Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.828
Peer-review started: December 25, 2023
First decision: January 10, 2024
Revised: February 1, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: May 15, 2024
Processing time: 137 Days and 4.1 Hours
Insulin therapy plays a crucial role in the management of type 2 diabetes as the disease progresses. Over the past century, insulin formulations have undergone significant modifications and bioengineering, resulting in a diverse range of available insulin products. These products show distinct pharmacokinetic and pharmacodynamic profiles. Consequently, various insulin regimens have em-erged for the management of type 2 diabetes, including premixed formulations and combinations of basal and bolus insulins. The utilization of different insulin regimens yields disparate clinical outcomes, adverse events, and, notably, patient-reported outcomes (PROs). PROs provide valuable insights from the patient’s perspective, serving as a valuable mine of information for enhancing healthcare and informing clinical decisions. Adherence to insulin therapy, a critical patient-reported outcome, significantly affects clinical outcomes and is influenced by multiple factors. This review provides insights into the clinical effectiveness of various insulin preparations, PROs, and factors impacting insulin therapy adherence, with the aim of enhancing healthcare practices and informing clinical decisions for individuals with type 2 diabetes.
Core Tip: Understanding the dynamics of insulin therapy in type 2 diabetes is crucial for the effective management of the disease. This review navigates through the evolution of insulin formulations, emphasizing the impact of different regimens on patient-reported outcomes. Adherence to insulin therapy, a pivotal factor for successful outcomes, is investigated alongside various insulin preparations and the influencing factors. Gaining insight into these dynamics can steer healthcare strategies, ultimately refining decision-making and boosting patient outcomes in type 2 diabetes management.
- Citation: Emad-Eldin M, Balata GF, Elshorbagy EA, Hamed MS, Attia MS. Insulin therapy in type 2 diabetes: Insights into clinical efficacy, patient-reported outcomes, and adherence challenges. World J Diabetes 2024; 15(5): 828-852
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/828.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.828
Diabetes mellitus (DM) is a noncommunicable disease that has become increasingly prevalent worldwide. It affects all age groups from children to older adults. According to the International Diabetes Federation Atlas 10th edition, the worldwide incidence of diabetes is estimated to be approximately 536.6 million people (representing 10.5% of the total population), which means that more than 1 in 10 adults suffer from this disease[1]. Type 2 DM (T2DM), accounting for over 90% of global diabetes cases, exhibits a persistent and rapid escalation in prevalence, which consequentially burden experienced at both individual and health system levels[2].
DM is a disease characterized by the disturbance of glucose hemostasis owing to insulin malfunction in the target tissues causing abnormalities in fat, protein, and carbohydrate metabolisms. The principal hallmark of the disease is elevated glucose level in the venous plasma, which is the gold standard for the diagnosis of DM[3]. The elevation of plasma glucose level is caused by absolute insulin deficiency as in type 1 diabetes (T1DM) or increased insulin resistance as in T2DM or both[4]. Consequently, prolonged insulin deficiency or resistance give rise to a spectrum of metabolic dysfunctions, precipitating insidious complications[5]. Insufficient or neglected management of hyperglycemia over an extended period can accelerate the onset of these complications[6]. In diabetes complications could precede the onset of pervasive impacts on various organs within the body, ultimately contributing to increased morbidity and mortality rates[7,8]. Therefore, detection of these complications during the initial diagnosis of diabetes is crucial.
The progressive nature of T2DM is a notable characteristic that leads to a gradual escalation of treatment options, starting from lifestyle modifications and oral antidiabetic medications and eventually culminating in insulin therapy[9,10]. Hence, many individuals with T2DM eventually discover that insulin therapy is an effective approach for managing their blood glucose levels. Among the extensive array of treatment modalities available for diabetes, insulin therapy is widely recognized as the most effective agent for lowering glucose levels, addressing acute and chronic elevations in blood glucose[11]. Over the past century, insulin therapy has undergone various stages of development, resulting in a diverse range of insulin types with pharmacokinetic (PK) and pharmacodynamic (PD) properties that are currently accessible for patients with diabetes. This diversity provides a broad spectrum of options and regimens that can be tailored to suit different patient-related conditions[12].
Patient-reported outcomes (PROs) emerge as invaluable instruments, particularly in the realm of chronic conditions such as diabetes, providing profound insights from the patient’s perspective across diverse facets of the disease. The true value of PROs was acknowledged in the early 1970s through studies that highlighted the significance of patients’ reports in enhancing overall patient care[13]. One application of PROs involves elucidating symptoms, treatment responsiveness, psychological well-being, and health-related quality of life (HRQOL)[14]. The multifaceted data derived from PROs serves as a pivotal resource, alongside clinical outcomes, for guiding adjustments in therapeutic approaches, informing clinical decisions, and facilitating the modulation of various aspects pertinent to patient care[15].
This review aimed to provide insights into diverse insulin preparations and regimens, considering their variable PK and PD properties for effective diabetes management. In addition, the review seeks to analyze PROs across clinical trials associated with distinct insulin regimens, particularly in individuals with type 2 diabetes, providing a comprehensive overview of the impact of insulin therapy on treatment satisfaction and HRQOL. Furthermore, the investigation of PROs aims to identify and understand factors influencing adherence to insulin therapy, offering guidance to patients and clinicians in addressing these factors to enhance overall adherence to insulin therapy.
Over the past century, insulin therapy has undergone significant development and innovation, from the isolation and purification of pancreatic extracts to the utilization of recombinant DNA technology to produce insulin analogs[16,17]. These advancements have significantly improved diabetic clinical care and patient outcomes. At present, there is a wide range of insulin formulations available, each with unique PK and PD properties. Understanding these properties is crucial for optimizing blood glucose control and avoiding adverse events such as hypoglycemia. Clinicians must possess knowledge of the tailored PK and PD properties of insulin to ensure safe and effective prescribing practices for their patients[18].
The physiology of endogenous insulin is substantial in understanding the rationale behind different exogenous insulin preparations. In a healthy individual, the pancreas regulates glucose homeostasis through continuous basal insulin secretion controlling adipocyte lipolysis and liver glucose production along with peaks of insulin secretion after meals to manage postprandial glucose levels[19]. Insulin, which is secreted as a prohormone by pancreatic beta-cells, undergoes proteolytic cleavage and is regulated by zinc and calcium ions for molecular assembly, structure, and stability. Endogenous insulin secretion is feedback-regulated based on blood glucose levels[20]. However, exogenous insulin adjustments lack this feedback mechanism, relying on blood glucose monitoring and considering carbohydrate intake[21]. This understanding forms the foundation for developing optimized basal and bolus insulin formulations through bioengineering advancements to enhance the PK and PD properties.
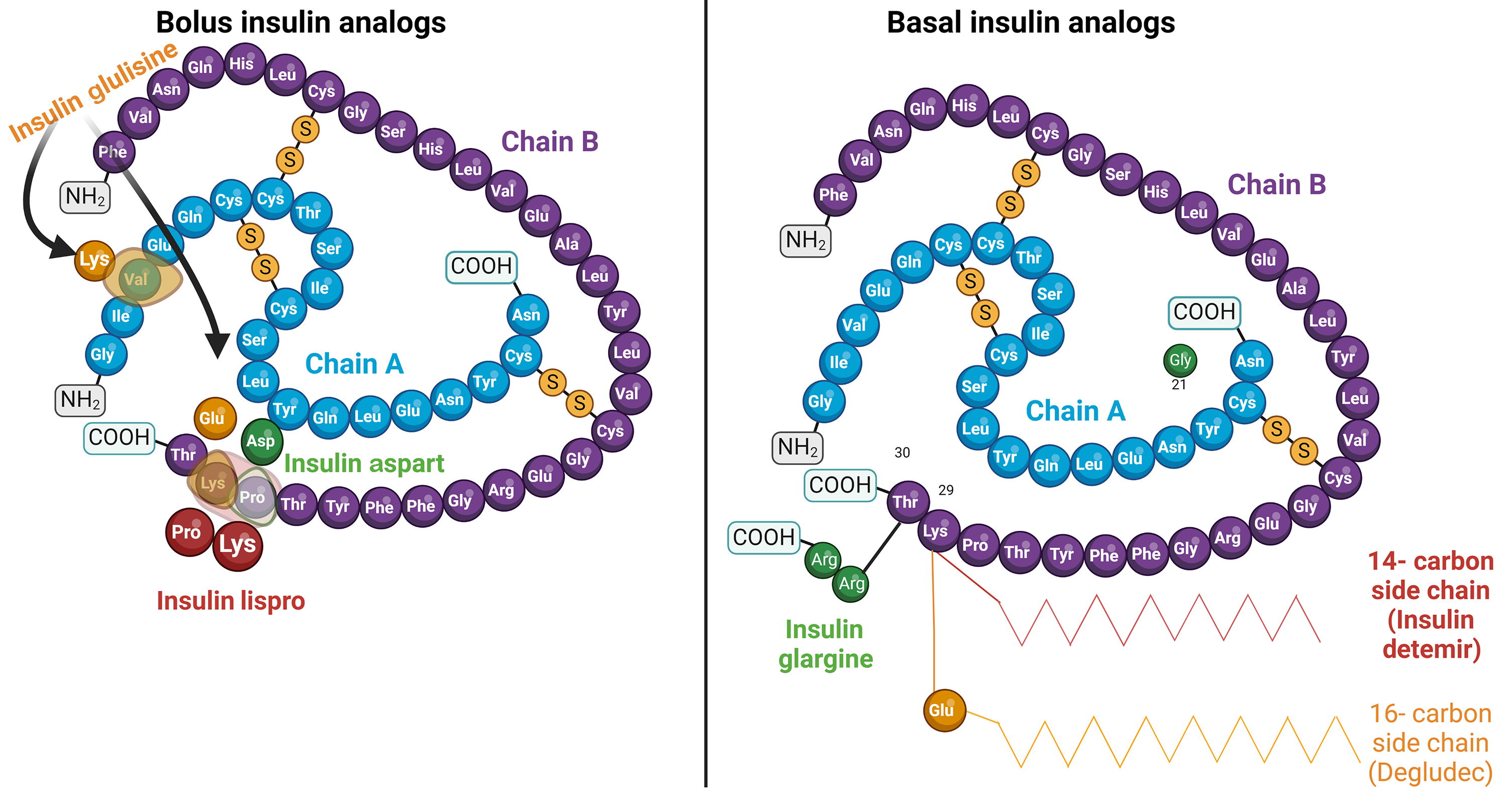
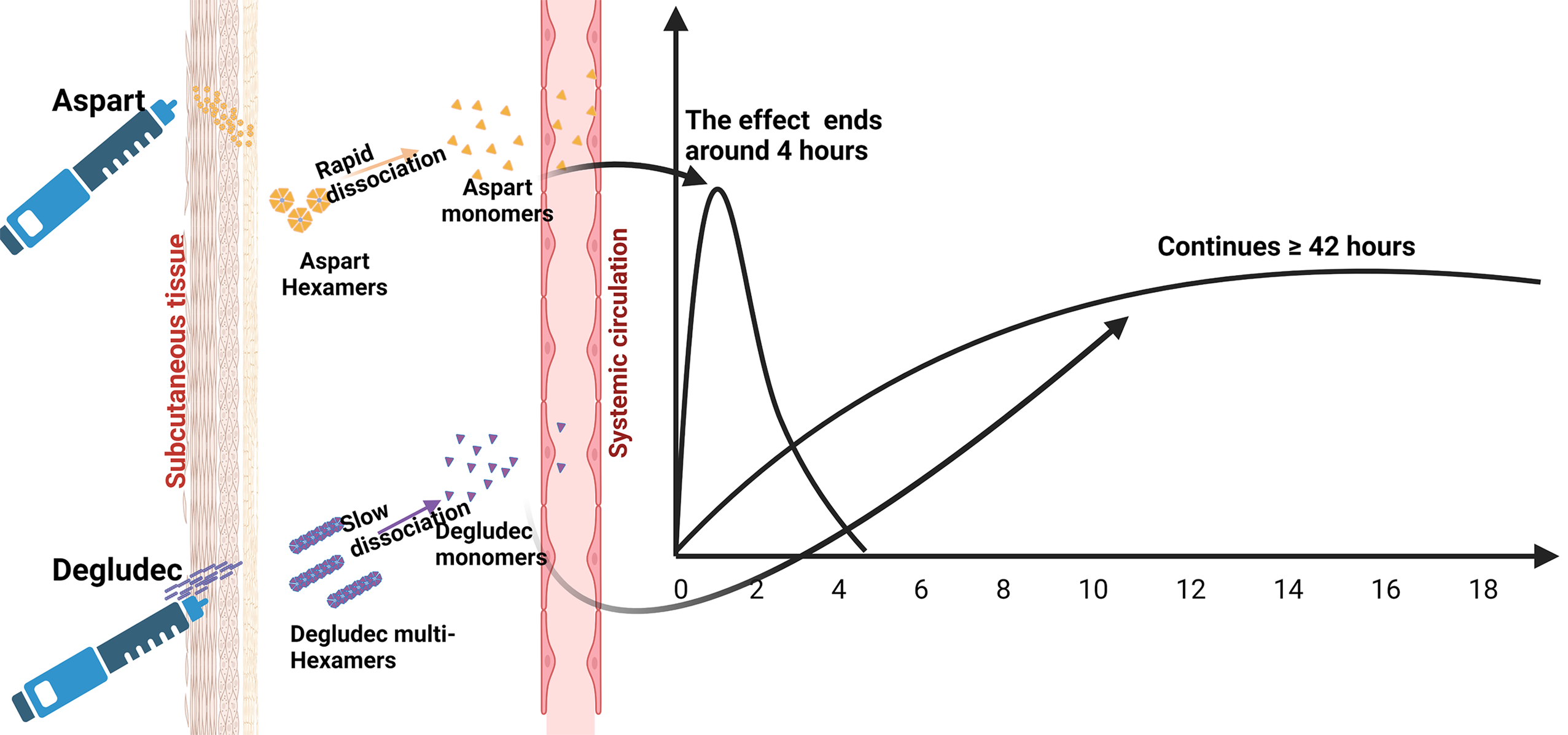
The time-action profiles of insulin preparations are determined by the absorption and distribution kinetics that occur after injection. When comparing different insulin formulations, parameters such as onset of action, duration of action, and peak time are crucial for pharmacometric evaluations. These parameters are influenced by various factors, including local blood flow to the injection sites in the subcutaneous (SC) tissue and the kinetics of insulin molecules transitioning from the SC depot to the bloodstream[12,22]. Manipulating the molecular features of insulin through recombinant DNA technology and the choice of formulations, including suspensions or hexameric solutions, play a role in determining the duration for insulin to enter systemic circulation[17,23]. For instance, insulin formulations in the hexameric form have interactions that bind the molecules together in the SC space. Stronger interactions delay the dissociation of hexamers into monomers, which results in a longer onset and duration of action. Conversely, weaker interactions lead to faster dissociation of hexamers and shorter onset and duration of action (Figure 1). In addition, it is possible to modulate insulin therapy in SC tissue by modifying insulin formulations with variable excipients and controlling the stability of hexamer molecules[24].
Bolus insulin analogs demonstrate a more rapid onset, shorter duration, and quicker peak time of action compared with regular human insulin (Table 1)[25]. These properties make them suitable for administration just before or during meals, effectively managing blood glucose levels. The reduced duration of action of bolus insulin analogs lowers the risk of postprandial hypoglycemia, which commonly occurs when blood sugar levels are lower after a meal[26]. The time-action profile of regular human insulin demonstrated limitations, characterized by a delayed onset and prolonged duration relative to endogenous insulin (Table 1)[27]. This resulted in an increased risk of hypoglycemia before and after meals, accompanied by variability among individuals and within the same individual, presenting challenges in dosing and a heightened probability of hypoglycemic events[27,28]. To address these issues, the development of bolus insulin analogs with tailored properties was crucial to enhance the clinical experience of mealtime insulin therapy and reduce the incidence of hypoglycemia[17,26]. It has become evident that rapid-acting insulin analogs yield superior outcomes in terms of reduced hypoglycemic events, improved postprandial blood glucose control, and lower HbA1c levels when compared with regular human insulin[29,30].
| Type of insulin | Brand name | Onset of action (min) | Peak time (h) | Duration of action (h) | Ref. | ||
| Short acting insulin | |||||||
| Human regular insulin | Humulin® R; Novolin® R; Actrapid® | 30-60 | 2-4 | 6-8 | [39] | ||
| Ultra-rapid insulin | |||||||
| Ultra-rapid aspart | Fiasp® | 2 | 1-3 | 3-5 | [40] | ||
| Ultra-rapid lispro | Lyumjev® | 20 | 1-3 | 3-4 | [41] | ||
| Rapid-acting insulin | |||||||
| Insulin lispro | Humalog® | 20-30 | 1-2 | ≤ 5 | [42] | ||
| Insulin aspart | Novolog® | 15 | 1-3 | 3-5 | [43] | ||
| Insulin glulisine | Apidra® | 12-30 | 1.5 | Approximately 4 | [44] | ||
| Intermediate-acting insulin | |||||||
| NPH insulin | Humulin®; Novolin® | 2-4 | 4-10 | Up to 18 | [45] | ||
| Long-acting insulin | |||||||
| Insulin glargine-100 | Lantus® | N/A | No peak | 24 | [46] | ||
| Insulin detemir | Levemir® | N/A | No peak | 14-24 | [47] | ||
| Insulin glargine-300 | Toujeo® | N/A | No peak | Up to 36 | [48] | ||
| Insulin degludac | Tresiba® | N/A | No peak | > 40 | [49] | ||
| Premixed insulins | |||||||
| Human insulin mix (70/30) | Mixtard® 30; Novolin® 70/30 | 30 | 2-10 | Up to 24 | [50] | ||
| Lispro mix (75/25) Lispro mix (50/50) | Humalog® Mix (75/25); Humalog® Mix (50/50) | 5-20 | 1-2 | Up to 24 | [51] | ||
| Aspart mix (70/30) | NovoMix® 30 | 10-20 | 1-4 | Up to 24 | [52] | ||
| Degludec/Aspart | Ryzodeg® | 15 | 2-4 | > 24 | [53] | ||
Bolus insulin analogs have been designed to accelerate self-disassociation and rapid release of monomers from the SC injection site. These analogs differ from human insulin by one or two amino acids, facilitating their faster onset of action (Figure 2)[31]. Insulin lispro and insulin aspart are examples of rapid-acting insulin analogs that have a quicker onset than regular human insulin. This is achieved by modulating the intermolecular interactions of insulin dimers, weakening the binding forces between them[32]. Consequently, the hexameric structures of insulin analogs dissociate more rapidly into monomers, facilitating their release into the bloodstream. Conversely, insulin glulisine, another rapid-acting insulin analog, uses polysorbate 20 as a stabilizer instead of zinc (Figure 2)[33]. Polysorbate 20 acts as a surfactant, preventing insulin molecules from aggregating. This unique stabilization mechanism enables insulin glulisine to be formulated as monomers or dimers, eliminating the delayed onset of action observed with hexameric insulin preparations[34,35].
In addition to modifications to insulin amino acids, the integration of excipients assumes a pivotal role in the development of insulin formulations featuring distinct time-action profiles and enhanced efficacy, particularly in the management of postprandial glucose, compared with rapid-acting insulins[36]. The incorporation of excipients aimed at improving SC blood flow, such as vitamin B3 and L-arginine, has given rise to formulations such as ultra-fast aspart (FIAsp®)[37]. Similarly, the inclusion of agents such as Treprostinil or BioChaperone, designed to augment vascular permeability or diffusion mechanisms as observed in ultra-rapid lispro (URLi), has yielded insulins with faster onset compared with rapid insulin analogs[38].
The main goal of developing basal insulin preparations is to achieve a consistent and steady time-action profile that closely resembles the behavior of endogenous basal insulin. Various strategies have been employed since the inception of insulin to extend its availability from the SC space into systemic circulation, resulting in diverse basal insulin formulations with distinct time-action profiles. Neutral Protamine Hagedorn (NPH) insulin, introduced in 1946, represents one such intermediate-acting formulation with a delayed onset (approximately 1–3 h), a late peak (approximately 4–6 h), and a duration of action lasting approximately 13–18 h (Table 1)[39-54]. Over time, the utilization of NPH insulin has exhibited notable drawbacks. Its limited duration of action, reaching a maximum of 18 h, impedes its feasibility for once-daily administration in a considerable patient population[55]. When administered at bedtime for nocturnal basal coverage, the delayed peak of NPH poses the risk of hypoglycemia during the early morning hours[56]. Furthermore, the inherent unpredictability in the absorption and activity of NPH contributes to intra-individual variability in insulin levels, leading to fluctuations in plasma insulin concentrations and, subsequently, unforeseen episodes of hypoglycemia and undesired hyperglycemia[20,57,58]. Furthermore, the wide variability in de-precipitation at the injection site further adds complexity to its predictability and absorption[59].
To address the limitations of NPH insulin in meeting basal insulin requirements, basal insulin analogs have been developed through recombinant DNA technology. Categorized into two generations, the first includes insulin glargine-100 U/mL (Gla-100) and insulin detemir (IDet), whereas the second comprises insulin glargine-300 U/mL (Gla-300) and insulin degludec (IDeg)[60]. Introduced in 2000 under the brand name Lantus® by Sanofi, Gla-100 is a synthetic analog of human insulin distinguished by modifications at positions A21 and B31/B32 (Figure 2), enhancing its solubility in acidic pH and resulting in an increased isoelectric point to 6.7. Upon SC injection, Gla-100 undergoes microprecipitation in the injection depot because of exposure to higher physiological pH, followed by slow dissolution into monomeric form before entering the circulation system[61]. This mechanism yields a time-action profile for Gla-100 that is flatter, more stable, and consistent without noticeable peaks over 24 h compared with NPH insulin[16]. Clinical trials have provided evidence that transitioning from NPH insulin to glargine leads to enhanced glycemic control, as indicated by decreased fasting blood glucose levels and reduced episodes of nocturnal hypoglycemia[62,63].
IDet, marketed as Levemir®, is another first-generation long-acting basal insulin analog that received Food and Drug Administration (FDA) approval in 2005. It structurally differs from human insulin by incorporating a 14-carbon myristoyl fatty acid to the lysine at B-29 while eliminating the C-terminal threonine amino acid at B-30 (Figure 2). Upon injection, IDet molecules self-associate into di-hexamers, prolonging their persistence in the SC space by delaying hexameric dissociation. Furthermore, the fatty acyl side chains of IDet enable its binding to albumin, resulting in delayed distribution to peripheral tissues and elimination from the body[47]. Compared with insulin glargine, IDet has a shorter duration of action, leading to reduced glucose-lowering activity in the second 12 h after administration[64]. In addition, IDet exhibits lower potency, requiring higher doses and more frequent injections in patients with obesity and T2DM, typically following a twice-daily regimen reflecting the lipophilic nature of IDet molecules[16,65].
The second generation of basal insulin analogs, including insulin Gla-300 and IDeg, surpasses the first generation by offering flatter, more predictable, and longer-lasting insulin profiles. FDA-approved in 2015 and marketed as Toujeo® by Sanofi, Gla-300 is a concentrated form of Gla-100 with an identical molecular structure[16]. Upon SC injection, Gla-300 exhibits equipotent dosing to Gla-100 but with a reduced volume, which results in a more compacted SC depot and a decelerated, gradual, and extended release of Gla-300 monomers into circulation, persisting for up to 36 h[23]. Clinical trials showed that Gla-300 offers a less-pronounced glucose-lowering effect than Gla-100 but with more stable profile, reduced variability, and enhanced physiological modulation capacity[66].Therefore, patients with T2DM may require a 12% higher dose of Gla-300 than Gla-100 for equivalent glycemic control, and a 20% reduction in dosage is recommended when transitioning from Gla-300 to Gla-100 to mitigate the risk of hypoglycemia[17,67].
IDeg, commercially known as Tresiba® (Novo Nordisk), was FDA-approved in 2015 as a synthetic analog of human insulin. Distinguished by its extended duration of action, lasting up to 42 h at a steady state, IDeg undergoes structural modifications by replacing the threonine amino acid at position B30 with a side chain containing glutamic acid and a 16-carbon fatty acid at position B29 (Figure 2)[19]. Such modifications, in the presence of zinc and phenol as preservatives, facilitate the formation of a soluble and stable di-hexamer. Upon SC injection, the phenolic preservative diffuses away, and di-hexamers self-associate to form multihexamers in the injected depot (Figure 1). Gradual dissociation of multi-hexamers into monomers, coupled with reversible attachment to albumin in the bloodstream, enables IDeg to maintain effectiveness for an extended period[68]. Studies have shown that IDeg achieves comparable glycemic control to insulin glargine, with a reduced incidence of nocturnal hypoglycemic episodes in individuals with T2DM[69,70].
Insulin Icodec, engineered by Novo Nordisk for once-weekly administration, undergoes distinct modifications relative to human insulin. These include the substitution of A14 with glutamic acid and both B16 and B25 with histidine, coupled with Thr30 deletion at B30. In addition, the acylation of B29 involves the addition of a 20-carbon fatty acid to the lysine amino acid[71]. These modifications significantly prolong its duration of action by improving proteolytic stability and solubility, concurrently reducing its affinity for insulin receptors and enhancing binding with serum albumin. This reduction in receptor binding and clearance effectively extends the overall action of Insulin Icodec[72]. A recent meta-analysis comparing Insulin Icodec with other basal insulin analogs revealed that they have similar efficacy in terms of glycemic indices, accompanied by a slight increase in the risk of hypoglycemia and weight gain associated with the use of Icodec[73].
Overall, different basal insulin analogs have demonstrated a more physiological insulin delivery profile, characterized by flattened peaks and longer durations of action, compared with intermediate-acting insulins such as NPH. This profile has been associated with a lower incidence of nocturnal hypoglycemic events[17,60,74]. Therefore, numerous studies consistently support the use of basal insulin analogs over NPH owing to their lower risk of hypoglycemia[26], reduced emergency department visits or hospitalizations related to hypoglycemia[75], and comparable glycemic control with fewer injections[76]. Therefore, for individuals aiming to minimize the occurrence of hypoglycemia while maintaining consistent glycemic control, the use of basal insulin analogs is recommended as a preferable alternative to NPH insulin[77].
Insulin therapy intensification becomes necessary when basal insulin alone is insufficient to achieve glycemic targets. Insulin mixtures, which combine basal and bolus insulins in a single vial or cartridge, offer a solution while preserving their respective PK properties. These mixtures can be formulated using either human insulin or insulin analogs. Biphasic human insulin comprises 70% of NPH insulin and 30% of soluble human insulin, whereas biphasic insulin analogs such as biphasic insulin aspart (Novomix®) and biphasic insulin lispro (Humalog® mix) are offered in varying concentrations (75/25, 50/50, 70/30), utilizing the protaminated form of each insulin for an extended action (Table 1)[78]. The widespread popularity of premixed insulin preparations in diabetes management can be attributed to their convenience as they offer both basal and bolus insulin coverage in a single injection while minimizing the risk of mixing errors[79-81].
The protaminated fraction within premixed insulin formulations introduces inherent limitations to their PK and PD properties, influencing clinical efficacy. interaction between protaminated fraction and soluble insulin yields the “shoulder effect”, resulting in undesirable prolonged glucose-lowering effects[82,83]. Moreover, variability in insulin action may occur due to the need for proper resuspension for accurate dosing and the release of insulin from insulin-protamine precipitates in the SC tissue[84-86]. To address these limitations, studies have investigated the use of a premixed analog co-formulation comprising a combination of two insulin analogs: Degludec as the basal insulin and aspart for bolus coverage (RYZOGEG®). When compared with other premixed formulations, particularly biphasic aspart, the premixed analog co-formulation (degludec/aspart) demonstrated comparable glycemic control while exhibiting lower rates of overall and nocturnal hypoglycemia, along with improved control of fasting blood glucose[53,87].
Biphasic human insulin, although widely used, has notable limitations such as delayed time to peak effect (1–5 h) and the need for administration 30–45 min before eating meals containing carbohydrates, which can restrict flexibility and patient compliance[79]. These regimens are also associated with a higher risk of hypoglycemia than basal–bolus regimens owing to fixed insulin proportions and less physiological PK[88,89]. Contrarily, premixed analog formulations have demonstrated reduced occurrences of nocturnal and overall hypoglycemic events, along with improved postprandial glycemic control while achieving comparable HbA1c levels[90-92]. When comparing premixed insulin analog regimens, both aspart and lispro have demonstrated similar PK and PD properties, resulting in similar glycemic control and hypoglycemic events[93-95].
In general, premixed insulin formulations generally allow for decreased frequency of injections compared with basal–bolus therapy, which may be advantageous for some patients. However, it is important to note that premixed insulin therapy requires strict adherence to consistent meal timing and carbohydrate intake as the insulin components within the formulation cannot be independently adjusted[96]. Despite their benefits, premixed insulin formulations have limitations that need to be addressed to optimize their effectiveness and improve patient outcomes in the management of diabetes. Further research is warranted to enhance the design and formulation of premixed insulin regimens, taking into account these limitations and striving to achieve optimal glycemic control and patient satisfaction.
In patients with T2DM, the initiation of insulin therapy typically encompasses the administration of basal insulin using either NPH or an analog[97].
If the initial insulin dosage proves inadequate for achieving glycemic targets, a gradual approach is typically used, commencing with lower doses and incrementally adjusting to achieve the desired glycemic control[98]. In cases of insufficient control, the treatment plan is further intensified by incorporating prandial insulin, focusing on meals with significant postprandial glucose excursions. Should the target A1c level remain unattained during follow-up, the treatment strategy undergoes escalation, progressively including prandial insulin doses until a comprehensive basal–bolus regimen is established. This escalation may involve considering premixed insulin regimens or introducing self-mixed/split insulin regimens, all aimed at glycemic control optimization[99].
The basal–bolus approach, widely regarded as the optimal regimen, closely mirrors physiological processes by providing basal insulin for overnight coverage and eliciting dynamic prandial insulin responses to meals[100-102]. However, a consensus on the most effective or optimal insulin regimen for patients with diabetes has not yet been reached. This assertion is supported by two meta-analyses that thoroughly investigated and compared premixed and basal–bolus insulin regimens in individuals with T2DM who did not achieve the desired outcomes with previous treatments[103]. One meta-analysis revealed similar effectiveness in lowering HbA1c levels between premixed and basal–bolus insulin regimens, particularly in patients with T2DM initiating insulin therapy for the first time. However, among individuals with previous insulin experience, basal–bolus therapy exhibited superior efficacy in HbA1c reduction, accompanied by heightened insulin requirements and increased body weight, whereas the risk of hypoglycemia remained unchanged[104]. Similarly, another meta-analysis revealed no clinically significant difference in HbA1c reduction between insulin-experienced or insulin-naive patients with T2DM, with similar event rates for overall daily insulin dose, hypoglycemia, and weight gain between the compared regimens[105].
A significant divergence in insulin regimens is the number of daily insulin injections that patients with diabetes approach with caution and careful consideration. However, a comparison of full basal–bolus (2–4 shots/d) with full premixed (2–3 shots/d) insulin regimens, based on the daily insulin injections, showed no significant difference (P = 0.095) in terms of reducing HbA1c levels, regardless of the total number of insulin injections per day[103]. At present, there is a lack of comprehensive research providing sufficient evidence to definitively determine or establish superiority between premixed and basal–bolus regimens for treating T2DM in patients who have not achieved the desired results with previous treatment approaches.
Notably, most studies comparing various insulin regimens in patients with T2DM highlight the importance of a patient-centered approach in selecting the appropriate regimen. This approach involves considering patient preferences and individualized factors such as insulin accessibility, complexity, and flexibility. Clinicians should consider these factors to tailor the treatment and adapt it according to the specific needs of each patient[103,106-109].
PROs are self-reported measures that provide valuable insights into the impact of chronic health conditions and treatments on individuals’ daily lives. They are used to assess various dimensions of health, including physical functioning, emotional well-being, treatment satisfaction, and HRQOL[110,111]. PROs are increasingly being incorporated into research studies and routine clinical practice[112], enabling better understanding of the patient experience and facilitating personalized care. PRO-related studies provide valuable information that can be implemented in clinical decision-making. This data serves a dual purpose: It informs and improves individual patient care and facilitates comparisons and enhancements in the overall quality of care delivered[113]. Embracing shared decision-making and considering patient preferences in treatment selection align with recent guidelines advocating for a patient-centered approach to diabetes care[114,115]. This emphasis on patient preferences is likely to have significant implications for the future use of PROs in healthcare service evaluation[111,116].
Patient-reported outcome measures (PROMs) offer a valuable approach for measuring PROs. PROMs consist of standardized and frequently validated questionnaires that enable the assessment of a patient’s health status at a specific moment, whether during the course of an illness or while managing a health condition[117]. Their valuable role emerges in guiding clinicians and researchers seeking to gain insights into the experiences of patients with diabetes[118]. These tools are used to assess various aspects of the lives of diabetic patients, including treatment satisfaction, medication adherence, self-management abilities, and overall quality of life (QOL)[119]. In the research field, questionnaires serve as effective tools for several reasons as they enable the convenient and efficient collection of data from a large number of individuals[120]. Furthermore, PROMs provide a standardized approach for gathering patient experiences, ensuring that results can be easily compared between different patient groups in a validated manner[121]. Unlike conventional measures such as clinical laboratory data, PROMs offer a more comprehensive understanding of patients’ overall life experiences[14]. In addition, they offer the opportunity to identify patients who may be struggling to manage their condition and to evaluate the effectiveness of new treatments and interventions[122].
Several questionnaires have been developed for the purpose of collecting PROs. The choice of questionnaire depends on the specific needs of the patient and the research objectives. In the context of diabetes and its treatments, numerous questionnaires exist to assess the impact of the disease on various aspects of QOL. For instance, a recent systematic review examined 17 questionnaires that target different life domains affected by diabetes, including physical, psychological, emotional, and social aspects[121]. The review showed significant variability among the available questionnaires in terms of validity and language availability. This diversity provides researchers with a range of options to measure QOL, allowing them to select the most appropriate questionnaire based on their research question and the characteristics of the population being studied. Aside from the aforementioned 17 questionnaires, there are several others used to assess the impact of diabetes on various life domains, which can be categorized as either generic or diabetes-specific questionnaires. The commonly utilized generic questionnaires include the Short Form 12 or 36 (SF-12 or SF-36), EuroQOL 5 dimensions, Hospital Anxiety and Depression Scale, International Physical Activity Questionnaire, and Work Productivity and Activity Impairment[123-128]. Diabetes-specific questionnaires such as the Diabetes Productivity Measure and the Diabetes Symptom Checklist–Revised have also been employed in various studies[129,130]. Compared with generic questionnaires, diabetes-specific questionnaires provide more standardized measures to evaluate the effects of diabetes on different aspects of life, including general well-being, mental health, productivity, and symptomatology.
When investigating the impact of different insulin types on QOL, researchers have a variety of options to assess patient satisfaction levels and the effects of treatment on QOL. Similar to the questionnaires used to assess the impact of diabetes on QOL, those used to evaluate patient satisfaction and the effects of insulin therapy can be generic, encompassing all diabetes medications, or specific to insulin treatments. The commonly used generic questionnaires for assessing the effects of insulin include the Patient-Perceived Difficulties in Diabetes Treatment, Patients’ Perceptions About Medications for Diabetes, and Diabetes Medication Satisfaction questionnaires[130-132]. Among questionnaires presented in table[3], the Diabetes Treatment Satisfaction Questionnaire is widely used to evaluate patients’ satisfaction with insulin therapy[133-141]. However, because researchers require sensitive tools capable of detecting differences arising from variations in insulin types or delivery devices, several questionnaires specifically designed to assess the effects of insulin therapy have been developed. For instance, the Insulin Treatment Satisfaction Questionnaire has exhibited validity and reliability in assessing satisfaction levels with different insulin therapies[142]. The Insulin Treatment Experience Questionnaire has proven effective in identifying QOL differences associated with various insulin therapies[143]. In addition, the Experience With Insulin Therapy Questionnaire and Expectations About Insulin Therapy Questionnaire were developed to provide insights into the expectations and experiences of insulin therapy specifically for individuals who are new to insulin treatment[144]. Furthermore, the Insulin Therapy-Related QOL questionnaire, which is validated and widely utilized, evaluates the effects of insulin therapy on the different aspects of life. Overall, the wide array of available questionnaires, along with other assessment tools, provides researchers and healthcare provides (HCPs) with valuable resources to enhance the QOL of individuals with diabetes by optimizing insulin therapy based on PROs[143].
QOL encompasses a comprehensive and multidimensional concept that comprises an individual’s subjective assessment of their physical, emotional, and social well-being[145]. The significance of QOL in managing chronic diseases, such as diabetes, is pivotal, impacting patients’ capacity to deal with their condition and sustain their overall well-being[146]. Therefore, when formulating treatment plans and interventions for diabetes management, it is important to consider the impact on QOL. Numerous studies and guidelines emphasize the importance of addressing QOL in diabetes man-agement. The recent guidelines from the American Diabetes Association (ADA) recommend HCPs to acknowledge the influence of diabetes on QOL and provide necessary support and resources to help patients overcome related challenges[147].
Diabetes has long been known to take a toll on the QOL of those who live with it[145,148]. This negative impact is not only limited to one aspect of the patients’ lifestyle but can also extend to deteriorate various dimensions, including physical, emotional, social, and financial sides[149]. Diabetes, as a chronic disease, is known for its association with various physical complications, such as atherosclerotic cardiovascular disease, nephropathy, neuropathy, and re-tinopathy, causing pain and limitations in daily activities, thus adversely affecting QOL[150-152]. Emotionally, managing diabetes can result in anxiety and stress due to the constant need for blood glucose management, regular medication intake, and symptom and complication monitoring. These demands can greatly impact the patients’ emotional and physical well-being[153]. The social functioning and relationships of diabetic patients may also be affected by changes in diet, exercise routines, and daily activities[154]. In addition, the financial burden associated with the costs of medications, supplies, and healthcare services for diabetes management can cause stress and limit access to resources, further negatively impacting QOL[155]. Considering these factors, recent guidelines emphasize the significance of prioritizing QOL as a key treatment objective, which is now recognized as the fifth component within the glycemic pentad framework, providing guidance for healthcare professionals in making informed treatment decisions[156]. It is important for clinicians and HCPs to recognize that the effects of diabetes extend beyond metabolic changes and disease complications, significantly impacting various aspects of the patients’ lifestyles.
The widely acknowledged understanding that living with diabetes, as a chronic condition, can have a negative impact on QOL becomes even more intricate when insulin therapy is incorporated into patients’ treatment regimens. Insulin therapy introduces an additional factor that has the potential to positively or negatively influence QOL[129,157]. Therefore, we conducted a comprehensive investigation of relevant literature in the PubMed database, with a particular focus on PRO-related studies . Predefined keywords, such as “insulin therapy,” “PROs,” “Questionnaire,” “type 2 diabetes,” and “quality of life,” were utilized, and variations and combinations were employed to ensure a comprehensive search. The inclusion criterion for this study encompassed research conducted since 2000, aligning with contemporary developments in diabetes management. The objective was to comprehensively evaluate the impact of insulin therapy on the QOL in individuals with T2DM. The results of this study, summarized in Table 2, revealed both the positive and negative effects of insulin therapy on QOL. On the one hand, it has proven to be effective in managing hyperglycemia symptoms and improving metabolic control, particularly among patients unresponsive to previous treatments[158-160]. Furthermore, it demonstrated effectiveness in mitigating the occurrence of diabetic complications, consequently leading to enhancements in various aspects of the QOL and overall well-being[161-163]. In addition, the initiation of insulin therapy has been shown to yield favorable outcomes across various domains related to QOL, encompassing psychological, physical, and social aspects (Table 2)[133,134,164-168]. A recent study proved that diabetic patients who received insulin therapy with treatment adherence and had more knowledge on diabetes showed improvement in QOL scores compared with those treated with oral medications alone[169]. Furthermore, studies have demonstrated that such enhancements extend beyond the realms of QOL, as they are accompanied by a notable increase in treatment satisfaction (Table 2)[129,133-135,170-172], thereby highlighting the significance of initiating insulin therapy as a comprehensive and effective intervention.
| Effect on QOL | Comparison | PROMs | Result | Ref. |
| Negative | Insulin vs non-insulin users at baseline | SF-12v2; ADDQoL | Stable insulin treatment was associated with worse health status and QOL in comparison to non-insulin users | [123] |
| Insulin vs non-insulin users at baseline and after 1 year | DHP; Rand-36 | Insulin users had more significant problems in social functioning, pain and mental state than non-insulin users | [166] | |
| Insulin vs non-insulin users | SF-36v.2; WHOQOL-BREF | Insulin treated patients were associated with lower mental score than non-insulin users with no significant difference in other domains | [180] | |
| Insulin vs non-insulin users | DQOL; Rosser index | Individuals with established insulin therapy showed lower satisfaction and QOL | [123] | |
| Insulin vs non-insulin users | EQ5D; DTSQ | Insulin treatment was associated with lower HRQOL and treatment satisfaction scores than non-insulin treatment | [136] | |
| Insulin vs non-insulin users | SF-36 | Insulin users had worse vitality and physical function domains than non-insulin users | [125] | |
| Insulin vs non-insulin users | EQ-5D | Insulin users had lower HRQOL scores than those not using any therapies | [126] | |
| Insulin vs non-insulin users | ADDQOL | Insulin therapy was associated with lower QOL scores | [181] | |
| Insulin vs non-insulin users | SF-12 | Insulin was associated with significant lower HRQOL scores (especially mental composite scores) | [174] | |
| Insulin vs non-insulin users | SF-36; DHP | Insulin users showed lower scores in six domains of SF-36 questionnaire (physical functioning, social functioning, role physical, mental health, vitality, and general health) and in 2 domains of DHP-1 (barriers to activity and psychological distress) than non-insulin users | [175] | |
| Insulin vs non-insulin users | DQOLBCI; PDDT; QOL-VAS | Insulin therapy was associated with lower overall QOL scores (especially pain associated with treatment scale) | [182] | |
| Insulin vs non-insulin users | ADDQOL | Insulin use was associated with lower scores of QOL especially in younger patients | [131] | |
| Insulin vs non-insulin users | SF-36; HADS | Insulin therapy negatively affected emotional state, bodily pain, and physical state | [176] | |
| Insulin vs non-insulin users | ADDQOL-18 | Insulin use was associated with negative impact on QOL | [183] | |
| Insulin vs non-insulin users | EQ-5D-5L; SF-12; EQ-5D-3L | Insulin users experienced more issues in self-care and usual activities, while individuals receiving combination therapy of insulin and oral drugs encountered greater difficulties in mobility, pain/discomfort, and anxiety/depression domains | [184] | |
| Insulin vs non-insulin users | SF-36; HADS; COPM; VAS-P; IPAQ | Insulin users exhibited more significant impairments in quality of life, functional capacity, and socialization, while also reporting higher levels of neuropathic pain and symptoms of anxiety and depression compared to non-insulin users | [127] | |
| Retrospective comparison | EQ-5D-5L | Longer duration of insulin use was associated with lower scores of QOL | [185] | |
| Positive | Baseline vs follow up for 4 years of insulin therapy | SF-12v2; ADDQOL | Initiation of insulin therapy did not significantly affect health status or QOL during 4 years of follow up | [123] |
| Baseline vs 4-8 wk short course of intensive insulin therapy | SF-36; DQOL; DSC-R | Early initiation of a short course of intensive insulin Therapy (IIT) associated with increased satisfaction and QOL | [129] | |
| Baseline vs one year of insulin treatment | DHP; Rand-36 | Significant improvements in psychological distress and disinhibited eating scores of DHP questionnaire. Also, scores of vitality, physical function, social functioning and health change domain related to Rand-36 showed significant improvements | [166] | |
| Insulin vs non-insulin users | SF-36 | Insulin users showed better quality of life in 2 domains (physical and emotional) out of 8 domains compared to non-insulin users | [167] | |
| Baseline vs 4 and 12 wk for insulin and non-insulin users | SF-36; DTSQ; GDS; GHQ-28 | Insulin was associated with improvement in QOL, treatment satisfaction and mood reflected in different domains (Mental Health-Vitality-Social functioning-Role emotional and physical) | [133] | |
| Baseline vs 6 months of follow up | SF-36; DTSQ; HADS | Significant improvement in health status of Basal/Bolus group over time (7 domains) and in comparison, to oral medication group (vitality, social function, and mental health domains). Also, Basal/Bolus showed improvement in depression scores (first 3 months) and anxiety. Treatment satisfaction improved in all groups | [134] | |
| Baseline vs 6 months of follow up | HFS-w; DSC-R; WHO-5; ITAS | Significant improvement of well-being and QOL especially vitality and general mood | [168] | |
| Insulin (analog vs human) ± OAD vs OAD alone | DAWN QOL | Insulin users showed higher scores related to diet compliance and 3 subscales related to QOL measurements in comparison to non-insulin users | [169] | |
| Baseline vs 18 months of insulin initiation or switching | EQ-VAS; PITQ; EITQ | Improvement of the patients’ satisfaction to insulin and QOL scores in comparison to baseline | [170,171] | |
| Baseline vs one year of follow up | SF-36v2; DQOLCTQ | Scores of treatment satisfaction, diabetes impact and worry improved over time for each group with no significant difference between them | [172] | |
| Baseline vs 12 vs 24 wk for insulin users and non-insulin-users | DTSQ; ADDQOL | Significant improvement of treatment satisfaction and QOL for insulin group better than oral antidiabetic group | [135] | |
| Baseline vs 24 wk after insulin initiation or insulin switching | EQ-5D | Significant improvements in all measured 5 domains (Mobility- Pain/discomfort-Anxiety/depression-Self-Care-Usual activities) related to QOL for both insulin naïve and experienced patients | [164] | |
| Baseline vs 24 wk after insulin initiation or insulin switching | EQ-5D | Significant improvement in 3 domains (Mobility-Pain/discomfort-Anxiety/depression) out of 5 domains related to QOL for both insulin naïve and experienced patients | [165] |
Conversely, studies comparing insulin users with noninsulin users consistently reported lower QOL scores among the former across mental, physical, social, and overall health dimensions (Table 2)[125,166,173-176]. Moreover, insulin users have reported lower levels of treatment satisfaction compared with those who do not incorporate insulin into their treatment regimens[177,178]. These unfavorable effects on QOL can be attributed to adverse events associated with insulin therapy, such as increased fear of hypoglycemia and negative impacts associated with weight gain[124,178,179]. Furthermore, the incorporation of insulin therapy may elicit feelings of stigma among individuals with diabetes, fostering a perceived loss of control over their own disease management[180].
Overall, an intriguing finding was obtained when examining various studies that explored the positive and negative effects of insulin therapy on QOL depending on PROMs, as shown in Table 2[181-185]. Studies that showed unfavorable QOL outcomes among insulin users mainly adopted a comparative approach, contrasting them with noninsulin users. Conversely, studies that reported favorable results in terms of satisfaction and QOL tended to focus on the impact of insulin therapy in a longitudinal follow-up manner, with only two studies including a direct comparison between insulin and noninsulin users[167,169]. These findings indicate that prolonged use of insulin therapy is associated with enhanced glycemic control, leading to a reduced incidence of diabetic complications and improved QOL[163,180].
The selection of an optimal insulin regimen tailored to individual patients’ needs is a crucial aspect of providing effective diabetes care. Guidelines highlight the importance of thoroughly assessing various factors when deciding to initiate insulin therapy, including age, overall well-being, mental status, life expectancy, presence of complications, and feasibility of different insulin formulations[186]. Patients with diabetes are also recommended to have a good understanding of different insulin regimens, diligent glycemic control monitoring, and the process of selecting the most suitable formulation for effective treatment[187].
When commencing insulin therapy, HCPs have the option to choose between premixed insulin and basal insulin depending on the specific clinical situation. While the ADA suggests starting with basal insulin, there are cases where patients may benefit from starting with premixed insulin. These situations can include when a patient’s HbA1c level is above 8.5%, when patients struggle with the complexity of a basal–bolus regimen, or when fasting blood glucose levels are below 150 mg/dL but HbA1c remains elevated[188,189]. Studies comparing the initiation of premixed and basal insulin therapies in patients with T2DM have demonstrated that the former provides better control of blood sugar levels than the latter. However, it is important to note that using premixed insulin increases the risk of hypoglycemia and weight gain[190,191].
Premixed insulin regimens offer a simplified approach to insulin therapy as they contain preformulated combinations of short-acting and intermediate- or long-acting insulin. These combinations demonstrate a safety and effectiveness profile comparable to basal–bolus regimens. In addition, they offer the advantage of necessitating fewer injections, thereby contributing to a reduction in the complexity of the overall insulin regimen[191]. It is an important option for patients who have regular and routine eating habits or who may have difficulty counting their carbohydrate intake. In addition, it offers advantages over self-mixed insulin, providing more precise dosages, greater effectiveness, and improved patient convenience[50,192]. It can also be a preferred option for patients who find it challenging to adhere to the demands of basal plus or basal–bolus regimens due to the reduced injection frequency and monitoring associated with premixed insulin[193]. Furthermore, premixed insulin can help alleviate insulin distress, which can hinder optimal glycemic control, as it involves less intrusion and improves patients’ willingness to adhere to the treatment regimen[154].
Despite these advantages, premixed insulin formulations have limitations in terms of dosing flexibility, as they do not allow for independent adjustment of insulin dosage between the long- and short-acting components. They are also found to be less effective than full basal–bolus regimens in achieving targeted HbA1c levels[107,194]. Furthermore, the use of premixed insulin is associated with an increased risk of hypoglycemia and weight gain as well as a higher likelihood of experiencing adverse events, which may make it unsuitable for regular use in elderly individuals[195-197]. In addition, the sole long-acting insulin used in most premixed formulations, NPH, has certain drawbacks in its time-action profile that can affect the efficacy and safety of the regimen[198].
Alternatively, the basal–bolus insulin regimen, also referred to as multiple daily injections, is commonly regarded as the preferred regimen owing to its ability to closely replicate the natural secretion pattern of insulin[199]. This regimen provides patients with flexibility in terms of varying mealtimes and carbohydrate content. It has also the potential to maintain a consistent 24-h glucose profile and achieve the same level of HbA1c as conventional insulin regimens[200]. However, the basal–bolus regimen can be complex, requiring patients to accurately count carbohydrates and adjust insulin dosages accordingly, which can be challenging and time-consuming[201]. Regular and diligent blood glucose monitoring is necessary for individuals on the basal–bolus regimen. It also requires patients to possess knowledge of insulin–carbohydrate ratios and correction factors, allowing them to make necessary adjustments to their insulin dosages to achieve the desired glycemic control. This regimen emphasizes the importance of patient involvement and understanding in managing their diabetes[189]. A recent study reported that patients who discontinued the basal–bolus regimen often cited its complexity as the main reason for their nonadherence. Challenges in calculating bolus doses, managing food intake in relation to bolus insulin, and keeping track of administering two different types of insulin were also reported[202].
Given the current lack of conclusive evidence regarding the superiority of premixed and basal–bolus insulin regimens in the treatment of T2DM, it becomes essential to explore PROs to shed light on the preferable regimen that minimizes the detrimental effects on patient QOL. Conducting investigations that prioritize PROs can provide valuable insights into the experiences and preferences of patients, facilitating the comparison of both insulin regimens and guiding the selection of the most suitable regimen for each patient. Our comprehensive study involved a systematic search on the PubMed database, targeting studies conducted since 2000 that focused on PROs comparing the premixed and basal–bolus insulin regimens. In the search, variations and combinations of specific terms were used, including “insulin therapy,” “PROs,” “Questionnaire,” “premixed,” and “basal–bolus.” The main goal was to conduct a thorough comparison of the outcomes of these two insulin regimens, and the detailed results of PROs are presented in (Table 3). Some studies have reported that patients treated with the basal–bolus regimen reported higher treatment satisfaction than those treated with the premixed regimen[137,203]. While some studies demonstrated that the basal–bolus regimen is associated with improvements in HRQOL and reduced psychological fear of hypoglycemia[137,138,203-205], other studies showed that patients reported favorable outcomes in HRQOL with premixed insulin regimens[128,206,207]. Nevertheless, several studies found no significant difference in treatment satisfaction and various domains of QOL between the two regimens[101,130,132,139,140,208]. The variations in the reported outcomes among patients with diabetes can be attributed to factors such as the utilization of diverse questionnaires in different studies, which differ in complexity, length, and focus, leading to a broad spectrum of conclusions. In addition, individual perceptions of inconvenience and pain associated with insulin injections can significantly vary, leading to variations in treatment satisfaction[209]. Patients’ perceptions of insulin therapy may be influenced by the complexity of various treatment regimens, contributing to variations in outcomes[210]. Furthermore, variations in metabolic responses to different insulin regimens among different ethnic and racial groups can directly impact patients’ reactions to these regimens[211].
| Result | Type of insulin therapy | Study design | PROMs | Comment | Ref. |
| Favor basal bolus therapy | Glargine + glulisine (BB) vs lispromix or aspartmix (PM) | Comparative 12 wk intervention (crossover) phases | Validated generic and diabetes-specific modules of treatment satisfaction and QOL | The basal-bolus insulin regimen showed better outcomes in terms of treatment satisfaction and quality of life scores compared to premixed insulin regimens | [203] |
| Premixed vs basal bolus | 6 months observational prospective study | EQ-5D-3L | Basal-bolus regimen showed the most significant potential to increase HRQOL in comparison to other regimens | [204] | |
| Glargine + glulisine (BB) vs aspartmix (PM) | BB vs PM after 24 wk of treatment | DTSQ; ITSQ; ADDQOL; EQ-5D | The basal-bolus group showed significantly higher overall satisfaction with treatment, satisfaction with insulin, perceived frequency of hyperglycemia, and overall present quality of life | [137] | |
| Glargine + glulisine (BB) vs aspartmix (PM) | Baseline vs 60 wk follow up | PAIS-SR; EQ-5D; HFS; DQOL | The basal-bolus regimen showed superior outcomes compared to the premixed group in terms of QOL. The basal-bolus group experienced significant improvements in psychological adjustment to illness and less fear of hypoglycemia. The premixed group reported significant increases in hypoglycemic worry and a significant decline in general health related QOL | [205] | |
| Glargine + lispro (BB) vs lispromix (PM) | Baseline vs 24 wk follow up | DTSQ; EWITQ | No significant difference between the two groups in term of DTSQ and EWITQ scores except that premixed group showed significantly higher perceived frequency of hypoglycemia than basal-bolus group | [138] | |
| Favor Premixed therapy | Glargine or NPH based BB regimen vs aspartmix (PM) | 24 wk prospective study after switching to AspartMix | EQ-5D | Switching to AspartMix showed significant improvement in HRQOL | [206] |
| Glargine + glulisine (BB) vs aspartmix (PM) | 26 months randomized trial | EQ-5D; DQOL; WPAI | No significant difference of different scales for three questionnaires between two groups except for work missed for health which is significantly lower in BB group | [128] | |
| NPH + lispro (BB) vs lispromix (PM) | 12 wk randomized comparative trial | ITR-QOL | The premixed group exhibited significantly higher scores in the total score and daily activities subscale score of ITR-QOL compared to the basal-bolus group | [207] | |
| Undetectable difference | Glargine + glulisine (BB) vs lispromix or aspartmix (PM) | Patients switched from PM to BB with follow up for 16 wk | DTSQ | No significant difference in treatment satisfaction between two groups | [139] |
| Detemir + aspart (BB) vs aspartmix (PM) | Baseline vs 50 wk of treatment | SF-36; DiabMedSat; DPM; TRIM-D | No statistically significant differences between premixed and basal-bolus group in term of patients’ satisfaction, productivity and quality of life | [130] | |
| Premixed vs basal bolus | Baseline vs 6 months Switching to basal bolus regimen | DQOL | No significant difference in satisfaction, impact of diabetes, social concern and concern related to diabetes after initiation of basal-bolus regimen | [101] | |
| Glargine + lispro (BB) vs lispromix (PM) | 48 wk open label randomized study | EQ-5D; DHP-18 | No significant difference of different scales for two questionnaires between two groups | [208] | |
| Glargine + lispro (BB) vs lispromix (PM) | Baseline vs 24 wk follow up | ITSQ; PAM-D21 | No significant difference between the two groups in terms of ITSQ domain and total scores, and PAM-D21 questionnaires domain scores | [132] | |
| Glargine + lispro (BB) vs lispromix (PM) | Baseline vs 48 wk follow up | EQ-5D; DTSQ | No significant difference between the two groups | [140] | |
| Glargine + glulisine (BB) vs aspartmix (PM) | Baseline vs 24 wk follow up | DTSQ | No significant difference between the two groups in term of treatment satisfaction | [141] |
It is imperative to acknowledge the distinction between clinical trials and real-world settings when evaluating the effectiveness of different insulin regimens. This distinction highlights the need to consider real-life factors and patient experiences alongside clinical trial findings to gain a comprehensive understanding of treatment outcomes. Clinical trials typically select participants with higher compliance rates and provide more intensive monitoring, which may not reflect the conditions faced by patients in real-world settings. Therefore, the outcomes observed in clinical trials may not always precisely reflect the performance of these insulin regimens when used by patients with varying levels of adherence and follow-up in real-world settings[107,188].
For patients with T2DM receiving insulin therapy, adhering to their prescribed medication regimen significantly influences their ability to effectively manage blood glucose levels[212]. Extensive research has shown that inadequate medication adherence and persistence, coupled with suboptimal glycemic control, can lead to adverse clinical outcomes, including an elevated risk of cardiovascular events, morbidity, and premature mortality[160,213]. Conversely, improved adherence to insulin therapy yields better glycemic control and reduced healthcare resource utilization[214]. Studies consistently demonstrate a positive correlation between insulin therapy adherence and achieving adequate glycemic control in patients with diabetes. Recent studies using novel technology also proved that nonadherence to insulin therapy results in poor glycemic control that causes higher HbA1c[215,216]. Despite this fact, many diabetic patients discontinue or interrupt insulin therapy shortly after commencement[217].
Achieving the target glycemic control in diabetes requires patients to exhibit two key behaviors: Adherence and persistence. Adherence to therapy refers to the degree to which patients follow the instructions and recommendations of their HCPs, including taking medications at the right dose, time, and duration[218]. It is the responsibility of HCPs to develop an insulin regimen feasible for patients to implement, whereas patients are responsible for complying with the treatment plan. Adherence to insulin therapy poses distinct challenges, with a significant proportion of patients with T2DM (44.3%) not adhering to this treatment, leading to suboptimal glycemic control and increased risks of complications[219]. Persistence, however, relates to the length of time patients adhere to their prescribed treatment regimen[220]. In simpler terms, HCPs must keep writing prescriptions for the medicines needed by the patients, and patients must keep getting those prescriptions filled and taking the medicines as prescribed. However, studies have reported that merely 20% of individuals who start basal insulin treatment continue with it for a full year[220]. Nonpersistence with insulin therapy can also have negative consequences, including inadequate glycemic control and elevated risk of diabetic complications, which is consistent with the impact of nonadherence[217]. Furthermore, studies have reported that poor adherence to insulin therapy is prevalent, with rates reaching up to 86% in certain patient populations, depending on the measurement methods used and the characteristics of the population being studied[10]. Moreover, adherence to insulin therapy may differ depending on the specific insulin regimen being utilized. For example, an investigation using insurance claims data in France showed distinct treatment persistence rates of 61.8% for basal insulin, 15.0% for fast-acting insulin, and 23.2% for other insulin regimens[221]. The frequency of insulin administration can also impact both persistence and adherence to insulin treatment[221,222]. A survey conducted on both patients and physicians highlighted that the most significant challenges and concerns affecting insulin treatment adherence were related to the frequency and precise timing of insulin injections[223]. Numerous studies have consistently reported that the complexity of insulin regimens, particularly those involving a higher number of injections, directly impairs adherence to insulin therapy[224-229]. In addition, the perception of insulin therapy by patients and HCPs can have a significant impact on adherence. Therefore, factors influencing adherence to insulin therapy can be categorized into three primary domains: Patient perceptions of insulin therapy, HCP perspectives on insulin therapy, and patient–HCP relationship.
Research indicates that patient adherence to insulin therapy is intricately associate with their perceptions and beliefs[230]. The determinants of these attitudes encompass a wide range of factors, including insulin-related beliefs, psychological considerations, hypoglycemic concerns, and barriers to therapy. These factors significantly impact patients’ willingness to use insulin as a treatment for diabetes. Beliefs about insulin, influenced by illness severity, cultural perspectives, and specific notions associated with insulin, can influence patients’ acceptance and utilization of this therapy[231,232]. Furthermore, cultural beliefs that do not align with prevailing beliefs about insulin and diabetes management can hinder adherence to insulin therapy[233].
Psychological factors also play a pivotal role in patients’ perception of insulin therapy. These factors include fear and anxiety related to hypoglycemia, pain from injections, and weight gain[234,235]. Studies have demonstrated that some diabetic patients experience shame and self-blame, believing that they caused their illness and that their need for insulin is a result of poor diabetes control. Depression has been found to have a significant impact on insulin use, particularly in relation to the fear of self-injection. Research suggests that depression is a strong predictor of this fear. Negative emotions have also been identified as obstacles to successful self-care strategies in patients with low activation in self-management[236]. Furthermore, patients reported difficulty in injecting insulin in front of others due to concerns about making them feel uncomfortable or offended. Some patients may experience anxiety or fear related to injections, resulting in the avoidance of insulin injections due to anxiety[211].
Many surveys conducted among patients with diabetes have identified hypoglycemia as a significant barrier affecting their emotional state, daily activities, and ability to adhere to insulin treatment[237,238]. As a result, patients may inject smaller doses of insulin to avoid hypoglycemia, which can lead to poor treatment adherence or omission[239,240]. In addition, the occurrence of hypoglycemic events, particularly nocturnal hypoglycemia, can significantly disrupt various aspects of diabetes self-management, including sleep quality, work performance, driving abilities, and overall personal well-being[241]. Moreover, research has shown that these events can lead to negative financial implications and a decline in overall QOL[238].
The complexity of managing insulin therapy poses challenges that can hinder adherence to insulin treatment. Numerous surveys have established links between nonadherence and practical obstacles, including difficulties with injections and inflexible treatment regimens[212,242]. Patients may omit insulin injections owing to uncertainty about whether a dose has already been administered[243]. Titration of insulin doses is another complex aspect that some patients struggle with. Studies have shown that patients often ignore instructions or adopt their own approach to titration[239,240,243,244]. Some patients preferred to have HCPs make insulin changes, whereas others preferred to have control over their own insulin regimen. In addition, patients with T2DM have been found to commonly experience a high medication regimen complexity index, which adversely affects medication adherence and leads to poor glycemic control[231]. Contrarily, patients with low or moderate medication regimen complexity have shown to have improved adherence and better glycemic control[225]. These findings indicate that patients may face varying challenges and barriers to adherence based on the type of insulin regimen prescribed.
Social influences play a pivotal role in adherence to insulin therapy. The perception of stigma associated with publicly injecting insulin can greatly impact adherence. Patients may be concerned about being judged by others who associate insulin injection with drug addiction[245]. Furthermore, the influence of family and friends on patients’ management and adherence of their insulin treatment. Some patients find it challenging to adhere to their insulin use due to family mealtime routines and requirements, whereas others recognize the positive impact of having family support in managing their diabetes[244]. Many patients perceive that insulin therapy negatively affects their participation in travel, leisure, and social activities. They express concerns about restrictions on their social interactions and feel that their insulin injection behavior is influenced in social settings due to the requirements of insulin therapy[232]. The social implications of insulin therapy are particularly notable in low- and middle-income countries, where individuals and healthcare systems may face great financial burdens due to limited access to affordable insulin. The rising cost of insulin in recent years has put a strain on patients relying on this medication to manage their diabetes[246]. It also causes them to ration their medication or skip doses, resulting in adverse health outcomes and escalating healthcare expenses over time[247]. In addition, there are other expenses related to insulin therapy, such as glucose monitoring supplies and HCP visits. Insulin-dependent patients may also experience indirect costs due to missed work or reduced productivity related to the management of their diabetes[248].
Numerous effective approaches have been identified to address factors that negatively impact patients’ perceptions of insulin therapy. Notably, research indicates that culturally tailored interventions within the context of diabetes management can successfully address cultural beliefs associated with insulin therapy. Such interventions specifically target cultural factors and beliefs, with the aim of improving patient understanding, acceptance, and adherence to insulin therapy[249,250]. Addressing the complexity and practical barriers associated with different insulin regimens and their impact on medication adherence is crucial for HCPs to customize interventions and support strategies for individuals receiving insulin therapy. Artificial intelligence shows promise in overcoming practical obstacles by identifying pro-blematic glycemic patterns and providing effective insulin dosage recommendations. This has the potential to improve adherence to insulin therapy, thereby preventing life-threatening complications associated with insulin treatment[251]. Furthermore, the presence of positive social and family support has been strongly associated with effective adherence to diabetes management. Addressing social barriers is essential and fostering social and family support systems is crucial in ensuring optimal adherence to insulin therapy[252].
Another area that mainly affects adherence to insulin therapy is the perception of HCPs to the insulin therapy and their relationships with the patients. One crucial aspect is the competence required by primary-care HCPs to effectively initiate and manage insulin therapy as well as provide ongoing support to patients with diabetes. Some HCPs expressed a lack of confidence in handling insulin-related issues and emphasized the importance of training and continuous guidance from diabetes specialists[253]. HCPs recognized that patient-level factors strongly influenced insulin usage and believed that patient education positively influenced adherence. Furthermore, HCPs have observed that insulin treatment can be perceived by certain patients as a symbol of failure or progression to a more advanced stage of their illness, potentially affecting their behavior. Patient behavior regarding insulin therapy is often influenced by subjective emotions and perceptions rather than solely focusing on treatment objectives[254]. Various reasons for low adherence to insulin therapy have been identified by HCPs, including patients’ busy schedules, travel commitments, challenges in timing meals, stress or emotional issues, fear of public embarrassment, and patients’ perception of their diabetes control. Understanding these factors and collaborating with patients to address them are crucial for HCPs to enhance adherence to insulin therapy[255].
Adherence to insulin therapy in diabetic patients is influenced by the quality of the rapport with HCPs. The nature of this rapport can have either positive or negative impacts on patients’ insulin behaviors, depending on factors such as the HCP’s communication skills when discussing insulin-related information, their responsiveness to patient concerns, the duration of consultations, and the accessibility and relevance of the support provided[231,256]. In addition, the divergent agenda between HCPs and patients is an important aspect of their relationship. While HCPs often prioritize achieving tight glycemic control, patients place greater emphasis on their overall QOL and broader life needs. Patients may modify their behavior to try to appease HCPs, reflecting the different priorities and concerns between the two groups[244].
Research has shown that HCPs can enhance patient adherence to insulin therapy through various means. The key strategies include effectively demonstrating the insulin injection process, adopting a collaborative approach in decision-making, emphasizing the benefits of insulin, and addressing patient concerns and misconceptions. Encouraging patient collaboration in developing a shared action plan is emphasized as a valuable approach, whereas an authoritarian communication style that repeatedly attempts to persuade patients to initiate insulin therapy is considered to be the least helpful. These findings provide important insights for HCPs aiming to improve patient adherence to insulin therapy[257].
Adherence to insulin therapy is impacted by the cost of insulin therapy, which patients and HCPs need to consider. Studies have shown that a significant proportion, up to two-thirds, of diabetic patients, particularly in low-middle-income countries, report skipping insulin doses due to the inability to afford the cost of insulin[258]. Addressing the cost-related barriers to insulin therapy adherence requires a multifaceted approach, which includes discussing affordability with patients, raising awareness about available resources, integrating cost considerations into practice guidelines, promoting the use of lower-cost biosimilars, and advocating for policies that ensure affordable access to insulin. By implementing these strategies, both patients and HCPs can work toward improving adherence to insulin therapy despite financial constraints[259].
This review acknowledges several significant limitations that should be taken into account when considering its findings and implications for future research. First, the absence of conclusive evidence favoring either premixed or basal–bolus insulin regimens based on PROs poses a challenge due to the inconsistent and inconclusive results observed across different studies. This diversity in outcomes hinders the formulation of definitive recommendations regarding the superiority of one regimen over the other. Second, the inclusion of studies using different questionnaires with varying focuses and complexities introduces heterogeneity and makes it difficult to effectively compare and synthesize the findings. The lack of standardized measurement tools across studies contributes to the limitations of the review. In addition, reliance on data mainly derived from clinical trials raises concerns about the applicability of the findings to real-world settings characterized by lower compliance and monitoring. The controlled environment of clinical trials may not fully capture the complexities and challenges experienced by individuals in their everyday lives. Furthermore, while the review identifies factors influencing adherence, the causal relationships and interplay between these factors remain unclear. Further research is warranted to gain a more comprehensive understanding of the intricate mechanisms governing patients’ adherence behaviors.
In summary, it is crucial to recognize that equipping healthcare professionals with essential skills, offering support, and embracing a patient-centered approach can help foster positive relationships and achieve improved outcomes in insulin therapy for patients. Collaborative efforts between HCPs and diabetic patients are essential in addressing adherence barriers and formulating strategies to improve adherence. This patient-centered approach plays a pivotal role in achieving optimal glycemic control and reducing the likelihood of diabetes-related complications. While the current understanding of adherence and PROs in insulin therapy for T2DM has yielded valuable insights, expanding the research scope is imperative. Future studies should explore a wider range of insulin options beyond the traditional focus on premixed and basal–bolus regimens. In addition, standardized PRO-questionnaires tailored to insulin users are crucial for ensuring consistent and comparable data across studies. This would facilitate robust comparisons and enhance the reliability of research findings. Understanding subjective factors that influence treatment adherence and QOL would inform personalized interventions and support strategies. Furthermore, real-world data from diverse clinical settings, obtained from electronic health records and patient registries, is necessary to accurately depict adherence patterns and translate research findings into practice. Evaluating the cost-effectiveness of different insulin regimens alongside their impact on both adherence and QOL is crucial for informing resource allocation and policy decisions. Finally, developing targeted interventions for specific patient groups, considering factors such as age, cultural background, and com-orbidities, can significantly improve adherence and patient-centered outcomes.
Insulin therapy has undergone significant advancements over the past century, resulting in a wide range of insulin preparations with distinct PK and PD properties. This diversity has given rise to various insulin regimens, contributing to a considerable variability in treatment approaches. However, there is currently no universally optimal insulin regimen that HCPs can use for all patients with T2DM. Consequently, it is imperative to investigate PROs using different PROMs to assess the impact of insulin therapy on patient satisfaction and QOL. Further research is warranted to address conflicting findings from diverse clinical trials and explore the influence of insulin therapy on QOL based on PROs. HCPs should exhibit a keen interest in individualized patient needs and preferences regarding insulin therapy while possessing a thorough understanding of factors affecting adherence to different insulin regimens, taking into consideration active feedback from patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jain R, India; Papazafiropoulou, Greece; Shah SIA, Pakistan S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4744] [Article Influence: 1581.3] [Reference Citation Analysis (36)] |
| 2. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1638] [Article Influence: 409.5] [Reference Citation Analysis (2)] |
| 3. | Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2019;127:S1-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 4. | Sargsyan A, Herman MA. Regulation of Glucose Production in the Pathogenesis of Type 2 Diabetes. Curr Diab Rep. 2019;19:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Kabadi UM. Diabetes Mellitus: Disorder of Cellular Dysfunction Due to Lack of Entry into Cell of Glucose. The Most Efficient Fuel for Cellular Function. Open J Endocr Metab Dis. 2021;11:79-101. [DOI] [Full Text] |
| 6. | Marcovecchio ML, Lucantoni M, Chiarelli F. Role of chronic and acute hyperglycemia in the development of diabetes complications. Diabetes Technol Ther. 2011;13:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 8. | An J, Nichols GA, Qian L, Munis MA, Harrison TN, Li Z, Wei R, Weiss T, Rajpathak S, Reynolds K. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Peyrot M, Bailey TS, Childs BP, Reach G. Strategies for implementing effective mealtime insulin therapy in type 2 diabetes. Curr Med Res Opin. 2018;34:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ceriello A, deValk HW, Guerci B, Haak T, Owens D, Canobbio M, Fritzen K, Stautner C, Schnell O. The burden of type 2 diabetes in Europe: Current and future aspects of insulin treatment from patient and healthcare spending perspectives. Diabetes Res Clin Pract. 2020;161:108053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care. 2009;32:S253-S259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Donnor T, Sarkar S. Insulin- Pharmacology, Therapeutic Regimens and Principles of Intensive Insulin Therapy. 2023 Feb 15. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 13. | Warsame R, D'Souza A. Patient Reported Outcomes Have Arrived: A Practical Overview for Clinicians in Using Patient Reported Outcomes in Oncology. Mayo Clin Proc. 2019;94:2291-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Weldring T, Smith SM. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights. 2013;6:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (1)] |
| 15. | Lucas CG, Villanueva-Meyer JE, Whipple N, Oberheim Bush NA, Cooney T, Chang S, McDermott M, Berger M, Cham E, Sun PP, Putnam A, Zhou H, Bollo R, Cheshier S, Poppe MM, Fung KM, Sung S, Glenn C, Fan X, Bannykh S, Hu J, Danielpour M, Li R, Alva E, Johnston J, Van Ziffle J, Onodera C, Devine P, Grenert JP, Lee JC, Pekmezci M, Tihan T, Bollen AW, Perry A, Solomon DA. Myxoid glioneuronal tumor, PDGFRA p.K385-mutant: clinical, radiologic, and histopathologic features. Brain Pathol. 2020;30:479-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Bolli GB, Cheng AYY, Owens DR. Insulin: evolution of insulin formulations and their application in clinical practice over 100 years. Acta Diabetol. 2022;59:1129-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE. The Evolution of Insulin and How it Informs Therapy and Treatment Choices. Endocr Rev. 2020;41:733-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 18. | Lee MKS, Liu Z, Quek TPL, Chew DEK. Insulin-related knowledge among health care professionals at a tertiary hospital. Diabetes Spectr. 2013;26:187-193. [DOI] [Full Text] |
| 19. | Retnakaran R, Zinman B. The ongoing evolution of basal insulin therapy over 100 years and its promise for the future. Diabetes Obes Metab. 2022;24:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Huising MO. Paracrine regulation of insulin secretion. Diabetologia. 2020;63:2057-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | McCarthy O, Schmidt S, Christensen MB, Bain SC, Nørgaard K, Bracken R. The endocrine pancreas during exercise in people with and without type 1 diabetes: Beyond the beta-cell. Front Endocrinol (Lausanne). 2022;13:981723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Pitt JP, McCarthy OM, Hoeg-Jensen T, Wellman BM, Bracken RM. Factors Influencing Insulin Absorption Around Exercise in Type 1 Diabetes. Front Endocrinol (Lausanne). 2020;11:573275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Mathieu C, Martens PJ, Vangoitsenhoven R. One hundred years of insulin therapy. Nat Rev Endocrinol. 2021;17:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Maikawa CL, Nguyen LT, Mann JL, Appel EA. Formulation Excipients and Their Role in Insulin Stability and Association State in Formulation. Pharm Res. 2022;39:2721-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Grunberger G. Insulin analogs-are they worth it? Yes! Diabetes Care. 2014;37:1767-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Oyer DS. The science of hypoglycemia in patients with diabetes. Curr Diabetes Rev. 2013;9:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia. 2004;47:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Guerci B, Sauvanet JP. Subcutaneous insulin: pharmacokinetic variability and glycemic variability. Diabetes Metab. 2005;31:4S7-4S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Melo KFS, Bahia LR, Pasinato B, Porfirio GJM, Martimbianco AL, Riera R, Calliari LEP, Minicucci WJ, Turatti LAA, Pedrosa HC, Schaan BD. Short-acting insulin analogues versus regular human insulin on postprandial glucose and hypoglycemia in type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Kim HS, Yu JM, Jang HC, Choi EK, Park JH, Shon HS, Chung CH, Park KG, Cho JH, Kim W, Lee KH, Lee JH, Yoo SJ. Real-World Analysis of Rapid-Acting Insulin Analog Use and Its Blood Glucose Lowering Effect in Patients with Type 2 Diabetes Mellitus: Results from PASSION Disease Registry in Korea. Diabetes Metab Syndr Obes. 2022;15:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 31. | Bode BW. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart, lispro, and glulisine. Endocr Pract. 2011;17:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Gast K, Schüler A, Wolff M, Thalhammer A, Berchtold H, Nagel N, Lenherr G, Hauck G, Seckler R. Rapid-Acting and Human Insulins: Hexamer Dissociation Kinetics upon Dilution of the Pharmaceutical Formulation. Pharm Res. 2017;34:2270-2286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Beals JM, DeFelippis MR, Paavola CD, Allen DP, Garg A, Baldwin DB. Insulin. In: Crommelin DJA, Sindelar RD, Meibohm B, Editors. 5th ed. Pharmaceutical Biotechnology Fundamentals and Applications. Berlin: Springer. 2019;403-427. |
| 34. | Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet. 2008;47:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Tibaldi JM. Evolution of insulin development: focus on key parameters. Adv Ther. 2012;29:590-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Avgerinos I, Papanastasiou G, Karagiannis T, Michailidis T, Liakos A, Mainou M, Matthews DR, Tsapas A, Bekiari E. Ultra-rapid-acting insulins for adults with diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2021;23:2395-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Owens DR, Bolli GB. The continuing quest for better subcutaneously administered prandial insulins: a review of recent developments and potential clinical implications. Diabetes Obes Metab. 2020;22:743-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Jinnouchi H, Imori M, Nishiyama H, Imaoka T. Ultra-Rapid Lispro Efficacy and Safety Compared to Humalog® in Japanese Patients With Type 2 Diabetes: PRONTO-T2D Subpopulation Analysis. Diabetes Ther. 2020;11:2075-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 39. | Gardner DF, Arakaki RF, Podet EJ, Nell LJ, Thomas JW, Field JB. The pharmacokinetics of subcutaneous regular insulin in type I diabetic patients: assessment using a glucose clamp technique. J Clin Endocrinol Metab. 1986;63:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17:682-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 41. | Leohr J, Dellva MA, LaBell E, Coutant DE, Klein O, Plum-Moerschel L, Zijlstra E, Linnebjerg H. Pharmacokinetic and Glucodynamic Responses of Ultra Rapid Lispro vs Lispro Across a Clinically Relevant Range of Subcutaneous Doses in Healthy Subjects. Clin Ther. 2020;42:1762-1777.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Puttagunta AL, Toth EL. Insulin lispro (Humalog), the first marketed insulin analogue: indications, contraindications and need for further study. CMAJ. 1998;158:506-511. [PubMed] |
| 43. | Setter SM, Corbett CF, Campbell RK, White JR. Insulin aspart: a new rapid-acting insulin analog. Ann Pharmacother. 2000;34:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Garg SK, Ellis SL, Ulrich H. Insulin glulisine: a new rapid-acting insulin analogue for the treatment of diabetes. Expert Opin Pharmacother. 2005;6:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Saleem F, Sharma A. NPH Insulin. 2023 Jun 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed] |
| 46. | Linnebjerg H, Lam EC, Zhang X, Seger ME, Coutant D, Chua L, Kapitza C, Heise T. Duration of action of two insulin glargine products, LY2963016 insulin glargine and Lantus insulin glargine, in subjects with type 1 diabetes mellitus. Diabetes Obes Metab. 2017;19:33-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Nordisk N. Levemir R® insulin detemir (rDNA origin) injection. Highlights of Prescribing Information. Feb 2015. [cited 3 March 2024]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021536s031s051lbl.pdf. |
| 48. | Brown MA, Davis CS, Fleming LW, Fleming JW. The role of Toujeo®, insulin glargine U-300, in the treatment of diabetes mellitus. J Am Assoc Nurse Pract. 2016;28:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Nordisk N. Center for drug evaluation and research. Pharmacology Review(S). 2015. [cited 3 March 2024]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/203313Orig1s000_203314Orig1s000PharmR.pdf. |
| 50. | Turner HE, Matthews DR. The use of fixed-mixture insulins in clinical practice. Eur J Clin Pharmacol. 2000;56:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Roach P, Woodworth J, Gudat U, Cerimele B, Diebler F, Pein M, Dreyer M. A 75% insulin lispro/25% NPL mixture provides a longer duration of insulin activity compared with insulin lispro alone in patients with Type 1 diabetes. Diabet Med. 2003;20:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Weyer C, Heise T, Heinemann L. Insulin aspart in a 30/70 premixed formulation. Pharmacodynamic properties of a rapid-acting insulin analog in stable mixture. Diabetes Care. 1997;20:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Hirose T, Awata T, Yamamoto Y, Hemmingsen MP. Clinical considerations for use of insulin degludec/insulin aspart in Japanese patients. Expert Opin Biol Ther. 2018;18:77-85. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 325] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 55. | Schober E, Schoenle E, Van Dyk J, Wernicke-Panten K; Pediatric Study Group of Insulin Glargine. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes. Diabetes Care. 2001;24:2005-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Linn T, Fischer B, Soydan N, Eckhard M, Ehl J, Kunz C, Bretzel RG. Nocturnal glucose metabolism after bedtime injection of insulin glargine or neutral protamine hagedorn insulin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:3839-3846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Lauritzen T, Faber OK, Binder C. Variation in 125I-insulin absorption and blood glucose concentration. Diabetologia. 1979;17:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 78] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Kelkar S, Muley S, Ambardekar P, Kelkar S. Insulin Pharmacodynamics, Pharmacokinetics, and Insulin Regimens. Optim Manag Diabetes Surg. 2019;275-289. [DOI] [Full Text] |
| 59. | Gilor C, Fleeman LM. One hundred years of insulin: Is it time for smart? J Small Anim Pract. 2022;63:645-660. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Cheng AYY, Patel DK, Reid TS, Wyne K. Differentiating Basal Insulin Preparations: Understanding How They Work Explains Why They Are Different. Adv Ther. 2019;36:1018-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 61. | Rodbard HW. An Evolutionary Perspective on Basal Insulin in Diabetes Treatment: Role of Insulin Therapy In Diabetes. J Fam Pract. 2016;65:S3-S7. [PubMed] |
| 62. | Chatterjee S, Jarvis-Kay J, Rengarajan T, Lawrence IG, McNally PG, Davies MJ. Glargine versus NPH insulin: efficacy in comparison with insulin aspart in a basal bolus regimen in type 1 diabetes--the glargine and aspart study (GLASS) a randomised cross-over study. Diabetes Res Clin Pract. 2007;77:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Sharplin P, Gordon J, Peters JR, Tetlow AP, Longman AJ, McEwan P. Improved glycaemic control by switching from insulin NPH to insulin glargine: a retrospective observational study. Cardiovasc Diabetol. 2009;8:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Porcellati F, Rossetti P, Busciantella NR, Marzotti S, Lucidi P, Luzio S, Owens DR, Bolli GB, Fanelli CG. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care. 2007;30:2447-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 65. | Porcellati F, Lucidi P, Rossetti P, Candeloro P, Andreoli AM, Marzotti S, Cioli P, Bolli GB, Fanelli CG. Differential effects of adiposity on pharmacodynamics of basal insulins NPH, glargine, and detemir in type 2 diabetes mellitus. Diabetes Care. 2011;34:2521-2523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Porcellati F, Lucidi P, Candeloro P, Cioli P, Marinelli Andreoli A, Curti G, Bolli GB, Fanelli CG. Pharmacokinetics, Pharmacodynamics, and Modulation of Hepatic Glucose Production With Insulin Glargine U300 and Glargine U100 at Steady State With Individualized Clinical Doses in Type 1 Diabetes. Diabetes Care. 2019;42:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Ritzel R, Roussel R, Bolli GB, Vinet L, Brulle-Wohlhueter C, Glezer S, Yki-Järvinen H. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 68. | Beals JM. Insulin Analogs‐Improving the Therapy of Diabetes. In: Fischer J, Rotella DP, editors. Successful Drug Discovery. Hoboken: Wiley, 2015: 35-60. [DOI] [Full Text] |
| 69. | Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. Insulin degludec compared with insulin glargine in insulin-naïve patients with type 2 diabetes: A 26-week, randomized, controlled, Pan-Asian, treat-to-target trial. J Diabetes Investig. 2013;4:605-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Ratner RE, Gough SC, Mathieu C, Del Prato S, Bode B, Mersebach H, Endahl L, Zinman B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 71. | Kjeldsen TB, Hubálek F, Hjørringgaard CU, Tagmose TM, Nishimura E, Stidsen CE, Porsgaard T, Fledelius C, Refsgaard HHF, Gram-Nielsen S, Naver H, Pridal L, Hoeg-Jensen T, Jeppesen CB, Manfè V, Ludvigsen S, Lautrup-Larsen I, Madsen P. Molecular Engineering of Insulin Icodec, the First Acylated Insulin Analog for Once-Weekly Administration in Humans. J Med Chem. 2021;64:8942-8950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 72. | Singh AK, Singh A, Singh R, Misra A. Once-weekly basal insulin icodec: Looking ONWARDS from pharmacology to clinical trials. Diabetes Metab Syndr. 2022;16:102615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Mukhopadhyay P, Chatterjee P, Pandit K, Sanyal D, Ghosh S. Once-weekly Insulin Icodec as Compared to Once-daily Basal Insulins: A Meta-analysis. Endocr Pract. 2024;30:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1042] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 75. | Bradley MC, Chillarige Y, Lee H, Wu X, Parulekar S, Muthuri S, Wernecke M, MaCurdy TE, Kelman JA, Graham DJ. Severe Hypoglycemia Risk With Long-Acting Insulin Analogs vs Neutral Protamine Hagedorn Insulin. JAMA Intern Med. 2021;181:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 76. | Lajara R, Cengiz E, Tanenberg RJ. The role of the new basal insulin analogs in addressing unmet clinical needs in people with type 1 and type 2 diabetes. Curr Med Res Opin. 2017;33:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Semlitsch T, Engler J, Siebenhofer A, Jeitler K, Berghold A, Horvath K. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;11:CD005613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Sinha B. Profile of Insulins. In: Kalra S, Sinha B, editors. Insulin Therapy Made Easy. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd, 2020. |
| 79. | Rizvi AA. Treatment of type 2 diabetes with biphasic insulin analogues. Eur Med J Diabetes. 2016;4:74-83. [PubMed] |
| 80. | He X, Chen L, Wang K, Wu H, Wu J. Insulin adherence and persistence among Chinese patients with type 2 diabetes: a retrospective database analysis. Patient Prefer Adherence. 2017;11:237-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Thomsen RW, Baggesen LM, Søgaard M, Pedersen L, Nørrelund H, Buhl ES, Haase CL, Johnsen SP. Effectiveness of intensification therapies in Danes with Type 2 diabetes who use basal insulin: a population-based study. Diabet Med. 2017;34:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Rave K, Heinemann L, Puhl L, Gudat U, Woodworth JR, Weyer C, Heise T. Premixed formulations of insulin lispro. Activity profiles in type 1 diabetic patients. Diabetes Care. 1999;22:865-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Heise T, Eckers U, Kanc K, Nielsen JN, Nosek L. The pharmacokinetic and pharmacodynamic properties of different formulations of biphasic insulin aspart: a randomized, glucose clamp, crossover study. Diabetes Technol Ther. 2008;10:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13:677-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999;354:1604-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 87. | Haahr H, Fita EG, Heise T. A Review of Insulin Degludec/Insulin Aspart: Pharmacokinetic and Pharmacodynamic Properties and Their Implications in Clinical Use. Clin Pharmacokinet. 2017;56:339-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK; 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 89. | Bellido V, Suarez L, Rodriguez MG, Sanchez C, Dieguez M, Riestra M, Casal F, Delgado E, Menendez E, Umpierrez GE. Comparison of Basal-Bolus and Premixed Insulin Regimens in Hospitalized Patients With Type 2 Diabetes. Diabetes Care. 2015;38:2211-2216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 90. | Margaritidis C, Karlafti E, Kotzakioulafi E, Kantartzis K, Tziomalos K, Kaiafa G, Savopoulos C, Didangelos T. Comparison of Premixed Human Insulin 30/70 to Biphasic Aspart 30 in Well-Controlled Patients with Type 2 Diabetes Using Continuous Glucose Monitoring. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Farshchi A, Aghili R, Oskuee M, Rashed M, Noshad S, Kebriaeezadeh A, Kia M, Esteghamati A. Biphasic insulin Aspart 30 vs. NPH plus regular human insulin in type 2 diabetes patients; a cost-effectiveness study. BMC Endocr Disord. 2016;16:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Davidson JA, Liebl A, Christiansen JS, Fulcher G, Ligthelm RJ, Brown P, Gylvin T, Kawamori R. Risk for nocturnal hypoglycemia with biphasic insulin aspart 30 compared with biphasic human insulin 30 in adults with type 2 diabetes mellitus: a meta-analysis. Clin Ther. 2009;31:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care. 2003;26:2027-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Kumar A. Efficacy and safety of biphasic insulin aspart and biphasic insulin lispro mix in patients with type 2 diabetes: A review of the literature. Indian J Endocrinol Metab. 2016;20:288-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Domeki N, Matsumura M, Monden T, Nakatani Y, Aso Y. A Randomized Trial of Step-up Treatment with Premixed Insulin Lispro-50/50 vs. Aspart-70/30 in Patients with Type 2 Diabetes Mellitus. Diabetes Ther. 2014;5:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Wu T, Betty B, Downie M, Khanolkar M, Kilov G, Orr-Walker B, Senator G, Fulcher G. Practical Guidance on the Use of Premix Insulin Analogs in Initiating, Intensifying, or Switching Insulin Regimens in Type 2 Diabetes. Diabetes Ther. 2015;6:273-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Aschner P. Insulin Therapy in Type 2 Diabetes. Am J Ther. 2020;27:e79-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 98. | Forst T, Choudhary P, Schneider D, Linetzky B, Pozzilli P. A practical approach to the clinical challenges in initiation of basal insulin therapy in people with type 2 diabetes. Diabetes Metab Res Rev. 2021;37:e3418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S140-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 535] [Article Influence: 267.5] [Reference Citation Analysis (1)] |
| 100. | Tran MNT, Dang KT, Ly LD, Tran NQ. Basal-bolus insulin therapy for the treatment of non-critically ill patients with type 2 diabetes in Vietnam: Effectiveness and factors associated with inpatient glycemic control. Int J Diabetes Dev Ctries. 2023;43:199-207. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 101. | Vinagre I, Sánchez-Hernández J, Sánchez-Quesada JL, María MÁ, de Leiva A, Pérez A. Switching to basal-bolus insulin therapy is effective and safe in long-term type 2 diabetes patients inadequately controlled with other insulin regimens. Endocrinol Nutr. 2013;60:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 103. | Maiorino MI, Bellastella G, Esposito K, Giugliano D. Premixed insulin regimens in type 2 diabetes: pros. Endocrine. 2017;55:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Wang C, Mamza J, Idris I. Biphasic vs basal bolus insulin regimen in Type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2015;32:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Giugliano D, Chiodini P, Maiorino MI, Bellastella G, Esposito K. Intensification of insulin therapy with basal-bolus or premixed insulin regimens in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2016;51:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 106. | Giugliano D, Sieradzki J, Stefanski A, Gentilella R. Personalized intensification of insulin therapy in type 2 diabetes - does a basal-bolus regimen suit all patients? Curr Med Res Opin. 2016;32:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Anyanwagu U, Mamza J, Gordon J, Donnelly R, Idris I. Premixed vs basal-bolus insulin regimen in Type 2 diabetes: comparison of clinical outcomes from randomized controlled trials and real-world data. Diabet Med. 2017;34:1728-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Kalra S, Czupryniak L, Kilov G, Lamptey R, Kumar A, Unnikrishnan AG, Boudiba A, Abid M, Akanov ZA, Latheef A, Araz M, Audehm R, Bahendeka S, Balde N, Chaudhary S, Deerochanawong C, Fasanmade O, Iraqi H, Latt TS, Mbanya JC, Rodriguez-Saldana J, Hyun KS, Latif ZA, Lushchyk M, Megallaa M, Naseri MW, Bay NQ, Ramaiya K, Randeree H, Raza SA, Shaikh K, Shrestha D, Sobngwi E, Somasundaram N, Sukor N, Tan R. Expert Opinion: Patient Selection for Premixed Insulin Formulations in Diabetes Care. Diabetes Ther. 2018;9:2185-2199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 109. | Altemimi MT, Odhaib SA, Imran HJ, Alhamza A, Almomin A, Mansour AA. Comparison of Human Premixed and Basal Plus Short-Acting Insulin Regimens for Individuals With Type 2 Diabetes During Ramadan Fasting. Cureus. 2020;12:e11976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 111. | Schneider D, Taddei-Allen P, Dougherty T. The importance of patient-reported outcomes in type 2 diabetes: insight from the PIONEER program with oral semaglutide. Am J Manag Care. 2020;26:S356-S367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 112. | Marrero DG, Hilliard ME, Maahs DM, McAuliffe-Fogarty AH, Hunter CM. Using patient reported outcomes in diabetes research and practice: Recommendations from a national workshop. Diabetes Res Clin Pract. 2019;153:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Lorenz EC, Egginton JS, Stegall MD, Cheville AL, Heilman RL, Nair SS, Mai ML, Eton DT. Patient experience after kidney transplant: a conceptual framework of treatment burden. J Patient Rep Outcomes. 2019;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 114. | Saisho Y. Use of Diabetes Treatment Satisfaction Questionnaire in Diabetes Care: Importance of Patient-Reported Outcomes. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 115. | Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 818] [Article Influence: 272.7] [Reference Citation Analysis (1)] |
| 116. | Gibbons E, Black N, Fallowfield L, Newhouse R, Fitzpatrick R. Patient-reported outcome measures and the evaluation of services. In: Challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Southampton (UK): NIHR Journals Library, 2016. |
| 117. | Terwee CB, Elders PJM, Blom MT, Beulens JW, Rolandsson O, Rogge AA, Rose M, Harman N, Williamson PR, Pouwer F, Mokkink LB, Rutters F. Patient-reported outcomes for people with diabetes: what and how to measure? A narrative review. Diabetologia. 2023;66:1357-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Langendoen-Gort M, Groeneveld L, Prinsen CAC, Beulens JW, Elders PJM, Halperin I, Mukerji G, Terwee CB, Rutters F. Patient-reported outcome measures for assessing health-related quality of life in people with type 2 diabetes: A systematic review. Rev Endocr Metab Disord. 2022;23:931-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 119. | Grigorescu ED, Lăcătușu CM, Crețu I, Floria M, Onofriescu A, Ceasovschih A, Mihai BM, Șorodoc L. Self-Reported Satisfaction to Treatment, Quality of Life and General Health of Type 2 Diabetes Patients with Inadequate Glycemic Control from North-Eastern Romania. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Svedbo Engström M, Johansson UB, Leksell J, Linder E, Eeg-Olofsson K. Implementing the Digital Diabetes Questionnaire as a Clinical Tool in Routine Diabetes Care: Focus Group Discussions With Patients and Health Care Professionals. JMIR Diabetes. 2022;7:e34561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 121. | Oluchi SE, Manaf RA, Ismail S, Kadir Shahar H, Mahmud A, Udeani TK. Health Related Quality of Life Measurements for Diabetes: A Systematic Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 122. | Greenhalgh J, Dalkin S, Gooding K, Gibbons E, Wright J, Meads D, Black N, Valderas JM, Pawson R. Functionality and feedback: a realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Southampton (UK): NIHR Journals Library; 2017 Jan-. [PubMed] |
| 123. | Davis TME, Bruce DG, Curtis BH, Barraclough H, Davis WA. The relationship between intensification of blood glucose-lowering therapies, health status and quality of life in type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Res Clin Pract. 2018;142:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Ludvigsson J. Insulin Adverse Events. Pediatr Endocrinol Rev. 2020;17:183-190. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 125. | Sepúlveda E, Poínhos R, Constante M, Pais-Ribeiro J, Freitas P, Carvalho D. Health-related quality of life in type 1 and type 2 diabetic patients in a Portuguese central public hospital. Diabetes Metab Syndr Obes. 2015;8:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Nguyen HTT, Moir MP, Nguyen TX, Vu AP, Luong LH, Nguyen TN, Nguyen LH, Tran BX, Tran TT, Latkin CA, Zhang MW, Ho RC, Vu HTT. Health-related quality of life in elderly diabetic outpatients in Vietnam. Patient Prefer Adherence. 2018;12:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 127. | Reis ACD, Cunha MV, Bianchin MA, Freitas MTR, Castiglioni L. Comparison of quality of life and functionality in type 2 diabetics with and without insulin. Rev Assoc Med Bras (1992). 2019;65:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Levin PA, Zhang Q, Mersey JH, Lee FY, Bromberger LA, Bhushan M, Bhushan R. Glycemic control with insulin glargine plus insulin glulisine versus premixed insulin analogues in real-world practices: a cost-effectiveness study with a randomized pragmatic trial design. Clin Ther. 2011;33:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 129. | Opsteen C, Qi Y, Zinman B, Retnakaran R. Effect of short-term intensive insulin therapy on quality of life in type 2 diabetes. J Eval Clin Pract. 2012;18:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Malek R, Ajili F, Assaad-Khalil SH, Shinde A, Chen JW, Van den Berg E. Similar glucose control with basal-bolus regimen of insulin detemir plus insulin aspart and thrice-daily biphasic insulin aspart 30 in insulin-naive patients with type 2 diabetes: Results of a 50-week randomized clinical trial of stepwise insulin intensification. Diabetes Metab. 2015;41:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Chung JO, Cho DH, Chung DJ, Chung MY. Assessment of factors associated with the quality of life in Korean type 2 diabetic patients. Intern Med. 2013;52:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 132. | Tinahones FJ, Gross JL, Onaca A, Cleall S, Rodríguez A. Insulin lispro low mixture twice daily versus basal insulin glargine once daily and prandial insulin lispro once daily in patients with type 2 diabetes requiring insulin intensification: a randomized phase IV trial. Diabetes Obes Metab. 2014;16:963-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 133. | Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract. 2002;55:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Hendra TJ, Taylor CD. A randomised trial of insulin on well-being and carer strain in elderly type 2 diabetic subjects. J Diabetes Complications. 2004;18:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Houlden R, Ross S, Harris S, Yale JF, Sauriol L, Gerstein HC. Treatment satisfaction and quality of life using an early insulinization strategy with insulin glargine compared to an adjusted oral therapy in the management of Type 2 diabetes: the Canadian INSIGHT Study. Diabetes Res Clin Pract. 2007;78:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolffenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care. 2002;25:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 349] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 137. | Vora J, Cohen N, Evans M, Hockey A, Speight J, Whately-Smith C. Intensifying insulin regimen after basal insulin optimization in adults with type 2 diabetes: a 24-week, randomized, open-label trial comparing insulin glargine plus insulin glulisine with biphasic insulin aspart (LanScape). Diabetes Obes Metab. 2015;17:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Jia W, Xiao X, Ji Q, Ahn KJ, Chuang LM, Bao Y, Pang C, Chen L, Gao F, Tu Y, Li P, Yang J. Comparison of thrice-daily premixed insulin (insulin lispro premix) with basal-bolus (insulin glargine once-daily plus thrice-daily prandial insulin lispro) therapy in east Asian patients with type 2 diabetes insufficiently controlled with twice-daily premixed insulin: an open-label, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 139. | Ito H, Abe M, Antoku S, Omoto T, Shinozaki M, Nishio S, Mifune M, Togane M. Effects of switching from prandial premixed insulin therapy to basal plus two times bolus insulin therapy on glycemic control and quality of life in patients with type 2 diabetes mellitus. Drug Des Devel Ther. 2014;8:391-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 140. | Giugliano D, Tracz M, Shah S, Calle-Pascual A, Mistodie C, Duarte R, Sari R, Woo V, Jiletcovici AO, Deinhard J, Wille SA, Kiljanski J. Initiation and gradual intensification of premixed insulin lispro therapy versus Basal {+/-} mealtime insulin in patients with type 2 diabetes eating light breakfasts. Diabetes Care. 2014;37:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Jin SM, Kim JH, Min KW, Lee JH, Ahn KJ, Park JH, Jang HC, Park SW, Lee KW, Won KC, Kim YI, Chung CH, Park TS, Lee MK. Basal-prandial versus premixed insulin in patients with type 2 diabetes requiring insulin intensification after basal insulin optimization: A 24-week randomized non-inferiority trial. J Diabetes. 2016;8:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 142. | Anderson RT, Skovlund SE, Marrero D, Levine DW, Meadows K, Brod M, Balkrishnan R. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 143. | Pouwer F, Hermanns N. Insulin therapy and quality of life. A review. Diabetes Metab Res Rev. 2009;25:S4-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Naegeli AN, Hayes RP. Expectations about and experiences with insulin therapy contribute to diabetes treatment satisfaction in insulin-naïve patients with type 2 diabetes. Int J Clin Pract. 2010;64:908-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 145. | Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205-218. [PubMed] [DOI] [Full Text] |
| 146. | Megari K. Quality of Life in Chronic Disease Patients. Health Psychol Res. 2013;1:e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 147. | American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S40-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 148. | Mauricio D. Quality of life and treatment satisfaction are highly relevant patient-reported outcomes in type 2 diabetes mellitus. Ann Transl Med. 2018;6:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 149. | Rajput M, Arivarasan Y, Khongsit A, Rajput R. Quality of Life among Diabetics: A Cross-Sectional Study in a Tertiary Care Center of Rohtak, Haryana. Indian J Community Med. 2020;45:283-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 150. | American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S125-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 318] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 151. | American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S151-S167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (1)] |
| 152. | Lu Y, Wang N, Chen Y, Nie X, Li Q, Han B, Xia F, Cang Z, Lu M, Meng Y, Lu Y. Health-related quality of life in type-2 diabetes patients: a cross-sectional study in East China. BMC Endocr Disord. 2017;17:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 153. | Halliday JA, Speight J, Bennet A, Beeney LJ, Hendrieckx C. The Diabetes and Emotional Health Handbook and Toolkit for Health Professionals Supporting Adults With Type 1 and Type 2 Diabetes: Formative Evaluation. JMIR Form Res. 2020;4:e15007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 154. | Kalra S, Jena BN, Yeravdekar R. Emotional and Psychological Needs of People with Diabetes. Indian J Endocrinol Metab. 2018;22:696-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 155. | Ganasegeran K, Hor CP, Jamil MFA, Loh HC, Noor JM, Hamid NA, Suppiah PD, Abdul Manaf MR, Ch'ng ASH, Looi I. A Systematic Review of the Economic Burden of Type 2 Diabetes in Malaysia. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 156. | Kalra S, Kalra B, Agrawal N. Oral insulin. Diabetol Metab Syndr. 2010;2:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 157. | Khalili M, Sabouhi F, Abazari P, Aminorroaya A. Comparing the quality of life in insulin recipient and refusal patients with type 2 diabetes. Iran J Nurs Midwifery Res. 2016;21:351-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 158. | Wexler DJ. Management of persistent hyperglycemia in type 2 diabetes mellitus. Jan 11, 2024. [cited 3 March 2024]. Available from: https://www.uptodate.com/contents/management-of-persistent-hyperglycemia-in-type-2-diabetes-mellitus. |
| 159. | Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 160. | Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 161. | Aschalew AY, Yitayal M, Minyihun A. Health-related quality of life and associated factors among patients with diabetes mellitus at the University of Gondar referral hospital. Health Qual Life Outcomes. 2020;18:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 162. | Hayes A, Arima H, Woodward M, Chalmers J, Poulter N, Hamet P, Clarke P. Changes in Quality of Life Associated with Complications of Diabetes: Results from the ADVANCE Study. Value Health. 2016;19:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 163. | Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 164. | Shah S, Zilov A, Malek R, Soewondo P, Bech O, Litwak L. Improvements in quality of life associated with insulin analogue therapies in people with type 2 diabetes: results from the A1chieve observational study. Diabetes Res Clin Pract. 2011;94:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 165. | Yang W, Zhuang X, Li Y, Wang Q, Bian R, Shen J, Hammerby E, Yang L. Improvements in quality of life associated with biphasic insulin aspart 30 in type 2 diabetes patients in China: results from the A1chieve® observational study. Health Qual Life Outcomes. 2014;12:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 166. | Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med. 1999;16:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 167. | Harahap A, Nasution M. Comparison quality of life patients treated with insulin and oral hypoglycemic drugs. IOP Conf Ser: Earth Environ Sci. 2018;125:012166. [DOI] [Full Text] |
| 168. | Hajos TR, Pouwer F, de Grooth R, Holleman F, Twisk JW, Diamant M, Snoek FJ. Initiation of insulin glargine in patients with Type 2 diabetes in suboptimal glycaemic control positively impacts health-related quality of life. A prospective cohort study in primary care. Diabet Med. 2011;28:1096-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 169. | Baruah MP, Bhowmick A, Bhuyan S, Bhuyan SB, Deka J, Bora SS. Impact of Anti-Diabetic Medications on Quality of Life in Persons with Type 2 Diabetes Mellitus. Indian J Endocrinol Metab. 2021;25:432-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 170. | Jabbar A, Mohamed WMIBW, Ozaki R, Mirasol R, Treuer T, Lew T, Qi R, Sheu WH, Deerochanawong C, Babineaux SM. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Western Pacific region. Curr Med Res Opin. 2018;34:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Jabbar A, Abdallah K, Hassoun A, Malek R, Senyucel C, Spaepen E, Treuer T, Bhattacharya I. Patterns and trends in insulin initiation and intensification among patients with Type 2 diabetes mellitus in the Middle East and North Africa region. Diabetes Res Clin Pract. 2019;149:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 172. | Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, Plunkett CD, LaPorte FB, Burke R, Brown MB, Halter JB, Raskin P. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28:1568-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 173. | Dąbrowski M, Filip W, Huc B. Association between insulin therapy and quality of life in diabetes. J Pre-Clin Clin Res. 2017;11:10-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 174. | Doubova SV, Mino-León D, Pérez-Cuevas R. Linking quality of healthcare and health-related quality of life of patients with type 2 diabetes: an evaluative study in Mexican family practice. Int J Qual Health Care. 2013;25:664-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 175. | Boels AM, Rutten G, Cleveringa F, van Avendonk M, Vos R. Insulin Therapy in Type 2 Diabetes Is Associated With Barriers to Activity and Worse Health Status: A Cross-Sectional Study in Primary Care. Front Endocrinol (Lausanne). 2021;12:573235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 176. | Mikailiūkštienė A, Juozulynas A, Narkauskaitė L, Žagminas K, Sąlyga J, Stukas R. Quality of life in relation to social and disease factors in patients with type 2 diabetes in Lithuania. Med Sci Monit. 2013;19:165-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 177. | Davis TM, Clifford RM, Davis WA; Fremantle Diabetes Study. Effect of insulin therapy on quality of life in Type 2 diabetes mellitus: The Fremantle Diabetes Study. Diabetes Res Clin Pract. 2001;52:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of Hypoglycemia in Children and Adolescents and Their Parents with Type 1 Diabetes. Curr Diab Rep. 2016;16:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 179. | Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr. 2010;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 180. | Chen HS, Wu TE, Kuo CS. Long-term glycemic control after 6 months of basal insulin therapy. Am J Manag Care. 2014;20:e369-e379. [PubMed] |
| 181. | PrasannaKumar HR, Mahesh MG, Menon VB, Srinath KM, Shashidhara KC, Ashok P. Patient Self-reported quality of life assessment in Type 2 diabetes mellitus: A pilot study. Niger J Clin Pract. 2018;21:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 182. | Tamir O, Wainstein J, Raz I, Shemer J, Heymann A. Quality of life and patient-perceived difficulties in the treatment of type 2 diabetes. Rev Diabet Stud. 2012;9:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 183. | Daher AM, AlMashoor SA, Winn T. Glycaemic control and quality of life among ethnically diverse Malaysian diabetic patients. Qual Life Res. 2015;24:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 184. | Jankowska A, Golicki D. EQ-5D-5L-based quality of life normative data for patients with self-reported diabetes in Poland. PLoS One. 2021;16:e0257998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 185. | Negash Z, Tadiwos A, Urgessa EM, Gebretekle GB, Abebe E, Fentie AM. Insulin injection practice and health related quality of life among individuals with diabetes at Tikur Anbessa Specialized Hospital, Ethiopia: a cross-sectional study. Health Qual Life Outcomes. 2023;21:38. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 186. | Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, Blonde L, Bush MA, DeFronzo RA, Garber JR, Garvey WT, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Perreault L, Rosenblit PD, Samson S, Umpierrez GE. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26:107-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 187. | Silver B, Ramaiya K, Andrew SB, Fredrick O, Bajaj S, Kalra S, Charlotte BM, Claudine K, Makhoba A. EADSG Guidelines: Insulin Therapy in Diabetes. Diabetes Ther. 2018;9:449-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 188. | Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal-bolus regimen versus premix insulin analogs: when and for whom? Diabetes Care. 2013;36:S212-S218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 189. | Ali M, Aung K, Young M, Eligar V, Davies J. Different insulin initiation regimens in patients with type 2 diabetes-a review article. Int J Diabetes Clin Res. 2018;5:083. [DOI] [Full Text] |
| 190. | Buse JB, Wolffenbuttel BH, Herman WH, Shemonsky NK, Jiang HH, Fahrbach JL, Scism-Bacon JL, Martin SA. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care. 2009;32:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 191. | Jude EB, Malecki MT, Gomez Huelgas R, Prazny M, Snoek F, Tankova T, Giugliano D, Khunti K. Expert Panel Guidance and Narrative Review of Treatment Simplification of Complex Insulin Regimens to Improve Outcomes in Type 2 Diabetes. Diabetes Ther. 2022;13:619-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 192. | Frias PF, Frias JP. New Basal Insulins: a Clinical Perspective of Their Use in the Treatment of Type 2 Diabetes and Novel Treatment Options Beyond Basal Insulin. Curr Diab Rep. 2017;17:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 193. | Wu T. Premixed insulin analogues: A new look at an established option. Diabetes Prim Care Aust. 2016;1:129-133. |
| 194. | Fritsche A, Larbig M, Owens D, Häring HU; GINGER study group. Comparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes-results of the GINGER study. Diabetes Obes Metab. 2010;12:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 195. | Gururaj Setty S, Crasto W, Jarvis J, Khunti K, Davies MJ. New insulins and newer insulin regimens: a review of their role in improving glycaemic control in patients with diabetes. Postgrad Med J. 2016;92:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 196. | Meece J. Basal Insulin Intensification in Patients with Type 2 Diabetes: A Review. Diabetes Ther. 2018;9:877-890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 197. | Koufakis T, Grammatiki M, Kotsa K. Type 2 diabetes management in people aged over seventy-five years: targets and treatment strategies. Maturitas. 2021;143:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 198. | Patel D, Triplitt C, Trujillo J. Appropriate Titration of Basal Insulin in Type 2 Diabetes and the Potential Role of the Pharmacist. Adv Ther. 2019;36:1031-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 199. | Nagao M, Harada T, Tanimura-Inagaki K, Kobayashi S, Fukuda I, Sugihara H, Oikawa S. Fasting plasma glucose level-based formula for estimating starting daily dose in basal-bolus insulin therapy. Sci Rep. 2023;13:1032. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 200. | Howard JY, Watts SA. Bolus Insulin Prescribing Recommendations for Patients With Type 2 Diabetes Mellitus. Fed Pract. 2017;34:S26-S31. [PubMed] |
| 201. | Kramer G, Kuniss N, Kloos C, Lehmann T, Müller N, Sanow B, Lorkowski S, Wolf G, Müller UA. Principles of self-adjustment of insulin dose in people with diabetes type 2 and flexible insulin therapy. Diabetes Res Clin Pract. 2016;116:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 202. | Pfeiffer KM, Basse A, Lee XY, Waldman LT. Diabetes Management and Healthcare Resource Use When Intensifying from Basal Insulin to Basal-Bolus: A Survey of Type 2 Diabetes Patients. Diabetes Ther. 2018;9:1931-1944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 203. | Testa MA, Gill J, Su M, Turner RR, Blonde L, Simonson DC. Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab. 2012;97:3504-3514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 204. | Zhang P, Bao Y, Chen M, Zhang H, Zhu D, Ji L, Li X, Ji J, Zhao F, Fisher EB, Zhao Y, Duolikun N, Wang D, Jia W. Changes of health-related quality of life after initiating basal insulin treatment among people with type 2 diabetes. Medicine (Baltimore). 2023;102:e34718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 205. | Polonsky WH, Thompson S, Wei W, Riddle MC, Chaudhari S, Jackson J, Bruno AS. Greater fear of hypoglycaemia with premixed insulin than with basal-bolus insulin glargine and glulisine: patient-reported outcomes from a 60-week randomised study. Diabetes Obes Metab. 2014;16:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 206. | Dieuzeide G, Chuang LM, Almaghamsi A, Zilov A, Chen JW, Lavalle-González FJ. Safety and effectiveness of biphasic insulin aspart 30 in people with type 2 diabetes switching from basal-bolus insulin regimens in the A1chieve study. Prim Care Diabetes. 2014;8:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 207. | Masuda H, Sakamoto M, Irie J, Kitaoka A, Shiono K, Inoue G, Atsuda K, Yamada S. Comparison of twice-daily injections of biphasic insulin lispro and basal-bolus therapy: glycaemic control and quality-of-life of insulin-naïve type 2 diabetic patients. Diabetes Obes Metab. 2008;10:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 208. | Bowering K, Reed VA, Felicio JS, Landry J, Ji L, Oliveira J. A study comparing insulin lispro mix 25 with glargine plus lispro therapy in patients with Type 2 diabetes who have inadequate glycaemic control on oral anti-hyperglycaemic medication: results of the PARADIGM study. Diabet Med. 2012;29:e263-e272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 209. | Mehmet S, Hussey C, Ibrahim S. Patients' perceptions of injecting insulin and self‐monitoring of blood glucose in the presence of others. Pract Diabetes. 2015;32:59-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 210. | Brod M, Kongsø JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 211. | do Vale Moreira NC, Ceriello A, Basit A, Balde N, Mohan V, Gupta R, Misra A, Bhowmik B, Lee MK, Zuo H, Shi Z, Wang Y, Montenegro RM, Fernandes VO, Colagiuri S, Boulton AJM, Hussain A. Race/ethnicity and challenges for optimal insulin therapy. Diabetes Res Clin Pract. 2021;175:108823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 212. | Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Factors associated with injection omission/non-adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab. 2012;14:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 213. | García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 467] [Article Influence: 38.9] [Reference Citation Analysis (2)] |
| 214. | Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33:74-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 215. | Munshi MN, Slyne C, Greenberg JM, Greaves T, Lee A, Carl S, Atakov-Castillo A, Toschi E. Nonadherence to Insulin Therapy Detected by Bluetooth-Enabled Pen Cap Is Associated With Poor Glycemic Control. Diabetes Care. 2019;42:1129-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 216. | Sendekie AK, Netere AK, Kasahun AE, Belachew EA. Medication adherence and its impact on glycemic control in type 2 diabetes mellitus patients with comorbidity: A multicenter cross-sectional study in Northwest Ethiopia. PLoS One. 2022;17:e0274971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 217. | Idris I, Gulati K, Perez-Nieves M, Hadjiyianni I, Cao D, Tahbaz A, Ivanova J, Hassan SW. Associated factors that influenced persistence with basal analog insulin therapy among people with type 2 diabetes: An exploratory analysis from a UK real-world sample. Prim Care Diabetes. 2019;13:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 218. | Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35:207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 219. | Yavuz DG, Ozcan S, Deyneli O. Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens. Patient Prefer Adherence. 2015;9:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 220. | Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1561] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 221. | Roussel R, Charbonnel B, Behar M, Gourmelen J, Emery C, Detournay B. Persistence with Insulin Therapy in Patients with Type 2 Diabetes in France: An Insurance Claims Study. Diabetes Ther. 2016;7:537-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 222. | Nau DP. Recommendations for improving adherence to type 2 diabetes mellitus therapy--focus on optimizing oral and non-insulin therapies. Am J Manag Care. 2012;18:S49-S54. [PubMed] |
| 223. | Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 224. | Chefik FH, Tadesse TA, Quisido BJE, Roba AE. Adherence to insulin therapy and associated factors among type 1 and type 2 diabetic patients on follow up in Madda Walabu University Goba Referral Hospital, South East Ethiopia. PLoS One. 2022;17:e0269919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 225. | Ayele AA, Tegegn HG, Ayele TA, Ayalew MB. Medication regimen complexity and its impact on medication adherence and glycemic control among patients with type 2 diabetes mellitus in an Ethiopian general hospital. BMJ Open Diabetes Res Care. 2019;7:e000685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 226. | Rwegerera GM. Adherence to anti-diabetic drugs among patients with Type 2 diabetes mellitus at Muhimbili National Hospital, Dar es Salaam, Tanzania- A cross-sectional study. Pan Afr Med J. 2014;17:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 227. | Bermeo-Cabrera J, Almeda-Valdes P, Riofrios-Palacios J, Aguilar-Salinas CA, Mehta R. Insulin Adherence in Type 2 Diabetes in Mexico: Behaviors and Barriers. J Diabetes Res. 2018;2018:3190849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 228. | Alsayed KA, Ghoraba MK. Assessment of diabetic patients' adherence to insulin injections on basal-bolus regimen in diabetic care center in Saudi Arabia 2018: Cross sectional survey. J Family Med Prim Care. 2019;8:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 229. | Sarbacker GB, Urteaga EM. Adherence to Insulin Therapy. Diabetes Spectr. 2016;29:166-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 230. | Liu C, De Roza J, Ooi CW, Mathew BK, Elya, Tang WE. Impact of patients' beliefs about insulin on acceptance and adherence to insulin therapy: a qualitative study in primary care. BMC Prim Care. 2022;23:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 231. | Abu Hassan H, Tohid H, Mohd Amin R, Long Bidin MB, Muthupalaniappen L, Omar K. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract. 2013;14:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 232. | Janes R, Titchener J, Pere J, Pere R, Senior J. Understanding barriers to glycaemic control from the patient's perspective. J Prim Health Care. 2013;5:114-122. [PubMed] |
| 233. | Brown K, Avis M, Hubbard M. Health beliefs of African-Caribbean people with type 2 diabetes: a qualitative study. Br J Gen Pract. 2007;57:461-469. [PubMed] |
| 234. | Cefalu WT, Mathieu C, Davidson J, Freemantle N, Gough S, Canovatchel W; OPTIMIZE Coalition. Patients' perceptions of subcutaneous insulin in the OPTIMIZE study: a multicenter follow-up study. Diabetes Technol Ther. 2008;10:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 235. | Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ. 2009;35:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 236. | Mollema ED, Snoek FJ, Adèr HJ, Heine RJ, van der Ploeg HM. Insulin-treated diabetes patients with fear of self-injecting or fear of self-testing: psychological comorbidity and general well-being. J Psychosom Res. 2001;51:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 237. | Willis WD, Diago-Cabezudo JI, Madec-Hily A, Aslam A. Medical resource use, disturbance of daily life and burden of hypoglycemia in insulin-treated patients with diabetes: results from a European online survey. Expert Rev Pharmacoecon Outcomes Res. 2013;13:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 238. | Fulcher G, Singer J, Castañeda R, Fraige Filho F, Maffei L, Snyman J, Brod M. The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: two international surveys. J Med Econ. 2014;17:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 239. | Brod M, Rana A, Barnett AH. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin. 2012;28:1933-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 240. | Leiter LA, Boras D, Woo VC. Dosing irregularities and self-treated hypoglycemia in type 2 diabetes: results from the Canadian Cohort of an International Survey of Patients and Healthcare Professionals. Can J Diabetes. 2015;39:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 241. | Brod M, Wolden M, Christensen T, Bushnell DM. A nine country study of the burden of non-severe nocturnal hypoglycaemic events on diabetes management and daily function. Diabetes Obes Metab. 2013;15:546-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 242. | Brod M, Rana A, Barnett AH. Impact of self-treated hypoglycaemia in type 2 diabetes: a multinational survey in patients and physicians. Curr Med Res Opin. 2012;28:1947-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 243. | Brod M, Pohlman B, Kongsø JH. Insulin administration and the impacts of forgetting a dose. Patient. 2014;7:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 244. | Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence. 2014;8:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 245. | Jenkins N, Hallowell N, Farmer AJ, Holman RR, Lawton J. Participants' experiences of intensifying insulin therapy during the Treating to Target in Type 2 Diabetes (4-T) trial: qualitative interview study. Diabet Med. 2011;28:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 246. | Moucheraud C, Lenz C, Latkovic M, Wirtz VJ. The costs of diabetes treatment in low- and middle-income countries: a systematic review. BMJ Glob Health. 2019;4:e001258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 247. | McAdam-Marx C, Ruiz-Negron N, Sullivan JM, Tucker JM. The effects of patient out-of-pocket costs on insulin use among people with type 1 and type 2 diabetes with Medicare Advantage insurance-2014-2018. Health Serv Res. 2024;59:e14152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 248. | Ellis K, Mulnier H, Forbes A. Perceptions of insulin use in type 2 diabetes in primary care: a thematic synthesis. BMC Fam Pract. 2018;19:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 249. | Sun AC, Tsoh JY, Saw A, Chan JL, Cheng JW. Effectiveness of a culturally tailored diabetes self-management program for Chinese Americans. Diabetes Educ. 2012;38:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 250. | Shiyanbola OO, Maurer M, Schwerer L, Sarkarati N, Wen MJ, Salihu EY, Nordin J, Xiong P, Egbujor UM, Williams SD. A Culturally Tailored Diabetes Self-Management Intervention Incorporating Race-Congruent Peer Support to Address Beliefs, Medication Adherence and Diabetes Control in African Americans: A Pilot Feasibility Study. Patient Prefer Adherence. 2022;16:2893-2912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 251. | Tyler NS, Mosquera-Lopez CM, Wilson LM, Dodier RH, Branigan DL, Gabo VB, Guillot FH, Hilts WW, El Youssef J, Castle JR, Jacobs PG. An artificial intelligence decision support system for the management of type 1 diabetes. Nat Metab. 2020;2:612-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 252. | Miller TA, Dimatteo MR. Importance of family/social support and impact on adherence to diabetic therapy. Diabetes Metab Syndr Obes. 2013;6:421-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 253. | Goderis G, Borgermans L, Mathieu C, Van Den Broeke C, Hannes K, Heyrman J, Grol R. Barriers and facilitators to evidence based care of type 2 diabetes patients: experiences of general practitioners participating to a quality improvement program. Implement Sci. 2009;4:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 254. | Lee YK, Ng CJ, Lee PY, Khoo EM, Abdullah KL, Low WY, Samad AA, Chen WS. What are the barriers faced by patients using insulin? A qualitative study of Malaysian health care professionals' views. Patient Prefer Adherence. 2013;7:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 255. | Adu MD, Malabu UH, Malau-Aduli AEO, Malau-Aduli BS. Enablers and barriers to effective diabetes self-management: A multi-national investigation. PLoS One. 2019;14:e0217771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 256. | Hortensius J, Kars MC, Wierenga WS, Kleefstra N, Bilo HJ, van der Bijl JJ. Perspectives of patients with type 1 or insulin-treated type 2 diabetes on self-monitoring of blood glucose: a qualitative study. BMC Public Health. 2012;12:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 257. | Balogh EG, Perez-Nieves M, Cao D, Hadjiyianni II, Ashraf N, Desai U, Snoek FJ, Sturt JA. Key Strategies for Overcoming Psychological Insulin Resistance in Adults with Type 2 Diabetes: The UK Subgroup in the EMOTION Study. Diabetes Ther. 2020;11:1735-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 258. | Herman WH, Kuo S. 100 years of Insulin: Why is Insulin So Expensive and What Can be Done to Control Its Cost? Endocrinol Metab Clin North Am. 2021;50:e21-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 259. | Rajkumar SV. The High Cost of Insulin in the United States: An Urgent Call to Action. Mayo Clin Proc. 2020;95:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |