Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.403
Peer-review started: August 24, 2023
First decision: November 21, 2023
Revised: December 8, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 15, 2024
Processing time: 204 Days and 7.8 Hours
Type 2 diabetes mellitus (T2DM), a fast-growing issue in public health, is one of the most common chronic metabolic disorders in older individuals. Osteoporosis and sarcopenia are highly prevalent in T2DM patients and may result in fractures and disabilities. In people with T2DM, the association between nutrition, sarco
To evaluate the connections among nutrition, bone mineral density (BMD) and body composition in patients with T2DM.
We enrolled 689 patients with T2DM for this cross-sectional study. All patients underwent dual energy X-ray absorptiometry (DXA) examination and were categorized according to baseline Geriatric Nutritional Risk Index (GNRI) values calculated from serum albumin levels and body weight. The GNRI was used to evaluate nutritional status, and DXA was used to investigate BMD and body composition. Multivariate forward linear regression analysis was used to identify the factors associated with BMD and skeletal muscle mass index.
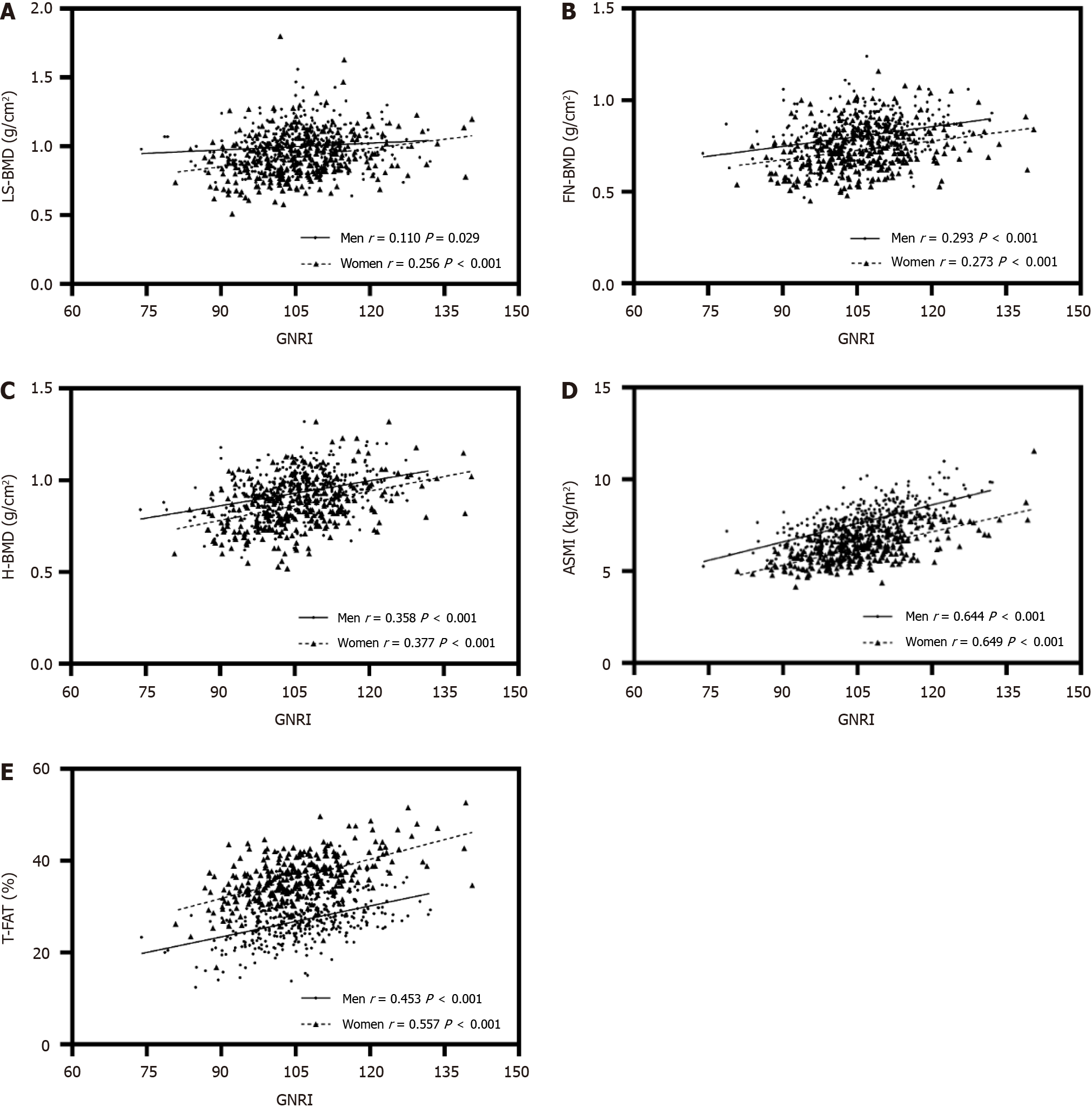
Of the total patients, 394 were men and 295 were women. Compared with patients in tertile 1, those in tertile 3 who had a high GNRI tended to be younger and had lower HbA1c, higher BMD at all bone sites, and higher appendicular skeletal muscle index (ASMI). These important trends persisted even when the patients were divided into younger and older subgroups. The GNRI was positively related to ASMI (men: r = 0.644, P < 0.001; women: r = 0.649, P < 0.001), total body fat (men: r = 0.453, P < 0.001; women: r = 0.557, P < 0.001), BMD at all bone sites, lumbar spine (L1-L4) BMD (men: r = 0.110, P = 0.029; women: r = 0.256, P < 0.001), FN-BMD (men: r = 0.293, P < 0.001; women: r = 0.273, P < 0.001), and hip BMD (men: r = 0.358, P < 0.001; women: r = 0.377, P < 0.001). After adjustment for other clinical parameters, the GNRI was still significantly associated with BMD at the lumbar spine and femoral neck. Additionally, a low lean mass index and higher β-collagen special sequence were associated with low BMD at all bone sites. Age was negatively correlated with ASMI, whereas weight was positively correlated with ASMI.
Poor nutrition, as indicated by a low GNRI, was associated with low levels of ASMI and BMD at all bone sites in T2DM patients. Using the GNRI to evaluate nutritional status and using DXA to investigate body composition in patients with T2DM is of value in assessing bone health and physical performance.
Core Tip: Osteoporosis and sarcopenia are highly prevalent in type 2 diabetes mellitus (T2DM) patients. In people with T2DM, the association between nutrition, sarcopenia, and osteoporosis has rarely been explored. We observed that poor nutrition, as indicated by a low Geriatric Nutritional Risk Index (GNRI), was associated with low levels of ASMI and bone mineral density at all bone sites in T2DM patients. Using the GNRI to evaluate nutritional status and using dual energy X-ray absorptiometry to investigate body composition in patients with T2DM is of value in assessing bone health and physical performance.
- Citation: Zhu XX, Yao KF, Huang HY, Wang LH. Associations between Geriatric Nutrition Risk Index, bone mineral density and body composition in type 2 diabetes patients. World J Diabetes 2024; 15(3): 403-417
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/403.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.403
Over the past few years, there has been a rise in the prevalence of osteoporosis and sarcopenia among the elderly population, leading to physical impairment, diminished quality of life and even death of patients[1,2]. Type 2 diabetes mellitus (T2DM), a rapidly growing public health problem, is one of the most common chronic metabolic disorders in older individuals[3]. For patients with T2DM, osteoporosis is one of the possible long-term complications[4]. Sarcopenia, or loss of muscle mass and function, is a major cause of disability in diabetes[5]. Therefore, it is imperative to identify early sarcopenia, osteoporosis and their risk factors in older individuals with T2DM. Subsequently, suitable measures should be taken to avert and manage this ailment.
As a multifactorial systemic disease, many factors contribute to sarcopenia, such as age, sex, body mass index (BMI), duration of diabetes, glycemic control, nutritional status, and lifestyle[6-8]. Sarcopenia is commonly believed to be a decline in skeletal muscle mass and reduced muscle function that occurs with age. In sarcopenia research, the Asia Working Group for Sarcopenia suggests the utilization of the skeletal muscle index (SMI). This index is calculated by dividing the appendicular skeletal muscle mass (ASMM) by the square of height, providing an adjusted measurement of muscle mass[9]. The factors associated with osteoporosis in T2DM include age, sex, BMI, serum vitamin D concentrations, lifestyle factors, duration of diabetes[10], and nutritional risk[11]. Since there are several common factors in osteoporosis and sarcopenia, many studies of the association between osteoporosis and skeletal muscle mass have been reported. The connection between low muscle mass and osteoporosis in patients with T2DM remains uncertain.
Malnutrition is frequently found in elderly individuals. Older adults with T2DM may face an increased risk of undernutrition due to excessively strict dietary habits aimed at managing blood sugar levels[12]. Various tools have been developed to assess malnutrition status, including the Malnutrition Screening Tool[13], Malnutrition Universal Screening Tool[14], Mini Nutritional Assessment Short Form[15], Nutrition Risk Score 2002[16], and Geriatric Nutritional Risk Index (GNRI)[17]. The GNRI has been utilized as a convenient and accessible method among these instruments for assessing outcomes, relying on serum albumin levels and the ratio of real body weight to ideal body weight.
The relationship between nutritional status and bone mass has been observed in different populations, such as individuals with chronic obstructive pulmonary disease[18], rheumatoid arthritis[19,20], and end-stage renal disease[21]. In people with T2DM, nutrition, sarcopenia, and osteoporosis are rarely explored. Therefore, in this study, we investigated associations between bone mineral density (BMD), the GNRI and body composition in patients with T2DM.
We conducted a retrospective cross-sectional study among T2DM patients admitted to the Department of Endocrinology, The Second Affiliated Hospital of Nantong University, between January 1, 2020, and March 1, 2022.
The main inclusion criterion in this study was T2DM. T2DM was defined as a fasting blood glucose level of > 7.0 mmol/L and/or a 2-h postprandial blood glucose level > 11.1 mmol/L in an oral glucose tolerance test, in accordance with the 1999 World Health Organization T2DM diagnosis and classification criteria. The patients were excluded based on the following criteria: (1) Malignant tumor and severe heart, cerebral, liver or kidney diseases; (2) pituitary, thyroid, parathyroid and adrenal diseases; (3) treatment with glucocorticoids or sex hormones in the past 6 mo; (4) concomitantly taking drugs affecting bone metabolism, such as calcium, vitamin D and bisphosphonates; and (5) unavailability of complete data on relevant variables and assessments. This study was approved by the ethics committee of The Second Affiliated Hospital of Nantong University and was in line with the Helsinki Declaration. The number for ethics approval was 2021KT063.
Collection of demographic, medical, and laboratory data: All demographic information and relevant medical histories of the participants were recorded from their medical records. Demographic data included age, sex, height, weight and BMI. Body weight and height were measured with the patient lightly clothed and without shoes. BMI (kg/m2) was calculated as body weight in kilograms divided by height in meters squared. Medical history included diabetes duration and history of hypertension. The duration of diabetes was calculated by months from the time that the patient was diagnosed with T2DM in their medical records to the date we took blood tests. We also collected the glucose-lowering therapy status among participants. Glucose-lowering therapies were categorized as lifestyle alone and drug therapy. Hypoglycemic agents included insulin, insulin secretagogues, insulin sensitizers, metformin, AGIs (α-glucosidase inhibitors), DPP-4Is (dipeptidyl peptidase-4 inhibitors), SGLT-2Is (sodium-glucose cotransporter-2 inhibitors) and GLP-1RAs (glucagon-like peptide-1 receptor agonists).
For laboratory data collection, the nurses in the ward took blood samples from the antecubital vein in the early morning hours after overnight fasting (at least 8 h). Triglycerides (TGs; colorimetric method), total cholesterol (TC; cholesterol oxidase method), low-density lipoprotein cholesterol (LDL-C; selective melting method) and high-density lipoprotein cholesterol (HDL-C; enzyme modification method) were measured by an automatic biochemical instrument (Model 7600, Hitachi). The level of HbA1c was assessed by ion exchange high-performance liquid chromatography. The levels of bone metabolism markers, including osteocalcin (OS), β-collagen special sequence (β-CTX) and total type I procollagen N-terminal extension peptide (TP1NP). Additionally, other biochemical markers, such as serum creatinine (Cr), uric acid (UA), albumin and total bilirubin (TBil), were measured according to standard methodology.
BMD and body composition measurements: BMD and body composition were measured using dual energy X-ray absorptiometry (DXA; Hologic-Discovery Wi, S/N86856). All of the patients were scanned, and calculations were performed by professionals in the corresponding medical and technical departments. According to the instrument manual, all operations were carried out in the standard mode: The patient lay flat and was scanned from head to feet. The measured indices included lumbar spine (L1-L4) BMD (LS-BMD), femoral neck BMD (FN-BMD), hip BMD, total (whole-body) BMD, total body fat, the android/gynoid ratio, fat mass index, lean mass index and appendicular SMI (ASMI). BMD (g/cm2) was calculated using the following formula: Bone mineral content (g)/area (cm2); ASMI was calculated by limb skeletal muscle mass: ASMM (kg)/height2 (m2); lean mass index was calculated using the following formula: Lean mass (kg)/height2 (m2); and fat mass index was calculated using the following formula: Fat mass (kg)/height2 (m2).
Based on the serum albumin level and baseline body weight, the GNRI is calculated as follows: GNRI = [1.489 albumin (g/L) + (weight/ideal weight)]. Ideal weight can be further calculated by the following equations: Men: Ideal weight = height (cm) – 100 – [(height - 150)/4]; Women: Ideal weight = height (cm) – 100 – [(height - 150)/2.5].
The patients were classified by GNRI tertiles with cutoff values of < 101.85, 101.85 to 109.52, and > 109.52. A descriptive analysis of the data was performed based on the type of data, including the mean and standard deviation, and frequency and percentage. The trends of continuous data and categorical data were detected using one-way ANOVA with linear polynomial contrasts, Kruskal-Wallis tests, and Chi-squared tests with linear-by-linear associations. Furthermore, we generated scatter plots using GraphPad Prism to show the correlation between the GNRI and BMD, ASMI, and total body fat (T-FAT). The factors associated with BMD and ASMI were identified using multiple stepwise linear regression analyses.
For the statistical analysis, we employed IBM SPSS Statistics (25.0) and GraphPad Prism (9.0). Statistical significance was determined using a P value less than 0.05. Normally distributed values are given as the mean ± SD, skewed distributed values are given as the median (25% and 75% interquartiles), and categorical variables are given as frequency (percentage).
In this study, we enrolled 689 patients (57.2% men and 42.8% women), with a mean age of 55.59 ± 10.88 years.
Table 1 shows comparisons of the characteristics of the patients classified by GNRI tertiles. Compared with patients in tertile 1, those in tertile 3 tended to be younger, had lower HbA1c and β-CTX, and had higher BMI, BMD, total body fat, android/gynoid ratio, fat mass index, lean mass index, ASMI, albumin, UA, TG, TC and TBil. These important trends persisted even when the patients were divided into younger and older subgroups (Tables 2-4).
| Characteristics | Total (n = 689) | GNRI tertile 1 (n = 230) | GNRI tertile 2 (n = 230) | GNRI tertile 3 (n = 229) | F/H/χ2 | P value |
| Women [n (%)] | 295 (42.8) | 109 (47.4) | 96 (41.7) | 90 (39.3) | 3.065 | 0.080 |
| Age (yr) | 55.59 ± 10.88 | 58.02 ± 10.49 | 56.00 ± 10.84 | 52.74 ± 10.69 | 28.071 | < 0.001 |
| Height (cm) | 166.89 ± 8.24 | 165.25 ± 7.88 | 167.10 ± 8.52 | 168.33 ± 8.06 | 16.410 | < 0.001 |
| Weight (kg) | 70.00 (62.00-80.00) | 62.00 (56.00-69.00) | 71.00 (64.75-78.85) | 80.00 (72.75-90.00) | 16.636 | < 0.001 |
| Diabetes duration (yr) | 7.33 ± 6.20 | 8.96 ± 6.42 | 7.58 ± 6.44 | 5.45 ± 5.15 | 38.741 | < 0.001 |
| BMI (kg/m2) | 25.39 (23.23-27.78) | 22.92 (21.31-24.49) | 25.53 (24.20-26.93) | 28.28 (26.62-30.47) | 19.284 | < 0.001 |
| SBP (mmHg) | 133.84 ± 15.34 | 132.96 ± 16.80 | 133.89 ± 15.98 | 134.67 ± 12.98 | 1.420 | 0.234 |
| DBP (mmHg) | 81.00 ± 9.88 | 79.44 ± 10.19 | 80.76 ± 9.50 | 82.79 ± 9.70 | 13.343 | < 0.001 |
| Hypertension [n (%)] | 333 (48.3) | 112 (48.7) | 117 (50.9) | 104 (45.4) | 0.492 | 0.483 |
| GNRI (score) | 105.61 (99.64-112.01) | 97.09 (93.02-99.66) | 105.62 (103.73-107.68) | 114.25 (112.01-119.37) | 27.818 | < 0.001 |
| Glucose-lowering therapies [n (%)] | ||||||
| Lifestyle alone | 121 (17.6) | 31 (13.5) | 36 (15.7) | 54 (23.6) | 8.071 | 0.004 |
| Insulin treatments | 248 (36.0) | 102 (44.3) | 89 (38.7) | 57 (24.9) | 18.817 | < 0.001 |
| Insulin secretagogues | 222 (32.2) | 83 (36.1) | 78 (33.9) | 61 (26.6) | 4.681 | 0.030 |
| Insulin sensitizers | 79 (11.5) | 25 (10.9) | 28 (12.2) | 26 (11.4) | 0.027 | 0.870 |
| Metformin | 322 (46.7) | 88 (38.3) | 110 (47.8) | 124 (54.1) | 11.622 | < 0.001 |
| AGIs | 105 (15.2) | 23 (10.0) | 33 (14.3) | 49 (21.4) | 11.519 | < 0.001 |
| DPP-4Is | 57 (8.3) | 18 (7.8) | 23 (10.0) | 16 (7.0) | 0.105 | 0.745 |
| SGLT-2Is | 93 (13.5) | 28 (12.2) | 35 (15.2) | 30 (13.1) | 0.085 | 0.771 |
| GLP-1RAs | 41 (6.0) | 3 (1.3) | 14 (6.1) | 24 (10.5) | 17.240 | < 0.001 |
| Statins | 122 (17.7) | 37 (16.1) | 45 (19.6) | 40 (17.5) | 0.151 | 0.698 |
| Laboratory findings | ||||||
| HbA1c (%) | 8.99 ± 1.85 | 9.50 ± 2.02 | 8.92 ± 1.63 | 8.53 ± 1.75 | 33.073 | < 0.001 |
| Albumin (g/L) | 38.50 (36.20-41.30) | 35.90 (33.88-37.63) | 38.60 (37.00-40.60) | 41.70 (39.45-44.00) | 17.954 | < 0.001 |
| Cr (µmol/L) | 58.51 ± 21.32 | 58.22 ± 25.22 | 57.85 ± 21.58 | 59.46 ± 16.26 | 0.386 | 0.535 |
| UA (µmol/L) | 312.68 ± 99.25 | 279.47 ± 103.09 | 314.02 ± 89.46 | 344.68 ± 94.19 | 53.222 | < 0.001 |
| TG (mmol/L) | 1.89 (1.18-3.11) | 1.46 (0.98-2.33) | 1.81 (1.15-2.83) | 2.38 (1.58-3.98) | 7.626 | < 0.001 |
| TC (mmol/L) | 4.41 ± 1.06 | 4.29 ± 1.01 | 4.33 ± 0.98 | 4.62 ± 1.15 | 10.890 | 0.001 |
| HDL-C (mmol/L) | 1.14 ± 0.27 | 1.15 ± 0.28 | 1.15 ± 0.25 | 1.10 ± 0.27 | 3.679 | 0.056 |
| LDL-C (mmol/L) | 2.81 ± 0.87 | 2.80 ± 0.88 | 2.80 ± 0.82 | 2.84 ± 0.91 | 0.309 | 0.578 |
| TBil (µmol/L) | 11.21 ± 4.71 | 10.25 ± 4.60 | 11.43 ± 4.68 | 11.95 ± 4.70 | 15.213 | < 0.001 |
| OS (ng/mL) | 11.85 ± 3.99 | 12.06 ± 4.26 | 11.88 ± 3.89 | 11.60 ± 3.82 | 1.508 | 0.220 |
| β-CTX (ng/mL) | 0.45 ± 0.22 | 0.51 ± 0.25 | 0.44 ± 0.21 | 0.41 ± 0.19 | 25.645 | < 0.001 |
| TP1NP (ng/mL) | 40.73 ± 14.53 | 41.00 ± 14.00 | 40.74 ± 14.66 | 40.45 ± 14.98 | 0.165 | 0.685 |
| DXA parameters (g/cm2) | ||||||
| LS-BMD | 0.97 ± 0.16 | 0.92 ± 0.14 | 0.99 ± 0.17 | 0.99 ± 0.15 | 22.118 | < 0.001 |
| FN-BMD | 0.77 ± 0.12 | 0.73 ± 0.12 | 0.79 ± 0.13 | 0.81 ± 0.11 | 53.333 | < 0.001 |
| H-BMD | 0.91 ± 0.13 | 0.85 ± 0.12 | 0.91 ± 0.13 | 0.95 ± 0.12 | 83.980 | < 0.001 |
| T-BMD | 1.10 ± 0.12 | 1.07 ± 0.12 | 1.10 ± 0.12 | 1.12 ± 0.11 | 21.875 | < 0.001 |
| Body composition | ||||||
| Total body fat (%) | 31.03 ± 6.56 | 29.03 ± 6.55 | 30.80 ± 5.83 | 33.26 ± 6.62 | 51.017 | < 0.001 |
| Android/gynoid ratio | 1.31 ± 0.22 | 1.23 ± 0.22 | 1.33 ± 0.21 | 1.36 ± 0.20 | 49.682 | < 0.001 |
| Fat mass index (kg/m2) | 7.53 (6.20-9.09) | 6.33 (5.08-7.53) | 7.54 (6.44-8.74) | 8.91 (7.51-10.75) | 13.010 | < 0.001 |
| Lean mass index (kg/m2) | 16.95 (15.53-18.54) | 15.66 (14.44-16.83) | 17.11 (15.92-18.39) | 18.60 (16.99-19.90) | 14.055 | < 0.001 |
| ASMI (kg/m2) | 7.09 ± 1.17 | 6.38 ± 0.91 | 7.09 ± 0.97 | 7.79 ± 1.1 | 218.066 | < 0.001 |
| Characteristics | Total (n = 689) | Younger1 (n = 219) | Older2 (n = 470) | χ2/t/z | P value |
| Women [n (%)] | 295 (42.8) | 111 (50.7) | 184 (39.1) | 8.120 | 0.004 |
| Age (yr) | 55.59 ± 10.88 | 43.71 ± 7.23 | 61.12 ± 7.25 | -29.374 | < 0.001 |
| Height (cm) | 166.89 ± 8.24 | 167.33 ± 8.55 | 166.69 ± 8.1 | 0.951 | 0.342 |
| Weight (kg) | 70.00 (62.00-80.00) | 71.00 (62.00-82.00) | 70.00 (62.00-80.00) | -1.178 | 0.239 |
| Diabetes duration (yr) | 7.33 ± 6.20 | 4.44 ± 3.82 | 8.68 ± 6.62 | -10.603 | < 0.001 |
| BMI (kg/m2) | 25.39 (23.23-27.78) | 25.42 (23.11-28.34) | 25.39 (23.32-27.55) | -1.001 | 0.317 |
| SBP (mmHg) | 133.84 ± 15.34 | 128.84 ± 13.78 | 136.16 ± 15.49 | -5.977 | < 0.001 |
| DBP (mmHg) | 81.00 ± 9.88 | 81.74 ± 10.15 | 80.65 ± 9.75 | 1.342 | 0.180 |
| Hypertension [n (%)] | 333 (48.3) | 66 (30.1) | 267 (56.8) | 42.556 | < 0.001 |
| GNRI (score) | 105.61 (99.64-112.01) | 107.2 (101.18-113.56) | 105.09 (98.93-110.98) | -2.880 | 0.004 |
| Glucose-lowering therapies | |||||
| Lifestyle alone [n (%)] | 121 (17.6) | 58 (26.5) | 63 (13.4) | 17.653 | < 0.001 |
| Insulin treatments [n (%)] | 248 (36.0) | 73 (33.3) | 175 (37.2) | 0.987 | 0.321 |
| Insulin secretagogues [n (%)] | 222 (32.2) | 44 (20.1) | 178 (37.9) | 21.627 | < 0.001 |
| Insulin sensitizers [n (%)] | 79 (11.5) | 20 (9.1) | 59 (12.6) | 1.722 | 1.189 |
| Metformin [n (%)] | 322 (46.7) | 92 (42.0) | 230 (48.9) | 2.880 | 0.090 |
| AGIs [n (%)] | 105 (15.2) | 29 (13.2) | 76 (16.2) | 0.992 | 0.319 |
| DPP-4Is [n (%)] | 57 (8.3) | 19 (8.7) | 38 (8.1) | 0.069 | 0.793 |
| SGLT-2Is [n (%)] | 93 (13.5) | 26 (11.9) | 67 (14.3) | 0.727 | 0.394 |
| GLP-1RAs [n (%)] | 41 (6.0) | 15 (6.8) | 26 (5.5) | 0.463 | 0.496 |
| Statins [n (%)] | 122 (17.7) | 38 (17.4) | 84 (17.9) | 0.028 | 0.868 |
| Laboratory findings | |||||
| HbA1c (%) | 8.99 ± 1.85 | 8.99 ± 1.9 | 8.98 ± 1.82 | 0.075 | 0.941 |
| Albumin (g/L) | 38.50 (36.20-41.30) | 39.00 (36.8-41.6) | 38.25 (35.8-41) | -2.470 | 0.014 |
| Cr (µmol/L) | 58.51 ± 21.32 | 52.09 ± 12.75 | 61.5 ± 23.74 | -6.750 | < 0.001 |
| UA (µmol/L) | 312.68 ± 99.25 | 317.68 ± 112.65 | 310.35 ± 92.39 | 0.903 | 0.367 |
| TG (mmol/L) | 1.89 (1.18-3.11) | 1.99 (1.28-3.41) | 1.78 (1.15-2.87) | -1.940 | 0.052 |
| TC (mmol/L) | 4.41 ± 1.06 | 4.5 ± 1.09 | 4.37 ± 1.04 | 1.475 | 0.141 |
| HDL-C (mmol/L) | 1.14 ± 0.27 | 1.09 ± 0.25 | 1.16 ± 0.27 | -3.049 | 0.002 |
| LDL-C (mmol/L) | 2.81 ± 0.87 | 2.85 ± 0.87 | 2.79 ± 0.87 | 0.803 | 0.422 |
| TBil (µmol/L) | 11.21 ± 4.71 | 10.99 ± 4.28 | 11.31 ± 4.89 | -0.836 | 0.404 |
| OS (ng/mL) | 11.85 ± 3.99 | 11.93 ± 3.43 | 11.81 ± 4.23 | 0.409 | 0.683 |
| β-CTX (ng/mL) | 0.45 ± 0.22 | 0.47 ± 0.21 | 0.45 ± 0.23 | 1.137 | 0.256 |
| TP1NP (ng/mL) | 40.73 ± 14.53 | 41.58 ± 13.84 | 40.34 ± 14.84 | 1.044 | 0.297 |
| DXA parameters (g/cm2) | |||||
| LS-BMD | 0.97 ± 0.16 | 1.00 ± 0.14 | 0.95 ± 0.16 | 4.126 | < 0.001 |
| FN-BMD | 0.77 ± 0.12 | 0.82 ± 0.12 | 0.75 ± 0.12 | 6.360 | < 0.001 |
| H-BMD | 0.91 ± 0.13 | 0.94 ± 0.12 | 0.89 ± 0.13 | 5.441 | < 0.001 |
| T-BMD | 1.10 ± 0.12 | 1.13 ± 0.1 | 1.08 ± 0.12 | 4.643 | < 0.001 |
| Body composition | |||||
| Total body fat (%) | 31.03 ± 6.56 | 31.76 ± 6.25 | 30.69 ± 6.68 | 2.001 | 0.046 |
| Android/gynoid ratio | 1.31 ± 0.22 | 1.3 ± 0.22 | 1.31 ± 0.21 | -0.107 | 0.915 |
| Fat mass index (kg/m2) | 7.53 (6.20-9.09) | 7.94 (6.55-9.26) | 7.32 (6.17-8.99) | -2.621 | 0.009 |
| Lean mass index (kg/m2) | 16.95 (15.53-18.54) | 17.01 (15.45-18.66) | 16.94 (15.56-18.51) | -0.952 | 0.341 |
| ASMI (kg/m2) | 7.09 ± 1.17 | 7.23 ± 1.31 | 7.02 ± 1.09 | 2.026 | 0.043 |
| Characteristics | Total (n = 219) | GNRI tertile 1 (n = 63) | GNRI tertile 2 (n = 68) | GNRI tertile 3 (n = 88) | F/H/χ2 | P value |
| Women [n (%)] | 111 (50.7) | 40 (63.5) | 33 (48.5) | 38 (43.5) | 5.786 | 0.016 |
| Age (yr) | 43.71 ± 7.23 | 45.87 ± 6.84 | 43.04 ± 6.45 | 42.67 ± 7.80 | 6.880 | 0.009 |
| Height (cm) | 167.33 ± 8.55 | 164.57 ± 8.13 | 167.1 ± 8.49 | 169.48 ± 8.38 | 12.759 | < 0.001 |
| Weight (kg) | 71.00 (62.00-82.00) | 60.00 (56.00-66.70) | 70.00 (64.00-76.85) | 83.05 (74.25-93.20) | 10.568 | < 0.001 |
| Diabetes duration (yr) | 4.44 ± 3.82 | 5.84 ± 3.91 | 4.82 ± 4.03 | 3.15 ± 3.14 | 20.524 | < 0.001 |
| BMI (kg/m2) | 25.42 (23.11-28.34) | 22.72 (21.10-23.88) | 25.32 (23.84-26.57) | 28.60 (26.97-31.73) | 11.641 | < 0.001 |
| SBP (mmHg) | 128.84 ± 13.78 | 126.62 ± 13.78 | 123.6 ± 13.08 | 134.49 ± 12.31 | 16.237 | < 0.001 |
| DBP (mmHg) | 81.74 ± 10.15 | 80.22 ± 10.41 | 77.99 ± 7.75 | 85.72 ± 10.30 | 14.496 | < 0.001 |
| Hypertension [n (%)] | 66 (30.1) | 17 (27.0) | 21 (30.9) | 28 (31.8) | 0.383 | 0.536 |
| GNRI (score) | 107.2 (101.18-113.56) | 97.28 (92.85-99.92) | 105.69 (104.20-107.71) | 114.90 (112.15-120.21) | 15.551 | < 0.001 |
| Glucose-lowering therapies [n (%)] | ||||||
| Lifestyle alone | 58 (26.5) | 14 (22.2) | 12 (17.6) | 32 (36.4) | 4.469 | 0.035 |
| Insulin treatments | 73 (33.3) | 28 (44.4) | 30 (44.1) | 15 (17.0) | 13.761 | < 0.001 |
| Insulin secretagogues | 44 (20.1) | 12 (19.0) | 18 (26.5) | 14 (15.9) | 0.382 | 0.536 |
| Insulin sensitizers | 20 (9.1) | 6 (9.5) | 6 (8.8) | 8 (9.1) | 0.006 | 0.936 |
| Metformin | 92 (42.0) | 20 (31.7) | 31 (45.6) | 41 (46.6) | 2.617 | 0.106 |
| AGIs | 29 (13.2) | 6 (9.5) | 7 (10.3) | 16 (18.2) | 11.519 | < 0.001 |
| DPP-4Is | 19 (8.7) | 4 (6.3) | 9 (13.2) | 6 (6.8) | 0.002 | 0.961 |
| SGLT-2Is | 26 (11.9) | 11 (17.5) | 8 (11.8) | 7 (8.0) | 3.118 | 0.077 |
| GLP-1RAs | 15 (6.8) | 0 (0.0) | 5 (7.4) | 10 (11.4) | 7.234 | 0.007 |
| Statins | 38 (17.4) | 12 (19.0) | 12 (17.6) | 14 (15.9) | 0.256 | 0.613 |
| Laboratory findings | ||||||
| HbA1c (%) | 8.99 ± 1.9 | 9.58 ± 2.26 | 9.07 ± 1.66 | 8.52 ± 1.69 | 12.151 | 0.001 |
| Albumin (g/L) | 39.00 (36.8-41.6) | 36.20 (34.60-37.90) | 39.15 (37.20-40.75) | 41.60 (39.00-44.23) | 9.497 | < 0.001 |
| Cr (µmol/L) | 52.09 ± 12.75 | 48.4 ± 13.54 | 51.31 ± 10.27 | 55.35 ± 13.2 | 11.693 | 0.001 |
| UA (µmol/L) | 317.68 ± 112.65 | 270.22 ± 140.24 | 313.84 ± 90.09 | 354.62 ± 92.24 | 22.682 | < 0.001 |
| TG (mmol/L) | 1.99 (1.28-3.41) | 1.50 (0.98-2.02) | 2.03 (1.12-3.52) | 2.46 (1.84-4.42) | 5.412 | < 0.001 |
| TC (mmol/L) | 4.5 ± 1.09 | 4.37 ± 0.94 | 4.3 ± 0.85 | 4.75 ± 1.30 | 5.389 | 0.021 |
| HDL-C (mmol/L) | 1.09 ± 0.25 | 1.15 ± 0.26 | 1.08 ± 0.21 | 1.06 ± 0.26 | 4.644 | 0.032 |
| LDL-C (mmol/L) | 2.85 ± 0.87 | 2.92 ± 0.91 | 2.86 ± 0.79 | 2.79 ± 0.92 | 0.850 | 0.358 |
| TBil (µmol/L) | 10.99 ± 4.28 | 9.78 ± 3.67 | 11.11 ± 4.15 | 11.76 ± 4.63 | 7.855 | 0.006 |
| OS (ng/mL) | 11.93 ± 3.43 | 12.05 ± 3.14 | 11.77 ± 3.07 | 11.98 ± 3.90 | 0.008 | 0.931 |
| β-CTX (ng/mL) | 0.47 ± 0.21 | 0.53 ± 0.24 | 0.45 ± 0.19 | 0.44 ± 0.20 | 5.436 | 0.021 |
| TP1NP (ng/mL) | 41.58 ± 13.84 | 41.95 ± 11.58 | 40.21 ± 13.02 | 42.37 ± 15.87 | 0.075 | 0.784 |
| DXA parameters (g/cm2) | ||||||
| LS-BMD | 1.00 ± 0.14 | 0.96 ± 0.14 | 1.01 ± 0.13 | 1.02 ± 0.15 | 7.426 | 0.007 |
| FN-BMD | 0.82 ± 0.12 | 0.77 ± 0.11 | 0.82 ± 0.12 | 0.85 ± 0.11 | 18.433 | < 0.001 |
| H-BMD | 0.94 ± 0.12 | 0.88 ± 0.11 | 0.94 ± 0.11 | 0.99 ± 0.11 | 34.357 | < 0.001 |
| T-BMD | 1.13 ± 0.1 | 1.1 ± 0.11 | 1.12 ± 0.09 | 1.15 ± 0.10 | 8.681 | 0.004 |
| Body composition | ||||||
| Total body fat (%) | 31.76 ± 6.25 | 29.85 ± 5.69 | 31.24 ± 5.90 | 33.53 ± 6.49 | 13.922 | < 0.001 |
| Android/gynoid ratio | 1.3 ± 0.22 | 1.21 ± 0.22 | 1.31 ± 0.19 | 1.37 ± 0.21 | 22.631 | < 0.001 |
| Fat mass index (kg/m2) | 7.94 (6.55-9.26) | 6.56 (5.61-7.63) | 7.89 (6.52-8.88) | 9.13 (7.71-11.21) | 7.905 | < 0.001 |
| Lean mass index (kg/m2) | 17.01 (15.45-18.66) | 15.25 (14.14-16.62) | 16.93 (15.82-18.37) | 18.65 (17.26-20.34) | 9.380 | < 0.001 |
| ASMI (kg/m2) | 7.23 ± 1.31 | 6.23 ± 0.90 | 7.09 ± 1.00 | 8.04 ± 1.24 | 105.442 | < 0.001 |
| Characteristics | Total (n = 470) | GNRI tertile 1 (n = 167) | GNRI tertile 2 (n = 162) | GNRI tertile 3 (n = 141) | F/H/χ2 | P value |
| Women [n (%)] | 184 (39.1) | 69 (41.3) | 63 (38.9) | 52 (36.9) | 0.636 | 0.425 |
| Age (yr) | 61.12 ± 7.25 | 62.6 ± 7.56 | 61.43 ± 7.01 | 59.02 ± 6.70 | 18.962 | < 0.001 |
| Height (cm) | 166.69 ± 8.1 | 165.5 ± 7.80 | 167.1 ± 8.55 | 167.62 ± 7.79 | 5.425 | 0.020 |
| Weight (kg) | 70.00 (62.00-80.00) | 62.00 (57.00-70.00) | 72.50 (65.00-80.00) | 80.00 (72.00-85.50) | 12.768 | < 0.001 |
| Diabetes duration (yr) | 8.68 ± 6.62 | 10.13 ± 6.79 | 8.74 ± 6.90 | 6.89 ± 5.62 | 18.945 | < 0.001 |
| BMI (kg/m2) | 25.39 (23.32-27.55) | 23.05 (21.34-24.57) | 25.71 (24.33-27.11) | 28.01 (26.51-29.75) | 15.296 | < 0.001 |
| SBP (mmHg) | 136.16 ± 15.49 | 135.35 ± 17.25 | 138.2 ± 15.13 | 134.78 ± 13.42 | 0.049 | 0.825 |
| DBP (mmHg) | 80.65 ± 9.75 | 79.15 ± 10.12 | 81.93 ± 9.93 | 80.96 ± 8.86 | 2.998 | 0.084 |
| Hypertension [n (%)] | 267 (56.8) | 95 (56.9) | 96 (59.3) | 76 (53.9) | 0.237 | 0.626 |
| GNRI (score) | 105.09 (98.93-110.98) | 97.09 (93.08-99.55) | 105.57 (103.59-107.63) | 113.94 (111.77-118.57) | 22.930 | < 0.001 |
| Glucose-lowering therapies [n (%)] | ||||||
| Lifestyle alone | 63 (13.4) | 17 (10.2) | 24 (14.8) | 22 (15.6) | 2.019 | 0.155 |
| Insulin treatments | 175 (37.2) | 74 (44.3) | 59 (36.4) | 42 (29.8) | 6.938 | 0.008 |
| Insulin secretagogues | 178 (37.9) | 71 (42.5) | 60 (37.0) | 47 (33.3) | 2.771 | 0.096 |
| Insulin sensitizers | 59 (12.6) | 19 (11.4) | 22 (13.6) | 18 (12.8) | 0.152 | 0.697 |
| Metformin | 230 (48.9) | 68 (40.7) | 79 (48.8) | 83 (58.9) | 10.012 | 0.002 |
| AGIs | 76 (16.2) | 17 (10.2) | 26 (16.0) | 33 (23.4) | 9.802 | 0.002 |
| DPP-4Is | 38 (8.1) | 14 (8.4) | 14 (8.6) | 10 (7.1) | 0.158 | 0.691 |
| SGLT-2Is | 67 (14.3) | 17 (10.2) | 27 (16.7) | 23 (16.3) | 2.509 | 0.113 |
| GLP-1RAs | 26 (5.5) | 3 (1.8) | 9 (5.6) | 14 (9.9) | 9.636 | 0.002 |
| Statins | 84 (17.9) | 25 (15.0) | 33 (20.4) | 26 (18.4) | 0.707 | 0.400 |
| Laboratory findings | ||||||
| HbA1c (%) | 8.98 ± 1.82 | 9.47 ± 1.93 | 8.86 ± 1.61 | 8.54 ± 1.79 | 21.198 | < 0.001 |
| Albumin (g/L) | 38.25 (35.8-41) | 35.80 (33.50-37.30) | 38.40 (36.70-40.60) | 41.70 (39.70-44.00) | 15.071 | < 0.001 |
| Cr (µmol/L) | 61.5 ± 23.74 | 61.93 ± 27.54 | 60.59 ± 24.35 | 62.03 ± 17.46 | 0.000 | 0.994 |
| UA (µmol/L) | 310.35 ± 92.39 | 282.96 ± 85.27 | 314.1 ± 89.47 | 338.47 ± 95.18 | 29.509 | < 0.001 |
| TG (mmol/L) | 1.78 (1.15-2.87) | 1.42 (0.97-2.44) | 1.75 (1.18-2.62) | 2.29 (1.41-3.83) | 5.347 | < 0.001 |
| TC (mmol/L) | 4.37 ± 1.04 | 4.27 ± 1.04 | 4.34 ± 1.03 | 4.53 ± 1.04 | 4.991 | 0.026 |
| HDL-C (mmol/L) | 1.16 ± 0.27 | 1.15 ± 0.29 | 1.18 ± 0.26 | 1.13 ± 0.27 | 0.348 | 0.555 |
| LDL-C (mmol/L) | 2.79 ± 0.87 | 2.75 ± 0.87 | 2.77 ± 0.84 | 2.87 ± 0.9 | 1.525 | 0.218 |
| TBil (µmol/L) | 11.31 ± 4.89 | 10.43 ± 4.91 | 11.56 ± 4.89 | 12.06 ± 4.75 | 8.890 | 0.003 |
| OS (ng/mL) | 11.81 ± 4.23 | 12.07 ± 4.63 | 11.93 ± 4.19 | 11.37 ± 3.76 | 1.992 | 0.159 |
| β-CTX (ng/mL) | 0.45 ± 0.23 | 0.5 ± 0.25 | 0.44 ± 0.22 | 0.39 ± 0.18 | 22.076 | < 0.001 |
| TP1NP (ng/mL) | 40.34 ± 14.84 | 40.65 ± 14.83 | 40.96 ± 15.33 | 39.26 ± 14.33 | 0.617 | 0.433 |
| DXA parameters (g/cm2) | ||||||
| LS-BMD | 0.95 ± 0.16 | 0.91 ± 0.15 | 0.98 ± 0.18 | 0.97 ± 0.14 | 12.015 | 0.001 |
| FN-BMD | 0.75 ± 0.12 | 0.71 ± 0.11 | 0.77 ± 0.13 | 0.78 ± 0.10 | 29.138 | < 0.001 |
| H-BMD | 0.89 ± 0.13 | 0.84 ± 0.12 | 0.9 ± 0.14 | 0.93 ± 0.11 | 45.242 | < 0.001 |
| T-BMD | 1.08 ± 0.12 | 1.06 ± 0.12 | 1.09 ± 0.13 | 1.1 ± 0.11 | 10.818 | 0.001 |
| Body composition | ||||||
| Total body fat (%) | 30.69 ± 6.68 | 28.72 ± 6.83 | 30.62 ± 5.81 | 33.09 ± 6.71 | 34.740 | < 0.001 |
| Android/gynoid ratio | 1.31 ± 0.21 | 1.24 ± 0.22 | 1.33 ± 0.21 | 1.36 ± 0.19 | 28.386 | < 0.001 |
| Fat mass index (kg/m2) | 7.32 (6.17-8.99) | 6.26 (4.97-7.51) | 7.38 (6.42-8.59) | 8.70 (7.20-10.56) | 10.212 | < 0.001 |
| Lean mass index (kg/m2) | 16.94 (15.56-18.51) | 15.82 (14.71-17.03) | 17.17 (15.92-18.43) | 18.56 (16.77-19.81) | 10.488 | < 0.001 |
| ASMI (kg/m2) | 7.02 ± 1.09 | 6.44 ± 0.91 | 7.09 ± 0.97 | 7.63 ± 1.08 | 112.733 | < 0.001 |
Figure 1 shows the correlation between GNRI, BMD, T-FAT and ASMI in T2DM patients; the average BMD at the lumbar spine, femur neck and total hip in men was higher than that in women (1.00 vs 0.92, 0.81 vs 0.73, 0.94 vs 0.86, respectively, and all P < 0.001); the GNRI was found to be positively and significantly associated with ASMI, T-FAT and BMD at all bone sites in men and women; Table 5 shows multiple linear regression models displaying associations of the GNRI with BMD; the fully adjusted Model 3 further adjusted for HbA1c, OS, β-CTX, TP1NP, albumin, Cr, UA, TG, TC, HDL-C, LDL-C, TBil, and the GNRI was significantly and positively associated with LS-BMD (b = 0.040, t = 2.492, P = 0.013, R2 = 0.197) and FN-BMD (b = 0.027, t = 2.345, P = 0.019, R2 = 0.341).
| Models | B (95%CI) | β | t value | P value | Adjusted R2 for model |
| Lumbar spine BMD | |||||
| Model 01 | 0.003 (0.002 to 0.004) | 0.186 | 4.952 | < 0.001 | 0.033 |
| Model 12 | -0.002 (-0.004 to 0.000) | -0.113 | -1.919 | 0.055 | 0.150 |
| Model 23 | -0.002 (-0.004 to 0.000) | -0.105 | -1.765 | 0.078 | 0.153 |
| Model 34 | 0.040 (0.008 to 0.071) | 2.402 | 2.492 | 0.013 | 0.197 |
| Femoral neck BMD | |||||
| Model 01 | 0.004 (0.003 to 0.005) | 0.281 | 7.664 | < 0.001 | 0.077 |
| Model 12 | -0.001 (-0.002 to 0.000) | -0.071 | -1.32 | 0.187 | 0.293 |
| Model 23 | -0.001 (-0.002 to 0.000) | -0.06 | -1.12 | 0.263 | 0.306 |
| Model 34 | 0.027 (0.004 to 0.049) | 2.047 | 2.345 | 0.019 | 0.341 |
| Total hip BMD | |||||
| Model 01 | 0.005 (0.004 to 0.006) | 0.363 | 10.213 | < 0.001 | 0.131 |
| Model 12 | 0.000 (-0.002 to 0.001) | -0.034 | -0.636 | 0.525 | 0.312 |
| Model 23 | 0.000 (-0.002 to 0.001) | -0.025 | -0.477 | 0.634 | 0.318 |
| Model 34 | 0.021 (-0.003 to 0.044) | 1.52 | 1.745 | 0.082 | 0.343 |
Table 6 shows the determinants of BMD using multivariate stepwise linear regression analysis after adjusting for age, sex, height, weight, diabetes duration, hypertension, systolic/diastolic blood pressure (SBP), diastolic blood pressure (DBP), GNRI, BMI, HbA1c, OS, β-CTX, TP1NP, albumin, Cr, UA, TG, TC, HDL-C, LDL-C, TBil, ASMI, total body fat, android/gynoid ratio, fat mass index and lean mass index; the lean mass index was positively correlated with BMD at all bone sites; age, diabetes duration and β-CTX were negatively correlated with BMD at all bone sites; height and Cr were positively correlated with lumbar spine BMD, whereas albumin and ASMI were negatively correlated with lumbar spine BMD; albumin and the android/gynoid ratio were negatively correlated with femoral neck BMD, whereas height was positively correlated with femoral neck BMD; weight was positively correlated with total hip BMD, whereas the android/gynoid ratio was negatively correlated with total hip BMD.
| Factors | B | SE | t value | P value | 95%CI | |
| Lower | Upper | |||||
| Lumbar spine BMD | ||||||
| Age | -0.002 | 0.001 | -3.754 | < 0.001 | -0.003 | -0.001 |
| Height | 0.003 | 0.001 | 3.669 | < 0.001 | 0.001 | 0.005 |
| Diabetes duration | -0.003 | 0.001 | -2.665 | 0.008 | -0.005 | -0.001 |
| Albumin | -0.004 | 0.001 | -2.864 | 0.004 | -0.007 | -0.001 |
| Cr | 0.001 | 0.000 | 1.983 | 0.048 | 0.000 | 0.001 |
| β-CTX | -0.089 | 0.033 | -2.724 | 0.007 | -0.153 | -0.025 |
| TP1NP | -0.001 | 0.000 | -1.633 | 0.103 | -0.002 | 0.000 |
| ASMI | -0.040 | 0.016 | -2.449 | 0.015 | -0.072 | -0.008 |
| Lean mass index | 0.036 | 0.008 | 4.498 | < 0.001 | 0.020 | 0.051 |
| Femoral neck BMD | ||||||
| Age | -0.003 | 0 | -6.8 | < 0.001 | -0.003 | -0.002 |
| Height | 0.003 | 0.001 | 4.672 | < 0.001 | 0.002 | 0.004 |
| Diabetes duration | -0.002 | 0.001 | -3.617 | < 0.001 | -0.004 | -0.001 |
| Albumin | -0.003 | 0.001 | -2.698 | 0.007 | -0.005 | -0.001 |
| β-CTX | -0.100 | 0.018 | -5.589 | < 0.001 | -0.135 | -0.065 |
| Android/gynoid ratio | -0.045 | 0.02 | -2.259 | 0.024 | -0.084 | -0.006 |
| ASMI | 0.011 | 0.012 | 0.963 | 0.336 | -0.012 | 0.034 |
| Lean mass index | 0.014 | 0.006 | 2.457 | 0.014 | 0.003 | 0.025 |
| Total hip BMD | ||||||
| Age | -0.002 | 0.000 | -5.451 | < 0.001 | -0.003 | -0.001 |
| Weight | 0.002 | 0.001 | 3.142 | 0.002 | 0.001 | 0.003 |
| Diabetes duration | -0.002 | 0.001 | -3.327 | 0.001 | -0.004 | -0.001 |
| β-CTX | -0.082 | 0.019 | -4.403 | < 0.001 | -0.118 | -0.045 |
| Android/gynoid ratio | -0.041 | 0.02 | -2.003 | 0.046 | -0.081 | -0.001 |
| Lean mass index | 0.020 | 0.003 | 6.266 | < 0.001 | 0.014 | 0.026 |
Table 7 shows the determinants of ASMI using multivariate forward linear regression analysis after adjusting for age, sex, height, weight, diabetes duration, hypertension, SBP, DBP, GNRI, BMI, HbA1c, OS, β-CTX, TP1NP, albumin, Cr, UA, TG, TC, HDL-C, LDL-C and TBil; in men, age, diabetes duration and HbA1c were negatively correlated with ASMI, whereas weight and BMI were positively correlated with ASMI; in women, weight and OS were positively correlated with ASMI, whereas age, height, TBil and β-CTX were negatively correlated with ASMI.
| Factors | B | SE | t value | P value | 95%CI | |
| Lower | Upper | |||||
| Men | ||||||
| Age | -0.012 | 0.003 | -3.721 | < 0.001 | -0.019 | -0.006 |
| Weight | 0.019 | 0.006 | 2.983 | 0.003 | 0.006 | 0.031 |
| Diabetes duration | -0.014 | 0.005 | -2.587 | 0.010 | -0.025 | -0.003 |
| BMI | 0.136 | 0.022 | 6.238 | < 0.001 | 0.093 | 0.178 |
| HbA1c | -0.057 | 0.017 | -3.449 | 0.001 | -0.090 | -0.025 |
| Women | ||||||
| Age | -0.008 | 0.003 | -2.811 | 0.005 | -0.014 | -0.003 |
| Weight | 0.066 | 0.003 | 20.846 | < 0.001 | 0.059 | 0.072 |
| Height | -0.030 | 0.007 | -4.491 | < 0.001 | -0.043 | -0.017 |
| TBil | -0.017 | 0.007 | -2.369 | 0.018 | -0.032 | -0.003 |
| OS | 0.019 | 0.009 | 2.147 | 0.033 | 0.002 | 0.037 |
| β-CTX | -0.536 | 0.175 | -3.059 | 0.002 | -0.881 | -0.191 |
This study investigated associations among GNRI, BMD, and ASMI in T2DM patients. In this research, we discovered that proper nutrition, as denoted by a high GNRI, was linked to a lower HbA1c, higher BMD at all bone sites, higher lean mass index and higher ASMI. Based on prior research, this study utilized the GNRI and found that the GNRI was positively related to ASMI and BMD at all bone sites in T2DM patients. Additionally, a low lean mass index and higher β-CTX were associated with low BMD at all bone sites. Age was negatively correlated with ASMI, whereas weight was positively correlated with ASMI.
Despite the appropriate consumption, the nutrition of patients with T2DM was significantly impacted[22]. Diabetes speeds up the decline of muscle power, quality and serum albumin, highlighting the importance of maintaining a proper balance of protein and energy in one’s diet. The current investigation demonstrated that a decreased GNRI posed a notable hazard for diminished BMD and ASMI among individuals with T2DM. This finding is consistent with previous studies[23]. Studies have demonstrated that the GNRI can be applied as a convenient and reliable indicator of the BMD and ASMI conditions of patients with chronic hepatitis C[24], postmenopausal women who have undergone total thyroidectomy[25] and patients receiving hemodialysis[26]. Therefore, the GNRI might be a convenient and reliable indicator of BMD and ASMI status in patients with T2DM. As albumin level reflects protein status and is a major component of the GNRI, the effect of protein on bone and muscle may help to explain the associations between GNRI, BMD and ASMI.
The second important finding of this study is that a low GNRI was associated with a higher HbA1c. This indicates that the presence of malnutrition is not conducive to blood sugar control. In addition to drug therapy, the basic treatment regimen for type 2 diabetes patients is diet restriction and exercise to achieve the goal of controlling blood sugar. Malnutrition can result if there is no strict and regular diet strategy. A previous study has proven that hyperglycemia contributes to the accelerated decline in muscle mass among patients with T2DM[27]. Higher HbA1c levels may lead to an increased risk of low muscle mass via a variety of mechanisms. The main causes include insulin resistance, inflammation, and the production of glycation end products. Therefore, nutritional balance is beneficial to control blood sugar and reduce the incidence of sarcopenia. Individuals with type 2 diabetes, especially the elderly, need individualized dietary strategies to reduce the incidence of malnutrition. Regular nutritional assessments are necessary. People with type 2 diabetes can avoid the adverse effects of malnutrition by adjusting their diet.
At all bone sites, there was a correlation between low BMD and a high level of β-CTX, which is the third significant discovery of this research. β-CTX is derived from the degradation of type I collagen, and its content in bone collagen is much higher than that in the rest of the tissue, so it can be more representative and more directly reflect the degradation of bone matrix collagen and be used as an indicator of bone resorption. Bone homeostasis depends on the resorption and formation of bones. Long-term hyperglycemia can affect the adhesion of osteoblasts to collagen, causing dysfunction of osteoblasts, inhibiting bone formation and accelerating bone resorption, causing an increase in PINP and β-CTX. This may explain our finding of an association between a high β-CTX level and low BMD. β-CTX plays a critical role in bone turnover and is a sensitive marker for the early diagnosis of osteoporosis.
Another important finding of this study is that age was negatively correlated with ASMI. Sarcopenia is the age-related loss of muscle mass, strength, and function[28]. Degenerative changes in the structure and function of the human neuromuscular system occur with age, and the presence of diabetes accelerates the decline in muscle mass and strength through changes such as high levels of reactive oxygen species produced by oxidative stress and dysfunctional mitochondria. In this study, we also found a significant association between weight and ASMI. The majority of studies have shown that low BMI is also associated with sarcopenia[29]. Malnutrition, a potent risk factor for sarcopenia, could potentially account for the higher occurrence and frequency of sarcopenia in individuals with reduced body weight. Malnutrition, a potent risk factor for sarcopenia, might well explain the increased prevalence and incidence of sarcopenia in individuals with lower weight.
This study had multiple limitations. Because the study had a cross-sectional design, it was not possible to establish causal relationships. Furthermore, the participants chosen for this research encompassed both males and females spanning a wide age bracket of 21 to 81 years. T2DM patients of the same gender and age range have not been studied, but this study is closer to the clinical situation. Also, we only included participants who were hospitalized; we did not evaluate muscle strength and quality. In the end, although we did not consider that environmental pollutants (mainly air pollutants) is able to significantly affect both the clinical features of T2DM (mainly onset of disease and blood sugar control) and the nutritional status, we selected participants who lived in the same area for a long time. In the future, we will consider selecting participants from different regions of China for further research.
Poor nutrition, as indicated by a low GNRI, was associated with low levels of ASMI and BMD at all bone sites in T2DM patients. Using the GNRI to evaluate nutritional status and using DXA to investigate body composition in patients with T2DM is of value in assessing bone health and physical performance.
In people with type 2 diabetes mellitus (T2DM), the association between nutrition, sarcopenia, and osteoporosis has rarely been explored.
The relationship between nutritional status and bone mass has been observed in different populations, including individuals with chronic obstructive pulmonary disease, rheumatoid arthritis, and end-stage renal disease.
Assess the associations among nutrition, bone mineral density (BMD) and body composition in patients with T2DM.
A total of 689 patients with T2DM were included to perform a retrospective analysis. The general information and biochemical indices of these patients were statistically analyzed.
Those who had a high Geriatric Nutritional Risk Index (GNRI) tended to be younger and had lower HbA1c, higher BMD at all bone sites, and higher appendicular skeletal muscle index.
Poor nutrition, as indicated by a low GNRI, was associated with low levels of ASMI and BMD at all bone sites in type 2 diabetes mellitus patients.
We used a retrospective study to explore the association between nutrition, sarcopenia, and osteoporosis in patients with T2DM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen GX, United States; Di Ciaula A, Italy; Gica N, Romania; Horowitz M, Australia; Yang MW, China S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
| 1. | Yin L, Xu Z, Wang L, Li W, Zhao Y, Su Y, Sun W, Liu Y, Yang M, Yu A, Blake GM, Wu X, Veldhuis-Vlug AG, Cheng X, Hind K, Engelke K. Associations of Muscle Size and Density With Proximal Femur Bone in a Community Dwelling Older Population. Front Endocrinol (Lausanne). 2020;11:503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Miyamoto K, Hirayama A, Sato Y, Ikeda S, Maruyama M, Soga T, Tomita M, Nakamura M, Matsumoto M, Yoshimura N, Miyamoto T. A Metabolomic Profile Predictive of New Osteoporosis or Sarcopenia Development. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Liu X, Wang L, Xing Y, Engel SS, Zeng L, Yao B, Xu W, Chen G, Zhang Y, Zhang R, Liu S, Weng J, Ji Q. Efficacy and safety of metformin and sitagliptin-based dual and triple therapy in elderly Chinese patients with type 2 diabetes: Subgroup analysis of STRATEGY study. J Diabetes Investig. 2020;11:1532-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yamamoto M. Insights into bone fragility in diabetes: the crucial role of bone quality on skeletal strength. Endocr J. 2015;62:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Bitar MS, Nader J, Al-Ali W, Al Madhoun A, Arefanian H, Al-Mulla F. Hydrogen Sulfide Donor NaHS Improves Metabolism and Reduces Muscle Atrophy in Type 2 Diabetes: Implication for Understanding Sarcopenic Pathophysiology. Oxid Med Cell Longev. 2018;2018:6825452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q, Hu X, Tao R, Li L, Qian F, Yu L. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep. 2016;6:38937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 7. | Izzo A, Massimino E, Riccardi G, Della Pepa G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 8. | Murata Y, Kadoya Y, Yamada S, Sanke T. Sarcopenia in elderly patients with type 2 diabetes mellitus: prevalence and related clinical factors. Diabetol Int. 2018;9:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Ono Y, Miyakoshi N, Kasukawa Y, Akagawa M, Kimura R, Nagahata I, Yuasa Y, Sato C, Shimada Y. Diagnosis of Presarcopenia Using Body Height and Arm Span for Postmenopausal Osteoporosis. Clin Interv Aging. 2020;15:357-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Chen HL, Deng LL, Li JF. Prevalence of Osteoporosis and Its Associated Factors among Older Men with Type 2 Diabetes. Int J Endocrinol. 2013;2013:285729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Xiu S, Chhetri JK, Sun L, Mu Z, Wang L. Association of serum prealbumin with risk of osteoporosis in older adults with type 2 diabetes mellitus: a cross-sectional study. Ther Adv Chronic Dis. 2019;10:2040622319857361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Umpierrez GE, Pasquel FJ. Management of Inpatient Hyperglycemia and Diabetes in Older Adults. Diabetes Care. 2017;40:509-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 634] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 14. | Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, King C, Elia M. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr. 2004;92:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 794] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 15. | Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. 2001;56:M366-M372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1652] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 16. | Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1758] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 17. | Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1520] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 18. | Lakhdar R, Rabinovich RA. Can muscle protein metabolism be specifically targeted by nutritional support and exercise training in chronic obstructive pulmonary disease? J Thorac Dis. 2018;10:S1377-S1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Reina D, Gómez-Vaquero C, Díaz-Torné C, Solé JMN; Rheumatology Service. Hospital Moisès Broggi. Assessment of nutritional status by dual X-Ray absorptiometry in women with rheumatoid arthritis: A case-control study. Medicine (Baltimore). 2019;98:e14361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Tokumoto H, Tominaga H, Arishima Y, Jokoji G, Akimoto M, Ohtsubo H, Taketomi E, Sunahara N, Nagano S, Ishidou Y, Komiya S, Setoguchi T. Association between Bone Mineral Density of Femoral Neck and Geriatric Nutritional Risk Index in Rheumatoid Arthritis Patients Treated with Biological Disease-Modifying Anti-Rheumatic Drugs. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Nakanishi K, Shishido K, Kumata C, Ito K, Nakashima Y, Wakasa M. Bone density of the femoral neck in patients on maintenance dialysis. PLoS One. 2018;13:e0197965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Donnelly A. Nutritional requirements in malnutrition and diabetes mellitus. Nurs Stand. 2018;33:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wang L, Zhang D, Xu J. Association between the Geriatric Nutritional Risk Index, bone mineral density and osteoporosis in type 2 diabetes patients. J Diabetes Investig. 2020;11:956-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Bering T, Diniz KGD, Coelho MPP, Vieira DA, Soares MMS, Kakehasi AM, Correia MITD, Teixeira R, Queiroz DMM, Rocha GA, Silva LD. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J Cachexia Sarcopenia Muscle. 2018;9:255-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Chiu TH, Chen SC, Yu HC, Hsu JS, Shih MC, Jiang HJ, Hsu WH, Lee MY. Association between Geriatric Nutrition Risk Index and Skeletal Muscle Mass Index with Bone Mineral Density in Post-Menopausal Women Who Have Undergone Total Thyroidectomy. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Chen SC, Chung WS, Wu PY, Huang JC, Chiu YW, Chang JM, Chen HC. Associations among Geriatric Nutrition Risk Index, bone mineral density, body composition and handgrip strength in patients receiving hemodialysis. Nutrition. 2019;65:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Fang L, Zhong S, Ma D, Li C, Hao Y, Gao Y, Zhang L, Shen L. A cross-sectional study: an assessment of low muscle mass and osteoporosis in type 2 diabetes mellitus patients with a high glycated hemoglobin level. Ther Adv Chronic Dis. 2021;12:20406223211026762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Rotini A, Martínez-Sarrà E, Duelen R, Costamagna D, Di Filippo ES, Giacomazzi G, Grosemans H, Fulle S, Sampaolesi M. Aging affects the in vivo regenerative potential of human mesoangioblasts. Aging Cell. 2018;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Choi CJ, Choi WS, Kim CM, Lee SY, Kim KS. Risk of Sarcopenia and Osteoporosis in Male Tuberculosis Survivors: Korea National Health and Nutrition Examination Survey. Sci Rep. 2017;7:13127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |