Published online Jan 15, 2024. doi: 10.4239/wjd.v15.i1.43
Peer-review started: August 24, 2023
First decision: November 9, 2023
Revised: November 25, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 15, 2024
Processing time: 141 Days and 9.9 Hours
Among older adults, type 2 diabetes mellitus (T2DM) is widely recognized as one of the most prevalent diseases. Diabetic nephropathy (DN) is a frequent com
To investigate the factors that impact T2DM complicated with DN and utilize this information to develop a predictive model.
The clinical data of 210 patients diagnosed with T2DM and admitted to the First People’s Hospital of Wenling between August 2019 and August 2022 were retrospectively analyzed. According to whether the patients had DN, they were divided into the DN group (complicated with DN) and the non-DN group (without DN). Multivariate logistic regression analysis was used to explore factors affecting DN in patients with T2DM. The data were randomly split into a training set (n = 147) and a test set (n = 63) in a 7:3 ratio using a random function. The training set was used to construct the nomogram, decision tree, and random forest models, and the test set was used to evaluate the prediction performance of the model by comparing the sensitivity, specificity, accuracy, recall, precision, and area under the receiver operating characteristic curve.
Among the 210 patients with T2DM, 74 (35.34%) had DN. The validation dataset showed that the accuracies of the nomogram, decision tree, and random forest models in predicting DN in patients with T2DM were 0.746, 0.714, and 0.730, respectively. The sensitivities were 0.710, 0.710, and 0.806, respectively; the specificities were 0.844, 0.875, and 0.844, respectively; the area under the receiver operating characteristic curve (AUC) of the patients were 0.811, 0.735, and 0.850, respectively. The Delong test results revealed that the AUC values of the decision tree model were lower than those of the random forest and nomogram models (P < 0.05), whereas the difference in AUC values of the random forest and column-line graph models was not statistically significant (P > 0.05).
Among the three prediction models, random forest performs best and can help identify patients with T2DM at high risk of DN.
Core Tip: Machine learning is widely used in medical prediction models. Logistic regression (nomogram), decision tree, and random forest models are three important machine learning techniques. However, few studies have compared the predictive efficacies of these three models in patients with type 2 diabetes mellitus and diabetic nephropathy. Here, we established three risk prediction models-nomogram, decision tree, and random forest-for comparison and found that random forest has the strongest combined predictive power.
- Citation: Cai SS, Zheng TY, Wang KY, Zhu HP. Clinical study of different prediction models in predicting diabetic nephropathy in patients with type 2 diabetes mellitus. World J Diabetes 2024; 15(1): 43-52
- URL: https://www.wjgnet.com/1948-9358/full/v15/i1/43.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i1.43
Type 2 diabetes mellitus (T2DM) is one of the most common diseases affecting the older population. However, its incidence among children, adolescents, and young adults is increasing because of obesity, physical inactivity, and poor dietary habits. According to the International Diabetes Federation, approximately 537 million people (20-79 years old) worldwide currently have diabetes, with more than 90% of cases being T2DM[1]. Approximately 4.2 million people died of diabetes and its complications in 2019. Diabetic nephropathy (DN) is a chronic kidney disease induced by DM and a frequent microvascular complication of DM[2]. Relevant research data show that approximately 1/100 patients with diabetes develop end-stage renal disease yearly, and approximately 3/50 patients with massive albuminuria eventually develop end-stage renal disease yearly[3]. Patients with DN have a higher risk of death than those with diabetes alone or without comorbid DN. However, recent studies have shown that the prevalence of DN in patients with T2DM ranges between 20% and 40%[4,5]. To reduce the death rate of patients, early identification, prevention, and slowing down the development of DN are important. However, random urine measurement of the urinary albumin/creatinine ratio and 24 h urinary albumin quantification have shortcomings in the diagnosis of DN. Renal biopsy is the gold standard for the diagnosis of DN, but the acceptance of the examination is often low, and the economic cost is high[6]. Therefore, constructing a diagnostic and predictive model of DN plays a significant role in auxiliary diagnosis. At present, machine learning has been widely used in medical prediction models. Logistic regression (nomogram), decision tree, and random forest models are three important techniques in machine learning, all of which can quickly mine effective information from data; However, their application effects differ for different data types[7]. Little research has compared the predictive efficacies of the three models for DN in patients with T2DM. Therefore, this study established a prediction model for DN in patients with T2DM based on a nomogram, decision tree, and random forest and compared the prediction efficacy of the three models, providing a basis for the clinical identification of high-risk populations.
First, this was a retrospective study. A total of 210 patients admitted to the First People’s Hospital of Wenling with a clear diagnosis of T2DM between August 2019 and August 2022 were selected for this study. According to the diagnostic information, 74 of the 210 patients with T2DM complicated by DN were defined as the DN group, and the remaining 136 patients with T2DM without concurrent DN were defined as the non-DN group. The inclusion criteria were as follows: (1) Age 18 to 75 years; (2) T2DM diagnosed according to the diagnostic criteria; and (3) Complete clinical data, including demographic data and laboratory test results. The exclusion criteria were as follows: (1) Definite diagnosis of primary kidney disease or secondary kidney disease of the immune system, blood system, or drug; (2) Complication with severe primary diseases of the digestive, respiratory, cardiovascular, hematological, and nervous systems, accompanied by more than one malignant tumor; and (3) Rapidly progressive hypertension or diseases other than cerebrovascular diseases within the last 3 mo.
Standards criteria for DM: Based on “Standards for the Diagnosis and Treatment of Diabetes (2023 Edition)” of ADA[8] and “Guidelines for the Diagnosis, Prevention and Treatment of Type 2 Diabetes”[9]. Diagnostic standards were developed as follows: (1) Common symptoms of diabetes such as polydipsia, polyuria, polyphagia, unexplained weight loss, and random blood sugar ≥ 11.1 mmol/L; (2) Fasting blood sugar ≥ 7.0 mmol/L; or (3) Blood glucose ≥ 11.1 mmol/L 2 h after dextrose load. Patients with no typical symptoms of diabetes need to be reexamined on another day to confirm. The fasting state refers to not eating any calories for > 8 h; random blood glucose levels were measured at any time of the day, regardless of the time of the last meal.
Diagnostic criteria for DN: The diagnostic criteria were established based on the ADA “Standards for the Diagnosis and Treatment of Diabetes (2023 Edition)”, Guidelines for the Diagnosis, Prevention and Treatment of Type 2 Diabetes”, and “Guidelines for primary management of diabetic kidney disease in China”[10]. That is, patients with diabetes with renal impairment and urinary microalbumin/creatinine ratio (ACR) ≥ 30 mg/g (or ≥ 3 mg/mmol) or glomerular filtration rate < 60 mL/min/1.73 m2 for a total duration of > 3 mo can be diagnosed with DN.
General information: Age, sex, body mass index (kg/m2), diabetes duration, and history of high blood pressure, diabetic retinopathy (DR), and coronary heart disease. Laboratory indicators (collected within 24 h after admission): Fasting blood glucose (FBG, mmol/L), serum creatinine (Scr, μmol/L), glycosylated hemoglobin (HbAlc, %, 7% = 53 mmol/mol), blood urea nitrogen (BUN, mmol/L), total cholesterol (mmol/L), triglyceride (mmol/L), high-density lipoprotein cholesterol (mmol/L), and low-density lipoprotein cholesterol (mmol/L).
Data were analyzed and processed using the SPSS software (version 23.0). Quantitative data in accordance with the normal distribution were expressed as mean ± SD, and the t-test was used for comparison among groups. Count data were expressed as (%), and the χ2 test was used for comparison among groups. The DN and non-DN groups were subjected to univariate analysis, and variables with statistically significant variances were incorporated into a multifactor logistic regression analysis model to screen for highly relevant predictive variables. The prediction model was con
A total of 210 patients with T2DM were included in this study, of whom 87 were men and 123 were women. Seventy-four patients had T2DM complicated with DN, and the incidence of DN was 35.24%. There were 74 patients in the DN group, with a mean age of 56.01 ± 9.41 years. There were 136 patients in the non-DN group, with a mean age of 57.42 ± 8.15 years.
The clinical data of the patients in the DN and non-DN groups were compared. The duration of diabetes, FBG, Scr, HbAlc, and BUN levels were higher in the DN group than in the non-DN group, and the proportion of patients with DR was also higher in the DN group than in the non-DN group (P < 0.05). More details are shown in Table 1.
| Indicators | DN group (n = 74) | Non-DN group (n = 136) | t/χ² | P value |
| Age | 56.01 ± 9.41 | 57.42 ± 8.15 | -1.129 | 0.260 |
| Sex | 1.150 | 0.284 | ||
| Male | 27 (36.49) | 60 (44.12) | ||
| Female | 47 (63.51) | 76 (55.88) | ||
| BMI (kg/m2) | 24.60 ± 3.92 | 23.73 ± 2.94 | 1.673 | 0.097 |
| Duration of diabetes (yr) | 5.28 ± 1.34 | 4.86 ± 0.76 | 2.507 | 0.014 |
| History of hypertension | 0.465 | 0.495 | ||
| Yes | 24 (32.43) | 38 (27.94) | ||
| No | 50 (67.57) | 98 (72.06) | ||
| DR | 8.761 | 0.003 | ||
| Yes | 34 (45.95) | 24 (17.65) | ||
| No | 40 (54.05) | 112 (82.35) | ||
| Coronary heart disease | 0.350 | 0.554 | ||
| Yes | 19 (25.68) | 30 (22.06) | ||
| No | 55 (74.32) | 106 (77.94) | ||
| FBG (mmol/L) | 8.18 ± 1.67 | 7.71 ± 1.14 | 2.160 | 0.033 |
| Scr (μmol/L) | 91.25 ± 14.72 | 84.61 ± 9.80 | 3.485 | 0.001 |
| HbAlc (%) | 7.04 ± 1.33 | 6.29 ± 1.05 | 4.239 | < 0.001 |
| BUN (mmol/L) | 7.10 ± 0.96 | 6.78 ± 1.13 | 2.111 | 0.036 |
| TC (mmol/L) | 4.81 ± 0.89 | 4.83 ± 0.97 | –0.189 | 0.851 |
| TG (mmol/L) | 1.78 ± 0.4 | 1.72 ± 0.38 | 1.090 | 0.277 |
| HDL-C (mmol/L) | 1.41 ± 0.31 | 1.45 ± 0.32 | −0.972 | 0.332 |
| LDL-C (mmol/L) | 3.31 ± 0.57 | 3.33 ± 0.86 | −0.185 | 0.853 |
Regression analysis was performed by taking the patients with T2DM complicated with DN to be the dependent variables and taking the duration of diabetes, FBG, Scr, HbAlc, BUN, and DR as the independent variables (there was no collinearity problem between the diagnosed variables), as shown in Tables 2 and 3. Multivariate analysis showed that the duration of diabetes, FBG, Scr, HbAlc, and DR were factors influencing DN in patients with T2DM (P < 0.05).
| Factors | Variables of interest | Assignment of value |
| Concurrent DN or not | Y | Yes = 1, No = 0 |
| Duration of diabetes (yr) | × 1 | Numerical value |
| FBG (mmol/L) | × 2 | Numerical value |
| Scr (μmol/L) | × 3 | Numerical value |
| HbAlc (%) | × 4 | Numerical value |
| BUN (mmol/L) | × 5 | Numerical value |
| DR | × 6 | Yes = 1, No = 0 |
| Factors | B | SE | Wald | P value | OR | 95%CI |
| Duration of diabetes (yr) | 0.352 | 0.164 | 4.619 | 0.032 | 1.421 | 1.031-1.959 |
| FBG (mmol/L) | 0.272 | 0.128 | 4.518 | 0.034 | 1.312 | 1.021-1.685 |
| Scr (μmol/L) | 0.063 | 0.015 | 16.745 | < 0.001 | 1.065 | 1.033-1.097 |
| HbAlc (%) | 0.707 | 0.161 | 19.31 | < 0.001 | 2.029 | 1.480-2.781 |
| BUN (mmol/L) | 0.250 | 0.158 | 2.507 | 0.113 | 1.283 | 0.942-1.748 |
| DR | 0.883 | 0.360 | 6.035 | 0.014 | 2.419 | 1.196-4.894 |
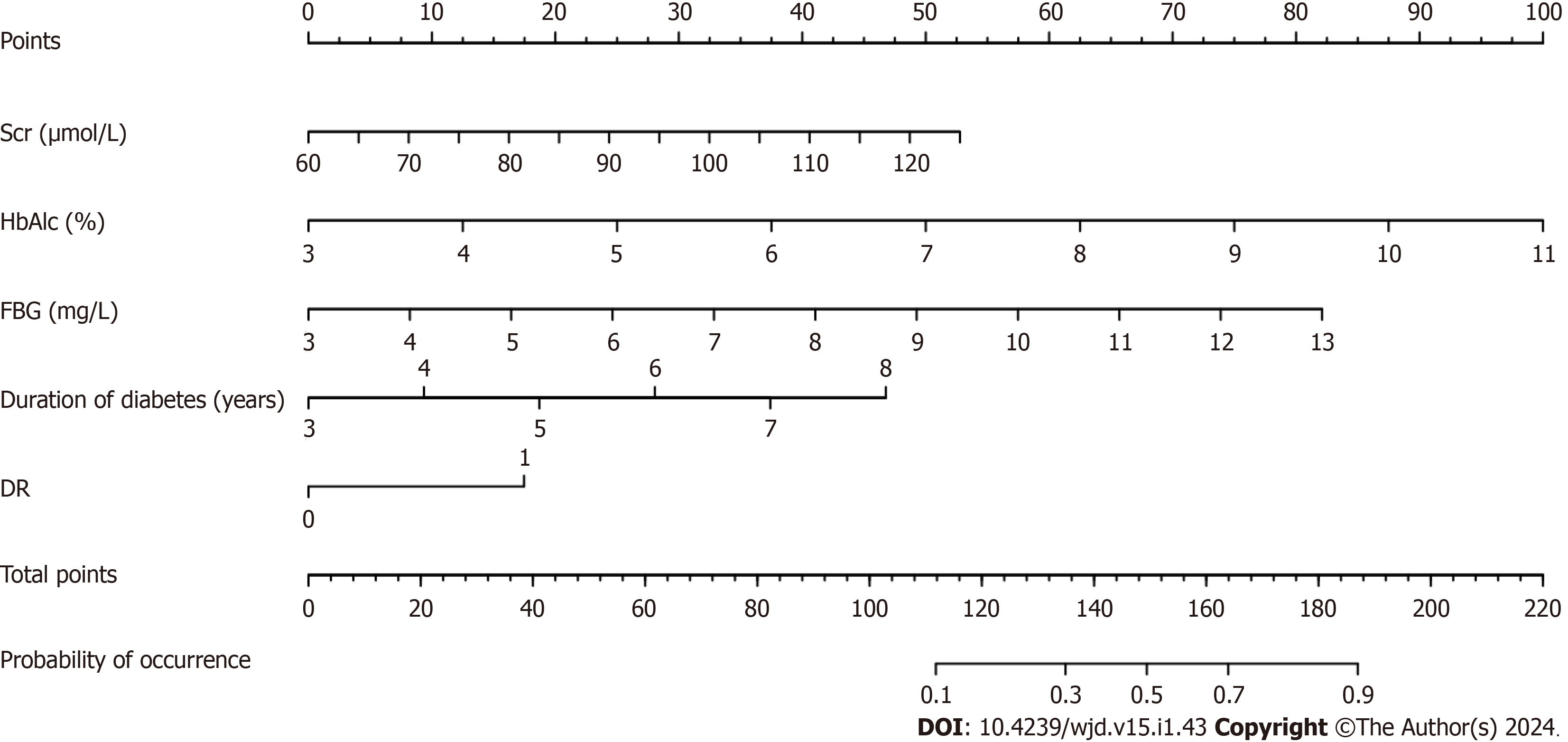
Based on the results of the multifactor logistic regression analysis, the obtained independent predictors (FBG, Scr, HbAlc, DR, and duration of diabetes) were used to construct a nomogram model for predicting DN in patients with T2DM (Figure 1).
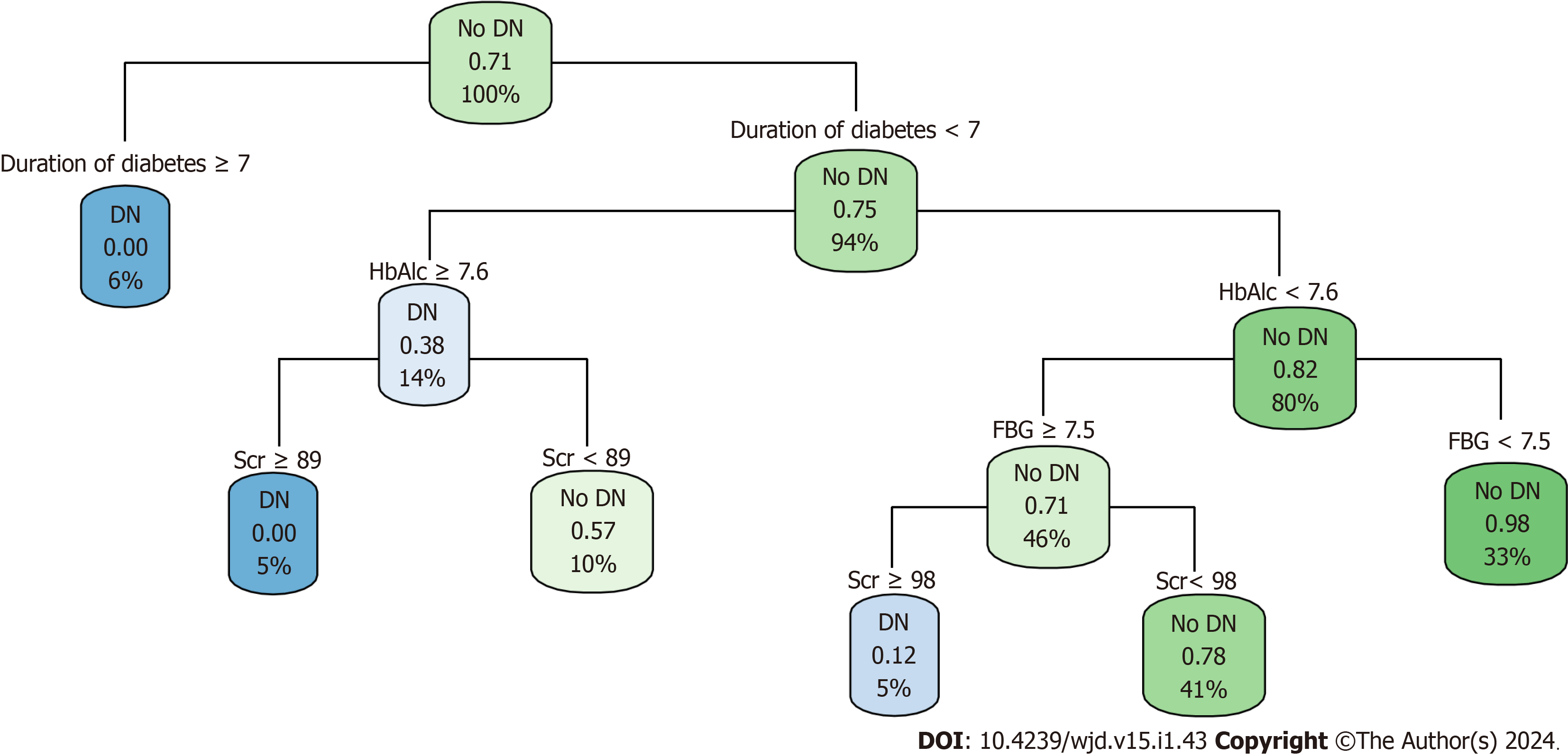
A decision-tree prediction model of DN in patients with T2DM was constructed, and four explanatory variables were screened: FBG, HbAlc, Scr, and duration of diabetes. The results showed that the duration of diabetes was the first factor influencing DN in patients with T2DM. The model identified six judgment rules, of which three judged concurrent DN and three judged no concurrent DN. The incidence of DN in patients with T2DM with duration of diabetes ≥ 7 years was 6%. The incidence of DN in patients with T2DM with duration of diabetes < 7 years, HbAlc ≥ 7.6%, and Scr ≥ 89 μmol/L was 5%. The incidence of DN in patients with T2DM with duration of diabetes < 7 years, HbAlc < 7.6%, FBG ≥ 7.5 mmol/L, and Scr ≥ 98 umol/L was 5% (Figure 2).
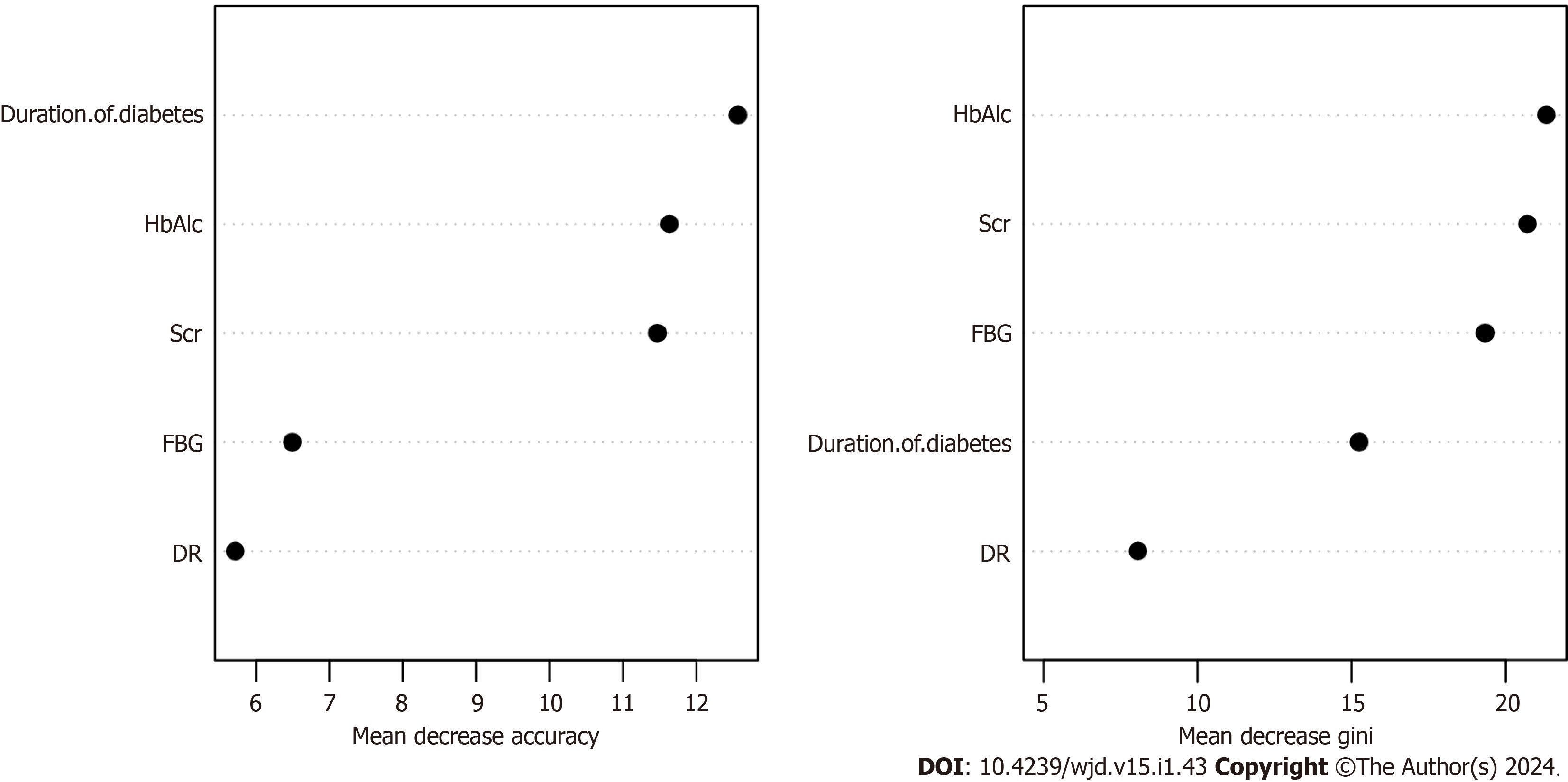
Based on the overall change in the prediction precision of the constructed random forest model, the variables affecting DN in patients with T2DM were HbAlc, Scr, FBG, duration of diabetes, and DR (Figure 3).
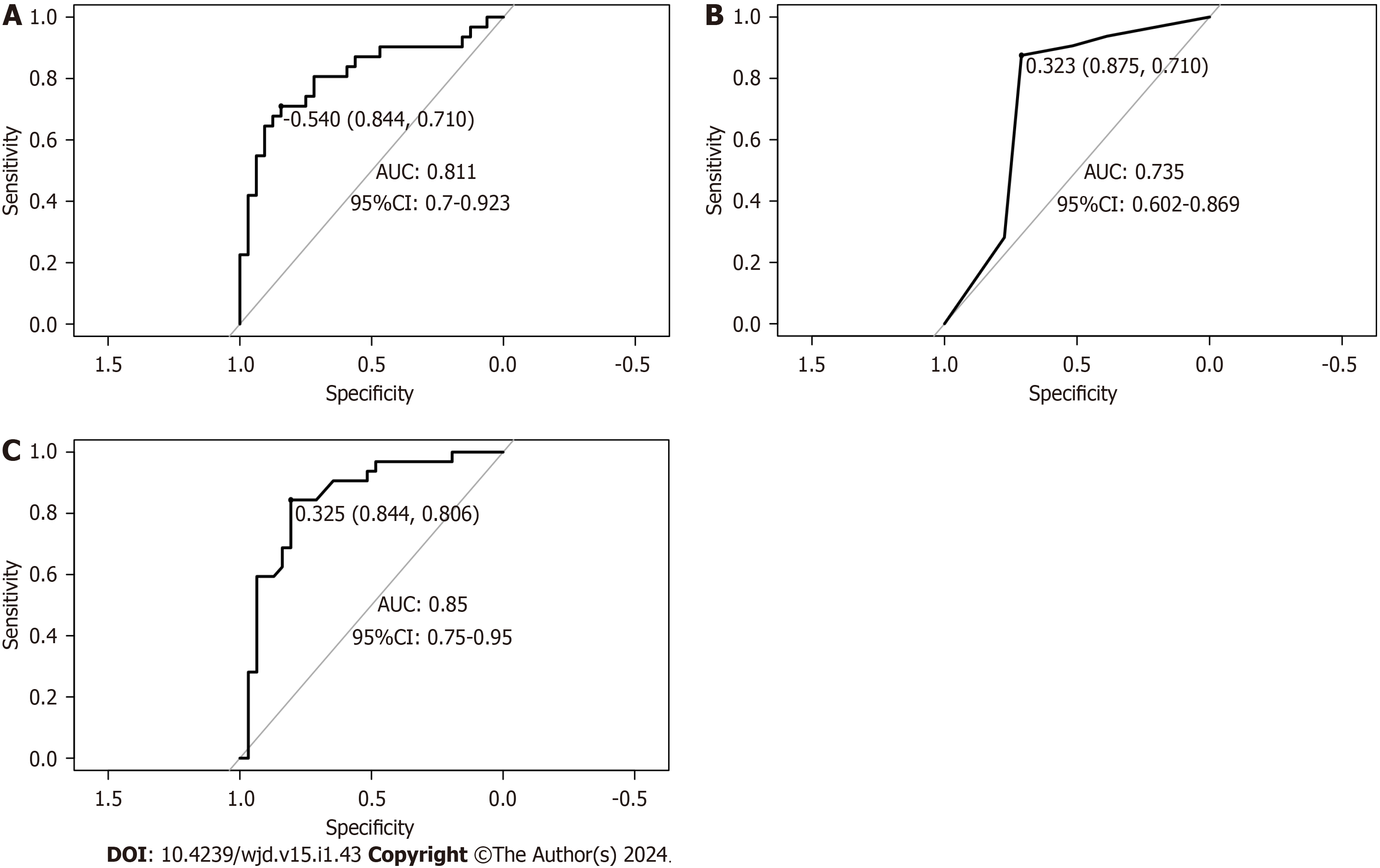
In the validation set, the overall evaluation of the efficacy of the nomogram in predicting concurrent DN in patients with T2DM was not significantly different from that of the random forest model. In contrast, the overall efficacy of the decision tree model was significantly lower than that of the nomogram and random forest model, with significant differences (all P > 0.05) (Table 4 and Figure 4).
| Model | Accuracy | Sensitivity | Specificity | Rate of recall | Rate of precision | AUC (95%CI) |
| Nomogram | 0.746 | 0.710 | 0.844 | 0.906 | 0.690 | 0.811 (0.700-0.923) |
| Decision tree | 0.714 | 0.710 | 0.875 | 0.906 | 0.659 | 0.735 (0.602-0.869) |
| Random forest | 0.730 | 0.806 | 0.844 | 0.906 | 0.674 | 0.850 (0.750-0.950) |
According to the National Kidney Foundation, persistent proteinuria is the primary indicator of kidney injury. ACR > 30 μg/mg in random urine samples was defined as renal injury, of which 30-300 μg/mg was the microalbuminuria stage, suggesting that the kidney had increased capillary permeability, which was the earliest sign of renal injury in patients with diabetes. In patients with T2DM, abnormal blood glucose levels frequently exist prior to diagnosis, and patients with T2DM may already have microalbuminuria (or clinical albuminuria) at the time of initial diagnosis[11,12]. Nephropathy is one of the most frequent complications of diabetes, and its insidious characteristics provide an intervention node for studying DN. Interventions for early detection, early detection diagnosis, and treatment can prevent the occurrence of DN and reduce or delay the occurrence of end-stage renal disease[13]. Therefore, identifying and intervening in clinically modifiable factors for the occurrence and progression of DN remains the primary strategy for its prevention and cure of DN.
The incidence of DN complications in patients with T2DM in this study was 35.24%. Wagnew et al[14] revealed that the incidence of DN was 35.3%, which is consistent with the results of this study. However, Zhang et al[15] revealed that the incidence of DN was only 21.8%, which was significantly lower than that reported in the present study. The incidence of DN varies greatly among studies and may be related to factors such as population characteristics and regions. The findings of this study revealed that FBG, Scr, HbAlc, DR, and duration of diabetes were factors affecting DN in patients with T2DM. The levels of FBG, Scr, HbAlc, and duration of diabetes in the DN group were higher than those in the non-DN group, and the proportion of DR was higher than that in the non-DN group, which was similar to the results of previous studies[16-18]. This suggests that patients with DN have worse glycemic control and poorer renal function[19,20]. Moreover, compared with patients with T2DM without DR, patients with DR are more likely to develop DN.
Nagel et al[21] followed high-risk populations for > 20 years and found that fasting hyperglycemia was a predictor of high albumin leakage rate. Hyperglycemia causes renal damage through the activation of multiple pathways, including the formation of glycosylated complexes by the interaction of glucose with proteins outside the cell, metabolism to sorbitol through the polyol pathway, and metabolism to glucosamine through the hexosamine biosynthesis process, thereby mediating hyperglucose-induced renal damage. HbAlc can cause microvascular damage. In a tissue environment with high glucose levels, the non-enzymatic catalytic process of the glycation reaction is accelerated, manifesting as a continuous increase in HbAlc. After glycation, abnormal hemoglobin levels cause chronic damage to the microcirculation vessels, damaging the basement membrane charge barrier and urine proteins. This indicates that microvascular damage to diabetic kidney tissues is a risk factor for microvascular or macrovascular lesions in patients with diabetes[22]. This study also confirmed that high blood glucose and HbAlc levels are associated with DN. Since Scr is mainly excreted in urine via glomerular filtration from the blood and is almost not reabsorbed by renal tubules, the output of creatinine is constant when renal function is normal or slightly damaged, and an increase in Scr indicates that renal function is damaged[23]. Diabetes duration is the main risk factor for various complications in patients with T2DM, especially the primary risk factor for renal complications. If the duration of diabetes is > 5 years, microalbuminuria can occur. Without active intervention, it can progress to DN[24]. Studies have shown that DN and DR have the same risk factors, such as diabetes course and FBG[25]. Diabetic retinal abnormalities are associated with glomerular injury. Studies have shown that changes in retinal arteriole and venule diameter are associated with renal histological changes, such as basement membrane thickness and mesangial matrix volume increase[26]. In patients with diabetes with macroalbuminuria, retinopathy is rare; Therefore, DR combined with macroalbuminuria is more likely to be diagnosed as DN. For patients with DR, early intervention of risk factors and renal pathology examination should be performed to control the risk of DN.
Nomograms, decision trees, and random forest prediction models were established based on the above indicators. The results showed that the random forest model performed better than the nomogram and decision tree models in terms of AUC, sensitivity, and other evaluation indicators. In contrast, the overall evaluation indicators of the nomogram model were better than those of the decision tree model. The statistical difference between the AUC of the random forest and nomogram model was not significant; However, the statistical difference between the AUC of the decision tree and nomogram and the random forest model was significant. In general, the comprehensive predictive abilities of the three prediction models for concurrent DN in patients with T2DM constructed in this study were as follows: Decision tree < nomogram < random forest. This may be because the decision tree model prefers variables with higher values; however, these variables are not necessarily the best predictive variables, which reduces the predictive ability of the decision tree model. In addition, eliminating some candidate variables in the pruning process of the decision-tree model reduces its predictive ability to a certain extent[27]. A nomogram is a model that integrates multiple related factors to predict the probability of an event; it is intuitive, visual, and has good accuracy[28]. However, owing to its easy form (very much like a linear model), it is difficult to capture complex relationships and handle the problem of data imbalance and is sensitive to multicollinear data. Therefore, the prediction efficiency was slightly lower than that of the random forest model. However, random forest integrates the output of an individual decision tree to produce the final prediction result, which has the characteristics of robust operation. It is not easily affected by collinearity between variables and has a significant effect on reducing the variance of the model[29]. Tseng et al[30] found that the nomogram and random forest models outperformed the decision tree model in the diagnosis of postoperative cardiac patients with acute kidney injury. Hu et al[31] showed that the random forest model has better predictive power than the nomogram model and can effectively predict the risk of cognitive impairment in older adults. These results further demonstrate that the random forest model has a strong comprehensive prediction ability. As this was a retrospective study, all data were obtained from the same hospital, and the sample representation was limited.
This study established nomogram, random forest, and decision tree prediction models for T2DM complicated by DN using a machine learning algorithm. The result showed that the random forest model had good prediction and stability, thus providing a reference for the clinical identification of T2DM complicated by DN. Future studies need to further validate the model through prospective multi-center data and include more variables and samples to further improve the predictive ability of the model to better guide clinical practice.
Hyperglycemia is the main pathophysiological feature of diabetes, and its complications are the key factors of death and disability in patients with diabetes. Diabetic nephropathy (DN) is a microvascular complication and is one of the main complications of diabetes. The initial prediction of DN is beneficial for taking measures to prevent and delay the occurrence and progression of corresponding complications. Machine learning has been widely used to construct predictive models for diabetic complications.
Patients with type 2 diabetes mellitus (T2DM) complicated by DN are at high risk of mortality. We explored the factors affecting the complications of DN to establish three prediction models commonly used in medicine, compared the prediction effects, and selected the optimal model to provide a basis for clinical identification of patients with T2DM complicated with DN.
This study aimed to explore the factors influencing T2DM complicated with DN and use these factors to construct a prediction model for DN. The prediction effect of random forest is the best among the three models of nomogram, decision tree, and random forest and may become a useful tool for the early recognition of the risk of DN.
We retrospectively analyzed the clinical data of 210 patients with T2DM treated at our hospital between August 2019 and August 2022. Factors influencing DN were analyzed, and nomograms, decision trees, and random forest prediction models were established to compare their prediction efficiency. These three prediction methods are widely used in the medical field and have advantages and limitations. At the same time, through research, we can select a more suitable model to predict the complication risk of DN.
Fasting blood glucose, serum creatinine, glycosylated hemoglobin, diabetic retinopathy, and the duration of diabetes were independent factors influencing DN. Among the established nomograms, decision trees, and random forest pre
In this study, the predictive performances of three models were compared. The random forest model performed best in predicting the risk of DN in patients with T2DM and may be a useful alternative tool for diagnosing T2DM.
Future studies should include larger and more comprehensive samples, conduct multi-center studies, further improve existing algorithms, and consider the combination of multiple algorithms to construct a more complete and accurate prediction model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dąbrowski M, Poland; Di Ciaula A, Italy; Mostafavinia A, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. Brussels: International Diabetes Federation; 2021–. [PubMed] |
| 2. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2132] [Article Influence: 426.4] [Reference Citation Analysis (0)] |
| 3. | Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol. 2011;22:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Luk AOY, Hui EMT, Sin MC, Yeung CY, Chow WS, Ho AYY, Hung HF, Kan E, Ng CM, So WY, Yeung CK, Chan KS, Chan KW, Chan PF, Siu SC, Tiu SC, Yeung VTF, Chan JCN, Chan FWK, Cheung C, Cheung NT, Ho ST, Lam KSL, Yu LWL, Chao D, Lau IT. Declining Trends of Cardiovascular-Renal Complications and Mortality in Type 2 Diabetes: The Hong Kong Diabetes Database. Diabetes Care. 2017;40:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 758] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 6. | Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014. JAMA. 2016;316:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 697] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 7. | Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284:603-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 497] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 8. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1322] [Article Influence: 661.0] [Reference Citation Analysis (70)] |
| 9. | Tsur A, Harman-Bohem I, Buchs AE, Raz I, Wainstein J. [The guidelines for the diagnosis prevention and treatment of type 2 diabetes mellitus--2005]. Harefuah. 2006;145:583-586, 630. [PubMed] |
| 10. | Li L, Yang Y, Zhu X, Xiong X, Zeng L, Xiong S, Jiang N, Li C, Yuan S, Xu H, Liu F, Sun L. Design and validation of a scoring model for differential diagnosis of diabetic nephropathy and nondiabetic renal diseases in type 2 diabetic patients. J Diabetes. 2020;12:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Qi C, Mao X, Zhang Z, Wu H. Classification and Differential Diagnosis of Diabetic Nephropathy. J Diabetes Res. 2017;2017:8637138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021;2021:1497449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 494] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 13. | Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 14. | Wagnew F, Eshetie S, Kibret GD, Zegeye A, Dessie G, Mulugeta H, Alemu A. Diabetic nephropathy and hypertension in diabetes patients of sub-Saharan countries: a systematic review and meta-analysis. BMC Res Notes. 2018;11:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Zhang XX, Kong J, Yun K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J Diabetes Res. 2020;2020:2315607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 16. | Tziomalos K, Athyros VG. Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis. Rev Diabet Stud. 2015;12:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Hu Y, Shi R, Mo R, Hu F. Nomogram for the prediction of diabetic nephropathy risk among patients with type 2 diabetes mellitus based on a questionnaire and biochemical indicators: a retrospective study. Aging (Albany NY). 2020;12:10317-10336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Shi R, Niu Z, Wu B, Zhang T, Cai D, Sun H, Hu Y, Mo R, Hu F. Nomogram for the Risk of Diabetic Nephropathy or Diabetic Retinopathy Among Patients with Type 2 Diabetes Mellitus Based on Questionnaire and Biochemical Indicators: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2020;13:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Zhou DM, Wei J, Zhang TT, Shen FJ, Yang JK. Establishment and Validation of a Nomogram Model for Prediction of Diabetic Nephropathy in Type 2 Diabetic Patients with Proteinuria. Diabetes Metab Syndr Obes. 2022;15:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Xi C, Wang C, Rong G, Deng J. A Nomogram Model that Predicts the Risk of Diabetic Nephropathy in Type 2 Diabetes Mellitus Patients: A Retrospective Study. Int J Endocrinol. 2021;2021:6672444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Nagel G, Zitt E, Peter R, Pompella A, Concin H, Lhotta K. Body mass index and metabolic factors predict glomerular filtration rate and albuminuria over 20 years in a high-risk population. BMC Nephrol. 2013;14:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 22. | Zhu Y, Wang X, Wang W, Wang H, Zhang F. Expression and influence of pentraxin-3, HbAlc and ApoA1/ApoB in serum of patients with acute myocardial infarction combined with diabetes mellitus type 2. Exp Ther Med. 2018;15:4395-4399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Hu H, Nakagawa T, Honda T, Yamamoto S, Okazaki H, Yamamoto M, Miyamoto T, Eguchi M, Kochi T, Shimizu M, Murakami T, Tomita K, Ogasawara T, Sasaki N, Uehara A, Kuwahara K, Kabe I, Mizoue T, Sone T, Dohi S; Japan Epidemiology Collaboration on Occupational Health Study Group. Low serum creatinine and risk of diabetes: The Japan Epidemiology Collaboration on Occupational Health Study. J Diabetes Investig. 2019;10:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Ninković V, Ninković S, Zivojinović D. [Cardiovascular autonomous dysfunction in diabetics: the influence of disease duration, glycoregulation degree and diabetes type]. Srp Arh Celok Lek. 2008;136:488-493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Saini DC, Kochar A, Poonia R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J Ophthalmol. 2021;69:3364-3368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Li Y, Su X, Ye Q, Guo X, Xu B, Guan T, Chen A. The predictive value of diabetic retinopathy on subsequent diabetic nephropathy in patients with type 2 diabetes: a systematic review and meta-analysis of prospective studies. Ren Fail. 2021;43:231-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Bamber JH, Evans SA. The value of decision tree analysis in planning anaesthetic care in obstetrics. Int J Obstet Anesth. 2016;27:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Lv J, Liu YY, Jia YT, He JL, Dai GY, Guo P, Zhao ZL, Zhang YN, Li ZX. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J Surg Oncol. 2021;19:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Uddin S, Khan A, Hossain ME, Moni MA. Comparing different supervised machine learning algorithms for disease prediction. BMC Med Inform Decis Mak. 2019;19:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 550] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 30. | Tseng PY, Chen YT, Wang CH, Chiu KM, Peng YS, Hsu SP, Chen KL, Yang CY, Lee OK. Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit Care. 2020;24:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 31. | Hu M, Shu X, Yu G, Wu X, Välimäki M, Feng H. A Risk Prediction Model Based on Machine Learning for Cognitive Impairment Among Chinese Community-Dwelling Elderly People With Normal Cognition: Development and Validation Study. J Med Internet Res. 2021;23:e20298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |