Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.988
Peer-review started: December 31, 2023
First decision: January 17, 2024
Revised: January 26, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: May 15, 2024
Processing time: 131 Days and 6.4 Hours
Visceral obesity is increasingly prevalent among adolescents and young adults and is commonly recognized as a risk factor for type 2 diabetes. Estrogen [17β-estradiol (E2)] is known to offer protection against obesity via diverse me-chanisms, while its specific effects on visceral adipose tissue (VAT) remain to be fully elucidated.
To investigate the impact of E2 on the gene expression profile within VAT of a mouse model of prediabetes.
Metabolic parameters were collected, encompassing body weight, weights of visceral and subcutaneous adipose tissues (VAT and SAT), random blood glucose levels, glucose tolerance, insulin tolerance, and overall body composition. The gene expression profiles of VAT were quantified utilizing the Whole Mouse Genome Oligo Microarray and subsequently analyzed through Agilent Feature Extraction software. Functional and pathway analyses were conducted employing Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses, respectively.
Feeding a high-fat diet (HFD) moderately increased the weights of both VAT and SAT, but this increase was mitigated by the protective effect of endogenous E2. Conversely, ovariectomy (OVX) led to a significant increase in VAT weight and the VAT/SAT weight ratio, and this increase was also reversed with E2 treatment. Notably, OVX diminished the expression of genes involved in lipid metabolism compared to HFD feeding alone, signaling a widespread reduction in lipid metabolic activity, which was completely counteracted by E2 administration. This study provides a comprehensive insight into E2's local and direct protective effects against visceral adiposity in VAT at the gene level.
In conclusion, the present study demonstrated that the HFD-induced over-nutritional challenge disrupted the gene expression profile of visceral fat, leading to a universally decreased lipid metabolic status in E2 deficient mice. E2 treatment effectively reversed this condition, shedding light on the mechanistic role and therapeutic potential of E2 in combating visceral obesity.
Core Tip: It is widely accepted that adipocyte hypertrophy arises from an increase in lipogenesis and/or a reduction in lipolysis. Our findings reveal that despite the presence of adipocyte hypertrophy and visceral adiposity, the expression of genes related to both fatty acid biosynthesis and oxidation in visceral adipose tissue was significantly reduced following ovariectomy (OVX) in mice fed a high-fat diet, suggesting a comprehensive decline in metabolic activity. Remarkably, estrogen treatment fully reversed the disrupted expression patterns of lipid metabolic genes in OVX mice, correcting their disordered metabolic phenotype. This study unveils a novel mechanism underlying visceral adiposity and highlights estrogen's protective role against visceral obesity.
- Citation: Liu SH, Shangguan ZS, Maitiaximu P, Li ZP, Chen XX, Li CD. Estrogen restores disordered lipid metabolism in visceral fat of prediabetic mice. World J Diabetes 2024; 15(5): 988-1000
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/988.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.988
The distinctive gynoid-type body shape, characterized by predominant fat distribution in the femoral-gluteal subcutaneous area, becomes apparent during puberty and diminishes after menopause. This change underscores the pivotal role of ovarian 17-β estradiol (E2) in regulating fat distribution[1,2]. Throughout their reproductive years, women exhibit a lower incidence of obesity, diabetes, and various metabolic disorders. However, after menopause, marked by reduced or ceased E2 production, women become increasingly susceptible to central or visceral obesity, marked by excess lipid accumulation primarily in the visceral cavity and abdominal subcutaneous area, which predisposes them to metabolic diseases such as obesity and diabetes[3,4].
The primary function of white adipose tissue (WAT) is to store excess energy in the form of triglycerides. Based on its anatomical deposition sites, WAT is generally divided into two major categories: Subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT), which exhibit significant differences in adipocyte size and cellularity, vascular distribution, innervation, immune and inflammatory cell compositions, and a noticeable gender dimorphism[5]. Women of re-productive age tend to accumulate more SAT in the gluteal-femoral region, whereas men accumulate more VAT in the abdominal compartment[6]. Generally, girls have less VAT than boys, and this sex difference in fat distribution becomes more pronounced with maturation, especially from late puberty to early adulthood, with girls and women having 5% and 48% less VAT, respectively, than their male counterparts[1,5,7]. VAT constitutes about 10-20% of total body fat in men and 5%-8% in pre-menopausal women, a percentage that increases significantly in post-menopausal women[7,8]. Excessive VAT accumulation is closely linked to insulin resistance, diabetes, nonalcoholic steatohepatitis, cardiovascular diseases (CVD), mental disorders, and various cancers[9-13]. Even among individuals of normal weight, post-menopausal women with central obesity face a higher risk of CVD mortality and cancer mortality, highlighting the detrimental consequences of E2 deficiency and aberrantly increased visceral fat volume and/or composition[14]. Hormone replacement therapy (HRT) has been shown to be protective in restoring sex hormones and preventing visceral obesity, diabetes, and many other chronic diseases in post-menopausal and ovarian insufficiency women[15,16]. E2 exerts its effects through its two major receptors - ERα and ERβ. Notably, there is a gender dimorphism in ER expression, specifically in VAT, where the expression of both ERα and ERβ is much higher in females than in males[17]. Compared to ERβ, ERα plays a more pivotal role in whole-body energy metabolism and in WAT. The absence of ERα leads to weight gain, particularly in VAT, emphasizing the crucial role of E2/ER signaling in VAT metabolism[18-20].
Regarding the longitudinal developmental process of an individual, disregarding genetic, ethnic, nutritional, and environmental factors, endocrine factors including growth hormone, IGF-1, leptin, androgens, and estrogens, play determining roles in adolescence body composition and fat distribution. This process is characterized by pronounced gender dimorphism, with males gaining more fat-free mass, while females accumulate more fat mass, primarily in the hip and thigh areas[21,22]. The attainment of a minimum level of fatness for the initiation (17% of body weight) and maintenance (22% of body weight) of menstrual cycles suggests a tight and necessary association among fat accumulation, reproduction, and sex hormones[23]. Early pubertal girls, with fat localized predominantly on the hips, exhibit the highest levels of sex steroids and gonadotropins, indicating that gynoid-type fat distribution results from ovarian activity[24]. Paradoxically, obese girls with predominantly abdominal fat accumulation show increased plasma E2 levels and decreased androgen/estrogen ratio, implying a compensatory increase in aromatization in VAT or a state of E2 resi-stance. In either case, a reciprocal relationship between body fat distribution and E2 levels is suggested[24].
While the effects of E2 in regulating fat expansion and distribution, fatty acid uptake and metabolism, vascularization, immunological cell composition, and adipokine expression profiles have been extensively studied, its direct effects in situ in VAT at the gene level are little known[25]. The present study was designed to delineate the local and direct actions of E2 in VAT in estrogen-deficient mice challenged with an over-nutritional high-fat diet (HFD).
Female C57BL/6 mice, aged 6-8 wk (corresponding to late puberty), were acquired from the Shanghai Center of Experimental Animals, Chinese Academy of Sciences (Shanghai, PR China). The mice were housed in a controlled environment with temperatures set at 22 ± 1°C and subjected to a 12-h light-dark cycle, and had free access to water and standard chow or HFD pellets. Metabolic parameters and gene expression profiles were investigated in mice consuming a lard-enriched HFD (comprising 20% lard, 4% sucrose, 2% milk, 1% cholesterol, and 73% standard chow), with findings further confirmed in a group fed a more calorie-dense HFD deriving 60% of its calories from fat (D12492; Research Diets, Inc., New Brunswick, NJ, United States). Estrogen deficiency was induced by bilateral ovariectomy (OVX) surgery under anesthesia. Post-surgery, the mice were treated immediately with E2 through the subdermal implantation of an E2 pellet (0.18 μg/pellet, 60-d release, Innovative Research of America, Sarasota, Florida, United States) beneath the neck skin, with additional pellets provided as necessary under anesthesia. Following a week-long recovery from surgery, the mice were placed on the HFD regimen.
At the end of the experiments, serum samples were obtained through cardiac puncture under anesthesia and cen-trifuged at 3000 rpm to separate the supernatant, which was then stored at -80 ºC. Leptin levels in the plasma were quantified using a Milliplex kit (Mouse Metabolic Magnetic Bead Panel, Cat.#MMHMAG-44K, EMD Millipore). Adipose tissues, harvested under anesthesia, were either fixed in 4% paraformaldehyde or immediately frozen in liquid nitrogen before being stored at -80 ºC for future analysis. All animal experiments and procedures received approval from the Xiamen University Animal Care and Use Committee and adhered to the ARRIVE guidelines.
Body weight was monitored weekly, while blood glucose levels were measured every 2 wk. Blood glucose measurements were obtained using the ONETOUCH UltraVue device (Johnson, China) from blood samples collected from the tail vein.
Intra-peritoneal glucose tolerance test (IPGTT) was performed following a 12-h overnight fast. Glucose was injected intraperitoneally at a dose of 1 g/kg body weight. Blood glucose levels were then measured from the tail vein at specific time intervals: Immediately before (0 min) and at 15, 30, 60, and 120 min after the glucose injection. The glucose area under the curve (AUC) values during the IPGTT were calculated employing the trapezoidal rule.
Intra-peritoneal insulin tolerance test (IPITT) was carried out after a 6-h fast. Insulin was administered via intra-peritoneal injection at a dose of 0.5 IU/kg body weight. Blood glucose levels were measured using blood samples from the tail vein at the time points of 0, 15, 30, 60, and 120 min post insulin injection. Glucose AUC values during the IPITT were calculated using the trapezoidal rule.
Body composition, including fat mass, lean mass, and body fluid, was measured using EchoMRI-100H (EchoMRI, United States).
Fixed VATs underwent dehydration and paraffin embedding, and were sectioned into 5 μm slices. The slices were dewaxed, rehydrated, and stained with hematoxylin and eosin (HE). Morphological changes were visualized using an EVOS™ M7000 microscope (Thermo Fisher Scientific).
Total RNA was extracted using TRIzol® Reagent (Invitrogen Life Technologies) following the manufacturer’s instructions. The procedure for RNA labeling and array hybridization followed the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology). Briefly, total RNA from each sample underwent linear amplification and was labeled with Cy3-UTP. The labeled cRNA was then purified using the RNeasy Mini Kit (Qiagen). For frag-mentation, 1 μg of labeled cRNA was mixed with 11 μL of 10 × Blocking Agent and 2.2 μl of 25 × Fragmentation Buffer, and the mixture was heated at 60 °C for 30 min. After fragmentation, 55 μL of 2 × GE Hybridization Buffer was added to the labeled cRNA, which was then applied to the gasket slide and assembled with the microarray slide. The slides were incubated for 17 h at 65 °C in an Agilent Hybridization Oven. Post-hybridization, the arrays were washed, fixed, and scanned with an Agilent DNA Microarray Scanner (part number G2505C). Gene expression data were extracted using the Agilent Feature Extraction Software.
The analysis of microarray images was performed utilizing Agilent Feature Extraction software (version 11.0.1.1). The GeneSpring GX v 12.1 software package (Agilent Technologies) was employed for quantile normalization and subsequent data processing. Only genes marked as Detected in at least 7 of the 14 samples proceeded to further analysis. Differentially expressed genes, determined by statistical significance between groups, were identified using Volcano Plot and Fold Change filtering. Hierarchical clustering was executed with R scripts. For understanding biological implications, Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed using the established enrichment computation methods.
Total RNA was extracted employing the RZ reagent (TIANGEN, DP419, China) in accordance with the guidelines provided by the manufacturer. The conversion of total RNA to cDNA was facilitated by the FastKing RT Kit - With gDNase (TIANGEN, KR116). Subsequent real-time quantitative PCR was performed utilizing the SuperReal PreMix Plus-SYBR Green (TIANGEN, FP205, China) on a Roche LightCycler 480 II system. The expression of target genes was normalized to the expression of the housekeeping gene β-Actin. The specific primers used for this analysis are: Fatty acid synthase (Fasn; forward): 5’-CATG ACCTCGTG ATGAA CGTGT-3’, (reverse) 5’-CGGG TGAGGACGTTTACAAAG-3’; acetyl-Coenzyme A carboxylase alpha (Acaca; forward): 5’-ACGCATCGCCCAATTTCTGA-3’, (reverse) 5’-CCAGACAGGGACATGGACTC-3’; 3-oxoacyl-ACP synthase (Oxsm; forward): 5’-CACAGGCATTGGCCTAGTGAC-3’, (reverse) 5’-CCTCTTGGTACATAAGCAGCTAC-3’; carnitine palmitoyl transferase 1a (Cpt1a; forward): 5’-CTCCGCCTGAGCCATGAAG-3’, (reverse) 5’-CACCAGTGATGATGCCATTCT-3’; carnitine palmitoyl transferase 2 (Cpt2; forward): 5’-CAAAAGACTCATCCGCTTTGTTC-3’, (reverse) 5’-CATCACGACTGGGTTTGGGTA-3’; lipase (LIPE; forward ): 5’-TGGCACACCATTTTGACCTG-3’, (reverse) 5’-TTGCGGTTAGAAGCCACATAG-3’; leptin (Lep; forward): 5’-TTCACACACGCAGTCGGTATC-3’, (reverse) 5’-GGCTGGTGAGGACCTGTTG-3’; β-Actin (forward): 5’-GGCTGTATTCCCCTCCATCG-3’, (reverse) 5’-CCAGTTGGTAACAATGCCATGT-3’.
Data are expressed as the mean ± SE and were analyzed using ANOVA. Statistical significance was considered when the P value was < 0.05.
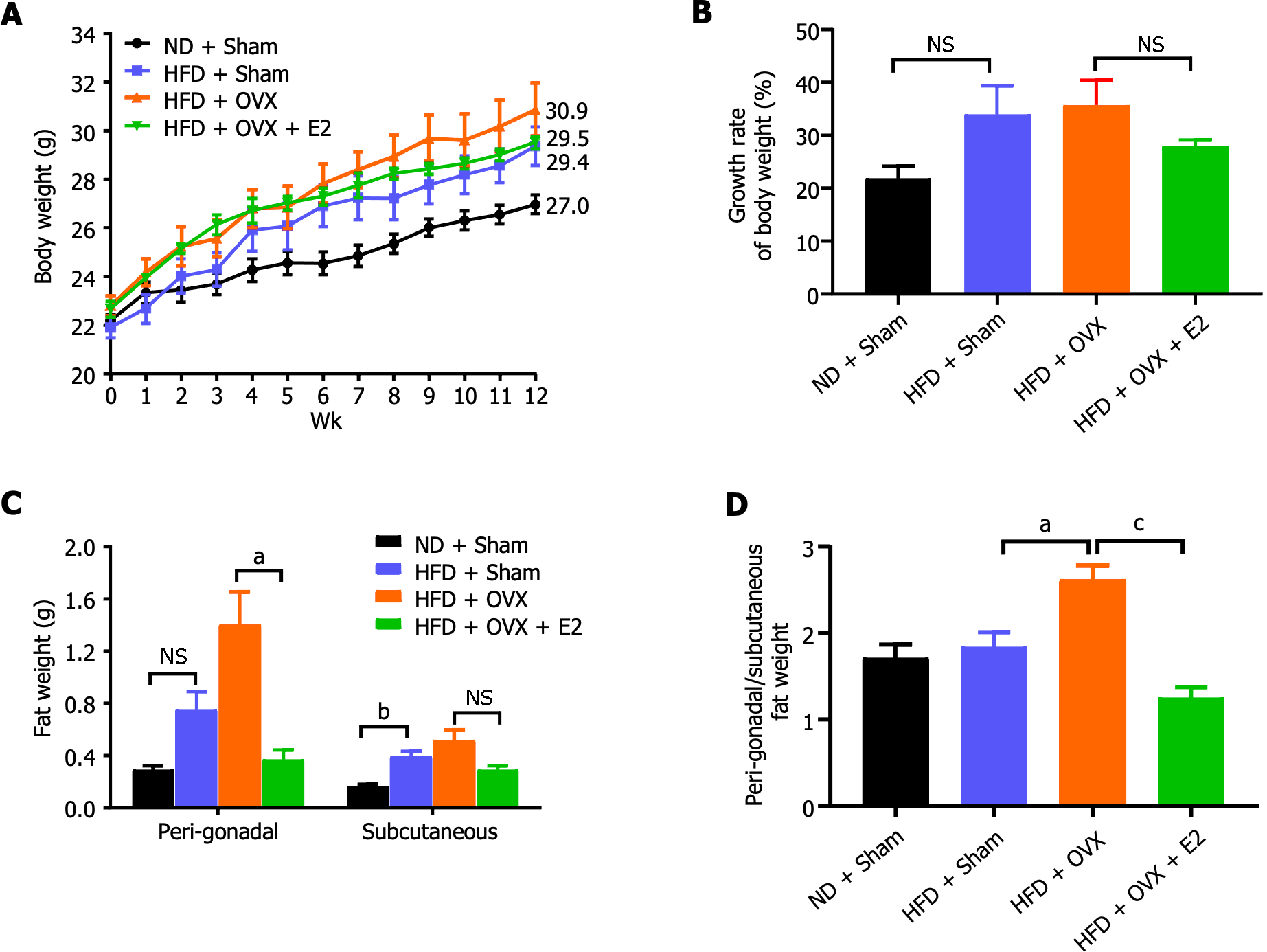
A diet enriched with 20% lard and OVX led to a slight increase in body weight among the mice, though the change was not statistically significant (P > 0.05; Figure 1A and B). E2 administration appeared to counteract the body weight gain induced by OVX, albeit without statistical significance (P > 0.05; Figure 1A and B). Both VAT and SAT weights were increased due to the HFD and were further exacerbated by OVX. This effect was completely reversed with E2 treatment (P < 0.05; Figure 1C). Central obesity, defined by excess fat accumulation in the abdominal area, was significantly deteriorated by OVX through the elimination of the protective effects of endogenous E2 against HFD-induced visceral adiposity. This was evidenced by a significant increase in the ratio of peri-gonadal to subcutaneous fat pad weight, which was fully restored by E2 treatment (P < 0.001; Figure 1D).
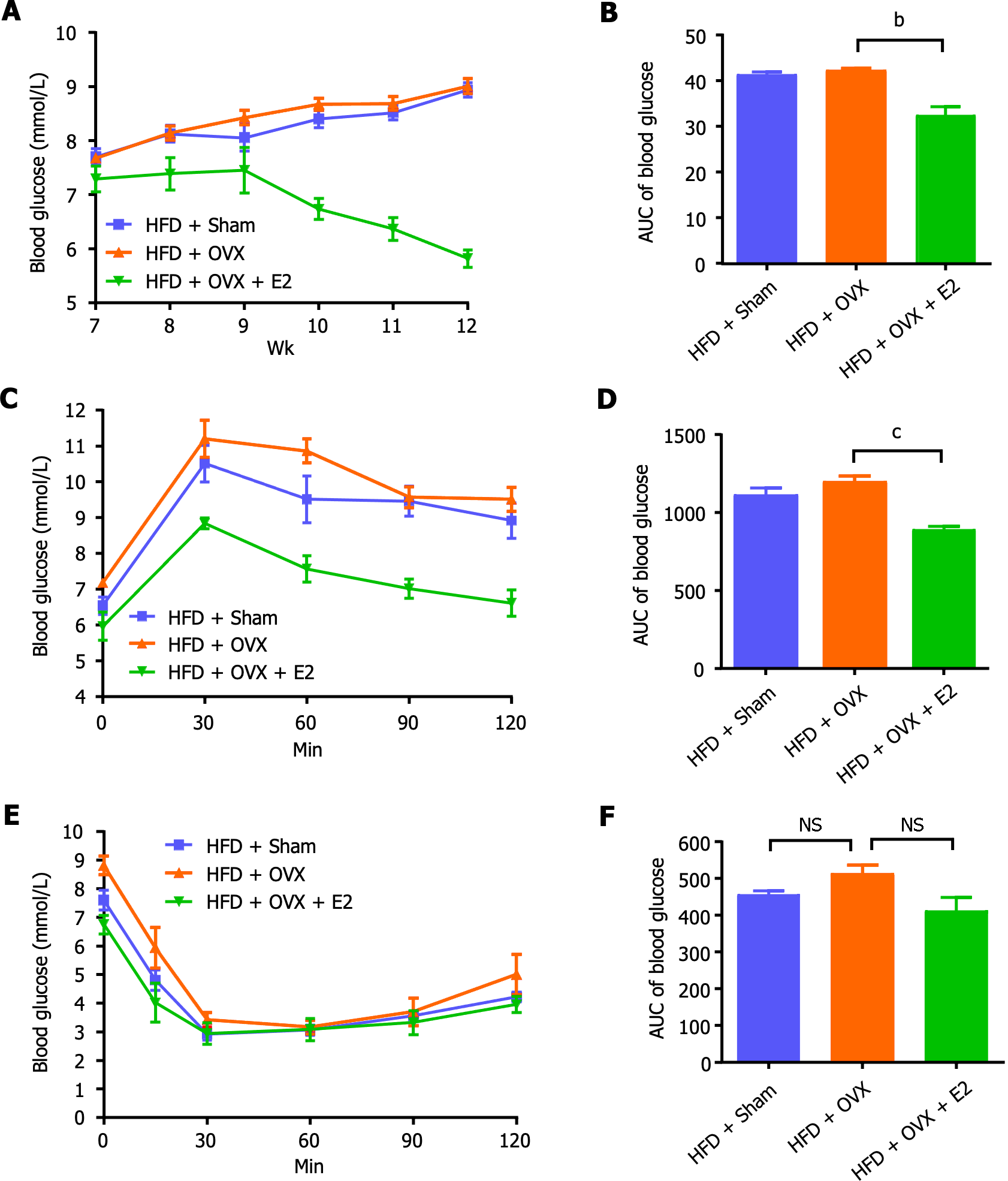
Compared to the control mice on a normal chow diet, sham-operated HFD mice displayed no significant changes in random blood glucose levels, glucose tolerance, and insulin tolerance, attributing to the protective effect of endogenous E2 on glucose metabolism (Figure 2). OVX impaired glucose tolerance, which was effectively reversed by E2 treatment (P < 0.001; Figure 2C and D). E2 also reduced random blood glucose levels (P < 0.01; Figure 2A and B) and tended to increase insulin sensitivity by improving insulin tolerance, though these changes did not achieve statistical significance (P > 0.05; Figure 2E and F).
To explore the impacts of OVX and E2 replacement therapy on VAT function and signaling in the context of an HFD, VAT gene expression profiles were assessed through microarray analysis. GO analysis revealed that OVX induced significant disruptions in cellular functions compared to HFD feeding alone, as evidenced by altered gene expression: Both decreased and increased expression of genes associated with biological processes, cellular components, and molecular functions in OVX mice primarily related to cellular and metabolic processes, intracellular organelles, and binding activities, suggesting functional disturbances in VAT. These alterations were almost entirely rectified by E2 treatment (data not shown).
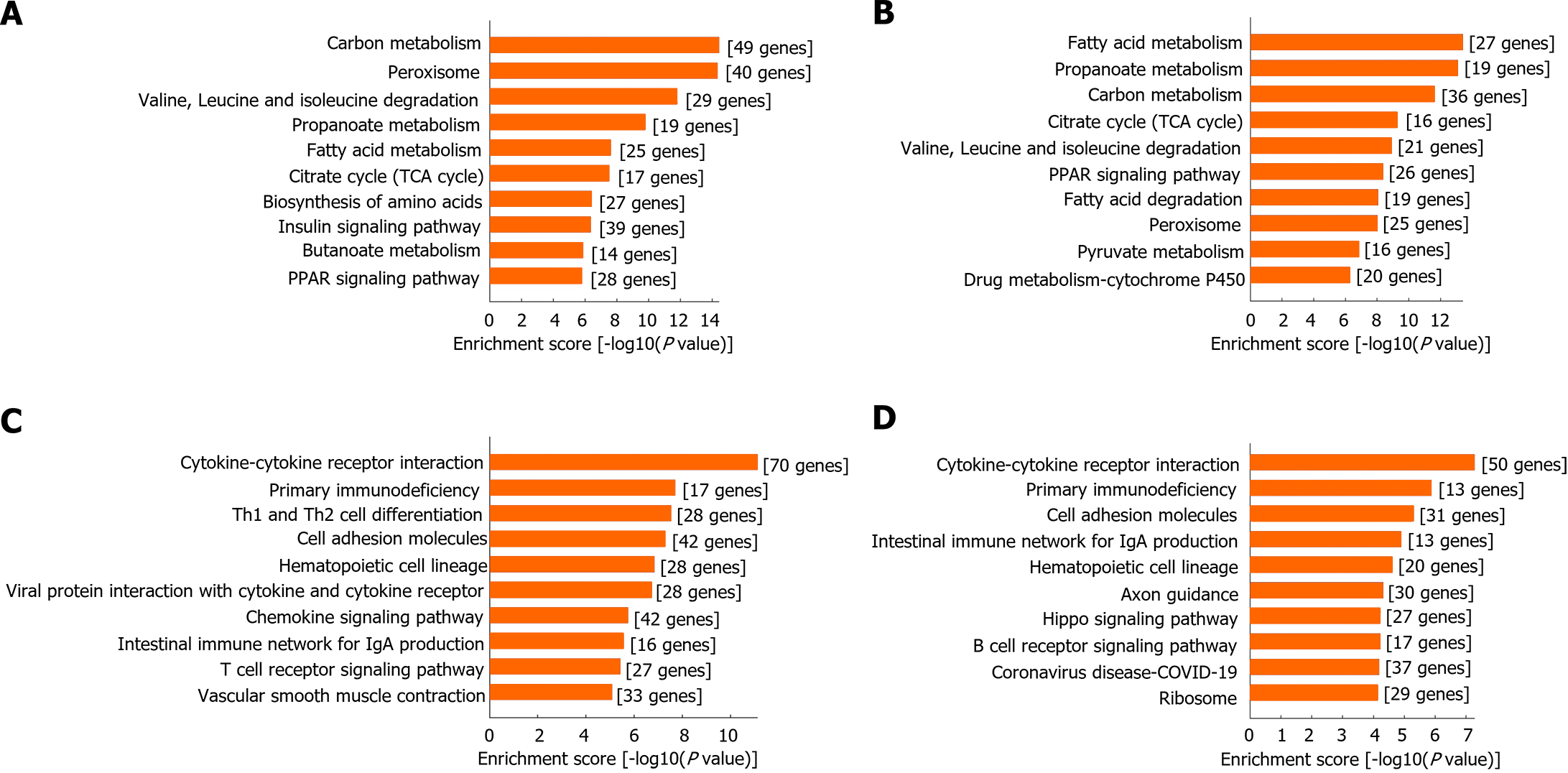
KEGG signaling pathway analysis highlighted that, relative to HFD feeding alone, the most significantly down-regulated pathways in OVX mice involved carbon metabolism, peroxisome, and fatty acid metabolism among others, indicating a metabolic dysfunction. E2 treatment effectively reversed the down-regulation of these pathways, underlining E2's capability in restoring metabolic balance affected by OVX (Figure 3A). For E2-treated HFD + OVX mice, the most significantly up-regulated pathways included fatty acid metabolism, carbon metabolism, and citrate cycle (TCA cycle), reflecting E2's potent role in ameliorating OVX-induced metabolic disorders and enhancing fatty acid metabolism (Figure 3B).
Moreover, compared to HFD feeding alone, OVX increased the expression of pathways related to cytokine-cytokine receptor interaction and various immune and inflammatory processes, highlighting an induced immune and inflammatory state. This condition was almost fully mitigated by E2 treatment, showing E2's potential in counteracting the immune and inflammatory responses triggered by OVX (Figure 3C and D).
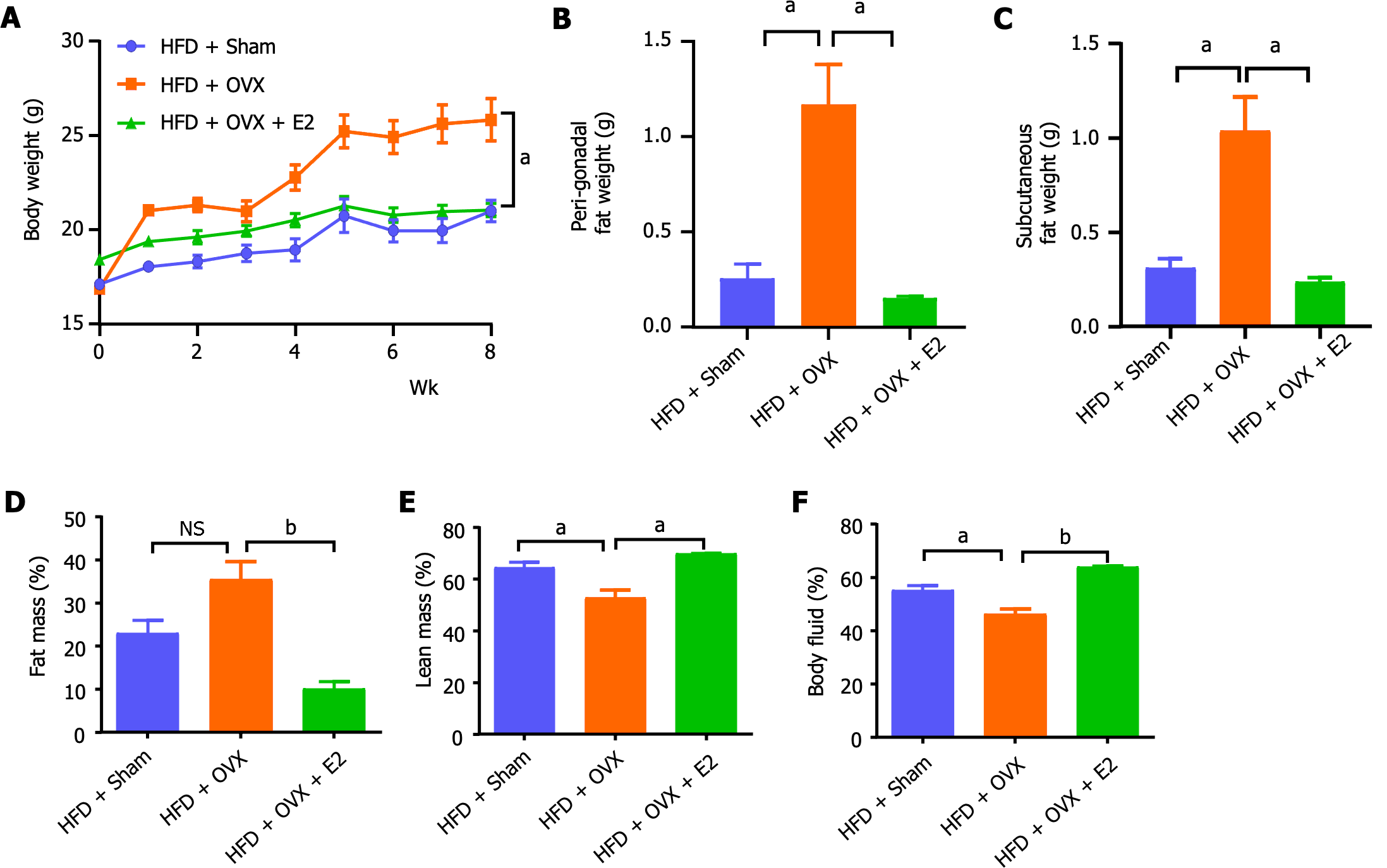
To corroborate the protective effects of E2 against visceral adiposity observed in mice fed a 20% lard-enriched HFD, we introduced an additional group of mice to a more calorically dense HFD, comprising 60% of calories from fat. This further examination confirmed that OVX inclined the mice towards HFD-induced adiposity, manifesting as increases in body weight, SAT, and VAT weights, changes that were completely mitigated by E2 treatment (P < 0.05; Figure 4A-C). Body composition analyses provided additional evidence that OVX led to an increase in fat mass while reducing lean mass and body fluid levels, with E2 treatment effectively reversing these effects (P < 0.05; Figure 4D-F).
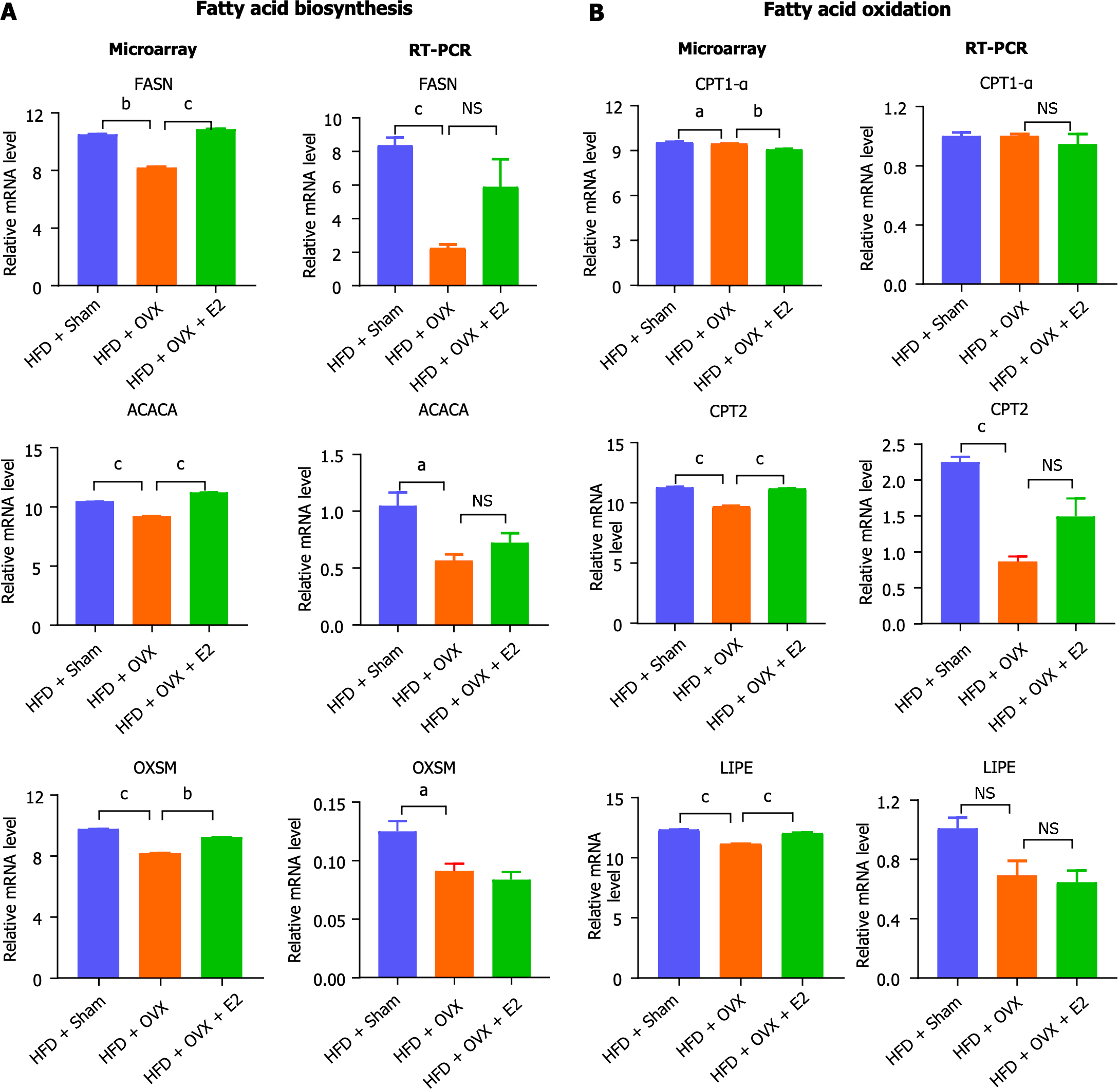
Quantitative real-time PCR was employed to validate the expression levels of select lipid metabolic genes, facilitating a comparison with RNA microarray findings. This analysis confirmed that key enzymes involved in lipid metabolism were down-regulated or showed a tendency to be down-regulated following OVX, a trend that was counteracted by E2 treatment. Specifically, genes involved in fatty acid biosynthesis, such as Fasn, Acaca, and Oxsm, were found to be down-regulated in OVX mice, a condition that was reversed with E2 treatment (Figure 5). Similarly, genes important for fatty acid oxidation, including Cpt1a, Cpt2, and LIPE, were down-regulated in OVX mice, and E2 treatment effectively reversed this down-regulation (Figure 5).
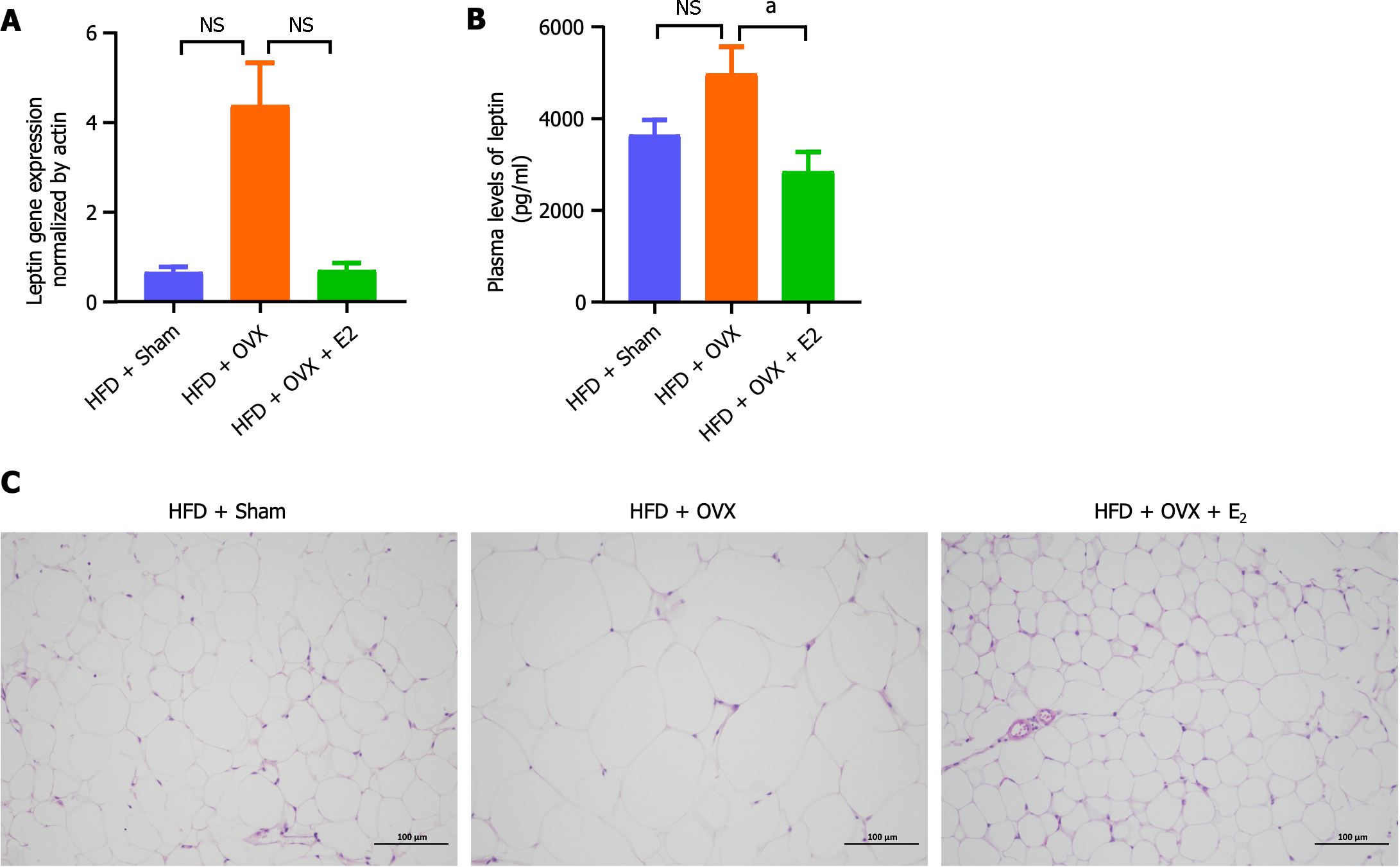
Plasma leptin level serves as an indicator of overall adiposity. Here, the plasma leptin levels were measured to assess their correlation with changes in adiposity due to dietary and hormonal manipulations. Compared to mice fed an HFD alone, OVX appeared to elevate plasma leptin levels, an effect that E2 treatment successfully reversed (P < 0.05; Figure 6A). Similarly, the gene expression of leptin in VAT showed a tendency to increase following OVX, which E2 treatment managed to normalize, although this change did not reach statistical significance (P > 0.05; Figure 6B). HE staining further revealed that, in comparison to HFD feeding alone, the addition of OVX led to an increase in adipocyte size, a phenomenon that was mitigated by E2 treatment (Figure 6C).
The rise of adolescent obesity incidence over past decades has been alarmingly linked to various metabolic disorders including diabetes[26-29]. In the present study, employing an HFD-fed obese mouse model with estrogen (E2) deficiency induced by OVX, we showed that E2 deficiency significantly worsened HFD-induced visceral obesity and disorders of glucose metabolism. Moreover, it specifically altered VAT gene expression, notably down-regulating genes associated with metabolism, especially fatty acid metabolism. Remarkably, E2 supplementation reversed these adverse effects, indicating E2's crucial role in maintaining VAT fatty acid metabolism and proper fat distribution. OVX led to a reduction in the expression of genes involved in both fatty acid synthesis and oxidation, significantly impairing VAT's metabolic function—a condition that E2 treatment fully restored. Furthermore, OVX induced an increase in cytokine and immune-signaling gene expression, suggesting an inflammatory state that E2 treatment also effectively mitigated. These findings underscore E2's significant protective and regulatory effects on lipid metabolism and inflammation within VAT.
Disrupted homeostasis, where energy intake surpasses consumption leading to fat storage, is a key factor in obesity. Notably, fat distribution rather than total fat mass serves as a better predictor of 'metabolically healthy' outcomes[30]. Excessive accumulation of fat in the visceral and abdominal subcutaneous areas significantly increases the risk of insulin resistance, diabetes, and various metabolic diseases[9-13]. Normal-weight post-menopausal women with central obesity have a heightened mortality risk[14]. Here we found that OVX markedly increased VAT weight and slightly increased body weight, pinpointing a major factor in disrupted VAT lipid metabolism. Body fat distribution exhibits a clear sexual dimorphism, evident from puberty, with males and females showing preferences for central and peripheral fat accumulation, respectively[1,8]. This divergence amplifies with maturation, influenced significantly by sex steroid hormones[1]. Key life stages—puberty and menopause—dictate fat distribution shifts in women, from predominantly lower body during puberty to more abdominal post-menopause, largely attributed to estrogen deficiency[7,8,21,22,31,32]. Aging contributes to increased total fat mass from pre- to post-menopause, but the drastic decline of E2 during menopause primarily drives fat redistribution[33]. Our findings that OVX significantly raised VAT weight and the VAT/SAT ratio, all reversible with E2 treatment, underscore the crucial role of E2 deficiency in visceral obesity development (Figure 1C and D). HRT with E2 has been shown to prevent post-menopausal obesity, especially in reducing visceral fat, thus maintaining a healthier fat distribution[34,35]. For adolescent girls with Polycystic Ovary Syndrome, long-term treatment with an estrogen-progestagen compound has been beneficial in reducing waist size and improving glucose metabolism[36], further highlighting E2's critical role in managing obesity and metabolic health.
In addition to obesity, particularly central obesity, muscle wasting is a significant concern in post-menopausal women, characterized by high fat mass coupled with low lean mass and body fluid levels[37,38]. Our study revealed that OVX led to an increase in fat mass and a decrease in lean mass and body fluid in mice, illustrating the impact of E2 deficiency on body composition (Figure 4D-F). This E2 deficiency not only worsened central obesity but also was evidenced by elevated plasma leptin levels and increased leptin gene expression in VAT, suggesting leptin resistance. These conditions were effectively reversed with E2 treatment (Figure 6A and B), highlighting E2's protective role against visceral adiposity and leptin resistance, a finding consistent with reports from obese animal models[39-41].
WAT expansion occurs through either hypertrophy, marked by an increase in adipocyte size, or hyperplasia, characterized by an increase in adipocyte number. In our study, HE staining revealed adipocyte hypertrophy in the VAT of OVX mice, indicating metabolic unhealthiness due to WAT expansion. This hypertrophy was corrected with E2 treatment (Figure 6C), suggesting E2's role in maintaining adipocyte health. Adipocyte hypertrophy is linked to metabolic complications like local inflammation and insulin resistance[42], which can be exacerbated by the presence of M1 macrophages or B-cell-mediated inflammation, further contributing to insulin resistance[43]. Our insulin tolerance tests indicated a trend toward decreased insulin sensitivity in OVX mice, an effect that E2 treatment tended to reverse, although without reaching statistical significance (Figure 2E and F). Additionally, OVX induced an up-regulation in the expression of immune and inflammatory-related genes within VAT, a state that E2 treatment effectively reversed (Figure 4C and D), highlighting E2's potential to enhance lipid metabolism and reduce inflammation in VAT. Research by Ohlsson et al[44] supports this, showing that selective overexpression of aromatase in WAT can diminish inflammation and improve insulin sensitivity in male mice, with increased aromatase activity also promoting adipogenesis. It is suggested that a HFD may worsen these inflammatory responses and adipocyte size due to the compounded effects of OVX[45], em-phasizing the complex interplay between diet, hormonal status, and adipose tissue health.
E2’s protective effects on visceral adiposity and glucose tolerance have been well-documented across both animal models and human studies[34,35,39,41]. Despite this, the specific mechanisms of E2's direct actions on VAT remain partially understood. Through microarray analysis and subsequent KEGG pathway exploration, we discovered that OVX significantly diminished global lipid metabolism in VAT, affecting both anabolic fatty acid synthesis and catabolic fatty acid oxidation pathways. This indicates a broad suppression of fatty acid metabolism, with the most significantly down-regulated genes primarily involved in metabolic functions, particularly fatty acid metabolism—a process notably restored by E2 treatment (Figure 3). Further validation via RT-PCR confirmed OVX's reduction in the expression of crucial enzymes for fatty acid biosynthesis (Fasn, Acaca, and Oxsm) and oxidation (Cpt1α, Cpt2, and LIPE), which E2 treatment effectively reversed (Figure 5). This aligns with findings from other researchers who reported decreased Fasn expression in VAT of OVX mice fed an HFD, although they noted further inhibition with E2 treatment[46]. Observations at the protein level by Boldarine et al[47] indicated that OVX in retroperitoneal white fat favored lipogenesis over fatty acid oxidation, leading to a proinflammatory state. Thus, the adipocyte hypertrophy observed in VAT could be attributed not solely to increased lipogenesis or impaired lipolysis but to a comprehensive disruption in transcriptional programs, adipokine secretion, insulin responses, and overall lipid metabolism and storage capacity[25,48].
In this study, utilizing a late pubertal estrogen deficiency prediabetic mouse model, we discovered that an HFD-induced over-nutritional challenge precipitates visceral adiposity, impairs glucose tolerance, and disrupts the gene expression profile of visceral fat, leading to a comprehensive down-regulation of lipid metabolism, encompassing both lipogenesis and lipolysis. Remarkably, these adverse effects were almost entirely reversed by E2 treatment. Our findings provide a comprehensive insight into the role of E2 deficiency in the development of visceral obesity under conditions of nutritional excess and highlight the necessity of systemically enhancing lipid metabolism, rather than merely targeting lipogenesis or lipolysis, for the effective treatment of visceral obesity. Moreover, this research unveils a novel mechanism by which estrogen deficiency contributes to the pathogenesis of visceral adiposity, underscoring the therapeutic potential of E2 in managing visceral obesity.
The prevalence of visceral obesity among adolescents and young adults is surging, significantly heightening their risk of metabolic diseases, such as type 2 diabetes. While estrogen [17β-estradiol (E2)] is known to offer protection against obesity through diverse mechanisms, its specific impact on visceral adipose tissue (VAT) remains to be fully elucidated.
To investigate the impact of E2 on the gene expression profile within VAT of late pubertal prediabetic mice.
To elucidate the local and direct effects of E2 on VAT and uncover the underlying molecular mechanisms in a prediabetic mouse model.
Female C57BL/6 mice were used to create an E2 deficient prediabetes model through ovariectomy (OVX) followed by high-fat diet (HFD) feeding. Metabolic parameters were monitored. Gene expression profiles in VAT were assessed using Whole Mouse Genome Oligo Microarray. Pathway analyses were conducted with the Kyoto Encyclopedia of Genes and Genomes. Expression of key lipid metabolic genes was confirmed by RT-PCR. Morphological alterations in VAT were examined via HE staining.
HFD modestly elevated the weights of visceral (VAT) and subcutaneous adipose tissue (SAT), a testament to the protective role of endogenous E2. In stark contrast, OVX markedly boosted VAT weight and the VAT/SAT weight ratio, effects that were mitigated by subsequent E2 treatment. OVX led to the down-regulation of genes implicated in both fatty acid biosynthesis and oxidation, signaling a comprehensive slowdown in lipid metabolism. Remarkably, E2 treatment fully reversed these alterations.
OVX intensified the visceral adiposity triggered by HFD feeding, leading to a universally diminished lipid metabolism in the absence of E2. Treatment with E2 effectively reversed this condition, shedding light on the mechanistic insights and the therapeutic promise of E2 in combating visceral obesity.
Mitochondria play a pivotal role in fatty acid elongation and oxidation. Investigating the influence of E2 on mitochondrial fatty acid metabolism is therefore crucial.
We are grateful to Ms. Xiao-Yan Liu and Mr. Han-Yu Liu for their proofreading and editing of the English manuscript. The authors express their gratitude to the Research Center for Translational Medicine at the First Affiliated Hospital of Xiamen University for offering a supportive research platform.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, South Korea S-Editor: Lin C L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring). 2010;18:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 462] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal Hormone Therapy and Type 2 Diabetes Prevention: Evidence, Mechanisms, and Clinical Implications. Endocr Rev. 2017;38:173-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 4. | Boldarine VT, Pedroso AP, Neto NIP, Dornellas APS, Nascimento CMO, Oyama LM, Ribeiro EB. High-fat diet intake induces depressive-like behavior in ovariectomized rats. Sci Rep. 2019;9:10551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1644] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 6. | Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, Miller KK, Bredella MA. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 7. | Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1485] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 8. | Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, Shimomura I, Tarui S, Matsuzawa Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207-202. [PubMed] |
| 9. | Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 847] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 10. | Saponaro C, Sabatini S, Gaggini M, Carli F, Rosso C, Positano V, Armandi A, Caviglia GP, Faletti R, Bugianesi E, Gastaldelli A. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 2022;42:2418-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Chen Q, Wu Y, Gao Y, Zhang Z, Shi T, Yan B. Effect of visceral adipose tissue mass on coronary artery disease and heart failure: A Mendelian randomization study. Int J Obes (Lond). 2022;46:2102-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Noh JW, Yang HK, Jun MS, Lee BC. Puerarin Attenuates Obesity-Induced Inflammation and Dyslipidemia by Regulating Macrophages and TNF-Alpha in Obese Mice. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Grumbach MM. Estrogen, bone, growth and sex: a sea change in conventional wisdom. J Pediatr Endocrinol Metab. 2000;13 Suppl 6:1439-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE, Neuhouser ML, Shadyab AH, Chlebowski RT, Manson JE, Bao W. Association of Normal-Weight Central Obesity With All-Cause and Cause-Specific Mortality Among Postmenopausal Women. JAMA Netw Open. 2019;2:e197337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Mattiasson I, Rendell M, Törnquist C, Jeppsson S, Hulthén UL. Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm Metab Res. 2002;34:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Armeni E, Paschou SA, Goulis DG, Lambrinoudaki I. Hormone therapy regimens for managing the menopause and premature ovarian insufficiency. Best Pract Res Clin Endocrinol Metab. 2021;35:101561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Luo H, Jiang M, Lian G, Liu Q, Shi M, Li TY, Song L, Ye J, He Y, Yao L, Zhang C, Lin ZZ, Zhang CS, Zhao TJ, Jia WP, Li P, Lin SY, Lin SC. AIDA Selectively Mediates Downregulation of Fat Synthesis Enzymes by ERAD to Retard Intestinal Fat Absorption and Prevent Obesity. Cell Metab. 2018;27:843-853.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Rodriguez-Cuenca S, Monjo M, Proenza AM, Roca P. Depot differences in steroid receptor expression in adipose tissue: possible role of the local steroid milieu. Am J Physiol Endocrinol Metab. 2005;288:E200-E207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Lim GE, Albrecht T, Piske M, Sarai K, Lee JTC, Ramshaw HS, Sinha S, Guthridge MA, Acker-Palmer A, Lopez AF, Clee SM, Nislow C, Johnson JD. 14-3-3ζ coordinates adipogenesis of visceral fat. Nat Commun. 2015;6:7671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Bjune JI, Strømland PP, Jersin RÅ, Mellgren G, Dankel SN. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front Endocrinol (Lausanne). 2022;13:828780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;31:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Baker ER. Body weight and the initiation of puberty. Clin Obstet Gynecol. 1985;28:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | de Ridder CM, Bruning PF, Zonderland ML, Thijssen JH, Bonfrer JM, Blankenstein MA, Huisveld IA, Erich WB. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J Clin Endocrinol Metab. 1990;70:888-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Steiner BM, Berry DC. The Regulation of Adipose Tissue Health by Estrogens. Front Endocrinol (Lausanne). 2022;13:889923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 26. | Steinbeck KS, Lister NB, Gow ML, Baur LA. Treatment of adolescent obesity. Nat Rev Endocrinol. 2018;14:331-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 27. | Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1105] [Cited by in RCA: 992] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 28. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4666] [Article Influence: 583.3] [Reference Citation Analysis (2)] |
| 29. | Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Banfield L, Toor PP, Zhou F, Guven A, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. The Prevalence of Obesity Among Children With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5:e2247186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 370] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 31. | Frank AP, de Souza Santos R, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2019;60:1710-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | Ambikairajah A, Walsh E, Tabatabaei-Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol. 2019;221:393-409.e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 33. | Papadakis GE, Hans D, Gonzalez Rodriguez E, Vollenweider P, Waeber G, Marques-Vidal P, Lamy O. Menopausal Hormone Therapy Is Associated With Reduced Total and Visceral Adiposity: The OsteoLaus Cohort. J Clin Endocrinol Metab. 2018;103:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Reubinoff BE, Wurtman J, Rojansky N, Adler D, Stein P, Schenker JG, Brzezinski A. Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: a prospective study. Fertil Steril. 1995;64:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001;39:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Pasquali R, Gambineri A, Anconetani B, Vicennati V, Colitta D, Caramelli E, Casimirri F, Morselli-Labate AM. The natural history of the metabolic syndrome in young women with the polycystic ovary syndrome and the effect of long-term oestrogen-progestagen treatment. Clin Endocrinol (Oxf). 1999;50:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Dmitruk A, Czeczelewski J, Czeczelewska E, Golach J, Parnicka U. Body composition and fatty tissue distribution in women with various menstrual status. Rocz Panstw Zakl Hig. 2018;69:95-101. [PubMed] |
| 38. | Geraci A, Calvani R, Ferri E, Marzetti E, Arosio B, Cesari M. Sarcopenia and Menopause: The Role of Estradiol. Front Endocrinol (Lausanne). 2021;12:682012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 39. | Weigt C, Hertrampf T, Zoth N, Fritzemeier KH, Diel P. Impact of estradiol, ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity. Mol Cell Endocrinol. 2012;351:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem Mol Biol. 2010;122:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 42. | Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 1010] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 43. | Verboven K, Wouters K, Gaens K, Hansen D, Bijnen M, Wetzels S, Stehouwer CD, Goossens GH, Schalkwijk CG, Blaak EE, Jocken JW. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep. 2018;8:4677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 44. | Ohlsson C, Hammarstedt A, Vandenput L, Saarinen N, Ryberg H, Windahl SH, Farman HH, Jansson JO, Movérare-Skrtic S, Smith U, Zhang FP, Poutanen M, Hedjazifar S, Sjögren K. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am J Physiol Endocrinol Metab. 2017;313:E450-E462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Ludgero-Correia A Jr, Aguila MB, Mandarim-de-Lacerda CA, Faria TS. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition. 2012;28:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, Sasahara M, Tsuneki H, Saito S, Sasaoka T. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab. 2012;303:E445-E456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Boldarine VT, Pedroso AP, Brandão-Teles C, LoTurco EG, Nascimento CMO, Oyama LM, Bueno AA, Martins-de-Souza D, Ribeiro EB. Ovariectomy modifies lipid metabolism of retroperitoneal white fat in rats: a proteomic approach. Am J Physiol Endocrinol Metab. 2020;319:E427-E437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN, O'Rourke RW. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity (Silver Spring). 2016;24:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |