Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.783
Peer-review started: December 5, 2023
First decision: December 18, 2023
Revised: December 28, 2023
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: April 15, 2024
Processing time: 128 Days and 18 Hours
Diabetic cardiomyopathy is considered as a chronic complication of diabetes mellitus (DM). Therefore, early detection of left ventricular systolic function (LVSF) damage in DM is essential.
To explore the use of the three-dimensional speckle tracking technique (3D-STI) for measuring LVSF in DM patients via meta-analysis.
The electronic databases were retrieved from the initial accessible time to 29 April 2023. The current study involved 9 studies, including 970 subjects. We carried out this meta-analysis to estimate myocardial function in DM compared with controls according to myocardial strain attained by 3D-STI.
Night articles including 970 subjects were included. No significant difference was detected in the left ventricular ejection fraction between the control and the diabetic group (P > 0.05), while differences in global longitudinal strain, global circumferential strain, global radial strain, and global area strain were markedly different between the controls and DM patients (all P < 0.05).
The 3D-STI could be applied to accurately measure early LVSF damage in patients with DM.
Core Tip: In this study, we found that three-dimensional speckle tracking technique (3D-STI) could precisely assess early left ventricular systolic dysfunction in diabetes mellitus (DM). Our meta-analysis indicated that global longitudinal strain (GLS), global radial strain, global circumferential strain, and global area strain (GAS) in DMs were lower than controls, suggesting that the left ventricular systolic function in DMs was impaired compared with controls. Among them, the decrease of GLS and GAS was more obvious, which may be since the left ventricular wall is composed of three layers of myocardial fibers. The assessment of left ventricular strain in DM patients through 3D-STI might estimate the damage of left ventricular systolic function in DM in the early stage.
- Citation: Li Z, Qian Y, Fan CY, Huang Y. Application of three-dimensional speckle tracking technique in measuring left ventricular myocardial function in patients with diabetes. World J Diabetes 2024; 15(4): 783-792
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/783.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.783
Diabetes mellitus (DM) is a common disease in China. Long-term poor blood glucose control can cause multisystem damage and a series of chronic complications[1]. Diabetic cardiomyopathy (DCM) is a chronic complication and is a serious cause of poor prognosis in individuals with DM[2]. Additionally, numerous reports have suggested that DM could elevate the occurrence of cardiac disorders, hypertension, and other illnesses and could worsen coronary artery illness[3]. Therefore, early detection of left ventricular systolic function (LVSF) damage in DM patients is essential.
Currently, the routine clinical factor for assessing cardiac function is left ventricular ejection fraction (LVEF). However, LVEF is strongly affected by subjectivity, and several publications have exposed that LVEF could not indicate the severity of LVSF in patients with earlier DM. Moreover, it is impossible to effectively predict patients with segmental wall motion abnormalities and ejection fraction retention by LVEF. Therefore, evaluating left ventricular myocardial function is highly important for the diagnosis, treatment, and prognosis of heart disease. The 3D-STI is an innovative approach for evaluating cardiac motor function. It tracks myocardial motion from three-dimensional space through detecting myocardial echo speckle signals, which breaks through the limitation of the two-dimensional plane of 2D-STI and can evaluate cardiac function more accurately.
The 3D-STI is of great value in the assessment of primary or secondary LVSF. However, the ability of the 3D-STI to evaluate the outcome of left ventricular myocardial contractile function (LVMCF) in DM remains uncertain, and additional reports are needed. The purpose of the current analysis was to examine the ability of the 3D-STI to early predict LVSF damage in DM via meta-analysis.
The meta-analysis was registered (202390079) in INPLASY and followed the preferred reporting criteria of PRISMA 2020. A comprehensive exploration of studies on 3D-STI assessment in DM patients was conducted based on the PRISMA 2020 recommendations. Through the PubMed, Embase, Scopus, and Cochrane Library databases, studies on 3D-STI assessment in DM patients were retrieved from the initial obtainable time to 29 April 2023. The exploration strategy was employed based on the following terms: (1) “Three-dimensional speckle tracking”, “3D-speckle tracking”, “3D-STI”, or “STE”; (2) “Diabetes mellitus” or “DM”; and (3) “left ventricular” or “LV”. The current study conducted a meta-analysis.
Full-text articles that included the main factors were eligible for inclusion in this study. The main factors were as follows: (1) Had a randomized controlled trial and cohort study; (2) had an article comparing LVMCF parameters between the DM group and control group; (3) had no history of cardiovascular syndromes; (4) had a diagnostic approach of 3D-STI; and (5) had at least one notable result, including global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS), and global area strain (GAS).
Repeated documents and publications that did not supply original descriptions of interest, such as case reports, meeting essays, reviews, fundamental studies, and nonrelevant publications, were eliminated. Two researchers individually assessed the selected articles followed the selection criteria. Divergences between researchers were resolved by an agreement obtained from the assist of a third author.
The quality of the publications was considered with the New-Ottawa Scale and evaluated based on the following features: The comparison of the case and control groups, and the estimation of the consequences. The quality of the chosen studies was estimated individually by two investigators, and the incongruity was determined by discussion.
Publication bias was estimated through Egger’s test for the included articles. The random effects approach was used to minimize the variability among the included publications. The stability of the strains was measured through sensitivity analysis by eliminating one by one from the article.
The weighted mean difference and 95% confidence interval (95%CI) were employed to depict the statistical consequences of continuous variables. Heterogeneity was measured through RevMan 5.3 software and the I2 test. An I2 < 25% indicated low heterogeneity, while a value > 50% implied high heterogeneity. The random-effects model was employed and checked with the fixed-effects model. Sensitivity analyses were carried out through the leave-one-out method. A difference was statistically significant at P < 0.05.
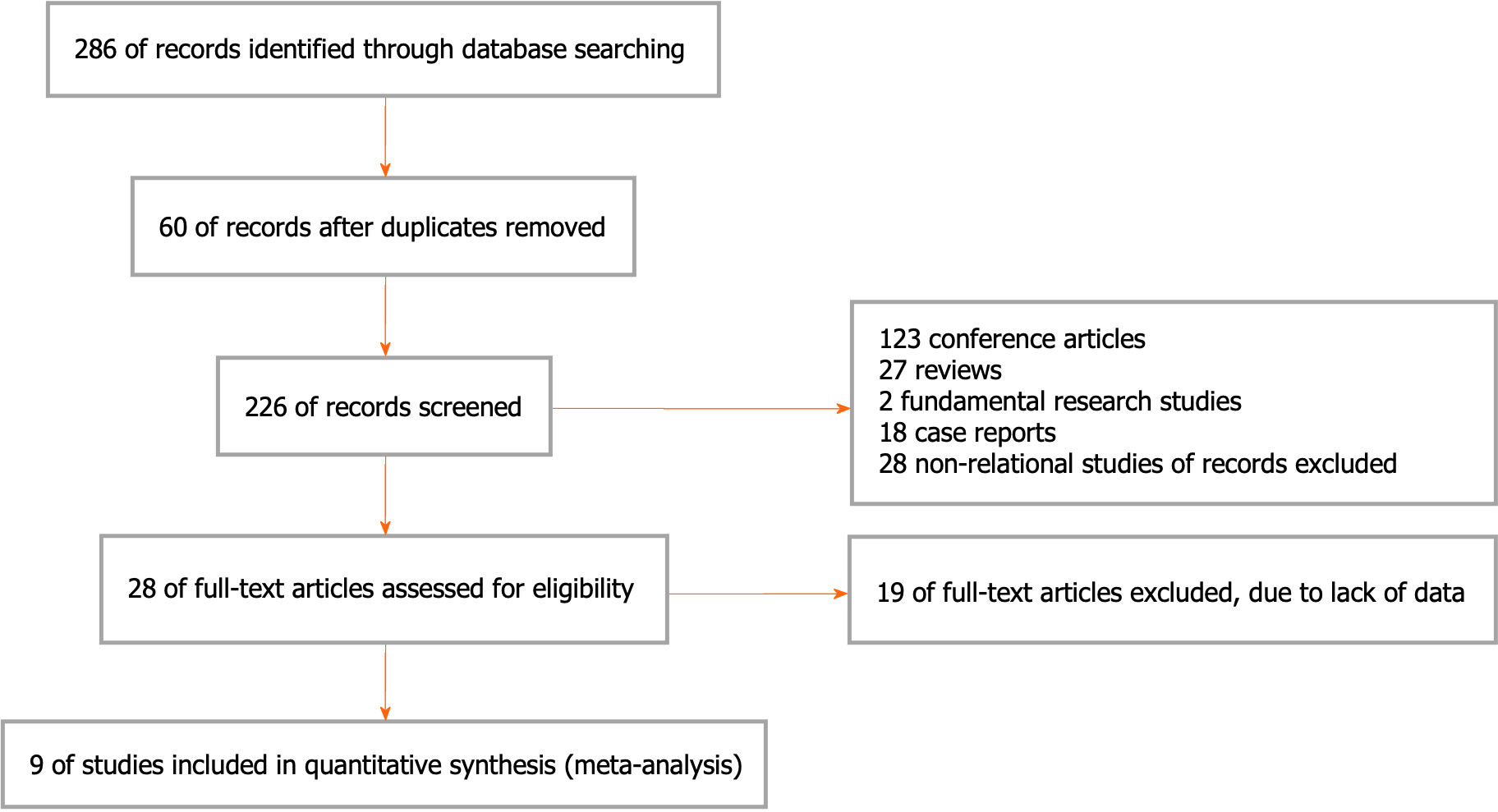
Two hundred and eighty-six articles were obtained from the above databases using a retrieval strategy. Sixty duplicated studies were excluded. Moreover, studies without suitable information, including meeting articles (123), reviews (27), fundamental studies (2), case reports (18), and nonrelational studies (28), were disqualified. After the full texts were read, 19 studies were rejected due to lack of statistics. Ultimately, the remaining 9 articles were involved. The article collection process is exhibited in Figure 1 and Table 1.
| Ref. | Country | Instrument | Groups | Number | Gender (male/female, n) | Age (yr) | 3D-STI parameters and LVEF | NOS score |
| Tadic et al[4], 2015 | Serbia | GE Vivid E7 | DM | 50 | 26/24 | 52.00 ± 8.00 | GLS, GCS, GRS, GAS, LVEF | 8 |
| NC | 50 | 24/26 | 50.00 ± 7.00 | |||||
| Wang et al[5], 2015 | China | GE Vivid E9 | DM | 46 | 24/22 | 63.10 ± 9.80 | GLS, GCS, GRS, GAS, LVEF | 7 |
| NC | 40 | 21/19 | 65.50 ± 5.90 | |||||
| Zhang et al[6], 2013 | China | GE Vivid E9 | DM-a1 | 31 | 15/16 | 61.00 ± 9.00 | GLS, GCS, GRS, GAS, LVEF | 8 |
| DM-b1 | 37 | 21/16 | 60.00 ± 10.00 | |||||
| NC | 63 | 30/33 | 58.00 ± 10.00 | |||||
| Enomoto et al[7], 2016 | Japan | Aplio-ArtidaTM | DM | 77 | 53/24 | 56.00 ± 15.00 | GLS, GRS, GCS, LVEF | 7 |
| NC | 35 | 18/17 | 52.00 ± 16.00 | |||||
| Wang et al[8], 2015 | China | GE Vivid E9 | DM-a2 | 36 | 18/18 | 64.40 ± 7.90 | GLS, GRS, GCS, GAS | 8 |
| DM-b2 | 41 | 21/20 | 65.70 ± 9.00 | |||||
| DM-c2 | 46 | 22/24 | 63.10 ± 9.80 | |||||
| NC | 36 | 18/18 | 66.80 ± 8.40 | |||||
| Yang et al[9], 2021 | China | GE Vivid E9 | DM-a3 | 28 | 19/9 | 51.42 ± 8.94 | GLS, GRS, GCS, LVEF | 8 |
| DM-b3 | 19 | 13/6 | 52.16 ± 9.22 | |||||
| NC | 27 | 18/9 | 49.93 ± 8.28 | |||||
| Conte et al[10], 2013 | Italy | GE Vivid E7 | DM-a4 | 44 | 23/21 | 60.90 ± 6.60 | GLS, GRS, GCS, GAS | 7 |
| DM-b4 | 27 | 17/10 | 56.20 ± 7.80 | |||||
| NC | 24 | 13/11 | 58.40 ± 9.40 | |||||
| Abomandour et al[11], 2022 | Egypt | Vivid E2013 | DM-a5 | 31 | 17/14 | 32.94 ± 5.56 | GLS, GRS, GCS, LVEF | 7 |
| DM-b5 | 31 | 18/13 | 28.74 ± 9.35 | |||||
| NC | 31 | 11/20 | 30.32 ± 9.53 | |||||
| Wang et al[12], 2022 | China | GE Vivid E9 | DM-a6 | 40 | 21/19 | 64.40 ± 7.90 | GLS, GRS, GCS, GAS, LVEF | 8 |
| DM-b6 | 40 | 21/19 | 60.80 ± 8.10 | |||||
| NC | 40 | 20/20 | 61.90 ± 6.90 |
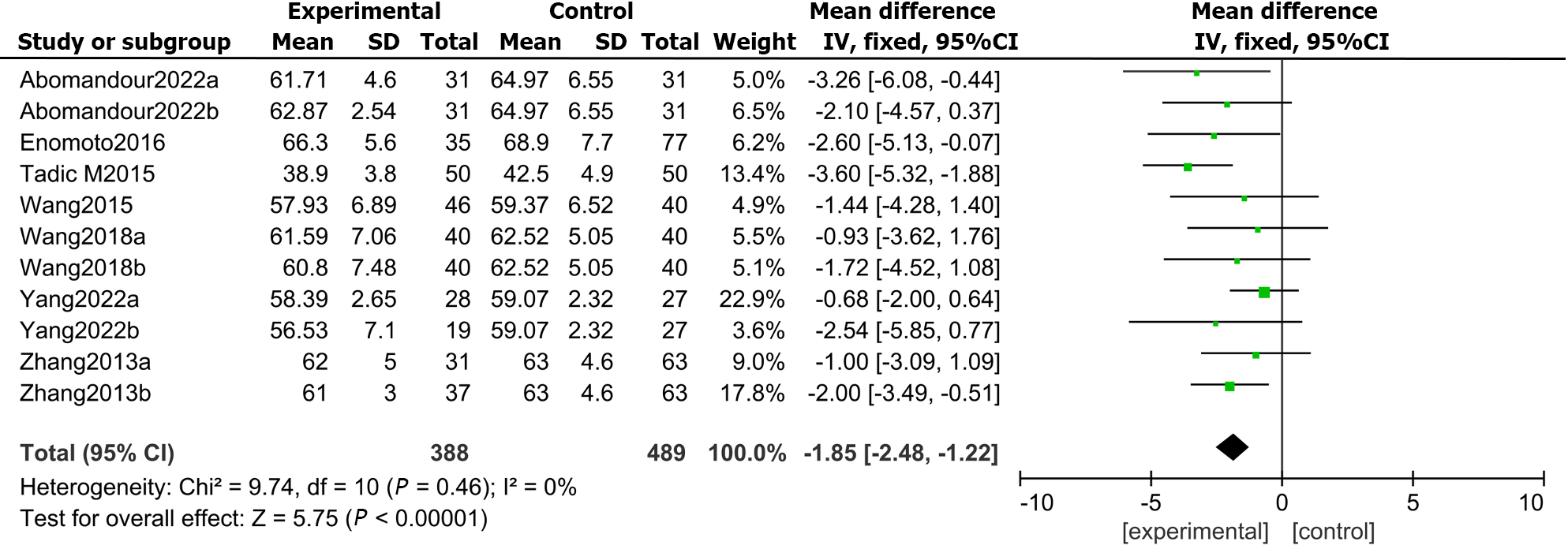
In total, 7 articles compared the LVEF measured by 3D-STI between the DMs and controls. The findings showed that the difference in LVEF between DMs and healthy controls was not statistically significant (MD: -1.85, 95%CI: -2.48 to -1.22, P = 0.46; I2 = 0%; Figure 2).
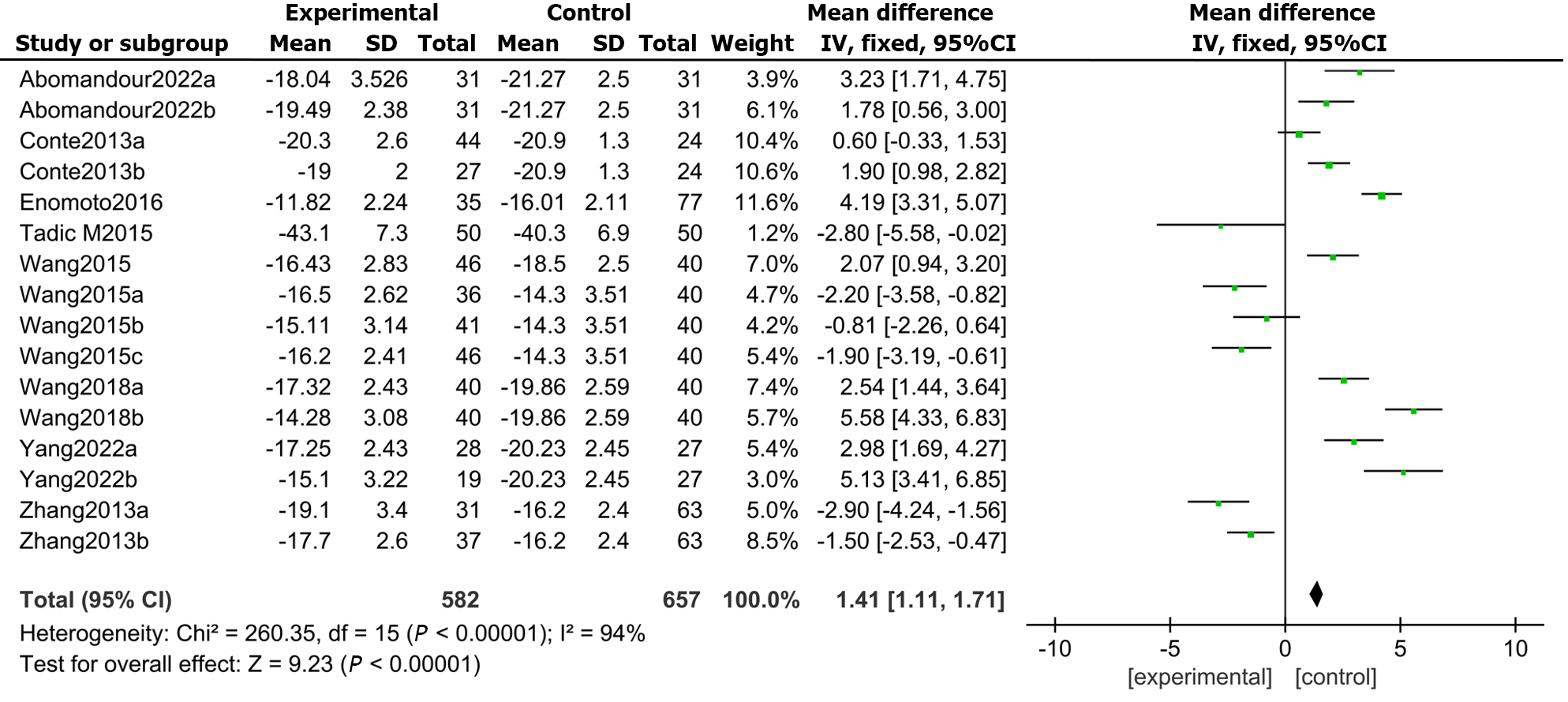
Furthermore, all 9 included studies reported GLS in DM patients and healthy controls. The results demonstrated that the LVGLS (MD: 1.41, 95%CI: 1.11 to 1.71, P = 0.000; I2 = 94%; Figure 3) was appreciably lower in the DMs than in the controls.
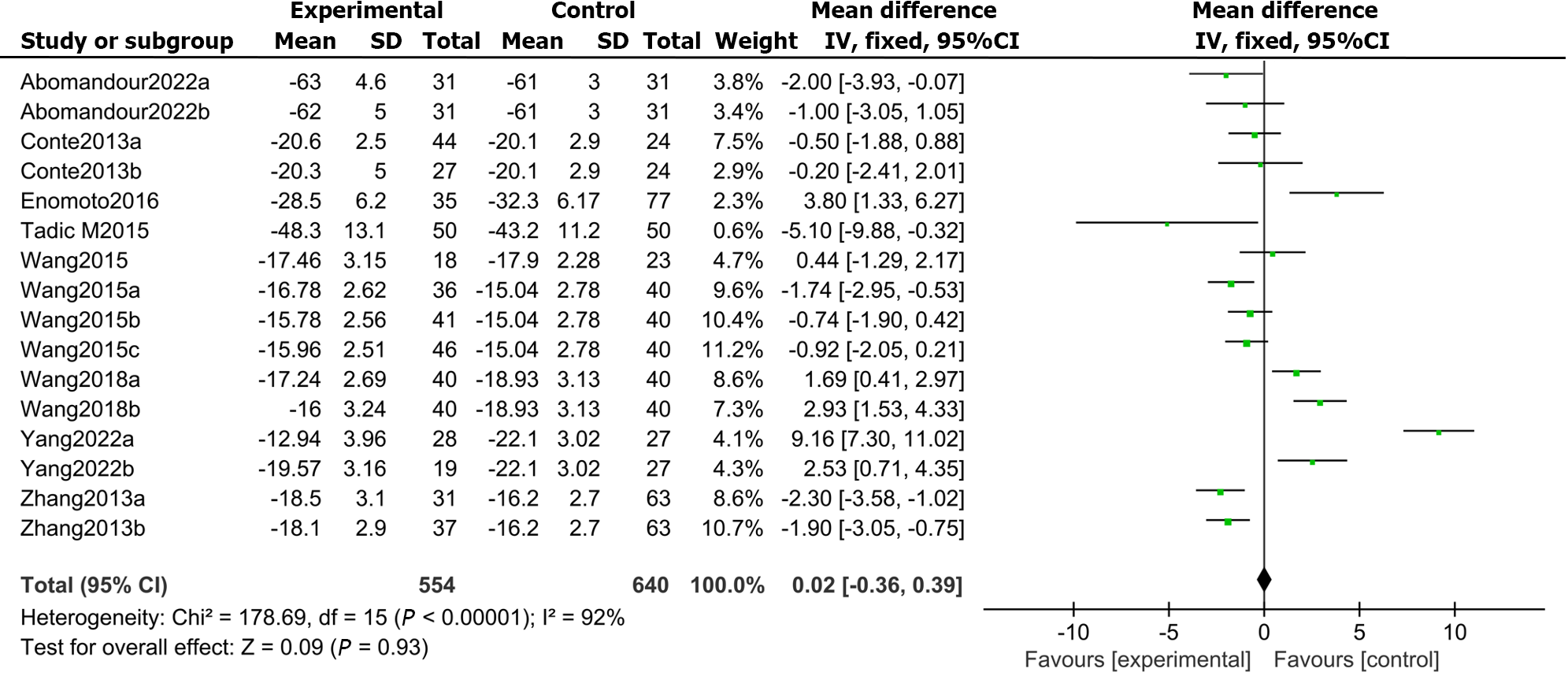
There were 9 articles recording the GCS score in patients with DM and controls. The results demonstrated that the LVGCS (MD: 0.02, 95%CI: -0.36 to 0.39, P = 0.000; I2 = 92%; Figure 4) was markedly lower in the DMs than in the controls.
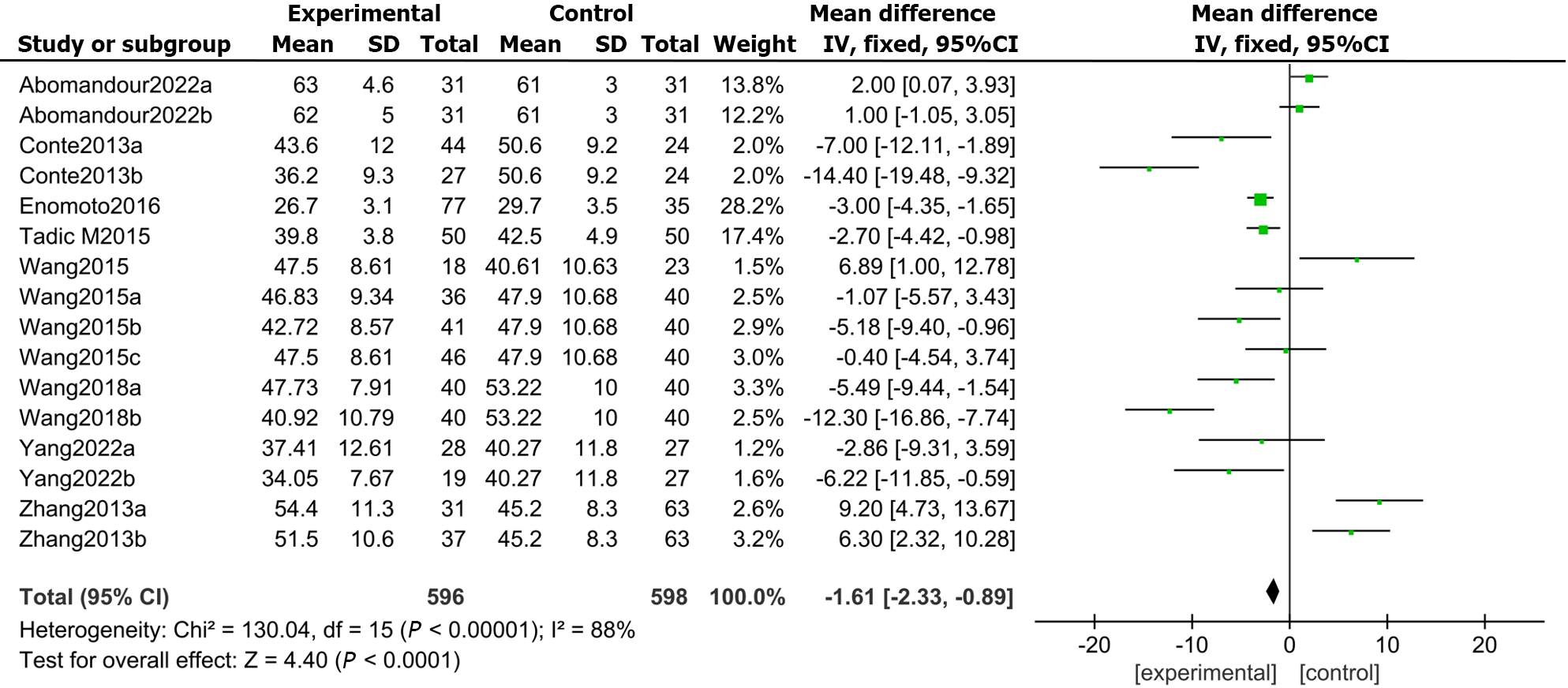
All 9 included studies reported GRSs in DM patients and healthy controls. The results demonstrated that the LVGRS (MD: -1.61, 95%CI: -2.33 to -0.89, P = 0.000; I2 = 88%; Figure 5) was markedly impaired in the DMs compared with the controls.
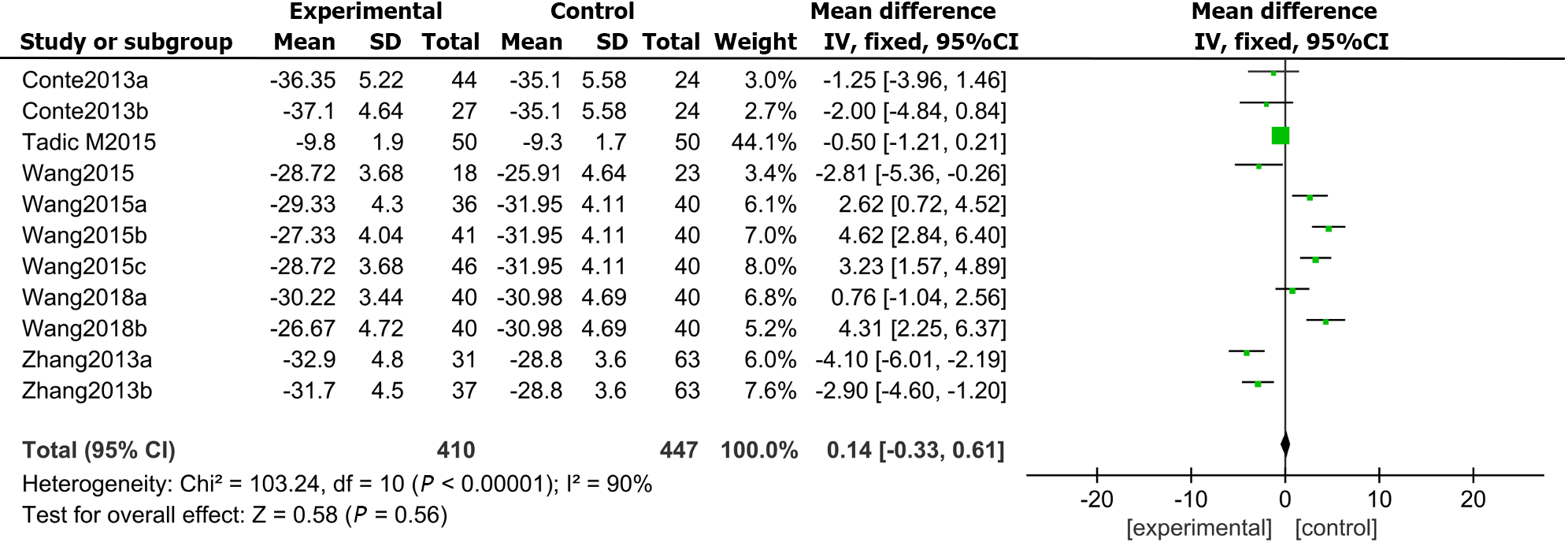
In total, 6 articles compared the GASs measured by 3D-STI between the DM and the control group. The results exposed that GAS was markedly impaired in DMs compared with controls (MD: 0.14, 95%CI: -0.33 to 0.61, P = 0.000; I2 = 90%; Figure 6).
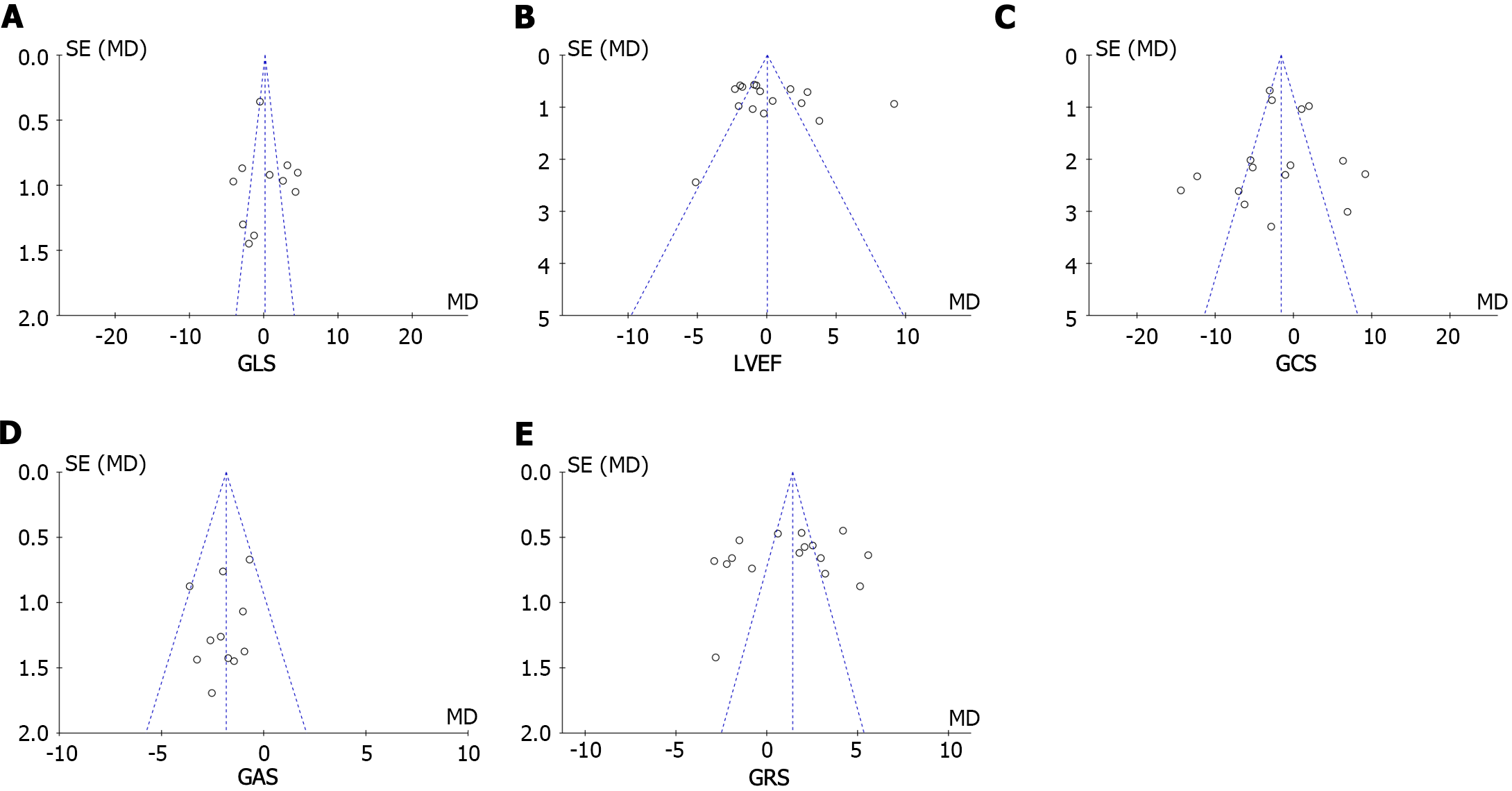
Publication bias was evaluated through Egger’s test for the involved studies (Figure 7). We detected no publication bias in GLS (P = 0.286) or LVEF (P = 0.825). Moreover, there was publication bias for the GRS (P = 0.022), GCS (P = 0.032), and GAS (P = 0.041). The trim-and-fill approach was further employed to find the modified merged values for the GRS, GCS, and GAS. A sensitivity analysis was also carried out to evaluate the stability of the strains. None of the articles confirmed a marked influence on the merged value, implying that the involved publications exhibited worthy stability.
In the early stage of DCM, due to abnormal metabolism of subendothelial cardiomyocytes and more severe hypoxia, myocardial fibers are first affected[13]. When damaged, the strain parameter GLS reflects myocardial mechanics abnormalities[14]. However, due to the inconsistency of the duration of disease and the degree of blood glucose control in different patients, GLS and other left ventricular systolic strain parameters (GCS, GRS, and GAS) can decrease at the same time or successively[15]. Therefore, the application of appropriate and accurate diagnostic tools to detect and monitor myocardial injury is an important aspect of managing DCM patients.
Conventional echocardiography is the most frequently used imaging approach for measuring and evaluating left ventricular function[16]. Evaluation of left ventricular function according to the LVEF has been widely used in the clinic[17]. The use of strain imaging of the myocardium opens a new window for the study of myocardial mechanics. Strain measurement according to the myocardial mechanics of the left ventricle can quickly and effectively evaluate changes in myocardial contractility[18]. Speckle tracking imaging (STI) tracks myocardial tissue signals frame by frame through the principle of “block matching”, without significant displacement between adjacent frames, and can evaluate myocardial motion and quantify changes in cardiac function without angle dependence[19]. With the emergence of 2D-STI, the abovementioned left ventricular myocardial strain parameters can be measured quantitatively[20]. 3D-STI combines real-time three-dimensional echocardiography and STI and can track myocardial tissue signals in three-dimensional space without the limitation of planes, which compensates for the deficiency of 2D-STI[21]. This is the first meta-analysis evaluating the clinical utility of the 3D-STI for assessing LVMCF in patients with DM.
The consensus is that the LVEF is the stroke volume after the standardized change in left ventricular volume. It is a frequently used parameter for the clinical assessment of LVSF. At present, magnetic resonance imaging is considered the gold standard for detecting LVSF[22]. The findings of this meta-analysis implied that there were anomalous alterations in left ventricular myocardial mechanics in DM patients without a significant decrease in LVEF. In addition, 3D-STI can yield parameters such as the GRS, GLS, GAS, GCS, and 3D-strain. Saeedi et al[23] reported that GLS, GRS, GCS and GAS were decreased in DM compared with those in the control group. Baber et al[24] reported that there was no marked difference in GGS or GRS between DMs and controls, but the GLS and GAS were considerably lower than those in controls.
The consequences of our meta-analysis indicated that the GLS, GRS, GCS, and GAS in DMs were decreased compared with those in controls, suggesting that the LVSF in DMs was impaired compared with that in controls. The decreases in GLS and GAS were more obvious, possibly because the left ventricular wall is composed of three layers of myocardial fibers. The subepicardial myocardial fibers are arranged counterclockwise oblique in the direction of the left ventricular longitudinal axis, approximately circular in the middle layer of the ventricular wall, and clockwise in the longitudinal axis to the innermost layer, namely, the subendocardial layer. The diversity of myocardial fiber arrangement determines the complexity of left ventricular three-dimensional motion[25]. GLS represents the ability of the heart to move in the long axis direction and is caused by the contraction of longitudinal muscle fibers under the endocardium. These muscle fibers have the characteristics of strong contractility and high demand for oxygen, which may be the reason why longitudinal strain is more sensitive than that in other directions of the left ventricle during mild hypoperfusion of the subendocardial myocardium in the early stage of diabetes[26]. The GRS and GCS are mainly affected by the contractility of annular fibers in the middle layer of the myocardium. The GAS reflects the rate of change in the endocardial area from the initial area to the area after deformation; this metric is a strain index integrated with longitudinal and circumferential strain, is inversely proportional to the radial strain, and can be regarded as the composite of GLS and GRS[27]. As reported previously, the GAS has the best correlation with LVSF[28]. Wang et al[29] suggested that the GAS could offer a more precise basis for quantifying global and local myocardial function with good reproducibility. Chen et al[30] reported that there was a negative correlation between GAS and GLS and between GAS and hemoglobin A1c, among which GAS had the strongest correlation. Additionally, another study confirmed that the GAS can provide a more accurate basis and good reproducibility for quantifying global and local myocardial function[31].
The present meta-analysis has several limitations. Firstly, the studies included in this meta-analysis included diseases such as hypertension or obesity in DM patients and good or poor control of blood glucose levels in DM patients. Therefore, there might be selection bias. Second, only 8 articles were involved in this study, and the sample size was rather small, so the results might be affected. However, we strictly established the selection criteria for the articles and strictly evaluated the quality of the studies to improve the overall quality of the meta-analysis and the credibility of the results.
In conclusion, the 3D-STI might be used to precisely calculate early LVSF in patients with DM. The measurement of left ventricular strain in DM patients through 3D-STI could estimate the LVSF damage in patients with early diabetes.
Diabetic cardiomyopathy is a chronic complication, which is a critical reason of poor prognosis and even death in patients with diabetes mellitus (DM). Additionally, numerous reports have implied that DM could raise the occurrence of heart disorder, hypertension, and other illnesses, and could worsen coronary artery illness. Therefore, early detection of left ventricular systolic function (LVSF) damage in DM, necessary treatment is especially essential. Three-dimensional speckle tracking technique (3D-STI) is of beneficial worth in the assessment of primary or secondary LVSF. However, the 3D-STI evaluating capability of left ventricular myocardial contractile function (LVMCF) in DM remains uncertain and further reports are needed.
To explore the application value of 3D-STI in assessing LVMCF in DM by meta-analysis.
To investigate the assessment of 3D-STI in estimating early left ventricular systolic dysfunction in DM by meta-analysis. 3D-STI provides a feasible and accurate new technique for clinical measurement of LVSF in left ventricular caused by DM, which might play an important role in the evaluation of cardiac function injury.
We carried out a meta-analysis to evaluate myocardial function in patients with DM compared with controls according to myocardial strain attained by 3D-STI. We searched he PubMed, Embase, Scopus databases, and the Cochrane library from the initial accessible time to 29 April 2023. PRISMA guidelines were used. Data for meta-analysis were pooled using a random-effects model. We extracted data and used the Cochrane “Risk of bias” tool to assess methodological quality. Effect was presented as mean difference with 95% confidence interval using RevMan 5.3. The current study is the first meta-analysis to report that 3D-STI could precisely assess early left ventricular systolic dysfunction in DM.
The findings of this meta-analysis implied that there existed anomalous alterations in left ventricular myocardial mechanics in DM without a significant decrease in LVEF. In addition, 3D-STI could obtain parameters such as GRS, GLS, GAS, GCS, 3D-Strain and so on. Among them, the decrease of GLS and GAS was more obvious, which may be since the left ventricular wall is composed of three layers of myocardial fibers: The subepicardial myocardial fibers are arranged counterclockwise oblique in the direction of the left ventricular longitudinal axis, approximately circular in the middle layer of the ventricular wall, and clockwise in the longitudinal axis to the innermost layer, namely the subendocardial layer.
Our data provided the first evidence for the essential role of 3D-STI in assessing the early left ventricle systolic dysfunction in DM precisely. The assessment of left ventricular strain in DM patients through 3D-STI might estimate the damage of LVSF in DM in the early stage.
We believe that with the continuous improvement of computer and three-dimensional ultrasound technology, the shortcomings will be overcome, and 3D-STI is expected to become the gold standard for clinical non-invasive determination of LVSF.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Crowther CA, New Zealand; Phoswa WN, South Africa; Wu QN, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Tomkins M, Lawless S, Martin-Grace J, Sherlock M, Thompson CJ. Diagnosis and Management of Central Diabetes Insipidus in Adults. J Clin Endocrinol Metab. 2022;107:2701-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Arow M, Waldman M, Yadin D, Nudelman V, Shainberg A, Abraham NG, Freimark D, Kornowski R, Aravot D, Hochhauser E, Arad M. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 2020;19:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 3. | Wang L, Cai Y, Jian L, Cheung CW, Zhang L, Xia Z. Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc Diabetol. 2021;20:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Tadic M, Ilic S, Cuspidi C, Stojcevski B, Ivanovic B, Bukarica L, Jozika L, Celic V. Left Ventricular Mechanics in Untreated Normotensive Patients with Type 2 Diabetes Mellitus: A Two- and Three-dimensional Speckle Tracking Study. Echocardiography. 2015;32:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Wang Q, Gao Y, Tan K, Xia H, Li P. Assessment of left ventricular function by three-dimensional speckle-tracking echocardiography in well-treated type 2 diabetes patients with or without hypertension. J Clin Ultrasound. 2015;43:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Wei X, Liang Y, Liu M, Li C, Tang H. Differential changes of left ventricular myocardial deformation in diabetic patients with controlled and uncontrolled blood glucose: a three-dimensional speckle-tracking echocardiography-based study. J Am Soc Echocardiogr. 2013;26:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Enomoto M, Ishizu T, Seo Y, Kameda Y, Suzuki H, Shimano H, Kawakami Y, Aonuma K. Myocardial dysfunction identified by three-dimensional speckle tracking echocardiography in type 2 diabetes patients relates to complications of microangiopathy. J Cardiol. 2016;68:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Wang Q, Gao Y, Tan K, Li P. Subclinical impairment of left ventricular function in diabetic patients with or without obesity: A study based on three-dimensional speckle tracking echocardiography. Herz. 2015;40 Suppl 3:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Yang QM, Fang JX, Chen XY, Lv H, Kang CS. The Systolic and Diastolic Cardiac Function of Patients With Type 2 Diabetes Mellitus: An Evaluation of Left Ventricular Strain and Torsion Using Conventional and Speckle Tracking Echocardiography. Front Physiol. 2021;12:726719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Conte L, Fabiani I, Barletta V, Bianchi C, Maria CA, Cucco C, De Filippi M, Miccoli R, Prato SD, Palombo C, Di Bello V. Early Detection of Left Ventricular Dysfunction in Diabetes Mellitus Patients with Normal Ejection Fraction, Stratified by BMI: A Preliminary Speckle Tracking Echocardiography Study. J Cardiovasc Echogr. 2013;23:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Abomandour HG, Elnagar AM, Aboleineen MW, Shehata IE. Subclinical Impairment of Left Ventricular Function assessed by Speckle Tracking in Type 2 Diabetic Obese and Non-Obese Patients: Case Control Study. J Cardiovasc Echogr. 2022;32:95-106. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 12. | Wang Q, Fu C, Xia H, Gao Y. Aggravating effect of obstructive sleep apnoea on left ventricular remodelling and function disorder in patients with type 2 diabetes mellitus: a case-control study by 3D speckle tracking echocardiography. Acta Cardiol. 2022;77:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Dillmann WH. Diabetic Cardiomyopathy. Circ Res. 2019;124:1160-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 478] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 14. | Wan H, Zhao S, Zeng Q, Tan Y, Zhang C, Liu L, Qu S. CircRNAs in diabetic cardiomyopathy. Clin Chim Acta. 2021;517:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Zhan J, Chen C, Wang DW, Li H. Hyperglycemic memory in diabetic cardiomyopathy. Front Med. 2022;16:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Niemann M, Herrmann S, Ertl G, Weidemann F. [Echocardiography in diabetic cardiomyopathy]. Herz. 2013;38:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Külahçıoğlu Ş, Karagöz IK, Bilen Y, Kültürsay B, Akbaş RB, Yücel E, Tokgöz HC, Uslu A, Karagöz A, Kaymaz C. Evaluation of the relationship between diabetic retinopathy and left atrial deformation parameters. Egypt Heart J. 2022;74:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Bogo MA, Pabis JS, Bonchoski AB, Santos DCD, Pinto TJF, Simões MA, Silva JC, Pabis FC. Cardiomyopathy and cardiac function in fetuses and newborns of diabetic mothers. J Pediatr (Rio J). 2021;97:520-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Feng J, Zhai Z, Wang Z, Huang L, Dong S, Liu K, Shi W, Lu G, Qin W. Speckle tracking imaging combined with myocardial comprehensive index to evaluate left ventricular function changes in patients with systemic lupus erythematosus. Echocardiography. 2021;38:1632-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Morariu VI, Arnautu DA, Morariu SI, Popa AM, Parvanescu T, Andor M, Abhinav S, David VL, Ionescu A, Tomescu MC. 2D speckle tracking: a diagnostic and prognostic tool of paramount importance. Eur Rev Med Pharmacol Sci. 2022;26:3903-3910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Yu Z, Pan H, Cheng Z, Lu K, Hu H. Evaluation of Left Ventricular Systolic Function in Patients with Coronary Microvascular Dysfunction by Three-Dimensional Speckle-Tracking Imaging. Braz J Cardiovasc Surg. 2022;37:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Kiko T, Yoshihisa A, Yokokawa T, Misaka T, Yamada S, Kaneshiro T, Nakazato K, Takeishi Y. Direct comparisons of left ventricular volume and function by simultaneous cardiac magnetic resonance imaging and gated 13N-ammonia positron emission tomography. Nucl Med Commun. 2020;41:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Saeedi M, Hadjiakhondi A, Nabavi SM, Manayi A. Heterocyclic Compounds: Effective α-Amylase and α-Glucosidase Inhibitors. Curr Top Med Chem. 2017;17:428-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Baber U, Stefanini GG, Giustino G, Stone GW, Leon MB, Sartori S, Aquino M, Steg PG, Windecker S, Wijns W, Serruys PW, Valgimigli M, Morice MC, Camenzind E, Weisz G, Smits PC, Kandzari DE, von Birgelen C, Dangas GD, Galatius S, Jeger RV, Kimura T, Mikhail GW, Itchhaporia D, Mehta L, Ortega R, Kim HS, Kastrati A, Chieffo A, Mehran R. Impact of Diabetes Mellitus in Women Undergoing Percutaneous Coronary Intervention With Drug-Eluting Stents. Circ Cardiovasc Interv. 2019;12:e007734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | El-Naggar HM, Osman AS, Ahmed MA, Youssef AA, Ahmed TAN. Three-dimensional echocardiographic assessment of left ventricular geometric changes following acute myocardial infarction. Int J Cardiovasc Imaging. 2023;39:607-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc Imaging. 2018;11:260-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 504] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 27. | Wang YB, Huang H, Lin S, Hao MJ, He LJ, Liu K, Bi XJ. Evaluation of Left Ventricular Function by Three-Dimensional Speckle-Tracking Echocardiography in Patients with Chronic Kidney Failure. Curr Med Sci. 2022;42:895-901. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Chisholm RH, Sonenberg N, Lacey JA, McDonald MI, Pandey M, Davies MR, Tong SYC, McVernon J, Geard N. Epidemiological consequences of enduring strain-specific immunity requiring repeated episodes of infection. PLoS Comput Biol. 2020;16:e1007182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Wang Q, Fu C, Xia H, Gao Y. Elevated Plasma Homocysteine Level Associated with Further Left Ventricular Structure and Function Damages in Type 2 Diabetic Patients: A Three-Dimensional Speckle Tracking Echocardiography Study. Metab Syndr Relat Disord. 2021;19:443-451. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Chen X, Guo H, Yang Q, Fang J, Kang X. Quantitative evaluation of subclinical left ventricular dysfunction in patients with type 2 diabetes mellitus by three-dimensional echocardiography. Int J Cardiovasc Imaging. 2020;36:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Chang TW, Hsu HC, Tsai WC. Association of left ventricular global area strain derived from resting 3D speckle-tracking echocardiography and exercise capacity in individuals undergoing treadmill exercise test. Int J Med Sci. 2022;19:1576-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |