Published online Dec 15, 2024. doi: 10.4239/wjd.v15.i12.2404
Revised: September 21, 2024
Accepted: October 15, 2024
Published online: December 15, 2024
Processing time: 92 Days and 6.5 Hours
In this article, we comment on an original article published in a recent issue of the World Journal of Diabetes. That observational cross-sectional study focused on investigating the function of the glymphatic system and its clinical correlates in patients with different glucose metabolism states by using diffusion tensor imaging along the perivascular space (DTI-ALPS) index. It was shown that the cerebral glymphatic system may be dysfunctional in patients with type 2 diabetes. Various clinical variables affected the DTI-ALPS index in different glucose metabolism states. In conclusion, the study by Tian et al improves the under
Core Tip: The study underscores the potential dysfunction of the glymphatic system in type 2 diabetes, highlighting its role in diabetic brain damage. Utilizing diffusion tensor imaging along the perivascular space can provide valuable insights into early diagnostic approaches.
- Citation: Velikova T, Vasilev G. Insights into glymphatic system dysfunction and glucose continuum. World J Diabetes 2024; 15(12): 2404-2408
- URL: https://www.wjgnet.com/1948-9358/full/v15/i12/2404.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i12.2404
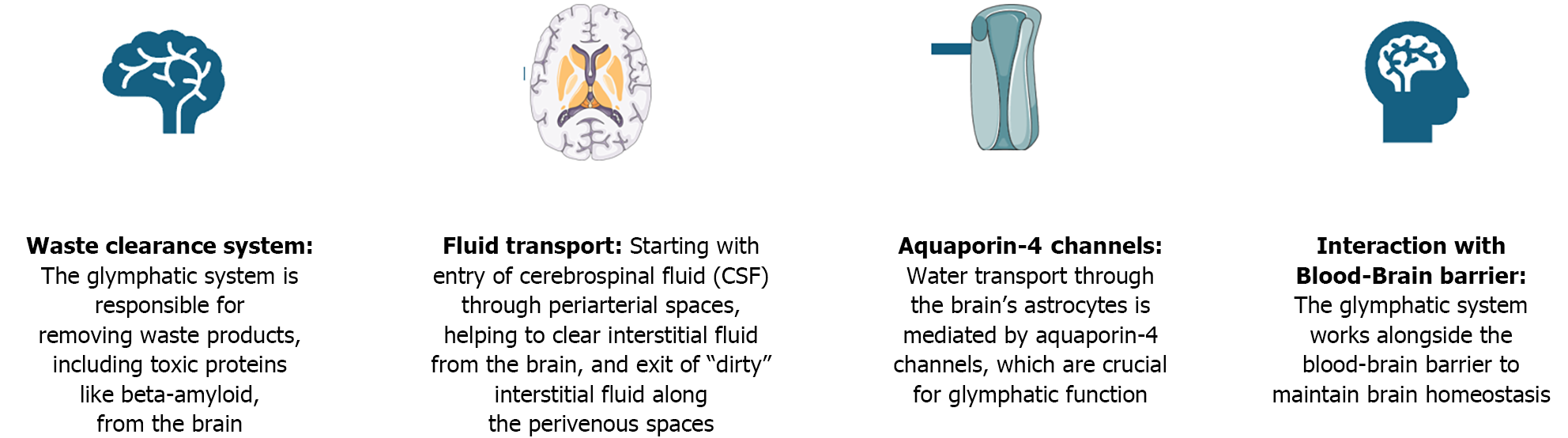
The glymphatic system, a macroscopic waste clearance system recently identified in the brain, is crucial for maintaining cerebral homeostasis. Functioning similarly to the peripheral lymphatic system, the glymphatic system facilitates the removal of metabolic waste products, including amyloid-, from the cerebral interstitial space[1]. The relationship between the glymphatic system and altered glucose metabolism extends across various stages of health and disease, influencing brain function and neurodegenerative processes. At different points in the spectrum of altered glucose metabolism, from normal function to insulin resistance and diabetes, the efficiency of the glymphatic system can be significantly affected[2]. The glymphatic system overview is presented in Figure 1.
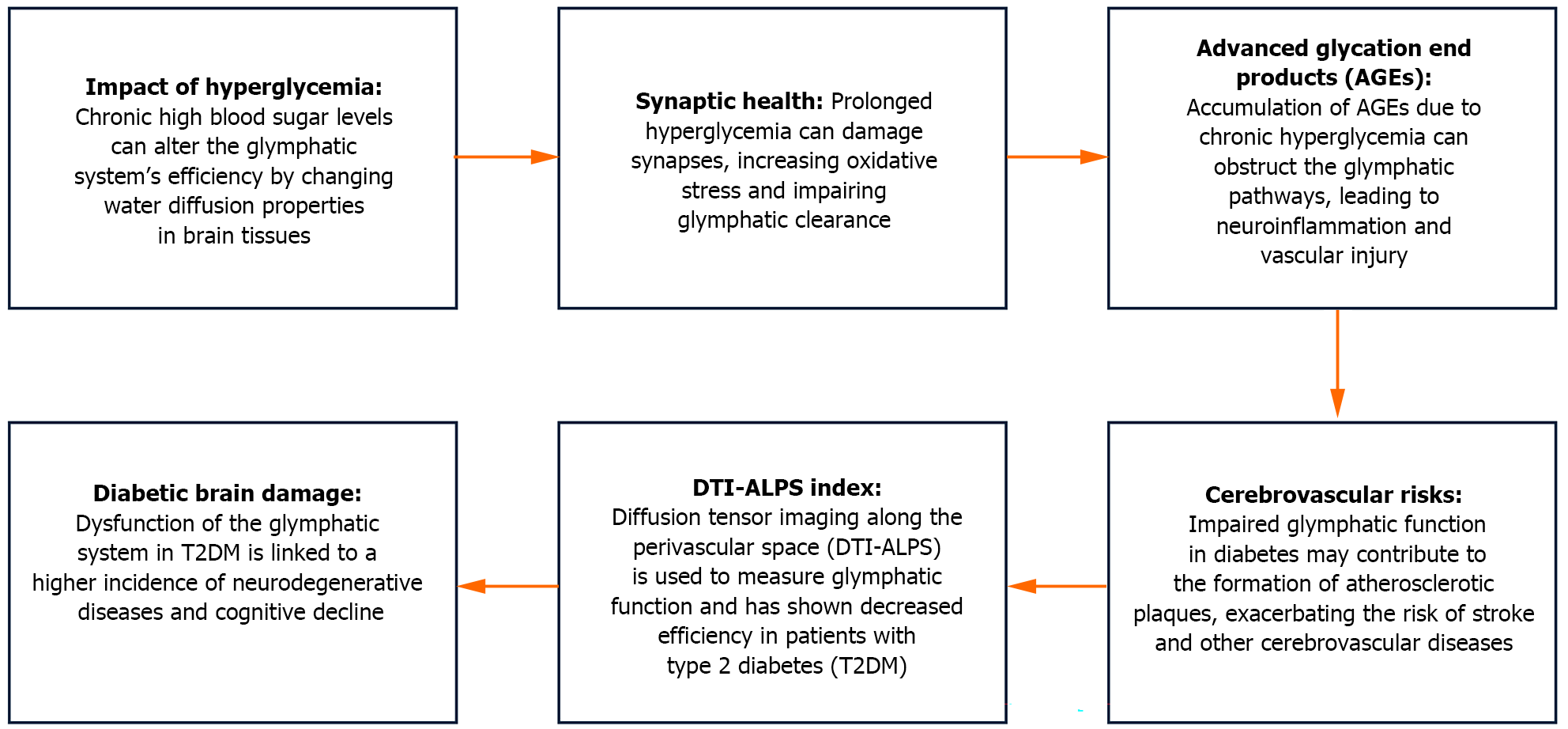
Glucose is the primary energy substrate in the brain, and its metabolism is tightly regulated to meet the high energy demands of neuronal activity. Alterations in glucose metabolism can significantly affect brain function. Hyperglycemia and hypoglycemia, a potential consequence of diabetes therapy, both pose risks to brain health. Prolonged hyperglycemia results in the buildup of advanced glycation end-products (AGEs) and increased oxidative stress, which hinders neuronal function and worsens neurodegenerative processes. Conversely, hypoglycemia can cause acute neuronal damage due to energy deprivation[2].
The glymphatic system functions as a critical waste clearance pathway in the central nervous system, relying on the flow of cerebrospinal fluid (CSF) to remove metabolic byproducts from the brain. This process is primarily facilitated by aquaporin (AQP)4 water channels, which are strategically positioned on the end-feet of astrocytes–star-shaped glial cells that play a pivotal role in maintaining brain homeostasis. In individuals with normal glucose metabolism, the glymphatic system operates optimally during periods of rest, particularly during deep sleep stages like slow-wave sleep, when the interstitial space in the brain expands, allowing for enhanced CSF influx and efficient waste product clearance. This clearance mechanism is vital for preventing the accumulation of neurotoxic substances, thus safeguarding neuronal function and integrity[3].
Hyperglycemia, a hallmark of diabetes mellitus, has been shown to disrupt the glymphatic system. Studies using diabetic animal models have demonstrated that chronic hyperglycemia leads to a reduction in AQP4 expression and mislocalization of these channels, impairing glymphatic clearance efficiency. This impairment may contribute to the accumulation of amyloid- and tau proteins, which are associated with Alzheimer's disease (AD) pathology. Diabetic patients are at a higher risk of developing vascular dementia, partly due to the interplay between impaired glymphatic function and vascular endothelial dysfunction[4].
Hypoglycemia, although less common, poses significant risks to brain function. Acute hypoglycemia can lead to neuronal injury due to insufficient glucose supply, resulting in energy failure and neuronal death. The impact of hypoglycemia on the glymphatic system is less well studied. It is plausible that energy deficits during hypoglycemic episodes could impair the active transport mechanisms required for adequate glymphatic clearance. This impairment could exacerbate the accumulation of neurotoxic waste products during prolonged or recurrent hypoglycemic episodes, potentially contributing to cognitive deficits observed in some diabetic patients undergoing intensive insulin therapy[4].
The glymphatic system is most active during sleep, particularly during slow-wave sleep. Sleep disturbances, which are common in diabetes, can, therefore, impair glymphatic function (Figure 2)[5,6]. Poor sleep quality and reduced slow-wave sleep duration have been associated with decreased glymphatic clearance, potentially linking sleep disorders with the exacerbation of neurodegenerative processes in diabetic patients[3,7]. Additionally, obstructive sleep apnea, frequently observed in individuals with obesity and type 2 diabetes mellitus (T2DM), has been shown to impair glymphatic clearance due to intermittent hypoxia and sleep fragmentation[8].
Impaired glymphatic function has been implicated in the pathogenesis of several neurodegenerative diseases, including AD and Parkinson's disease. In the context of diabetes, the chronic metabolic imbalance exacerbates the impairment of the glymphatic system, accelerating the accumulation of toxic proteins. This cumulative effect not only increases the risk of neurodegeneration but also contributes to the severity and progression of cognitive decline in diabetic patients[2].
Understanding the relationship between the glymphatic system and glucose metabolism opens new avenues for therapeutic interventions to preserve brain health in metabolic disorders. Strategies to enhance glymphatic clearance, such as optimizing sleep quality, regulating glucose levels, and potentially targeting AQP4 channels, could mitigate the risk of neurodegenerative diseases in diabetic patients. Additionally, interventions to improve insulin sensitivity and reduce chronic hyperglycemia may enhance glymphatic function and provide neuroprotective benefits[9].
The case observational cross-sectional study by Tian et al[10], "Glymphatic function and its influencing factors in di
The glymphatic system remains poorly understood. Nonetheless, there is evidence regarding its dysfunction in the brain across various phases of disturbed glucose metabolism. However, the specific factors associated with the glymphatic system are not well defined. The authors enrolled 22 patients with normal glucose metabolism, 20 with prediabetes, and 22 with T2DM. Glymphatic system function was examined by 3.0T magnetic resonance imaging. The authors used the diffusion tensor imaging along the perivascular space (DTI-ALPS) index to assess the glymphatic system, and evaluated overall cognitive function using the mini-mental state examination. After analyzing the results, the authors concluded that the cerebral glymphatic system dysfunction was likely to manifest predominantly during T2DM. The study identified several clinical factors that influence the DTI-ALPS index across different states of glucose metabolism. Overall, the research improves the comprehension of the mechanisms underlying brain damage associated with diabetes and offers potential biological markers for early diagnosis.
Tian et al[10] found that the most notable findings included a positive correlation between the left-side and mean DTI-ALPS index (after adjusting for sex) and 2-h postprandial blood glucose levels in the prediabetes group. The left-side DTI-ALPS index showed a negative correlation with total cholesterol and low-density lipoprotein levels. Conversely, in the prediabetes group, the right-side and mean DTI-ALPS index were negatively associated with glycosylated hemoglobin levels and the waist-to-hip ratio. In the T2DM group, the left-side, right-side and mean DTI-ALPS index showed a positive correlation with height. However, the left-side and mean DTI-ALPS index were negatively correlated with high-density lipoprotein levels in the T2DM group.
The study had some limitations, including a small sample size and some methodological concerns (e.g., the use of multiple b-value acquisitions suggests that the DSI acquisition method might be considered the most optimal protocol for DTI-ALPS scanning). However, the limitations were not serious, but they could inform future research.
The authors highlighted that chronic hyperglycemia may alter the water diffusion properties within brain tissue. Over time, sustained high blood sugar levels can affect synapse formation, exacerbate oxidative stress responses, and result in the buildup of AGEs. This can accelerate the formation of atherosclerotic plaques, leading to neuronal and vascular damage. These processes contribute to a higher risk of peripheral vascular complications[11,12].
Consequently, further research on this topic will help to elucidate the role of the glymphatic system in glucose me
The study by Tian et al[10] enhances our comprehension of the role of the glymphatic system in T2DM-related brain damage. To build on these findings, we need further research, that is, longitudinal studies to explore the long-term effects of glymphatic dysfunction in T2DM and clinical integration by incorporating DTI-ALPS imaging in routine assessments for patients with T2DM to identify early signs of brain damage. Additionally, personalized interventions and targeted strategies based on glymphatic dysfunction to prevent or mitigate brain-related complications in diabetes would benefit patients with T2DM. Nevertheless, a cross-disciplinary collaboration between endocrinologists and neurologists can foster integrating glymphatic system assessments into diabetes management protocols.
| 1. | Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2426] [Cited by in RCA: 3689] [Article Influence: 307.4] [Reference Citation Analysis (0)] |
| 2. | Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide. Neurochem Res. 2015;40:2583-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 1260] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 3. | Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2433] [Cited by in RCA: 3294] [Article Influence: 274.5] [Reference Citation Analysis (0)] |
| 4. | Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37:1326-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 5. | Voumvourakis KI, Sideri E, Papadimitropoulos GN, Tsantzali I, Hewlett P, Kitsos D, Stefanou M, Bonakis A, Giannopoulos S, Tsivgoulis G, Paraskevas GP. The Dynamic Relationship between the Glymphatic System, Aging, Memory, and Sleep. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 6. | Kim YK, Nam KI, Song J. The Glymphatic System in Diabetes-Induced Dementia. Front Neurol. 2018;9:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Andica C, Kamagata K, Takabayashi K, Kikuta J, Kaga H, Someya Y, Tamura Y, Kawamori R, Watada H, Taoka T, Naganawa S, Aoki S. Neuroimaging findings related to glymphatic system alterations in older adults with metabolic syndrome. Neurobiol Dis. 2023;177:105990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 8. | Huang Y, Yang C, Yuan R, Liu M, Hao Z. Association of obstructive sleep apnea and cerebral small vessel disease: a systematic review and meta-analysis. Sleep. 2020;43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Lohela TJ, Lilius TO, Nedergaard M. The glymphatic system: implications for drugs for central nervous system diseases. Nat Rev Drug Discov. 2022;21:763-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 105] [Reference Citation Analysis (0)] |
| 10. | Tian B, Zhao C, Liang JL, Zhang HT, Xu YF, Zheng HL, Zhou J, Gong JN, Lu ST, Zeng ZS. Glymphatic function and its influencing factors in different glucose metabolism states. World J Diabetes. 2024;15:1537-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Shen T, Yue Y, Ba F, He T, Tang X, Hu X, Pu J, Huang C, Lv W, Zhang B, Lai HY. Diffusion along perivascular spaces as marker for impairment of glymphatic system in Parkinson's disease. NPJ Parkinsons Dis. 2022;8:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 12. | Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 1044] [Article Influence: 149.1] [Reference Citation Analysis (0)] |