Published online Nov 15, 2024. doi: 10.4239/wjd.v15.i11.2157
Revised: August 29, 2024
Accepted: September 26, 2024
Published online: November 15, 2024
Processing time: 195 Days and 21 Hours
In this editorial, we comment on an article by Tang et al published in the World Journal of Diabetes. Obesity and diabetes are two pathological situations that are intrinsically related. Neither lifestyle changes nor pharmacological treatments have achieved diabetes remission. From this perspective, bariatric surgery has been widely used as an approach for weight loss in obese patients and as a strategy to promote metabolic modulation. The main effects of bariatric surgery involve direct action in improving cardiovascular function and endothelial function and reducing insulin resistance, leading to diabetes remission in the short term following surgery. In this context, it has been observed that hormones from the gastrointestinal tract and endothelium play a prominent role in this process. By reversing endothelial dysfunction, it is possible to balance pro-inflammatory cytokine production, improving the availability of nitric oxide and inhibiting vascular oxidative stress. Furthermore, it can be considered an efficient anti-inflammatory strategy, alleviating interferon-gamma-mediated adipose tissue inflammation. The current challenge must be to unravel the pathophysiological mechanisms and potential targets for treating metabolic diseases.
Core Tip: In this editorial, we address the pathophysiological mechanisms associated with bariatric surgery in type 2 diabetes remission. Within this perspective, we discuss gastrointestinal tract hormones, mainly peptide-1, which is a hormone secreted by gastrointestinal L cells and released immediately after food intake, as well as the benefits obtained after reversing endothelial syndrome via bariatric surgery.
- Citation: Tatmatsu-Rocha JC, Lima da Silva MR. Diabetes and obesity: A debate on bariatric interventions and its implications. World J Diabetes 2024; 15(11): 2157-2161
- URL: https://www.wjgnet.com/1948-9358/full/v15/i11/2157.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i11.2157
Obesity is a disease characterized by excessive accumulation of body fat, and it has been associated with adverse metabolic conditions[1]. It directly contributes to increased cardiovascular risk with increased mortality[2]. Among the main changes caused by obesity are insulin resistance, arterial hypertension, dyslipidemia and chronic inflammation[3,4]. Diabetes and obesity have generated a great burden on the health system, and today they are considered epidemic diseases[1] and have incapacitated some economically active individuals[5]. The impacts on the quality of life of this group must be taken into consideration, given that obesity and diabetes are health conditions generally diagnosed simultaneously[6]. The literature has shown that obesity promotes a significant increase in mortality related to metabolic diseases[7]. Insulin resistance, its high rates, decreased hormonal regulation and systemic inflammation are patho-physiological characteristics that make obesity and diabetes close realities[8]. Insulin resistance occurs due to the increased release of fatty acids, lipids and other advanced factors from adipose tissue, leading to numerous complications[9]. In this context, the need for disease control is clear, as diabetes management goes far beyond glycemic control. Maintaining an ideal weight is essential to prevent or reduce the incidence of macro and microvascular diseases and their complications[2,10]. Even in the face of the constantly developing pharmaceutical industry, with numerous promising drugs, studies point to bariatric surgery as a promising and potentially curative approach for the treatment of obesity and type 2 diabetes mellitus[11]. Also described as metabolic surgery, bariatric surgery has shown efficacy in providing prolonged remission of diabetes without the need for continuous pharmacological treatment[12]. Among patients undergoing bariatric surgery, approximately 75%-80% of patients showed significant improvement in type 2 diabetes control at 1-year follow-up appointments[12,13]. The proportion of patients who achieved diabetes remission across studies was estimated to be between 30 and 77%[13]. However, this proportion decreased when a longer follow-up period was performed in patients with chronic diabetes[14]. There are validated tools that have been used preoperatively to predict whether the patient will have diabetes remission after bariatric surgery. These include the DiaRem score, which has predictive power based on variables such as age, glycated hemoglobin values, insulin use, and use of other blood glucose- reducing agents[15].
Over the decades, bariatric surgery has proven to be very effective in achieving significant and sustainable weight loss in many patients[16]. Laparoscopic techniques have contributed to access to new techniques in bariatric surgery in the last decade. Some techniques have been transformative in the field. These include vertical banded gastroplasty[17], which decreased at the end of the 1990s and the adjustable laparoscopic gastric band[18], which emerged around 2012. From then on, biliopancreatic bypass[19] decreased compared to Roux-en-Y gastric bypass (RYGB)[20]. Finally, the use of laparoscopic sleeve gastrectomy[21] has rapidly increased in recent years.
The described mechanisms regarding the effects of bariatric interventions on glycemic index remission are still not clear. Most studies indicate that the weight loss provided by surgery is a determining factor in the resolution of type 2 diabetes[22]. Therefore, patients who experience greater weight loss are more likely to control or even remit the disease compared to those with less weight loss. Other variables include the patient’s age, duration of illness and glycemic control, which are mechanisms independent of weight loss[4]. The main marker of diabetes resolution for years has been weight loss. However, there is a growing trend towards the use of metabolic markers, such as an increase in cardiovascular risk factors, endothelial function and lipid profile[23].
RYGB surgery has been increasingly used because it is known to be beneficial in weight loss and in reducing deaths from cardiovascular complications[24]. Glucagon-like peptide-1 (GLP-1) has been considered a key metabolic variable to verify clinical improvement after RYGB, whose effects (GLP-1) are independent of weight. This peptide has protective effects on the endothelium in addition to its metabolic actions. After RYGB surgery, there is a marked increase in GLP-1, which does not occur with weight loss due to dietary restriction. In this sense, a hypothesis has been formulated that GLP-1 is altered in the face of rapid metabolic changes and that this would only occur after RYGB, given that insulin resistance occurs even before weight loss after bariatric intervention[25,26].
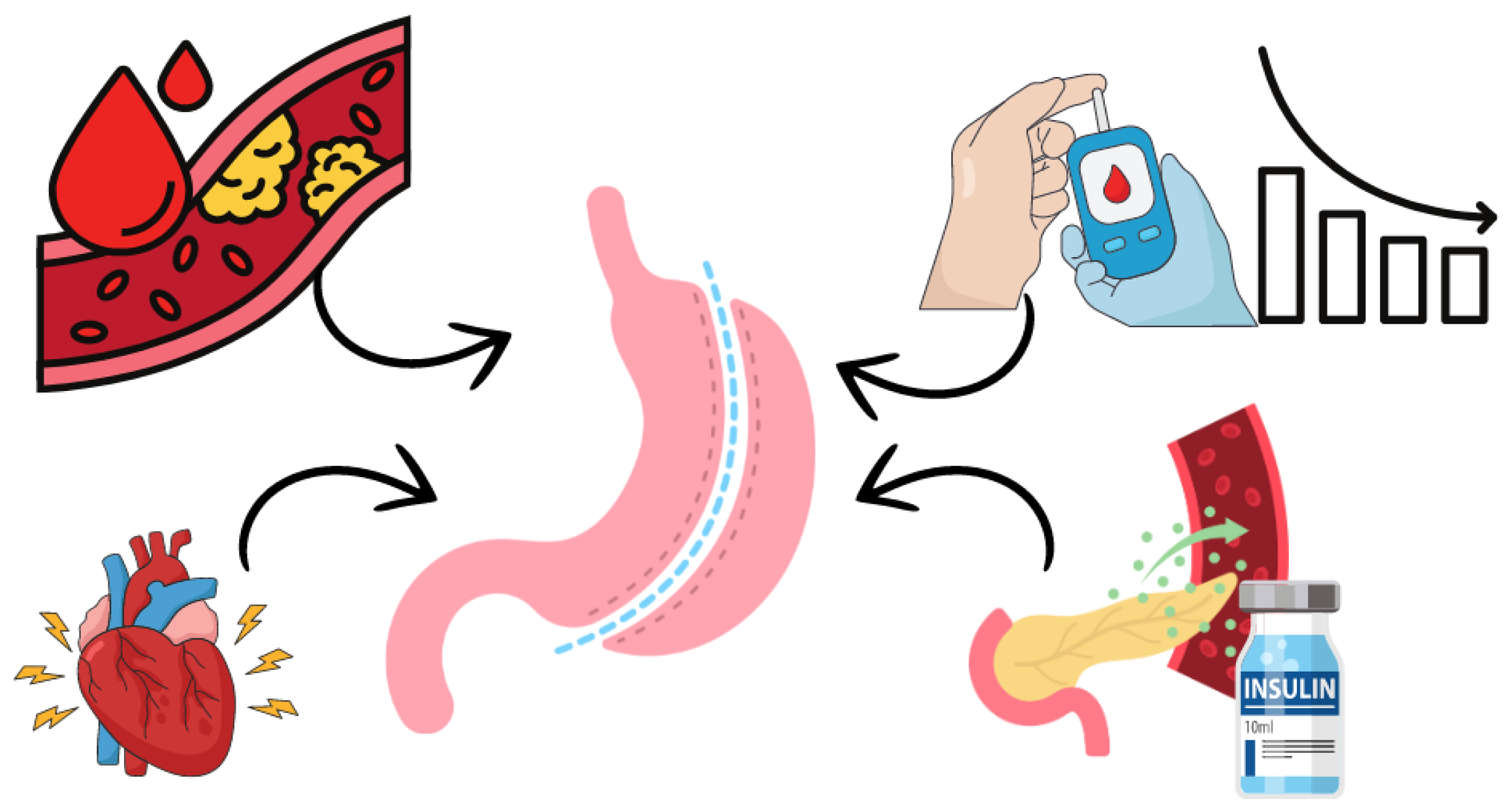
There are reports that the visceral adipose tissue of patients with obesity presents gene expression related to inflammation, oxidative stress and production of pro-inflammatory cytokines. This leads to impaired arteriolar function and possibly endothelial dysfunction[27], which can cause important cardiovascular changes. When stimulated, the endo-thelium can release agents that cause vasomotor function and hemostasis[27,28]. In contrast, the endothelium in healthy conditions presents low levels of oxidative stress and endothelial smooth muscle with relaxed tone due to the release of some markers that act on this vascular smooth muscle. This balance of vasodilators and vasoconstrictors as well as procoagulant and anticoagulant agents is essential for the ideal physiological balance of the endothelium. Endothelial dysfunction occurs due to an imbalance of these agents and evolves into some cardiovascular pathologies, such as cerebrovascular diseases, as well as peripheral arterial disease, coronary artery disease and type 2 diabetes[29,30].
Bariatric surgeries may be beneficial in protecting against cardiovascular disease, especially when performed early, before the development of endothelial lesions (Figure 1). However, hormonal changes play a crucial role in diabetes remission. There is an urgent need for in-depth research that fully explains the metabolic and hormonal effects of the gastrointestinal tract and the different perspectives on the mechanisms underlying this approach.
| 1. | Yin M, Wang Y, Han M, Liang R, Li S, Wang G, Gang X. Mechanisms of bariatric surgery for weight loss and diabetes remission. J Diabetes. 2023;15:736-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Tatmatsu-Rocha JC, Mendes-Costa LS. Inflammatory markers, oxidative stress, and mitochondrial dynamics: Repercussions on coronary artery disease in diabetes. World J Diabetes. 2024;15:1853-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e984-e1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1580] [Article Influence: 395.0] [Reference Citation Analysis (0)] |
| 4. | Xu H. Obesity and metabolic inflammation. Drug Discov Today Dis Mech. 2013;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Segateli L, Oliveira MAD, Coca MLL, Fogaça ECBSES, Silva LIB, Silva PL, Rocha Junior PR, Lopes LDG. Estratégias de promoção à saúde para pacientes com diabetes mellitus na atenção primária à saúde: revisão integrativa. Braz J Hea Rev. 2024;7:e68794. [DOI] [Full Text] |
| 6. | Pantanetti P, Cangelosi G, Alberti S, Di Marco S, Michetti G, Cerasoli G, Di Giacinti M, Coacci S, Francucci N, Petrelli F, Ambrosio G, Grinta R. Changes in body weight and composition, metabolic parameters, and quality of life in patients with type 2 diabetes treated with subcutaneous semaglutide in real-world clinical practice. Front Endocrinol (Lausanne). 2024;15:1394506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Chen J, Zhou H, Liu K. Normal weight and central obesity as predictors of increased all-cause mortality in metabolic dysfunction associated steatotic liver disease. J Hepatol. 2024;80:e143-e145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Park B, Yoon J, Tran TXM. Accounting for time-varying exposures and covariates in the relationship between obesity and diabetes: analysis using parametric g-formula. J Epidemiol Community Health. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Zhou XD, Shapiro MD, Lip GYH, Tilg H, Valenti L, Somers VK, Byrne CD, Targher G, Yang W, Viveiros O, Opio CK, Mantzoros CS, Ryan JD, Kok KYY, Jumaev NA, Perera N, Robertson AG, Abu-Abeid A, Misra A, Wong YJ, Ruiz-Úcar E, Ospanov O, Kızılkaya MC, Luo F, Méndez-Sánchez N, Zuluaga M, Lonardo A, Al Momani H, Toro-Huamanchumo CJ, Adams L, Al-Busafi SA, Sharara AI, Chan WK, Abbas SI, Sookoian S, Treeprasertsuk S, Ocama P, Alswat K, Kong AP, Ataya K, Lim-Loo MC, Oviedo RJ, Szepietowski O, Fouad Y, Zhang H, Abdelbaki TN, Katsouras CS, Prasad A, Thaher O, Ali A, Molina GA, Sung KC, Chen QF, Lesmana CRA, Zheng MH. Global burden of metabolic diseases, 1990-2021. Metabolism. 2024;160:155999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 10. | Ghusn W, Salame M, Sayegh L, Hage K, Storm AC, Dayyeh BKA, Ghanem OM. The association between microvascular and macrovascular diseases and diabetes remission after bariatric surgery. Surg Endosc. 2024;38:1835-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Li S, Zhang P, Di J, Han X, Tu Y, Yang D, Xu R, Xiao Y, Zhou J, Bao Y, Yin J, Yu H, Jia W, Han J. Associations of change in body fat percentage with baseline body composition and diabetes remission after bariatric surgery. Obesity (Silver Spring). 2024;32:871-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Courcoulas AP, Patti ME, Hu B, Arterburn DE, Simonson DC, Gourash WF, Jakicic JM, Vernon AH, Beck GJ, Schauer PR, Kashyap SR, Aminian A, Cummings DE, Kirwan JP. Long-Term Outcomes of Medical Management vs Bariatric Surgery in Type 2 Diabetes. JAMA. 2024;331:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 96] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 13. | Yazici H, Ozturk AM, Cekic A, Yasar AC, Yildirim M. Laparoscopic Sleeve Gastrectomy as One-Step Procedure for Patients with Obesity: Long-Term Outcomes of a Single Center. Bariatr Surg Pract P. 2024;19:62-68. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Gregg EW, Chen H, Bancks MP, Manalac R, Maruthur N, Munshi M, Wing R; Look AHEAD Research Group. Impact of remission from type 2 diabetes on long-term health outcomes: findings from the Look AHEAD study. Diabetologia. 2024;67:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 15. | Saberdoust F, Salehabadi G, Sheykholeslamy S, Noroozi E, Moradi M, Pazouki A, Kabir A. Diagnostic Value of Advanced-DiaRem for Predicting Diabetic remission after One Anastomosis Gastric Bypass/Minigastric Bypass. Obes Surg. 2024;34:3467-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Scott AW, Amateau SK, Leslie DB, Ikramuddin S, Wise ES. Rates and Risk Factors for 30-Day Morbidity After One-Stage Vertical Banded Gastroplasty Conversions: A Retrospective Analysis. Am Surg. 2024;31348241248817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Carbonaro J, McLaughlin T, Seip R, Staff I, Wu Y, Santana C, Bond D, Tishler D, Benbrahim A, Papasavas P. Five-year outcomes of revisional bariatric surgery: gastric band to sleeve gastrectomy or to Roux-en-Y gastric bypass. Surg Endosc. 2024;38:2719-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Papadia FS, Adami G, Razzetta A, Florenzano A, Longo G, Rubartelli A, Carlini F, De Cian O, Camerini G. Biliopancreatic diversion for severe obesity: long-term weight maintenance and occurrence of nutritional complications are two facets of the same coin. Br J Surg. 2024;111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Hedberg S, Thorell A, Österberg J, Peltonen M, Andersson E, Näslund E, Hertel JK, Svanevik M, Stenberg E, Neovius M, Näslund I, Wirén M, Ottosson J, Olbers T; BEST Study Group. Comparison of Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass: A Randomized Clinical Trial. JAMA Netw Open. 2024;7:e2353141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 20. | Winckelmann LA, Gribsholt SB, Bødkergaard K, Rejnmark L, Madsen LR, Richelsen B. Risk of fractures following bariatric surgery with Roux-en-Y gastric bypass or sleeve gastrectomy: a Danish population-based cohort study. Eur J Endocrinol. 2024;191:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Rodbard HW, Barnard-Kelly K, Pfeiffer AFH, Mauersberger C, Schnell O, Giorgino F. Practical strategies to manage obesity in type 2 diabetes. Diabetes Obes Metab. 2024;26:2029-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Craig Wood G, Horwitz D, Still CD, Mirshahi T, Benotti P, Parikh M, Hirsch AG. Performance of the DiaRem Score for Predicting Diabetes Remission in Two Health Systems Following Bariatric Surgery Procedures in Hispanic and non-Hispanic White Patients. Obes Surg. 2018;28:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Hainer V, Toplak H, Mitrakou A. Treatment modalities of obesity: what fits whom? Diabetes Care. 2008;31 Suppl 2:S269-S277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Sanches E, Timmermans M, Topal B, Celik A, Sundbom M, Ribeiro R, Parmar C, Ugale S, Proczko M, Stepaniak PS, Pujol Rafols J, Mahawar K, Buise MP, Neimark A, Severin R, Pouwels S. Cardiac remodeling in obesity and after bariatric and metabolic surgery; is there a role for gastro-intestinal hormones? Expert Rev Cardiovasc Ther. 2019;17:771-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Osto E, Doytcheva P, Corteville C, Bueter M, Dörig C, Stivala S, Buhmann H, Colin S, Rohrer L, Hasballa R, Tailleux A, Wolfrum C, Tona F, Manz J, Vetter D, Spliethoff K, Vanhoutte PM, Landmesser U, Pattou F, Staels B, Matter CM, Lutz TA, Lüscher TF. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation. 2015;131:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Faria G, Preto J, da Costa EL, Guimarães JT, Calhau C, Taveira-Gomes A. Acute improvement in insulin resistance after laparoscopic Roux-en-Y gastric bypass: is 3 days enough to correct insulin metabolism? Obes Surg. 2013;23:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol. 2012;32:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol Rev. 2017;97:495-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 504] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 29. | Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 30. | Tang HH, Wang D, Tang CC. Effect of bariatric surgery on metabolism in diabetes and obesity comorbidity: Insight from recent research. World J Diabetes. 2024;15:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |