Endocrinol (Lausanne). 2019;10:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Faramia J, Ostinelli G, Drolet-Labelle V, Picard F, Tchernof A. Metabolic adaptations after bariatric surgery: adipokines, myokines and hepatokines. Curr Opin Pharmacol. 2020;52:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, De Luca M, Faria SL, Goodpaster KPS, Haddad A, Himpens JM, Kow L, Kurian M, Loi K, Mahawar K, Nimeri A, O'Kane M, Papasavas PK, Ponce J, Pratt JSA, Rogers AM, Steele KE, Suter M, Kothari SN. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2022;18:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 394] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 7. | Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 636] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 8. | Torres AJ. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): Surgical risk and long-term results. Surg Obes Relat Dis. 2019;15:243-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Patel RT, Shukla AP, Ahn SM, Moreira M, Rubino F. Surgical control of obesity and diabetes: the role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring). 2014;22:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Liu L, Shi Z, Ji X, Zhang W, Luan J, Zahr T, Qiang L. Adipokines, adiposity, and atherosclerosis. Cell Mol Life Sci. 2022;79:272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Sahu B, Bal NC. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie. 2023;204:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 12. | de Medeiros SF, Rodgers RJ, Norman RJ. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Hum Reprod Update. 2021;27:771-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27:68-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 14. | Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1493] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 15. | Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Cheng ZQ, Liu TM, Ren PF, Chen C, Wang YL, Dai Y, Zhang X. Duodenal-jejunal bypass reduces serum ceramides via inhibiting intestinal bile acid-farnesoid X receptor pathway. World J Gastroenterol. 2022;28:4328-4337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1830] [Cited by in RCA: 2000] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 18. | Warnke I, Goralczyk R, Fuhrer E, Schwager J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr Metab (Lond). 2011;8:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129:3990-4000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 434] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 20. | Gómez-Banoy N, Guseh JS, Li G, Rubio-Navarro A, Chen T, Poirier B, Putzel G, Rosselot C, Pabón MA, Camporez JP, Bhambhani V, Hwang SJ, Yao C, Perry RJ, Mukherjee S, Larson MG, Levy D, Dow LE, Shulman GI, Dephoure N, Garcia-Ocana A, Hao M, Spiegelman BM, Ho JE, Lo JC. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat Med. 2019;25:1739-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 21. | Pereira S, Cline DL, Glavas MM, Covey SD, Kieffer TJ. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr Rev. 2021;42:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 22. | Straub LG, Scherer PE. Metabolic Messengers: Adiponectin. Nat Metab. 2019;1:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 23. | Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1650] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 24. | Hu C, Zhang G, Sun D, Han H, Hu S. Duodenal-jejunal bypass improves glucose metabolism and adipokine expression independently of weight loss in a diabetic rat model. Obes Surg. 2013;23:1436-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol. 2020;16:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 26. | Nilsson L, Binart N, Bohlooly-Y M, Bramnert M, Egecioglu E, Kindblom J, Kelly PA, Kopchick JJ, Ormandy CJ, Ling C, Billig H. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun. 2005;331:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Chaves VE, Júnior FM, Bertolini GL. The metabolic effects of growth hormone in adipose tissue. Endocrine. 2013;44:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |