Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.919
Peer-review started: December 19, 2022
First decision: January 5, 2023
Revised: January 11, 2023
Accepted: May 11, 2023
Article in press: May 11, 2023
Published online: June 15, 2023
Processing time: 177 Days and 17.5 Hours
Type 2 diabetes mellitus (T2DM) is a metabolic disease of impaired glucose utilization. Imbalance in generation and elimination of free radicals generate oxidative stress which modulates glucose metabolism and insulin regulation, resulting in the occurrence and progression of diabetes and associated complications. Antioxidant supplements in T2DM can be seen as a potential preventive and effective therapeutic strategy.
To compare randomized controlled trials (RCTs) in which antioxidants have been shown to have a therapeutic effect in T2DM patients.
We systematically searched the electronic database PubMed by keywords. RCTs evaluating the effect of antioxidant therapy on glycaemic control as well as oxidant and antioxidant status as primary outcomes were included. The outcomes considered were: A reduction in blood glucose; changes in oxidative stress and antioxidant markers. Full-length papers of the shortlisted articles were assessed for the eligibility criteria and 17 RCTs were included.
The administration of fixed-dose antioxidants significantly reduces fasting blood sugar and glycated hemoglobin and is associated with decreased malondialdehyde, advanced oxidation protein products, and increased total antioxidant capacity.
Antioxidant supplements can be a beneficial approach for the treatment of T2DM.
Core Tip: Antioxidant supplementation reduces oxidative stress in diabetes. Antioxidant supplementation is a potential therapeutic approach for type 2 diabetes mellitus.
- Citation: Shrivastav D, Dabla PK, Sharma J, Viswas A, Mir R. Insights on antioxidant therapeutic strategies in type 2 diabetes mellitus: A narrative review of randomized control trials. World J Diabetes 2023; 14(6): 919-929
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/919.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.919
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycaemia which arises from resistance or deficiency of insulin secreted from pancreatic beta cells[1]. Obesity and physical inactivity are general well-known risk factors for T2DM as well as its micro (nephropathy and retinopathy) and macrovascular (atherosclerotic cardiovascular disease) complications[2]. According to the World Health Organization, the prevalence and death rate was 470 million and 1.37 million in 2017, respectively, and expected to increase continuously, and the estimated prevalence and death rate in 2025 will be 570.9 million and 1.59 million, respectively[3]. In India, the prevalence of T2DM and impaired fasting glucose was 9.3% and 24.5%, respectively, in 2022. Approximately 45.8% of T2DM patients are aware of their diabetes, 6.1% are taking diabetes medication, and 15.1% have diabetes under control.
Oxidative stress is the excess production or insufficient clearance of highly reactive molecules like reactive oxygen species (ROS) and reactive nitrogen species. In physiological conditions, it is generated in the non-enzymatic, enzymatic, and mitochondrial processes. Enzymes of respiratory chain, phagocytosis, prostaglandin synthesis, and mitochondrial cytochrome P450 system and purine degradation produce free radicals[4]. In diabetes, due to hyperglycaemia, the formation of free radicals is increased, resulting in an increase in oxidative stress which promotes the rate of protein glycation (non-enzymatic), oxidation of glucose, lipid peroxidation, and ultimately impairment of cellular machinery, enzymes, and insulin pathways[5].
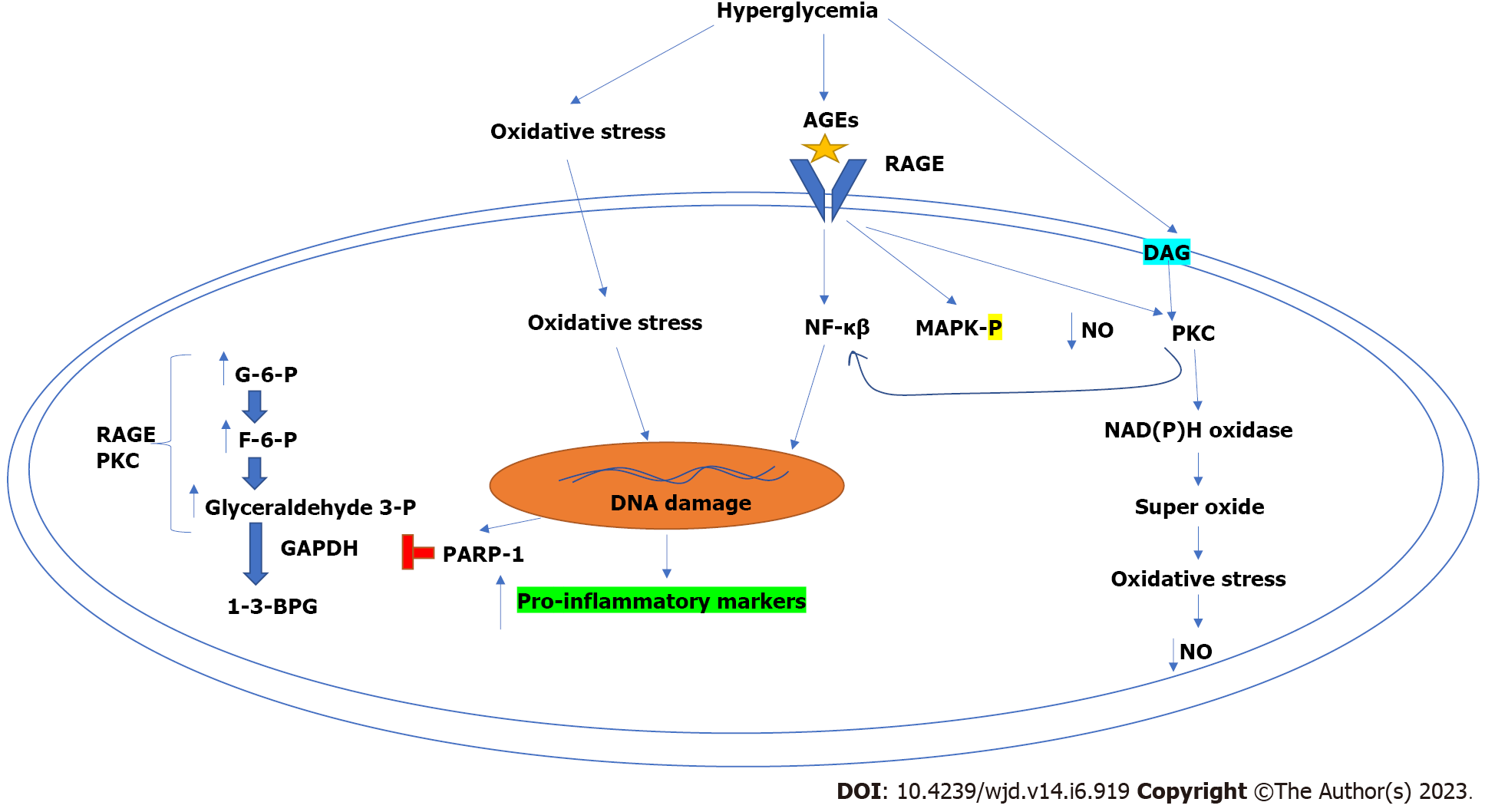
In T2DM, the prolonged exposure to high glucose and free fatty acid levels significantly contributes to the dysfunction of beta cells. These beta cells are highly sensitive to free radicals (due to low quenching and antioxidant activity). Consequently, the oxidative stress can harm mitochondria and significantly decrease insulin secretion and may cause insulin resistance (Figure 1). Under physiological conditions, cellular metabolic processes like glucose oxidation, generate superoxide anion radical [O2(-)] inside the mitochondria which is combated by the antioxidant defence system of the body at a certain level[6]. However, in hyperglycaemic conditions, the production of O2(-) is elevated, which decreases the body’s antioxidant capacity and consequently generates oxidative stress and damage to several biomolecules including DNA[7]. DNA damage activates poly-ADP-ribose polymerase-1 (PARP-1) (DNA damage repair enzyme). Since this PARP-1 enzyme is a potent inhibitor of glyceraldehyde 3-phosphate dehydrogenase of the glycolysis pathway, the intracellular concentration of glycolytic intermediates including glyceraldehyde 3-phosphate, fructose-6-phosphate, and glucose-6-phosphate increases[8]. As a result, glycolytic intermediates accumulate inside the cell and promote some other pro-oxidant pathways like protein kinase C and the advanced glycation end products hexosamine and polyol related pathways[9].
To counteract the oxidative stress, the human body produce antioxidants at a low concentration which significantly delay or inhibit cellular damage[4]. Humans have extremely complex antioxidant systems that protect the body's cells and organ systems from free radicals. Antioxidants can be categorized as antioxidant enzymes and substrates[10], natural substances[11], combination medications[12], synthetic antioxidants[13], and pharmaceuticals[14]. In the antioxidant enzyme and substrate system, superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase, and catalase can combat the oxidative stress either directly or sequentially and abolish its excessive development of deleterious effects[15]. The non-enzymatic antioxidant system is endogenously produced and scavenges free radicals. It includes vitamin C, vitamin D, vitamin E, carotenoids, lipoic acid, selenium, and other dietary derivatives such as glutathione and ubiquinol[16].
Exogenous antioxidant supplementation may reduce oxidative stress in T2DM by increasing antioxidant levels and decreasing free radical formation[17]. This supplementation potentially improves the metabolic pathways including nitric oxide (NO) production, endothelial dysfunction, mitochondrial function, and vascular NAD(P)H oxidase activity[18,19]. According to recent clinical data in diabetic patients, supplementation of antioxidants improves glycaemic status [glycated hemoglobin (HbA1c) and random blood sugar], reduces oxidative stress biomarkers [malondialdehyde (MDA)], and increases serum levels of antioxidant enzymes including SOD, catalase, and glutathione peroxidase[5]. Golbidi et al[20] investigated the therapeutic use of antioxidants as an adjuvant to standard diabetes treatment. Those authors searched the clinical trial studies over the last ten years using terms vitamin E, vitamin C, coenzyme Q10 (CoQ10), alpha lipoic acid, L-carnitine, ruboxistaurin, or LY 333531 and diabetes and concluded that vitamin supplementation is not beneficial for managing diabetes complications. In this study, we tried to compare interventional randomized control trials (RCTs) in which antioxidants have been shown to have a therapeutic effect in the treatment of T2DM.
Search methodology: The literature search was carried out in the PubMed NCBI database. The search strategy was carried out by combination of (“Diabetes Mellitus, Type 2”[MeSH]) AND “Antioxidants”[MeSH]) AND “Oxidative Stress”[MeSH]) using Boolean operators. The fixed dose of antioxidant was the inclusion criterion for eligibility.
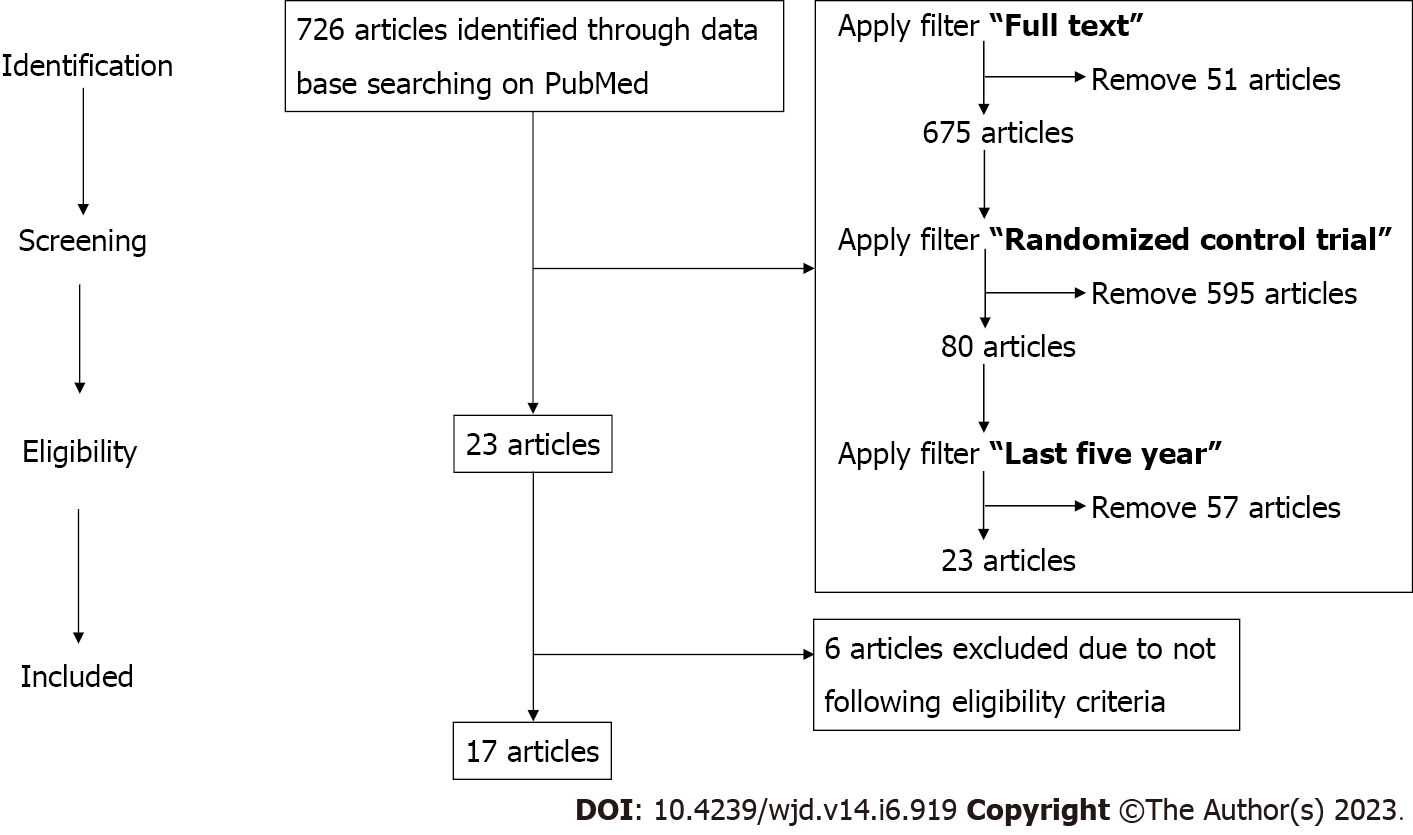
At the beginning of the literature search, the NCBI PubMed database showed 726 articles. After applying filters and limiting the search with “full text”, “five years” (2017 to 2022), and “human randomized controlled trials”, 23 RCTs were obtained. Full-length papers of the shortlisted articles were assessed for the eligibility criteria and 17 RCTs that fulfilled the inclusion criteria were finally included in the study (Figure 2 and Table 1).
| No. | Study design | Setting | Population | Sample size | Intervention | Duration | Effect of treatment | Ref. |
| 1 | Randomized controlled trial | Primary Health Care Centre in Podgorica | T2DM patients | Total: n = 130; Group I: n = 65; Group II: n = 65 | Group I: 14000 IU vitamin D + metformin; Group II: Metformin only | First for 3 mo and later on for 6 mo | Improves blood HbA1c and reduces advanced oxidation protein products | Cojic et al[22], 2021 |
| 2 | Randomized controlled trial | Prince of Wales Hospital, the Teaching Hospital of The Chinese University of Hong Kong, Shatin, Hong Kong | T2DM patients | Total: n = 20; Group I: n = 10; Group II: n = 10 | Group I: 1.4 g/d bilberry (Vaccinium myrtillus | 3 wk | Reduces serum HbA1c level by 4.6% and ascorbic acid by 14% | Chan et al[43], 2021 |
| 3 | Randomized controlled trial | Department of Anesthesia, Isfahan University of Medical Sciences, Isfahan | T2DM patients | Total: n = 54; Group I: n = 27; Group II: n = 27 | Group I: Three-gram citrulline daily; Group II: Placebo | 2 mo | Reduces serum fasting blood glucose and MDA level by 16% and 25%, respectively; Increases serum levels of NOx, SOD, and GPx by 27%, 2%, and 2.2%, respectively | Azizi et al[45], 2021 |
| 4 | Randomized controlled trial | Khon Kaen University, China | T2DM patients | Total: n = 24; Group I: n = 12: Group II: n = 12 | Group I: 1000 mg vitamin C; Group II: Placebo daily | 6 wk | Improves blood pressure regulation, increases NO release, and significantly lowers serum MDA and F2-IsoPs levels | Boonthongkaew et al[23], 2021 |
| 5 | Randomized controlled trial | Department of Clinical Pharmacology and Therapeutics, Nizam's Institute of Medical Sciences, Hyderabad, India | T2DM patents | Total: n = 60 patients; Group I: n = 20; Group II: n = 20; Group III: n = 20 | Group I: One capsule of T. chebula 250 mg twice daily; Group II: One capsule of T. chebula 500 mg twice daily; Group III: Placebo | 12 wk | Improves serum NO level and reduces oxidative stress markers (GSH and MDA) | Pingali et al[31], 2020 |
| 6 | Randomized controlled trial | Endocrinology and Metabolism Clinics of Golestan Hospital at Ahvaz Jundishapur University of Medical Science, Iran (IRCT registration number: IRCT20120704010181N12) | T2DM patients | Total: n = 42; Group I: n = 21; Group II: n = 21 | Group I: One-gram Anethum graveolens (dill) powder; Group II: Placebo | 8 wk | Decreases serum insulin, HOMA-IR, LDL-C, TC, and MDA and increases serum level of HDL and total antioxidant level | Haidari et al[33], 2020 |
| 7 | Randomized controlled trial | Tan Tock Seng Hospital, Singapore (registration number: NCT02776397) | T2DM | Total: n = 187; Group I: Type 2 diabetes individuals with haptoglobin 2-2 (Hp 2-2); Group II: Type 2 diabetes individuals without haptoglobin 2-2 (Hp 2-2) | Group I: Total 400 IU of vitamin E daily; Group II: Placebo | 24 wk | Increases reactive hyperaemia index, LDL, and ox-LDL concentrations | Dalan et al[24], 2020 |
| 8 | Randomized controlled trial | Isfahan University Endocrine and Metabolism Research Centre, Isfahan, Iran (IRCT registration number: IRCT20180818040827N1 | T2DM | Total: n = 80; Group I: n = 40; Group II: n = 40 | Group I: 20 g wheat germ; Group II: Placebo | 12 wk | Significant change in serum TC level | Mohammadi et al[47], 2020 |
| 9 | Randomized controlled trial | Velayat Hospital of Qazvin University of Medical Sciences, Qazvin, Iran (IRCT registration number: IRCT2017041019669N4) | T2DM | Total: n = 62; Group I: n = 31; Group II: n = 31 | Group I: 500 mg of propolis 3 times in a day; Group II: Placebo | 8 wk | Decreases FBS, 2-hp, insulin, HbA1c, and HOMA-IR and upregulates TAC, SOD, and GSH | Afsharpour et al[40], 2019 |
| 10 | Double-blind randomized, placebo-controlled clinical trial | Diabetes Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Kermanshah University of Medical Sciences, Tehran, Iran (IRCT registration number: IRCT20140413017254N5) | T2DM | Total: n = 80; Group I: n = 40; Group II: n = 40 | Group I: 80 mg Nano curcumin capsules once a day; Group II: Placebo | 8 wk | Improves serum HbA1c, RBS, total neuropathy score, and total reflex score | Asadi et al[35], 2019 |
| 11 | Double-blind, randomized, parallel, placebo-controlled trial | Yeh, Chung Shan Medical University Taiwan (registration number: NCT02622672) | T2DM | Total: n = 50; Group I: n = 25; Group II: n = 25 | Group I: Liquid ubiquinol (100 mg/d); Group II: Placebo | 12 wk | Reductions in blood HbA1c and fasting glucose, and increase in SOD activity | Yen et al[29], 2018 |
| 12 | Single-blinded randomized controlled clinical trial | Medical Laboratories of the Central Blood Bank Society, and the Medical Relief Society, Gaza Strip, Palestine | T2DM | Total: n = 40 patients; Group I: n = 10; Group II: n = 10; Group III: n = 10; Group IV: n = 10 | Group I: 500 mg of metformin + placebo twice daily; Group II: 500 mg of metformin + 500 mg of vitamin C twice daily; Group III: 500 mg of metformin + 400 mg of vitamin E twice daily; Group IV: 500 mg of metformin + 500 mg of vitamin C + 400 mg of vitamin E twice daily | 90 d | Regulates FBS, HbA1c, HOMA-IR, and QISCI and improves GST, MDA, G6PD, GSH-PX, GSHE, and GSHW | El-Aal et al[25], 2018 |
| 13 | Randomized double-blind placebo-controlled trial | Baqiyatallah University of Medical Sciences, Iran (IRCT registration number: IRCT201505301165N4) | T2DM | Total: n = 100; Group I: n = 50; Group II: n = 50 | Group I: 500 mg curcumin + 5 mg piperine/day; Group II: Placebo | 3 mo | Controls insulin, HbA1c, and HOMA-IR index | Panahi et al[36], 2018 |
| 14 | Randomized, double blind, parallel group design | Clinics Hospital of Porto Alegre | T2DM | Total: n = 30; Group I: n = 15; Group II: n = 15 | Group I: n-3 PUFAs (capsules containing 180 mg of eicosapentaenoic acid and 120 mg of docosahexaenoic acid; Group II: Placebo | 8 wk | Reduces serum level of TBARS, F2-isoprostanes, and triglycerides | Fayh et al[27], 2018 |
| 15 | Randomized double-blind placebo-controlled trial | Tehran University of Medical Sciences (IRCT registration number: IRCT2015072523336N1) | T2DM | Total: n = 48; Group I: n = 24; Group II: n = 24 | Group I: 800 mg/d resveratrol daily; Group II: Placebo | 2 mo | Decreases MDA and carbonyl protein and increases total thiol, NOS, and catalase | Seyyedebrahimi et al[49], 2018 |
| 16 | Randomized double-blind placebo-controlled clinical trial | Diabetic Clinic of Golestan Hospital, Jundishapur University of Medical Science, in Ahvaz, Iran (IRCT registration number: IRCT2015081810181N6) | T2DM | Total: n = 64; Group I: n = 32; Group II: n = 32 | Group I: 500 mg hesperidin/daily; Group II: Placebo | 6 wk | Increases total antioxidant concentration and reduces serum concentrations of fructosamine, 8-OHDG, and MDA | Homayouni et al[38], 2017 |
| 17 | Randomized double-blind placebo-controlled clinical trial | Toho University Medical Center | T2DM | Total: n = 50; Group I: n = 25; Group II: n = 25 | Group I: Resveratrol oligo-stilbene 27.97 mg/100 mg/d; Group II: Placebo | 12 wk | Decreases SBP and reactive oxygen metabolite significantly and also reduces risk of atherosclerosis in T2DM patients | Imamura et al[50], 2017 |
This study was performed to find the effect of antioxidants on oxidative stress in T2DM patients by comparing RCT studies. After a literature search in the PubMed database, it was found that the antioxidants, including vitamins, free fatty acids, natural products, etc., play diverse roles in combating oxidative stress in T2DM patients[21]. It is well known that non-enzymatic antioxidants like vitamins A, C, and E, glutathione, lipoic acid, mixed carotenoids, CoQ10, a number of bioflavonoids, antioxidant minerals like copper, zinc, manganese, and selenium, as well as cofactors like albumin, folic acid, uric acid, and vitamins B1, B2, B6, and B12 are involved in diverse biological functions. Antioxidants have shown promise as a potential therapy for the prevention and treatment of cancer, diabetic complications, and cardiovascular disease (CVD) since ROS have been linked to these diseases. In a study by Cojic et al[22], vitamin D supplements were given to proven T2DM patients with an average history of 4-6 years during a 6-mo follow-up period, and it was found that vitamin D supplementation (14000 IU weekly or 4 drops daily for 6 mo) improved blood HbA1c and reduced advanced oxidation protein products (AOPP). The triglyceride/thiobarbituric acid-reactive substances (TG/TBARS) index, homeostasis model assessment of insulin resistance (HOMA-IR) index, and MDA level were likewise affected by this vitamin D treatment. Boonthongkaew et al[23] studied the effect of vitamin C supplementation (1000 mg daily for 6 wk) on blood pressure (BP), oxidative stress, and NO release in T2DM patients and revealed that vitamin C supplementation improves blood pressure regulation, increases NO release, and significantly lowers serum MDA and F2-isoprostanes (IsoPs) levels. In another study, after supplementation of vitamin E (alpha-tocopherol-400 IU) in T2DM patients (duration of diabetes, 9-11 years), change in the reactive hyperaemia index (RHI) and augmentation index as the primary outcome, and pulse-wave velocity (PWV), carotid intima media thickness (CIMT), inflammation (hsCRP), derivatives of reactive-oxygen metabolites (dROMs), biological antioxidant potential (BAPs), HbA1c, low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and oxidised LDL-C (ox-LDL) as the secondary outcomes were measured. Dalan et al[24] concluded that vitamin E supplementation does not significantly improves RHI, PWV, CIMT, hsCRP, dROMS, BAPs, HDL-C, and HbA1c though a significant fall in ox-LDL levels was observed. Further in subgroup analysis, vitamin E supplementation can increase reactive hyperaemia index, LDL, and ox-LDL concentrations in the non-Hp-2-2 group. Similarly, El-Aal et al[25] revealed that supplementation of vitamin C and/or vitamins E for 90 consecutive days to T2DM patients regulates fasting blood sugar (FBS), HbA1c, HOMA-IR, and quantitative insulin sensitivity check index (QUICKI). Further, it also improves serum levels of glutathione-S-transferase, MDA, glucose-6-phosphate dehydrogenase, glutathione (GSH)-peroxidase, reduced glutathione in erythrocyte lysate, and reduced glutathione in whole blood. Polyunsaturated fatty acids (n-3 PUFAs) are long-chain polyunsaturated fatty acids that have antioxidant properties. Indeed, n-3 PUFA supplementation has been demonstrated to reduce oxidative stress-related mitochondrial dysfunction and endothelial cell mortality, with the benefit mediated by increased endogenous antioxidant enzyme activity[26]. In another study conducted by Fayh et al[27], supplementation of n-3 PUFAs (capsules containing 180 mg of eicosapentaenoic acid and 120 mg of docosahexaenoic acid) to T2DM patients (diabetes history of 6-8 years) non-significantly reduces serum levels of TBARS, F2-IsoPs, and triglycerides. CoQ10 is a powerful antioxidant found naturally in the mitochondria that is endogenously synthesised and fat soluble. Because of its antioxidant properties, it can effectively inhibit the oxidation of fat, protein, and DNA in the body. Deficiency in CoQ10, particularly ubiquinol (the reduced form of CoQ10), is common in T2DM patients[28]. Yen et al[29] revealed that supplementing T2DM patients with ubiquinol (100 mg/d for 12 wk) resulted in a significant reduction in blood HbA1c, fasting glucose, and anti-glycaemic agent use (thiazolidinediones by 25% to 83%), and increased SOD activity. However, there were no significant changes in the levels of serum MDA and ox-LDL. After 12 wk of supplementation, there was a further substantial association between the plasma CoQ10 level and the insulin level, HOMA-IR, and anti-hyperglycaemic medication effect scores.
Plant-based natural antioxidants are mostly composed of polyphenols (phenolic acids, flavonoids, anthocyanins, lignans, and stilbenes), carotenoids (xanthophylls and carotenes), and phenolic acids. These naturally occurring antioxidants, particularly polyphenols and carotenoids, have a variety of biological effects, including anti-inflammatory, antibacterial, antiviral, anti-aging, and anticancer properties[30]. Terminalia chebula, a traditional ayurvedic herb, is well-known for its antioxidant and antihyperlipidemic properties. Pingali et al[31] suggested that the supplementation of aqueous extract of Terminalia chebula (250 mg and 500 mg twice daily for 12 wk) to T2DM patients significantly improved endothelial function, serum NO level, lipid profile, hsCRP levels, and oxidative stress markers (GSH and MDA)[31]. Dill, also known as Anethum graveolens L (A. graveolens), is a herb that is frequently used as a spice and a remedy. The oils of A. graveolens are also a source of antioxidants, have antibacterial and antispasmodic qualities, and are also a source of minerals, proteins, and fibres. According to research, A. graveolens exhibits anticancer, antibacterial, anti-gastric-irritation, anti-inflammatory, and antioxidant effects[32]. The interventional study of Haidari et al[33] suggested that the supplementation of A. graveolens (dill) powder (3 capsules per day, 1 g each daily) to T2DM patients (duration of diabetes, 8-9 years) significantly decreases serum insulin, HOMA-IR, LDL-C, total cholesterol (TC), and MDA and increases the serum level of HDL and total antioxidant level. However, a non-significant difference was observed in serum hsCRP (an inflammatory marker) level. Curcumin (C21H2OO6) is a lipophilic substance and polyphenol in nature. Due to its chemical structure and presence of hydroxyl and methoxy groups, it has many properties, in particular antioxidant, antimicrobial, anti-inflammatory, anti-angiogenic, and antimutagenic ones. Curcumin regulates cyclooxygenase-2, lipoxygenase, xanthine oxidase, and inducible nitric oxide synthase (NOS), and reduces serum level of MDA[34]. In another trial, Asadi et al[35] suggested that the supplementation of nano-curcumin (80 mg per day for 8 wk) to T2DM patients (diabetes history of 10-11 years) significantly improves serum HbA1c, random blood sugar, total neuropathy score, and total reflex score. Similarly, the administration of curcuminoids (daily dose of 500 mg/d) co-administered with piperine (5 mg/d for 3 mo) can control insulin, HbA1c, and HOMA-IR index. Further, it also reduces serum hsCRP and creatinine levels in T2DM patients[36]. Hesperidin (30,5,7-trihydroxy-40-methoxy-flavanone-7-rhamnglucoside), a bioflavonoid, is a well-known antioxidant that can reduce risk of cardiovascular disease and T2DM[37]. The oral administration of hesperidin at 500 mg/d for 6 wk in T2DM patients (disease history of 3-11 years) increases total antioxidant concentration (mean percent change 13.35% ± 19.21%) and reduces the serum concentration of fructosamine (mean percent change 10.10% ± 16.84%), 8-hydroxy-2’-deoxyguanosine (mean percent change 25.11% ± 28.23%), and MDA (mean percent change 16.46% ± 18.04%)[38]. Various studies evidently prove that propolis (a resin like material synthesized by honey bee) has antioxidant properties and is sufficiently capable of scavenging free radicals[39]. The oral supplementation of propolis (500 mg, three times a day for 8 wk) to T2DM patients (disease history of 3-11 years) decreases FBS, 2-h postprandial glucose, insulin, HbA1c by 14%, and HOMA-IR by 25%, and upregulates total antioxidant capacity (TAC) by 19%, SOD by 3%, and GSH by 17%[40]. Anthocyanin is one of the major secondary metabolites which have antioxidant properties. Bilberry (Vaccinium myrtillus L.) is a natural and big source of anthocyanins[41]. Although bilberry is most typically used to improve vision, it has also been shown to lower blood sugar, have anti-inflammatory and lipid-lowering properties, increase antioxidant defense, and reduce oxidative stress. As a result, bilberry may be useful in the treatment or prevention of inflammation, dyslipidaemia, hyperglycaemia, and elevated oxidative stress, as well as CVD, cancer, diabetes, dementia, and other age-related disorders[42]. The oral supplementation of bilberry (1.4 g/d of extract) daily for 4 wk reduces serum HbA1c level by 4.6% and ascorbic acid by 14%. Further, it decreases serum level of lipid standardized vitamin E, allantoin, glutathione peroxidase, and superoxide dismutase non-significantly[43]. The non-essential α-amino acid L-citrulline plays a major role in liver and kidney regulations. L-citrulline is also beneficial for NO production and endothelial cell regulation[44]. The supplementation of L-citrulline (3 g daily for 2 mo) to T2DM patients (history of 3.5 years) significantly reduces serum fasting blood glucose and MDA levels by 16% and 25%, respectively. However, it significantly increases serum levels of NOx, SOD, and GPx level by 27%, 2% and 2.2%, respectively[45]. Wheat germ (WGEs) is a by-product of the wheat milling process that contains a variety of bioactive chemicals. Wheat germ exracts (WGEs) show potential as antioxidants since they include a variety of bioactive components. According to the findings of a previous study, bioactive compounds present in WGEs lower plasma lipid and oxidation levels[46]. Supplementation of WGEs (20 g per day for 8 wk) to T2DM patients results in a significant change in serum TC level, but it affects neither serum levels of FBS, HbA1C, TG, LDL-C, HDL-C, VLDL, MDA, and TAC, nor HOMA-IR, HOMA-B, QUICKI, TG/HDL ratio, LDL/HDL ratio, systolic blood pressure, and diastolic blood pressure[47].
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a polyphenolic compound and a type of plant secondary metabolite, is a potent antioxidant which potentially scavenges the free radicals[48]. Oral supplementation of 800 mg/d resveratrol for 2 mo to T2DM patients decreases MDA by 8%, and carbonyl protein by 18.54%. However, it increases total thiol by 12%, NOS by 3%, and catalase 12%. Further, it also upregulates the expression of nuclear factor erythroid 2-related factor 2 (oxidative stress responsive transcription factor)[49]. Similarly, administration of 100 mg resveratrol tablets (total resveratrol:oligo-stilbene 27.97 mg/100 mg/d) daily for 12 wk effectively regulates arterial stiffness. Resveratrol supplementation not only decreases systolic BP and reactive oxygen metabolite significantly but also reduces risk of atherosclerosis in T2DM patients[50]. In this study, we tried to analyze that how imbalance between the production and inactivation of ROS leads to the development of insulin resistance and metabolic syndrome. Therefore, preventing the damage caused by oxidation can prove to be an effective therapeutic strategy in diabetes. We conducted a comparison of RCTs comparison and performed a review of the available literature to summarize the evidence covering the patho
The literature search revealed that non-enzymatic antioxidants such as vitamins A, C, and E, glutathione, lipoic acid, mixed carotenoids, CoQ10, and antioxidant minerals have diverse biological functions that can potentially prevent and treat cancer, diabetic complications, and cardiovascular diseases. The studies reviewed demonstrated that supplementation of vitamins D, C, and E, n-3 PUFAs, and CoQ10 can regulate FBS, HbA1c, and oxidative stress biomarkers such as AOPP, TBARS, and MDA. In particular, vitamin D supplementation significantly improved blood HbA1c and reduced AOPP, while vitamin C supplementation improved blood pressure regulation and significantly lowered serum MDA and F2-IsoPs levels. On the other hand, vitamin E supplementation did not significantly improve RHI, PWV, CIMT, hsCRP, dROMS, BAPs, HDL-C, and HbA1c, but it caused a significant decrease in ox-LDL levels. Furthermore, supplementation of n-3 PUFAs non-significantly reduced serum levels of TBARS, F2-IsoPs, and triglycerides, while ubiquinol supplementation resulted in a significant reduction in blood HbA1c, fasting glucose, and anti-glycaemic agent use, and increased SOD activity. However, there were no significant changes in the levels of serum MDA and ox-LDL. These studies highlight the potential benefits of antioxidant supplementation in managing T2DM and the importance of further research to establish optimal dosages, treatment durations, and patient populations.
The modern lifestyle, which includes an unhealthy diet, a lack of physical activity, and exposure to a variety of chemicals from various sources such as pesticides, heavy metals, food additives, and environmental pollution, can all influence the appearance of oxidative stress. Oxidative stress plays an important role in the pathogenesis of various metabolic disorders including pre-obesity, obesity, and T2DM. The production of ROS endogenously and/or exogenously is a significant contributor to the development of T2DM and its complications. Constant efforts have been made by researchers globally to develop the therapeutic model to treat T2DM which can ameliorate oxidative stress. In general, oxidative stress can be reduced by adopting a balanced lifestyle and healthy diet. Although nutrition plays a critical role, the supplementation of a diet with antioxidants like vitamins and natural products has the sufficient capacity to downregulate oxidative stress by quenching free radicals and enzymatic and non-enzymatic reactions. It is also suggested that these antioxidants may mitigate T2DM via various mechanisms like synchronizing or controlling insulin related cell signalling which can regulate gene replication, transcription, and translation and increase insulin secretion, and improve function of hepatic β cells and glucose reabsorption. Ideally, antioxidant rich food can be taken as part of life in early age. Further, it is also clear that antioxidants are sufficiently capable to reduce low grade inflammation with associated diseases. Also, antioxidant therapy might prove to be beneficial while being supplemented at the late stage of T2DM.
Type 2 diabetes mellitus (T2DM) is a condition that affects how the glucose is metabolized for energy. When there is an imbalance between the creation and removal of free radicals, oxidative stress can occur, which affects how the body regulates glucose and insulin, leading to the development and worsening of diabetes and related complications. Taking antioxidant supplements may be a promising way to prevent and treat T2DM.
T2DM is a chronic metabolic disorder with increasing prevalence worldwide, and oxidative stress is implicated in its complications. Antioxidants may counteract this process and can help in improving the metabolic pathways.
To review the current evidence on the role of oxidative stress in the pathogenesis of T2DM and to evaluate the effectiveness of antioxidants as a potential therapy for managing diabetes and its complications.
We systematically searched the electronic database PubMed by keywords. Randomized control trials (RCTs) evaluating the effect of antioxidant therapy on glycemic control and oxidant and antioxidant status as primary outcomes were included. The outcomes considered were: A reduction in blood glucose; changes in oxidative stress and antioxidant markers. Full-length papers of the shortlisted articles were assessed for the eligibility criteria and 17 RCTs were included.
The administration of fixed-dose antioxidants significantly reduced fasting blood sugar and glycated hemoglobin, and was associated with decreased malondialdehyde and advanced oxidation protein products and increased total antioxidant capacity.
The modern lifestyle and environmental factors can contribute to oxidative stress, which plays a significant role in the development of metabolic disorders such as pre-obesity, obesity, and T2DM. The use of antioxidants through a balanced diet and/or supplementation can reduce oxidative stress, which may mitigate the development and complications of T2DM. Antioxidants can also reduce low-grade inflammation associated with various diseases. Further follow-up research is needed to determine the optimal timing and dosage of antioxidant therapy for diabetic patients.
Future research should focus on identifying new antioxidants and their mechanisms of action in reducing oxidative stress and preventing or managing T2DM. Additionally, studies on the effectiveness of antioxidant supplementation in combination with other therapies, such as exercise and medication, should be conducted. Further investigation is also needed to determine the optimal timing and dosage of antioxidant supplementation for diabetes prevention and treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Nasrallah O, Lebanon S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Petrov MS, Basina M. Diagnosis of endocrine disease: Diagnosing and classifying diabetes in diseases of the exocrine pancreas. Eur J Endocrinol. 2021;184:R151-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 472] [Cited by in RCA: 558] [Article Influence: 50.7] [Reference Citation Analysis (6)] |
| 3. | Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (1)] |
| 4. | Liu T, Stern A, Roberts LJ, Morrow JD. The isoprostanes: novel prostaglandin-like products of the free radical-catalyzed peroxidation of arachidonic acid. J Biomed Sci. 1999;6:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 375] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 6. | Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3066] [Cited by in RCA: 2579] [Article Influence: 171.9] [Reference Citation Analysis (0)] |
| 7. | Nita M, Grzybowski A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid Med Cell Longev. 2016;2016:3164734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 927] [Cited by in RCA: 913] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 8. | Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 505] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Mendonca HR, Carpi-Santos R, da Costa Calaza K, Blanco Martinez AM. Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res. 2020;15:625-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 824] [Cited by in RCA: 1084] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 11. | Collins AE, Saleh TM, Kalisch BE. Naturally Occurring Antioxidant Therapy in Alzheimer's Disease. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Shekh-Ahmad T, Lieb A, Kovac S, Gola L, Christian Wigley W, Abramov AY, Walker MC. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol. 2019;26:101278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 746] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 14. | Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006;113:189-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, Zachary MD, Remacle J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 534] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front Pharmacol. 2018;9:1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 606] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 17. | Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24:547-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 856] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 18. | Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal. 2011;15:1517-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1267] [Cited by in RCA: 1091] [Article Influence: 109.1] [Reference Citation Analysis (1)] |
| 20. | Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev. 2011;7:106-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 21. | Amini L, Chekini R, Nateghi MR, Haghani H, Jamialahmadi T, Sathyapalan T, Sahebkar A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res Manag. 2021;2021:5529741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 22. | Cojic M, Kocic R, Klisic A, Kocic G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front Endocrinol (Lausanne). 2021;12:610893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 23. | Boonthongkaew C, Tong-Un T, Kanpetta Y, Chaungchot N, Leelayuwat C, Leelayuwat N. Vitamin C supplementation improves blood pressure and oxidative stress after acute exercise in patients with poorly controlled type 2 diabetes mellitus: A randomized, placebo-controlled, cross-over study. Chin J Physiol. 2021;64:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Dalan R, Goh LL, Lim CJ, Seneviratna A, Liew H, Seow CJ, Xia L, Chew DEK, Leow MKS, Boehm BO. Impact of Vitamin E supplementation on vascular function in haptoglobin genotype stratified diabetes patients (EVAS Trial): a randomised controlled trial. Nutr Diabetes. 2020;10:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | El-Aal AA, El-Ghffar EAA, Ghali AA, Zughbur MR, Sirdah MM. The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: A single-blinded randomized controlled clinical trial. Diabetes Metab Syndr. 2018;12:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 26. | Oppedisano F, Macrì R, Gliozzi M, Musolino V, Carresi C, Maiuolo J, Bosco F, Nucera S, Caterina Zito M, Guarnieri L, Scarano F, Nicita C, Coppoletta AR, Ruga S, Scicchitano M, Mollace R, Palma E, Mollace V. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 27. | Fayh APT, Borges K, Cunha GS, Krause M, Rocha R, de Bittencourt PIH Jr, Moreira JCF, Friedman R, da Silva Rossato J, Fernandes JR, Reischak-Oliveira A. Effects of n-3 fatty acids and exercise on oxidative stress parameters in type 2 diabetic: a randomized clinical trial. J Int Soc Sports Nutr. 2018;15:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Ernster L, Forsmark-Andrée P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71:S60-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 155] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Yen CH, Chu YJ, Lee BJ, Lin YC, Lin PT. Effect of liquid ubiquinol supplementation on glucose, lipids and antioxidant capacity in type 2 diabetes patients: a double-blind, randomised, placebo-controlled trial. Br J Nutr. 2018;120:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2412] [Cited by in RCA: 2548] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 31. | Pingali U, Sukumaran D, Nutalapati C. Effect of an aqueous extract of Terminalia chebula on endothelial dysfunction, systemic inflammation, and lipid profile in type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical study. Phytother Res. 2020;34:3226-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Jana S, Shekhawat GS. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn Rev. 2010;4:179-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Haidari F, Zakerkish M, Borazjani F, Ahmadi Angali K, Amoochi Foroushani G. The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials. 2020;21:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Jakubczyk K, Drużga A, Katarzyna J, Skonieczna-Żydecka K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 35. | Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement Ther Med. 2019;43:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 36. | Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, Sahebkar A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res (Stuttg). 2018;68:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 37. | Man MQ, Yang B, Elias PM. Benefits of Hesperidin for Cutaneous Functions. Evid Based Complement Alternat Med. 2019;2019:2676307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 38. | Homayouni F, Haidari F, Hedayati M, Zakerkish M, Ahmadi K. Hesperidin Supplementation Alleviates Oxidative DNA Damage and Lipid Peroxidation in Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytother Res. 2017;31:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Kocot J, Kiełczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid Med Cell Longev. 2018;2018:7074209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 40. | Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Haghighian HK. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement Ther Med. 2019;43:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Urbonaviciene D, Bobinaite R, Viskelis P, Bobinas C, Petruskevicius A, Klavins L, Viskelis J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Pires TCSP, Caleja C, Santos-Buelga C, Barros L, Ferreira ICFR. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications - A Review. Curr Pharm Des. 2020;26:1917-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | Chan SW, Chu TTW, Choi SW, Benzie IFF, Tomlinson B. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in Chinese patients with type 2 diabetes. Phytother Res. 2021;35:3236-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 45. | Azizi S, Ebrahimi-Mameghani M, Mobasseri M, Karamzad N, Mahdavi R. Oxidative stress and nitrate/nitrite (NOx) status following citrulline supplementation in type 2 diabetes: a randomised, double-blind, placebo-controlled trial. J Hum Nutr Diet. 2021;34:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Liaqat H, Kim KJ, Park SY, Jung SK, Park SH, Lim S, Kim JY. Antioxidant Effect of Wheat Germ Extracts and Their Antilipidemic Effect in Palmitic Acid-Induced Steatosis in HepG2 and 3T3-L1 Cells. Foods. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Mohammadi H, Karimifar M, Heidari Z, Zare M, Amani R. The effects of wheat germ supplementation on metabolic profile in patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Phytother Res. 2020;34:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, Sharifi-Rad J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 630] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 49. | Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018;55:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 50. | Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. Int Heart J. 2017;58:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |