Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.617
Peer-review started: December 19, 2022
First decision: February 28, 2023
Revised: March 1, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 15, 2023
Processing time: 146 Days and 17 Hours
Breast milk is the best and principal nutritional source for neonates and infants. It may protect infants against many metabolic diseases, predominantly obesity and type 2 diabetes. Diabetes mellitus (DM) is a chronic metabolic and microvascular disease that affects all the body systems and all ages from intrauterine life to late adulthood. Breastfeeding protects against infant mortality and diseases, such as necrotizing enterocolitis, diarrhoea, respiratory infections, viral and bacterial infection, eczema, allergic rhinitis, asthma, food allergies, malocclusion, dental caries, Crohn's disease, and ulcerative colitis. It also protects against obesity and insulin resistance and increases intelligence and mental development. Gestational diabetes has short and long-term impacts on infants of diabetic mothers (IDM). Breast milk composition changes in mothers with gestational diabetes.
To investigate the beneficial or detrimental effects of breastfeeding on the cardiometabolic health of IDM and their mothers.
We performed a database search on different engines and a thorough literature review and included 121 research published in English between January 2000 and December 15, 2022, in this review.
Most of the literature agreed on the beneficial effects of breast milk for both the mother and the infant in the short and long terms. Breastfeeding protects mothers with gestational diabetes against obesity and type 2 DM. Despite some evidence of the protective effects of breastfeeding on IDM in the short and long term, the evidence is not strong enough due to the presence of many confounding factors and a lack of sufficient studies.
We need more comprehensive research to prove these effects. Despite many obstacles that may enface mothers with gestational diabetes to start and maintain breastfeeding, every effort should be made to encourage them to breastfeed.
Core Tip: Breast milk is the ideal nutritional source for all neonates. It protects against many cardiometabolic disorders for babies and their mothers in the presence or absence of gestational diabetes. It protects against overweight, obesity, insulin resistance, prediabetes, diabetes, and metabolic syndrome in offspring regardless of gestational diabetes status. Therefore, it prevents significant risk factors predisposing to cardiovascular diseases during childhood and adulthood. Every effort should be made to encourage breastfeeding.
- Citation: Elbeltagi R, Al-Beltagi M, Saeed NK, Bediwy AS. Cardiometabolic effects of breastfeeding on infants of diabetic mothers. World J Diabetes 2023; 14(5): 617-631
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/617.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.617
Breast milk is the best and principal nutritional source for neonates, providing them with the needed protein, fat, carbohydrate, vitamins, and minerals requirements. In addition, it provides them with different substances and bioactive agents that help protect them against infections and inflammation by contributing to a healthy microbiome, organ development, and an efficient immune system[1]. Breast milk is rich in growth factors that support the development and growth of the newborn's brain, gut, endocrine, and vascular systems[2]. Many studies suggested that breast milk protects infants against many metabolic diseases, predominantly obesity and type 2 diabetes[3]. Breast milk is continuously changing with dynamic and bioactive composition modification from colostrum to late stages of lactation. It often varies diurnally, within feeds, between different populations, and even between mothers from the same population to meet the metabolic needs of their babies[4]. The amount of breastmilk needed at one month of age is about 650 mL/d, increased to 770 mL/d at three months and 800 mL/d at six months, then dropped to 520 mL/d by one year of age. In addition, the duration and frequency of breastfeeding also change with infant development and maturation, starting with 20 to 40 min, up to six times/d, which is reduced to 10-20 min when the infant reaches three months of age. The frequency of breastfeeding decreases as the weaning starts[5].
Diabetes mellitus (DM) is a chronic metabolic and microvascular disease that affects all the body systems and all ages from intrauterine life to late adulthood. DM that occurs during pregnancy could have its onset before or arise as de novo for the first-time during pregnancy (gestational DM), which could disappear or persist after delivery[6]. Impaired glucose tolerance occurs in 3%-10% of pregnancies and correlates positively with the average diabetes incidence in the general population. The risk of gestational diabetes increases with advanced maternal age, obesity, non-white ancestry, and physical inactivity[7]. Gestational diabetes has short and long-term effects on infants of diabetic mothers (IDM). Neonates have a higher risk of post-natal hypoglycemia, macrosomia, respiratory problems, hy-pertrophic cardiomyopathy, congenital disabilities, and various metabolic and hematologic disorders. At the same time, there is an increased risk of obesity during childhood and type 2 DM in adulthood[8]. Breastfeeding is well known to have many beneficial effects on both mothers and infants. However, the breast milk of mothers with diabetes has altered composition. Therefore, it is expected to have different effects than those from non-diabetic mothers[9]. This review investigates the beneficial or detrimental effects of breastfeeding on the cardiometabolic health of IDM and their mothers.
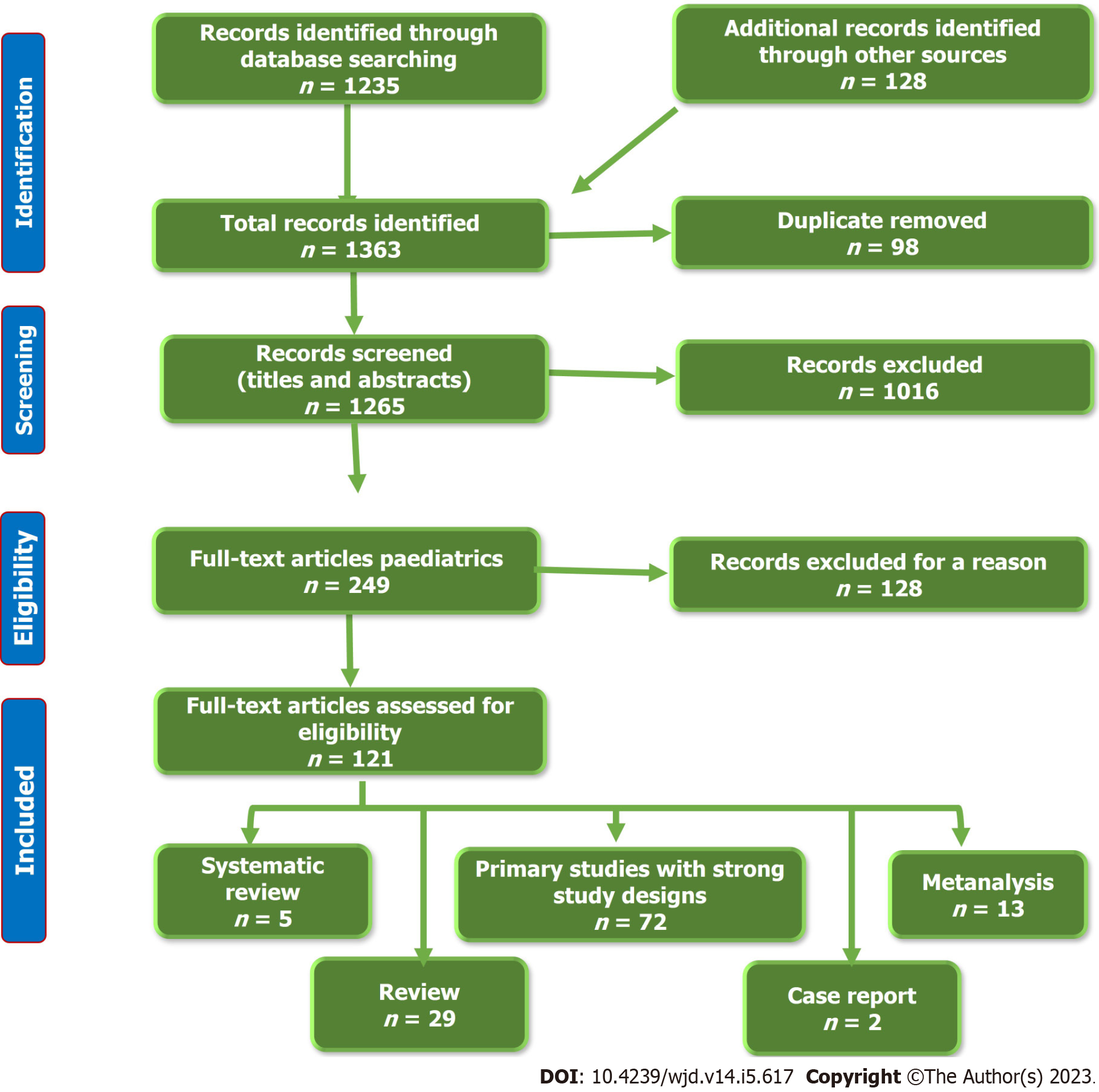
To establish an evidence-based vision of this aim, we performed a thorough literature review by searching the available electronic databases, including Cochrane Library, PubMed, PubMed Central, Cumulative Index to Nursing and Allied Health Literature, Embase, Web of Science, Library and Information Science Abstracts, Scopus, and the National Library of Medicine catalog up until December 15, 2022, using the keywords: Diabetes Mellitus, Gestational Diabetes, Cardiometabolic, Breastfeeding, Breast milk. We identified 1363 articles, 98 of which were removed due to duplication. After the screening of the titles and abstract, we excluded 1016 articles. From the remaining 249 full-text articles, only 121 articles fulfilled the eligibility criteria.
We included full-text research articles (72 articles), metanalysis (13 articles), systematic reviews (5 articles), reviews (29 articles), and Case reports (2 articles). We included articles that were written in English and concerned with the effects of breastfeeding on the cardiometabolic effects in IDM. Figure 1 shows the study flow chart. Reference lists were checked, and citation searches were performed on the included studies. We also reviewed the articles that are available as abstracts only. We excluded articles with a commercial background.
Most of the literature agreed on the beneficial effects of breast milk for both the mother and the infant in the short and long terms. Breastfeeding protects mothers with gestational diabetes against obesity and type 2 DM. Despite some evidence of the protective effects of breastfeeding on IDM in the short and long term, the evidence is not strong enough due to the presence of many confounding factors and a lack of sufficient studies.
Breast milk is the ideal nutrition source for the infant, especially in the first six months, as it provides the baby with everything they need in the proper proportions for the first six months of life. Its composition modifies according to the infants’ changing requirements, particularly in the first few weeks of life. Colostrum is the wonder of breastfeeding in the first post-natal days, with thick yellowish color, high protein, low sugar, and many beneficial compounds. It helps develop the baby's immature gut to be ready to receive the increasing amount of breastfeeding in the following days[1]. In addition, early breastfeeding in the delivery room may prevent the development of post-natal hypoglycemia in IDMs[10]. However, breastfeeding has low vitamin D. Breastfed babies should be supplemented with vitamin D[11]. The low iron profile in breast milk could be beneficial in decreasing the risk of bacterial growth. Iron supplementation in breastfed babies should be considered to improve brain and cognitive development, especially those born prematurely or at low birth weight [12].
Breastfeeding performs crucial effects on the programming activity during early life. Many recent meta-analyses of several studies provide strong evidence that breastfeeding benefits neonates, infants, children, and lactating mothers considerably. The degrees of these beneficial effects vary according to different settings’ background environmental and hygienic conditions[13]. According to 28 metanalyses, breastfeeding protects against infant mortality, especially in low-income settings, by 4-10 times and by 36% in high-income settings[14]. Breastfeeding also protects against many diseases, such as diarrhea by 75% and respiratory infections by 57%, particularly in young children[15]. Breast milk has plenty of antibodies that protect the baby against many viruses and bacteria, which is particularly important during the early critical months of life. Colostrum provides the baby with many different antibodies, especially immunoglobulin A, a crucial element of the baby portal immunity, protecting the nose, throat, and gastrointestinal tract[16].
Breastmilk may also give some protection against eczema, allergic rhinitis, asthma, and food allergies, but with weak evidence[17]. Even though breastfeeding protects against malocclusion and dental caries, more prolonged breastfeeding (beyond one year of age) and nocturnal breastfeeding increase dental caries by two to three folds[18]. It can decrease the incidence of necrotizing enterocolitis in preterm babies. A meta-analysis by Altobelli et al[19] showed that premature infants who received both their own and donated breastmilk had a statistically significant reduced risk of necrotizing enterocolitis, possibly due to the reduced microbial contamination, its pre, and probiotic effects, and its unique immunological components. Breastfed infants are less liable to develop Crohn’s disease and ulcerative colitis. A meta-analysis by Xu et al[20] showed that breastfeeding is associated with a reduced risk of Crohn’s disease and ulcerative colitis in all ethnicities, particularly among Asians. Breastfeeding has dose-dependent protection against Crohn’s disease and ulcerative colitis, with the most potent effect when breastfeeding continues for at least one year.
Breastfeeding causes a mild reduction of body mass index (BMI) without significant differences in growth outcome. However, a meta-analysis by Giugliani et al[21] showed a 13% reduction in the risk of later obesity. Grube et al[22] showed that breastfeeding for longer than four months significantly reduces the risk of developing overweight and obesity than in non-breastfed babies or those with a shorter breastfeeding period. The weight-reducing effects of breastfeeding could be related to the development of specific strains of gut microbiota that could impact fat storage[23]. At the same time, breast milk contains more leptin hormone than formula milk, if present. Leptin is a vital hormone that regulates the baby’s appetite and controls fat storage[24]. Meanwhile, breastfed infants have more self-regulation of their feeding habits, especially those in on-demand feeding, which supports them in developing healthy feeding patterns[25].
Therefore, the risk of type 2 DM can be reduced by 24% to 32% and to a lesser degree with type 1 DM[26]. Meanwhile, six months or longer of breastfeeding decreases the risk of childhood leukemia by 14%-20%[27]. Breastfeeding also helps to alleviate the clinical course and the severity of urolithiasis identified during infancy. Infants who had prolonged breastfeeding are more liable for reduced size and/or the number of urinary stones. Infants receiving breastfeeding for the first six months need less treatment and have less growth impairment[28].
There is also an association between breastfeeding and increased intelligence by at least 2-3 points after adjusting for the home environment and average parental intelligence quotient (IQ). This increase may be related to the nutritional components, non-nutritional bioactive factors, maternal-infant bonding with physical intimacy, interactions, touch, and eye-to-eye contact. Infants with breastfeeding are less liable to have behavioral problems or learning difficulties than bottle-feeding infants. This breastfeeding-promoting effect on the baby’s optimal brain development during early life can have long-lasting impacts on infant neurodevelopmental function[29,30]. This effect is more pronounced in preterm babies than in term infants. Belfort et al[31] showed that predominant breastfeeding in the first four weeks of life is related to a larger volume of deep nuclear gray matter volume at term equivalent age and improved IQ, working memory, academic achievement, and motor function at the age of seven in the very preterm infants. Increasing breastfeeding duration is positively correlated with enhancing cognitive development. Ribas-Fitó et al[32] observed a linear dose-response relationship between breastfeeding and cognition at the age of four in children with a history of antenatal exposure to dichlorodiphenyltrichloroethane (DDT) despite the risk of breastfeeding pollution with DDT. Breastfeeding protects against indoor and outdoor air pollution exposure and adverse outcomes due to the effects of long-chain polyunsaturated fatty acids (LC-PUFA), carotenoids, antioxidant vitamins, flavonoids, cytokines, and immunoglobins. Though breastfeeding may be polluted with many pollutants, its protective effects outweigh its potential health hazards to the infant[33] (Table 1).
| Beneficial effects of breastfeeding |
| Neonates |
| Infants and children |
| Lactating mothers |
Breastfeeding has significant impacts on lactating mothers. It may help overweight mothers to lose weight. Numerous studies described a positive correlation between postpartum weight loss and breastfeeding, while others studied did not find a significant association. Several possible mechanisms, determinants, and metabolic pathways may play a role in this weight reduction[34]. Lactating mother burns about 20 calories/ounce of breastmilk she produces. Therefore, one day of breastfeeding may help burn up to 900 calories and more fat. Jarlenski et al[35] showed that exclusive breastfeeding for at least three months or more has a minimal but considerable effect on postpartum weight reduction among American women. Schalla et al[36] showed that returning to a pre-pregnancy body shape is an important feature that encourages mothers to continue breastfeeding. One of the other immediate benefits that lactating mothers have with breastfeeding is the rapid involution of the gravid uterus to return to its pre-gravid size due to oxytocin release in response to the sucking of the breastfed baby, which boosts uterine contractions and lessens bleeding. In addition, oxytocin helps to increase maternal-infant bonding[37].
Breastfeeding is correlated with a significantly reduced risk of ovarian cancer in general and, in particular, for the most lethal high-grade serous subtype of ovarian cancer. This finding suggests that breastfeeding is a possibly modifiable factor that may decrease the risk of ovarian cancer regardless of the effect of pregnancy. The longer the breastfeeding duration, the more the risk is reduced[38]. A meta-analysis by Unar-Munguía et al[39] showed that exclusive breastfeeding significantly reduces breast cancer risk compared to non-breastfeeding parous women. Breastfeeding mothers are less likely to suffer postpartum depression than mothers who do not breastfeed or discontinue it early. These effects are maintained for the first four postpartum months. Conversely, postpartum depression reduces the breastfeeding rate in a reciprocal mechanism[40].
The longer the duration of breastfeeding, the less the risk of developing type 2 diabetes in lactating women. Schwarz et al[41] showed that breastfeeding is associated with improved maternal glucose metabolism. They also showed an increased risk of developing type 2 diabetes in later life when the parous women lactate for less than a month after term pregnancy, regardless of the women’s BMI or physical activity. Breastfeeding also decreases the risk of hypertension, hypercholesteremia, and arthritis. In addition, breastfeeding protects mothers who breastfeed their children for five months or more in at least one pregnancy against coronary artery disease (CAD), with a 30% risk reduction later in life. Conversely, parous women who never breastfed or stopped breastfeeding early have a two-fold increased risk of CAD[42].
Many possible mechanisms are proposed to explain the protective effects of breastfeeding. These mechanisms include the beneficial effects of breastfeeding on the respiratory, nervous, and immune systems, which are related to breast milk's anti-inflammatory, antioxidant, neuroprotective, and immunomodulatory features[33]. The high cholesterol content of breast milk during infancy inversely suppresses endogenous cholesterol synthesis in adulthood by suppressing the regulation of hydroxymethyl-glutaril liver coenzyme A[43,44]. Therefore, breast milk protects against the development of hypercholesteremia, especially low-density lipoprotein cholesterol which is a significant risk factor for coronary heart diseases[45]. This cholesterol-regulating effect of breast milk can explain its protective effects against atherosclerosis, hypertension, and coronary heart diseases. The low sodium and the high LC-PUFA contents of breast milk compared to formula milk might give more protection against the future development of hypertension during childhood and adulthood[46,47]. LC-PUFA is a crucial element of the tissue membrane system, such as the coronary endothelial system, therefore reducing the risk of coronary heart disease and stroke during adulthood[48]. Breastfeeding can also reduce fasting insulin and insulin resistance in infancy, childhood, and adulthood[49,50].
Breastfeeding has a behavioral modifying effect on the infant's appetite, satiety, and feeding pattern due to its unique micro- and macro-nutrients and hormonal contents. These unique features of breastmilk explain its protective role against obesity[51,52]. Breastmilk contains leptin, which is not present in formula milk, and less protein and fat than formula milk, so breastfeeding is likely to adequately stimulate the secretion of insulin growth factor-type 1. Subsequently, it can induce adequate insulin secretion, fewer adipocytes stimulation and size, and balancing fat reserve, which eventually results in adequate weight gain and is less likely to cause overweight and obesity[51,53]. Breastmilk can also modulate the expression of obesity-predisposing genes, preventing the development of obesity and other non-communicable diseases[54]. Breastmilk has a significant regulating effect on the blood glucose level due to its high content of LC-PUFA. The LC-PUFA amount in the skeletal muscle membranes is inversely proportional to the fasting blood glucose level, insulin resistance, subsequent hyperinsulinemia, and type 2 diabetes[55,56]. The low protein content, the lower volume of breastmilk consumed by the infant, and the differences in the levels of hormones of insulin, neurotensin, intro-glucagon, motilin, and pancreatic polypeptide, and lower subcutaneous fat deposition are additive protective factors against developing type 2 diabetes[57].
Breastfeeding has well-known immune-modulating and protective effects against many immune and allergic disorders, especially in low-income countries[58]. Breastfeeding supports passive and active immunity in infants and young children[59]. Breastfeeding also protects against many infectious diseases in infancy and early childhood. These protective effects are dose-dependent, increasing with exclusive breastfeeding and more prolonged duration[60]. These protective and immune-enhancing effects of breastfeeding are due to its richness of many compounds that enhance both innate (such as various cellular components, lysozyme, oligosaccharides, lactoferrin, the cluster of differentiation 14, and probiotics components)[61-63] and active (such as immunoglobins A, M, and G) immunity[59]. In addition, breastmilk contains many immune-modulating ingredients, such as cytokines or nutritional components, such as LC-PUFA; vitamins A, B12, and D, and zinc[58]. Omega-3 LC-PUFA, abundant in breast milk, helps T-cell membrane stabilization, T-cell signaling, improvement, and reduction of many pro-inflammatory substances production. On the contrary, omega-6 LC-PUFAs stimulate their production[64]. Exclusive breastfeeding modulates the inflammatory status by promoting an anti-inflammatory cytokine milieu and decreases gut inflammation that persists throughout infancy, adolescence, and adulthood[65-67]. The anti-inflammatory effect of breastfeeding is due to the presence of various immunoreactive and immunomodulator factors such as lactoperoxidase, lactoferrin, immunoglobins, osteopontin, superoxide dismutase, platelet-activating factor acetylhydrolase, alkaline phosphatase, antioxidant compounds, bioactive factors, and many growth factors that have anti-inflammatory effects[62].
The anti-inflammatory and immune-modulating effects of breastmilk boost lung development and function. In addition, the breastmilk cytokines, growth factors, and maternal immunoglobins may stimulate lung growth and development, inhibit airway inflammation, and decrease the risk of developing asthma. Breastfeeding is also associated with a reduced risk of being overweight and obese and, consequently, better lung function[68-70]. Breastmilk nutrients such as β-carotene, lutein/zeaxanthin, polyphenol, and anthocyanin also affect lung efficiency[71]. Breastfeeding effects on DNA methylation provide an additional protective mechanism for the respiratory tract and improve lung development and maturation[72]. Moreover, sucking during breastfeeding stimulates the development of the diaphragm and the respiratory muscles, enhances the coordination between swallowing and respiration, and, thus, improves lung capacity[73].
The better structural and physiological neurodevelopment and cognitive and psychomotor performance associated with breastfeeding are related to many factors. Breast milk is rich in LC PUFAs, antioxidants (such as carotenoids and flavonoids), and other nutrients and bioactive factors that can induce immunomodulation and reduce oxidative stress and neuroinflammation[29,71,74]. Breast milk also contains many compounds essential for proper brain development, neurotransmitters synthesis, synaptogenesis, and intracellular communication. Breast milk is rich in LC PUFAs, glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor (BDNF), gangliosides, sialic acid, lutein, choline, zeaxanthin, and flavonoids. These nutrients are essential in the human brain's gross and functional development[75-77]. Other social and environmental factors associated with breastfeeding, such as mother-infant bonding and educational and socioeconomic levels, may also play a role in better neurodevelopment[78].
Effects on the mothers: Breastfeeding induces more favorable metabolic parameters in lactating women. It initiates a metabolic shift from pregnancy to postpartum with the alteration of resource allocation from the caloric storage stage to the milk production phase with lipid transport facilitation to the mammary gland to help in milk synthesis[79]. Stuebe[80] showed that early, high-intensity breastfeeding might help to reset the endocrine balance to shift from the insulin-resistant state in pregnancy to insulin sensitive state; thus, lactation may protect against long-term cardiometabolic health consequences. Breastfeeding induces improved glucose utilization through reduced insulin production, enhanced insulin sensitivity, and decreased β-cell proliferation[81]. Therefore, lactating women are less liable to have atherogenic blood lipids and have better glucose and lipid metabolism, lower fasting and postprandial blood glucose, low insulin levels, and more insulin sensitivity than non-lactating women, especially in the first four postpartum months[82]. In addition, lactation reduces the risk of obesity, metabolic syndrome, cardiovascular diseases, and type 2 diabetes during mid to late life[83,84]. The liver, white adipose tissues and skeletal muscles are responsible for about 50% of the mammals' metabolic rate. Breastfeeding increases hepatic mitochondrial respiration, therefore increasing the metabolic rate. In a study of rats, Hyatt et al[81] showed that lactation induces PPARδ protein level changes in the liver, white adipose tissue, and skeletal muscle, which may partially clarify the observed lower blood glucose levels. A large study from Japan showed that the longer the duration of breastfeeding, the less risk for developing metabolic syndrome in women under 55 years of age[85]. The long-term protective effects of breastfeeding were also confirmed by Wiklund et al[86], who showed that breastfeeding longer than six months gives protection against obesity, impaired glucose tolerance, insulin resistance, hypercholesteremia, and hypertension that could persist for 16 to 20 years later.
Effects on the infants: Breastfeeding has significant effects on infant metabolism through different mechanisms. Breast milk has numerous beneficial compounds that can cause epigenetic changes in genes that control metabolism or predispose to insulin resistance, diabetes, or obesity. For example, breastmilk downregulates phosphatase and tensin homolog and acetyl-CoA carboxylase beta genes, protecting against developing insulin resistance and DM[87-89]. Meanwhile, breastfeeding appears to counter the deleterious effect of the peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism on anthropometrical parameters in adolescents[90]. The liver X receptors gene expression is also modulated by breastfeeding. Activating these receptors stimulates a set of target genes needed for the de novo synthesis of triglycerides and cholesterol transport in many tissues[91]. In addition, infant serum lysophosphatidylcholine, which is positively associated with obesity risk, is affected by breast milk fatty acids composition and, interestingly, milk protein content and composition in early but not late lactation[92]. Therefore, breastfed infants are metabolically different from the infant formula regarding the lipid and energy metabolism levels (ketone bodies, carnitines, and Krebs cycle)[93]. Breastfeeding positively affects metabolic variables, anthropometric indices, and diabetes-prompting genes compared to bottle feeding[87]. Breastfeeding also increases BDNF, which enhances synaptogenesis and neuronal development in infants between 4-6 mo of age[94]. This neurotrophic factor impacts numerous metabolic pathways by modifying the hypothalamus or specific neurotransmitters that facilitate food intake[95]. The effects of breastfeeding on the infant metabolism are dose-dependent. Corona et al[96] showed that the duration of breastfeeding is inversely related to the Z-score of triceps skinfold-for-age till the age of three years. On the other hand, Martin et al[97] showed that even with a long duration, exclusive breastfeeding failed to reduce insulin resistance or cardiometabolic risk parameters at 11.5 years. Therefore, the breastfeeding effect on the body's metabolism still demands additional analysis and research. We need to study why there are differences in the results of these studies and confounding factors that may impact their results.
Breast milk is a biologically-active, continuously dynamic fluid that significantly differs from woman to woman and from one stage to another. It is affected by various maternal factors such as term-preterm labor, maternal diet, metabolic disorders, and diseases[2,98]. DM is a chronic systemic metabolic disorder that could affect pregnant ladies with pregestational or gestational (a de novo) onset[99]. Mother with gestational DM has a 15 to 24 h delay in lactogenesis II (initiation of lactation) markers such as citrate, lactose, and total nitrogen to reach levels similar to healthy women[100]. This delay in breast milk initiation in women with gestational diabetes could be related to low levels of circulating human placental lactogen in the latter stages of pregnancy, which is positively correlated with mammary gland growth during pregnancy[101].
In addition, Arthur et al[102] and Azulay Chertok et al[103] found a significant delay in the timing of the lactose increase in the colostrum in lactating women with type 1 or gestational DM, accompanied by a reduced milk volume in the first three postpartum days, as lactose is the main osmotic ingredient in the human milk. The observed delay in citrate concentration rise in colostrum may cause a delay in the de novo medium-chain fatty acids synthesis, as citrate is essential for acetyl CoA generation from glucose[104]. Avellar et al[105] showed that women with gestational DM had higher colostrum contents of cytokines and chemokines, with increased levels of interleukin 6 (IL-6), IL-15, interferon-γ, reduced IL-1ra levels, and a decreased granulocyte-macrophage colony-stimulating factor (GM-CSF), causing altered immune composition of the colostrum. Bitman et al[106] found that women with type 1 DM who started to pump milk at 72 h postpartum firstly gave breast milk with reduced total fat, medium-chain fatty acids, and total cholesterol but increased linoleic, oleic, and polyunsaturated long-chain fatty acid content than healthy women. These fatty acid profile changes are related to changes in specific endogenous metabolic pathways[107]. Women with type 1 DM also have impaired mammary gland lipid metabolism and high glucose and sodium contents in mature milk. However, no significant differences exist in the free amino acid profile in women with and without gestational DM[108]. The high amino acid levels in the colostrum and high levels of saturated and non-saturated fatty acid levels in mature milk in lactating women with and without gestational DM are crucial for neonatal development in the early period of life[109]. In addition, Suwaydi et al[110] showed that gestational DM has significant relationships with metabolic hormone concentrations, including ghrelin, insulin, and adiponectin. However, these relationships might be restricted to the early lactation stage.
Breastfeeding is associated with reduced risk of type 2 DM in women with gestational DM for up to two postpartum years. In addition, breastfeeding may have long-lasting protective effects beyond two years after delivery, especially with greater lactation intensity and prolonger duration[111]. A meta-analysis by Pathirana et al[112] showed that breastfeeding might protect against some cardiovascular risk factors, such as Type 2 DM, in mothers with a history of gestational DM. Breastfeeding for over three months is associated with the least postpartum diabetes risk in women with gestational DM[113]. Lactation enhances glucose tolerance in mothers with gestational DM, especially in the early postpartum period. Reduced estrogen levels in breastfeeding mothers might protect against impaired glucose metabolism and consequently decrease the risk of diabetes. In addition, breastfeeding decreases the risk of obesity and further reduces the risk of type 2 DM[114].
Children born to mothers with gestational DM are more prone to prediabetes, metabolic syndrome, and obesity later in life. Gestational DM is correlated with excessive fetal growth, macrosomia, and overnutrition in utero[115]. The growth pattern in children born to women with DM (including gestational DM) is slower than controls in the first two years of life, followed by rapid weight gain and consequently increased risk of being overweight, obese, and having other metabolic disorders[116]. There is a double risk of being overweight in breastfeeders from mothers with DM compared to banked breast milk feeders at the age of two years[117]. Therefore, every effort should be made to decrease these risks. However, despite the altered breast milk composition of mothers with gestational DM, e.g., reduced milk protein, there is some evidence of the beneficial effects of breastfeeding on the car-diometabolic health of their offspring. The Nurse Health study showed a significantly reduced risk of being overweight at 9-14 years in the offspring of mothers with gestational DM who breastfed for the first six months of life[118]. In addition, Ong et al[119] showed that breastfeeding might give some protection against undesirable fat distribution and hypertriglyceridemia in children born to mothers with gestational DM and consequently help in reducing childhood cardiometabolic risks. In the Prima Indian study, the prevalence of DM among the offspring of mothers with gestational DM was significantly lower in those with exclusive breastfeeding than those without breastfeeding at age 10-39 years after adjustment for age, sex, and birth weight[120]. Another study assessed the effects of breastfeeding and gestational DM on Hispanic children between 8 and 13 years. They found that breastfeeding protects against developing prediabetes and metabolic syndrome in the offspring with or without gestational DM[121]. However, a meta-analysis by Pathirana et al[112] failed to prove any protective effects of breastfeeding in IDM due to a lack of sufficient studies.
As breastfeeding provides adequate nutritious, easily digestible nutrients for infants, every effort should be made to encourage breastfeeding and to support the mothers to complete their mission successfully. Despite many obstacles that may enface mothers with gestational DM to start and maintain breastfeeding, every effort should be made to encourage them to breastfeed. Exclusive breastfeeding should be encouraged for 4-6 mo and complemented or supplemented for two years when possible. Information about breastfeeding, including techniques, frequency, duration, and how to overcome potential obstacles, should be available and understandable. The parents should learn and practice responsive feeding and understand the baby's cues when hungry or satisfied. The mother should also know the potential benefits of breastfeeding for her and her baby, especially when she has DM. The government should encourage and implement paid maternity leaves for at least three months to help mothers stay with their babies at home and breastfeed them. In addition, we still need to study the protective effects of breastfeeding on IDM in the short and long term. In addition, many factors are responsible for the variation of the results of the different studies, including the different methodological procedures and the differences in the target populations. Therefore, we need more extensive and multicentre studies for a longer duration and different races to ensure the beneficial roles of breastfeeding on various items of metabolic and cardiovascular health and disorders both in paediatrics and adulthood. In addition, we should request infant formula companies to do their best to mimic breast milk and reduce the gap between the advantages of breast milk and the disadvantages of infant formula. For example, these companies should revise and optimize the protein content, the amount and types of fat, and the impacts of adding probiotics, prebiotics, human milk oligosaccharides, and other well-established breast milk components.
Breastfeeding has many beneficial effects for both lactating mothers and their offspring. It protects against overweight, obesity, insulin resistance, prediabetes, DM, and metabolic syndrome in offspring regardless of gestational diabetes status. In addition, it prevents significant risk factors predisposing to cardiovascular diseases in childhood and adulthood. Therefore, every effort should be made to educate mothers about the benefits of breastfeeding for controlling DM, cardiovascular diseases, and hypertension in women and their offspring.
Breast milk is the best and principal nutritional source for neonates. Breast milk is the best and principal nutritional source for neonates. Breast milk is the best and principal nutritional source for neonates. Breast milk is the best and principal nutritional source for neonates. Gestational diabetes has short and long-term effects on infants of diabetic mothers (IDM). Gestational diabetes has short and long-term effects on IDM.
Breast milk of mothers with diabetes has different compositions. Therefore, it is expected to have different effects than those from non-diabetic mothers.
We aimed to investigate the positive or negative cardiometabolic effects of breastfeeding on the health of IDM and their mothers.
We searched different search engines and conducted a thorough literature review of the cardiometabolic effects of breastfeeding on the health of IDM and their mothers. We included 121 articles published in English between January, 2000 and December 15, 2022 in this review.
Most of the literature agreed that breast milk has many beneficial effects for both the mother and their infant in the short and long terms. Breastfeeding protects mothers with gestational diabetes against obesity and type 2 diabetes mellitus (DM). There is some evidence that breastfeeding has protective effects on IDM in the short and long term. However, this evidence is not strong enough due to the presence of many confounding factors and a lack of sufficient studies.
Breastfeeding has numerous favorable effects for both breastfeeding mothers and their infants, protecting the offspring against overweight, obesity, insulin resistance, prediabetes, DM, and metabolic syndrome regardless of gestational diabetes status. In addition, it prevents major risk factors that predispose to cardiovascular diseases in childhood and adulthood. Every effort should be made to teach mothers the benefits of breastfeeding in controlling DM, cardiovascular diseases, and hypertension in women and their offspring.
We need to study the protective effects of breastfeeding on IDM in the short and long term. We have to perform more extensive and multicentre studies for a longer duration and different races to ensure the beneficial roles of breastfeeding on various items of metabolic and cardiovascular health and disorders both in paediatrics and adulthood. We should request that those infant formula companies perform their best to mimic breast milk and reduce the gap between the advantages of breast milk and the disadvantages of infant formula.
We thank the anonymous referees for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gica N, Romania; Wu QN, China S-Editor: Zhang H L-Editor: A P-Editor: Chen YX
| 1. | Martin CR, Ling PR, Blackburn GL. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 2. | Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1389] [Cited by in RCA: 1692] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 3. | Dieterich CM, Felice JP, O'Sullivan E, Rasmussen KM. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am. 2013;60:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 4. | Samuel TM, Zhou Q, Giuffrida F, Munblit D, Verhasselt V, Thakkar SK. Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front Nutr. 2020;7:576133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Saki A, Eshraghian MR, Tabesh H. Patterns of daily duration and frequency of breastfeeding among exclusively breastfed infants in Shiraz, Iran, a 6-month follow-up study using Bayesian generalized linear mixed models. Glob J Health Sci. 2012;5:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Al-Biltagi M, Tolba OA, Rowisha MA, Mahfouz Ael-S, Elewa MA. Speckle tracking and myocardial tissue imaging in infant of diabetic mother with gestational and pregestational diabetes. Pediatr Cardiol. 2015;36:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Bogo MA, Pabis JS, Bonchoski AB, Santos DCD, Pinto TJF, Simões MA, Silva JC, Pabis FC. Cardiomyopathy and cardiac function in fetuses and newborns of diabetic mothers. J Pediatr (Rio J). 2021;97:520-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Peila C, Gazzolo D, Bertino E, Cresi F, Coscia A. Influence of Diabetes during Pregnancy on Human Milk Composition. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Chertok IR, Raz I, Shoham I, Haddad H, Wiznitzer A. Effects of early breastfeeding on neonatal glucose levels of term infants born to women with gestational diabetes. J Hum Nutr Diet. 2009;22:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Boquien CY. Human Milk: An Ideal Food for Nutrition of Preterm Newborn. Front Pediatr. 2018;6:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Friel J, Qasem W, Cai C. Iron and the Breastfed Infant. Antioxidants (Basel). 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Prentice AM. Breastfeeding in the Modern World. Ann Nutr Metab. 2022;78 Suppl 2:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, Bahl R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 494] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 15. | Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;1-186. [PubMed] |
| 16. | Czosnykowska-Łukacka M, Lis-Kuberka J, Królak-Olejnik B, Orczyk-Pawiłowicz M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front Pediatr. 2020;8:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, Bowatte G, Allen KJ, Dharmage SC. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 365] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 18. | Peres KG, Cascaes AM, Nascimento GG, Victora CG. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Altobelli E, Angeletti PM, Verrotti A, Petrocelli R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 20. | Xu L, Lochhead P, Ko Y, Claggett B, Leong RW, Ananthakrishnan AN. Systematic review with meta-analysis: breastfeeding and the risk of Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;46:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Giugliani ER, Horta BL, Loret de Mola C, Lisboa BO, Victora CG. Effect of breastfeeding promotion interventions on child growth: a systematic review and meta-analysis. Acta Paediatr. 2015;104:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Grube MM, von der Lippe E, Schlaud M, Brettschneider AK. Does breastfeeding help to reduce the risk of childhood overweight and obesity? PLoS One. 2015;10:e0122534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ngowi EE, Wang YZ, Khattak S, Khan NH, Mahmoud SSM, Helmy YASH, Jiang QY, Li T, Duan SF, Ji XY, Wu DD. Impact of the factors shaping gut microbiota on obesity. J Appl Microbiol. 2021;131:2131-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Rosenblad AK, Funkquist EL. Self-efficacy in breastfeeding predicts how mothers perceive their preterm infant's state-regulation. Int Breastfeed J. 2022;17:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 696] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 27. | Amitay EL, Keinan-Boker L. Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review. JAMA Pediatr. 2015;169:e151025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Bozkurt HB, Çetin T, Sarıca K. The Possible Beneficial Effect of Breastfeeding on the Clinical Course of Urolithiasis Detected During Infancy. Breastfeed Med. 2020;15:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 30. | Brown Belfort M. The Science of Breastfeeding and Brain Development. Breastfeed Med. 2017;12:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Belfort MB, Anderson PJ, Nowak VA, Lee KJ, Molesworth C, Thompson DK, Doyle LW, Inder TE. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks' Gestation. J Pediatr. 2016;177:133-139.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 32. | Ribas-Fitó N, Júlvez J, Torrent M, Grimalt JO, Sunyer J. Beneficial effects of breastfeeding on cognition regardless of DDT concentrations at birth. Am J Epidemiol. 2007;166:1198-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Zielinska MA, Hamulka J. Protective Effect of Breastfeeding on the Adverse Health Effects Induced by Air Pollution: Current Evidence and Possible Mechanisms. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Lambrinou CP, Karaglani E, Manios Y. Breastfeeding and postpartum weight loss. Curr Opin Clin Nutr Metab Care. 2019;22:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Jarlenski MP, Bennett WL, Bleich SN, Barry CL, Stuart EA. Effects of breastfeeding on postpartum weight loss among U.S. women. Prev Med. 2014;69:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Schalla SC, Witcomb GL, Haycraft E. Body Shape and Weight Loss as Motivators for Breastfeeding Initiation and Continuation. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2142] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 38. | Babic A, Sasamoto N, Rosner BA, Tworoger SS, Jordan SJ, Risch HA, Harris HR, Rossing MA, Doherty JA, Fortner RT, Chang-Claude J, Goodman MT, Thompson PJ, Moysich KB, Ness RB, Kjaer SK, Jensen A, Schildkraut JM, Titus LJ, Cramer DW, Bandera EV, Qin B, Sieh W, McGuire V, Sutphen R, Pearce CL, Wu AH, Pike M, Webb PM, Modugno F, Terry KL. Association Between Breastfeeding and Ovarian Cancer Risk. JAMA Oncol. 2020;6:e200421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 39. | Unar-Munguía M, Torres-Mejía G, Colchero MA, González de Cosío T. Breastfeeding Mode and Risk of Breast Cancer: A Dose-Response Meta-Analysis. J Hum Lact. 2017;33:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Hamdan A, Tamim H. The relationship between postpartum depression and breastfeeding. Int J Psychiatry Med. 2012;43:243-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Schwarz EB, Brown JS, Creasman JM, Stuebe A, McClure CK, Van Den Eeden SK, Thom D. Lactation and maternal risk of type 2 diabetes: a population-based study. Am J Med. 2010;123:863.e1-863.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Rajaei S, Rigdon J, Crowe S, Tremmel J, Tsai S, Assimes TL. Breastfeeding Duration and the Risk of Coronary Artery Disease. J Womens Health (Larchmt). 2019;28:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Wong WW, Hachey DL, Insull W, Opekun AR, Klein PD. Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J Lipid Res. 1993;34:1403-1411. [PubMed] |
| 44. | Devlin AM, Innis SM, Shukin R, Rioux MF. Early diet influences hepatic hydroxymethyl glutaryl coenzyme A reductase and 7alpha-hydroxylase mRNA but not low-density lipoprotein receptor mRNA during development. Metabolism. 1998;47:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 761] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 46. | Martin RM, Ness AR, Gunnell D, Emmett P, Davey Smith G; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? Circulation. 2004;109:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Owen CG, Whincup PH, Gilg JA, Cook DG. Effect of breast feeding in infancy on blood pressure in later life: systematic review and meta-analysis. BMJ. 2003;327:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Forsyth JS, Willatts P, Agostoni C, Bissenden J, Casaer P, Boehm G. Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ. 2003;326:953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Hui LL, Kwok MK, Nelson EAS, Lee SL, Leung GM, Schooling CM. The association of breastfeeding with insulin resistance at 17 years: Prospective observations from Hong Kong's "Children of 1997" birth cohort. Matern Child Nutr. 2018;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet. 2003;361:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 360] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 51. | Metzger MW, McDade TW. Breastfeeding as obesity prevention in the United States: a sibling difference model. Am J Hum Biol. 2010;22:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Dietz WH. Breastfeeding may help prevent childhood overweight. JAMA. 2001;285:2506-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Bell KA, Wagner CL, Feldman HA, Shypailo RJ, Belfort MB. Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr. 2017;106:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 54. | Cheshmeh S, Nachvak SM, Rezvani N, Saber A. Effects of Breastfeeding and Formula Feeding on the Expression Level of FTO, CPT1A and PPAR-α Genes in Healthy Infants. Diabetes Metab Syndr Obes. 2020;13:2227-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Baur LA, O'Connor J, Pan DA, Kriketos AD, Storlien LH. The fatty acid composition of skeletal muscle membrane phospholipid: its relationship with the type of feeding and plasma glucose levels in young children. Metabolism. 1998;47:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Aynsley-Green A. The endocrinology of feeding in the newborn. Baillieres Clin Endocrinol Metab. 1989;3:837-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Lucas A, Sarson DL, Blackburn AM, Adrian TE, Aynsley-Green A, Bloom SR. Breast vs bottle: endocrine responses are different with formula feeding. Lancet. 1980;1:1267-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 150] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Munblit D, Treneva M, Peroni DG, Colicino S, Chow LY, Dissanayeke S, Pampura A, Boner AL, Geddes DT, Boyle RJ, Warner JO. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Niers L, Stasse-Wolthuis M, Rombouts FM, Rijkers GT. Nutritional support for the infant's immune system. Nutr Rev. 2007;65:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 60. | Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 418] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 61. | Rajani PS, Seppo AE, Järvinen KM. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front Pediatr. 2018;6:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Cacho NT, Lawrence RM. Innate Immunity and Breast Milk. Front Immunol. 2017;8:584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 63. | Hou TY, McMurray DN, Chapkin RS. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol. 2016;785:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Kainonen E, Rautava S, Isolauri E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br J Nutr. 2013;109:1962-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Moodley-Govender E, Mulol H, Stauber J, Manary M, Coutsoudis A. Increased Exclusivity of Breastfeeding Associated with Reduced Gut Inflammation in Infants. Breastfeed Med. 2015;10:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | González-Jiménez E, Schmidt-RioValle J, Sinausía L, Perona JS. Association of Exclusive Breastfeeding Duration With Systemic Inflammation Markers in Adolescents: A Cross-Sectional Study. Biol Res Nurs. 2017;19:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | McDade TW, Metzger MW, Chyu L, Duncan GJ, Garfield C, Adam EK. Long-term effects of birth weight and breastfeeding duration on inflammation in early adulthood. Proc Biol Sci. 2014;281:20133116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Waidyatillake NT, Simpson JA, Allen KJ, Lodge CJ, Dharmage SC, Abramson MJ, De Livera AM, Matheson MC, Erbas B, Hill DJ, Lowe AJ. The effect of breastfeeding on lung function at 12 and 18 years: a prospective cohort study. Eur Respir J. 2016;48:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | van Meel ER, de Jong M, Elbert NJ, den Dekker HT, Reiss IK, de Jongste JC, Jaddoe VWV, Duijts L. Duration and exclusiveness of breastfeeding and school-age lung function and asthma. Ann Allergy Asthma Immunol. 2017;119:21-26.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Waidyatillake NT, Allen KJ, Lodge CJ, Dharmage SC, Abramson MJ, Simpson JA, Lowe AJ. The impact of breastfeeding on lung development and function: a systematic review. Expert Rev Clin Immunol. 2013;9:1253-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Tsopmo A. Phytochemicals in Human Milk and Their Potential Antioxidative Protection. Antioxidants (Basel). 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Rossnerova A, Tulupova E, Tabashidze N, Schmuczerova J, Dostal M, Rossner P Jr, Gmuender H, Sram RJ. Factors affecting the 27K DNA methylation pattern in asthmatic and healthy children from locations with various environments. Mutat Res. 2013;741-742:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 73. | Goldfield EC, Richardson MJ, Lee KG, Margetts S. Coordination of sucking, swallowing, and breathing and oxygen saturation during early infant breast-feeding and bottle-feeding. Pediatr Res. 2006;60:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Blesa M, Sullivan G, Anblagan D, Telford EJ, Quigley AJ, Sparrow SA, Serag A, Semple SI, Bastin ME, Boardman JP. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage. 2019;184:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 75. | Zielinska MA, Hamulka J, Grabowicz-Chądrzyńska I, Bryś J, Wesolowska A. Association between Breastmilk LC PUFA, Carotenoids and Psychomotor Development of Exclusively Breastfed Infants. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Li R, Xia W, Zhang Z, Wu K. S100B protein, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in human milk. PLoS One. 2011;6:e21663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LB, Ciappolino V, Agostoni C. DHA Effects in Brain Development and Function. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 78. | Walfisch A, Sermer C, Cressman A, Koren G. Breast milk and cognitive development--the role of confounders: a systematic review. BMJ Open. 2013;3:e003259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Hamosh M, Clary TR, Chernick SS, Scow RO. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970;210:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 199] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Stuebe AM. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin Perinatol. 2015;39:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 81. | Hyatt HW, Zhang Y, Hood WR, Kavazis AN. Lactation has persistent effects on a mother's metabolism and mitochondrial function. Sci Rep. 2017;7:17118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Gunderson EP. Impact of breastfeeding on maternal metabolism: implications for women with gestational diabetes. Curr Diab Rep. 2014;14:460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 84. | Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 85. | Matsunaga T, Kadomatsu Y, Tsukamoto M, Kubo Y, Okada R, Nagayoshi M, Tamura T, Hishida A, Takezaki T, Shimoshikiryo I, Suzuki S, Nakagawa H, Takashima N, Saito Y, Kuriki K, Arisawa K, Katsuura-Kamano S, Kuriyama N, Matsui D, Mikami H, Nakamura Y, Oze I, Ito H, Murata M, Ikezaki H, Nishida Y, Shimanoe C, Takeuchi K, Wakai K. Associations of breastfeeding history with metabolic syndrome and cardiovascular risk factors in community-dwelling parous women: The Japan Multi-Institutional Collaborative Cohort Study. PLoS One. 2022;17:e0262252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Wiklund P, Xu L, Lyytikäinen A, Saltevo J, Wang Q, Völgyi E, Munukka E, Cheng S, Alen M, Keinänen-Kiukaanniemi S. Prolonged breast-feeding protects mothers from later-life obesity and related cardio-metabolic disorders. Public Health Nutr. 2012;15:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Cheshmeh S, Nachvak SM, Hojati N, Elahi N, Heidarzadeh-Esfahani N, Saber A. The effects of breastfeeding and formula feeding on the metabolic factors and the expression level of obesity and diabetes-predisposing genes in healthy infants. Physiol Rep. 2022;10:e15469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Li YY, Xiao R, Li CP, Huangfu J, Mao JF. Increased plasma levels of FABP4 and PTEN is associated with more severe insulin resistance in women with gestational diabetes mellitus. Med Sci Monit. 2015;21:426-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Riancho JA, Vázquez L, García-Pérez MA, Sainz J, Olmos JM, Hernández JL, Pérez-López J, Amado JA, Zarrabeitia MT, Cano A, Rodríguez-Rey JC. Association of ACACB polymorphisms with obesity and diabetes. Mol Genet Metab. 2011;104:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Verier C, Meirhaeghe A, Bokor S, Breidenassel C, Manios Y, Molnár D, Artero EG, Nova E, De Henauw S, Moreno LA, Amouyel P, Labayen I, Bevilacqua N, Turck D, Béghin L, Dallongeville J, Gottrand F; Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Study Group. Breast-feeding modulates the influence of the peroxisome proliferator-activated receptor-gamma (PPARG2) Pro12Ala polymorphism on adiposity in adolescents: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) cross-sectional study. Diabetes Care. 2010;33:190-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Berlanga-Macías C, Sánchez-López M, Solera-Martínez M, Díez-Fernández A, Ballesteros-Yáñez I, Castillo-Sarmiento CA, Martínez-Ortega IA, Martínez-Vizcaíno V. Relationship between exclusive breastfeeding and brain-derived neurotrophic factor in children. PLoS One. 2021;16:e0248023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 92. | Hellmuth C, Uhl O, Demmelmair H, Grunewald M, Auricchio R, Castillejo G, Korponay-Szabo IR, Polanco I, Roca M, Vriezinga SL, Werkstetter KJ, Koletzko B, Mearin ML, Kirchberg FF. The impact of human breast milk components on the infant metabolism. PLoS One. 2018;13:e0197713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Martin FP, Moco S, Montoliu I, Collino S, Da Silva L, Rezzi S, Prieto R, Kussmann M, Inostroza J, Steenhout P. Impact of breast-feeding and high- and low-protein formula on the metabolism and growth of infants from overweight and obese mothers. Pediatr Res. 2014;75:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Grinman DY, Careaga VP, Wellberg EA, Dansey MV, Kordon EC, Anderson SM, Maier MS, Burton G, MacLean PS, Rudolph MC, Pecci A. Liver X receptor-α activation enhances cholesterol secretion in lactating mammary epithelium. Am J Physiol Endocrinol Metab. 2019;316:E1136-E1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, Yanovski JA, El Gharbawy A, Han JC, Tung YC, Hodges JR, Raymond FL, O'rahilly S, Farooqi IS. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366-3371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 96. | Corona LP, Conde WL. The effect of breastfeeding in body composition of young children. J Hum Growth Development. 2013;23:276-281. [DOI] [Full Text] |
| 97. | Martin RM, Patel R, Kramer MS, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer T, Thompson J, Gillman MW, Smith GD, Oken E. Effects of promoting longer-term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster-randomized, controlled trial. Circulation. 2014;129:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3232] [Cited by in RCA: 3217] [Article Influence: 247.5] [Reference Citation Analysis (0)] |
| 99. | Sibiak R, Jankowski M, Gutaj P, Mozdziak P, Kempisty B, Wender-Ożegowska E. Placental Lactogen as a Marker of Maternal Obesity, Diabetes, and Fetal Growth Abnormalities: Current Knowledge and Clinical Perspectives. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 100. | Al-Biltagi M, El Razaky O, El Amrousy D. Cardiac changes in infants of diabetic mothers. World J Diabetes. 2021;12:1233-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (5)] |
| 101. | Hartmann P, Cregan M. Lactogenesis and the effects of insulin-dependent diabetes mellitus and prematurity. J Nutr. 2001;131:3016S-3020S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Arthur PG, Smith M, Hartmann PE. Milk lactose, citrate, and glucose as markers of lactogenesis in normal and diabetic women. J Pediatr Gastroenterol Nutr. 1989;9:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Azulay Chertok IR, Haile ZT, Shuisong N, Kennedy M. Differences in Human Milk Lactose and Citrate Concentrations Based on Gestational Diabetes Status. Breastfeed Med. 2020;15:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Azulay Chertok IR, Haile ZT, Eventov-Friedman S, Silanikove N, Argov-Argaman N. Influence of gestational diabetes mellitus on fatty acid concentrations in human colostrum. Nutrition. 2017;36:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Avellar ACS, Oliveira MN, Caixeta F, Souza RCVE, Teixeira A, Faria AMC, Silveira-Nunes G, Faria ES, Maioli TU. Gestational Diabetes Mellitus Changes Human Colostrum Immune Composition. Front Immunol. 2022;13:910807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 106. | Bitman J, Hamosh M, Hamosh P, Lutes V, Neville MC, Seacat J, Wood DL. Milk composition and volume during the onset of lactation in a diabetic mother. Am J Clin Nutr. 1989;50:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Neubauer SH. Lactation in insulin-dependent diabetes. Prog Food Nutr Sci. 1990;14:333-370. [PubMed] |
| 108. | Klein K, Bancher-Todesca D, Graf T, Garo F, Roth E, Kautzky-Willer A, Worda C. Concentration of free amino acids in human milk of women with gestational diabetes mellitus and healthy women. Breastfeed Med. 2013;8:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Wen L, Wu Y, Yang Y, Han TL, Wang W, Fu H, Zheng Y, Shan T, Chen J, Xu P, Jin H, Lin L, Liu X, Qi H, Tong C, Baker P. Gestational Diabetes Mellitus Changes the Metabolomes of Human Colostrum, Transition Milk and Mature Milk. Med Sci Monit. 2019;25:6128-6152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 110. | Suwaydi MA, Zhou X, Perrella SL, Wlodek ME, Lai CT, Gridneva Z, Geddes DT. The Impact of Gestational Diabetes Mellitus on Human Milk Metabolic Hormones: A Systematic Review. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 111. | Gunderson EP, Hurston SR, Ning X, Lo JC, Crites Y, Walton D, Dewey KG, Azevedo RA, Young S, Fox G, Elmasian CC, Salvador N, Lum M, Sternfeld B, Quesenberry CP Jr; Study of Women, Infant Feeding and Type 2 Diabetes After GDM Pregnancy Investigators. Lactation and Progression to Type 2 Diabetes Mellitus After Gestational Diabetes Mellitus: A Prospective Cohort Study. Ann Intern Med. 2015;163:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 112. | Pathirana MM, Ali A, Lassi ZS, Arstall MA, Roberts CT, Andraweera PH. Protective Influence of Breastfeeding on Cardiovascular Risk Factors in Women With Previous Gestational Diabetes Mellitus and Their Children: A Systematic Review and Meta-Analysis. J Hum Lact. 2022;38:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Ziegler AG, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachmann L, Maier J, Winkler C, Hummel S. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61:3167-3171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 114. | Taylor JS, Kacmar JE, Nothnagle M, Lawrence RA. A systematic review of the literature associating breastfeeding with type 2 diabetes and gestational diabetes. J Am Coll Nutr. 2005;24:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 115. | Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 116. | Touger L, Looker HC, Krakoff J, Lindsay RS, Cook V, Knowler WC. Early growth in offspring of diabetic mothers. Diabetes Care. 2005;28:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. 2002;25:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 118. | Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. 2006;29:2231-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 119. | Ong YY, Pang WW, Huang JY, Aris IM, Sadananthan SA, Tint MT, Yuan WL, Chen LW, Chan YH, Karnani N, Velan SS, Fortier MV, Choo J, Ling LH, Shek L, Tan KH, Gluckman PD, Yap F, Chong YS, Godfrey KM, Chong MF, Chan SY, Eriksson JG, Wlodek ME, Lee YS, Michael N. Breastfeeding may benefit cardiometabolic health of children exposed to increased gestational glycemia in utero. Eur J Nutr. 2022;61:2383-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care. 1998;21:B138-41. [PubMed] |
| 121. | Vandyousefi S, Goran MI, Gunderson EP, Khazaee E, Landry MJ, Ghaddar R, Asigbee FM, Davis JN. Association of breastfeeding and gestational diabetes mellitus with the prevalence of prediabetes and the metabolic syndrome in offspring of Hispanic mothers. Pediatr Obes. 2019;14:e12515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |