Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1126
Peer-review started: April 17, 2023
First decision: May 15, 2023
Revised: May 22, 2023
Accepted: June 2, 2023
Article in press: June 2, 2023
Published online: July 15, 2023
Processing time: 86 Days and 23.9 Hours
An association between cardiorespiratory fitness (CRF) and insulin resistance in obese adolescents, especially in those with various obesity categories, has not been systematically studied. There is a lack of knowledge about the effects of CRF on insulin resistance in severely obese adolescents, despite their continuous rise.
To investigate the association between CRF and insulin resistance in obese adolescents, with special emphasis on severely obese adolescents.
We performed a prospective, cross-sectional study that included 200 pubertal adolescents, 10 years to 18 years of age, who were referred to a tertiary care center due to obesity. According to body mass index (BMI), adolescents were classified as mildly obese (BMI 100% to 120% of the 95th percentile for age and sex) or severely obese (BMI ≥ 120% of the 95th percentile for age and sex or ≥ 35 kg/m2, whichever was lower). Participant body composition was assessed by bioelectrical impedance analysis. A homeostatic model assessment of insulin resistance (HOMA-IR) was calculated. Maximal oxygen uptake (VO2max) was determined from submaximal treadmill exercise test. CRF was expressed as VO2max scaled by total body weight (TBW) (mL/min/kg TBW) or by fat free mass (FFM) (mL/min/kg FFM), and then categorized as poor, intermediate, or good, according to VO2max terciles. Data were analyzed by statistical software package SPSS (IBM SPSS Statistics for Windows, Version 24.0). P < 0.05 was considered statistically significant.
A weak negative correlation between CRF and HOMA-IR was found [Spearman’s rank correlation coefficient (rs) = -0.28, P < 0.01 for CRFTBW; (rs) = -0.21, P < 0.01 for CRFFFM]. One-way analysis of variance (ANOVA) revealed a significant main effect of CRF on HOMA-IR [F(2200) = 6.840, P = 0.001 for CRFTBW; F(2200) = 3.883, P = 0.022 for CRFFFM]. Subsequent analyses showed that obese adolescents with poor CRF had higher HOMA-IR than obese adolescents with good CRF (P = 0.001 for CRFTBW; P = 0.018 for CRFFFM). Two-way ANOVA with Bonferroni correction confirmed significant effect of interaction of CRF level and obesity category on HOMA-IR [F(2200) = 3.292, P = 0.039 for CRFTBW]. Severely obese adolescents had higher HOMA-IR than those who were mildly obese, with either good or poor CRF. However, HOMA-IR did not differ between severely obese adolescents with good and mildly obese adolescents with poor CRF.
CRF is an important determinant of insulin resistance in obese adolescents, regardless of obesity category. Therefore, CRF assessment should be a part of diagnostic procedure, and its improvement should be a therapeutic goal.
Core Tip: The association between obesity and insulin resistance is well established. However, data concerning the relationship between cardiorespiratory fitness (CRF) and insulin resistance in obese adolescents, especially in those with varying obesity categories, are quite limited. The results of present study show that obese adolescents with good CRF have lower homeostatic model assessment of insulin resistance (HOMA-IR) than obese adolescents with poor CRF. Moreover, there is no difference in HOMA-IR between severely obese adolescents with good CRF and mildly obese adolescents with poor CRF. Thus, the improvement of CRF in obese adolescents, including those with severe obesity, should be a therapeutic goal.
- Citation: La Grasta Sabolic L, Pozgaj Sepec M, Valent Moric B, Cigrovski Berkovic M. Association between cardiorespiratory fitness level and insulin resistance in adolescents with various obesity categories. World J Diabetes 2023; 14(7): 1126-1136
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1126.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1126
The global obesity epidemic is accompanied by rapid increase in the prevalence of cardiometabolic disorders. The association between obesity and insulin resistance is well established, along with the fact that insulin resistance represents a pivotal step in the progression towards prediabetes and type 2 diabetes[1,2]. The phenomenon of pubertal insulin resistance has been confirmed in cross-sectional and longitudinal studies[3]. Therefore, obese adolescents who are in puberty should be regarded as a particularly vulnerable group for glucose metabolism dysregulation. Growing evidence supports the notion that young-onset type 2 diabetes has a more aggressive disease phenotype, leading to early development of complications, adversely affecting quality of life and long-term outcomes. As more than half of the world’s population is expected to be overweight or obese within the next 12 years, expanding the options to manage adolescent obesity is essential to treat the epidemic.
Cardiorespiratory fitness (CRF) refers to ability of the circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production during sustained physical activity. In adults, poor CRF is associated with the risk of insulin resistance, irrespective of body weight[4]. Moreover, health benefits are most apparent at the low end of the CRF continuum, providing the evidence that interventions aimed at CRF improvement of the least fit individuals should be encouraged[5]. In children and adolescents, CRF is an important marker of health which shows an inverse relationship with obesity, insulin resistance and cardiometabolic risk[6-8]. Available data confirm that association between obesity and cardiometabolic risk scores could be partially decreased with improvements in fitness levels[9]. It seems that early intervention and prevention strategies targeting youth CRF may be associated with reduced risk for obesity and cardiometabolic disease later in life[10].
However, the relationship between obesity, CRF, and insulin resistance in the adolescent population is still insufficiently explored. According to a recently published study, high CRF was associated with lower total and regional fat and higher insulin sensitivity in overweight and obese adolescents[11]. Also, obese adolescents with low CRF had higher insulin resistance indices and insulin secretion response than adolescents with normal CRF, irrespective of body mass index (BMI) z-score[12]. According to another study which included children aged 8 years to 11 years, as BMI categories rose, CRF attenuated the metabolic risk score, with the biggest differences observed in the most obese children, although the attenuation was significant only in mild obesity[13].
The aim of our study was to investigate the association between CRF and insulin resistance in obese adolescents, with special emphasis on those with severe obesity, for whom the data about this topic are scarce.
Two hundred adolescents who had been referred to the Department of Pediatric Endocrinology and Diabetology at the University Hospital Center “Sestre milosrdnice” due to obesity from February 2019 to July 2022 participated in this cross-sectional study. Prior to enrolment, all the participants and their parents provided written informed consent. The study was approved by the University Hospital Ethics Committee and complied with the Declaration of Helsinki.
The inclusion criteria were: 10 years to 18 years of age, presence of puberty, and BMI ≥ of the 95th percentile for age and sex according to the Centers for Disease Control and Prevention BMI-for-age growth charts[14]. The exclusion criteria were: Chronic diseases which prevent CRF assessment or affect either body mass or body composition (hypothyroidism, hypercortisolism, and syndromes associated with obesity), history of disorders of glucose metabolism, and the use of drugs affecting glucose metabolism or body composition.
According to obesity category, adolescents were classified into groups with mild (class I obesity, BMI 100% to 120% of the 95th percentile for age and sex) or severe obesity (class II obesity, BMI 120% to 140% of the 95th percentile for age and sex or ≥ 35 kg/m2, whichever was lower; class III obesity, BMI more than 140% of the 95th percentile for age and sex or BMI ≥ 40 kg/m2, whichever was lower), and according to the terciles of CRF into groups with poor, intermediate, or good CRF.
During anthropometric measurements, adolescents were wearing minimal clothing and no shoes. Height was measured using a wall stadiometer (Holtain Ltd., Harpenden, United Kingdom) with a precision of 0.1 cm. Weight was determined using an electronic scale (Seca 704; BIS, Hamburg, Germany) with a precision of 0.1 kg. BMI (kg/m2) was calculated as weight (kg) divided by height (m) squared. Waist circumference was measured in a standing position, with a flexible, non-elastic measuring tape, midway between the most inferior rib and the top of the iliac crest, with a precision of 0.1 cm. Body composition was assessed by bioelectrical impedance analysis (BIA) (MC-780 analyzer; Tanita, Nagano, Japan). The pubertal stages were determined using Tanner’s criteria, based on breast size and contour in girls and testicular volume in boys.
Plasma glucose in mmol/L (Abbott Architect c8000; Abbott Laboratories, Chicago, IL, United States) and insulin concentrations in mU/L (ECLIA, Cobas e601; Roche Diagnostics, Basel, Switzerland) were measured after a 10 h-12 h overnight fast. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5.
CRF was assessed using a submaximal treadmill walking test, according to a validated protocol for overweight and obese adolescents[15]. After a 4-min warm-up at a self-selected comfortable walking speed (treadmill incline 0%), the participants were asked to maintain this speed for 4 min while the treadmill incline increased to 5%. Heart rate was recorded at rest (HR 0’) and at the end of the 4 minutes on a 5% incline (HR 4’), as well as the self-selected speed. Based on these two variables, maximal oxygen uptake (VO2max) was estimated from the equation that also included sex (female-F, male-M), weight and height.
VO2max (mL/min) = -1772.81 + 318.64 × sex (F = 0, M = 1) + 18.34 × weight (kg) + 24.45 × height (cm) - 8.74 × HR 4’-0.15 × weight (kg) × (HR 4’ - HR 0’) + 4.41 × speed (km/h) × 0.6213711922 × (HR 4’-HR 0’).
To facilitate comparison among adolescents of different sizes, VO2max was scaled by total body weight (TBW) (mL/min/kg TBW) and by fat free mass (FFM) (mL/min/kg FFM).
All statistical analyses were conducted using SPSS version 24.0 (IBM Corp., Armonk, NY, United States). Descriptive statistics were employed to summarize the demographic characteristics of the study population, and the variables being investigated. The normality of data distribution was tested with Shapiro-Wilk test. For data deviating from normal distribution, Levene’s test of homogeneity of variances was used. For comparisons, a t-test for independent samples was employed, and if Levene’s test was statistically significant, the corrected value of the t-test and the associated P were used. Spearman’s correlation coefficient was calculated as a measure of association between continuous variables. A chi-square test was employed for categorical variables. A one-way analysis of variance (ANOVA) was used to test the influence of CRF on HOMA-IR, while the differences between the groups of adolescents with mild and severe obesity were analyzed using two-way ANOVA with Bonferroni correction. All analyses were adjusted for age and sex. P values < 0.05 were considered statistically significant.
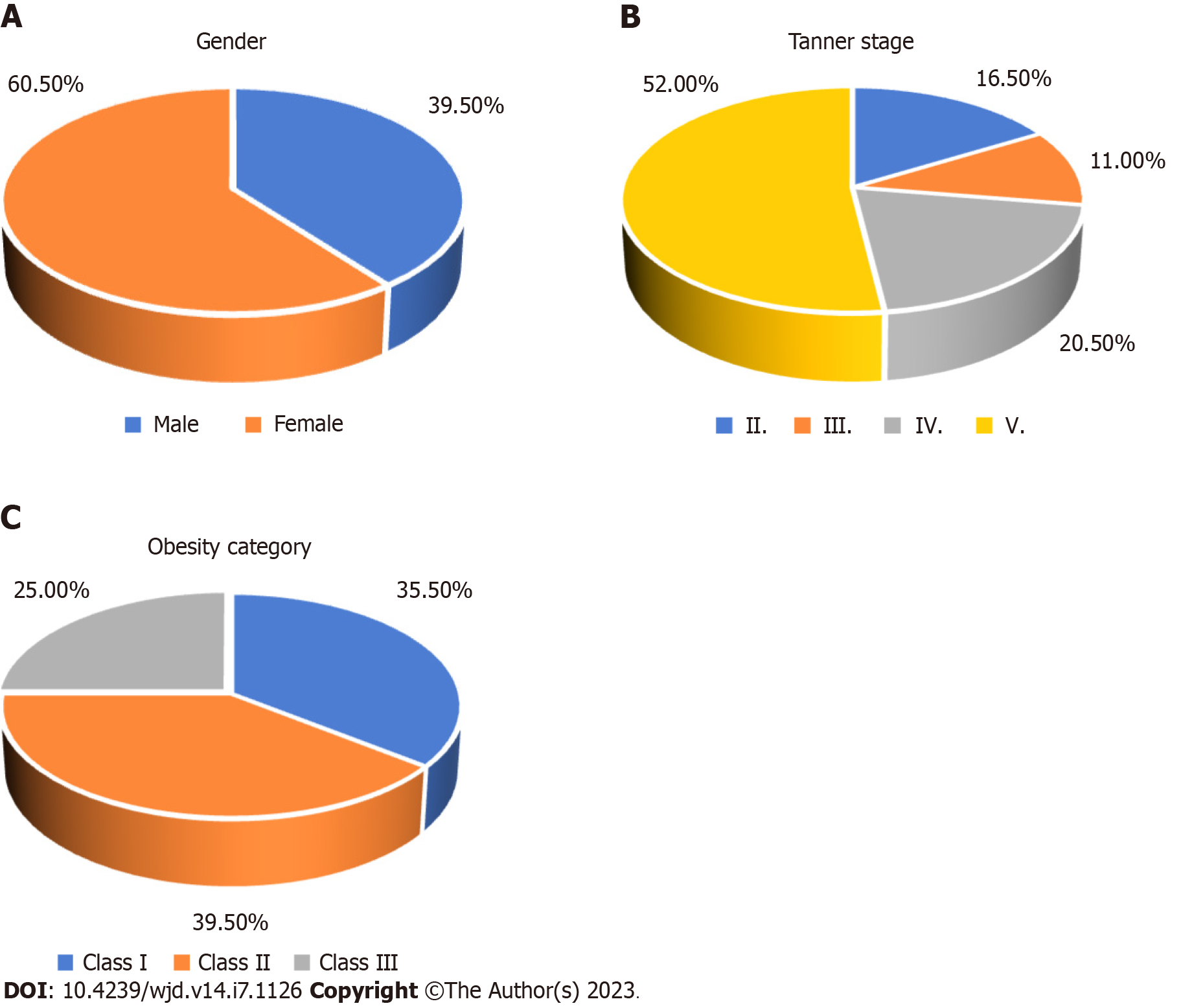
Two hundred obese, pubertal adolescents (average age of 14.54 years ± 1.90 years) were included in the study. There were more girls (60.5%) than boys. The majority of adolescents were in advanced puberty (72.5% Tanner stages IV and V), and were severely obese (64.5% class II and class III obesity) (Figure 1). More adolescent girls were in advanced puberty, while more adolescent boys were severely obese (Table 1).
| Variable | All | Females | Males | 1P value |
| Tanner stage | < 0.001 | |||
| II | 33 (16.5) | 7 (5.8) | 26 (32.9) | |
| III | 22 (11.0) | 9 (7.4) | 13 (16.5) | |
| IV | 41 (20.5) | 21 (17.4) | 20 (25.3) | |
| V | 104 (52.0) | 84 (69.4) | 20 (25.3) | |
| Obesity category | 0.004 | |||
| Class I | 71 (35.5) | 54 (44.6) | 17 (21.5) | |
| Class II | 79 (39.5) | 42 (34.7) | 37 (46.8) | |
| Class III | 50 (25.0) | 25 (20.7) | 25 (31.6) |
Groups of adolescents with various obesity categories differed according to majority of the measured variables (Table 2). All 3 groups differed with respect to BMI, BMI z-score, and CRFTBW (expressed as VO2max in ml/min/kg TBW) in a way that BMI and BMI z-score increased and CRFTBW decreased from class I to class III obesity group (P < 0.001). Subjects with class I and class II obesity had lower fasting insulin and HOMA-IR than subjects with class III obesity (P < 0.001 for fasting insulin, P = 0.001 for HOMA-IR). Finally, the class I obesity group had lower waist circumference and waist to height ratio, and higher CRFFFM (expressed as VO2max in mL/min/kg FFM) than class III obesity group (P = 0.017 for waist circumference, P = 0.019 for waist to height ratio, P = 0.003 for CRFFFM).
| Variable | Class I obesity, n = 71 (35.5%) | Class II obesity, n = 79 (39.5%) | Class III obesity, n = 50 (25.0%) | 1P |
| Height, cm | 166.65 ± 9.49 | 168.11 ± 10.10 | 167.32 ± 8.64 | 0.646 |
| Weight, kg | 88.51 ± 15.29 | 99.38 ± 18.59 | 106.52 ± 16.64 | < 0.001 |
| WC, cm | 102.42 ± 10.05 | 107.00 ± 16.82 | 110.39 ± 18.37 | 0.017 |
| WC/height | 0.61 ± 0.05 | 0.64 ± 0.10 | 0.66 ± 0.11 | 0.019 |
| BMI, kg/m2 | 30.65 ± 2.29 | 34.34 ± 2.54 | 41.02 ± 4.44 | < 0.001 |
| BMI z-score | 1.97 ± 0.19 | 2.35 ± 0.15 | 2.68 ± 0.24 | < 0.001 |
| VO2max, L/min | 2.55 ± 0.44 | 2.66 ± 20.54 | 2.77 ± 0.53 | 0.064 |
| VO2max, mL/min/kg TBW | 29.68 ± 3.53 | 27.69 ± 3.83 | 23.95 ± 3.60 | < 0.001 |
| VO2max, mL/min/kg FFM | 46.48 ± 4.46 | 45.38 ± 5.90 | 43.63 ± 6.71 | 0.003 |
| Fasting glucose, mmol/L | 4.99 ± 0.41 | 4.99 ± 0.47 | 5.05 ± 0.38 | 0.708 |
| Fasting insulin, mU/L | 25.00 ± 12.80 | 28.45 ± 16.22 | 37.31 ± 21.11 | < 0.001 |
| HOMA-IR | 5.78 ± 3.09 | 6.31 ± 3.68 | 8.49 ± 5.23 | 0.001 |
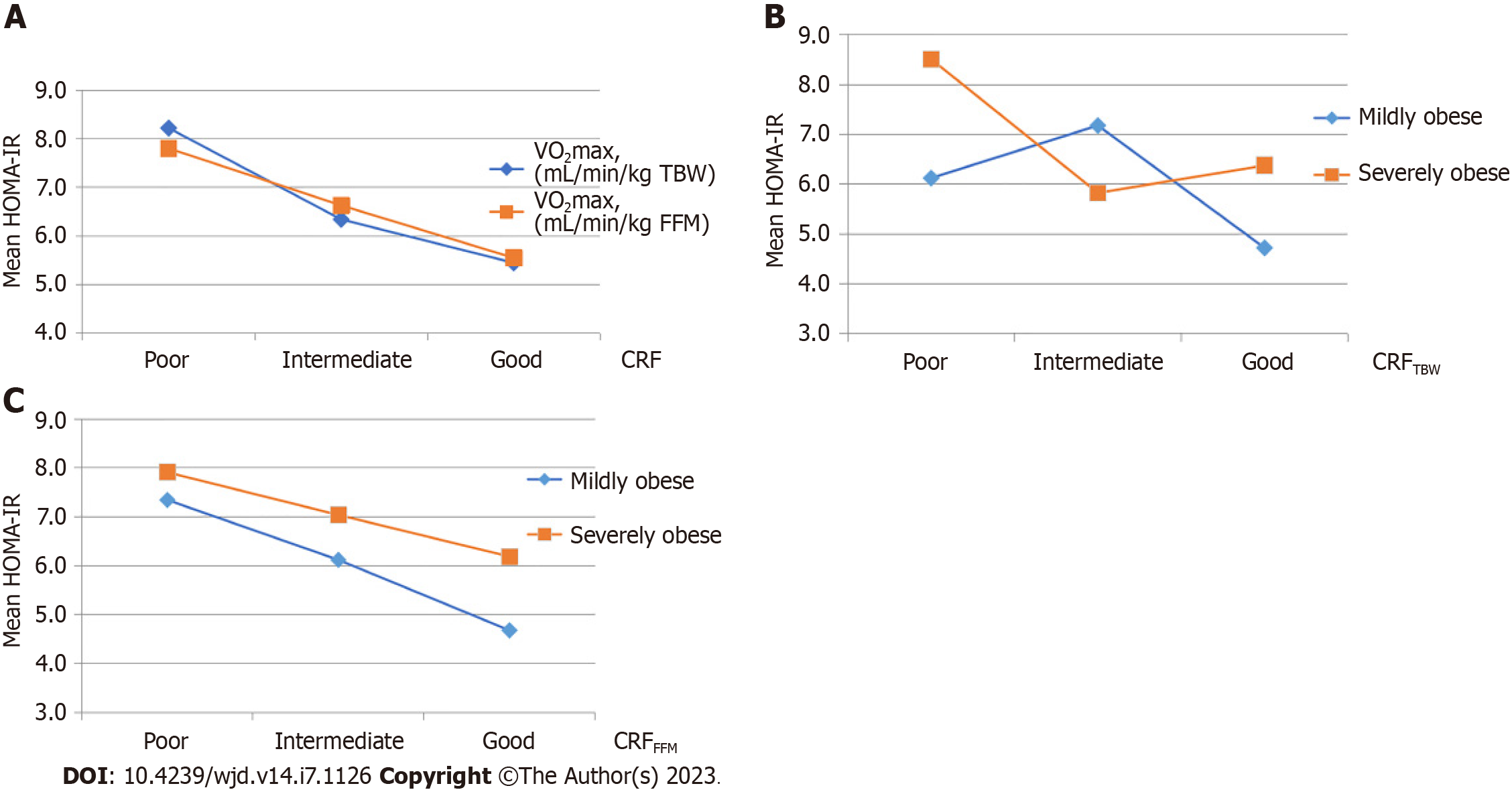
In obese adolescents, a weak negative Spearman’s correlation between CRFTBW and HOMA-IR (rs =-0.28, P < 0.01), and CRFFFM and HOMA-IR (rs =-0,21, P < 0.01) was found.
A statistically significant main effect of CRFTBW on HOMA-IR was detected [F(2200) = 6.840, P = 0.001]. Subsequent comparisons revealed that HOMA-IR was higher in the group of adolescents with poor than in the groups of adolescents with intermediate (P = 0.021) or good CRFTBW (P = 0.001) (Figure 2A).
Furthermore, a statistically significant main effect of CRFFFM on HOMA-IR was determined [F(2200) = 3.883, P = 0.022]. Subsequent comparisons revealed that HOMA-IR was higher in the group of adolescents with poor compared to the group with good CRFFFM (P = 0.018) (Figure 2A).
Separate main effects of CRFTBW level and obesity category (class I-mild obesity, class II and III-severe obesity) on HOMA-IR were not statistically significant, but their interaction was [F(2200) = 3.292, P = 0.039] (Table 3).
| CRF level | Mildly obese | Severely obese | F | P | ||||
| Mean | SD | n | Mean | SD | n | |||
| Poor CRFTBW | 6.12 | 2.97 | 8 | 8.51 | 5.36 | 58 | Main effect CRFTBW, F (2200) = 1.249 | 0.289 |
| Intermediate CRFTBW | 7.18 | 3.67 | 26 | 5.82 | 3.04 | 42 | Main effect obesity, F (2200) = 1.746 | 0.188 |
| Good CRFTBW | 4.72 | 2.23 | 37 | 6.37 | 3.41 | 29 | Interaction CRFTBW and obesity, F (2200) = 3.292 | 0.039 |
Adolescents with mild obesity had lower HOMA-IR than severely obese adolescents, regardless of their poor or good CRFTBW, while adolescents with intermediate CRFTBW did not differ significantly with regard to HOMA-IR (Figure 2B). In severely obese adolescents, HOMA-IR was the highest in subjects with poor CRFTBW. HOMA-IR of mildly obese adolescents with poor CRFTBW did not differ significantly from HOMA-IR of severely obese adolescents with good CRFTBW (Figure 2B).
The separate main effect of CRFFFM level on HOMA-IR was not statistically significant, while the influence of obesity category was of borderline statistical significance [F(2200) = 3.846, P = 0.051] (Table 4). HOMA-IR of mildly obese adolescents with poor CRFFFM was not significantly different from HOMA-IR of severely obese adolescents with good CRFFFM (Figure 2C).
| CRF level | Mildly obese | Severely obese | F | P | ||||
| Mean | SD | n | Mean | SD | n | |||
| Poor CRFFFM | 7.35 | 3.20 | 13 | 7.91 | 5.65 | 54 | Main effect CRFFFM, F (2200) = 2.027 | 0.135 |
| Intermediate CRFFFM | 6.12 | 3.77 | 30 | 7.04 | 3.23 | 37 | Main effect obesity, F (2200) = 3.846 | 0.051 |
| Good CRFFFM | 4.68 | 1.58 | 28 | 6.19 | 3.33 | 38 | Interaction CRFFFM and obesity, F (2200) = 0.167 | 0.846 |
In people living with obesity, the current widely accepted management strategies are based on diet and lifestyle modifications. However, the therapeutic emphasis is most often on calorie restriction and weight reduction, while the importance of regular physical activity and CRF improvement is insufficiently stressed. Moreover, physical activity is perceived primarily as a means to create a negative energy balance. Such an approach overlooks the important health benefits of CRF improvement, independent of weight loss[16].
Rates of obesity among children and adolescents are high and the prevalence of severe obesity in pediatric population is increasing[17]. Obese young people tend to participate in less physical activity than youths of healthier weight[18].
In this study, among 200 adolescents, 129 (64.5%) met the criteria for severe obesity. This should not come as a surprise, given that adolescents were referred for obesity evaluation to a tertiary care center. The proportion of participants with severe obesity was higher in adolescent boys than in adolescent girls, which is in line with other published data[17,19].
The hyperinsulinemic-euglycemic clamp is the gold standard for insulin sensitivity assessment, but it is expensive and labor-intensive. Alternative tests, including the frequently sampled intravenous glucose tolerance test, insulin tolerance test, insulin sensitivity test, and continuous infusion of glucose with model assessment are also quite impractical for routine use. The oral glucose tolerance test is easier to perform, but still time consuming. Fasting methods for assessment of insulin resistance such as fasting insulin, glucose/insulin ratio, quantitative insulin sensitivity check index, and HOMA-IR are inexpensive and less difficult to apply in clinical practice, although each of them has its merits and deficiencies[20]. In this study, HOMA-IR was used as a surrogate marker of insulin resistance, due to its correlation with clamp techniques and wide employment in clinical research.
Previous research revealed positive association between BMI and HOMA-IR in adults and in children[21,22]. In this study, adolescents with class I and class II obesity had lower HOMA-IR than adolescents with class III obesity, which is in line with already published data showing that HOMA-IR rose linearly throughout the whole spectrum of BMI from underweight to severely obese children[13].
Although obesity and increased proportion of body fat are strongly associated with cardiometabolic risk, some individuals with excess body fat have HOMA-IR in the normal range and no metabolic abnormalities[23]. Factors responsible for preserved insulin sensitivity are not clear, but could be related to their lifestyle and alterations in adipose tissue biology. The results from a meta-analysis with pooled data from 15 studies found that CRF, assessed as VO2max, was higher in obese people without than in obese people with metabolic abnormalities[24].
Scaling of VO2max by TBW leads to a considerable underestimation of CRF in obese individuals[25]. Some authors suggest lean body mass to be the strongest determinant of VO2max, while fat mass does not significantly affect VO2max after adjustment for lean mass[26]. To eliminate the confounding factor of adiposity, it is recommended to express CRF in relation to FFM[27]. However, body composition analysis is not routinely available nor performed in everyday practice. Therefore, in the present study, CRF was expressed in both ways, scaled by TBW and FFM.
When otherwise healthy obese children and adolescents and their peers with appropriate BMI were compared, despite being expressed in relation to lean mass, CRF was still significantly lower in the obese group[28]. According to our knowledge, there are no published data regarding the comparison of CRF between the groups of adolescents with different obesity categories. In this study adolescents from the class I obesity group had, in comparison with subjects from class III obesity group, significantly higher values of both CRFTBW and CRFFFM.
Also, in the entire study population, a weak negative correlation of HOMA-IR with CRFTBW and CRFFFM was found. Similar results were obtained in several studies. In a cross-sectional, multi-ethnic study, which included 1445 children aged 9 years to 10 years, a negative association between CRF and HOMA-IR was established. After adjustment for fat mass index the association still remained statistically significant. The adjustment for FFM index did not further reduce the negative association between CRF and HOMA-IR[29]. In 1710 children with an average age of 11.4 years ± 2.4 years, VO2max expressed in relation to lean and total body mass were correlated with HOMA-IR as follows: r = -0.076, P < 0.002; r = -0.264, P < 0.001[27]. However, somewhat different results were obtained from a study including 452 children aged 6 years to 8 years. CRF expressed in relation to TBW was negatively associated with HOMA-IR, while CRF appropriately controlled for body size and composition using lean mass was not related to HOMA-IR[30]. It is worth mentioning that participants included in our study were, in comparison with subjects from all the aforementioned studies, older and exclusively pubertal.
Although the association between CRF and insulin resistance is weak, it is not negligible. Prospective, longitudinal studies indicate a negative association of CRF in childhood with fasting insulin levels and HOMA-IR in adulthood, suggesting that CRF during adolescence is important for preserving insulin sensitivity in later life. A prospective study, which followed 317 adolescents from the age of 15 years for up to a maximum of 12 years, showed that CRF and isometric muscle strength in adolescence are negatively related to fasting insulin and HOMA-IR in young adulthood, regardless of obesity[31]. In a study with more than 2000 involved subjects, CRF and muscle fitness in children aged 7 years to 15 years were negatively associated with fasting insulin and HOMA-IR 20 years later. The association remained statistically significant after adjustment for childhood abdominal circumference[32].
To examine in more detail the association of CRF with HOMA-IR, participants were divided according to terciles of VO2max into groups with poor, intermediate and good CRFTBW and CRFFFM.
The group of adolescents with poor CRF had a significantly higher HOMA-IR than the group of adolescents with good CRF, both for CRFTBW and CRFFFM. Also, HOMA-IR was higher in the group with poor, compared to the group with intermediate CRFTBW. Other researchers came to similar results after the analysis of data collected for overweight or obese children (n = 115, average age 10.6 years ± 1.1 years, 54% girls), although they used a different protocol for CRF assessment. Namely, children with good CRF assessed by the beep test had lower HOMA-IR than children with poor CRF[33]. The results of our study are to the greatest extent comparable with the results of a large study which included 1710 children (mean age of 11.4 years ± 2.4 years; 920 normal-weight, 340 overweight and 450 obese). A progressive increase in HOMA-IR was found with decreasing CRFTBW, while HOMA-IR scores remained similar between the groups with moderate and low CRFFFM[27]. The stronger association between CRFTBW and HOMA-IR is partially due to the significant association of obesity with HOMA-IR. The influence of obesity on HOMA-IR is however reduced if CRF is scaled by FFM, but still remains significant as shown in this study.
Although the number of severely obese youth continues to grow, studies that explore modifying factors for cardiometabolic risk and insulin resistance in such a group of adolescents are lacking. Therefore, the secondary goal of our study was to examine the association of CRF level with HOMA-IR in adolescents with different obesity categories, including those with severe obesity.
In this study, mildly obese participants had lower HOMA-IR than severely obese, both in the groups with good and poor CRF, regardless of scaling. HOMA-IR was the highest in severely obese adolescents with poor CRF. Interestingly, HOMA-IR of severely obese participants with good CRF did not differ significantly from HOMA-IR of mildly obese subjects with poor CRF. Therefore, it seems that CRF attenuates the adverse effects of obesity on insulin resistance. This is in line with findings of a pooled study suggesting that CRF may play an important role in lowering the risk of cardiometabolic diseases in obese children[13].
One of the main limitations of our study is its cross-sectional design, which makes it impossible to establish a causal relationship between CRF and insulin resistance. Also, body composition including FFM was assessed by BIA, but the hydration status could not be fully controlled for all the participants.
In obese adolescents, independent of obesity category, poor CRF is associated with the highest HOMA-IR. This highlights the need to include the assessment of CRF in routine diagnostic algorithm and to encourage lifestyle-based strategies, with special emphasis on CRF improvement in obese adolescents, including those with severe obesity. Further research is needed to determine which interventions should be implemented in obese youth with low CRF in order to achieve optimal cardiometabolic effects. Current recommendations include combined aerobic and resistance training, as well as high-intensity interval training.
The global obesity epidemic, not sparing children and adolescents, is accompanied by rapid increase in the prevalence of cardiometabolic disorders. The association between obesity and insulin resistance is well established, along with the fact that insulin resistance represents a pivotal step in the progression towards prediabetes and type 2 diabetes. Obese adolescents who are in puberty should be regarded as a particularly vulnerable group for glucose metabolism dysregulation. Growing evidence supports the notion that young-onset type 2 diabetes has a more aggressive disease phenotype, leading to early development of complications, and adversely affecting quality of life and long-term outcomes. As more than half of the world’s population is expected to be overweight or obese within the next decade, expanding the options to manage adolescent obesity is essential to treat the epidemic.
Cardiorespiratory fitness (CRF), referring to ability of the circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production during sustained physical activity has been associated with the insulin resistance, irrespective of body weight. In children and adolescents, CRF is an important marker of health which shows an inverse relationship with obesity, insulin resistance and cardiometabolic risk. Available data confirm that association between fatness and cardiometabolic risk scores could be partially decreased with improvements in fitness levels. It seems that early intervention and prevention strategies targeting youth CRF may be associated with reduced risk for obesity and cardiometabolic disease later in life.
To investigate the association between CRF and insulin resistance in obese adolescents, with special emphasis on severely obese adolescents.
This was a prospective, cross-sectional study including 200 pubertal adolescents, 10 years to 18 years of age. According to body mass index (BMI), adolescents were classified as mildly obese (BMI 100% to 120% of the 95th percentile for age and sex) or severely obese (BMI ≥ 120% of the 95th percentile for age and sex or ≥ 35 kg/m2, whichever was lower). Participant body composition was assessed by bioelectrical impedance analysis (BIA). A homeostatic model assessment of insulin resistance (HOMA-IR) was calculated. Maximal oxygen uptake (VO2max) was determined from submaximal treadmill exercise test. CRF was expressed as VO2max scaled by total body weight (mL/min/kg TBW) or by fat free mass (mL/min/kg FFM), and then categorized as poor, intermediate or good, according to VO2max terciles. Data were analyzed by statistical software package SPSS (IBM SPSS Statistics for Windows, Version 24.0). P value < 0.05 was considered statistically significant.
We observed a weak negative correlation between CRF and HOMA-IR [Spearman’s rank correlation coefficient (rs) = -0.28, P < 0.01 for CRFTBW; (rs) =-0.21, P < 0.01 for CRFFFM]. A one-way analysis of variance (ANOVA) revealed a significant main effect of CRF on HOMA-IR [F(2200) = 6.840, P = 0.001 for CRFTBW; F(2200) = 3.883, P = 0.022 for CRFFFM]. Subsequent analyses showed that obese adolescents with poor CRF had higher HOMA-IR than obese adolescents with good CRF (P = 0.001 for CRFTBW; P = 0.018 for CRFFFM). Two-way ANOVA with Bonferroni correction confirmed significant effect of interaction of CRF level and obesity category on HOMA-IR [F(2200) = 3.292, P = 0.039 for CRFTBW]. Severely obese adolescents had higher HOMA-IR than mildly obese, with either good or poor CRF. However, HOMA-IR did not differ between severely obese adolescents with good and mildly obese adolescents with poor CRF.
CRF is important determinant of insulin resistance in obese adolescents, regardless of obesity category. Therefore, CRF assessment should be a part of diagnostic procedure, and its’ improvement should be a therapeutic goal.
Large scale prospective studies are needed to expand the knowledge of CRF, IR, and cardiometabolic health. Also, determination of participants’ body composition by using different methods (such as abdominal MR scans) would offer more precise insight into type and distribution of body fat.
We thank all the collaborators for their effort. We also thank parents for allowing participation of their children in this study and adolescents for their cooperation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y, China; Wu K, United States S-Editor: Chen YL L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Miao Z, Alvarez M, Ko A, Bhagat Y, Rahmani E, Jew B, Heinonen S, Muñoz-Hernandez LL, Herrera-Hernandez M, Aguilar-Salinas C, Tusie-Luna T, Mohlke KL, Laakso M, Pietiläinen KH, Halperin E, Pajukanta P. The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance. PLoS Genet. 2020;16:e1009018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Polidori N, Mainieri F, Chiarelli F, Mohn A, Giannini C. Early Insulin Resistance, Type 2 Diabetes, and Treatment Options in Childhood. Horm Res Paediatr. 2022;95:149-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 481] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Clarke SL, Reaven GM, Leonard D, Barlow CE, Haskell WL, Willis BL, DeFina L, Knowles JW, Maron DJ. Cardiorespiratory Fitness, Body Mass Index, and Markers of Insulin Resistance in Apparently Healthy Women and Men. Am J Med. 2020;133:825-830.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653-e699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1539] [Article Influence: 171.0] [Reference Citation Analysis (0)] |
| 6. | Raghuveer G, Hartz J, Lubans DR, Takken T, Wiltz JL, Mietus-Snyder M, Perak AM, Baker-Smith C, Pietris N, Edwards NM; American Heart Association Young Hearts Athero, Hypertension and Obesity in the Young Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young. Cardiorespiratory Fitness in Youth: An Important Marker of Health: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e101-e118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 7. | Schmidt MD, Magnussen CG, Rees E, Dwyer T, Venn AJ. Childhood fitness reduces the long-term cardiometabolic risks associated with childhood obesity. Int J Obes (Lond). 2016;40:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Agbaje AO, Haapala EA, Lintu N, Viitasalo A, Barker AR, Takken T, Tompuri T, Lindi V, Lakka TA. Peak oxygen uptake cut-points to identify children at increased cardiometabolic risk - The PANIC Study. Scand J Med Sci Sports. 2019;29:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Cristi-Montero C, Courel-Ibáñez J, Ortega FB, Castro-Piñero J, Santaliestra-Pasias A, Polito A, Vanhelst J, Marcos A, Moreno LM, Ruiz JR; HELENA study group. Mediation role of cardiorespiratory fitness on the association between fatness and cardiometabolic risk in European adolescents: The HELENA study. J Sport Health Sci. 2021;10:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | García-Hermoso A, Ramírez-Vélez R, García-Alonso Y, Alonso-Martínez AM, Izquierdo M. Association of Cardiorespiratory Fitness Levels During Youth With Health Risk Later in Life: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020;174:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 11. | Lee S, Pooni R, Arslanian S, Han M, Kuk JL. Separate and combined relationships for cardiorespiratory fitness and muscular strength with visceral fat and insulin sensitivity in adolescents with obesity. Appl Physiol Nutr Metab. 2021;46:945-951. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Maggio AB, Bou Puigdefabregas JW, Schwitzgebel VM, Chamay-Weber C, Beghetti M, Farpour-Lambert NJ. Insulin secretion response during oral glucose tolerance test is related to low cardiorespiratory fitness in obese adolescents. J Pediatr Endocrinol Metab. 2015;28:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Nyström CD, Henriksson P, Martínez-Vizcaíno V, Medrano M, Cadenas-Sanchez C, Arias-Palencia NM, Löf M, Ruiz JR, Labayen I, Sánchez-López M, Ortega FB. Does Cardiorespiratory Fitness Attenuate the Adverse Effects of Severe/Morbid Obesity on Cardiometabolic Risk and Insulin Resistance in Children? A Pooled Analysis. Diabetes Care. 2017;40:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Centers for Disease Control and Prevention, National Center for Health Statistics. CDC growth charts. United States. [cited 3 May 2023]. Available from: https://www.cdc.gov/growthcharts/cdc_charts.htm. |
| 15. | Nemeth BA, Carrel AL, Eickhoff J, Clark RR, Peterson SE, Allen DB. Submaximal treadmill test predicts VO2max in overweight children. J Pediatr. 2009;154:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Gaesser GA, Angadi SS. Obesity treatment: Weight loss vs increasing fitness and physical activity for reducing health risks. iScience. 2021;24:102995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 17. | Pinhas-Hamiel O, Hamiel U, Bendor CD, Bardugo A, Twig G, Cukierman-Yaffe T. The Global Spread of Severe Obesity in Toddlers, Children, and Adolescents: A Systematic Review and Meta-Analysis. Obes Facts. 2022;15:118-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Olds TS, Ferrar KE, Schranz NK, Maher CA. Obese adolescents are less active than their normal-weight peers, but wherein lies the difference? J Adolesc Health. 2011;48:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity and Metabolism, and Council on Clinical Cardiology. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 751] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 20. | Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1:36-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 390] [Cited by in RCA: 428] [Article Influence: 28.5] [Reference Citation Analysis (2)] |
| 21. | Martinez KE, Tucker LA, Bailey BW, LeCheminant JD. Expanded Normal Weight Obesity and Insulin Resistance in US Adults of the National Health and Nutrition Examination Survey. J Diabetes Res. 2017;2017:9502643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Lim SM, Choi DP, Rhee Y, Kim HC. Association between Obesity Indices and Insulin Resistance among Healthy Korean Adolescents: The JS High School Study. PLoS One. 2015;10:e0125238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 24. | Ortega FB, Cadenas-Sanchez C, Migueles JH, Labayen I, Ruiz JR, Sui X, Blair SN, Martínez-Vizcaino V, Lavie CJ. Role of Physical Activity and Fitness in the Characterization and Prognosis of the Metabolically Healthy Obesity Phenotype: A Systematic Review and Meta-analysis. Prog Cardiovasc Dis. 2018;61:190-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Königstein K, Klenk C, Rossmeissl A, Baumann S, Infanger D, Hafner B, Hinrichs T, Hanssen H, Schmidt-Trucksäss A. The Obesity Factor: How Cardiorespiratory Fitness is Estimated More Accurately in People with Obesity. Obesity (Silver Spring). 2018;26:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Ahn B, McMurray R, Harrell J. Scaling of VO2max and its relationship with insulin resistance in children. Pediatr Exerc Sci. 2013;25:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Cooper DM, Leu SY, Taylor-Lucas C, Lu K, Galassetti P, Radom-Aizik S. Cardiopulmonary Exercise Testing in Children and Adolescents with High Body Mass Index. Pediatr Exerc Sci. 2016;28:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Nightingale CM, Rudnicka AR, Kerry-Barnard SR, Donin AS, Brage S, Westgate KL, Ekelund U, Cook DG, Owen CG, Whincup PH. The contribution of physical fitness to individual and ethnic differences in risk markers for type 2 diabetes in children: The Child Heart and Health Study in England (CHASE). Pediatr Diabetes. 2018;19:603-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Haapala EA, Wiklund P, Lintu N, Tompuri T, Väistö J, Finni T, Tarkka IM, Kemppainen T, Barker AR, Ekelund U, Brage S, Lakka TA. Cardiorespiratory Fitness, Physical Activity, and Insulin Resistance in Children. Med Sci Sports Exerc. 2020;52:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Grøntved A, Ried-Larsen M, Ekelund U, Froberg K, Brage S, Andersen LB. Independent and combined association of muscle strength and cardiorespiratory fitness in youth with insulin resistance and β-cell function in young adulthood: the European Youth Heart Study. Diabetes Care. 2013;36:2575-2581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Fraser BJ, Blizzard L, Schmidt MD, Juonala M, Dwyer T, Venn AJ, Magnussen CG. Childhood cardiorespiratory fitness, muscular fitness and adult measures of glucose homeostasis. J Sci Med Sport. 2018;21:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Medrano M, Arenaza L, Migueles JH, Rodríguez-Vigil B, Ruiz JR, Labayen I. Associations of physical activity and fitness with hepatic steatosis, liver enzymes, and insulin resistance in children with overweight/obesity. Pediatr Diabetes. 2020;21:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |