Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.1001
Peer-review started: December 22, 2023
First decision: January 10, 2024
Revised: January 19, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: May 15, 2024
Processing time: 139 Days and 16 Hours
Type 2 diabetes is a chronic, non-communicable disease with a substantial global impact, affecting a significant number of individuals. Its etiology is closely tied to imbalanced dietary practices and sedentary lifestyles. Conversely, increasing die-tary fiber (DF) intake has consistently demonstrated health benefits in numerous studies, including improvements in glycemic control and weight management.
To investigate the efficacy of DF interventions in the management of type 2 diabetes mellitus (T2DM).
A systematic literature review was conducted to explore the association between DF intake and the management of T2DM. Following the inclusion and exclusion criteria, a total of 26 studies were included in this review.
The main strategies implied to increased DF intake were: High DF diet plus acarbose (2 studies); DF supplements (14 studies); and high DF diets (10 studies). Overall, most studies indicated that increased DF intake resulted in im-provements in glycemic control and weight management in T2DM patients.
DF represents a valuable strategy in the treatment of type 2 diabetes, improving health outcomes. DF intake offers the potential to improve quality of life and reduce complications and mortality associated with diabetes. Likewise, through supplements or enriched foods, DF contributes significantly to the control of several markers such as HbA1c, blood glucose, triglycerides, low-density lipoprotein, and body weight.
Core Tip: Dietary fiber (DF) represents a valuable strategy in the treatment of type 2 diabetes, improving health outcomes. Achieving a daily fiber intake of 35 g is feasible and holds substantial potential for reducing the risk of premature mortality by 10% to 48% in individuals with diabetes. DF intake offers the potential to improve quality of life and reduce complications and mortality associated with diabetes. Likewise, through supplements or enriched foods, DF contributes significantly to the control of several markers such as HbA1c, blood glucose, triglycerides, low-density lipoprotein, and body weight. However, weight loss is more influenced by calorie restriction than by the amount of fiber in the diet. Hence, future clinical studies should further explore the combination of increased DF intake and calorie restriction, as this strategy presents the most valuable results in type 2 diabetes mellitus management.
- Citation: Nitzke D, Czermainski J, Rosa C, Coghetto C, Fernandes SA, Carteri RB. Increasing dietary fiber intake for type 2 diabetes mellitus management: A systematic review. World J Diabetes 2024; 15(5): 1001-1010
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/1001.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.1001
Type 2 diabetes mellitus (T2DM) is a chronic, non-communicable disease (NCD) characterized by changes in metabolism that result in high blood glucose levels. Notably, this occurs due to insufficient insulin production by the beta cells of the pancreas or the inefficiency of insulin receptors in the cells, impairing the uptake and use of glucose[1,2]. Strikingly, projections suggest that by 2030, the global incidence of T2DM may reach a concerning level, impacting up to 439 million individuals, culminating in a troubling trend in the global prevalence of T2DM, with a projected 69% surge in affected adults in developing countries and a somewhat less pronounced 20% rise in already developed nations by the same year[3,4]. Therefore, it is essential to continue research to develop new approaches and strategies that reduce the incidence and prevalence of this disease, promoting an improvement in health and quality of life.
In this context, lack of physical activity and unbalanced nutrition characterized by glycemic index (GI) and low dietary fiber (DF) intake are risk factors for DM2 development[5]. DF refers to a diverse group of compounds that are resistant to digestion by human enzymes in the small intestine, which include non-starch polysaccharides, oligosaccharides, lignin, and associated plant substances such as cellulose, hemicellulose, beta-glucans, pectins, fructans, gums, and mucilages[6,7]. Importantly, soluble fibers undergo fermentation by colonic bacteria in the large intestine, leading to the production of short-chain fatty acids (SCFAs) and fostering the growth of beneficial bacteria[6]. Also, these fibers also have the capacity to absorb water, forming a gel that extends the transit time of food through the intestine. Consequently, this process delays gastric emptying, diminishes the absorption of specific nutrients, and encourages a slower and more gradual process of digestion[6,7]. On the other hand, insoluble fibers, which include hemicelluloses, cellulose, and lignin, travel through the digestive tract intact and are less fermented. Specifically, these fibers speed up intestinal transit, increase fecal volume, and help prevent constipation. Insoluble fibers are commonly found in whole grains, wheat, bran, nuts, seeds, and some fruits and vegetables[6].
T2DM is a chronic NCD with a substantial global impact, affecting a significant number of individuals. Moreover, T2DM etiology is closely tied to imbalanced dietary practices, sedentary lifestyles, and the increased consumption of ultra-processed foods, which are characterized by a deficiency in DF, albeit often abundant in refined carbohydrates and additives. Conversely, a sufficient intake of DF has consistently demonstrated health benefits in numerous studies and research endeavors over time. Against this backdrop, the present study aimed to investigate the efficacy of DF interventions in the management of T2DM.
This systematic literature review explores the association between DF and the management of T2DM. The Scopus, PubMed, and Web of Science databases were comprehensively searched up to July 2023 by two independent researchers. The keywords incorporated the terms "dietary fiber" AND "diabetes mellitus".
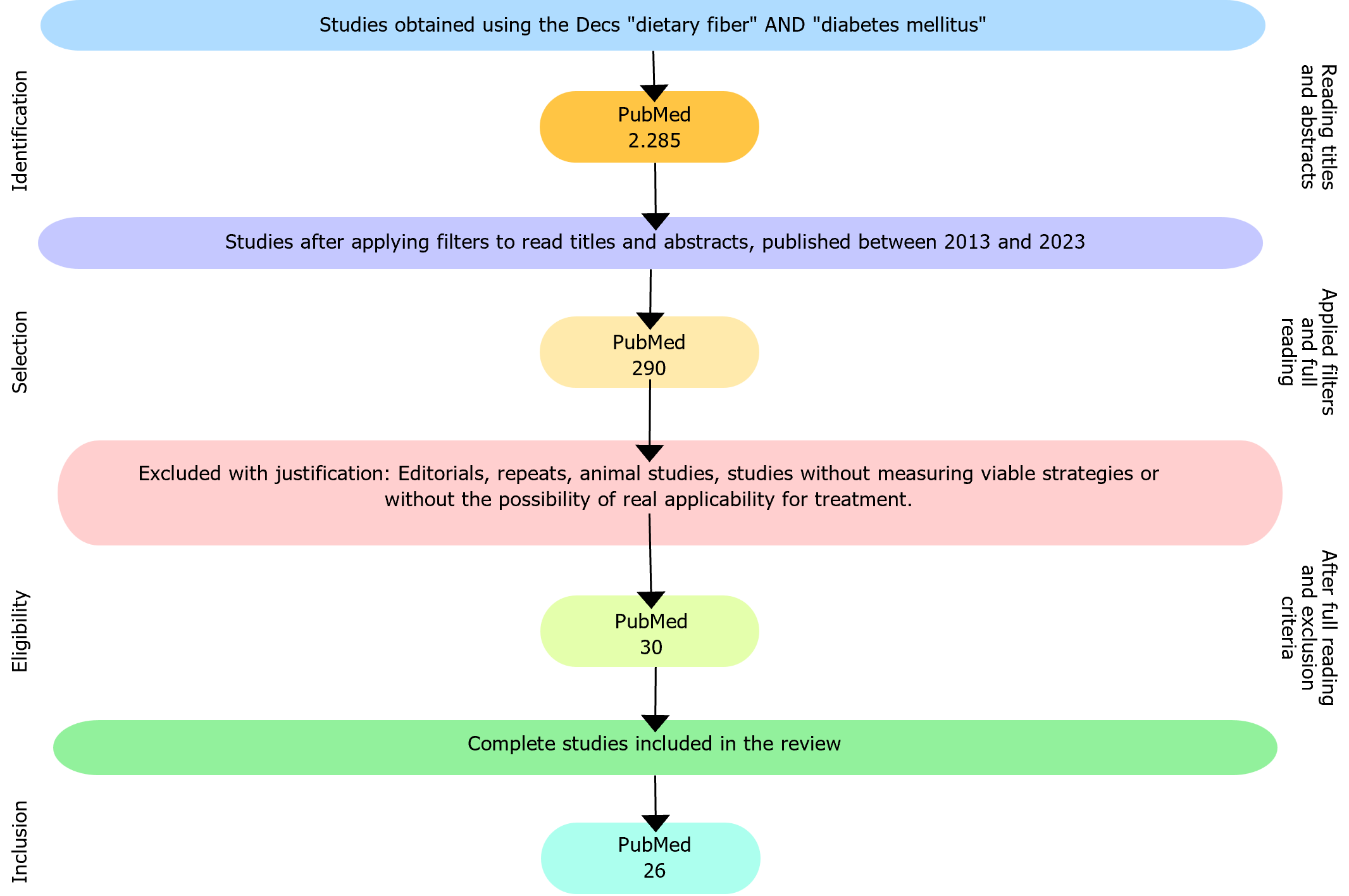
Article selection adhered to specific criteria, encompassing original research articles, published in English and freely accessible. The population should include T2DM patients, and the intervention should include DF, provided that they offered pertinent insights into the correlation between DF and T2DM, significantly contributing to the understanding of DF utilization in treating this condition. To facilitate the analysis and review of potential interventions, articles lacking a direct or indirect link to T2DM and DF were excluded. Review articles, animal studies, and those lacking information from reliable sources were also omitted. Studies evaluating the effect of increased DF intake on glycemic control and weight management as primary outcomes were included. Full-length papers of the shortlisted articles were assessed for the eligibility criteria and 26 studies were finally included.
The initial search yielded 2285 articles, and after applying filters and reviewing titles and abstracts, we narrowed down our selection to 290 articles for full text reading. Following a thorough analysis and the application of the exclusion criteria, 30 articles were ultimately included in this review. Four articles were excluded due to the unavailability of the full text, resulting in a final analysis of 26 articles, as depicted in Figure 1.
Initially, the 26 articles investigated diverse populations, spanning ages from 9 to 80 years, with a primary focus on individuals exhibiting symptoms related to T2DM and its consequences. Changes in glucose levels, HbA1c, overweight, obesity, inflammatory markers, and body mass index (BMI) were parameters consistently evaluated throughout the studies to identify significant improvements in the health of individuals diagnosed with T2DM.
Noteworthily, the included studies were conducted in various locations, encompassing populations with distinct food cultures, including countries like China, Norway, Brazil, Iran, Canada, Vietnam, Indonesia, Italy, Germany, and Japan. This diversity allowed for the observation of region-specific results, particularly concerning food culture and the types of DF typically consumed.
Regarding DF consumption, the 26 studies employed three primary methods. First, drugs, such as amylase enzyme inhibitors, were utilized in two articles. Second, the DF supplements, including inulin, fructans, guar gum, resistant starch, resistant dextrin, galacto-oligosaccharides, and psyllium, were ingested in 14 articles. The third method included increasing the intake of fiber-rich foods in the diet, as discussed in 10 articles. Most studies had an average duration of 6 wk, although some varied between 8 and 12 wk, and in a few cases, extended beyond 1 year.
Two studies (Table 1), encompassing a total of 60 patients, underscored the advantageous impact of acarbose in enhancing glycemic control, leading to a reduction in HbA1c levels. In 2018, a study in China compared two groups: An intervention group and a control group, both receiving acarbose as standard medication. The intervention group adopted a high-fiber diet, incorporating whole grains, traditional Chinese medicine foods, and prebiotics, while the control group adhered to a conventional diet. The results demonstrated superior glycemic control in the intervention group, with a significantly higher proportion of patients achieving HbA1c levels below 7% compared to the control group (89% vs 50%). This positive effect is attributed to enhanced control over starch (glucose) digestion and absorption, where a portion of starch transforms into fermentable carbohydrates, stimulating fermentation in collagen and promoting the production of SCFAs, lactobacilli, and bifidobacteria[8,9].
| Ref. | Population | Methods | Outcomes |
| Chen et al[8], 2023 | 17 Chinese T2DM patients, enrolled for 8 wk | Acarbose (100 mg; 3 times/d) + DF-rich diet; control group: Standard diet | Intervention resulted in decreased HbA1c, fasting glucose, and insulin sensitivity. No significant changes in the control group |
| Zhao et al[9], 2018 | 43 Chinese T2DM patients, enrolled for 11 wk | Acarbose (100 mg; 3 times/d) + DF-rich diet; control group: Standard diet | Intervention group showed sustained better glycemic control (HbA1c < 7%) for 89% of participants compared to the control. No significant differences in fasting glucose levels |
Consequently, the inclusion of acarbose emerges as a pivotal element in treating T2DM, contributing to reduced glycemia and enhanced control for patients. Notably, the study underscores that when coupled with a high-fiber diet, acarbose yields more specific outcomes compared to its isolated use. Therefore, co-administration of acarbose with a high-fiber diet is deemed beneficial for achieving positive results. Moreover, a potential etiological factor in T2DM is linked to a deficiency of SCFAs, arising from low DF intake and the consequent degradation of these fibers. Research also indicates that increased DF intake can instigate improvements in intestinal microbiota and insulin sensitivity, and the establishment of a protective intestinal barrier, resulting in a notable enhancement in the immune response of individuals with T2DM[8,9]. In summary, these findings suggest that combining acarbose, an amylase inhibitor, with a diet rich in fiber represents a promising therapeutic approach for identified T2DM patients. This combination holds the potential to yield substantial benefits in glycemic control and overall health for individuals with T2DM.
The 14 studies outlined in Table 2 encompassed a total sample size of 490 individuals. They explored the effects of consuming DF supplements, including inulin, fructans, resistant starch, resistant dextrin, psyllium, and certain combinations from specific brands containing DF. These studies conducted a comparative analysis between the group subjected to the DF intervention and the control group that received a placebo and showed mixed results[10-13].
| Ref. | Population | Methods | Outcomes |
| Dall'Alba et al[10], 2013 | 44 Brazilian T2DM patients, enrolled for 6 wk | PHGG (10 g/d) + standard diet; control group: Standard diet | No significant changes in TG, HDL, SBP, or FG. Decreased waist circumference and HbA1c in the intervention group |
| Dehghan et al[11], 2014 | 49 Iranian T2DM patients, enrolled for 8 wk | Inulin (10 g/d) + standard diet; control group: Standard diet + maltodextrin (10 g/d) | Significant decreases in HbA1c (6.82 mmol/mol; 10.4%), and insulin in the intervention group |
| Dehghan et al[12], 2014 | 52 Iranian T2DM patients, enrolled for 8 wk | Oligofructose + inulin (10 g/d) + standard-diet; control group: Standard diet + maltodextrin (10 g/d) | HbA1c, FG, weight, and BMI significantly decreased in the intervention group |
| Gargari et al[13], 2015 | 60 Iranian T2DM patients, enrolled for 8 wk | Resistant starch (10 g/d) + standard diet; control group: Standard diet + maltodextrin (10 g/d) | Intervention group showed significantly decreased HbA1c (-0.3%; -3.6%), TG (-33.4 mg/dL, -15.4%), and SBP. HDL increased significantly only in the intervention group |
| Aliasgharzadeh et al[14], 2015 | 55 Iranian T2DM patients, enrolled for 8 wk | Resistant dextrin (10 g/d) + standard diet; control group: Standard diet + maltodextrin (10 g/d) | Fasted insulin, weight (-3.1 kg)z, and BMI (-1.4) significantly decreased in the intervention group. Decreased HbA1c was not significant in the intervention group |
| Farhangi et al[15], 2016 | 54 Iranian T2DM patients, enrolled for 8 wk | Resistant dextrin (10 g/d) + standard diet; control group: Standard diet + maltodextrin (10 g/d) | HbA1c and SBP decreased significantly in the intervention group. No significant changes were observed for BMI |
| Abutair et al[16], 2016 | 40 T2DM patients from Palestine, enrolled for 8 wk | Psyllium (10.5 g/d) + standard diet; control group: Standard diet + maltodextrin (10 g/d) | Significant changes were observed for glycemic control, including Hb1Ac and FG |
| Pedersen et al[17], 2016 | 40 T2DM patients from Ucraine, enrolled for 12 wk | Galacto-oligosaccharide (5.5 mg/d) + standard diet; control group: Standard diet + maltodextrin (5.5 g/d) | The prebiotic group showed increased HbA1c and fasting glucose |
| Farhangi et al[21], 2018 | 55 Iranian T2DM patients, enrolled for 8 wk | Resistant dextrin (10 g/d) + standard diet; control group: Standard diet + maltodextrin (10 g/d) | No significant changes were observed for glycemic control variables, and BMI, except for fasting insulin. Pro-inflammatory markers were significantly decreased in the intervention group |
| Birkeland et al[18], 2020 | 25 Norwegian T2DM patients, enrolled for 6 wk | Inulin fructans (16 g/d) + standard diet; control group: Standard diet + maltodextrin (16 g/d) | No changes in glycemic control were reported, with positive effects on fecal microbiome composition |
| Vuksan et al[22], 2020 | 26 Canadian patients (11 T2DM), enrolled for 3 wk | Viscous fiber blend + dietary fiber + standard diet; control group: Dietary fiber + standard diet + maltodextrin (16 g/d) | No significant effects on FG between groups. SBP and cardiovascular risk were reduced in the intervention group |
| Birkeland et al[19], 2021 | 25 Norwegian T2DM patients, enrolled for 6 wk | Inulin fructans (16 g/d) + standard diet; control group: Standard diet + maltodextrin (16 g/d) | No positive changes in glycemic control following a standard meal were reported |
| Birkeland et al[20], 2021 | 29 Norwegian T2DM patients, enrolled for 6 wk | Inulin fructans (16 g/d) + standard diet; control group: Standard diet + maltodextrin (16 g/d) | No positive changes in glycemic control or weight were reported |
| Su et al[22], 2022 | 13 Chinese T2DM patients, enrolled for 12 wk | Fiber consisted of probiotics, prebiotics, and whole grains, including three ready-to-consume prepared foods (44 g/d) + standard diet | The fiber formulation reduced Hb1Ac, weight, blood glucose, and blood pressure in T2D patients |
For example, Aliasgharzadeh et al[14] investigated the effectiveness of resistant dextrin supplements administered at a dosage of 10 g/d over 8 wk. The study revealed significant reductions in body weight levels, with a decrease of 1.6 kg in the control group and 3.1 kg in the intervention group. Likewise, BMI showed reductions of 0.9 in the control group and 1.4 in the intervention group. Additionally, fasting insulin concentration was reduced by 20.1 pmol/L, representing a 22.8% decrease in the intervention group compared to the control group. Using a similar setting, Farhangi et al[15] demonstrated positive effects on Hb1Ac and systemic blood pressure. These studies suggest that resistant dextrin presents itself as a promising alternative for incorporation into foods, offering the potential to replace sugar and fat in meal preparation. This potential opens up opportunities for its inclusion in commercially available products in su-permarkets[14].
Furthermore, in the work of Abutair et al[16], the effects of fiber supplementation in patients with DM2 were investigated. Some relevant results include the evaluation of psyllium in the intervention group, which demonstrated significant reductions in weight (2.7 kg, P < 0.001), BMI (0.98 kg/m2, P < 0.001), waist circumference (WC; 2.6 cm, P < 0.001), and hip circumference (HC; 2.5 cm, P < 0.001). Despite a slight decrease in waist-hip ratio, this change did not reach statistical significance. The inclusion of 10.5 g of psyllium in the daily diet resulted in notable reductions in weight (from 91.7 kg to 88.8 kg, P < 0.001), BMI (from 31.8 kg/m2 to 30.9 kg /m2, P < 0.001), WC (from 106.2 cm to 107.3 cm, P < 0.001), and HC (from 109.9 cm to 107.3 cm, P < 0.001). These results reflect the positive impact of psyllium on glycemia and weight control, notably evidenced by the reduction in HbA1c and anthropometric measurements[16]. Surprisingly, the study of Pedersen et al[17] reported an unexpected finding, indicating an increase in HbA1c in both groups in addition to an increase in fasting glucose within the prebiotic group. This suggests that short-term treatment with a low-dose prebiotic fiber does not prevent further deterioration of key clinical parameters in T2DM, albeit considering the other studies included in this review, we can speculate that the composition of the DF supplement also plays an important role in the results.
Subsequent studies have unveiled promising outcomes in inulin supplementation compared to the placebo-administered control group[11,12,15,18]. Strikingly, DF supplements can cause a noteworthy 1.1% reduction in HbA1c, equivalent to 6.82 mmol/mol, indicating a 10.4% decrease compared to the control group. Furthermore, fasting insulin levels exhibited a notable decline of 4.1 mU/mL, reflecting a 34.3% reduction in comparison to the control group[11]. However, the study by Birkeland et al[19,20], utilizing fructans, including inulin, in the intervention group to assess postprandial levels of GLP-1 (glucagon like peptide-1) and GLP-2 (glucagon like peptide-2), insulin, and glucose, revealed that a dosage of 16 g/d failed to sufficiently modify the glycemic control, and the responses of GLP-1 and GLP-2, or other appetite-regulating parameters. This underscores the necessity to contemplate increasing the dosage and conducting additional studies for a more in-depth exploration of this approach[18-20]. Notably, DF supplementation appears beneficial not only in T2D control but also in regulating inflammatory markers[21] and regulating gut microbiome, which could also result in significant long-term benefits[22].
Among the 14 scrutinized studies, five fell short of documenting significant and conclusive improvements related to specific aspects of T2DM[23]. This underscores the imperative for additional investigations to address outstanding issues. One of these studies employed a 10g/d mixture of oligofructose and inulin in the intervention, yet the results were limited due to the small sample size available during the research period, impacting the robustness and comprehensiveness of the findings[12]. Likewise, another study focused mainly on analyzing the appetite of individuals with T2DM and used a mixture of oligofructose and inulin, showing no significant changes concerning reduced appetite[19,20,22]. These results highlight the complexity of the interactions between inulin and metabolic responses, suggesting that factors such as dosage and specific combinations may be decisive for the success of the intervention[17]. However, considering the studies included in this review, it can be inferred that DF supplements benefit T2DM patients.
Increasing DF through nutritional counseling is another important strategy (Table 3). In this context, two different trials encompassed a total of 89 patients submitted to ingestion of foods containing DF in conjunction with calorie restriction aimed at weight loss. The data reveal that the preponderant factor in weight reduction was calorie restriction, without discarding, however, the potential benefits associated with the use of DF in this process.
| Ref. | Population | Methods | Outcomes |
| Nowotny et al[24], 2015 | 59 German T2DM patients, enrolled for 8 wk | Intervention was increased DF (30-50 g/d); control group: Diet composed of ≤ 10 g/d DF | Both groups showed improved glycemic control and reduced weight |
| Belalcazar et al[35], 2014 | 1.701 American T2DM patients, enrolled for 48 wk | Intensive program increasing dietary fiber and physical activity, while also promoting caloric restriction | Positive changes in glycemic control, weight reduction, and other health parameters |
| Ziegler et al[25], 2015 | 30 German T2DM patients, enrolled for 8 wk | Intervention was increased DF (30-50 g/d); control group: Diet composed of ≤ 10 g/d DF | Both groups showed improved glycemic control and reduced weight. The magnitude of reduced Hb1Ac was higher in the DF intervention group |
| Li et al[36], 2016 | 298 Chinese T2DM patients, enrolled for 4 wk | Three intervention groups: Healthy diet (total DF = 33 g); 50 g oats (total DF = 36 g); and 100 g oats (total DF = 39 g); control group: Usual care without dietary changes | All intervention groups showed improved glycemic control and reduced weight. The magnitude of reduced Hb1Ac, FG, and BMI was higher in the DF intervention groups with increased DF intake |
| Gomes et al[26], 2017 | 20 Brazilian T2DM patients, enrolled for 4 wk | Compared two interventions: High glycemic diet and low GI diet | DF intake was not different between groups; the low glycemic diet induced reduced body fat, without changes in glycemic control in this study |
| Kondo et al[27], 2017 | 28 Japanese T2DM patients, enrolled for 8 wk | Compared two interventions: High fiber diet with brown rice and diet with white rice | Total DF intake increased only in the brown rice group. No differences in weight, body fat, and blood pressure were observed between the two groups. Fasting plasma glucose levels and Hb1Ac decreased in the brown rice diet group, but there was no statistically significant difference between the two groups |
| Tessari and Lante[28], 2017 | 22 Italian T2DM patients, enrolled for 96 wk | The intervention consisted in consuming a functional bread inducing increased DF intake. The control group received a regular bread | The intervention group showed improved Hb1Ac and post-prandial glucose. Body weight was increased in the intervention group, with no significant effects on other variables |
| Nguyen et al[29], 2019 | 49 Vietnamese T2DM patients, enrolled for 2 wk | Intervention consisted in Okara intake (resulting in 6 g of DF). The control received a standard diet | Intervention resulted in increased DF intake, promoting decreased FG and body weight |
| Yen et al[30], 2022 | 84 Indonesian T2DM patients, enrolled for 12 wk | Intervention consisted in increasing vegetable intake and DF intake. Control group received no counselling | Intervention with low glycemic diet induced reductions in HbA1C and body weight, and blood pressure parameters |
| Jenkins et al[31], 2022 | 134 Canadian T2DM patients, enrolled for 144 wk | Compared two interventions: Low GI diet and high fiber diet | Both interventions resulted in increased DF intake. However, low GI diet improved HB1Ac and body weight |
In this context, researchers encourage the adoption of this combination to explore other possible benefits of DF, especially concerning glycemic levels[24,25]. For example, the study by Nowotny et al[24] carried out through randomization, followed 59 participants for 5 wk, who received an individually calculated daily hypocaloric diet, with a constant distribution of macronutrients (50% of energy from carbohydrates, 30% from fat, and 20% from protein). The diet was rich in cereal fiber, without red meat, with increased coffee intake, containing 30-50 g/d of wheat and rye cereal fiber. On the other hand, the low-fiber diet, rich in red meat, contained 10 g/d of whole fiber and 150 g/d of red meat (beef), excluding coffee or tea. Both dietary interventions resulted in weight reduction and improved health parameters in individuals with T2DM. Similarly, Ziegler et al[25] using the same study design, showed that energy restriction for over 8 wk contributed to improved oxidative glucose utilization and weight reduction, which also resulted in better outcomes in T2DM, and indicated that the magnitude of HB1Ac changes was correlated with BMI changes.
Increasing DF intake through diet may imply changing food groups or specific food types, as proposed by Gomes et al[26], comparing a high glycemic diet with a low glycemic diet, without specific meal planning. Although the DF intake was not different between groups, the low glycemic diet resulted in body fat reduction and improved inflammatory markers, without changes in glycemic control. Similarly, Kondo et al[27], compared two dietary interventions differing in rice type (brown vs white), aiming to improve fiber intake. Unsurprisingly, fiber intake increased only in the brown rice group, though no significant improvements were seen in weight or glycemic control. Notably, fiber intake among groups was below 20 g/d in both of the studies above, arguably insufficient to promote health benefits in the general population. Interestingly, the study by Tessari and Lante[28] evaluated the addition of a functional bread to a regular diet, aiming to promote the intake of at least 40 g of starch equivalents per day, improving Hb1Ac and post-prandial glucose, regardless of the increased body weight of 1 kg. Likewise, beneficial effects of specific food preparations were also observed for okara, the pulp consisting of insoluble parts of the soybean[29], and with the increased intake of vegetables[30]. Examining the connection between high cereal fiber and low GI diets and their association with reduced cardiovascular disease (CVD) risk in cohort studies, Jenkins et al[31] conducted a randomized study involving 169 men and women with well-controlled type 2 diabetes. The participants followed either a low GI diet or a whole-grain wheat-fiber diet for 3 years. Both groups demonstrated an increase in total DF intake during the study, although the difference was not statistically significant. Notably, the low glycemic diet proved more effective in reducing HbA1c and body weight when compared to the wheat-fiber diet.
Therefore, the collective findings indicated that calorie restriction (hypocaloric diet) remains the primary factor identified for weight loss in T2DM patients. Additionally, the studies mentioned lend support to the notion that glycemic control can be improved by a diet incorporating DF, with a lower GI, and lower intake of saturated fat and cholesterol. The studies also reinforce that stimulating long-term dietary changes and increasing physical activity are pivotal for promoting positive changes during T2DM management.
Thus, the studies analyzed demonstrate that increasing DFs can play a relevant role in managing T2DM, albeit individualization of treatment, appropriate doses, and additional research are necessary to optimize the potential benefits and fully understand the mechanisms underlying these effects. The recommended intake of DF for the management of T2DM according to different guidelines and health organizations is approximately 25-30 g. As observed in the studies cited in this review, these recommendations are based on evidence that demonstrates the benefits of fiber in managing glycemia, reducing body weight and the risk of CVDs. Nevertheless, achieving this recommendation of daily fiber intake is feasible and holds substantial potential for reducing the risk of premature mortality by 10% to 48% in individuals with diabetes. For individuals with T2DM, maintaining adequate fiber intake, whether through supplements or fortified foods, significantly contributes to the control of various markers such as HbA1c, blood glucose, triglycerides, low-density lipoprotein (LDL), and body weight. It is worth noting, however, that the impact on weight loss is more influenced by calorie restriction than by the quantity of fiber in the diet. Significantly, the compilation of studies emphasizes that increasing fiber intake also mitigates an individual's inflammatory profile, through specific inflammatory mediators that adversely affect peripheral insulin sensitivity, such as tumor necrosis factor-α and interleukin-6[13]. The production of these pro-inflammatory mediators is directly linked to the amount of fat accumulated in the body, primarily within adipocytes. Therefore, the reduction in weight, particularly body fat, leads to a decrease in the production of these mediators, thereby contributing to the enhancement of peripheral insulin sensitivity[25,26].
The results of this systematic literature review expand the current literature, indicating promising results regarding the isolated and combined strategy of calorie restriction and increased DF intake, which can synergistically benefit T2DM. A recent meta-analysis, encompassing 42 studies and 1789 patients with diabetes, revealed that both DF from food and supplements, such as psyllium or viscous sources, can enhance glycemic control and mitigate cardiovascular risk factors, as current dietary guidelines have underscored the advantages of soluble forms of fiber[32]. The robust findings from intervention trials and cohort studies in this meta-analysis strongly advocate for nutritional recommendations, suggesting that individuals with all types of diabetes, including type 2 diabetes, should strive for adequate fiber intake from sources like vegetables, pulses, whole fruits, and whole grains. These foods are excellent dietary choices. Moreover, the data suggests that those opting for reduced total carbohydrate intake should consider fiber supplements as a means to ensure adequate consumption of fiber, whether from food or supplements, yielding benefits in blood glucose control and T2DM management[33]. These findings align with another meta-analysis of 14 studies involving 32699 patients, indicating that fiber consumption can lower the risk of T2DM, improving insulin sensitivity and glucose tolerance in individuals with T2DM or impaired glucose tolerance[34]. The positive effects of fiber on insulin resistance are attributed to the reduction in the GI of foods, the absorption of ingested lipids, and the gradual absorption of nutrients. This, in turn, reduces the risk of obesity, improves glucose homeostasis, regulates hormonal responses, manages inflammatory cytokines, and enhances the health and diversity of the intestinal microbiota[34]. Taking these findings collectively, it can be inferred that, when coupled with a personalized diet for those with T2DM, such supplements yield substantial advantages, contributing to glycemic control, weight management, and modulation of the inflammatory profile. Noteworthily, most of the analyzed trials were short-lasting (up to 12 wk), which does not allow far-reaching conclusions, as to achieve a significant reduction of T2DM incidence at a population level, a fiber-rich diet should be maintained throughout the lifespan.
DF represents a valuable strategy in the treatment of type 2 diabetes, improving health outcomes. DF intake offers the potential to improve quality of life and reduce complications and mortality associated with diabetes. Likewise, through supplements or enriched foods, DF contributes significantly to the control of several markers, such as HbA1c, blood glucose, triglycerides, LDL, and body weight. However, weight loss is more influenced by calorie restriction than by the amount of fiber in the diet. Hence, future clinical studies should further explore the combination of increased DF intake and calorie restriction, as this strategy presents the most valuable results in T2DM management.
Type 2 diabetes mellitus (T2DM) is a chronic, non-communicable disease characterized by changes in metabolism that result in high blood glucose levels. Therefore, it is essential to continue research to develop new approaches and strategies that reduce the incidence and prevalence of this disease, promoting an improvement in health and quality of life.
T2DM etiology is closely tied to imbalanced dietary practices. Conversely, a sufficient intake of dietary fiber (DF) has consistently demonstrated health benefits in numerous studies and research endeavors over time.
To investigate the efficacy of DF interventions in the management of T2DM.
We searched the Scopus, PubMed, and Web of Science databases up to July 2023, using the terms "dietary fiber" AND "diabetes mellitus".
Following a thorough analysis and the application of the exclusion criteria, 26 articles were ultimately included in this review. Regarding DF consumption, we identified three methods: Utilizing drugs, such as amylase enzyme inhibitors, in two articles; ingestion of DF supplements, including inulin, fructans, guar gum, resistant starch, resistant dextrin, galacto-oligosaccharides, and psyllium; and increasing the intake of fiber-rich foods in the diet, as discussed in 10 articles.
The collective findings indicate that glycemic control can be improved by a diet incorporating DF, using the aforementioned methods.
The results of this systematic literature review expand the current literature, indicating promising results regarding the isolated and combined strategy of calorie restriction and increased DF intake, which can synergistically contribute to notably benefiting T2DM.
We thank the referees for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dąbrowski M, Poland; Lie Z, China S-Editor: Lin C L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Adefegha SA. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J Diet Suppl. 2018;15:977-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Goyal R, Singhal M, Jialal I. Type 2 Diabetes. 2023 Jun 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed] |
| 3. | Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 4. | Sharma A, Mittal S, Aggarwal R, Chauhan MK. Diabetes and cardiovascular disease: inter-relation of risk factors and treatment. Future J Pharm Sci. 2020;6:130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Silva FM, Steemburgo T, de Mello VD, Tonding SF, Gross JL, Azevedo MJ. High dietary glycemic index and low fiber content are associated with metabolic syndrome in patients with type 2 diabetes. J Am Coll Nutr. 2011;30:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | McRorie JW Jr, McKeown NM. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J Acad Nutr Diet. 2017;117:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 314] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 7. | Soliman GA. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 8. | Chen L, Liu B, Ren L, Du H, Fei C, Qian C, Li B, Zhang R, Liu H, Li Z, Ma Z. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front Cell Infect Microbiol. 2023;13:1069954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 9. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1536] [Article Influence: 219.4] [Reference Citation Analysis (68)] |
| 10. | Dall'Alba V, Silva FM, Antonio JP, Steemburgo T, Royer CP, Almeida JC, Gross JL, Azevedo MJ. Improvement of the metabolic syndrome profile by soluble fibre - guar gum - in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr. 2013;110:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Dehghan P, Gargari BP, Jafar-Abadi MA, Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int J Food Sci Nutr. 2014;65:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Dehghan P, Pourghassem Gargari B, Asghari Jafar-abadi M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition. 2014;30:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Gargari BP, Namazi N, Khalili M, Sarmadi B, Jafarabadi MA, Dehghan P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement Ther Med. 2015;23:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Aliasgharzadeh A, Dehghan P, Gargari BP, Asghari-Jafarabadi M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: a randomised controlled clinical trial. Br J Nutr. 2015;113:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Farhangi MA, Javid AZ, Dehghan P. The effect of enriched chicory inulin on liver enzymes, calcium homeostasis and hematological parameters in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Prim Care Diabetes. 2016;10:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Abutair AS, Naser IA, Hamed AT. Soluble fibers from psyllium improve glycemic response and body weight among diabetes type 2 patients (randomized control trial). Nutr J. 2016;15:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Pedersen C, Gallagher E, Horton F, Ellis RJ, Ijaz UZ, Wu H, Jaiyeola E, Diribe O, Duparc T, Cani PD, Gibson GR, Hinton P, Wright J, La Ragione R, Robertson MD. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br J Nutr. 2016;116:1869-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Birkeland E, Gharagozlian S, Birkeland KI, Valeur J, Måge I, Rud I, Aas AM. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur J Nutr. 2020;59:3325-3338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 19. | Birkeland E, Gharagozlian S, Birkeland KI, Holm OKS, Thorsby PM, Aas AM. Effect of inulin-type fructans on appetite in patients with type 2 diabetes: a randomised controlled crossover trial. J Nutr Sci. 2021;10:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Birkeland E, Gharagozlian S, Gulseth HL, Birkeland KI, Hartmann B, Holst JJ, Holst R, Aas AM. Effects of prebiotics on postprandial GLP-1, GLP-2 and glucose regulation in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled crossover trial. Diabet Med. 2021;38:e14657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Farhangi MA, Javid AZ, Sarmadi B, Karimi P, Dehghan P. A randomized controlled trial on the efficacy of resistant dextrin, as functional food, in women with type 2 diabetes: Targeting the hypothalamic-pituitary-adrenal axis and immune system. Clin Nutr. 2018;37:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Su L, Hong Z, Zhou T, Jian Y, Xu M, Zhang X, Zhu X, Wang J. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep. 2022;12:1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 23. | Vuksan V, Sievenpiper JL, Jovanovski E, Jenkins AL, Komishon A, Au-Yeung F, Zurbau A, Ho HVT, Li D, Smircic-Duvnjak L. Effect of soluble-viscous dietary fibre on coronary heart disease risk score across 3 population health categories: data from randomized, double-blind, placebo-controlled trials. Appl Physiol Nutr Metab. 2020;45:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Nowotny B, Zahiragic L, Bierwagen A, Kabisch S, Groener JB, Nowotny PJ, Fleitmann AK, Herder C, Pacini G, Erlund I, Landberg R, Haering HU, Pfeiffer AF, Nawroth PP, Roden M. Low-energy diets differing in fibre, red meat and coffee intake equally improve insulin sensitivity in type 2 diabetes: a randomised feasibility trial. Diabetologia. 2015;58:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Ziegler D, Strom A, Nowotny B, Zahiragic L, Nowotny PJ, Carstensen-Kirberg M, Herder C, Roden M. Effect of Low-Energy Diets Differing in Fiber, Red Meat, and Coffee Intake on Cardiac Autonomic Function in Obese Individuals With Type 2 Diabetes. Diabetes Care. 2015;38:1750-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Gomes JMG, Fabrini SP, Alfenas RCG. Low glycemic index diet reduces body fat and attenuates inflammatory and metabolic responses in patients with type 2 diabetes. Arch Endocrinol Metab. 2017;61:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kondo K, Morino K, Nishio Y, Ishikado A, Arima H, Nakao K, Nakagawa F, Nikami F, Sekine O, Nemoto KI, Suwa M, Matsumoto M, Miura K, Makino T, Ugi S, Maegawa H. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS One. 2017;12:e0179869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Tessari P, Lante A. A Multifunctional Bread Rich in Beta Glucans and Low in Starch Improves Metabolic Control in Type 2 Diabetes: A Controlled Trial. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Nguyen LT, Nguyen TH, Nguyen LT, Kamoshita S, Tran TP, LE HT, Shimura F, Yamamoto S. Okara Improved Blood Glucose Level in Vietnamese with Type 2 Diabetes Mellitus. J Nutr Sci Vitaminol (Tokyo). 2019;65:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Yen TS, Htet MK, Lukito W, Bardosono S, Setiabudy R, Basuki ES, Wibudi A, Martianto D, Subekti I, Fahmida U. Increased vegetable intake improves glycaemic control in adults with type 2 diabetes mellitus: a clustered randomised clinical trial among Indonesian white-collar workers. J Nutr Sci. 2022;11:e49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Jenkins DJA, Chiavaroli L, Mirrahimi A, Mitchell S, Faulkner D, Sahye-Pudaruth S, Paquette M, Coveney J, Olowoyeye O, Patel D, Pichika SC, Bashyam B, Maraj T, Gillett C, de Souza RJ, Augustin LSA, Blanco Mejia S, Nishi SK, Leiter LA, Josse RG, McKeown-Eyssen GE, Berger AR, Connelly PW, Srichaikul K, Kendall CWC, Sievenpiper JL, Moody AR. Glycemic Index Versus Wheat Fiber on Arterial Wall Damage in Diabetes: A Randomized Controlled Trial. Diabetes Care. 2022;45:2862-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 32. | Thompson HJ. The Dietary Guidelines for Americans (2020-2025): Pulses, Dietary Fiber, and Chronic Disease Risk-A Call for Clarity and Action. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020;17:e1003053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 34. | Chen JP, Chen GC, Wang XP, Qin L, Bai Y. Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. Nutrients. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 35. | Belalcazar LM, Anderson AM, Lang W, Schwenke DC, Haffner SM, Yatsuya H, Rushing J, Vitolins MZ, Reeves R, Pi-Sunyer FX, Tracy RP, Ballantyne CM; Look AHEAD (Action for Health in Diabetes) Research Group. Fiber intake and plasminogen activator inhibitor-1 in type 2 diabetes: Look AHEAD (Action for Health in Diabetes) trial findings at baseline and year 1. J Acad Nutr Diet. 2014;114:1800-10.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Li X, Cai X, Ma X, Jing L, Gu J, Bao L, Li J, Xu M, Zhang Z, Li Y. Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2 Diabetics: A Randomized Control Trial. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |