Published online Nov 15, 2024. doi: 10.4239/wjd.v15.i11.2203
Revised: September 3, 2024
Accepted: September 18, 2024
Published online: November 15, 2024
Processing time: 227 Days and 5.4 Hours
Gestational diabetes mellitus (GDM) women require prenatal care to minimize short- and long-term complications. The mechanism by which exercise during pregnancy affects organ development and whether glucose transporter (GLUT) 1 plays a role in GDM offspring organ development remains unknown.
To determine the effect of exercise during pregnancy on the cardiac, hepatic and renal development of GDM mother’s offspring.
Placenta samples were collected from humans and mice. GDM mouse models were created using streptozotocin along with a GDM with exercise group. The hearts, livers and kidneys of 3- and 8-week-old offspring were collected for body composition analysis and staining. The effects of high glucose levels and hypoxia were investigated using HTR8/SVneo. Transwell and wound-healing assays were performed to assess cell migration. Immunofluorescence accompanied with TUNEL and Ki67 staining was used to explore apoptosis and proliferation.
Exercise during pregnancy downregulated the GLUT1 and hypoxia inducible factor-1α expression in placenta from individuals with GDM. Cobalt chloride-induced hypoxia and high glucose levels also significantly decreased migration and apoptosis of HTR8/SVneo cells. In addition, exercise reduced inflammatory cell infiltration in the liver and decreased the tubular vacuolar area in the kidneys of offspring.
GDM affects the growth and development of organs in offspring. Exercise during pregnancy can reverse adverse effects of GDM on the development of the heart, liver, and kidney in offspring.
Core Tip: We established a mouse model of gestational diabetes mellitus (GDM) and an exercise model during pregnancy. We discussed placental glucose transporter (GLUT) 1 expression and the hypoxic microenvironment, and the correlation between them. We also investigated organ development in the heart, liver, and kidney of the offspring. Our findings suggest that exercise during pregnancy is beneficial for organ development in the offspring of GDM. This benefit may be realized by modulating GLUT 1 expression and improving placental hypoxia. We hope that this study will emphasize the importance of exercise during pregnancy in women with GDM to improve its effects on organ development in the offspring.
- Citation: Tang YB, Wang LS, Wu YH, Zhang LX, Hu LY, Wu Q, Zhou ML, Liang ZX. Effect of exercise during pregnancy on offspring development through ameliorating high glucose and hypoxia in gestational diabetes mellitus. World J Diabetes 2024; 15(11): 2203-2219
- URL: https://www.wjgnet.com/1948-9358/full/v15/i11/2203.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i11.2203
Gestational diabetes mellitus (GDM) is defined as a diabetic or pre-diabetic state characterized by the onset or first detection of diabetes during pregnancy. GDM increases the risk of adverse pregnancy outcomes, such as macrosomia, preeclampsia, and premature delivery[1,2]. Barker’s hypothesis “Origins of Health and Disease Development” suggests that prenatal factors affect the fetal development and, thus, exert long-term effects on the physical and mental health of the offspring[3,4]. Placenta formation is critical for the intrauterine environment and prenatal factors. Hyperinsulinemia and hyperglycemia affect placenta formation and trophoblast invasion[5]. These processes are essential for establishing adequate oxygen supply to the placental unit. Clinical studies have shown a strong correlation between placental dysfunction, especially hypoxia, and cardiac dysplasia in offspring[6]. All women diagnosed with GDM require prenatal care to minimize short- and long-term complications.
Exercise intervention is an important clinical tool in the management and treatment of pregnant women with GDM to improve maternal and neonatal prognoses. A recent meta-analysis highlighted the key role of exercise, which is thought to alter the structure and physiological mechanisms of the placenta, in the management of GDM. Studies have shown that exercise during pregnancy affects placental oxidative stress, nutrient transport proteins, and angiogenic factors[7]. Although there are numerous benefits of exercise during pregnancy, the biological mechanisms by which these benefits are passed on from patients with GDM to their offspring are not well understood. Numerous studies have shown that maternal exercise improves the metabolic health of offspring by enhancing mitochondrial biosynthesis in the gut and liver[8-12]. In addition, maternal exercise is beneficial for proximal and long-term neurological development of offspring[13]. Although the effects of exercise on metabolism-related diseases in GDM progeny have been explored, the mechanism by which exercise during pregnancy affects organ development remains unclear.
Placental glucose flow during pregnancy is mediated by glucose transporters (GLUT). To date, six GLUT proteins (GLUT 1, GLUT 3, GLUT 4, GLUT 8, GLUT 9, and GLUT 12) have been identified in the human placenta[14]. GLUT 1 is a prevalent isoform in humans, and its increased expression in the placenta is associated with the development of macrosomia[15]. Fetal organ development is finely regulated by various factors, including body fluid signals and mechanical forces, many of which are controlled by the placenta. GLUT 1 is specifically expressed in the heart during the embryonic and early postnatal periods. Decreased GLUT 1 expression in the adult heart is associated with decreased myocardial contractile function[16]. GLUT 1 expression in hepatocytes promotes gluconeogenesis at low glucose concentrations[14]. However, whether GLUT 1 plays a role in GDM offspring organ development remains unknown.
In this study, we aimed to investigate the effect of exercise during gestation on the heart, liver, and kidney of GDM offspring during different growth periods and the role of GLUT 1. Our findings lay a foundation for the clinical management of females with GDM to ameliorate its adverse effects on offspring organ development.
Placenta samples were collected from women with GDM (n = 20) and those with normal pregnancies (n = 20) at the Women’s Hospital School of Medicine , Zhejiang University. None of the participants enrolled in this study had history of preeclampsia, hypertensive disorders, chronic diseases, smoking or drinking habits, fetal anomalies, intrauterine fetal growth restriction, or infection. The placenta samples were obtained within 30 min of cesarean delivery and transported to the laboratory in dry ice. A portion of the samples was stored in a refrigerator at −80 °C and the remainder were placed in 4% paraformaldehyde fixative for storage at room temperature. All participants provided written informed consent, and the experiment was approved by the Local Ethics Committee at the Women’s Hospital School of Medicine, Zhejiang University.
All experimental procedures were approved by the Institutional and Local Committee on the Care and Use of Animals of Zhejiang University (Hangzhou, China). All animals received humane care in accordance with the National Institutes of Health (United States) guidelines. Sexually mature male Institute of Cancer Research mice were purchased from the Zhejiang Academy of Medical Sciences (Hangzhou, China). The presence of a vaginal plug indicated embryonic day 0.5 (denoted as E0.5) of gestation. Intraperitoneal injection with streptozotocin (STZ) (50 mg/kg in 0.4 mmol/L citrate buffer; pH 4.5) or citrate buffer (200 μL) was administered on E1.5 of gestation for four consecutive days. Random plasma glucose (RPG) was measured using a one touch Ultra2 glucose meter (Yuyue) to determine the diabetic status at E7.5. Mice with RPG levels > 11 mmol/L were considered to have GDM (mGDM group). Voluntary wheel running in rodents is a model of locomotion that is more similar to natural human patterns than forced locomotion and has been widely used in studies of responses to exercise[17]. Therefore, GDM mouse models with exercise (mGE) had ad libitum access to a treadmill wheel in their cages throughout the gestational period until delivery. The mice in the control and mGDM groups were also placed on a static treadmill wheel and exposed to identical environmental conditions. Five mice from each group were euthanized for placental extraction. The remaining mice gave birth after 18–20 d of gestation. Offspring from all experimental groups were fed a normal diet without exercise intervention and sacrificed at 3 weeks (weaning period) and 8 weeks (sexually mature period). Anesthetized animals (5% isoflurane, 1 L/m O2-induced; 2.5% isoflurane, 0.4 L/m O2) were fasted overnight between 8: 00 pm and 8: 00 am. The thoracic and abdominal cavities were opened and the heart, liver, and kidney were removed, and subsequently rinsed with ice-cold PBS, weighed, and separated for histochemical and reverse-transcriptase polymerase chain reaction (RT-PCR) analyses. A flowchart of the mouse model and sampling is shown in Supplementary Figure 1.
The mice were subjected to an oral glucose tolerance test (OGTT) on embryonic day E13.5 to confirm GDM-related glucose intolerance during pregnancy. Glucose was administered at a dose of 2 g/kg of body weight. Moreover, the animals were fasted for 12-hour prior to the OGTT given that prolonged hypoglycemia in pregnancy could influence fetal development. Tail vein blood samples were collected at 0, 30, 60, and 120 minute after injection. The plasma glucose concentration was measured using the One Touch Ultra2 glucose meter (Yuyue, China). The area under the curve of glucose vs time was calculated to analyze glucose tolerance.
The human placental trophoblast cell line, HTR8/SVneo, purchased from Procell Life Science and Technology Co., Ltd. (Wuhan, China), was cultured were cultivated in RPMI-1640 medium (Procell, China) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics. To mimic gestational diabetes environment in vitro, 25 mmol/L high glucose was applied to culture the HTR8/SVneo cells for 24 hours. Subsequently, HTR8/SVneo cells were treated with cobalt chloride (CoCl2) to induce a hypoxic environment for 24 hours. The HTR8/SVneo cells maintained in media containing 11 mmol/L glucose were used as the control group.
Small interfering RNA (siRNA) plasmids targeting GLUT 1 (si-GLUT1) and empty siRNA plasmid (siRNA-NC) were synthesized by Gene Pharma (Shanghai, China). GP transfection mate (Gene Pharma, China) was applied to transfect the above recombinants into HTR8/SVneo cells according to the manufacturer’s instructions.
The migration capability of HTR8/SVneo cells was evaluated using a transwell assay. The cells were trypsinized and suspended at a final concentration of 2 × 105 cells/mL. Cell suspensions were placed in upper wells, while medium containing 10% FBS was placed in the bottom chamber. Subsequently, cell cultivation was performed for 8h. Subsequently, HTR8/SVneo cells on the lower surface were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The invading cells were finally counted under a microscope. Five randomly selected fields were counted for each group.
HTR8/SVneo cells were seeded in 6-well plates and cultivated to achieve 80%–90% confluence. Cell monolayers were wounded using a white pipette tip, and then rinsed with serum-free medium. Subsequently, the number of cultivated cells were recorded at 0 and 24 hours. Five randomly chosen fields were analyzed in each well.
Using the 2-Deoxy-2- [(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose (2-NBDG) probe, the cell glucose intake capacity was evaluated according to a standard protocol. Initially, PBS-rinsed cells were resuspended in a binding buffer. After being subjected to 5 μL of 2-NDBG operating fluid for 30 min, cells were washed twice by PBS. Cyto FlexS was used to analyze the cell glucose intake capacity.
Total RNA was extracted using a commercial TRIzol RNA isolation kit (Takara Bio, Japanese). The RNA concentration and purity were measured with a NanoDrop ND1000 spectrophotometer (Thermo Fisher Scientific, Australia). RNA was converted to cDNA using the RT Master Mix for qPCR with gDNA remover (Abclonal, China). RT-qPCR was performed with a SYBR Green Fast qPCR Mix (Abclonal, China). The primer sequences used for RT-qPCR are presented in Supplementary Table 1. The mean threshold cycle (CT) values were normalized to those of β-actin, and the relative mRNA levels of GLUT1 (slc2a1), GLUT3 (slc2a3), GLUT4 (slc2a4), GLUT8 (slc2a8), GLUT9 (slc2a9), and GLUT12 (slc2a12) were calculated using the theory and method of double standard curves. Calculation formula: A= CT value - CT value of β-actin; B=2-A; Relative mRNA expression(fold)=B/B of control group.
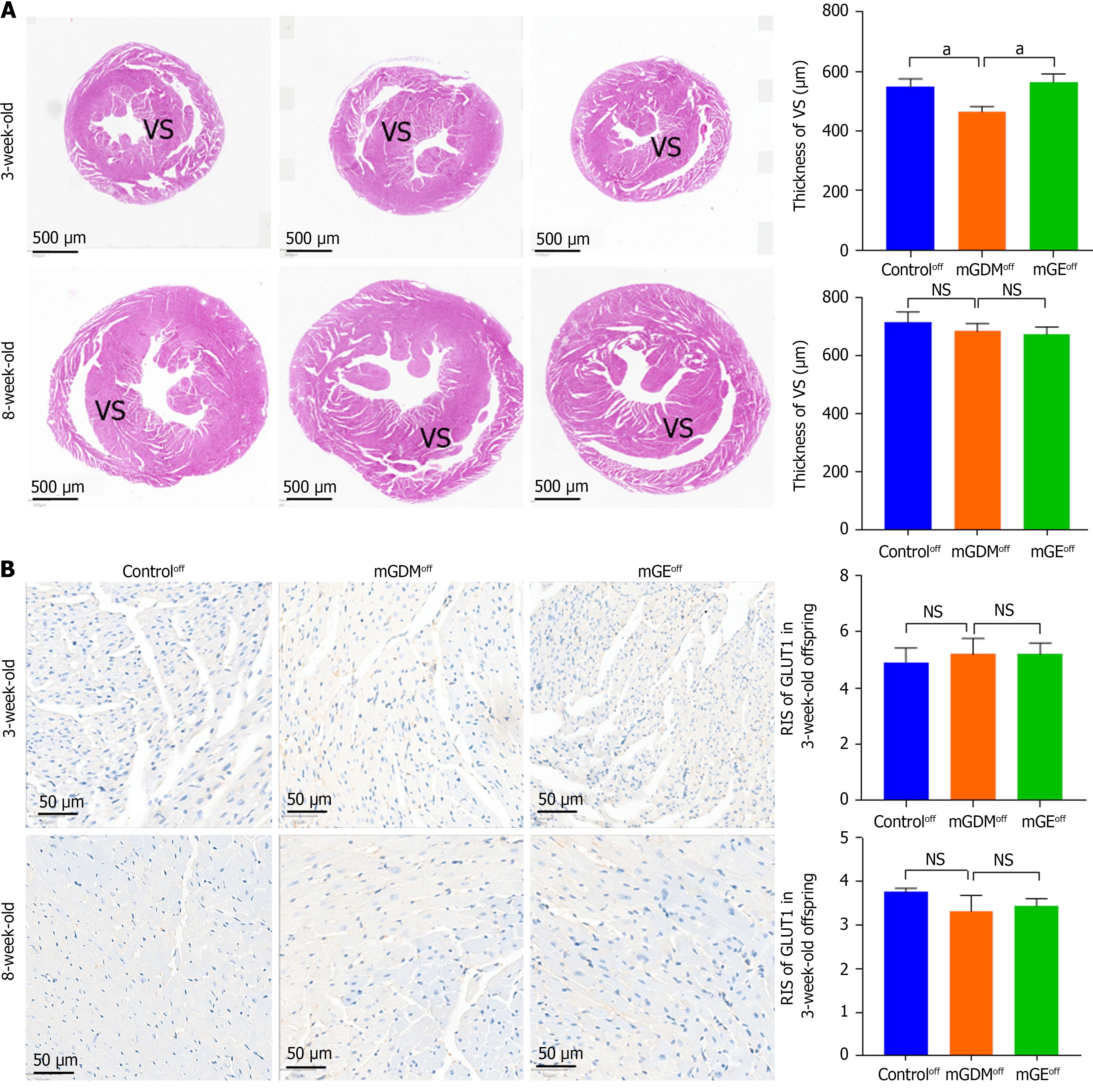
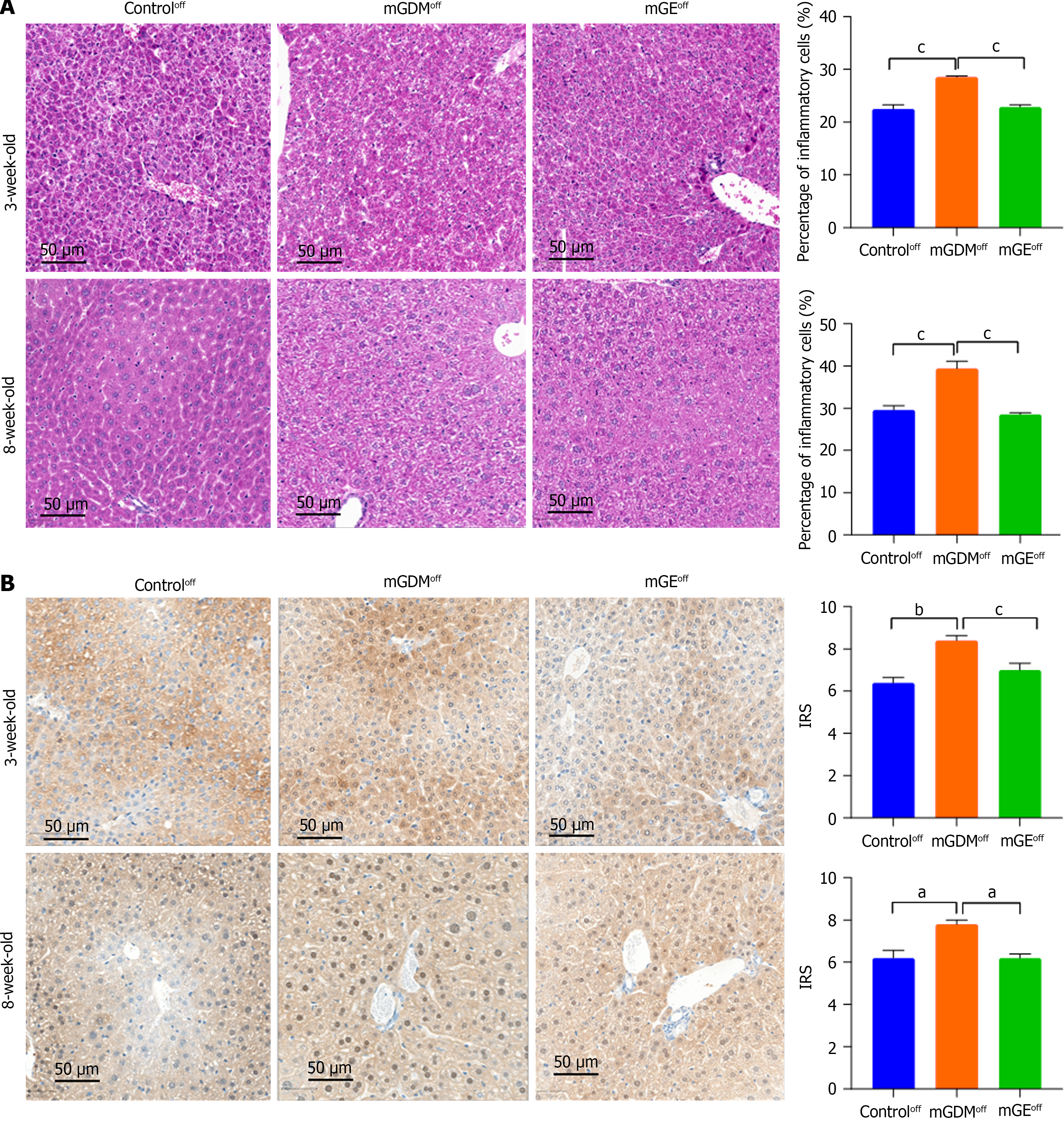
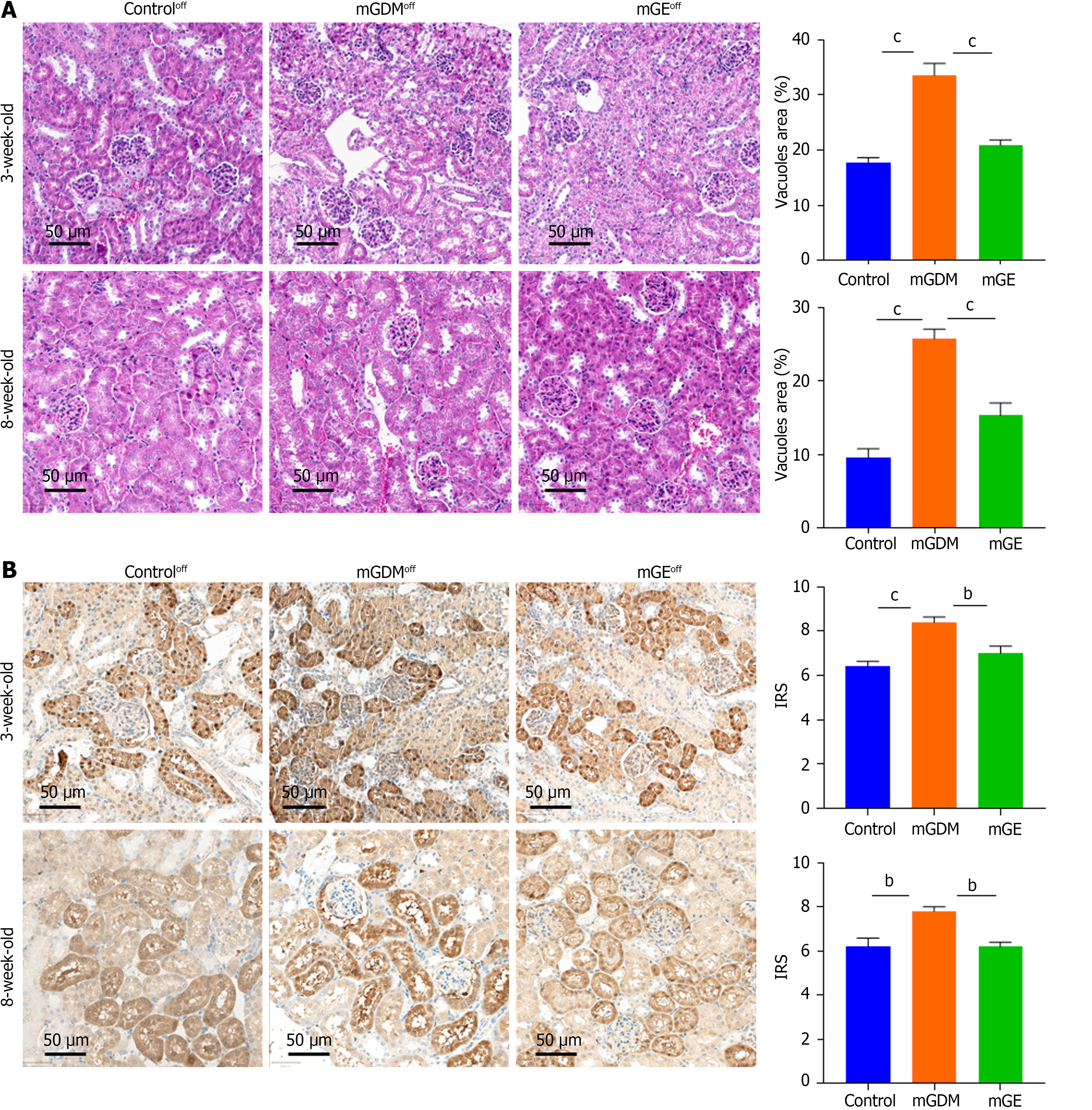
The hearts of weaning and sexually mature mice were fixed in 4% paraformaldehyde and PBS to examine whether maternal hyperglycemia altered the morphology of the heart, liver, and kidney. The heart and liver samples were dehydrated, embedded in paraffin wax, and cut into of 3-μm thick sections for hematoxylin and eosin (HE) staining. Images of the slices were obtained using a microscope (Ocus, Grundium) equipped with Qupath software (version 1.2). The average thickness of the ventricular septum (VS), average percentage of inflammatory cells, and vacuole area of the renal tissue were measured using image J software.
Protein expression of GLUT1 was detected by immunohistochemistry (IHC) analysis in paraffin-embedded placental tissues from female humans and mice with or without GDM as well as in the hearts of from mouse offspring. Sections were incubated overnight with 1:1000 anti-GLUT1 antibody (Proteintech, Wuhan, China) at 4 °C and then incubated with horseradish peroxidase (HRP)-coupled secondary antibody (dilution ratio of 1:2000; Proteintech, Wuhan, China) for 1 hour at room temperature. Positive signals were observed under a light microscope (Thermo Fisher Scientific, United States). Signal intensity was classified as 1 = weak, 2 = medium, and 3 = strong, and the number of positive cells was classified as 0% = negative, 1%–25% = 1, 26%–50% = 2, and 51%–75% = 3. The level of GLUT1 expression was semi-quantified according to the immunohistochemical score (IRS = intensity × frequency) and classified as low or high expression according to the median.
Quantification of total proteins, which were isolated from the placenta and HTR8/SVneo cells in RIPA buffer (Fude, China) was performed using the BCA Protein Assay Kit (Fude, China). After the separation by 10% SDS-PAGE, the proteins were transferred onto a polyvinylidene fluoride membrane (Biorad, United States). Next, the membranes were cultivated overnight with 5% non-fat milk in 0.1% tris-buffered saline with Tween-20, and primary antibodies targeting hypoxia inducible factor-1α (HIF-1α) (Proteintech, China), GLUT 1 (Proteintech, China), and β-Actin at 4 °C. The membranes were then incubated with secondary antibody for 1 h the following day. Finally, an enhanced chemiluminescence detection system (Fude, China) was used to visualize protein bands according to the manufacturer’s instructions. Image J was used for quantification.
The apoptosis and proliferation levels of HTR8/Sveo were detected by TUNEL and Ki67 immunofluorescence staining, respectively. Cell crawls were prepared first, and then cells were fixed with 4% paraformaldehyde. The cells were permeabilized with 1% tritonX100 for 20 min, and then incubated with goat serum for 30 min at room temperature. Sections were incubated overnight with 1:200 anti-Ki67 antibody (Proteintech, Wuhan, China) at 4 °C and then incubated with Alexa594-coupled secondary antibody (dilution ratio of 1:500; Abcam, United States) for 1 hour at room temperature. Another batch of sections was incubated dropwise with TUNEL (Roche, German) working solution at 37 °C for 2 hours. A small amount of DAPI-containing anti-fluorescence quenching sealer was added dropwise, and the coverslip was covered to seal the section. Positive signals were developed and observed under Confocal microscopy. The cell proliferation or apoptosis ratio was analyzed by Image J Software.
The results are presented as the mean ± SEM. Statistical analyses were performed using GraphPad Prism (version 8.0). Data were analyzed using a t-test or one-way analysis of variance, with the significance level set at P < 0.05.
The clinical characteristics of the study participants are listed in Table 1. The 1- and 2-hour plasma glucose levels in the GDM group were significantly higher than those in the normal group. No differences were observed in maternal age, gravidity, parity, pre-pregnancy weight, gestational weight gain (GWG), pre-pregnancy body mass index, fasting plasma glucose (FPG), third-trimester HbA1c, neonatal birth weight, and placental weight.
| Normal (n = 20) | GDM (n = 20) | P value | |
| Age (years) | 29.6 ± 0.646 | 31.7 ± 0.915 | 0.07 |
| Gravidity | 1.2 ± 0.156 | 1.3 ± 0.128 | 0.62 |
| Parity | 1.15 ± 0.082 | 1.35 ± 0.109 | 0.15 |
| Pre-pregnancy weight (kg) | 68.19 ± 2.202 | 65.32 ± 1.45 | 0.28 |
| Gestational weight gain (kg) | 14.025 ± 0.806 | 12.39 ± 0.901 | 0.18 |
| Pre-pregnancy BMI (kg/cm2) | 26.269 ± 0.706 | 25.466 ± 0.395 | 0.43 |
| OGTT during 24-28 week (mmol/L) | |||
| Fasting plasma glucose | 4.353 ± 0.068 | 4.509 ± 0.094 | 0.19 |
| 1-hour plasma glucose | 7.338 ± 0.328 | 9.285 ± 0.422 | < 0.001 |
| 2-hour plasma glucose | 6.371 ± 0.266 | 8.612 ± 0.381 | < 0.001 |
| Third trimester HbA1c (%) | 5.25 ± 0.074 | 5.46 ± 0.108 | 0.12 |
| Fetal sex | 0.74 | ||
| Male | 6 (30) | 7 (35) | |
| Female | 14 (70) | 13 (65) | |
| Neonatal birthweight (g) | 3254.5 ± 110.307 | 3176 ± 105.732 | 0.61 |
| Placental weight (g) | 531.579 ± 12.502 | 522.143 ± 14.645 | 0.63 |
The characteristics of the mice are summarized in Table 2. Thirty pregnant mice, 10 each from the control, mGDM, and mGE groups, were used in the experiments. RPG was significantly higher in mGDM group compared than in the control group, indicating successful modeling. Exercise during pregnancy can partially control RPG in pregnant GDM mice. Moreover, significant differences were observed in the OGTT results among the control, mGDM, and mGE groups (FPG, 1-hour plasma glucose, and 2-hour plasma glucose). Plasma insulin and HOMA-IR showed insulin resistance in GDM mice, and exercise during pregnancy significantly alleviated this symptom. Additionally, mGDM mice also exhibited lower GWG than the control group, and running exercise partially reversed this phenomenon. Other factors, such as gestational days, number of litters, pre-pregnancy weight, and offspring (F1) weight at 0, 3 and 8 weeks, were similar and demonstrated no significant difference.
| Control (n = 10) | mGDM (n = 10) | mGE (n = 10) | P value | |
| Gestational days | 19.3 ± 0.483 | 19.3 ± 0.483 | 19.4 ± 0.516 | 0.99 |
| Number of litters | 12.9 ± 2.132 | 11.9 ± 2.846 | 12.4 ± 1.897 | 0.39 |
| Pre-pregnancy weight (g) | 31.105 ± 2.578 | 31.826 ± 3.608 | 30.93 ± 3.180 | 0.61 |
| Gestational weight gain (g) | 19.295 ± 3.884 | 9.184 ± 3.623 | 14.54 ± 4.358 | < 0.001 |
| RPG (mmol/L)1 | 9.68 ± 2.411 | 30.2 ± 3.008 | 21.48 ± 7.229 | < 0.001 |
| OGTT at E13.5 (mmol/L)2 | ||||
| Fasting plasma glucose2 | 5.24 ± 0.875 | 17.05 ± 5.368 | 9.83 ± 3.522 | < 0.001 |
| 0.5-hour plasma glucose2 | 15.13 ± 4.004 | 29.84 ± 3.59 | 26.990 ± 6.033 | < 0.001 |
| 1-hour plasma glucose2 | 12.18 ± 3.260 | 32.0 ± 1.928 | 27.03 ± 6.045 | < 0.001 |
| 2-hour plasma glucose2 | 9.75 ± 2.396 | 27.56 ± 4.567 | 21.83 ± 9.758 | < 0.001 |
| Plasma insulin (μU/mL)2 | 1.756 ± 0.795 | 3.129 ± 1.013 | 3.761 ± 0.866 | < 0.05 |
| HOMA-IR2 | 0.416 ± 0.215 | 2.531 ± 1.545 | 1.70 ± 0.872 | < 0.001 |
| F1-weight at 0 week | 0.98 ± 0.019 | 0.910 ± 0.059 | 0.840 ± 0.022 | 0.29 |
| F1-weight at 3 weeks | 6.790 ± 0.734 | 5.212 ± 0.614 | 6.602 ± 0.595 | 0.14 |
| F1-weight at 8 weeks | 30.222 ± 1.294 | 29.658 ± 0.864 | 30.370 ± 1.129 | 0.73 |
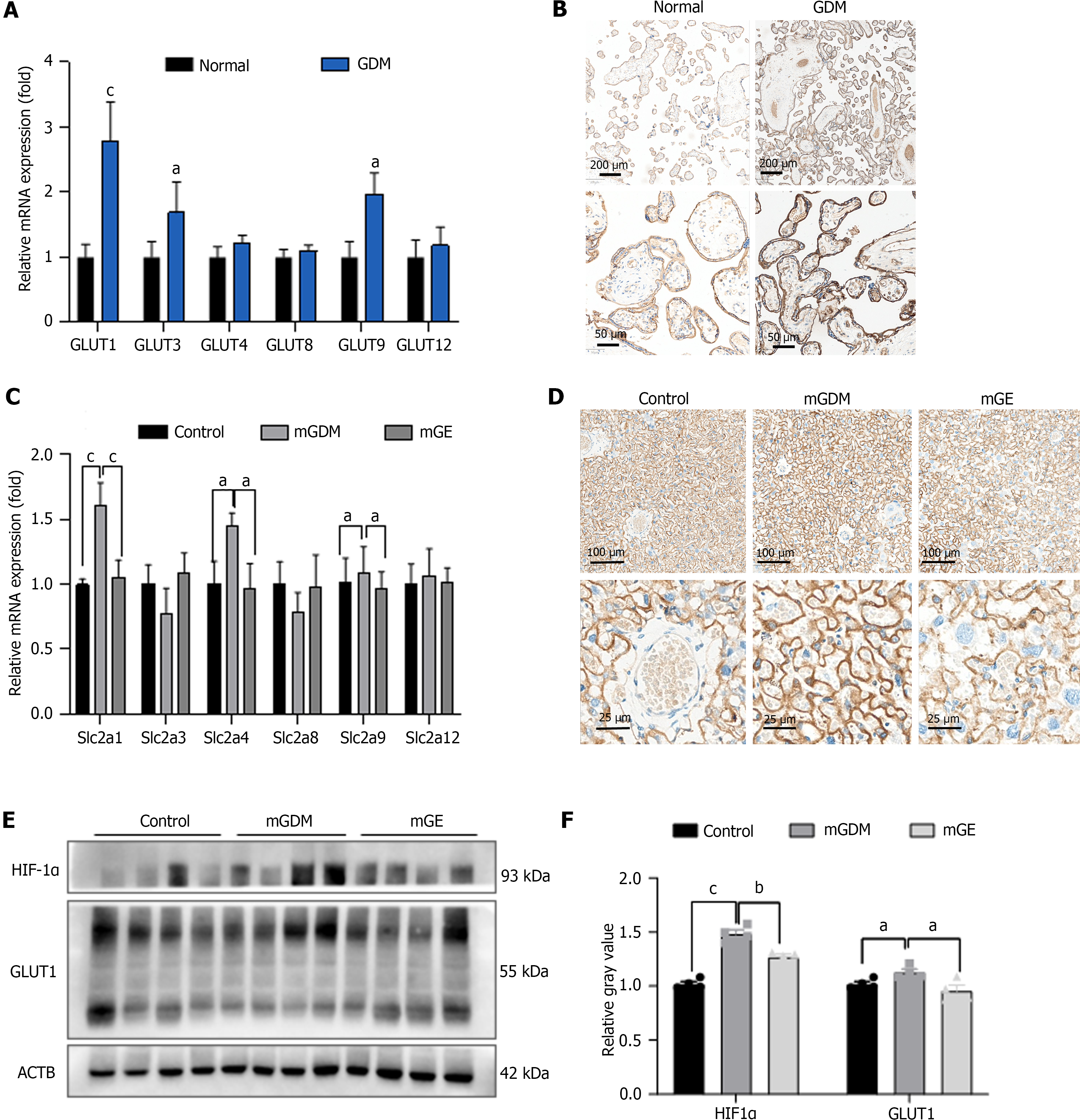
Relative differences in the placental mRNA expression of GLUT-related genes are shown in Figure 1A. GDM placentas showed upregulated GLUT1 gene expression compared to normal placentas and the difference in GLUT3, GLUT4, GLUT8, GLUT9, and GLUT12 mRNA expression between the normal and GDM groups was insignificant. We also performed IHC analysis on human placentas to examine the protein expression of GLUT1, which was increased in the GDM group (Figure 1B).
We then evaluated the expression of GLUT1(slc2a1), GLUT3(slc2a3), GLUT4(slc2a4), GLUT8 (slc2a8), GLUT9 (slc2a9), and GLUT12 (slc2a12) in the mouse placenta using RT-PCR and IHC (Figure 1C and D). The results showed that the mRNA and protein expression of GLUT1 (slc2a1) was upregulated in placentas of the mGDM group compared to that in the control group, whereas it was downregulated in placentas of the mGE group.
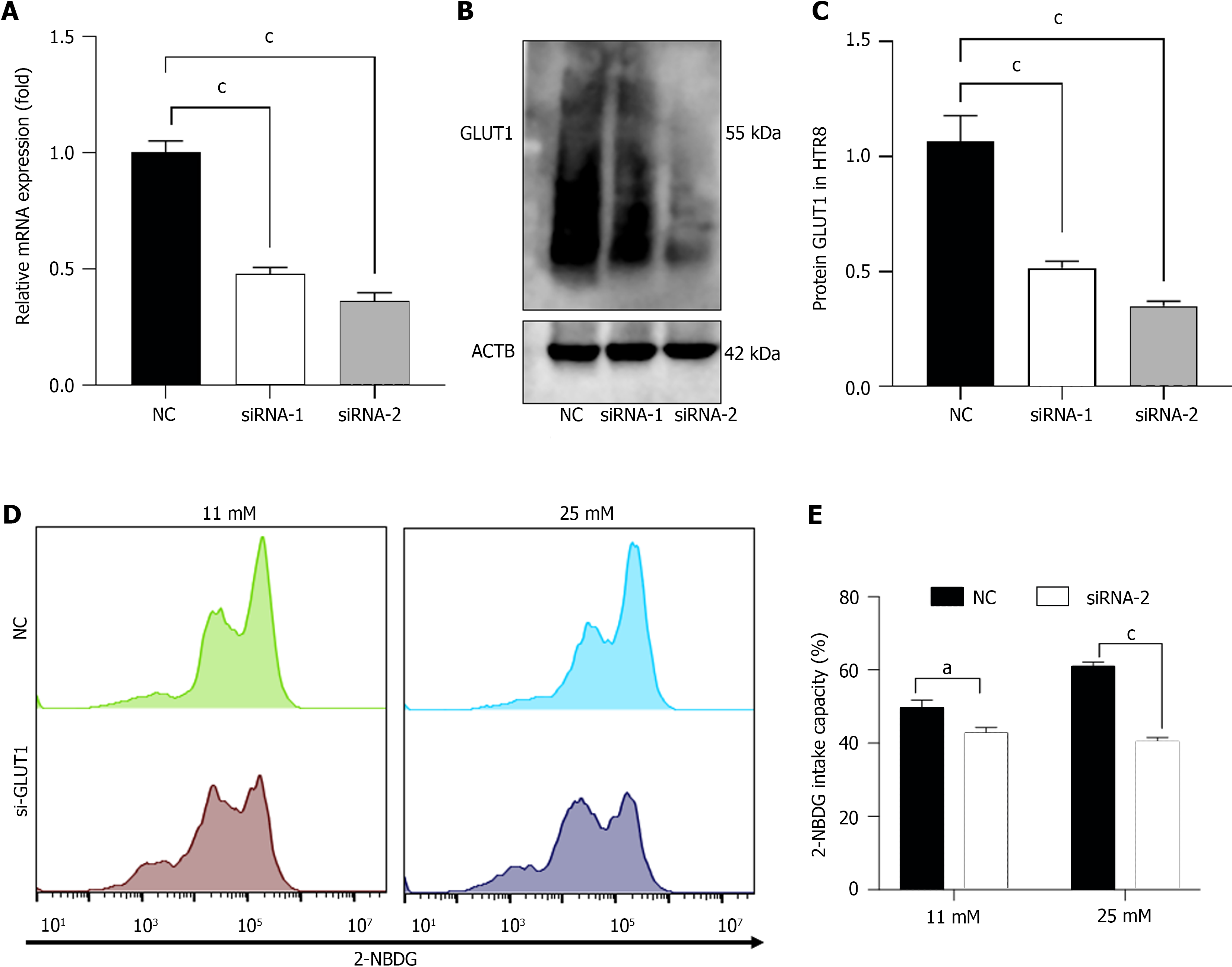
To determine the role that exercise played in improving hypoxia in placenta of individuals with GDM, we evaluated the expression of HIF-1α and GLUT1 in placenta tissue from the control, mGDM, and mGE mice. As shown in Figure 1E, the protein levels of HIF-1α and GLUT1 was remarkably elevated in GDM mice compared to that in healthy mice. Fur-thermore, the protein expression of HIF-1α and GLUT1 was significantly decreased in the mGE group. We silenced GLUT1 expression in HTR8/SVneo cells by transfection with si-GLUT1-1/2, and the transfection efficiency was examined by RT-PCR and western blotting (Figure 2A and B). siRNA-2 showed better transfection efficiency; thus, siRNA-2 was selected for subsequent analyses (named si-GLUT1). To determine the role of GLUT1 mediated glucose intake, 2-NBDG and flow cytometry were performed. As shown presented in Figure 2C and D, GLUT1 silencing significantly inhibited the high-glucose-induced glucose intake capacity of HTR8/SVneo cells.
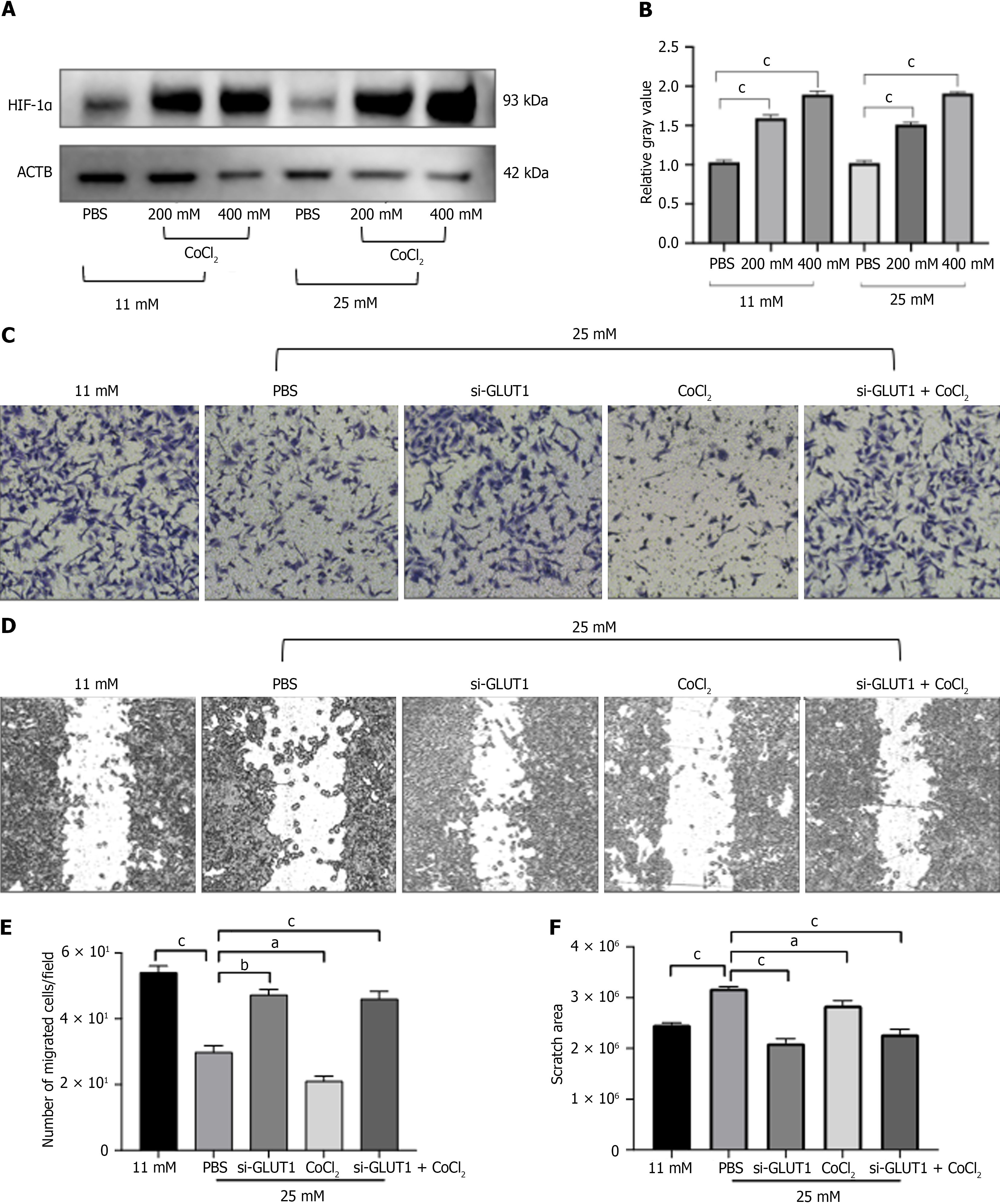
To mimic the hypoxic environment of the placenta, we used CoCl2 to induce hypoxia. As shown in Figure 3A, hypoxic stimulation with CoCl2 significantly up-regulated expression of HIF-1α in both normal and high glucose levels. Then, transwell and wound healing assays were performed to explore the role of high glucose and hypoxia in regulating cell migration capacity. Figure 3B-E showed that high glucose with hypoxia repressed the ability of HTR8/SVneo cells to migrate, whereas it was promoted after silencing GLUT1 expression.
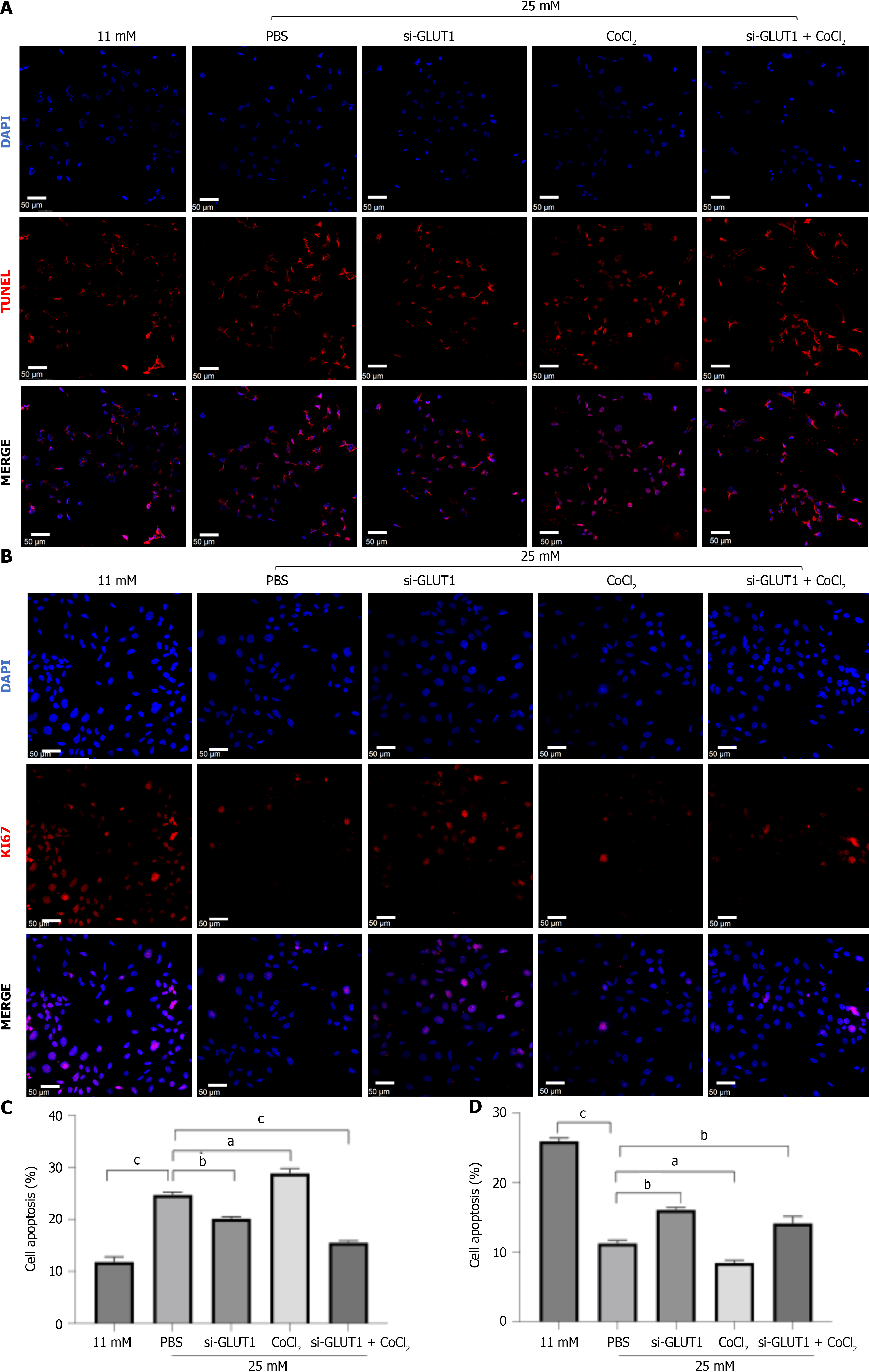
To further explore the effects of high glucose and hypoxia mediated cell injury, in situ TUNEL and Ki67 staining were performed. As shown in Figure 4A and C, the number of TUNEL staining-positive cells was greatly increased after the high glucose and CoCl2 treatment, while it was decreased by GLUT1 silencing. In addition, a clear decrease in the cell proliferation rate was observed following high glucose and hypoxic condition treatments when compared to the control group. However, GLUT1 knockdown attenuated cell proliferation (Figure 4B and D).
HE staining was used to monitor the thickness of the heart VS of weaning and sexually mature offspring to determine whether exercise during pregnancy positively affects cardiac development (Figure 5A). We demonstrated that the VS of the offspring of mGDM group (mGDMoff) group is significantly thinner than that of the control group. The VS of the weaning offspring in the offspring of mGE group (mGEoff) group was thicker than that of the mGDMoff group. The VS thickness of the three groups showed no differences in the sexually mature offspring period.
IHC analysis was used to identify GLUT1 in the heart tissues of weaning and sexually mature offspring (Figure 5B). We revealed that the GLUT1 expression in the heart of offspring in the offspring of control group (controloff), mGDMoff, and mGEoff in both weaning and sexually mature periods was similar.
We used HE staining to determine whether GDM and exercise during pregnancy affect hepatic development and chronic inflammation and to monitor the percentage of inflammatory cells in liver of weaning and sexually mature offspring (Figure 6A). The results showed that the inflammatory cell infiltration in the liver of the mGDMoff group was more intense than that in the controloff group during the weaning period (Figure 6B). Exercise significantly decreased the percentage of inflammatory cells in the liver. The percentage of inflammatory cells in the livers of the mGDMoff, controloff, and mGEoff groups during the sexual maturity period showed the same trend as that in the weaning period.
IHC was performed to analyze GLUT1 expression in the liver tissues of the offspring. The IRS of the mGDMoff group was higher than that of the controloff group during the weaning period, whereas the GLUT1 expression in the mGEoff group was significantly lower than that in the mGDMoff group, as shown in Figure 6B. Moreover, the GLUT1 expression in the livers of sexually mature offspring in the mGDMoff group was significantly higher than that in the controloff and mGEoff groups.
To investigate exercise-induced regulation of the kidneys of GDM offspring, the GLUT1 protein levels and the morphology of the kidneys of weaning offspring were detected by IHC and HE staining. As shown in Figure 7, exercise during gestation significantly downregulated GLUT1 expression and ameliorated renal tubular vacuolar degeneration. The same trend was observed in the kidneys of the 8-week-old offspring.
A study of children born to mothers with GDM and type 1 diabetes showed that the breastfed children had a higher risk of being overweight at the age of 2 than children breastfed by mothers without diabetes[18]. However, other studies have shown that breastfeeding reduces fat deposition in children due to intrauterine exposure to GDM[19,20]. In our study, mice offspring were breastfed after birth until 3 weeks of age, but were found to have significantly higher weight gain up to 8 weeks of age in the mGDMoff than in the controloff and mGEoff. Accordingly, we believe that breastfeeding can temporarily prevent offspring growth and development.
The results of this study showed that GLUT1 was the most significantly upregulated GLUT expressed in the placentas of females with GDM. Animal experiments have shown that exercise during gestation significantly reduces placental GLUT1 expression and ameliorates placental hypoxia. High glucose-stimulated glucose uptake was blocked by GLUT1 siRNA, indicating the direct involvement of GLUT1 in trophoblast glucose uptake. However, our study was limited by the fact that hypoxic conditions were chemically induced using CoCl2 instead of a hypoxia chamber[21]. Nevertheless, the hypoxic response was similar as shown by the increased HIF-1α expression in GDM placentas. Under hypoxic conditions, HIF-1α upregulated in trophoblast cells, which triggers degradation of Glial cell missing 1, thereby inhibiting trophoblast migration and invasion[22]. Our results show that GLUT1 silencing partially reversed the decrease in trophoblast migration induced by high glucose and hypoxia. GLUT1 is an important protein involved in the growth, development, and energy metabolism of the heart, liver, and kidney. We innovatively linked the expression of GLUT1 in the heart, liver, and kidney of the offspring to their organ structure and pathological alterations to explore the effects of exercise on the growth and development of GDM offspring.
Substantial epidemiological evidence has demonstrated that maternal hyperglycemia increases the risk of metabolic disorders in offspring and affects their organ growth and development. The cardiovascular system may be more susceptible to harmful factors in utero because it develops in the early stages of embryonic development[23,24]. Fetal cardiac metabolism is dependent on glucose levels and is supported by high GLUT1 expression[24]. Cardiac metabolism after birth shifts toward oxidative phosphorylation and decreases the GLUT1 expression. Cardiomyocytes isolated from mice with heart failure have shown increased glycolytic capacity and upregulated GLUT1 mRNA and protein expression after exercise[16]. This phenomenon was absent in the offspring of GDM mice subjected to wheel-running exercise during pregnancy. Exercise during pregnancy resulted in the downregulation of GLUT1 in the hearts of offspring with GDM during the weaning period but only at the transcriptional level. Neither cardiac GLUT1 transcription nor translation was are significantly regulated by sexual maturity. Thus, we concluded that the effect of GDM and prenatal exercise on postnatal cardiac GLUT1 expression is insignificant given the conversion of cardiac energy metabolism substrates after fetal birth. The weight of offspring in the mGE group were slightly higher than that of the offspring in the mGDM group during growth and development. The HE staining results also showed that the heart development of the offspring in mGE group was closer to that of the normal offspring. However, He et al[25] reported contradictory findings regarding myocardial hypertrophy in the offspring of mice with hyperglycemia during pregnancy. This inconsistency may have been caused by embryotoxicity due to the different concentrations of STZ. The wheel-running exercise may have eliminated some of the toxic effects of STZ on embryonic development, and exercise-induced upregulation of GLUT1 can help maintain cardiomyocyte respiration in heart failure and improve cardiac function[16]. This condition was also demonstrated by the effect of exercise on early heart development in the offspring. Previous animal studies have shown that hyperglycemia accelerates cardiomyocyte growth but reduces the number of cardiomyocytes[26]. This may also explain the thinner heart VS in the weaning offspring of GDM mice than in the normal group.
If the changes in maternal lifestyle lead to the excessive accumulation of liver fuel, then the fetus may be exposed to harmful changes in hepatic β-oxidation and ab initio adipogenesis. Ultimately, this pathological condition may increase fetal liver lipid accumulation, increasing the likelihood of fetal liver failure and the risk of liver disease in adulthood[27]. GLUT1 is an important marker of metabolic liver disease[28,29]. GLUT1 expression is increased in mouse and human liver fiber samples and is an important marker of hepatocarcinogenesis and metabolic liver disease[28,29]. Studies have shown that rodent exposure to alcohol and a high-fat diet leads to increased GLUT1 expression in hepatocytes[28]. Moreover, the inflammatory sites in the liver stained positive for GLUT1[29]. Our study also revealed that STZ-induced GDM increased inflammatory cell infiltration and enhanced the expression of both GLUT1 mRNA and protein in the liver of offspring, thereby increasing the likelihood of developing metabolic liver diseases in the future. Wheel-running exercise during pregnancy effectively reduced hepatic GLUT1 expression and inflammatory cell infiltration during weaning and sexual maturation in GDM offspring. The critical role of the liver in glucose metabolic homeostasis and inflammation may explain the intensified long-term effects of maternal GDM and exercise during pregnancy on GLUT1 expression in the liver rather than those in the heart.
Offspring born to mothers with GDM exhibited severe structural changes in the kidneys and renal dysfunction. A correlation between GLUT1 expression and the glycolytic activity of renal elements and the collection of tubular segments has been observed[30]. Pronounced glomerular dysfunction in mice with diabetic nephropathy is also closely associated with GLUT1 alterations[31]. In addition, our findings suggested that GDM offspring present a higher degree of tubular vacuolation in renal tissue during weaning compared with that in the control and mGE groups. Structural changes in the renal tubules persisted until sexual maturity although differences in kidney weight during growth and development were insignificant. Hence, sexually mature GDM offspring presented more severe renal tubular vacuolization than normal offspring, and wheel-running exercise during pregnancy effectively ameliorated this adverse effect of GDM. Chronic hyperglycemia in patients with diabetes increases GLUT1 expression in proximal tubules[32]. In addition, GLUT1 expression is significantly altered in glomeruli of patients with diabetic nephropathy[33]. Our IHC analysis results suggested that GDM causes increased GLUT1 expression in glomeruli and tubules during weaning and sexual maturation of the offspring. Exercise during gestation downregulated GLUT1 expression in offspring of GDM mothers; thus, it is evidently important for the structural and functional development of the kidney in GDM offspring. A popu-lation study showed that children exposed to a hyperglycemia intrauterine environment during gestation present low renal functional reserves[34]. This condition may be related to the decrease in number of kidney elements acquired by the offspring during gestation due to GDM. Studies on GLUT1 and kidney development, especially the influence of parents on offspring kidneys, are limited. Therefore, GLUT1-related mechanisms in offspring kidney development will be the focus of our future investigation.
The effects of GDM are associated with metabolic disorders and the induction of epigenetic changes[35]. Epigenetics affect specific placental gene expression and may influence the fetal epigenome, thereby ultimately exacerbating the development of complications in the offspring[36]. Growing evidence has shown that beneficial exercise-mediated effects are heritable[37]. Exercise during gestation can alter the development of offspring and is a powerful regulator of intergenerational inheritance. As previously mentioned, altered expression, location, and activity of GLUT1 may affect its function. We observed the consistency of GLUT1 expression levels in the heart and liver of GDM offspring considering developmental status and access too wheel-running exercise during pregnancy to partially improve poor developmental outcomes of GDM offspring. Therefore, we hypothesized that exercise may regulate placental function and offspring growth and development through GLUT1 epigenetic alterations. Although its specific mechanism was not investigated in our study, we will consider it in our future investigation. In addition, exercise may be a mechanism by which exercise ameliorates GDM complications by decreasing excess GDM-mediated placental fuel transfer; thus, reducing stress on fetal organ development[38].
Our study revealed that exercise during gestation reverses the adverse effects of GDM on the heart, liver, and kidney of offspring during both the weaning and sexual maturation periods. We also demonstrated that this favorable regulatory effect may be achieved through the regulation of hypoxia and GLUT1 mediated glucose uptake. However, this study has a limitation. We only considered heart, liver, and kidney development of weaning and sexually mature mouse offspring, but the condition of organs in adult and older GDM offspring is an important aspect of this research; therefore, in our future investigation we will attempt to perform a clear mechanistic study on the findings of this paper.
GDM affects the growth and development of the heart, liver, and kidney in offspring during the postnatal period. The regulation of the liver and kidney by GDM may be related to changes in GLUT1-induced glucose uptake and hypoxic conditions in the placenta. However, exercise during gestation can reverse these adverse effects of GDM on the heart, liver, and kidney of offspring. This study highlights the importance of exercise during pregnancy for females with GDM to ameliorate its effect on offspring organ development.
| 1. | Lende M, Rijhsinghani A. Gestational Diabetes: Overview with Emphasis on Medical Management. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Saravanan P; Diabetes in Pregnancy Working Group; Maternal Medicine Clinical Study Group; Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 3. | Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Wang YQ, Zhang HJ, Quinn MJ. Fetal Hypertension and the "Barker Hypothesis". Angiology. 2020;71:92-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Hoch D, Gauster M, Hauguel-de Mouzon S, Desoye G. Diabesity-associated oxidative and inflammatory stress signalling in the early human placenta. Mol Aspects Med. 2019;66:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Diniz MS, Hiden U, Falcão-Pires I, Oliveira PJ, Sobrevia L, Pereira SP. Fetoplacental endothelial dysfunction in gestational diabetes mellitus and maternal obesity: A potential threat for programming cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Mohammad S, Bhattacharjee J, Tzaneva V, Hutchinson KA, Shaikh M, Fernandes da Silva D, Burger D, Adamo KB. The Influence of Exercise-Associated Small Extracellular Vesicles on Trophoblasts In Vitro. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Kusuyama J, Alves-Wagner AB, Makarewicz NS, Goodyear LJ. Effects of maternal and paternal exercise on offspring metabolism. Nat Metab. 2020;2:858-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Zheng J, Alves-Wagner AB, Stanford KI, Prince NB, So K, Mul JD, Dirice E, Hirshman MF, Kulkarni RN, Goodyear LJ. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Harris JE, Baer LA, Stanford KI. Maternal Exercise Improves the Metabolic Health of Adult Offspring. Trends Endocrinol Metab. 2018;29:164-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Stanford KI, Rasmussen M, Baer LA, Lehnig AC, Rowland LA, White JD, So K, De Sousa-Coelho AL, Hirshman MF, Patti ME, Rando OJ, Goodyear LJ. Paternal Exercise Improves Glucose Metabolism in Adult Offspring. Diabetes. 2018;67:2530-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Chae SA, Son JS, de Avila JM, Du M, Zhu MJ. Maternal exercise improves epithelial development of fetal intestine by enhancing apelin signaling and oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2022;323:R728-R738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Vitor-de-Lima SM, Figueira de Oliveira ML, Tavares IS, Leandro CVG, Guedes RCA. Maternal voluntary physical exercise in the adult rat: evidence of exercise-associated differences in maternal food intake, and in brain effects on the progeny. Nutr Neurosci. 2024;27:120-131. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Stanirowski PJ, Lipa M, Bomba-Opoń D, Wielgoś M. Expression of placental glucose transporter proteins in pregnancies complicated by fetal growth disorders. Adv Protein Chem Struct Biol. 2021;123:95-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 915] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 16. | Jiang H, Jia D, Zhang B, Yang W, Dong Z, Sun X, Cui X, Ma L, Wu J, Hu K, Sun A, Ge J. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression. Basic Res Cardiol. 2020;115:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Wells JCK. Life history trade-offs and the partitioning of maternal investment: Implications for health of mothers and offspring. Evol Med Public Health. 2018;2018:153-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Shearrer GE, Whaley SE, Miller SJ, House BT, Held T, Davis JN. Association of gestational diabetes and breastfeeding on obesity prevalence in predominately Hispanic low-income youth. Pediatr Obes. 2015;10:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Crume TL, Ogden L, Maligie M, Sheffield S, Bischoff KJ, McDuffie R, Daniels S, Hamman RF, Norris JM, Dabelea D. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34:641-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Zhong H, Zhang J, Xia J, Zhu Y, Chen C, Shan C, Cui X. Influence of gestational diabetes mellitus on lipid signatures in breast milk and association with fetal physical development. Front Nutr. 2022;9:924301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Starska K, Forma E, Jóźwiak P, Bryś M, Lewy-Trenda I, Brzezińska-Błaszczyk E, Krześlak A. Gene and protein expression of glucose transporter 1 and glucose transporter 3 in human laryngeal cancer-the relationship with regulatory hypoxia-inducible factor-1α expression, tumor invasiveness, and patient prognosis. Tumour Biol. 2015;36:2309-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Kohan-Ghadr HR, Armistead B, Berg M, Drewlo S. Irisin Protects the Human Placenta from Oxidative Stress and Apoptosis via Activation of the Akt Signaling Pathway. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Nakano H, Minami I, Braas D, Pappoe H, Wu X, Sagadevan A, Vergnes L, Fu K, Morselli M, Dunham C, Ding X, Stieg AZ, Gimzewski JK, Pellegrini M, Clark PM, Reue K, Lusis AJ, Ribalet B, Kurdistani SK, Christofk H, Nakatsuji N, Nakano A. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 24. | Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 379] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 25. | He L, Wang X, Jin Y, Xu W, Guan Y, Wu J, Han S, Liu G. Identification and validation of the miRNA-mRNA regulatory network in fetoplacental arterial endothelial cells of gestational diabetes mellitus. Bioengineered. 2021;12:3503-3515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Li Z, Wu Y, Du B, Yu X, Wang H, Niu Y, Wang J, Chen S, Sun K. Associations of maternal gestational diabetes mellitus with alterations in cardiovascular system in early childhood. Diabetes Metab Res Rev. 2022;38:e3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Baker PR 2nd, Friedman JE. Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease. J Clin Invest. 2018;128:3692-3703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Cho SJ, Moon JS, Nikahira K, Yun HS, Harris R, Hong KS, Huang H, Choi AMK, Stout-Delgado H. GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax. 2020;75:227-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Zhou MY, Cheng ML, Huang T, Hu RH, Zou GL, Li H, Zhang BF, Zhu JJ, Liu YM, Liu Y, Zhao XK. Transforming growth factor beta-1 upregulates glucose transporter 1 and glycolysis through canonical and noncanonical pathways in hepatic stellate cells. World J Gastroenterol. 2021;27:6908-6926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Gronda E, Jessup M, Iacoviello M, Palazzuoli A, Napoli C. Glucose Metabolism in the Kidney: Neurohormonal Activation and Heart Failure Development. J Am Heart Assoc. 2020;9:e018889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Huang Y, Jin L, Yu H, Jiang G, Tam CHT, Jiang S, Zheng C, Jiang F, Zhang R, Zhang H, Chan JCN, Ma RCW, Jia W, Hu C, Liu Z. SNPs in PRKCA-HIF1A-GLUT1 are associated with diabetic kidney disease in a Chinese Han population with type 2 diabetes. Eur J Clin Invest. 2020;50:e13264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Sędzikowska A, Szablewski L. Human Glucose Transporters in Renal Glucose Homeostasis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Ni WJ, Guan XM, Zeng J, Zhou H, Meng XM, Tang LQ. Berberine regulates mesangial cell proliferation and cell cycle to attenuate diabetic nephropathy through the PI3K/Akt/AS160/GLUT1 signalling pathway. J Cell Mol Med. 2022;26:1144-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Aisa MC, Cappuccini B, Barbati A, Clerici G, Torlone E, Gerli S, Di Renzo GC. Renal Consequences of Gestational Diabetes Mellitus in Term Neonates: A Multidisciplinary Approach to the DOHaD Perspective in the Prevention and Early Recognition of Neonates of GDM Mothers at Risk of Hypertension and Chronic Renal Diseases in Later Life. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics. 2019;14:215-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 36. | Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta. 2016;48 Suppl 1:S54-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Barrès R, Zierath JR. The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nat Rev Endocrinol. 2016;12:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Stevanović-Silva J, Beleza J, Coxito P, Rocha H, Gaspar TB, Gärtner F, Correia R, Fernandes R, Oliveira PJ, Ascensão A, Magalhães J. Exercise performed during pregnancy positively modulates liver metabolism and promotes mitochondrial biogenesis of female offspring in a rat model of diet-induced gestational diabetes. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |