Published online Sep 15, 2023. doi: 10.4239/wjd.v14.i9.1369
Peer-review started: May 24, 2023
First decision: June 12, 2023
Revised: July 6, 2023
Accepted: August 2, 2023
Article in press: August 2, 2023
Published online: September 15, 2023
Processing time: 112 Days and 10.9 Hours
Diabetic skin ulcers, a significant global healthcare burden, are mainly caused by the inhibition of cell proliferation and impaired angiogenesis. XB130 is an adaptor protein that regulates cell proliferation and migration. However, the role of XB130 in the development of diabetic skin ulcers remains unclear.
To investigate whether XB130 can regulate the inhibition of proliferation and vascular damage induced by high glucose. Additionally, we aim to determine whether XB130 is involved in the healing process of diabetic skin ulcers, along with its molecular mechanisms.
We conducted RNA-sequencing analysis to identify the key genes involved in diabetic skin ulcers. We investigated the effects of XB130 on wound healing using histological analyses. In addition, we used reverse transcription-quantitative polymerase chain reaction, Western blot, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining, immunofluorescence, wound healing, and tubule formation experiments to investigate their effects on cellular processes in human umbilical vein endothelial cells (HUVECs) stimulated with high glucose. Finally, we performed functional analysis to elucidate the molecular mechanisms underlying diabetic skin ulcers.
RNA-sequencing analysis showed that the expression of XB130 was up-regulated in the tissues of diabetic skin ulcers. Knockdown of XB130 promoted the healing of skin wounds in mice, leading to an accelerated wound healing process and shortened wound healing time. At the cellular level, knockdown of XB130 alleviated high glucose-induced inhibition of cell proliferation and angiogenic impairment in HUVECs. Inhibition of the PI3K/Akt pathway removed the proliferative effects and endothelial protection mediated by XB130.
The findings of this study indicated that the expression of XB130 is up-regulated in high glucose-stimulated diabetic skin ulcers and HUVECs. Knockdown of XB130 promotes cell proliferation and angiogenesis via the PI3K/Akt signalling pathway, which accelerates the healing of diabetic skin ulcers.
Core Tip: The role of XB130 in the occurrence and development of diabetic skin ulcers healing is unclear. This study showed that the expression of XB130 was up-regulated in tissues of diabetic skin ulcers and human umbilical vein endothelial cells (HUVECs) stimulated by high glucose. Knockdown of XB130 promote the healing of skin wounds in mice, leading to an accelerated wound healing process and shortened wound healing time and alleviated hyperglycemia-induced cell proliferation inhibition and angiogenic impairment in HUVECs.
- Citation: Zhu XL, Hu DY, Zeng ZX, Jiang WW, Chen TY, Chen TC, Liao WQ, Lei WZ, Fang WJ, Pan WH. XB130 inhibits healing of diabetic skin ulcers through the PI3K/Akt signalling pathway. World J Diabetes 2023; 14(9): 1369-1384
- URL: https://www.wjgnet.com/1948-9358/full/v14/i9/1369.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i9.1369
Diabetes mellitus (DM) is a chronic metabolic disease in which the body does not produce enough insulin or reacts abnormally to the hormone, leading to typically high blood sugar levels. In 2021, more than 37 million Americans have diabetes. Of those, 28.7 million have been diagnosed and 8.5 million are undiagnosed[1]. Impaired wound healing often results in severe skin ulcers[2]; a high percentage (15%-27%) of diabetic foot skin ulcer cases require lower extremity amputation owing to treatment failure[3]. Therefore, developing effective strategies to prevent and treat cutaneous wounds in patients with diabetes is crucial.
Impaired healing of diabetic wounds is characterised by significant deficits in cellular proliferation and migration, and a notable decrease in protein synthesis[4,5]. These impairments result in delayed re-epithelialisation, angiogenesis, and granulation tissue formation[6]. Numerous molecular pathways play crucial roles in cell proliferation and protein synthesis during the wound healing process[7]. Laplante et al[8] discovered that mTOR signalling could effectively treat diabetic skin damage by promoting cell proliferation, inhibiting skin cell apoptosis, and accelerating epithelial regeneration.
XB130 is a bridging protein that serves as the mediator of multiple tyrosine kinases, playing an important role in regulating cell proliferation, survival, migration, and invasion[9,10]. As a tumor suppressor in carcinogen-induced skin tumorigenesis, knockout of XB130 significantly up-regulated epidermal tumor cell proliferation[11]. XB130 also found in stomach, oesophagus, and thyroid epithelial cells, and has been studied as a signal transduction protein[12-14]. The expression of XB130 were up-regulated in cholangiocarcinoma, non-small cell-lung cancer, prostate cancer, esophageal squamous cell carcinoma and gastric cancer, regulating XB130 expression may inhibit the development and progression of tumors[12,13,15-17]. However, whether XB130 regulate wound healing in patients with diabetes and the underlying molecular mechanisms have not been reported.
In this study, we first confirmed that the expression of XB130 was up-regulation in tissues of diabetic skin ulcer mice. Knockdown of XB130 can accelerate the healing process and shorten the healing time of skin wound in mice. At the cellular level, down-regulation of XB130 alleviated hyperglycaemia-induced inhibition of cell proliferation and angiogenic impairment in human umbilical vein endothelial cells (HUVECs). Mechanistically, down-regulation of XB130 accelerates healing of diabetic skin ulcers by promoting cell proliferation and angiogenesis through the PI3K/Akt signalling pathway.
C57BL/6 mice (20-25 g, 6 wk old) were obtained from Beijing Weitong Lihua Experimental Animal Technology, China. The mice were provided with free access to food and water; the temperature and relative humidity of the room were maintained at 20 °C-24 °C and 40%-60%, respectively. The mice were allowed to acclimatize to their surroundings for 1 wk prior to the experiment.
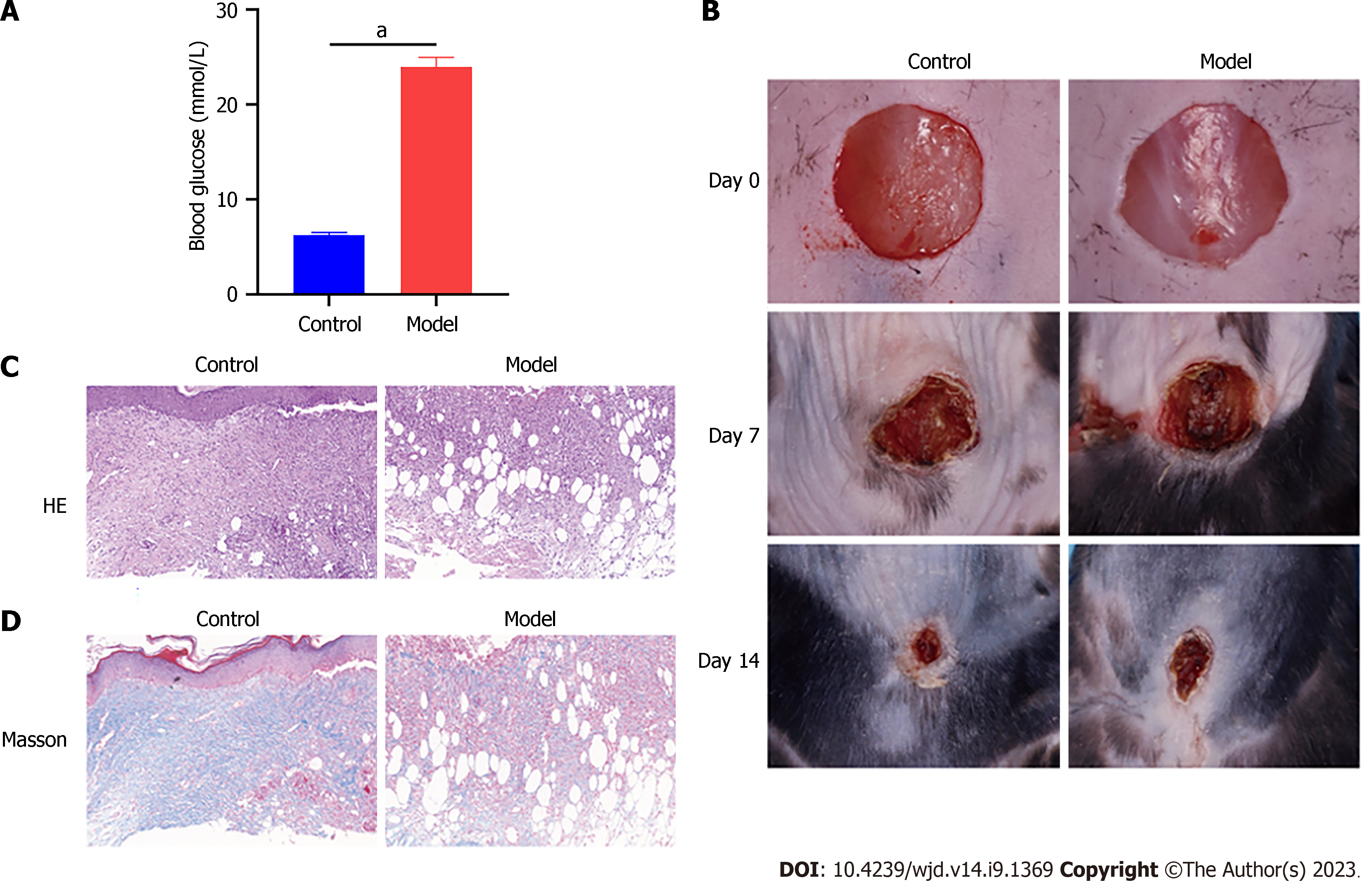
Diabetes was induced in mice by injecting them with streptozotocin (STZ; 40 mg/kg) for 5 consecutive days. The control group received an equivalent dose of citrate buffer solution[18]. Diabetic status was determined in mice if their blood glucose levels exceeded 250 mg/dL following STZ injection. Both diabetic and normal mice were anaesthetised with 2% isoflurane. Full-thickness excisional wounds measuring 8 mm in diameter were created on the dorsal skin of the mice.
Adenovirus knockdown of XB130 targeting sequence and its corresponding control were constructed by ABM (Nanjing, China). Mice were given tail vein injections of adenovirus at the concentration of 5 × 1011 genome-equivalents five days before surgery.
On day 14 post-treatment, the mice were euthanised and their wound tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Haematoxylin & eosin (HE) and Masson staining were performed using kits from Solarbio (China) and Sigma (United States), respectively, following the manufacturer’s protocols. Images were captured using a Ci-L microscope (Nikon, Japan) and analysed using the Image J software (version 1.8.0).
HUVECs were sourced from Guangzhou Saliai Stemcell Co., Ltd. And cultured using EGM-2 BulletKit (Lonza). Subconfluent cells from passages five to seven were used for the experiments. Before cell culture procedures, stock media were replaced with phenol red-free low-glucose DMEM (Gibco, United States) supplemented with 1% calf serum (Gibco, CA, United States) and left for 12 h. HUVECs were then exposed to EGM-2 supplemented with either normal glucose (NG, 5.5 mmol/L) or high glucose (HG, 33 mmol/L) for 72 h, while using D-mannitol as an osmotic control for the HG condition. The knockdown plasmid of XB130 and its corresponding control plasmid were obtained from Guangdong Ruibo Biotechnology Co., Ltd. Transfection efficiency was confirmed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blot.
Transferase-mediated dUTP nick end labeling (TUNEL) staining was performed according to the instruction of TUNEL apoptosis detection kit (Promega, United States). The cells were seeded onto 24-well plates and incubated at 37 °C for 12 h. After staining, cell apoptosis was counted by photographing. The apoptosis cells were brown considered as the TUNEL-positive cells. Apoptotic rate was evaluated.
Upon completion of the experimental protocol, HUVECs were stained with calcein (Corning, United States). The stained cells were then replated onto Matrigel-precoated 24-well plates containing growth-factor reduced Matrigel (150 µL/well) and incubated at 37 °C for 12 h. To assess capillary-like tubule formation, a computer-assisted microscope was used to detect tube-like structures that were at least four times longer than their widths.
To evaluate cell migration, a scratch assay was performed as described previously[19]. Following overnight incubation, the cells were seeded onto a 3.5-cm-diameter dish and allowed to form a confluent monolayer. Subsequently, a wound was created by scratching with a 200-mL pipette tip. The wounded cell monolayers were then imaged using a CoolSNAP HQ CCD (Nippon Roper Japan) at 0 h and 24 h post-wounding.
Well-grown HUVECs were fixed with 4.0% paraformaldehyde in phosphate-buffered saline for 10 min and permeabilised with 0.5% Triton for 15 min. Antibodies against PCNA, Ki67, Bcl2, Bax, and Cleaved caspase-3 obtained from Abcam (United States) were then used to incubate the cells overnight, followed by a 1 h incubation with or without Alexa Fluor 488-conjugated anti-mouse IgG secondary antibody (Abcam, United States) at room temperature. Nuclei were labelled with the fluorescent dye DAPI after 5 min of incubation. The cells were then observed under a confocal microscope (Leica, Germany) under each experimental condition.
Total RNA was extracted from skin tissues or HUVECs using TRIzol reagent (Invitrogen, United States) and reverse-transcribed into cDNA using a Tiangen Biotechnology (China) kit. RT-qPCR was conducted with β-Actin as an internal control, using 2 × SYBR Green QPCR Master Mix (Shanghai Dongsheng Biotechnology, China). The relative gene expression was determined using the 2-ΔΔCt method, and the primer sequences are provided in Table 1.
| Gene | Forward (5’→3’) | Reverse (5’→3’) |
| XB130 | AAGCAGCAGCTCTGATGAGG | GGTCTGGAAGGCTCTTCTGA |
| β-Actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
The protein from cell or tissue was extracted by RIPA and PMSF (Shanghai Life Mode Engineering, Shanghai, China), and the concentration of protein was determined by BCA kit (Shanghai Dongsheng Biotechnology, shanghai, China). The PVDF membrane (0.22 µm, Millipore ISEQ00010, United States) was then incubated with primary antibodies (Table 2) overnight at 4 °C, followed by incubation with HRP-conjugated secondary antibodies (Abcam, United States). Protein bands were detected using Prime Western Blot Detection Reagent (Cytiva, United Kingdom). A ChemiDoc MP imaging system (Tanon 4800, China) was used to detect chemiluminescence, and the ImageJ software was used to analyse the grey values of the bands.
| Gene | Brand | Provenance |
| XB130 | Abcam | Rabbit |
| Cleaved-caspase-3 | Abcam | Rabbit |
| Bcl2 | Abcam | Rabbit |
| Bax | Abcam | Rabbit |
| Ki67 | Abcam | Rabbit |
| PCNA | Abcam | Rabbit |
| AKT | Abcam | Rabbit |
| p-AKT | Abcam | Rabbit |
| p85α | Abcam | Rabbit |
| p-p85α | Abcam | Rabbit |
| β-Actin | Sigma | Mouse |
Experimental data were analysed using GraphPad Prism 9.0, SPSS 24.0, and R software 4.2.0. Statistical tests were chosen based on the distribution and variance homogeneity of the data. For normally distributed and homogeneous variance data, t-tests were used to compare two groups. For multiple group comparisons Dunnett’s-T3 test was used based on the distribution and variance. Data are presented as mean ± SD, and statistical significance was set at P < 0.05.
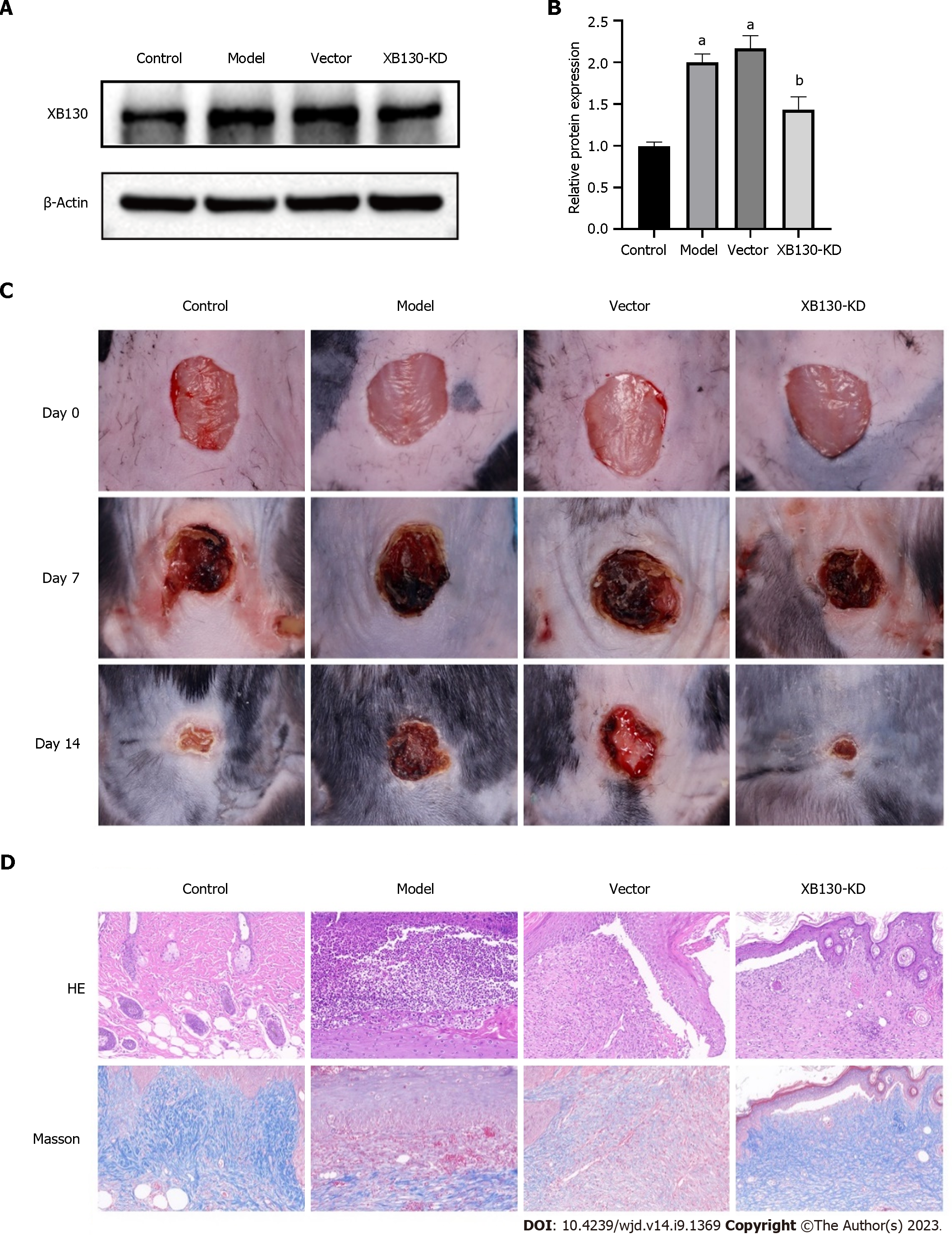
The toxicity of STZ to pancreatic cells makes it a popular choice for inducing diabetes mellitus in mice and rats[20]. After administering STZ for 5 consecutive days, the model group showed significantly elevated fasting blood glucose levels compared with the control group (Figure 1A). As shown in Figure 1B, the model group exhibited slower wound healing time and rate. Tissue samples from the model and control groups were collected on day 14 for HE and Masson staining. The wounds of the model mice showed severe tissue damage, disorganised tissue granulation, and reduced collagen deposition (Figure 1C and D).
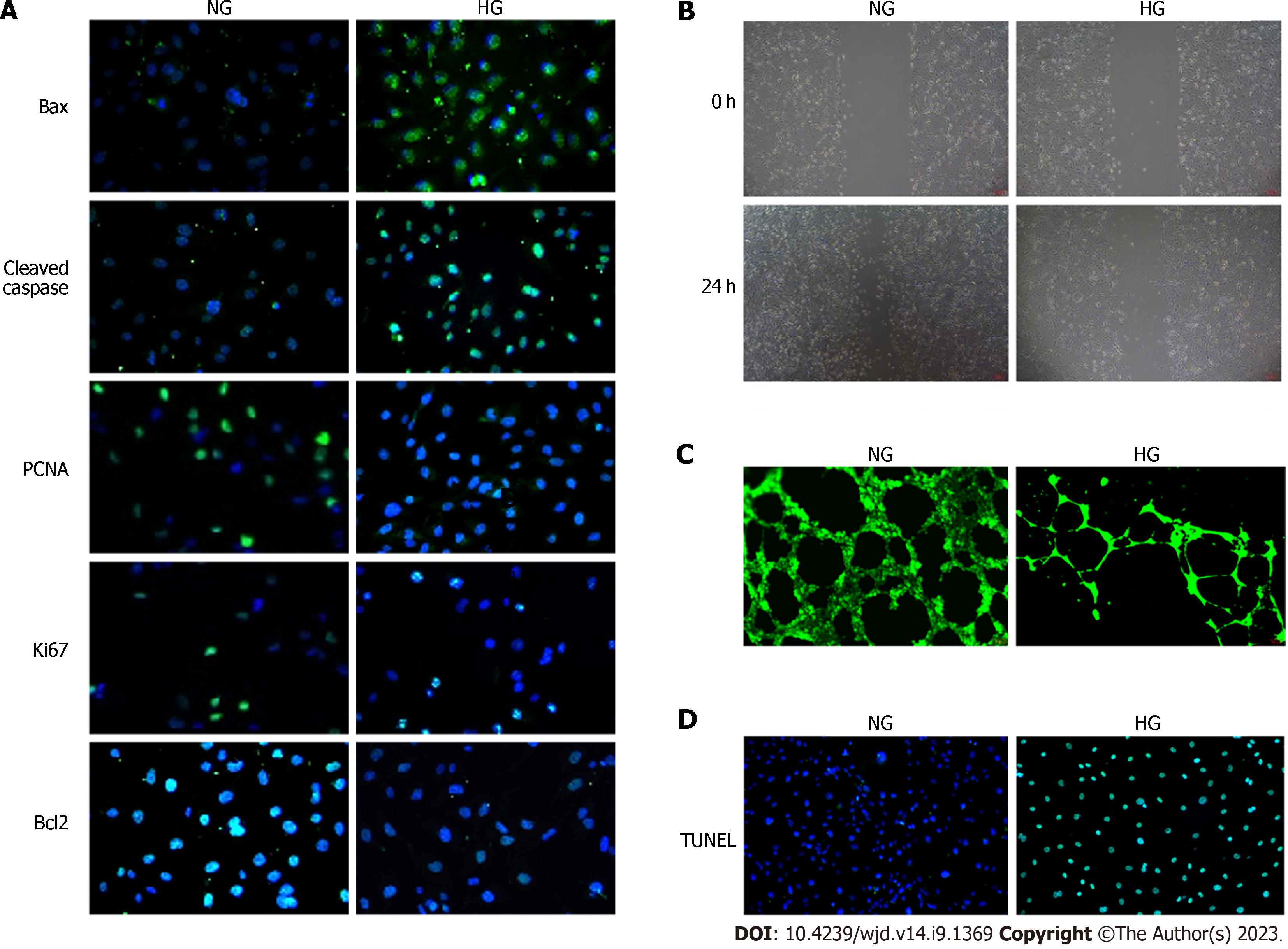
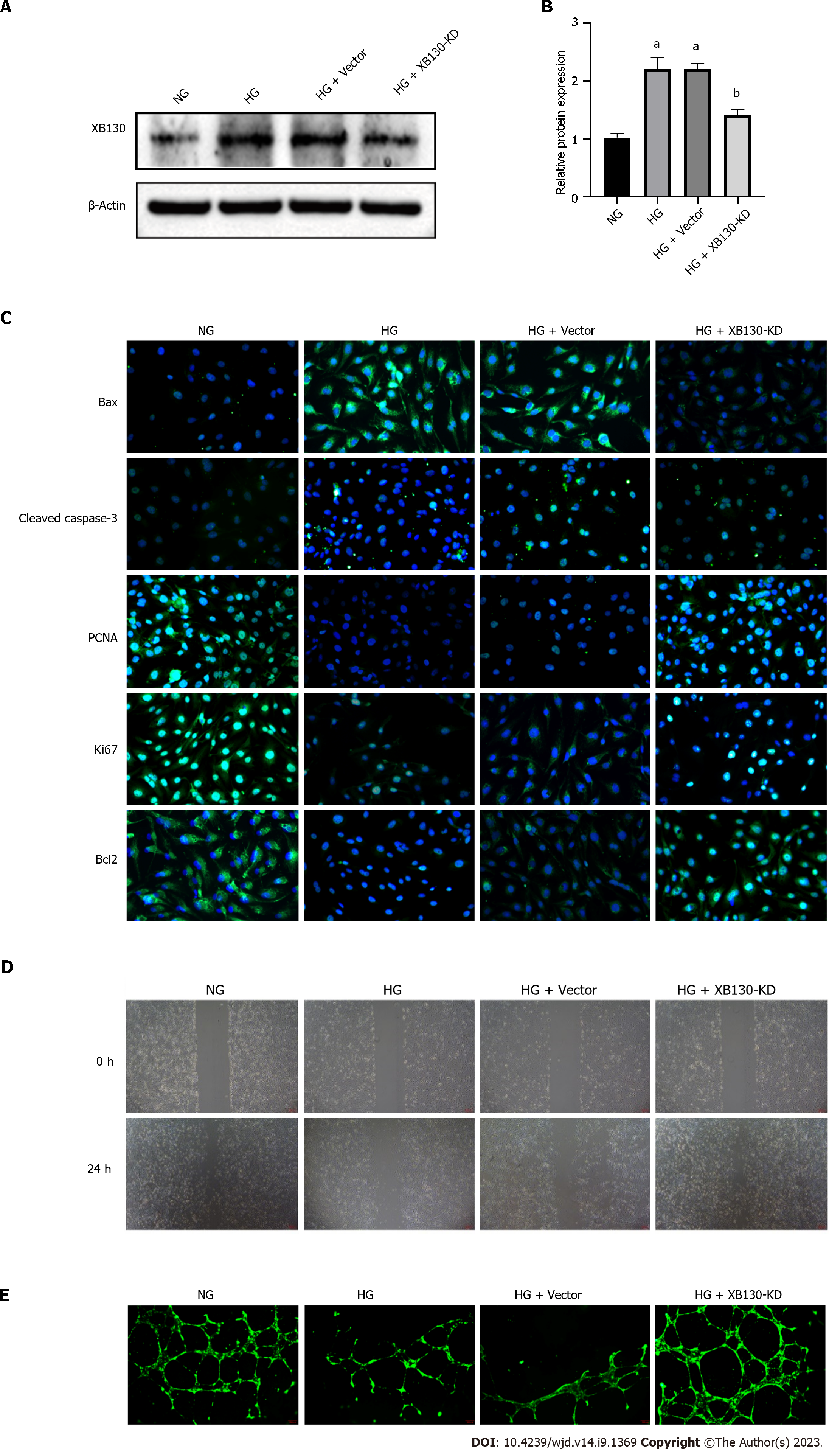
To simulate the HG environment during diabetic wound healing, we incubated HUVECs in an HG medium. Immunofluorescence staining for Bax and Cleaved caspase-3 in HG-treated HUVECs was higher than that in the NG group, whereas staining for PCNA, Ki67, and Bcl2 was lower (Figure 2A). The results of immunofluorescence analysis were confirmed by Western blot analysis (Supplementary Figure 1A and B). Moreover, HG treatment significantly impaired HUVEC migration and tube-forming activity (Figure 2B and C). We used the TUNEL assay to assess apoptosis in different groups and revealed an increased number of TUNEL-positive cells in the HG group (Figure 2D).
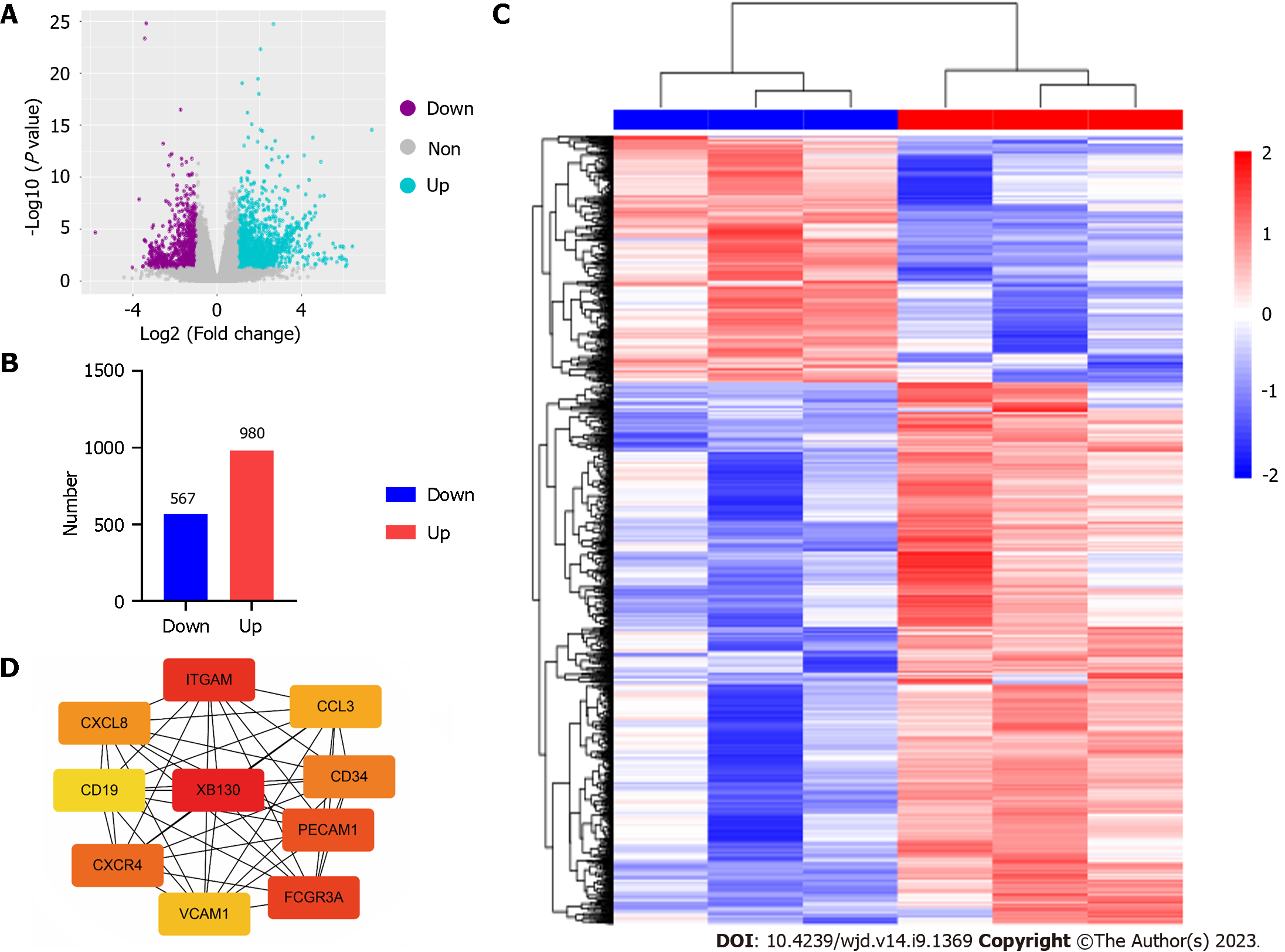
To investigate the genes involved in diabetic wound healing, RNA sequencing was performed on the ulcer tissues from control and model mice group. Prior to differential expression analysis, we conducted background correction, normalisation, and gene filtering. Total 1547 differentially expressed genes (DEGs) were yielded (Figure 3A-C). We employed the STRING database for protein-protein interaction (PPI) network analysis to better understand the interactions between DEGs. Using the CytoHubba plug-in in Cytoscape, we identified hub genes based on their degree, betweenness, and closeness centrality, and found XB130 to be the most significant hub gene (Figure 3D).
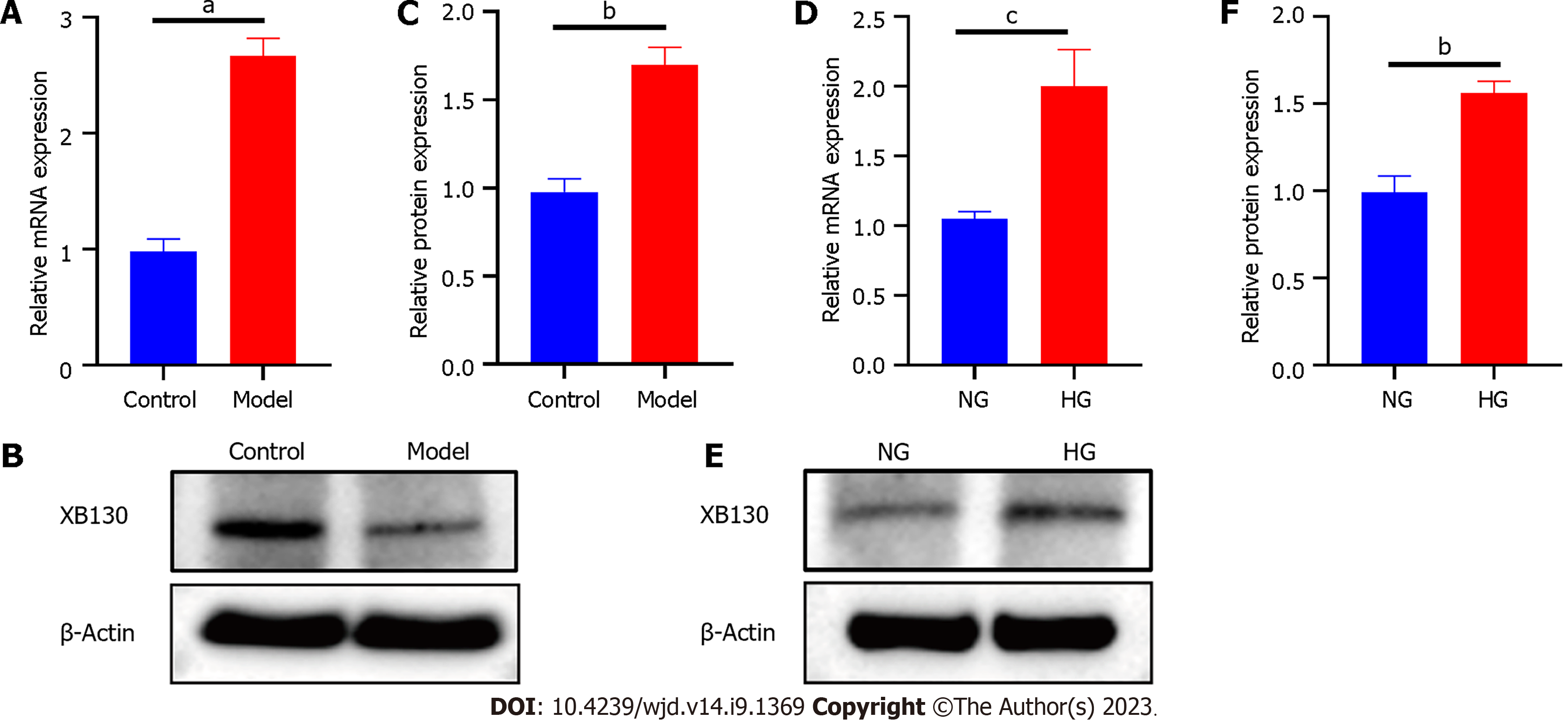
To confirm the accuracy of the microarray results, we assessed XB130 mRNA and protein levels using RT-qPCR and Western blot. Compared with the control group, the mRNA level of XB130 was significantly increased in the model group (Figure 4A). HG also resulted in increased mRNA expression of XB130 in HUVECs (Figure 4D). Western blot analysis validated the RT-qPCR results (Figure 4B, C, E, and F).
Next, we established a mouse model knockdown XB130 (XB130-KD) in diabetic mice by injection of adenovirus vectors carrying short hairpin RNA targeting XB130. The XB130-KD group showed a significant decrease in protein expression of XB130 compared with the vector group (Figure 5A and B). Subsequently, we evaluated the wound healing process and found that knocking down XB130 Led to a shorter wound healing time and a higher wound healing rate (Figure 5C). Furthermore, histological analysis revealed that knocking down XB130 resulted in better granulation formation, collagen deposition, and denser alignment compared with the model group (Figure 5D).
We evaluated the potential function of XB130 in HUVECs. After transfection, the expression of XB130 was significantly reduced in the XB130-KD group (Figure 6A and B). Immunofluorescence results showed that XB130 down-expression had a restraining effect on cell apoptosis and promoted cell proliferation in HUVECs (Figure 6C). These findings were further confirmed by Western blot analysis (Supplementary Figure 2A and B). Additionally, the TUNEL assay indicated that knocking down of XB130 reduced HG-induced apoptosis in HUVECs (Supplementary Figure 2C). Furthermore, XB130 down-expression counteracted the negative impact of hyperglycaemia on the migration and tube-forming activity of HUVECs (Figure 6D-E).
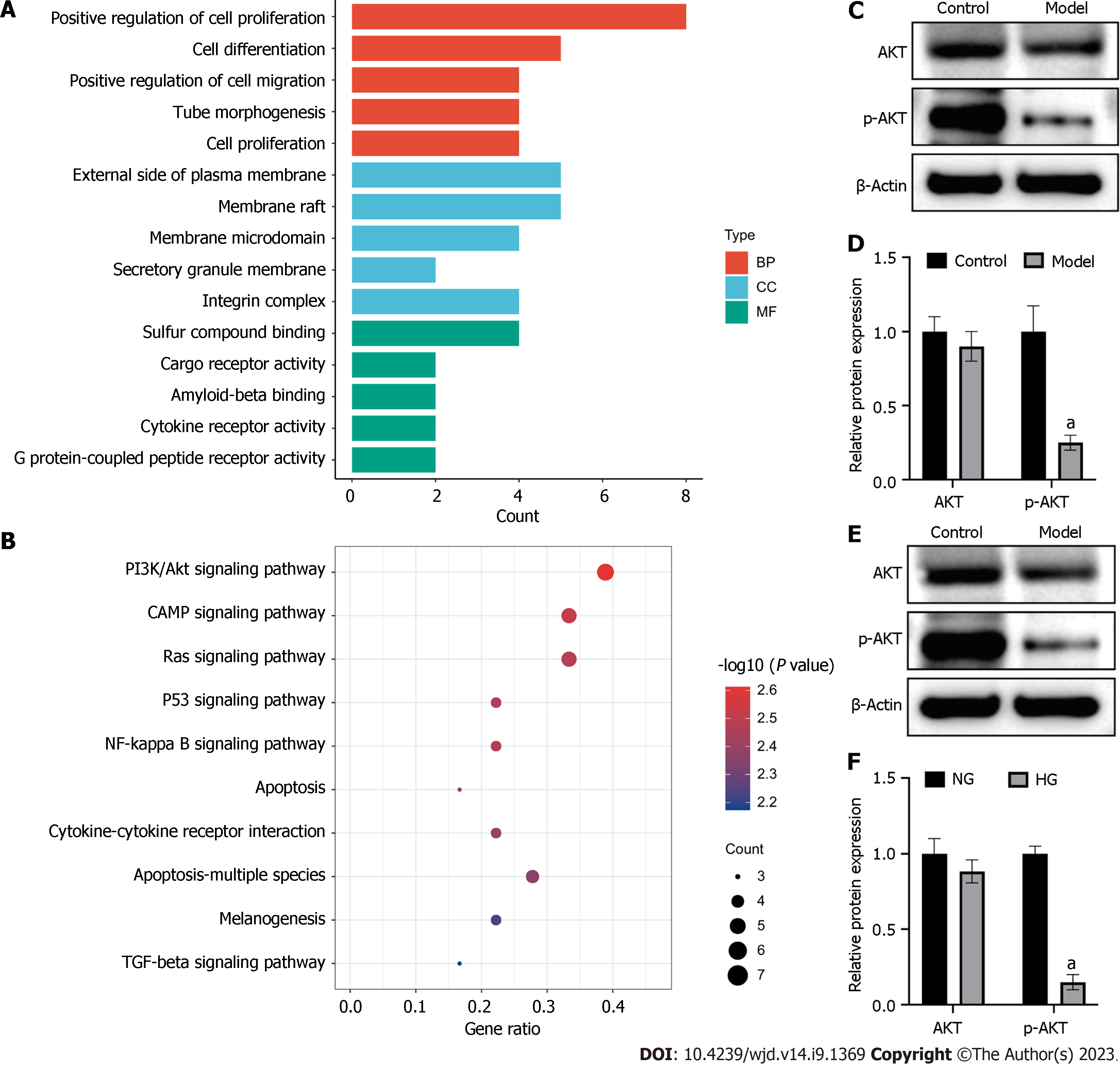
To investigate the mechanism underlying delayed wound healing in mice patients with diabetes, we conducted a functional enrichment analysis of the previously obtained hub genes. Gene Ontology (GO) analysis revealed that the hub genes were involved in the of cell proliferation, differentiation, and tube morphogenesis (Figure 7A). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated that these genes were predominantly associated with the PI3K/Akt, Ras, p53, and NF-kappa B signalling pathways. Notably, the PI3K/Akt signalling pathway had the highest number of differentially expressed genes (Figure 7B).
To validate this finding, we performed Western blot analysis and confirmed that the ratio of p-AKT to AKT was significantly decreased in diabetic mice (Figure 7C and D). Similar to results in vivo, HG levels inherited the PI3K/Akt signalling pathway in HUVECs (Figure 7E and F).
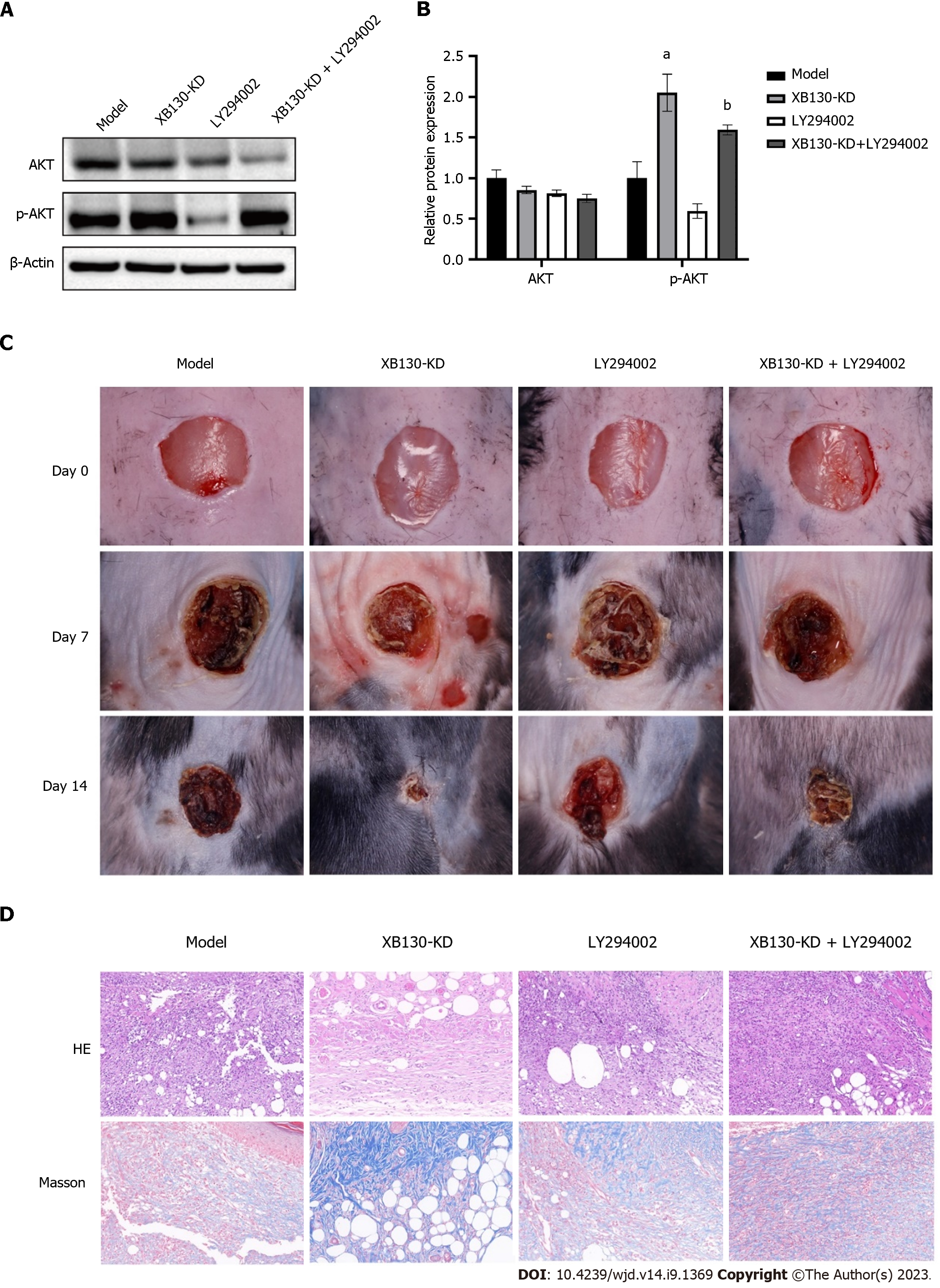
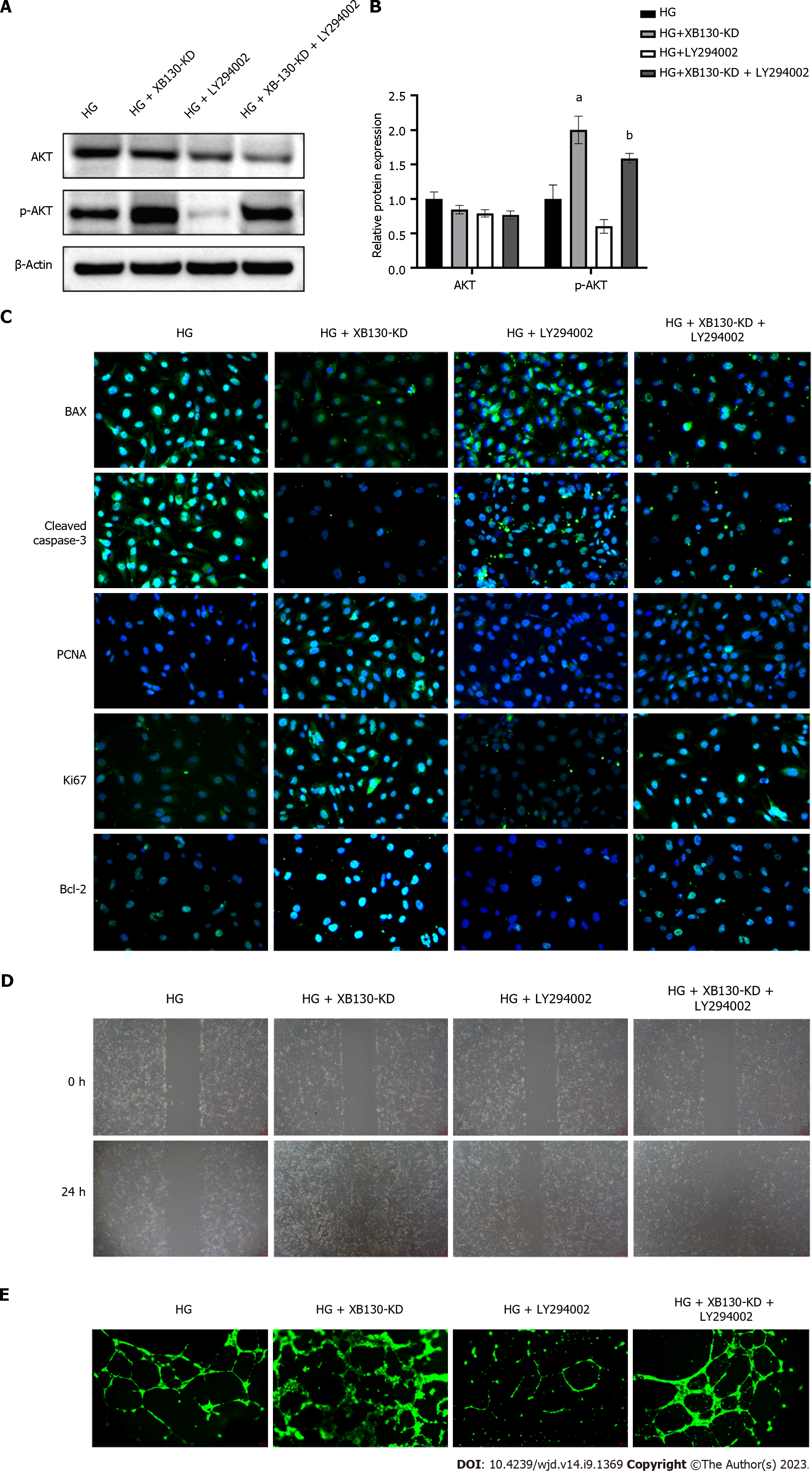
To investigate the effect of XB130 on wound healing via this pathway, diabetic mice with reduced XB130 expression were treated with LY294002, an inhibitor of the PI3K/Akt signalling pathway. Western blot analysis revealed that knocking down XB130 stimulated the Akt phosphorylation, however, the AKT phosphorylation was suppressed by LY294002 treatment (Figure 8A and B). Additionally, wound healing time was significantly longer in the LY294002 group than in the XB130-KD group (Figure 8C). Histological analysis showed that LY294002 treatment decreased collagen deposition in the wound area and angiogenesis in the granulation tissue (Figure 8D).
Western blot analysis showed that knocking down XB130 stimulated the PI3K/Akt signalling pathway, which was subsequently inhibited by LY294002 in HUVECs (Figure 9A and B). In LY294002-treated HUVECs, the protective effect of knocking down XB130 against HG was lost, resulting in increased apoptosis and decreased proliferation (Figure 9C). Western blot analysis yielded similar results (Supplementary Figure 3A and B). The TUNEL assay further demonstrated that LY294002 reversed the inhibitory effect of knocking down XB130 on HUVEC apoptosis (Supplementary Figure 3C). Moreover, in LY294002-treated HUVECs, the effects of knocking down XB130 on the pro-migration and tube-forming activities against HG impairment were removed (Figure 9D and E).
Diabetic skin ulcers, resulting from both internal and local pathological changes caused by diabetes, are a serious complication that can lead to amputation, despite therapeutic management[21,22]. The process of wound healing is complex, dynamic, and orderly, and is characterised by cell proliferation and migration[23,24]. In this study, we chose STZ, a well-established method for chemically inducing diabetes, to construct diabetic mice as an experimental model[18]. HUVECs were cultured in vitro in a high-glucose medium to mimic diabetic conditions. Our findings demonstrated that a high-glucose environment prolongs wound healing by inhibiting cell proliferation and tubule formation.
Aberrant gene expression is associated with various pathological conditions, including diabetic skin ulcers[25]. However, key driver genes that trigger and exacerbate this condition are not fully understood. We performed RNA sequencing the ulcer tissues from control and model mice group and identified 980 upregulated and 567 downregulated genes. Moreover, by performing PPI network analysis, we identified ten hub genes associated with diabetic skin ulcers (XB130, ITGAM, FCGR3A, PECAM1, CXCR4, CD34, CXCL8, CCL3, VCA11, and CD19). Among them, XB130 had the highest score based on topological algorithms. The expression of XB130 was up-regulated in HG induced diabetic skin ulcers tissue and HUVECs using RT-qPCR and Western blot.
XB130 is vital for regulating signal transduction and affects the cell cycle, proliferation, survival, and migration[14,16,17]. Although limited research has explored the relationship between XB130 and diabetic wound healing, our study aimed to address this knowledge gap by creating a model of XB130-downexpressed diabetic mice. Our findings indicate that knockdown of XB130 can enhance the healing of diabetic wounds, as evidenced by shorter epithelialisation time and rapid wound contraction. Moreover, histological analysis revealed that knockdown of XB130 not only improved diabetic wound healing, but also significantly promoted angiogenesis. To investigate the mechanism and function of XB130, we establish a knockdown of XB130 with HUVCEs. Our results revealed that the expression of PCNA, Ki67 and Bcl2 were significantly increased, while decreasing the expression of Bax and Cleaved caspase-3 in XB130-KD HUVECs treated with HG. Furthermore, knockdown of XB130 enhanced the migration and tube-forming activities of HUVECs. These findings indicate that knockdown of XB130 promotes diabetic wound healing by enhancing cell proliferation and tubule formation.
To investigate how HG environment affects wound healing, functional analysis of hub genes was performed. GO analysis revealed that the hub genes were enriched in cell proliferation, differentiation, migration, apoptosis, and tube morphogenesis. These results confirmed that HG levels hindered wound healing by affecting these cellular processes. Meanwhile, KEGG pathway analysis showed that these genes were involved in PI3K/Akt, Ras, p53, and NF-kappa B signalling pathways, and in apoptosis. Among these, the PI3K/Akt signalling pathway was the most important, with seven enriched genes. Our findings further prove that HG conditions reduce the ratio of p-AKT/AKT, leading to inhibition of the PI3K/Akt pathway.
The PI3K/AKT pathway plays a pivotal role in regulating cell proliferation and metabolism and is involved in the progression of various diseases[26-29]. When RTKs and GPCRs are activated, PI3K phosphorylates phosphatidylinositol 3,4-diphosphate, resulting in the production of PIP3. PIP3 activates AKT, which in turn phosphorylates and activates the mTORC complexes. In turn, activated mTORC complexes promote cell proliferation by phosphorylating and activating matrix metalloproteinase[30-32]. Our results indicate that knockdown of XB130 increases the levels of phosphorylated AKT. LY294002 and Wortmannin are commonly used inhibitors of the PI3K/Akt pathway. Our findings indicate that LY294002 delays wound healing in diabetic mice. Furthermore, inhibition of the PI3K/Akt signalling pathway reverses the negative effects of XB130 on proliferation and tubule formation in HUVECs.
This study shows that knockdown of XB130 can prevent high glucose-induced inhibition of proliferation and angiogenic impairment through the PI3K/Akt pathway. Based on our results, decreasing the expression of XB130 could serve as a promising therapeutic approach to accelerate the healing of diabetic skin ulcers.
Diabetic skin ulcers are mainly caused by the inhibition of cell proliferation and impaired angiogenesis, a high percentage (15%-27%) of diabetic foot skin ulcer cases require lower extremity amputation owing to treatment failure. XB130 is an adaptor protein that regulates cell proliferation and migration.
To explore the role of XB130 in the development of diabetic skin ulcers.
To investigate whether XB130 can regulate the inhibition of proliferation and vascular damage induced by high glucose. Additionally, we aim to determine whether XB130 is involved in the healing process of diabetic skin ulcers, along with its molecular mechanisms.
We conducted RNA-sequencing analysis to identify the key genes. The RT-qPCR, Western blot, TUNEL staining, immunofluorescence, wound healing, and tubule formation experiments were used to investigate their effects on cellular processes in human umbilical vein endothelial cells (HUVECs) stimulated with high glucose. Finally, we performed functional analysis to elucidate the molecular mechanisms underlying diabetic skin ulcers.
RNA-sequencing analysis showed that the expression of XB130 was up-regulated in the tissues of diabetic skin ulcers. Knockdown of XB130 promoted the healing of skin wounds in mice, leading to an accelerated wound healing process and shortened wound healing time. At the cellular level, knockdown of XB130 alleviated high glucose-induced inhibition of cell proliferation and angiogenic impairment in HUVECs. Inhibition of the PI3K/Akt pathway removed the proliferative effects and endothelial protection mediated by XB130.
The expression of XB130 is up-regulated in high glucose-stimulated diabetic skin ulcers and HUVECs. Knockdown of XB130 promotes cell proliferation and angiogenesis via the PI3K/Akt signalling pathway, which accelerates the healing of diabetic skin ulcers.
Decreasing the expression of XB130 could serve as a promising therapeutic approach to accelerate the healing of diabetic skin ulcers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee CG, United States; Mohamed M, United States S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Azevedo FF, Cantarutti TA, Remiro PFR, Barbieri B, Azoubel RA, Nagahara MHT, Moraes ÂM, Lima MHM. Histological and Molecular Evidence of the Positive Performance of Glycerol-Plasticized Chitosan-Alginate Membranes on Skin Lesions of Hyperglycemic Mice. Polymers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Yue C, Guo Z, Luo Y, Yuan J, Wan X, Mo Z. c-Jun Overexpression Accelerates Wound Healing in Diabetic Rats by Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int. 2020;2020:7430968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Situm M, Kolić M, Redzepi G, Antolić S. [Chronic wounds as a public health problem]. Acta Med Croatica. 2014;68 Suppl 1:5-7. [PubMed] |
| 4. | Huang H, Cui W, Qiu W, Zhu M, Zhao R, Zeng D, Dong C, Wang X, Guo W, Xing W, Li X, Li L, Tan Y, Wu X, Chen L, Fu X, Luo D, Xu X. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J Dermatol Sci. 2015;79:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Wagner T, Traxler D, Simader E, Beer L, Narzt MS, Gruber F, Madlener S, Laggner M, Erb M, Vorstandlechner V, Gugerell A, Radtke C, Gnecchi M, Peterbauer A, Gschwandtner M, Tschachler E, Keibl C, Slezak P, Ankersmit HJ, Mildner M. Different pro-angiogenic potential of γ-irradiated PBMC-derived secretome and its subfractions. Sci Rep. 2018;8:18016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Singh K, Sinha M, Pal D, Tabasum S, Gnyawali SC, Khona D, Sarkar S, Mohanty SK, Soto-Gonzalez F, Khanna S, Roy S, Sen CK. Cutaneous Epithelial to Mesenchymal Transition Activator ZEB1 Regulates Wound Angiogenesis and Closure in a Glycemic Status-Dependent Manner. Diabetes. 2019;68:2175-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Goren I, Müller E, Schiefelbein D, Gutwein P, Seitz O, Pfeilschifter J, Frank S. Akt1 controls insulin-driven VEGF biosynthesis from keratinocytes: implications for normal and diabetes-impaired skin repair in mice. J Invest Dermatol. 2009;129:752-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5794] [Cited by in RCA: 6591] [Article Influence: 507.0] [Reference Citation Analysis (1)] |
| 9. | Moodley S, Hui Bai X, Kapus A, Yang B, Liu M. XB130/Tks5 scaffold protein interaction regulates Src-mediated cell proliferation and survival. Mol Biol Cell. 2015;26:4492-4502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Lodyga M, Bai XH, Kapus A, Liu M. Adaptor protein XB130 is a Rac-controlled component of lamellipodia that regulates cell motility and invasion. J Cell Sci. 2010;123:4156-4169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Cho HR, Wang Y, Bai X, Xiang YY, Lu C, Post A, Al Habeeb A, Liu M. XB130 deficiency enhances carcinogen-induced skin tumorigenesis. Carcinogenesis. 2019;40:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Shi M, Huang W, Lin L, Zheng D, Zuo Q, Wang L, Wang N, Wu Y, Liao Y, Liao W. Silencing of XB130 is associated with both the prognosis and chemosensitivity of gastric cancer. PLoS One. 2012;7:e41660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Shiozaki A, Kosuga T, Ichikawa D, Komatsu S, Fujiwara H, Okamoto K, Iitaka D, Nakashima S, Shimizu H, Ishimoto T, Kitagawa M, Nakou Y, Kishimoto M, Liu M, Otsuji E. XB130 as an independent prognostic factor in human esophageal squamous cell carcinoma. Ann Surg Oncol. 2013;20:3140-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Li GM, Liang CJ, Zhang DX, Zhang LJ, Wu JX, Xu YC. XB130 Knockdown Inhibits the Proliferation, Invasiveness, and Metastasis of Hepatocellular Carcinoma Cells and Sensitizes them to TRAIL-Induced Apoptosis. Chin Med J (Engl). 2018;131:2320-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Poosekeaw P, Pairojkul C, Sripa B, Sa Ngiamwibool P, Iamsaard S, Sakonsinsiri C, Thanan R, Ungarreevittaya P. Adaptor protein XB130 regulates the aggressiveness of cholangiocarcinoma. PLoS One. 2021;16:e0259075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Wang Q, Yang G, Jiang Y, Luo M, Li C, Zhao Y, Xie Y, Song K, Zhou J. XB130, regulated by miR-203, miR-219, and miR-4782-3p, mediates the proliferation and metastasis of non-small-cell lung cancer cells. Mol Carcinog. 2020;59:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Chen B, Liao M, Wei Q, Liu F, Zeng Q, Wang W, Liu J, Hou J, Yu X. XB130 is overexpressed in prostate cancer and involved in cell growth and invasion. Oncotarget. 2016;7:59377-59387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1:e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 426] [Article Influence: 106.5] [Reference Citation Analysis (1)] |
| 19. | Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Treviño-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an Endothelial NAD(+)-H(2)S Signaling Network Is a Reversible Cause of Vascular Aging. Cell. 2018;173:74-89.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 20. | Qaed E, Wang J, Almoiliqy M, Song Y, Liu W, Chu P, Alademi S, Alademi M, Li H, Alshwmi M, Al-Azab M, Ahsan A, Mahdi S, Han G, Niu M, Ali A, Shopit A, Wang H, Li X, Qaid A, Ma X, Li T, Peng J, Ma J, Zhang J, Tang Z. Phosphocreatine Improves Cardiac Dysfunction by Normalizing Mitochondrial Respiratory Function through JAK2/STAT3 Signaling Pathway In Vivo and In Vitro. Oxid Med Cell Longev. 2019;2019:6521218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Kuai L, Fei XY, Xing JQ, Zhang JT, Zhao KQ, Ze K, Li X, Li B. An Efficacy Predictive Method for Diabetic Ulcers Based on Higher-Order Markov Chain-Set Pair Analysis. Evid Based Complement Alternat Med. 2020;2020:5091671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Wang D, Chen H, Lei L, Chen J, Gao J, Liu J, Li Q, Xie Y, Hu Y, Ni Y. Biofabricated macrophage and fibroblast membranes synergistically promote skin wound healing. Bioeng Transl Med. 2022;7:e10344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Vaid B, Chopra BS, Raut S, Sagar A, Badmalia MD, Ashish, Khatri N. Antioxidant and Wound Healing Property of Gelsolin in 3T3-L1 Cells. Oxid Med Cell Longev. 2020;2020:4045365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Qian L, Xia Z, Zhang M, Han Q, Hu D, Qi S, Xing D, Chen Y, Zhao X. Integrated Bioinformatics-Based Identification of Potential Diagnostic Biomarkers Associated with Diabetic Foot Ulcer Development. J Diabetes Res. 2021;2021:5445349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986-3997. [PubMed] |
| 27. | Lu H, Kang F. Down-regulating NEAT1 inhibited the viability and vasculogenic mimicry formation of sinonasal squamous cell carcinoma cells via miR-195-5p/VEGFA axis. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Wu Y, Li Y, Jiang T, Yuan Y, Li R, Xu Z, Zhong X, Jia G, Liu Y, Xie L, Xu K, Zhang H, Li X, Xiao J. Reduction of cellular stress is essential for Fibroblast growth factor 1 treatment for diabetic nephropathy. J Cell Mol Med. 2018;22:6294-6303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Yudushkin I. Getting the Akt Together: Guiding Intracellular Akt Activity by PI3K. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15:273-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 770] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 31. | Wu YJ, Neoh CA, Tsao CY, Su JH, Li HH. Sinulariolide Suppresses Human Hepatocellular Carcinoma Cell Migration and Invasion by Inhibiting Matrix Metalloproteinase-2/-9 through MAPKs and PI3K/Akt Signaling Pathways. Int J Mol Sci. 2015;16:16469-16482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Rodríguez-Cortez VC, Del Pino-Molina L, Rodríguez-Ubreva J, Ciudad L, Gómez-Cabrero D, Company C, Urquiza JM, Tegnér J, Rodríguez-Gallego C, López-Granados E, Ballestar E. Monozygotic twins discordant for common variable immunodeficiency reveal impaired DNA demethylation during naïve-to-memory B-cell transition. Nat Commun. 2015;6:7335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |