Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.463
Peer-review started: October 28, 2023
First decision: December 29, 2023
Revised: January 2, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 15, 2024
Processing time: 138 Days and 12.8 Hours
Sodium glucose cotransporter-2 inhibitors (SGLT-2i) are a class of drugs with modest antidiabetic efficacy, weight loss effect, and cardiovascular benefits as proven by multiple randomised controlled trials (RCTs). However, real-world data on the comparative efficacy and safety of individual SGLT-2i medications is sparse.
To study the comparative efficacy and safety of SGLT-2i using real-world clinical data.
We evaluated the comparative efficacy data of 3 SGLT-2i drugs (dapagliflozin, canagliflozin, and empagliflozin) used for treating patients with type 2 diabetes mellitus. Data on the reduction of glycated hemoglobin (HbA1c), body weight, blood pressure (BP), urine albumin creatinine ratio (ACR), and adverse effects were recorded retrospectively.
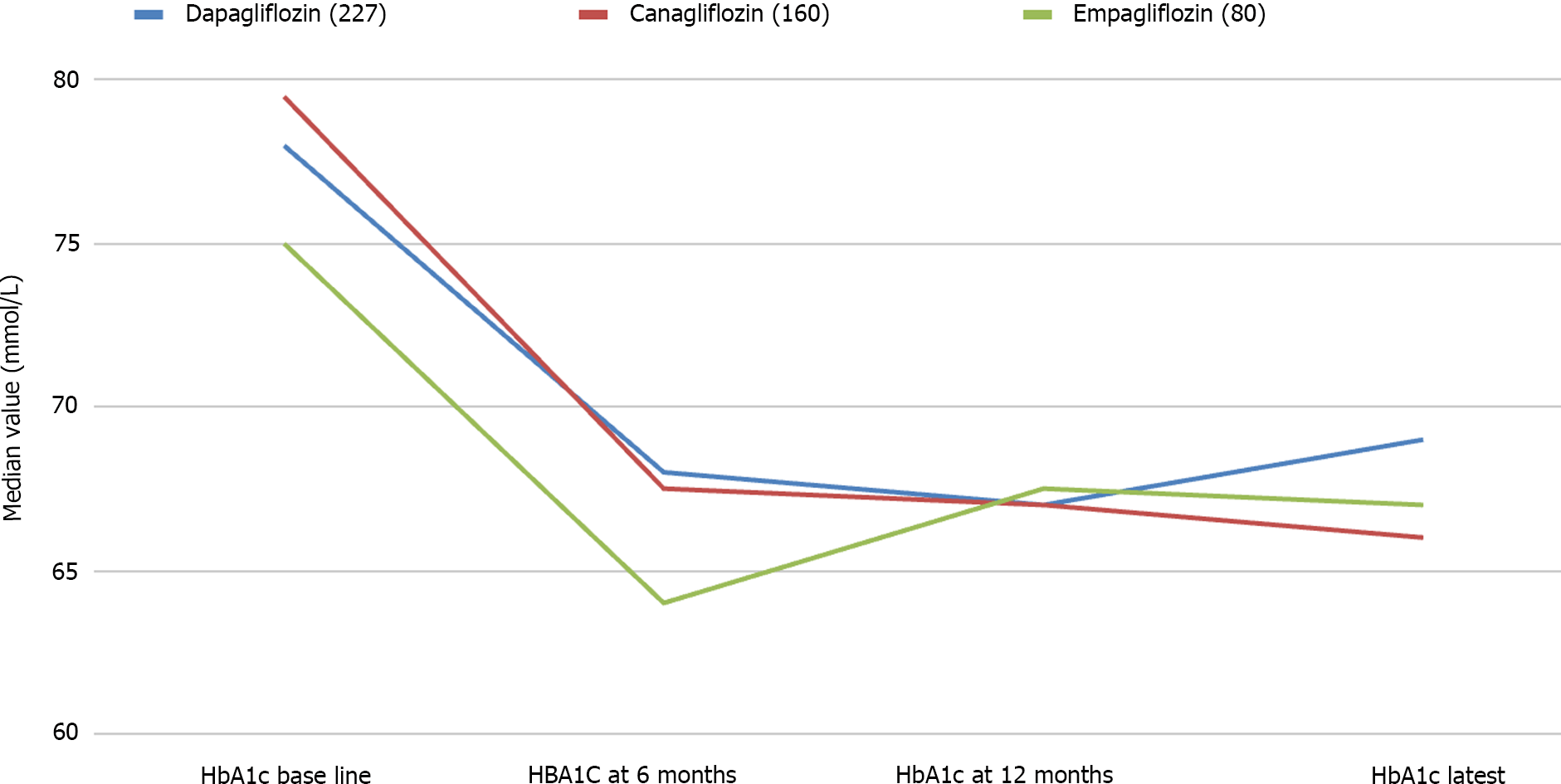
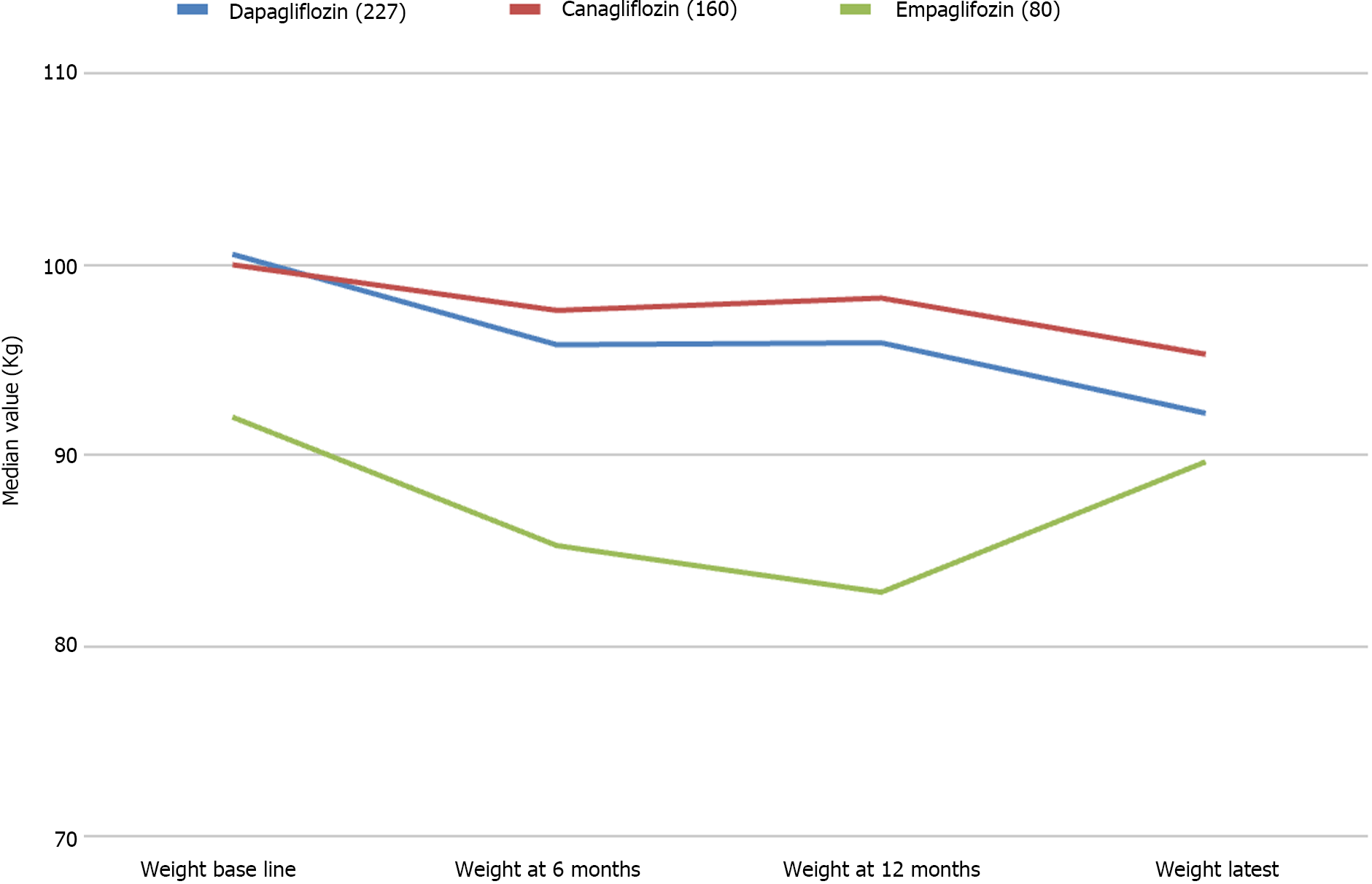
Data from 467 patients with a median age of 64 (14.8) years, 294 (62.96%) males and 375 (80.5%) Caucasians were analysed. Median diabetes duration was 16.0 (9.0) years, and the duration of SGLT-2i use was 3.6 (2.1) years. SGLT-2i molecules used were dapagliflozin 10 mg (n = 227; 48.6%), canagliflozin 300 mg (n = 160; 34.3%), and empagliflozin 25 mg (n = 80; 17.1). Baseline median (interquartile range) HbA1c in mmol/mol were: dapagliflozin - 78.0 (25.3), canagliflozin - 80.0 (25.5), and empagliflozin - 75.0 (23.5) respectively. The respective median HbA1c reduction at 12 months and the latest review (just prior to the study) were: 66.5 (22.8) & 69.0 (24.0), 67.0 (16.3) & 66.0 (28.0), and 67.0 (22.5) & 66.5 (25.8) respectively (P < 0.001 for all comparisons from baseline). Significant improvements in body weight (in kilograms) from baseline to study end were noticed with dapagliflozin - 101 (29.5) to 92.2 (25.6), and canagliflozin 100 (28.3) to 95.3 (27.5) only. Significant reductions in median systolic and diastolic BP, from 144 (21) mmHg to 139 (23) mmHg; (P = 0.015), and from 82 (16) mmHg to 78 (19) mmHg; (P < 0.001) respectively were also observed. A significant reduction of microalbuminuria was observed with canagliflozin only [ACR 14.6 (42.6) at baseline to 8.9 (23.7) at the study end; P = 0.043]. Adverse effects of SGLT-2i were as follows: genital thrush and urinary infection - 20 (8.8%) & 17 (7.5%) with dapagliflozin; 9 (5.6%) & 5 (3.13%) with canagliflozin; and 4 (5%) & 4 (5%) with empagliflozin. Diabetic ketoacidosis was observed in 4 (1.8%) with dapagliflozin and 1 (0.63%) with canagliflozin.
Treatment of patients with SGLT-2i is associated with statistically significant reductions in HbA1c, body weight, and better than those reported in RCTs, with low side effect profiles. A review of large-scale real-world data is needed to inform better clinical practice decision making.
Core Tip: Treatment of type 2 diabetes mellitus with sodium glucose cotransporter-2 inhibitors (SGLT-2i) is associated with significant glycated hemoglobin (HbA1c) reduction, body weight loss, and cardiovascular benefits as proven by multiple randomised controlled trials (RCTs). Our real-world data analysis of the efficacy and safety of individual SGLT-2i revealed better reduction of HbA1c with dapagliflozin, canagliflozin, and empagliflozin, while better body weight reduction was seen only with dapagliflozin and canagliflozin when compared to RCTs. Blood pressure reduction, and side effect profiles were comparable to previous studies. A significant improvement of albuminuria was obvious only with canagliflozin, presumably because of the low number of participants in this study. Analyses of various large-scale real-world data are expected to inform better clinical practice decision making in future.
- Citation: Islam L, Jose D, Alkhalifah M, Blaibel D, Chandrabalan V, Pappachan JM. Comparative efficacy of sodium glucose cotransporter-2 inhibitors in the management of type 2 diabetes mellitus: A real-world experience. World J Diabetes 2024; 15(3): 463-474
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/463.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.463
Sodium glucose cotransporter-2 inhibitors (SGLT-2i) belong to a class of new antidiabetic drugs with mainly extra-pancreatic mechanism of actions to control hyperglycemia predominantly by enhancing renal glycosuria. Even in nondiabetic individuals, these agents can cause a daily loss of about 80 g of glucose through the kidneys, which is much higher in individuals with hyperglycemia, and therefore, SGLT-2i possess moderate antidiabetic efficacy[1]. Glycosuria also results in a net body energy deficit (the reason why SGLT-2i causes weight loss), and osmotic diuresis as consequence - the additional benefits of these medications. The weight loss benefit makes this class of drugs an attractive treatment option for patients with type 2 diabetes mellitus (T2DM) and diabesity - diabetes because of obesity[2,3]. Moderate improvement in glycemic control with a reduction in glycated hemoglobin (HbA1c) is seen with the use of these drugs[1-11]. Multiple randomised controlled trials (RCTs) and meta-analyses have proven that SGLT-2i use is associated with cardiovascular benefits not only for primary prevention but also for the management of established cardiovascular disease[4-11]. Multiple studies also revealed the remarkable nephroprotective effects of this class of medications[10-14].
Although there are multiple clinical trials and prospective cohort studies examining the cardiovascular, renal, and diabesity outcomes of individual SGLT-2i molecules, making them drugs of choice in day-to-day management of patients with T2DM and multiple comorbidities related to diabesity, the comparative data on the efficacy of each molecule are rather sparse in the global scientific literature. As clinical trial and prospective cohort study settings are appropriately supervised and often rigorously controlled by the study teams, the data captured by these methods may not always reflect the actual real-world clinic picture when we consider the study outcomes. Therefore, we should have adequate real-world data from our own day-to-day clinical practice to truly appraise the actual long-term benefits of SGLT-2i and to help therapeutic decision making for our T2DM patients with multi-comorbidities. The current study was designed to suit this purpose in a large academic teaching hospital in the United Kingdom.
This is a retrospective clinical study by review of the data of all patients treated with any one of the SGLT-2i drug, empagliflozin, canagliflozin, or dapagliflozin between 1st July 2016 and 30th June 2022 at Lancashire Teaching Hospitals NHS Trust. The diabetes service of this hospital provides comprehensive diabetic care for patients in the Central Lancashire and South Cumbria regions of the United Kingdom, catering a population of about 0.4 million people. The study was approved by the institutional research committee (No: KB/PB/SE-387/2022).
The standard practice of using the SGLT-2i molecules in the hospital was to start with lower doses (daily dose of dapagliflozin at 5 mg, empagliflozin at 10 mg and canagliflozin at 100 mg) and subsequently to increase the dose to full strength of the drug (dapagliflozin 10 mg, empagliflozin 25 mg, and canagliflozin 300 mg, respectively) after a month with monitoring of renal functions just prior to the dose increment.
All adult patients (age ≥ 18 years) with T2DM treated by one of the above three SGLT-2i molecule for management of their diabetes were considered for inclusion in the study.
Electronic records were searched for all patients with a diagnosis of DM treated with antidiabetic medications of the SGLT-2i class during the study period. The total number of cases in this category was further reviewed for inclusion in the study.
Patients with a diagnosis of T2DM managed with one of the SGLT-2i. Participants with predefined primary outcome measures (HbA1c alteration from baseline values at follow-up) and/or secondary outcomes such as alterations in body weight ± body mass index (BMI), blood pressure (BP), renal functions, and urine albumin creatinine ratio (ACR) - all these outcomes with meaningful data in at least one of the follow-up period (6 months, 12 months and/or at the last follow up just prior to the study), and/or the development of adverse events [such as genital thrush, urinary tract infections (UTI) or diabetic ketoacidosis (DKA)].
Patients with T1DM. Incomplete study data to obtain meaningful outcome measures as specified above. Follow up duration less than 6 months. SGLT-2i commenced primarily for patients for other indications (such as heart failure or CKD) without T2DM or diet controlled T2DM. Discontinuation of SGLT-2i within 6 months of initiation for any reason including major adverse events such as DKA, recurrent genital thrush, allergy reactions or UTI.
Two junior doctors, under the supervision of a senior academic, recorded the relevant study information using a Microsoft Exel data sheet by entering the demographic, clinical, biochemical, and follow-up data of all study subjects from their electronic case records retrieved with the help of a senior data scientist of the hospital.
Initially, each quantitative trait was tested for normality of distribution using the Shapiro-Wilk normality test. Continuous variables were expressed as median and interquartile range (IQR), and categorical variables as numbers and percentages. The nonparametric Wilcoxon signed-rank test was used for within-group quantitative differences, whereas the two-tailed Fisher’s exact test was used to compare proportions. Spearman’s rank correlation analysis was used to explore the correlation between the safety and efficacy of SGLT-2 inhibitors with clinical and biochemical parameters. Kruskal Wallis nonparametric test was used to compare the relationship between continuous variables in different SGLT-2i drugs. The χ2 test is used for finding the association between two categorical variables. A P-value of < 0.05 (two-tailed) was considered statistically significant. Data were analyzed with Jamovi Software, Version 2.3.21.0 (based on the R program) and BlueSky Statistics Version 10.0, R package version 8.0.
Among a total of 562 patients treated with SGLT-2i during the study period, 467 T2DM patients had meaningful data for analysis of primary and or secondary outcomes. Table 1 shows the main clinical and biochemical characteristics of these subjects. Out of these patients, 160 (34.3%) were on canagliflozin, 227 (48.6%) on dapagliflozin, and 80 (17.1%) on empagliflozin. All these patients were using the full standard recommended doses of individual SGLT-2i molecules.
| Baseline features | n (%) or median (IQR) |
| Female gender | 173 (37.04%) |
| Age (yr) | 64 (14.8) |
| Ethnicity | |
| British white | 338 (72.37%) |
| Diabetes duration (yr) | 16 (9) |
| Diabetes duration ≥ 10 yr | 335 (71.73%) |
| Years of treatment with SGLT2i | 3.6 (2.1) |
| Body weight (kg) | 99 (29) |
| BMI (kg/m2) | 34 (7.58) |
| BP (mmHg) | |
| Systolic | 144 (21) |
| Diastolic | 82 (16) |
| HbA1c (mmol/mol) | 78 (25.5) |
| Serum creatinine (μmol/L) | 74 (21) |
| eGFR (mL/min/m2) | 88.5 (17) |
| Urine ACR | 2.1 (3.95) |
| GGT | 38 (0) |
| Concomitant medications | |
| Basal-bolus (novorapid + long-acting insulin) | 32 (18.7) |
| Mixed insulin (novomix-30 insulin) | 53 (26.4) |
| Long-acting insulin (lemevir, toujeo, lantus, tresiba, absalgar) | 83 (38.1) |
| Metformin | 377 (83.9) |
| Sulfonylurea (glimepride/glyburide/glipizide) | 199 (44.7) |
| DPP4 inhibitors (sitagliptin, saxagliptin, linagliptin, or alogliptin) | 130 (29.1) |
| Pioglitazone | 22 (5) |
| GLP1 inhibitor (dulaglutide, exanatide, semaglutide, liraglutide) | 94 (20.9) |
The median duration of SGLT-2i therapy was 3.6 (2.1) years. An overall median HbA1c reduction from 78 (26) mmol/mol to 68 (26) mmol/mol (P < 0.001) was observed with the drug treatment. In other words, we observed a median HbA1c reduction of -10 (IQR 25) mmol/mol, with a percentage decrease of 12.82% from the baseline value, indicating moderate antidiabetic efficacy of this class of oral agents. During this treatment period, a significant reduction in HbA1c levels was observed, with a more pronounced decrease in HbA1c during the first six months of SGLT-2i use.
Considering the secondary outcomes, a median body weight reduction from 99 (29) kg to 95.5 (30.3) kg; (P < 0.001) in the first 6 months, with a further reduction to 92.4 kg (median with IQR 27.3) at the latest measurement, and this decrease paralleled the median reduction in BMI: from 34 (7.58) to 33 (7.47) kg/m2 (P < 0.001). Significant reductions in median systolic BP, from 144 (21) mmHg to 139 (23) mmHg (P = 0.015), and median diastolic BP from 82 (16) mmHg to 78 (19) mmHg (P < 0.001) were also observed. Although a significant reduction in median estimated glomerular filtration rate (eGFR) from 88 (17) mL/min to 86.5 (18) mL/min (P < 0.001) at the 6 months’ follow up after the SGLT-2i commencement, an improvement to 87 (18) mL/min at 12 months, and maintenance at that level median eGFR: 87 (21) mL/min at latest follow up was noticed. No statistically significant differences were observed in other biochemical parameters like serum creatine and gamma glutamyl transpeptidase were observed at any follow up periods.
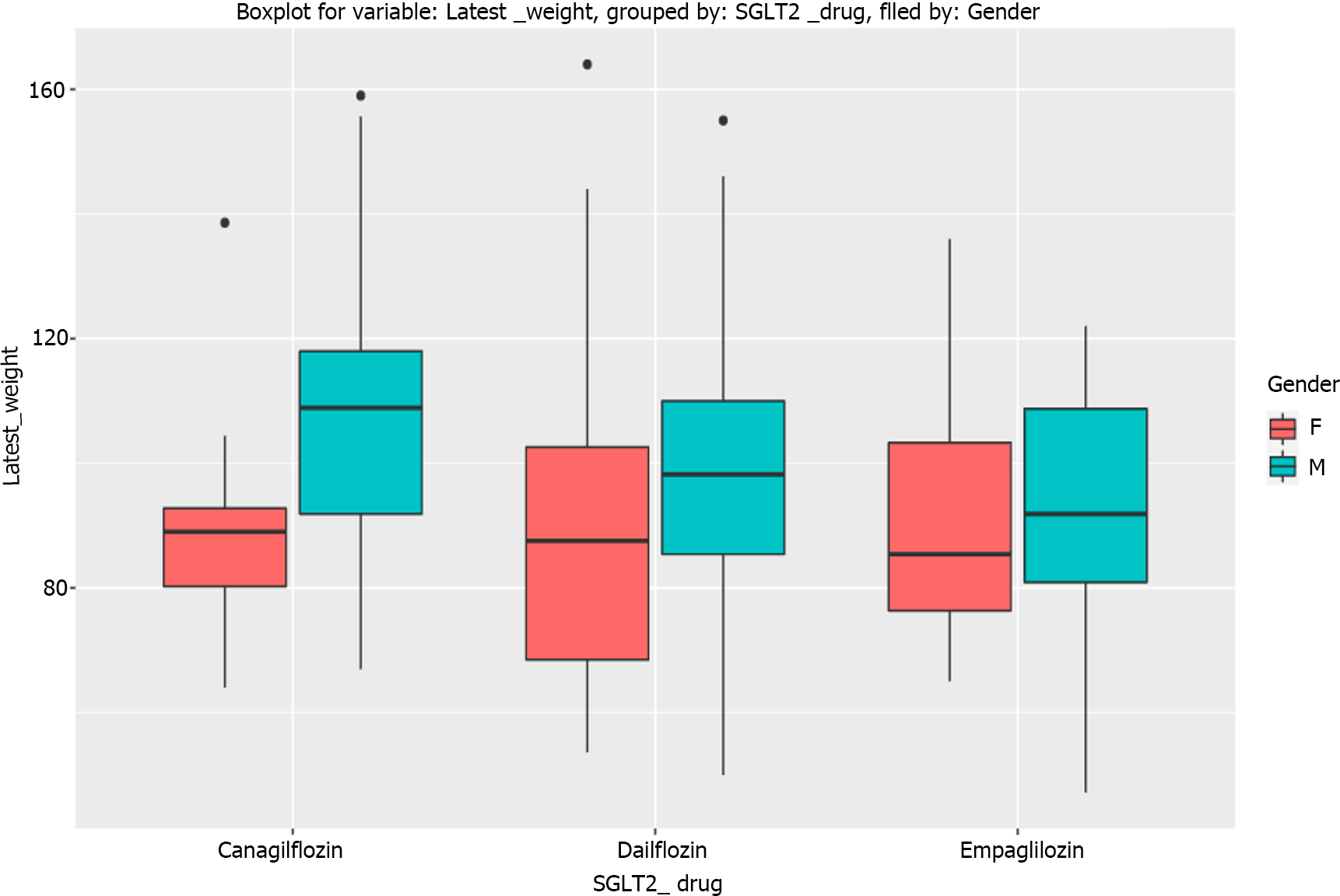
The primary outcome (HbA1c reduction) with each SGLT-2i drug is shown in Figure 1 and the composite treatment outcomes in Tables 2-4. The median weight changes with the individual SGLT-2i drug molecule are shown in Figure 2 and the gender-specific latest weight is shown in the Figure 3.
| Clinical/lab/parameter | Baseline, median (IQR; n) | 6 months F/U, median (IQR; n) | 12 months, median (IQR; n) | Latest, median (IQR; n) | P value (baseline vs latest) |
| HbA1c | 78 (25.3; 227) | 68 (25; 204)a | 66.5 (22.8; 190)b | 69 (24; 191) | < 0.001 |
| Weight | 101 (29.5; 198) | 95.4 (28.2; 150)a | 95.9 (29.2; 144)a | 92.2 (25.6; 93) | < 0.001 |
| BMI | 33.7 (6.6; 81) | - | - | 33.9 (6.38; 44) | 0.002 |
| Systolic BP | 145 (21.3; 185) | - | 142 (23; 159) | 142 (23.3; 112) | 0.080 |
| Diastolic BP | 84 (14; 185) | - | 80 (14; 159)b | 80 (19.3; 112) | < 0.001 |
| Albuminuria (> 3 mmol/mg) | 5.2 (17.4; 39) | 3 (14.9; 24)a | 2.1 (6.38; 18)a | 4.4 (11.9; 30) | 0.063 |
| Clinical/lab/parameter | Baseline, median (IQR; n) | 6 months F/U, median (IQR; n) | 12 months, median (IQR; n) | Latest, median (IQR; n) | P value (baseline vs latest) |
| HbA1c | 80 (25.5; 160) | 67.5 (20; 132)b | 67 (16.3; 128)b | 66 (28; 135) | < 0.001 |
| Weight | 100 (28.3; 125) | 97.6 (27.5; 91)a | 98.3 (30.4; 74) | 95.3 (27.5; 58) | < 0.001 |
| BMI | 35.1 (8.1; 73) | - | - | 32.5 (9.38; 50) | < 0.001 |
| Systolic BP | 145 (18; 121) | - | 145 (21.3; 84) | 138 (22.8; 64) | 0.041 |
| Diastolic BP | 84 (18; 121) | - | 80.5 (11.2; 84)a | 79.5 (19; 64) | 0.013 |
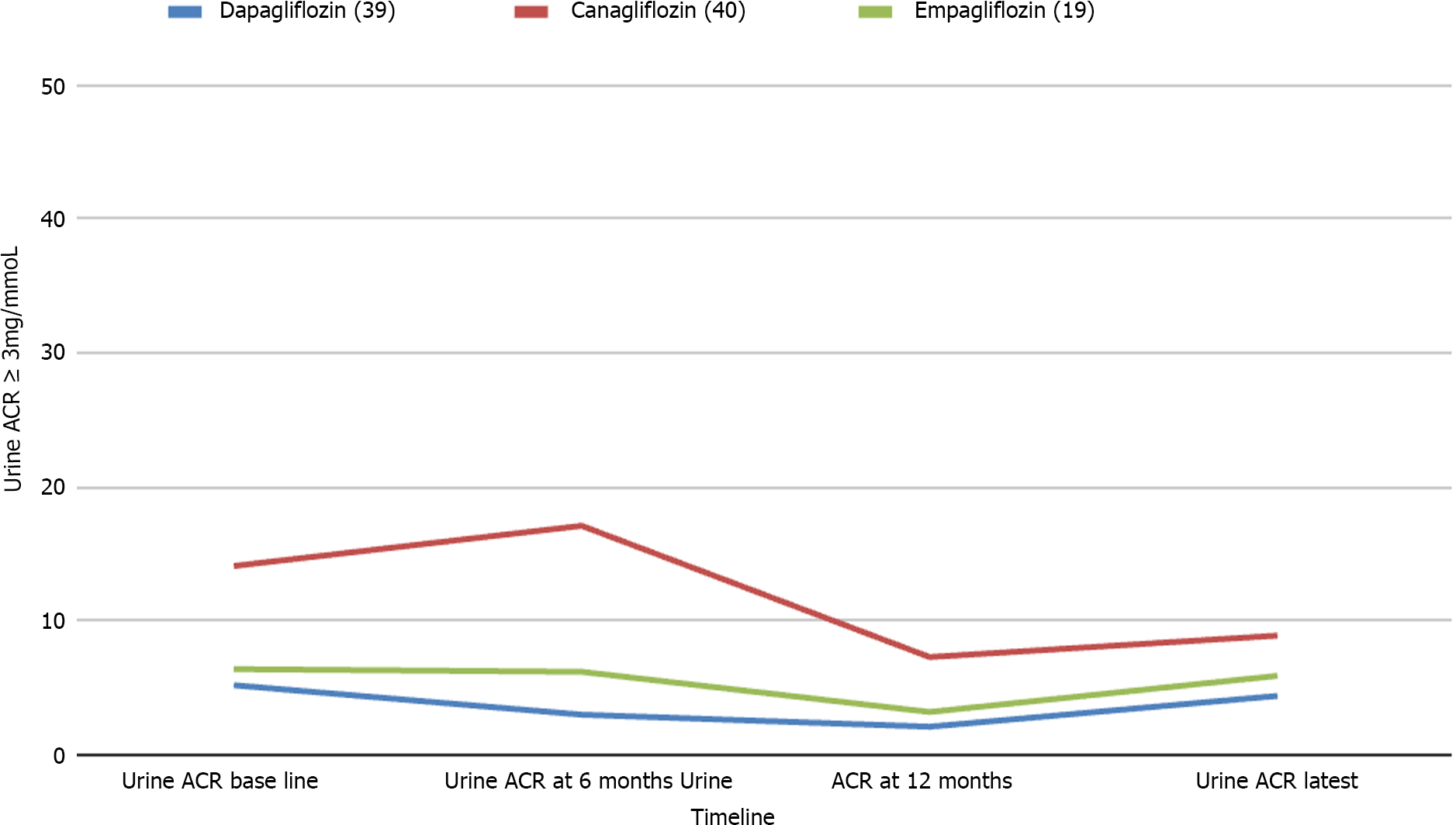
| Albuminuria (> 3 mmol/mg) | 14.1 (42.6; 40) | 17.1 (30.4; 12) | 7.3 (12.3; 21)a | 8.9 (23.7; 28) | 0.043 |
| Clinical/lab/parameter | Baseline, median (IQR; n) | 6 months F/U, median (IQR; n) | 12 months, median (IQR; n) | Latest, median (IQR; n) | P value (baseline vs latest) |
| HbA1c | 75 (23.5; 80) | 64.5 (25; 64)b | 67 (22.5; 59)a | 66.5 (25.8; 66) | 0.002 |
| Weight | 92 (27.5; 57) | 86 (22.8; 29)a | 82.8 (20.9; 20)b | 89.7 (27.5; 20) | 0.207 |
| BMI | 33.7 (9.92; 24) | - | - | 32.4 (5.57; 6) | 0.10 |
| Systolic BP | 136 (24.3; 52) | - | 137 (26; 23) | 130 (25.3; 32) | 0.096 |
| Diastolic BP | 74 (14.5; 52) | - | 74 (16; 23) | 75.5 (12.5; 32) | 0.187 |
| Albuminuria (> 3 mmol/mg) | 6.4 (15; 19) | 6.2 (11.8; 7) | 3.2 (2.98; 6) | 5.9 (2.35; 11) | 0.79 |
Only 98 participants had microalbuminuria at baseline and meaningful follow up data of urine ACR for analysis. Of these subjects, only those on canagliflozin showed a statistically significant reduction of albuminuria at the latest follow up, though dapagliflozin showed a significant reduction in ACR at 6 and 12 months follow up periods (Figure 4).
A total of 64 (13.7%) participants developed adverse events with the use of SGLT-2i therapy [genital thrush in 46 (9.9%), UTI in 11 (2.36%), and DKA in 5 (1.07%) cases]. Adverse effects of individual SGLT-2i molecules were as follows: Genital thrush and urinary infection - 20 (8.8%) & 17 (7.5%) participants with dapagliflozin; 9 (5.6%) & 5 (3.13%) participants with canagliflozin; and 4 (5%) & 4 (5%) participants with empagliflozin. DKA was observed in 4 (1.8%) participants with dapagliflozin and 1 (0.63%) participant with canagliflozin. No participant on empagliflozin developed DKA.
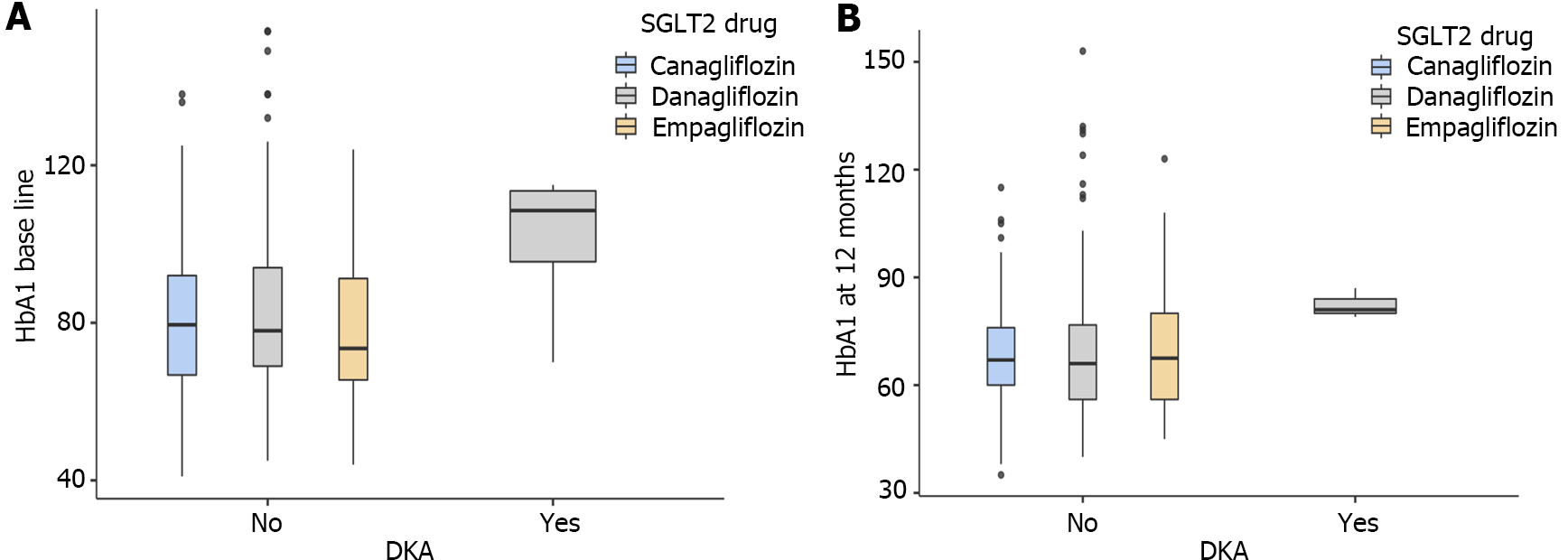
Explanatory data analysis using box plot showed that a high baseline and follow-up HbA1c levels were correlated with higher risk for DKA (Figure 5). While a baseline median HbA1C of ≥ 109 (18) mmol/mol was associated with higher risk of DKA, an HbA1C of < 78 (25) mmol/mol is appeared to confer protection from DKA. Similarly, median HbA1c at 12 months ≥ 81 (4) mmol/mol was associated with DKA, HbA1c level at 12 months < 67 (20) mmol/mol was associated with protection from DKA.
In this retrospective cohort study involving 467 participants with a median duration of T2DM of 16 (9) years treated for a median duration of 3.6 (2.1) years with various SGLT2i medications, we observed statistically significant improvements in glycemic, metabolic, and other cardiovascular outcomes known to occur with the use of this class of drugs in previous clinical studies. The median HbA1c reduction observed from baseline was 10-14 mmol/mol with these drug use at various follow up periods. Significant and persistent improvements in body weight from baseline to the study end were noticed with dapagliflozin (8.8 kg) and canagliflozin (4.7 kg). Though empagliflozin use was associated with significant weight loss at six (6 kg) and 12 months (9.2 kg) there was a regain of weight (see Table 4) at latest follow up. Significant reduction of microalbuminuria was observed with canagliflozin only [ACR 14.6 (42.6) at baseline to 8.9 (23.7) at study end]. The observed adverse effects with the use of SGLT-2i were not unacceptably high in our study. High baseline and follow up HbA1c levels predicted the risk of ketoacidosis.
Dapagliflozin was the commonest SGLT-2i molecule used in our cohort. A recent meta-analysis using pooled data from 8 clinical trials involving 3747 patients showed that its use is associated with a mean HbA1c reduction of 0.59% (approximately 7 mmol/mol)[15] though the first clinical trial (as an add-on therapy to metformin) with the molecule showed a better HbA1c reduction of 0.84% (approximately 9.5 mmol/mol)[16]. We observed a median HbA1c reduction of 10, 11.5 and 9 mmol/mol respectively at 6, 12 and last (median 3.6 years) follow up periods indicating a better treatment response than that were seen in the clinical trials. The better efficacy in glycemic control we observed may be related to the poorer baseline glycemic control (high baseline HbA1c) in our patients, a usual feature in the real-world settings compared to the strictly controlled clinical trial settings where some degree of selection bias is expected with participants likely having better baseline glycemic parameters. Moreover, a significant chunk of our patients was already on multiple antidiabetic agents at the time of addition of dapagliflozin where some drug synergism is expected. The efficacy of dapagliflozin appears to be better even in the clinical trial settings when baseline glycemic control is poor (with an observed mean HbA1c reduction of 12 mmol/mol at 24 wk), and the drug is used as an add-on therapy as observed in a recent study[17].
We observed much better weight reduction with the use of dapagliflozin in our cohort (median -8.8 kg at last follow up) compared to a weight reduction observed in the meta-analysis (-1.88 kg) by Pinto et al[15]. More profound weight loss observed in our study may be related to the poor baseline glycemic control in our cohort and multicombination antidiabetic drug therapy. Patients with poorer diabetes control are likely to have more profound glycosuria and the related negative energy balance from SGLT-2i as evidenced by previous studies with greater weight loss and better average HbA1c reduction as a consequence[18-20], supporting our observations. Although the systolic BP reduction following treatment did not reach statistical significance (P = 0.08) possibly because of the low number of participants in our cohort, a tendency towards significance in this study supports the beneficial effects of dapagliflozin as shown by other studies[21,22]. The significant reduction in diastolic BP observed in this cohort is also in agreement with the observations from other studies[23,24]. Although we observed significant reduction of albuminuria as observed by the DAPA-CKD trial[25], at 6 months and 12 months of follow up, the ACR reduction at the latest follow up did not reach statistical significance (P = 0.06) presumably because of the relatively low number of participants in the cohort. The adverse events such as genital thrush and UTI were relatively high from dapagliflozin use as shown by a recent meta-analysis[26]. The tendency for DKA in those with poorer glycemic control observed in the dapagliflozin cohort is highly important in choosing this molecule for managing patients with T2DM. Patients with poor T2DM control are usually insulinopenic and often behave metabolically like those with T1DM[27]. Dapagliflozin use in T1DM patients was associated with a 3.4-fold higher risk of DKA compared to those T2DM patients treated with drug[28]. Therefore, as we observed in our cohort, anyone with a high HbA1c indicating poor glycemic control are at a greater risk of DKA, and clinicians should exercise cation in prescribing dapagliflozin in such patients.
As shown in various studies, canagliflozin use was associated with the maximum HbA1c reduction compared to the other 2 SGLT-2i agents in our study[15,29,30]. However, the median HbA1c reduction was higher than that observed in clinical trials and meta-analyses presumably from the reasons as mentioned above (see dapagliflozin section). Similarly, the weight reduction was also higher than those seen in various studies[15,29-31]. The reason for this also could same as explained above under dapagliflozin section. Interestingly, we found statistically significant reductions in both the systolic and diastolic BP in the canagliflozin group compared to the other 2 molecules.
Canagliflozin use also showed better and consistent reduction in albuminuria compared to the other two SGLT-2i molecules in our study. This could partly be explained by higher baseline ACR in those with albuminuria in the canagliflozin sub-cohort and low numbers of participants in the individual cohorts of the other two drugs in our study (would not have reached significance). Canagliflozin also possesses some inhibitory effect on SGLT-1 receptors which would have potentially helped to improve albuminuria in this cohort as suggested by a recent study[32]. Although ketoacidosis related to SGLT-2i therapy was found to be highest with canagliflozin use compared to other drugs in this class in a recent study[33], we found a lower risk compared to dapagliflozin use in our cohort. Data from more real-world studies might shed light on to the reason for this interesting new observation.
Compared to the other two SGLT-2i molecules, the number of participants in the empagliflozin cohort was lower, and therefore some of the outcomes might not have reached statistical significance in the present study. Even then the HbA1c reduction was better for empagliflozin compared to the previous studies[15,29,34], possibly for the same reasons mentioned in the previous sections. For some unexplained reason, the tendency for weight reduction observed in this group at 6 and 12 months of follow up periods did not sustain at the latest follow up. The absence of significant BP reduction, improvement of albuminuria, and DKA in this group may be explained by low numbers in the sub-cohort.
Our study has several limitations as seen in any retrospective data analysis. High attrition rate of cases during follow-up, incomplete data of several of the clinical and biochemical parameters studied, dissimilar numbers of participants in the treatment arms with the three SGLT-2i drugs (downgrading quality of statistical comparisons), and higher baseline HbA1c compared to many previously reported studies, might have reduced the overall quality at least some of the outcome analyses.
Although SGLT-2i drugs have shown to alter lipid parameters with a reduction in triglycerides and improvement of high-density lipoprotein, with a resultant improvement of metabolic syndrome[35], which may be one among the several reasons for their cardiovascular benefits, we could not procure adequate data on lipid parameters (baseline and during follow up period) for inclusion in this study. However, significant improvements in other cardiometabolic parameters like BP, body weight, BMI, and albuminuria make our study results impressive in predicting remarkable future cardiovascular benefits for our cohort.
Moreover, the study results show several unique features such as relatively long period of follow up of our cohort, reasonable record of many of the study parameters and the real-world data with some good comparative and contrasting features with previously reported studies makes our study reasonably robust and should help to inform evidence-based clinical decision-making process by physicians.
The real-world use of SGLT-2i medications dapagliflozin, canagliflozin and empagliflozin is associated with significant improvements diabesity, cardiovascular outcomes such as BP reduction and improvement of microalbuminuria, all of which are expected to improve the cardiovascular morbidity and possibly mortality. The adverse effects are relatively low as observed in various clinical trials and meta-analyses. Caution should be exercised while treating T2DM patients with poor glycemic control and high baseline HbA1c with SGLT-2i because of higher risk of developing DKA. Data from larger real-world clinical studies are expected to inform clinical decision making in using SGLT-2i by practitioners more judiciously.
Sodium glucose cotransporter-2 inhibitors (SGLT-2i) are antidiabetic medications with moderate efficacy in glycemic control, and a potential for weight loss through their glycosuric effects, which are helpful for patients with diabesity - diabetes consequent to obesity. Marked cardiovascular and reno-protective effects with the use of SGLT-2i were proven by multiple randomised controlled trials (RCTs), observational studies and various systematic reviews.
Head-to-head comparison of the real-world data on the comparative efficacy and safety of individual SGLT-2i medications is rather sparse and needs more reports to appraise the benefits shown by the above studies.
To procure the comparative efficacy, safety, and adverse effect profiles of SGLT2i drugs in the clinical practice settings to make better therapeutic decision making as RCTs and prospective observational studies are often biased with rigorous monitoring of patients and the study results that wouldn’t always reflect the real-world medical practice.
We evaluated the comparative efficacy data of 3 SGLT-2i drugs (dapagliflozin, canagliflozin, and empagliflozin) at full doses, used for treating patients with type 2 diabetes mellitus (T2DM) only. Reduction of glycated hemoglobin, body weight, blood pressure, and urine albumin creatinine ratio were recorded, and the adverse effects were documented retrospectively from the clinical records.
This real-world data from 467 patients with T2DM showed remarkable improvements in diabesity and cardiometabolic outcomes with all the three SGLT-2i agents with a tendency for renal protection (improvement of albuminuria) in those on canagliflozin. These drugs are reasonably safe with acceptably mild side effects profiles when used judiciously in patients with diabesity.
We found that SGLT-2i class of medications are very useful for the management of diabesity with improvements cardiometabolic outcomes in the real-world settings as proven by previous RCTs and observational studies.
Management of diabesity is often a clinical dilemma in the context of optimal glycemic control as several antidiabetic agents including insulins tend to cause weight gain with a potential for worsening of diabesity in a vicious cycle. SGLT-2i group of drugs improves glycemic control and cause weight loss when used in patients with diabesity. Moreover, SGLT-2i medications are useful in improving cardiometabolic outcomes and renal protection in patients with T2DM. We studied the comparative efficacy of dapagliflozin, empagliflozin and canagliflozin using a retrospective data from our hospital which revealed significant improvements in body weight, blood pressure, and glycated hemoglobin, with canagliflozin also showing improvement in albuminuria. They were also reasonably safe with acceptable side effect profile when used in the appropriate clinical context.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Liu Y, China S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Wanner C, Marx N. SGLT2 inhibitors: the future for treatment of type 2 diabetes mellitus and other chronic diseases. Diabetologia. 2018;61:2134-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Khor XY, Pappachan JM, Jeeyavudeen MS. Individualized diabetes care: Lessons from the real-world experience. World J Clin Cases. 2023;11:2890-2902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Michaelidou M, Pappachan JM, Jeeyavudeen MS. Management of diabesity: Current concepts. World J Diabetes. 2023;14:396-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (4)] |
| 4. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8306] [Article Influence: 830.6] [Reference Citation Analysis (1)] |
| 5. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5407] [Article Influence: 675.9] [Reference Citation Analysis (0)] |
| 6. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4261] [Article Influence: 710.2] [Reference Citation Analysis (0)] |
| 7. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4451] [Article Influence: 741.8] [Reference Citation Analysis (0)] |
| 8. | Giugliano D, Bellastella G, Longo M, Scappaticcio L, Maiorino MI, Chiodini P, Esposito K. Relationship between improvement of glycaemic control and reduction of major cardiovascular events in 15 cardiovascular outcome trials: A meta-analysis with meta-regression. Diabetes Obes Metab. 2020;22:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, McMurray JJV, Solomon SD. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 514] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 10. | McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra SG, Masiukiewicz U, Cannon CP. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021;6:148-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 790] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 11. | Mavrakanas TA, Tsoukas MA, Brophy JM, Sharma A, Gariani K. SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: a systematic review and meta-analysis. Sci Rep. 2023;13:15922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 12. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3989] [Article Influence: 664.8] [Reference Citation Analysis (0)] |
| 13. | Heerspink HJL, Karasik A, Thuresson M, Melzer-Cohen C, Chodick G, Khunti K, Wilding JPH, Garcia Rodriguez LA, Cea-Soriano L, Kohsaka S, Nicolucci A, Lucisano G, Lin FJ, Wang CY, Wittbrodt E, Fenici P, Kosiborod M. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 14. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3142] [Article Influence: 628.4] [Reference Citation Analysis (1)] |
| 15. | Pinto LC, Rados DV, Remonti LR, Viana MV, Leitão CB, Gross JL. Dose-ranging effects of SGLT2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. Arch Endocrinol Metab. 2022;66:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 17. | Hong JH, Moon JS, Seong K, Lim S. Comparison of therapeutic efficacy and safety of sitagliptin, dapagliflozin, or lobeglitazone adjunct therapy in patients with type 2 diabetes mellitus inadequately controlled on sulfonylurea and metformin: Third agent study. Diabetes Res Clin Pract. 2023;203:110872. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Goring S, Hawkins N, Wygant G, Roudaut M, Townsend R, Wood I, Barnett AH. Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes Obes Metab. 2014;16:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Wilding JPH, Rigney U, Blak BT, Nolan ST, Fenici P, Medina J. Glycaemic, weight, and blood pressure changes associated with early versus later treatment intensification with dapagliflozin in United Kingdom primary care patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;155:107791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Olagunju A, Yamani N, Kenny D, Mookadam M, Mookadam F, Unzek S. Potential for sodium-glucose cotransporter-2 inhibitors in the management of metabolic syndrome: A systematic review and meta-analysis. World J Cardiol. 2022;14:599-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Zheng XD, Qu Q, Jiang XY, Wang ZY, Tang C, Sun JY. Effects of Dapagliflozin on Cardiovascular Events, Death, and Safety Outcomes in Patients with Heart Failure: A Meta-Analysis. Am J Cardiovasc Drugs. 2021;21:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Tsapas A, Karagiannis T, Kakotrichi P, Avgerinos I, Mantsiou C, Tousinas G, Manolopoulos A, Liakos A, Malandris K, Matthews DR, Bekiari E. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Obes Metab. 2021;23:2116-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 24. | Shi FH, Li H, Shen L, Fu JJ, Ma J, Gu ZC, Lin HW. High-dose sodium-glucose co-transporter-2 inhibitors are superior in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2021;23:2125-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, Rossing P, Sjöström CD, Stefansson BV, Toto RD, Wheeler DC, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 26. | Li CX, Liu LY, Zhang CX, Geng XH, Gu SM, Wang YQ, Liu H, Xie Q, Liang S. Comparative safety of different sodium-glucose transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2023;14:1238399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 27. | Perry RJ, Shulman GI. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem. 2020;295:14379-14390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | di Mauro G, Mascolo A, Gaio M, Rafaniello C, De Angelis A, Berrino L, Paolisso G, Rossi F, Capuano A. The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Chen MB, Wang H, Zheng QH, Xu HL, Cui WY. Effect of sodium-dependent glucose transporter inhibitors on glycated hemoglobin A1c after 24 weeks in patients with diabetes mellitus: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e24101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Lautsch D, Alsumali A, McLeod E, Kuang Y, He J, Singh R, Nevo A, Arnet U, Uyei J, Rajpathak S. Comparative Efficacy of Dual and Single Initiation of Add-On Oral Antihyperglycemic Agents in Type 2 Diabetes Uncontrolled on Metformin Alone: A Systematic Literature Review and Network Meta-Analysis. Diabetes Ther. 2021;12:389-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Cheong AJY, Teo YN, Teo YH, Syn NL, Ong HT, Ting AZH, Chia AZQ, Chong EY, Chan MY, Lee CH, Lim AYL, Kong WKF, Wong RCC, Chai P, Sia CH. SGLT inhibitors on weight and body mass: A meta-analysis of 116 randomized-controlled trials. Obesity (Silver Spring). 2022;30:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 32. | Clemmer JS, Yen TE, Obi Y. Modeling the renoprotective mechanisms of SGLT2 inhibition in hypertensive chronic kidney disease. Physiol Rep. 2023;11:e15836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Yang S, Liu Y, Zhang S, Wu F, Liu D, Wu Q, Zheng H, Fan P, Su N. Risk of diabetic ketoacidosis of SGLT2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Front Pharmacol. 2023;14:1145587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 34. | Lingvay I, Capehorn MS, Catarig AM, Johansen P, Lawson J, Sandberg A, Shaw R, Paine A. Efficacy of Once-Weekly Semaglutide vs Empagliflozin Added to Metformin in Type 2 Diabetes: Patient-Level Meta-analysis. J Clin Endocrinol Metab. 2020;105:e4593-e4604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Sánchez-García A, Simental-Mendía M, Millán-Alanís JM, Simental-Mendía LE. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol Res. 2020;160:105068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |