Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1051
Revised: March 6, 2024
Accepted: April 22, 2024
Published online: June 15, 2024
Processing time: 191 Days and 18.3 Hours
Monogenic diabetes, constituting 1%-2% of global diabetes cases, arises from single gene defects with distinctive inheritance patterns. Despite over 50 ass-ociated genetic disorders, accurate diagnoses and management of monogenic diabetes remain inadequate, underscoring insufficient clinician awareness. The disease spectrum encompasses maturity-onset diabetes of the young (MODY), characterized by distinct genetic mutations affecting insulin secretion, and neo
Core Tip: Monogenic diabetes forms a spectrum of single gene disorders, characterized by distinct patterns of inheritance, accounting for about 1%–2% of diabetes mellitus burden in the world. Although more than 50 unique genetic mutations associated with these disorders have been identified to date, the diagnosis and management of monogenic diabetes are still inadequate globally because of the poor awareness and professional inertia among physicians. Prompt suspicion and diagnostic evaluation of these disorders are of paramount importance from the management and genetic counselling perspectives. A clinical update review in the World Journal of Diabetes empowers physicians to have a better understanding about this disease.
- Citation: Bhattacharya S, Pappachan JM. Monogenic diabetes in children: An underdiagnosed and poorly managed clinical dilemma. World J Diabetes 2024; 15(6): 1051-1059
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1051.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1051
Monogenic diabetes encompasses a spectrum of clinical disorders resulting from single genetic defects with a distinct pattern of inheritance, constituting about 1%-2% of the global burden of diabetes mellitus (DM)[1,2]. Despite over 50 types of genetic disorders linked to diabetes development, a limited number of patients receive accurate diagnoses and management, highlighting insufficient awareness among clinicians[2,3]. Accounting for over 3% of diabetes cases in young individuals, monogenic diabetes is categorized into maturity-onset diabetes of the young (MODY), neonatal DM (NDM), and rare syndromic forms, based on genetic abnormalities and clinical presentation[3]. Correct diagnoses of these disorders are of paramount importance to ensure proper prognostication, appropriate management, and counseling on inheritance. Identifying the genetic defect is essential for precision medicine in monogenic DM. The adoption of a nomenclature using the standard abbreviation for the pathogenic gene followed by the clinical phenotype (e.g., GCK-MODY and KCNJ11-PNDM) underscores the importance of recognizing genetic defects in defining MODY, NDM, and other monogenic forms of DM.
Monogenic diabetes is relatively less prevalent than the commonly recognized type 1 DM (T1DM) or type 2 DM (T2DM). Additionally, many cases of monogenic diabetes are mistakenly classified as either T1DM or T2DM, leading to underreporting of its prevalence[4]. MODY, the most frequent form of monogenic diabetes, constitutes 1%-5% of diabetes cases, with varying estimates depending on clinical criteria, population, and testing methods[5-8].
Population-based studies to define the prevalence of monogenic diabetes are few. Data from diabetes registries in the United Kingdom where provisions for centralized genetic testing exist, reveal a prevalence of 2.5% for monogenic dia
Monogenic diabetes registries of the University of Chicago revealed GCK mutations in 59% followed by HNF1A mu
Though data from Asia, Africa and South America is relatively sparse, monogenic diabetes registries have recently been established in several countries of these continents. GCK mutation is again most common, followed by HNF1A mutation in Brazil[12]. The most common varieties identified in a tertiary care center in India were HNF1A-MODY, followed by HNF4A-MODY, ABCC8-MODY, GCK-MODY and HNF1B-MODY[13].
Cost-effectiveness studies indicate that testing young adults with a diagnosis of T2DM for the HNF-1A, HNF-4A and GCK, the three common MODY mutations, might be worthwhile[14]. While centralized genetic testing remains the effective strategy, implementing population-based genetic testing poses significant financial challenges, emphasizing the need for increased physician awareness and appropriate screening strategies, particularly in resource-limited settings.
Monogenic diabetes refers to a group of uncommon forms of diabetes characterized by a single gene defect that affects insulin secretion or action. The frequently encountered groups are MODY and NDM, with several other rare varieties also identified (Table 1).
| MODY | NDM | Mitochon-drial DM | Autoimmune monogenic DM | Insulin resistance syndromes | Lipodystrophy |
| MODY1 -HNF4A | Abnormal pancreatic development | MELAS | IPEX | Type A insulin resistance syndrome | Congenital generalized lipodystrophy |
| PLAGL1 | DMDF | FOXP3 | Donohue and Rabson-Mendenhall syndromes – INSR | ||
| HYMAI | MIDD | ||||
| ZFP57 | Leigh syndrome SNHL | ||||
| PDX1 | MM - m.3243A>G | ||||
| PTF1A | |||||
| PTF1A | |||||
| enhancer | |||||
| HNF1B | |||||
| RFX6 | |||||
| GATA6 | |||||
| GLIS3 | |||||
| NEUROG3 | |||||
| NEUROD1 | |||||
| PAX6 | |||||
| MODY2 -GCK | MNX1 | CGL1 - AGPAT2 | |||
| MODY3 - HNF1A | NKX2-2 | MELAS; possible atherosclerosis risk - m.3256C>T | CGL2 – BSCL2 | ||
| MODY4 -PDX1 | CNOT1 | MMC/MELAS - m.3260A>G | CGL3 – CAV1 | ||
| MODY5 - HNF1B | ONECUT1 | MELAS/DM - m.3271T>C | Autoimmune polyglandular syndrome type 1 -AIRE | SHORT syndrome - PIK3R1 | CGL4 - PTRF |
| MODY6 - NEUROD1 | Abnormal beta-cell function | MERRF - m.8344A>G | IPEX-like” phenotype CTLA4 | Other genetic abnormalities | Familial partial lipodystrophy |
| KCNJ11 | IL2RA | AKT2 TBC1D4 | |||
| ABCC8 | ITCH | PRKCE | |||
| INSC | LRBA | ||||
| MODY7 -KLF11 | GCK | Other autoimmune diabetes | FPLD2 - LMNA | ||
| MODY8 -CEL | SCLC2A2 (GLUT2) | MIDD, renal insufficiency - m.9155A>G | STAT1 | FPLD3 - PPARG | |
| MODY9 -PAX4 | SLC19A2 | STAT3 | FPLD4 – PLN1 | ||
| MODY10 -INS | KCNMA1 | STAT5B | FPLD5 - CIDEC | ||
| MODY11 -BLK | Destruction of beta-cells | DMDF/RP + SNHL - m.12258C>A | FPLD6 - LIPE | ||
| INS | |||||
| IER3IP1 | |||||
| EIF2B1 | |||||
| YIPF5 | |||||
| MODY12 -ABCC8 | Wolcott-Rallison syndrome -EIF2AK3 | MM + DMDF/Encephalomyopathy/Dementia + diabetes + ophthalmoplegia - m.14709T>C | FPLD7 – CAV1 | ||
| MODY13 -KCNJ11 | Wolfram syndrome - WFS1 | ||||
| MODY14 -APPL1 |
MODY, the most prevalent form of monogenic diabetes, typically demonstrates autosomal dominant inheritance. Current MODY classification identifies 14 distinct group of genes[15]. The mutations in MODY are diverse and can broadly affect transcription, glucose sensing, protein folding, ion channel function and signal transduction[16].
Most individuals with MODY fall into two phenotypic groups. The first group comprises HNF1A-MODY and HNF4A-MODY, characterized by beta-cell dysfunction with insulin secretory defects, impaired glucose-mediated insulin secretion, initial therapeutic response to low-dose sulfonylurea, gradual progression to insulin dependency, and susceptibility to typical DM complications. In contrast, GCK-MODY is characterized by mild fasting hyperglycemia with preserved insulin secretion. Individuals with GCK mutations typically do not require treatment, except during pregnancy[15,16].
The next broad group of monogenic diabetes comprise permanent or transient varieties of NDM. NDM is again a heterogenous group of disorders characterized by severe hyperglycemia usually occurring before six months of age. The frequency of NDM is approximately 1 in 90000-160000 live births, and around 80% are identified to have a specific genetic defect[17,18]. Nearly 40 genetic mutations inducing abnormal pancreatic development, beta-cell dysfunction, or beta-cell destruction have been identified in infants with NDM[19].
Permanent NDM is commonly caused by mutation in the KATP channel genes and the insulin (INS) genes. Activating mutations in KCNJ11 or ABCC8, coding for the inner subunit (Kir6.2) and the outer subunit (SUR1), respectively, prevent KATP channel closure and diminish insulin response to hyperglycemia. Babies with the disease often develop intrauterine growth retardation (IUGR), and are small-for-gestational age. The classic neurological association with KCNJ11 includes developmental delay and epilepsy, and along with NDM, labelled as DEND syndrome, although milder forms with less developmental delay and without epilepsy are more common. Diabetic ketoacidosis is present at diagnosis in 30%-75%, and initial stabilization often requires insulin[18].
Around two-thirds of transient NDM can be attributed to abnormalities in an imprinted region on chromosome 6q24. Severe IUGR is a hallmark, and one-third of these cases exhibit macroglossia. Early-onset, severe but nonketotic hyperglycemia is typically observed within the first week of life. Although the need for insulin or sulfonylurea is short-term, there is a high risk of developing diabetes in the future. The majority of the remaining cases of transient NDM are caused by activating mutations of the KCNJ11 or ABCC8 genes[19].
Mitochondrial diabetes, a form of maternally inherited diabetes, commonly arises from MT-TL1 (m.3243A>G) genotype, presenting as maternally inherited diabetes and deafness or mitochondrial encephalopathy with lactic acidosis and stroke-like episodes. Diminished glucose-stimulated insulin secretion results from reduced amount of oxidative phosphorylation due to defects in the tricarboxylic acid cycle and the mitochondrial electron transport chain[20].
Mutations in several immune regulatory genes such as AIRE, CTLA4, FOXP3, IL2RA, ITCH, LRBA, STAT1, STAT3, and STAT5B can lead to early-onset DM including NDM[19]. The prototype disorder is immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome resulting from mutation of FOXP3 gene[21]. Mutations in the INS receptor (INSR) gene cause insulin-resistance syndromes of varying severity, such as Donohue syndrome, Rabson-Mendenhall syndrome, and type A insulin resistance[22]. Single gene mutations are also responsible for generalized and partial lipodystrophies characterized by generalized or localized fat loss, insulin resistance, and youth-onset diabetes[23]. Additionally, rare syndromic forms like Wolcott-Rallison syndrome, characterized by early-onset non-autoimmune insulin-requiring diabetes associated with skeletal dysplasia and growth retardation, have been described[24].
A universal genetic screening strategy for all early-onset diabetes cases is unfortunately impractical, and identifying eligible candidates is best achieved through a combination of phenotypic and biochemical variables.
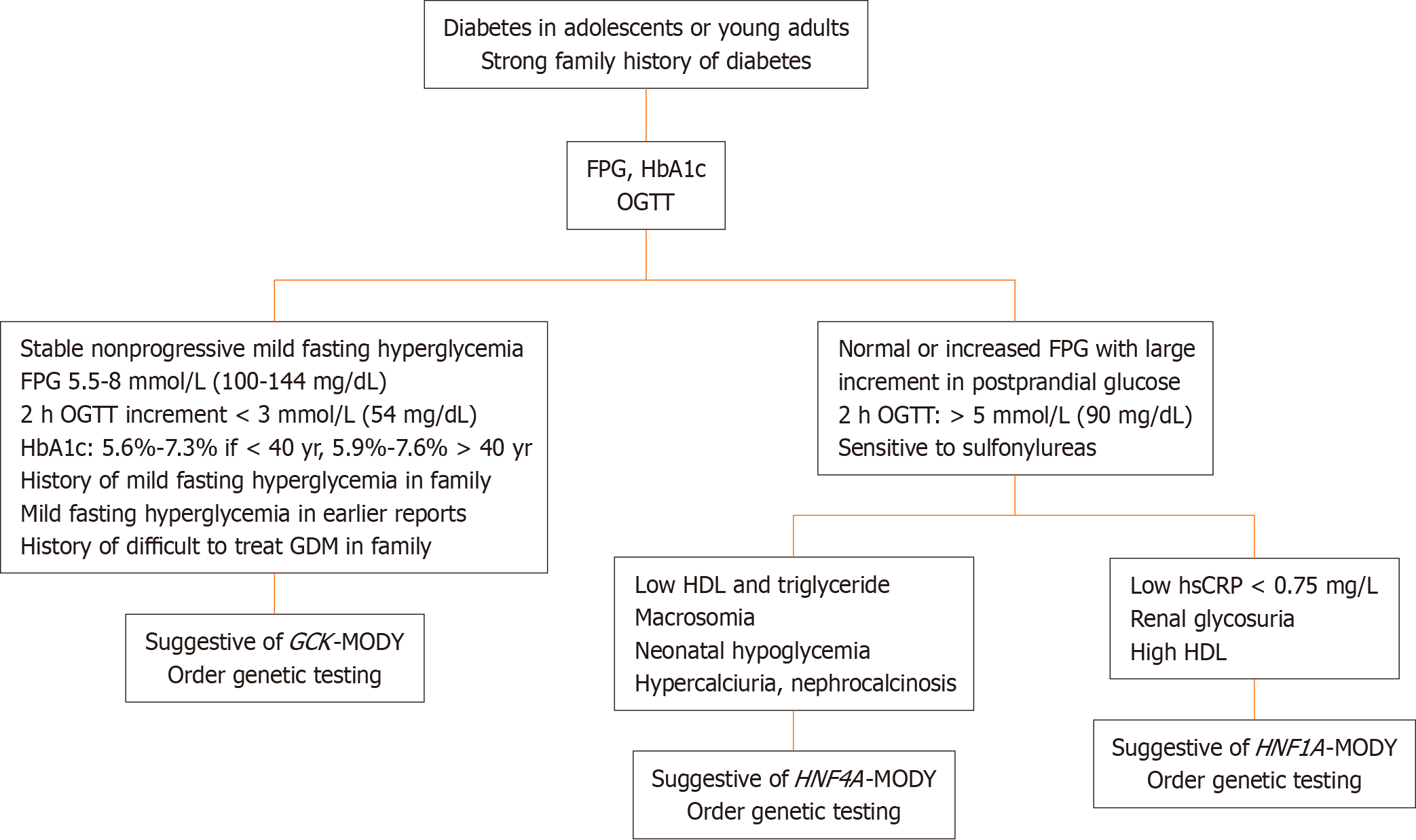
Several deviations from the original diagnostic triad of MODY, based on onset before 25 years of age, autosomal dominant inheritance, and noninsulin-dependent physiology, are now recognized. Given the diversity in clinical features, phenotypic characterization of MODY into homogeneous subgroups for identifying candidates for screening is prudent. A diagnostic algorithm for the three common types of MODY is presented in the Figure 1.
Infants diagnosed with diabetes in the first six months of life should undergo immediate molecular genetic testing. For those diagnosed between 6 and 12 months, genetic testing may be considered, especially if lacking islet autoantibodies or showing features suggestive of a monogenic cause. Genetic diagnosis of NDM provides crucial information on treatment options, associated comorbidities, and the course of diabetes. Genetic testing is indicated in the conditions summarized in Table 2[18,19]. Urine ketones, serum glucose, C-peptide, and insulin estimations, along with a pancreatic ultrasound, should be performed in all cases. Screening for antibodies will not alter the management approach before six months but is recommended for infants between 6 to 12 months. Additionally, congenital anomalies should be ruled out based on their association with genetic anomalies[18].
| Rank | Clinical or laboratory context |
| 1 | Infant before 6 months of age with plasma glucoses persistently > 250 mg/dL (13.9 mmol/L) without an alternative etiology |
| 2 | Hyperglycemia persisting after seven to ten days of birth |
| 3 | Infants with extreme hyperglycemia i.e., plasma glucose > 1000 mg/dL (55.6 mmol/L) |
| 4 | Infants with diabetes diagnosed between 6 and 12 months, especially in those without islet autoantibodies or who have other features suggestive of a monogenic cause |
| 5 | Infants with transient diabetes mellitus1 |
Insulin resistance syndromes are characterized by hyperinsulinemia (fasting serum insulin level > 30 μU/mL) accompanied by juvenile-onset glucose intolerance, acanthosis nigricans, hypertrichosis, polycystic ovary, low birth weight, IUGR, and distinctive facial features. The diagnosis is confirmed by abnormalities in the insulin receptor (INSR) or genes related to insulin receptor signaling (PIK3R1, AKT2, TBC1D4, PRKCE)[25]. Maternal transmission, along with bilateral hearing impairment, progressive external ophthalmoplegia, and other organ involvement, are hallmarks of mitochondrial diabetes. The detection of the m.3243A>G mutation in mitochondrial DNA is used for confirmation of this most prevalent variety[26].
Monogenic diabetes presents a unique opportunity for the application of precision medicine.
Individuals with GCK-MODY experience mild and stable fasting hyperglycemia, rarely developing chronic complications, and typically require no treatment, except during pregnancy[19]. For those with HNF1A-MODY or HNF4A-MODY, low doses of glibenclamide (or other sulphonylureas) show an excellent glucose-lowering response. A randomized controlled trial demonstrated similar efficacy of liraglutide and glimepiride in HNF1A-MODY, with liraglutide causing less hypoglycemia[27]. HNF1A inhibits sodium–glucose cotransporter 2 (SGLT2) expression in the kidney and causes glycosuria despite near-normal blood glucose levels. Given the effects of HNF1A on SGLT2 expression, caution should be observed in administering SGLT2 inhibitors in HNF1A-MODY[28].
Approximately 90% of children with KATP channel gene mutations can transition from insulin to sulfonylurea tablets, particularly glibenclamide. This switch significantly improves long-term glycemic control with minimal hypoglycemia. The dose requirement is higher compared to T2DM, typically around 0.5 mg/kg/d, occasionally reaching 2.3 mg/kg/d. Insulin requirement often goes down when glycemic control is maintained[19].
Individuals with genetic insulin resistance exhibit a high requirement for insulin, and the administration of metformin and SGLT-2 inhibitors by insulin-independent mechanisms allow for a reduction in insulin dose[25]. Mitochondrial diabetes has its onset around fourth decade and though initially responds to oral agents, the need for insulin arises earlier than in T2DM. Metformin is contraindicated because of the risk of lactic acidosis[19].
A review by Sun and Lin[29] in the recent issue of the World Journal of Diabetes[29], provides us a comprehensive overview of the genetic aspects, diagnosis, and management of monogenetic diabetes. The authors emphasize the challenges in identifying this condition due to its clinical and genetic diversity but highlights the promise of advanced genetic testing methods such as next-generation sequencing (NGS) and machine learning algorithms. The shift towards personalized treatments, including gene-based therapies like CRISPR-Cas9, represents a significant advancement in managing monogenic diabetes. The review discusses breakthroughs in diagnosis, emphasizing the role of targeted gene sequencing, whole-exome sequencing (WES), and NGS. While Sanger sequencing remains crucial, WES has proven effective in identifying novel causal genes, and NGS allows for simultaneous analysis of multiple genes. The incorporation of nanotechnology in diabetes care, particularly in glucose sensing and non-invasive insulin delivery, is also explored. The review underscores that studying monogenic DM has the potential to uncover new gene mutations, understand disease subtypes, and drive precision medicine strategies, signaling a transformative era in pediatric diabetes care.
Monogenic diabetes research has significantly advanced our understanding of diabetes pathophysiology and the genetics of common diabetes. Notably, it has unveiled heterozygous pathogenic mutations in genes like ABCC8, KCNJ11, INS, HNF1B, GATA4, and GATA6, demonstrating their roles in both MODY and NDM, as well as shaping the risk for type 2 diabetes[30]. The recognition of a potential spectrum for non-autoimmune diabetes challenges traditional distinctions, suggesting a more continuous disease progression. However, uncertainties persist, particularly in the variability of penetrance and expressivity of mutations, emphasizing the need for further research to comprehend the full spectrum of associated diseases[31,32].
While advancements in genetic diagnosis through affordable NGS have enabled precise identification of monogenic diabetes, drug development directly resulting from this research remains elusive. However, induced pluripotent stem cells (iPSCs) generated from monogenic diabetes models is an important breakthrough. By differentiating iPSCs into pancreatic beta-like cells, researchers can delve into disease mechanisms such as impaired insulin secretion or beta-cell dysfunction in a controlled laboratory setting[33]. This understanding can serve as a cornerstone for developing novel treatments, not only for monogenic diabetes but also for advancing personalized medicine approaches in the broader context of type 1 and type 2 diabetes[34].
The current clinical knowledge of monogenic diabetes demands a graded approach to diagnosis, emphasizing the judicious use of genetic methods. Future priorities include developing cost-effective gene screening methods, advancing treatments for monogenic diabetes, and deepening our understanding of pancreatic beta-cell biology with appropriate use of iPSCs generated from monogenic diabetes. Regardless of the successes, continued exploration of genomic medicine and gene-based therapies is crucial to bridge existing knowledge gaps and enhance the quality of life for individuals with monogenic diabetes.
Despite significant advancements in understanding the genetic basis of monogenic diabetes, challenges persist in identifying these conditions, leading to misclassification as type 1 or type 2 diabetes. Screening strategies to identify candidates for genetic testing based on phenotype and biochemical parameters are needed. Management strategies tailored to specific genetic defects highlight the opportunities for precision medicine in monogenic diabetes. Ongoing research into advanced genetic testing methods and gene-based therapies fuels optimism for continued progress in our understanding and management of this diverse spectrum of diabetes disorders. The article by Sun and Lin[29] in the Journal issue is a good step towards filling our knowledge gaps of the genetic, diagnostic, and therapeutic landscapes of monogenic diabetes in children.
We thank Dr. Marina G Kudiyirickal for providing audio clip for the Core Tip of this paper.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country of origin: United Kingdom
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Upadhya D, India S-Editor: Li L L-Editor: A P-Editor: Zheng XM
| 1. | IDF Diabetes Atlas. IDF Diabetes Atlas 2022 Reports. [cited 6 March 2024]. Available from: https://diabetesatlas.org/atlas-reports/?report-year=2022. |
| 2. | Murphy R, Colclough K, Pollin TI, Ikle JM, Svalastoga P, Maloney KA, Saint-Martin C, Molnes J; ADA/EASD PMDI, Misra S, Aukrust I, de Franco E, Flanagan SE, Njølstad PR, Billings LK, Owen KR, Gloyn AL. The use of precision diagnostics for monogenic diabetes: a systematic review and expert opinion. Commun Med (Lond). 2023;3:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Maloney KA, Mizerik E, King RH, McGinnis EM, Perkowitz S, Diamonstein CJ, Schmanski AA, Saliganan S, Shipper AG, Udler MS, Guan Y, Pollin TI. Genetic counseling in diabetes mellitus: A practice resource of the National Society of Genetic Counselors. J Genet Couns. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 5. | Johnson SR, Ellis JJ, Leo PJ, Anderson LK, Ganti U, Harris JE, Curran JA, McInerney-Leo AM, Paramalingam N, Song X, Conwell LS, Harris M, Jones TW, Brown MA, Davis EA, Duncan EL. Comprehensive genetic screening: The prevalence of maturity-onset diabetes of the young gene variants in a population-based childhood diabetes cohort. Pediatr Diabetes. 2019;20:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K, Oram RA, Knight B, Hyde C, Cox J, Mallam K, Moudiotis C, Smith R, Fraser B, Robertson S, Greene S, Ellard S, Pearson ER, Hattersley AT; UNITED Team. Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes. Diabetes Care. 2016;39:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, Ellard S, Farmer AJ, McCarthy MI, Owen KR. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Kropff J, Selwood MP, McCarthy MI, Farmer AJ, Owen KR. Prevalence of monogenic diabetes in young adults: a community-based, cross-sectional study in Oxfordshire, UK. Diabetologia. 2011;54:1261-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Davis TM, Makepeace AE, Ellard S, Colclough K, Peters K, Hattersley A, Davis WA. The prevalence of monogenic diabetes in Australia: the Fremantle Diabetes Study Phase II. Med J Aust. 2017;207:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Bowden TL, Letourneau-Freiberg LR, Kandasamy B, Sanyoura M, Tian P, Harris AG, Bell GI, Philipson LH, Naylor RN, Greeley SAW. Insight on Diagnosis and Treatment From Over a Decade of Research Through the University of Chicago Monogenic Diabetes Registry. Front Clin Diabetes Healthc. 2021;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kleinberger JW, Pollin TI. Undiagnosed MODY: Time for Action. Curr Diab Rep. 2015;15:110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Giuffrida FMA, Moises RS, Weinert LS, Calliari LE, Manna TD, Dotto RP, Franco LF, Caetano LA, Teles MG, Lima RA, Alves C, Dib SA, Silveiro SP, Dias-da-Silva MR, Reis AF; Brazilian Monogenic Diabetes Study Group (BRASMOD). Maturity-onset diabetes of the young (MODY) in Brazil: Establishment of a national registry and appraisal of available genetic and clinical data. Diabetes Res Clin Pract. 2017;123:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Aarthy R, Aston-Mourney K, Amutha A, Mikocka-Walus A, Anjana RM, Unnikrishnan R, Jebarani S, Venkatesan U, Gopi S, Radha V, Mohan V. Prevalence, clinical features and complications of common forms of Maturity Onset Diabetes of the Young (MODY) seen at a tertiary diabetes centre in south India. Prim Care Diabetes. 2023;17:401-407. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Naylor RN, John PM, Winn AN, Carmody D, Greeley SA, Philipson LH, Bell GI, Huang ES. Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care. 2014;37:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Antal Z. Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options. Endocrines. 2021;2:485-501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Nkonge KM, Nkonge DK, Nkonge TN. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin Diabetes Endocrinol. 2020;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 17. | Grulich-Henn J, Wagner V, Thon A, Schober E, Marg W, Kapellen TM, Haberland H, Raile K, Ellard S, Flanagan SE, Hattersley AT, Holl RW. Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPV). Diabet Med. 2010;27:709-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Lemelman MB, Letourneau L, Greeley SAW. Neonatal Diabetes Mellitus: An Update on Diagnosis and Management. Clin Perinatol. 2018;45:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Greeley SAW, Polak M, Njølstad PR, Barbetti F, Williams R, Castano L, Raile K, Chi DV, Habeb A, Hattersley AT, Codner E. ISPAD Clinical Practice Consensus Guidelines 2022: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2022;23:1188-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Yeung RO, Al Jundi M, Gubbi S, Bompu ME, Sirrs S, Tarnopolsky M, Hannah-Shmouni F. Management of mitochondrial diabetes in the era of novel therapies. J Diabetes Complications. 2021;35:107584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Parker VE, Semple RK. Genetics in endocrinology: genetic forms of severe insulin resistance: what endocrinologists should know. Eur J Endocrinol. 2013;169:R71-R80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Lightbourne M, Brown RJ. Genetics of Lipodystrophy. Endocrinol Metab Clin North Am. 2017;46:539-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis. 2010;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Ogawa W, Araki E, Ishigaki Y, Hirota Y, Maegawa H, Yamauchi T, Yorifuji T, Katagiri H. New classification and diagnostic criteria for insulin resistance syndrome. Endocr J. 2022;69:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Yee ML, Wong R, Datta M, Fazlo TN, Ebrahim MM, Mcnamara EC, De Jong G, Gilfillan C. Mitochondrial disease: an uncommon but important cause of diabetes mellitus. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Østoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, Knop FK, Vilsbøll T. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care. 2014;37:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Stride A, Ellard S, Clark P, Shakespeare L, Salzmann M, Shepherd M, Hattersley AT. Beta-cell dysfunction, insulin sensitivity, and glycosuria precede diabetes in hepatocyte nuclear factor-1alpha mutation carriers. Diabetes Care. 2005;28:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Sun HY, Lin XY. Genetic perspectives on childhood monogenic diabetes: Diagnosis, management, and future directions. World J Diabetes. 2023;14:1738-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 30. | Bonnefond A, Boissel M, Bolze A, Durand E, Toussaint B, Vaillant E, Gaget S, Graeve F, Dechaume A, Allegaert F, Guilcher DL, Yengo L, Dhennin V, Borys JM, Lu JT, Cirulli ET, Elhanan G, Roussel R, Balkau B, Marre M, Franc S, Charpentier G, Vaxillaire M, Canouil M, Washington NL, Grzymski JJ, Froguel P. Pathogenic variants in actionable MODY genes are associated with type 2 diabetes. Nat Metab. 2020;2:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Bonnefond A, Unnikrishnan R, Doria A, Vaxillaire M, Kulkarni RN, Mohan V, Trischitta V, Froguel P. Monogenic diabetes. Nat Rev Dis Primers. 2023;9:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (1)] |
| 32. | Flannick J, Johansson S, Njølstad PR. Common and rare forms of diabetes mellitus: towards a continuum of diabetes subtypes. Nat Rev Endocrinol. 2016;12:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Heller S, Melzer MK, Azoitei N, Julier C, Kleger A. Human Pluripotent Stem Cells Go Diabetic: A Glimpse on Monogenic Variants. Front Endocrinol (Lausanne). 2021;12:648284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Burgos JI, Vallier L, Rodríguez-Seguí SA. Monogenic Diabetes Modeling: In Vitro Pancreatic Differentiation From Human Pluripotent Stem Cells Gains Momentum. Front Endocrinol (Lausanne). 2021;12:692596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |