Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.623
Peer-review started: December 28, 2023
First decision: January 20, 2024
Revised: January 29, 2024
Accepted: March 6, 2024
Article in press: March 6, 2024
Published online: April 15, 2024
Processing time: 105 Days and 7.3 Hours
Youth-onset type 2 diabetes mellitus (T2DM), influenced by an increase in obesity, is a rising problem worldwide. Pathophysiological mechanisms of this early-onset T2DM include both peripheral and hepatic insulin resistance, along with increa
Core Tip: Youth-onset type 2 diabetes mellitus (T2DM) is a growing health problem. The incidence of youth-onset T2DM is especially high in overweight/obese individuals, in some ethnic groups with higher preponderance in female sex. Due to more aggressive course of T2DM in youth and earlier development of chronic complications, the stricter metabolic control targeting both glycemia and other vascular risk factors might be necessary. Newer agents with cardiovascular benefits and weight losing potential might become increasingly important in treatment of youth-onset T2DM. Moreover, future studies should focus on different approaches according to gender and use of new technologies in glucose monitoring.
- Citation: Krnic N, Sesa V, Mrzljak A, Berkovic MC. Are treatment options used for adult-onset type 2 diabetes mellitus (equally) available and effective for children and adolescents? World J Diabetes 2024; 15(4): 623-628
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/623.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.623
Youth-onset type 2 diabetes mellitus (T2DM) is increasing in incidence and prevalence. There is a strong predilection for its development in certain ethnic groups[1] and evermore so, the development of T2DM is related to the cumulative effect of early onset persistent obesity[2]. Despite male predominance among adolescents with prediabetes[3], there is a higher prevalence of T2DM in females[4]. Due to specific and unfavorable metabolic profiles encompassing insulin resistance and early β-cell decline, youth-onset T2DM is often accompanied by chronic end-stage complications, which become apparent much earlier in life[4,5]. Especially important are cardiovascular complications, which are not only a consequence of early-onset hyperglycemia but also of accompanying comorbidities, making treatment decisions difficult[6]. Currently, only a few therapeutic agents gained approval from the regulatory agencies for the treatment of youth-onset T2DM. Decision on their use and combinations often depends on the presenting clinical features, hemoglobin A1c (HbA1c) and hyperglycemia level, and the presence of catabolism (diabetic ketoacidosis or hyperosmolar hyperglycemic state). Moreover, it also depends on the long-term efficacy and, in the newer time, due to the availability of agents with cardiovascular and renal benefits, on the patient’s risk stratification and presence of comorbidities. Although there is a female gender predilection for T2DM development and a tendency for resolution of dysglycemia in male patients following puberty[7], there are no recommendations for different treatment approaches in male and female patients. Most treatment practice for youth-onset T2DM is derived from adult patients. However, they do not necessarily have the same pathophysiological features and therapeutic effects might not be the same as in adults. The aim of treatment in youth with T2DM is agreed at HbA1c < 7.0%[8], but several studies demonstrated a more aggressive course of disease in younger patients, suggesting stricter metabolic control might be necessary[9]. All available treatment modalities have comparable glucose-lowering effects[8] of HbA1c reduction by 1%-2%, but no data regarding favorable effects on other metabolic complications of T2DM and obesity. Furthermore, pharmacological and nonpharmacological therapy failure is more common in adolescents (first-line treatment with metformin and intensive lifestyle changes are suboptimal in more than 50% of youth within 2 years of diagnosis), necessitating the need for treatment intensification[10,11].
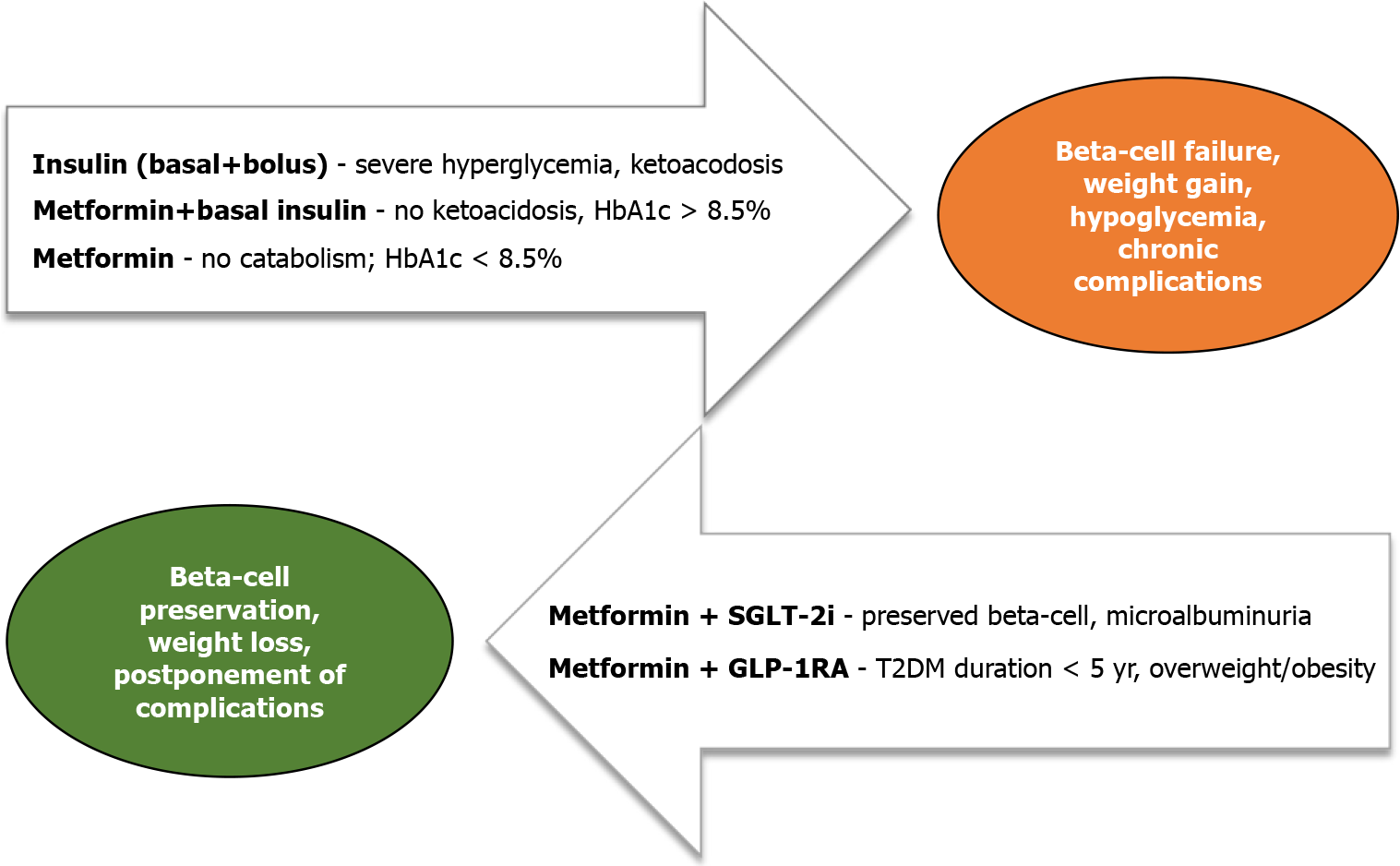
Metformin remains the pharmacological cornerstone of T2DM treatment in children and adolescents with T2DM, according to international guidelines[8,12]. It is advised as a first-line treatment in case of newly diagnosed T2DM in children and youth who present without ketoacidosis or hyperosmolar hyperglycemic state and if HbA1c < 8.5%. In contrast, it can be combined with basal insulin as a starting therapy when HbA1c exceeds 8.5%, with no signs of hypoinsulinemia[13].
Improvement in HbA1c ranges from 1 to 2%, but metabolic effects also include improved insulin sensitivity and potential beneficial effects on cardiovascular function, while the effects on reduction of body weight and waist circumference are limited[14]. In adults, metformin increases insulin sensitivity and delays β-cell decline. Compared to adults, metformin therapy does not favor residual β-cell function in children and adolescents[15], presumably due to more apparent hepatic insulin resistance earlier in the disease course[16]. Therefore, there is no current data to support the use of metformin in adolescents with prediabetes for the prevention of T2DM development[17]. Moreover, expectations of its long-term effects on glucose lowering in youth-onset T2DM alone or in combination with lifestyle interventions or insulin are modest, while its modulation of the entero-insular axis via gut microbiome is of a neglectable size. In addition, up to date, there is no evidence of its effect on reducing visceral or hepatic fat or lipolysis in children and adolescents with T2DM[11] (Figure 1).
Rosiglitazone was evaluated in youth with T2DM as an add-on therapy to metformin[18], with superior improvement in insulin sensitivity and fewer adverse effects[19] as compared to metformin alone, but so far, it has not gained approval for T2DM treatment in children and adolescents. In addition, rosiglitazone has been strongly restricted or even withdrawn from the market in most countries due to concerns about its cardiovascular safety; therefore, its use in adult T2DM is limited[20]. On the other hand, pioglitazone, yet another thiazolidinedione, has an important role in the treatment of adult T2DM patients with metabolic syndrome and cardiovascular risk, but its use in youth-onset T2DM is scarce and not approved[21].
Several therapy modalities using different types of insulin are used in the treatment of youth-onset T2DM. Insulin can be used as initial therapy in severe presentation of T2DM (ketosis/ketoacidosis or HbA1c > 8.5%)[8] or additional therapy if other therapeutic options fail to achieve improvement in metabolic control. However, insulin can lead to weight gain, which can perpetuate obesity and subsequent metabolic complications. The addition of insulin to youth with T2DM already treated with different treatment modalities (metformin alone/metformin + rosiglitazone/metformin + lifestyle interventions) led to variable therapeutic effects (only 33.2% of participants had consistent HbA1c decrease of ≥ 0.5%)[22], in addition to the need of treatment intensification with more complex regimens (basal bolus)[13]. Insulin is advised primarily in the form of multiple daily injections. At the same time, there are no studies done on youth-onset T2DM with insulin delivered via pump therapy with hybrid closed-loop technology, although data from adult T2DM are promising[23].
Glucagon-like polypeptide 1 receptor agonists (GLP-1RA), liraglutide, exenatide and dulaglutide, once daily or once weekly, have recently been approved for the treatment of T2DM in children and adolescents. The therapeutic benefit includes glucose-lowering effect (HbA1c lowering by 0.85% to 1.4% after 24 to 26 wk of therapy), while effects on body mass index and waist circumference are less pronounced than that seen in the adult population with T2DM and potentially require more prolonged duration of treatment (more than 52 wk)[24-26]. Moreover, a rebound is seen after discontinuation of treatment[27-29]. Nonetheless, one might speculate that early introduction of GLP-1RAs, in combination with metformin, might be beneficial for youth-onset T2DM, especially in case of early-stage disease, lasting for less than 5 years, while it would improve β-cell function, in addition to targeting insulin resistance and obesity.
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i), empagliflozin, has been approved for treating children and adolescents with T2DM since June 2023, alone or in combination with metformin[29]. The reduction in HbA1c of 1.13% was demonstrated in clinical trials, and similar to GLP-1RAs, the weight-lowering effect is minimal compared to the one seen in adults with T2DM[30]. Although no data is available in youth with T2DM, it could be assumed that beneficial effect on cardiovascular function and renal disease, as seen in adults, would also be demonstrated in youth with T2DM[31,32]. SGLT-2i promote urinary glucose excretion with compensatory increases in rates of gluconeogenesis and ketosis. How this transcribes in glucose management of young-onset T2DM is to be seen, while there is a defect in gluconeogenesis regulation. Moreover, the problem of euglycemic ketosis must not be neglected.
As compared to the frequent use of different technological devices among children and adolescents with type 1 diabetes, there are no current recommendations for similar use among youth with T2DM. Some studies have proven that continuous or intermittent glucose monitoring devices (CGM) are beneficial for lifestyle modification interventions[33]. Furthermore, it was demonstrated that greater fasting glucose variability during the first year following the diagnosis of T2DM is highly predictive of deterioration of β-cell function and development of comorbidities in following years[34]. CGM might help to detect those patients and intensify their treatment in the early phase of T2DM. The indications for using devices for CGM with different types of treatment or cost-effectiveness have not yet been established, and health insurance policies do not cover expenses in most countries.
Metabolic surgery is becoming an important tool in treating obesity in youth with type 2 diabetes, although numbers of treated patients are rather small and data on long-term safety and durability of treatment success still lacking[35]. The most commonly used methods Roux-en-Y gastric bypass, and vertical sleeve gastrectomy are highly effective, and weight reduction up to 73% is reported, together with T2DM remission after 3-years of follow-up, which is similar to that observed in adult T2DM population[36]. Timing of metabolic surgery is an important issue, and is still debatable, but many authorities argue that intervention earlier during adolescence, would be more beneficial in terms of offering comprehensive metabolic remission and fewer and/or postponed vascular complications in the future[37]. Finally, meticulous surveillance and strong family support is necessary to prevent nutritional deficiencies and potential regain of weight after bariatric surgery[38,39].
Although pharmacotherapy of adult-onset T2DM is diverse and efficient both in glucose management and offering cardiovascular and renovascular benefits, currently, there are only four medication classes for youth-onset T2DM: Historical agents such as metformin, insulin, and newer GLP-1RAs (liraglutide, exenatide and dulaglutide) and SGLT-2i empagliflozin. The poor long-term durability of metformin and insulin with the risk of hypoglycemia, weight gain and no potential for β-cell preservation might lead to a paradigm shift and more robust use of agents beyond glycemic effects. Whether their efficiency and long-term safety will be comparable to that seen in adult T2DM and the preferable timing and best combinations for youth-onset T2DM remain to be seen.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK, India; Zhu L, China S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Perng W, Conway R, Mayer-Davis E, Dabelea D. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care. 2023;46:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (1)] |
| 2. | Owora AH, Allison DB, Zhang X, Gletsu-Miller N, Gadde KM. Risk of Type 2 Diabetes Among Individuals with Excess Weight: Weight Trajectory Effects. Curr Diab Rep. 2022;22:471-479. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005-2016. JAMA Pediatr. 2020;174:e194498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 4. | Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, Marcovina SM, Mayer-Davis EJ, Hamman RF, Dolan L, Dabelea D, Pettitt DJ, Liese AD; SEARCH for Diabetes in Youth Study Group. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA. 2021;326:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 372] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 5. | Savic Hitt TA, Katz LEL. Pediatric Type 2 Diabetes: Not a Mini Version of Adult Type 2 Diabetes. Endocrinol Metab Clin North Am. 2020;49:679-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2648-2668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 7. | Mehreen TS, Kamalesh R, Pandiyan D, Kumar DS, Anjana RM, Mohan V, Ranjani H. Incidence and Predictors of Dysglycemia and Regression to Normoglycemia in Indian Adolescents and Young Adults: 10-Year Follow-Up of the ORANGE Study. Diabetes Technol Ther. 2020;22:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, Chang N, Fu J, Dabadghao P, Pinhas-Hamiel O, Urakami T, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:872-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 9. | TODAY Study Group; Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 320] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 10. | Yen FS, Wei JC, Liu JS, Hsu CC, Hwu CM. Clinical course of adolescents with type 2 diabetes mellitus: A nationwide cohort study in Taiwan. J Diabetes Investig. 2022;13:1905-1913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chung ST, Davis F, Patel T, Mabundo L, Estrada DE. Reevaluating First-line Therapies in Youth-Onset Type 2 Diabetes. J Clin Endocrinol Metab. 2024;109:e870-e872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65:1925-1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 484] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 13. | Hitt TA, Hannon TS, Magge SN. Approach to the Patient: Youth-Onset Type 2 Diabetes. J Clin Endocrinol Metab. 2023;109:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hosey CM, Halpin K, Yan Y. Considering metformin as a second-line treatment for children and adolescents with prediabetes. J Pediatr Endocrinol Metab. 2022;35:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | RISE Consortium; RISE Consortium Investigators. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on β-Cell Function: Comparison of Responses In Youth And Adults. Diabetes. 2019;68:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care. 2018;41:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Magge SN, Silverstein J, Elder D, Nadeau K, Hannon TS. Evaluation and Treatment of Prediabetes in Youth. J Pediatr. 2020;219:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 19. | TODAY Study Group. Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care. 2013;36:1765-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Xu B, Xing A, Li S. The forgotten type 2 diabetes mellitus medicine: rosiglitazone. Diabetol Int. 2022;13:49-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | de Jong M, van der Worp HB, van der Graaf Y, Visseren FLJ, Westerink J. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc Diabetol. 2017;16:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Bacha F, El Ghormli L, Arslanian S, Zeitler P, Laffel LM, Levitt Katz LE, Gandica R, Chang NT, Sprague JE, Macleish SA; TODAY Study Group. Predictors of response to insulin therapy in youth with poorly-controlled type 2 diabetes in the TODAY trial. Pediatr Diabetes. 2019;20:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Karol AB, O'Malley G, Fallurin R, Levy CJ. Automated Insulin Delivery Systems as a Treatment for Type 2 Diabetes Mellitus: A Review. Endocr Pract. 2023;29:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T; Ellipse Trial Investigators. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019;381:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 25. | Tamborlane WV, Bishai R, Geller D, Shehadeh N, Al-Abdulrazzaq D, Vazquez EM, Karoly E, Troja T, Doehring O, Carter D, Monyak J, Sjöström CD. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care. 2022;45:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Pérez M, Bismuth E, Dib S, Cho JI, Cox D; AWARD-PEDS Investigators. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med. 2022;387:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 27. | Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, Mastrandrea LD, Prabhu N, Arslanian S; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N Engl J Med. 2020;382:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 28. | Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes Rev. 2021;22:e13177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, Neubacher D, Marquard J; DINAMO Study Group. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023;11:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 30. | Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, Karlsson C, Norjavaara E. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 31. | Clark BC, Arnold WD. Strategies to Prevent Serious Fall Injuries: A Commentary on Bhasin et al A Randomized Trial of a Multifactorial Strategy to Prevent Serious Fall Injuries. N Engl J Med. 2020;383(2):129-140. Adv Geriatr Med Res. 2021;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4445] [Article Influence: 740.8] [Reference Citation Analysis (0)] |
| 33. | Manfredo J, Lin T, Gupta R, Abiola K, West M, Busin K, Tracey J, Brown EA, Magge SN, Wolf RM. Short-term use of CGM in youth onset type 2 diabetes is associated with behavioral modifications. Front Endocrinol (Lausanne). 2023;14:1182260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | TODAY Study Group. Long-term Outcomes Among Young Adults With Type 2 Diabetes Based on Durability of Glycemic Control: Results From the TODAY Cohort Study. Diabetes Care. 2022;45:2689-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Stefater MA, Inge TH. Bariatric Surgery for Adolescents with Type 2 Diabetes: an Emerging Therapeutic Strategy. Curr Diab Rep. 2017;17:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, Harmon CM, Zeller MH, Chen MK, Xanthakos SA, Horlick M, Buncher CR; Teen-LABS Consortium. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 509] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 37. | Shenoy A, Schulman AR. Advances in endobariatrics: past, present, and future. Gastroenterol Rep (Oxf). 2023;11:goad043. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Xanthakos SA, Khoury JC, Inge TH, Jenkins TM, Modi AC, Michalsky MP, Chen MK, Courcoulas AP, Harmon CM, Brandt ML, Helmrath MA, Kalkwarf HJ; Teen Longitudinal Assessment of Bariatric Surgery Consortium. Nutritional Risks in Adolescents After Bariatric Surgery. Clin Gastroenterol Hepatol. 2020;18:1070-1081.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 39. | Anekwe CV, Knight MG, Seetharaman S, Dutton WP, Chhabria SM, Stanford FC. Pharmacotherapeutic options for weight regain after bariatric surgery. Curr Treat Options Gastroenterol. 2021;19:524-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |