Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.299
Peer-review started: October 1, 2022
First decision: December 12, 2022
Revised: December 21, 2022
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 15, 2023
Processing time: 165 Days and 8.1 Hours
The dynamic characteristics of glucose metabolism and its risk factors in patients living with human immunodeficiency virus (PLWH) who accepted primary treatment with the efavirenz (EFV) plus lamivudine (3TC) plus tenofovir (TDF) (EFV + 3TC + TDF) regimen are unclear and warrant investigation.
To study the long-term dynamic characteristics of glucose metabolism and its contributing factors in male PLWH who accepted primary treatment with the EFV + 3TC + TDF regimen for 156 wk.
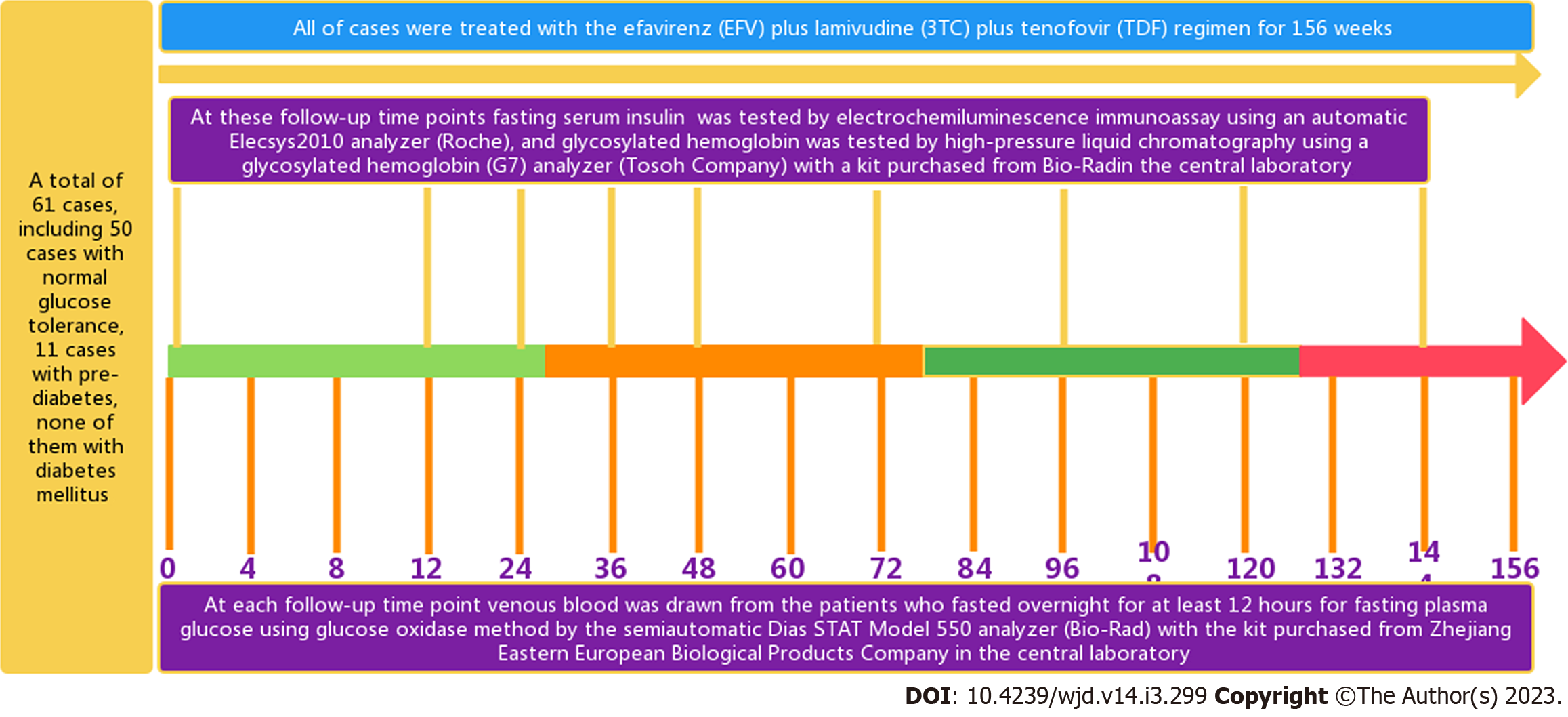
This study was designed using a follow-up design. Sixty-one male treatment-naive PLWH, including 50 cases with normal glucose tolerance and 11 cases with prediabetes, were treated with the EFV + 3TC + TDF regimen for 156 wk. The glucose metabolism dynamic characteristics, the main risk factors and the differences among the three CD4+ count groups were analyzed.
In treatment-naive male PLWH, regardless of whether glucose metabolism disorder was present at baseline, who accepted treatment with the EFV + 3TC + TDF regimen for 156 wk, a continuous increase in the fasting plasma glucose (FPG) level, the rate of impaired fasting glucose (IFG) and the glycosylated hemoglobin (HbA1c) level were found. These changes were not due to insulin resistance but rather to significantly reduced islet β cell function, according to the homeostasis model assessment of β cell function (HOMA-β). Moreover, the lower the baseline CD4+ T-cell count was, the higher the FPG level and the lower the HOMA-β value. Furthermore, the main risk factors for the FPG levels were the CD3+CD8+ cell count and viral load (VL), and the factors contributing to the HOMA-β values were the alanine aminotransferase level, VL and CD3+CD8+ cell count.
These findings provide guidance to clinicians who are monitoring FPG levels closely and are concerned about IFG and decreased islet β cell function during antiretroviral therapy with the EFV + 3TC + TDF regimen for long-term application.
Core Tip: To our knowledge, this prospective cohort study is the first to report the long-term dynamic effects of the tenofovir plus lamivudine plus efavirenz regimen and the baseline CD4+ T cell count on glucose metabolism in male patients living with human immunodeficiency virus. The result showed that gradual increases in the fasting plasma glucose, impaired fasting glucose rate and glycosylated hemoglobin, due to insulin resistance but rather to significantly reduced islet β cell function, regardless of glucose metabolism disorder or not at baseline. Baseline CD4+ T cell count could impact on fasting plasma glucose and homeostasis model assessment of β cell function.
- Citation: Liu DF, Zhang XY, Zhou RF, Cai L, Yan DM, Lan LJ, He SH, Tang H. Glucose metabolism continuous deteriorating in male patients with human immunodeficiency virus accepted antiretroviral therapy for 156 weeks. World J Diabetes 2023; 14(3): 299-312
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/299.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.299
Patients with human immune deficiency virus (HIV) infection or acquired immune deficiency syndrome (AIDS) have sharply increased in recent years. According to the World Health Organization, since HIV was discovered in 1981, nearly 84.2 million people have been infected worldwide, approximately 40.1 million people have died of AIDS, and 38.4 million people are living with HIV worldwide by the end of 2021. By the end of 2022, there were 1053000 patients living with HIV (PLWH) and 351000 cumulative reported deaths nationwide in China[1].
Antiretroviral therapy (ART) is currently the most effective treatment for AIDS, as it can prolong life expectancy and improve quality of life[2,3]. Studies have shown that a normal life expectancy will be acquired by AIDS patients when their CD4+ count is higher than 350 cells/mm3 and undetectable levels of the viral load (VL) are reached in one year after ART[2,3]. However, non-AIDS-related diseases, such as metabolic abnormalities, osteoporosis and cardiovascular diseases, have become essential factors affecting the prognosis of AIDS patients[4-7].
Disorders of glucose metabolism, including prediabetes and diabetes mellitus (DM), are common metabolic diseases and risk factors for cardiovascular disease. There have been some reports in the literature about the prevalence of abnormal glucose metabolism in patients with HIV/AIDS after ART[8-11]. The most important risk factors for prediabetes and diabetes were a family history of diabetes, aging, Hispanic heritage or black, obesity/overweight, lipodystrophy, central obesity, metabolic syndrome, dyslipidemia, treatment with certain ART regimens, and increased baseline fasting plasma glucose (FPG)[12-18]. No reports about the long-term dynamic characteristics of glucose metabolism after ART with a specific regimen were found in the literature.
As one of the first-line ART programs since the National Twelfth Five-Year Plan in China, the efavirenz (EFV) plus lamivudine (3TC) plus tenofovir (TDF) (EFV + 3TC + TDF) regimen has been used for more than ten years and has a reduced effect on metabolism, and there are few reports about its effect on glucose metabolism in the literature. Our previous study showed that FPG level increased within four weeks and then returned to the baseline level at 12 wk after ART with the EFV + 3TC + TDF regimen, especially in patients with CD4+ counts less than 350 cells/μL. However, the long-term dynamic characteristics of glucose metabolism and its contributing factors in such patients treated with the EFV + 3TC + TDF regimen are unclear and warrant further examination.
A prospective follow-up cohort study for three years was conducted with sixty-one male PLWH who were treatment-naive and visited the Public and Health Clinic Centre of Chengdu from October 1, 2012, to December 31, 2017[19].
The inclusion criteria were as follows: 18-65 years old; either sex; HIV-1 antibody positivity according to an enzyme-linked immunosorbent assay with confirmation by Western blotting; CD4+ T cell count < 500 cells/μL within 30 d before enrollment; voluntary provision of signed informed consent and agreement to receive follow-up; no plan to relocate from the current address during the trial; no DM, FPG < 7.0 mmol/L and glycosylated hemoglobin (HbA1c) < 6.5%; and no ART before the trial[19].
The exclusion criteria were as follows: Opportunistic infections or acute infections, malignant tumors related to AIDS at enrollment; opportunistic infections within 3 mo of enrollment or an unstable condition within 2 wk of enrollment; detection of any of the following: Hemoglobin lower than 9 g/dL, white blood cell count lower than 2000/μL, neutrophil count lower than 1000/μL, platelet count lower than 75000/μL, serum creatinine higher than 1.5 times the upper limit of normal (ULN), alanine aminotransferase (ALT)/alkaline phosphatase (ALP)/aspartate aminotransferase (AST) higher than 3 times the ULN, total bilirubin higher than 2 times the ULN, serum creatine phosphokinase higher than 2 times the ULN, or a creatinine clearance rate lower than 60 mL/min; DM, FPG > 7.0 mmol/L, or HbA1c > 6.5%; current drug use; pregnancy or lactation; severe neurological or mental disease; severe digestive tract ulcers; and a history of alcoholism[19].
The diagnostic criteria for AIDS, impaired FPG (IFG) and DM were obtained from the published guidelines[20,21].
The patients were divided into three groups based on baseline CD4 counts (23, 12, 26 cases in the > 350, 200-350 and < 200 cells/μL groups, respectively)[2,3,20].
At 8:00 am, venous blood was drawn from the patients who fasted overnight for at least 12 h for FPG, fasting serum insulin (FINS), HbA1c, T lymphocyte subsets, and HIV viral nucleic acid. All blood samples were collected and analyzed in the same central laboratory. The glucose oxidase method was used for glucose measurement by the semiautomatic Dias STAT Model 550 analyzer (Bio-Rad) with a kit purchased from Zhejiang Eastern European Biological Products Company. Insulin was tested by electrochemiluminescence immunoassay using an automatic Elecsys2010 analyzer (Roche); HbA1c was tested by high-pressure liquid chromatography using a HbA1c (G7) analyzer (Tosoh Company) with a kit purchased from Bio-Rad. HIV RNA was tested by fluorescence quantitative polymerase chain reaction. A flow cytometer from Beckman Coulter was used to measure the lymphocyte subset parameters (including the CD8+ count, CD4+ count, CD3+ count, CD8+%, CD4+%, and CD3+%). The formulas for calculating the homeostasis model assessment of β cell function (HOMA-β) value and the homeostasis model assessment of insulin resistance (HOMA-IR) value were as follows: HOMA-β = 20 × FINS/(FPG - 3.5) and HOMA-IR = (FPG × FINS)/22.5[22].
Before and after accepting ART for 4, 8, 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144 and 156 wk were the follow-up time points (Figure 1). The FPG levels were measured at each follow-up time point, and the FINS and HbA1c levels were detected at 0, 12, 24, 36, 48, 72, 96, 120 and 144 wk (Figure 1)[20].
Two researchers simultaneously collected, entered and checked the data to ensure data accuracy, authenticity, and integrity.
Patients and the public were not involved in the development of the research questions or in the design of the study. Patients received verbal and written information about the study; however, they were not involved in the recruitment of subjects or the conduct of the study. In addition, the burden of the intervention was assessed by the investigators. After signing an informed consent form, the participants were assessed for eligibility, and data collection was performed. Dissemination of the general results (without personally identifying data) will occur on demand.
GraphPad Prism version 8 (GraphPad Inc. United States) and Social Sciences software version 17.0 (IBM Inc., Armonk, NY, United States) were used. The FPG and FINS levels, HOMA-IR values and HOMA-β values had normal distributions, and the statistical analyses were conducted directly. The natural VL, as indicated by the HIV RNA level, was logarithmically transformed because of abnormal distributions. The measured data are expressed as the mean ± SD for measurement data, and ANOVA was used for multigroup comparisons. SNK analysis was used for further comparison of two groups. Independent-sample t tests were used for comparison of two groups. The comparison for the enumerated data expressed as rates used the chi-square test. A P value < 0.05 was considered statistically significant.
At the Public and Health Clinic Centre of Chengdu from October 1, 2012, to December 31, 2017, sixty-one male PLWH who were treatment-naive were enrolled into three groups based on baseline CD4+ counts (23, 12, 26 cases in the > 350, 200 to 350, and < 200 cells/μL groups, respectively). Of them, 13 were infected by heterosexual contact, 42 were infected by homosexual contact, and 5 were infected by both types of sexual contact. The baseline virological and immunological indicators, glucose metabolism indicators and general information of 61 cases are shown in Table 1[19]. At baseline, the HOMA-β values in the male PLWH were significantly reduced, with an average of 52.37 mIU/mmol. The HOMA-IR values were slightly elevated, and the average FPG, average FINS and average HbA1c levels were normal (Table 1[19]). Of them, the IFG percentage was 18.03% (11/61), and there was no patient with DM.
| Viable | Total (n = 61) | NGT (n = 50) | ||
| mean ± SD or case (%) | Range | mean ± SD or case (%) | Range | |
| Age (yr) | 32.05 ± 8.38 | 20-58 | 31.93 ± 8.59 | 20-60 |
| Infection duration (mo) | 11.16 ± 1.19 | 1-86 | 11.16 ± 1.19 | 1-86 |
| T lymphocyte subsets | ||||
| CD3+ (cells/μL) | 1433.98 ± 595.35 | 470-3074 | 1489.7 ± 603.75 | 561-3202 |
| CD3+CD4+ (cells/μL) | 313.87 ± 118.473 | 54-499 | 324.74 ± 150.37 | 10-833 |
| CD3+CD4+% (%) | 19.78 ± 6.83 | 1.4-43.4 | 20.46 ± 8.25 | 1.4-56.8 |
| CD3+CD8+ (cells/μL) | 1119.70 ± 605.0 | 360-2456 | 1100.70 ± 498.72 | 440-2456 |
| CD3+CD8+% (%) | 69.97 ± 13.80 | 36.1-97.2 | 69.31 ± 14.72 | 35.8-97.2 |
| 1Virus load of HIVRNA | 41772.77 ± 10.38 | 895.0-505987.0 | 91126.14 ± 2.64 | 895-970103 |
| Glycose metabolic parameters | ||||
| FPG (mmol/L) | 5.50 ± 0.508 | 3.90-6.53 | 5.39 ± 0.40 | 3.9-6.0 |
| HbA1c (%) | 5.35 ± 0.34 | 4.5-6.2 | 5.25 ± 0.37 | 4.1-6.2 |
| FINS (mIU/L) | 4.55 ± 3.03 | 0.5-11.09 | 2.39 ± 1.16 | 0.5-10.24 |
| HOMA-IR(mIU × mmol/L2) | 1.30 ± 0.84 | 0.81-2.31 | 1.21 ± 0.17 | 0.81-1.74 |
| HOMA-β (mIU/mmol) | 52.37 ± 36.25 | 14.8-182.46 | 64.10 ± 8.44 | 30.8-182.46 |
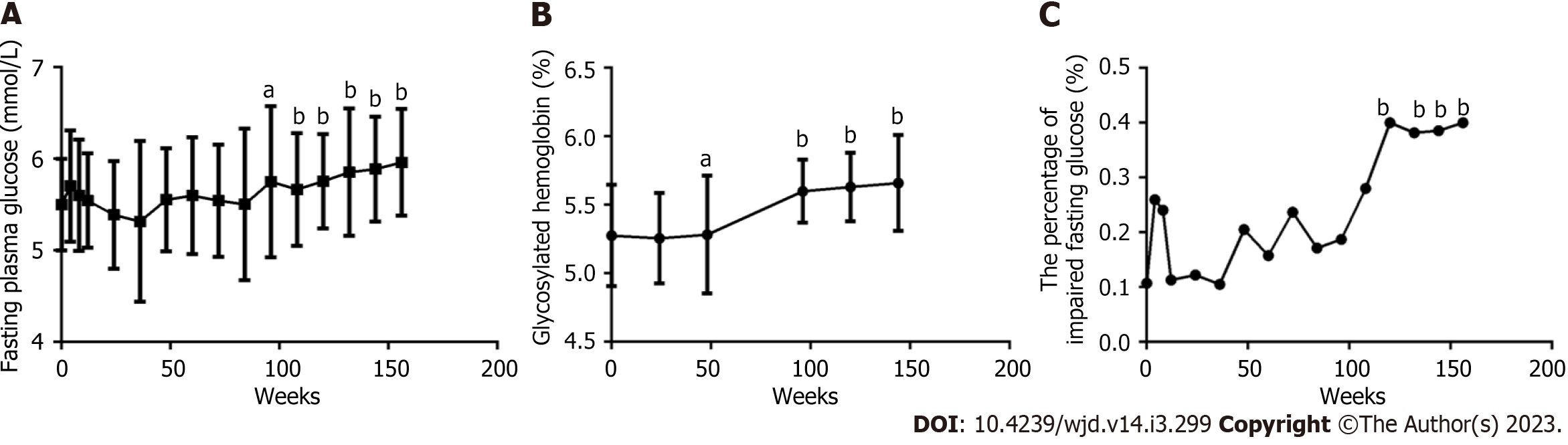
The FPG level in 61 patients (Figure 2A) continuously and gradually increased with prolonged ART, comparing each follow-up time point from 84 wk and at baseline, and a significant difference was found (all P < 0.05), especially at 108, 144 and 156 wk (all P < 0.0001). The HbA1c levels in 61 patients (Figure 2B) slightly decreased over the first 48 wk and then continuously and gradually increased with prolonged ART. A significant difference was found between each follow-up time point from 48 wk and at baseline (all P < 0.05), especially from 84 wk to 156 wk (all P < 0.0001). The percentage of IFG (Figure 2C) initially quickly increased, peaked at 4 wk, gradually fell until the trough was reached at 36 wk, and then increased again until it was measured at 156 wk, especially from 132 wk to 156 wk (P < 0.01). There were seven cases with newly diagnosed DM, including two cases with prediabetes and five cases with normal glucose tolerance (NGT) at baseline, and of them, 1, 1, 2, 1, and 2 cases were diagnosed with DM after 4, 8, 12, 72 and 96 wk, respectively. The patients with prediabetes received diet prescriptions and exercise prescriptions and did not use hypoglycemic drugs. Patients with diabetes received metformin for hypoglycemia with FPG controlled within 7.0 mmol/L and HbA1c controlled within 6.5%.
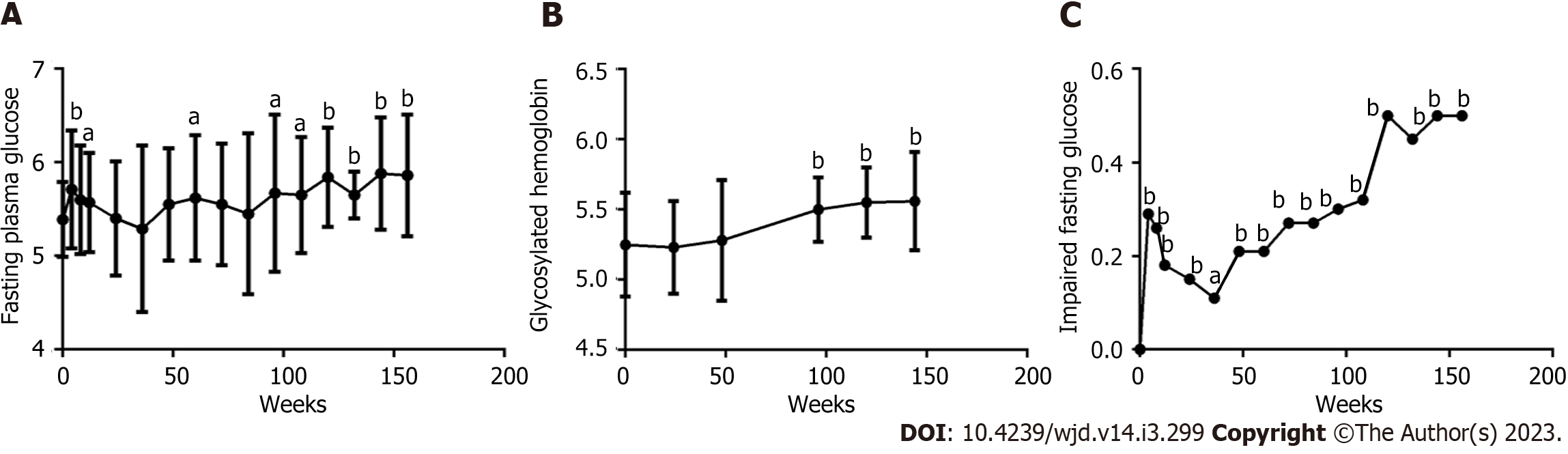
Except for 11 patients with prediabetes, the FPG level in 50 patients with NGT (Figure 3A) also continuously and gradually increased with prolonged ART. A significant difference was found between each follow-up time point from 96 wk and at baseline (all P < 0.05), especially from 120 wk to 156 wk (all P < 0.001). The HbA1c levels in 50 patients with NGT (Figure 3B) also continuously and gradually increased with prolonged ART, comparing each follow-up time point from 96 wk and at baseline, and a significant difference was found (all P < 0.0001). The percentage of IFG (Figure 3C) initially quickly increased at 4 wk, gradually fell until the trough was reached at 36 wk, and then increased again until it was measured at 156 wk, especially from 120 wk to 156 wk (all P < 0.0001).
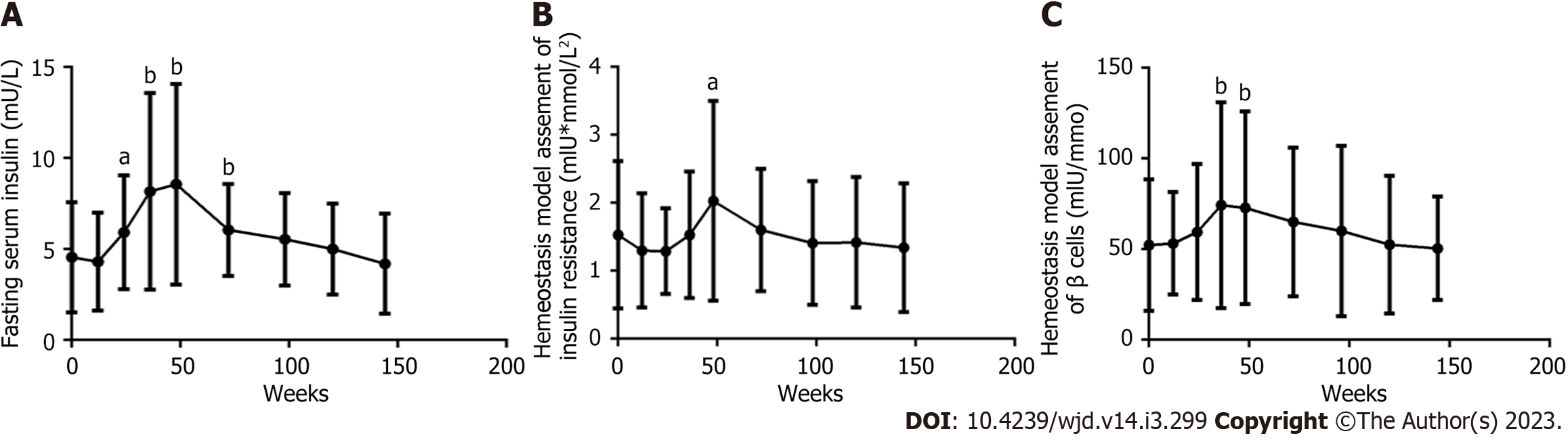
In 61 patients, the FINS level (Figure 4A), HOMA-IR value (Figure 4B), and HOMA-β value (Figure 4C) gradually rose to peak values at 48 wk and then decreased from 48 wk to 156 wk. At each follow-up time point and at baseline, significant differences were found in the FINS level at 24, 36, 48, and 60 wk, in the HOMA-IR value at 48 wk, and in the HOMA-β value at 36 and 48 wk (all P < 0.05).
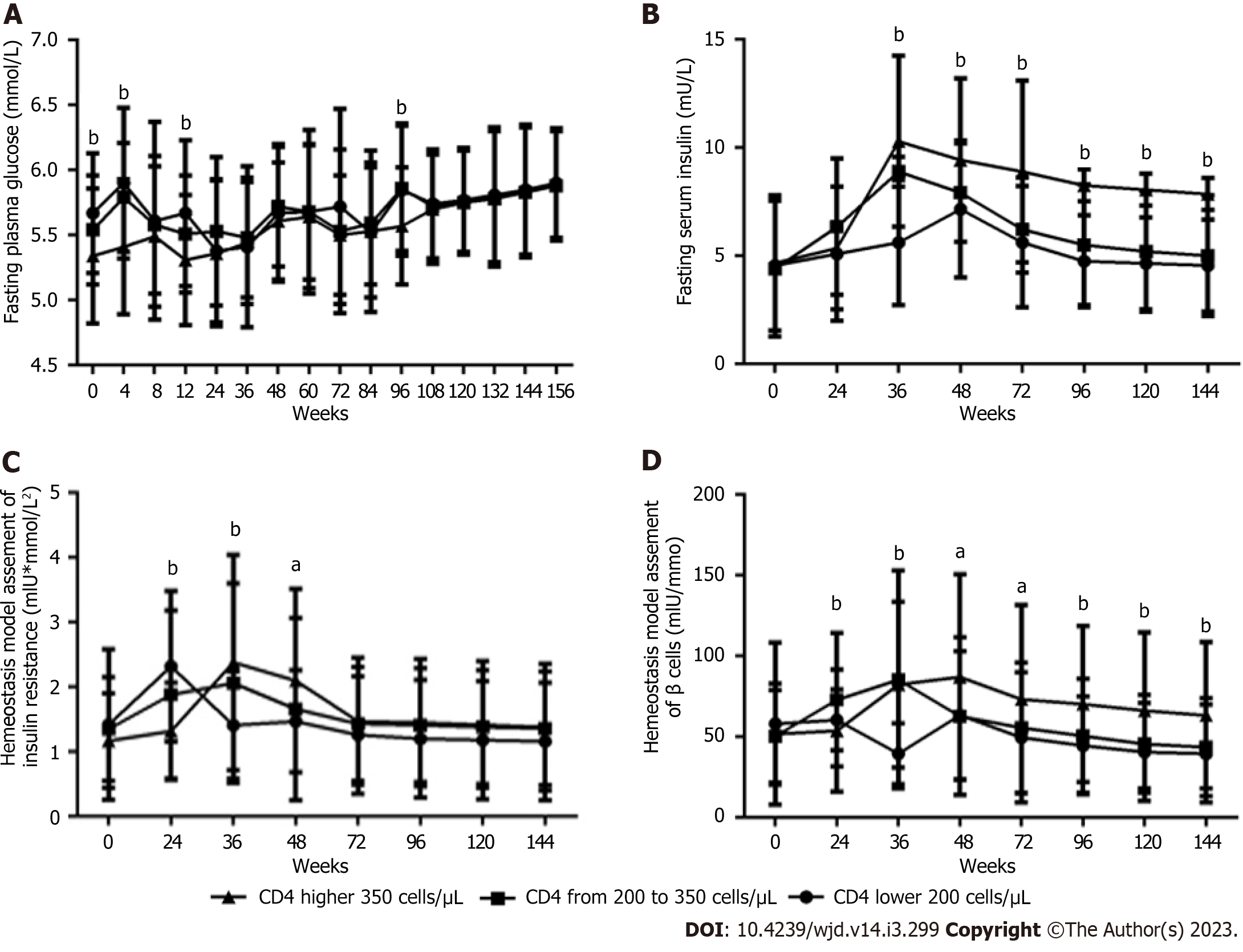
The lower the baseline CD4+ count was, the higher the FPG levels (Figure 5A) at baseline and at 4, 12 and 96 wk (all P < 0.05). In contrast, the lower the baseline CD4+ count, the lower the FINS levels (Figure 5B) from 36 and 144 wk, the HOMA-IR value (Figure 5C) at 36 and 48 wk, and the HOMA-β value (Figure 5D) from 36 wk to 144 wk were (all P < 0.05), but the higher the HOMA-IR value (Figure 5C) and the HOMA-β value (Figure 5D) at 24 wk was (all P < 0.05).
By Spearman correlation analysis, nonalcoholic fatty liver disease (NAFLD), age, body fat percentage, body mass index, lean body mass, body fat, AST level, ALT level, ALP level, γ-glutamyl transpeptidase (GGT) level, CD3+CD8+ percentage and serum creatinine level were all positively correlated, while CD3+CD8+ count, CD3+ count, CD3+ percentage, CD3+CD4+ count, VL and immunoglobulin M level were negatively correlated with FPG level (Table 2). By multiple stepwise regression analysis, the CD3+CD8+ cell count and VL were the main factors associated with the FPG level (Table 3).
| FPG | FINS | HOMA-IR | HOMA-β | |||||
| r | P value | r | P value | r | P value | r | P value | |
| Age (yr) | 0.120 | 0.008 | ||||||
| NAFLD (0 = without, 1 = with) | 0.155 | 0.001 | ||||||
| Follow-up weeks | 0.280 | 0.005 | ||||||
| BMI (kg/m2) | 0.291 | 0.004 | ||||||
| Body fat percentage (%) | 0.311 | 0.002 | ||||||
| Body fat weight (kg) | 0.297 | 0.003 | ||||||
| Lean body mass weight (kg) | 0.138 | 0.008 | ||||||
| ALT (g/L) | 0.126 | 0.006 | 0.221 | 0.031 | ||||
| AST (g/L) | 0.081 | 0.029 | 0.352 | < 0.0001 | ||||
| GGT (g/L) | 0.127 | 0.005 | 0.230 | 0.025 | ||||
| ALP (g/L) | 0.106 | 0.004 | 0.253 | 0.009 | 0.164 | 0.002 | ||
| Cr (μmol/L) | 0.198 | < 0.0001 | 0.378 | 0.027 | ||||
| Cysc (mg/L) | -0.280 | 0.005 | ||||||
| UA (mmol/L) | 0.175 | 0.001 | 0.179 | < 0.0001 | ||||
| CD3+ count (cells/μL) | -0.146 | < 0.0001 | ||||||
| CD4+count (cells/μL) | -0.107 | 0.030 | ||||||
| CD8+count (cells/μL) | -0.120 | 0.002 | -0.236 | 0.022 | -0.121 | 0.026 | ||
| CD3+% | -0.140 | 0.002 | ||||||
| CD4+% | 0.2363 | 0.023 | 0.111 | 0.042 | 0.118 | 0.030 | ||
| CD8+% | 0.134 | 0.007 | -0.293 | 0.004 | ||||
| CD4+/CD8+ | 0.312 | 0.002 | ||||||
| IgM (g/L) | -0.263 | 0.003 | ||||||
| LV (copes/L) | -0.266 | 0.033 | -0.374 | 0.045 | -0.427 | 0.024 | ||
| CD4+ count groups | -0.340 | 0.049 | ||||||
| Independent variable | Dependent variable | B | SE | Beta | t | P value |
| FPG | Constant | 5.416 | 0.233 | - | 23.206 | < 0.0001 |
| VL | -0.040 | 0.016 | -0.269 | -2.470 | 0.015 | |
| CD8+% | 0.008 | 0.004 | 0.241 | 2.215 | 0.029 | |
| FINS | Constant | 83.179 | 27.700 | - | 3.003 | 0.007 |
| ALT | 0.348 | 0.081 | 0.670 | 4.302 | < 0.0001 | |
| VL | -6.654 | 2.375 | -0.734 | -2.801 | 0.011 | |
| Follow-up weeks | -5.951 | 2.196 | -0.711 | -2.710 | 0.013 | |
| CD8+ count (cells/μL) | -0.010 | 0.005 | -0.325 | -2.122 | 0.046 | |
| HOMA-IR | Constant | 18.025 | 7.686 | - | 2.345 | 0.029 |
| ALT | 0.090 | 0.023 | 0.642 | 3.855 | 0.001 | |
| VL | -1.664 | 0.685 | -0.405 | -2.429 | 0.024 | |
| HOMA-β | Constant | 695.830 | 224.509 | - | 3.099 | 0.016 |
| ALT | 2.740 | 0.656 | 0.649 | 4.179 | < 0.0001 | |
| VL | -54.256 | 19.253 | -0.440 | -2.818 | 0.011 | |
| CD8+ count (cells/μL) | -0.090 | 0.039 | -0.345 | -2.271 | 0.034 |
According to Spearman correlation analysis, follow-up duration, ALT level, AST level, GGT level, ALP level, CD3+CD4+ percentage, and the ratio of the CD3+CD4+ count to the CD3+CD8+ count were positively correlated, while the serum cystatin C level, CD3+CD8+ count and percentage, and VL were negatively correlated with the FINS level (Table 2). By multiple stepwise regression analysis, the ALT level, follow-up duration, VL, and CD3+CD8+ count were the main factors associated with the FINS level (Table 3).
According to Spearman correlation analysis, the ALP level, the creatinine level, the uric acid (UA) level and the CD3+CD4+ percentage were positively correlated, while the group stratified by baseline CD3+CD4+ count and VL were negatively correlated with the HOMA-IR values (Table 2). By multiple stepwise regression analysis, the ALT level and VL were the main factors associated with the HOMA-IR value (Table 3).
According to Spearman correlation analysis, only the UA level and CD3+CD4+ percentage were positively correlated with the HOMA-β value, and none of the anthropometric parameters or biochemical, immunological and virological indicators were negatively correlated with the HOMA-β value. By multiple stepwise regression analysis, the ALT level, VL and CD3+CD8+ count were the main factors associated with the HOMA-β value (Table 3).
Our previous studies found that islet β cell dysfunction is common even in young male PLWH with normal weight and NGT and without significant IR. The EFV + 3TC + TDF regimen can lead to glucose impairment in the short term[23]. The purpose of this study was to identify the dynamic characteristics of glucose metabolism and its contributing factors after ART. We found that in male PLWH after ART, glucose metabolism continuously deteriorated, as indicated by the gradual increase in the FPG level, whereas the IFG rate increased rapidly, followed by a gradual decrease and then another gradual increase. The average FPG level of 61 cases was 5.50 mmol/L at baseline and increased to 5.88 mmol/L at 156 wk. The HbA1c level was 5.35% at baseline and gradually increased to 5.90% at 156 wk, but the percentage of IFG was 18.03% at baseline and volatically increased to 45% at 156 wk. Of 50 patients with NGT, the average FPG level was 5.39 mmol/L at baseline and increased to 5.86 mmol/L at 156 wk, the HbA1c level was 5.25% at baseline and gradually increased to 5.56% at 156 wk, and the percentage of IFG was 0.00% at baseline and volatically increased to 45% at 156 wk. There were seven patients with newly diagnosed DM within 96 wk, and the prevalence of newly diagnosed DM was 11.47% (7/61) in this cohort, slightly higher than the 11.2% overall prevalence in the general population in China[21]. However, the proportion of PLWH with prediabetes who progressed to diabetes reached 63.63% within two years, and the progress rate was 31.815% annually, higher than the literature reporting that 25.00% of individuals with prediabetes progressed to type 2 DM in three to five years[23]. These changes reflected the continuous deterioration of glucose metabolism in treatment-naive male patients with human immunodeficiency virus after treatment with the TDF plus lamivudine plus EFV regimen, especially after 96 wk, regardless of whether glucose metabolism disorder was present at baseline.
There have been some reports in the literature about the prevalence of abnormal glucose metabolism in patients with HIV/AIDS after ART. There have been no reports about the long-term dynamics in glucose metabolism after ART with a specific regimen. One cross-sectional study on DM among ART-naive PLWH in Guinea-Bissau showed that the prevalence of DM was 5.8% (52/893) and the prevalence of IFG was 5.6% (50/893)[8]. Another cross-sectional study of risk factors for IFG or DM among PLWH in Zambia receiving long-term combined antiretroviral treatment showed that ten percent (26/270) had IFG and 5% (14/270) had DM[9]. A large-sample epidemiological study showed that 24.8% of 262 PLWH and 38.2% of 1583 PLWH receiving ART treatment had elevated FPG levels[10,11] and that 2.1% of 1095 PLWH and 7.1% of 425 PLWH had DM[24-26]. The annual increase in the prevalence of DM in PLWH was 4.1%, with a 2.27-fold higher prevalence in 2011 than in 1999, while in the non-HIV-positive population, the annual increase was 3.9%, which represented a 1.62-fold increase in the prevalence[27]. Follow-up cohort study results also showed that the crude prevalence of DM ranged from 10.8 to 13.7 per 1000 patients annually[12-14], and the pooled prevalence of prediabetes was 125 per 1000 patients annually[14]. The most important risk factors for prediabetes and diabetes were a family history of diabetes, aging, Hispanic heritage or black, obesity/overweight, lipodystrophy, central obesity, metabolic syndrome, dyslipidemia, treatment with certain ART regimens, and increased baseline FPG[12-18]. No reports about the long-term dynamic characteristics of glucose metabolism after ART with a specific regimen were found in the literature.
In the general population abnormal glucose metabolism is associated with insulin resistance and impaired islet beta cell function. The results showed that in male PLWH, the HOMA-IR value first increased to a peak of 2.03 Um × mmol/L2 at 48 wk and then decreased until it was measured again at 156 wk. The HOMA-IR value was always lower than the reference value of 2.3 Um × mmol/L2, which indicates insulin resistance. The baseline HOMA-β value was 52.37 Um/mmol, which was almost half of the reference value of 100 mIU/mmol, and the peak HOMA-β value was 74.30 Um/mmol at 36 wk, which was also clearly lower than the reference value. Therefore, the continuous deterioration of glucose metabolism was not caused by insulin resistance but rather by significantly decreased islet β cell function.
Our studies have also shown that in male PLWH, the lower the baseline CD4+ count, the lower the HOMA-IR value and HOMA-β value were during the entire 3-year follow-up period. No reports of the impacts of the baseline CD4+ count on the HOMA-IR value and HOMA-β value after long-term ART with a specific regimen were found in the literature.
In this study, it was also demonstrated that age, follow-up duration, NAFLD, most of the biochemical indicators, and the CD3+CD8+ percentage values had positive correlations, while the CD3+CD4+ cell count and virological indicators had negative correlations with the FPG level. The main factors associated with the FPG level were the CD3+CD8+ cell count and VL. Although none of the anthropometric parameters or the biochemical, virological and immunological indicators had a direct correlation with the HOMA-β values representing islet β cell function, the ALT level, VL and CD3+CD8+ cell count were the main factors associated with the HOMA-β value.
Although CD4+ T cells are recognized for assessing immunity, there was an association not between glucose metabolism parameters and CD4+ count but between glucose metabolism parameters and CD8+ count.
A previous study found that CD8+ infiltration into adipose tissue promoted macrophage recruitment[31] and resulted in local TNF-α, other inflammatory mediators and IL-6 increases, which acted on adipocyte surface receptors and by other mechanisms to reduce insulin receptor substrate-1 (IRS-1), glucose transporter type 4 (GLUT4) and phosphoinositide 3-kinase p85α expression, thereby inhibiting insulin signaling[28-33] and leading to islet beta cell dysfunction, insulin resistance, and hyperglycemia.
Some reports have stated that FPG levels are inversely and significantly correlated with NADH dehydrogenase (C1) enzyme activity in oxidative phosphorylation[18]; youth infected with HIV and with IR have lower levels of markers of mitochondrial respiration than those without IR. Mitochondrial respiration dysfunction may contribute to IR in the population[34]. HIV infection can cause insulin sensitivity decline, and the replication of HIV has important effects on accessory proteins (Tat, Vpr) and may also cause insulin resistance. Vpr affects insulin transcription by inhibiting PPAR-gamma activity, whereas Tat activates nuclear factor-kappa B, inhibits insulin receptor signal translocation, and reduces GLUT4 translocation and phosphorylated IRS-1 expression[35], which leads to insulin resistance, islet beta cell dysfunction and elevated plasma glucose levels.
There was a lack of female patients who met the inclusion criteria during the enrollment duration of the study from October 1, 2012, to December 31, 2013. Only sixty-one male PLWH who were treatment-naive were enrolled in this study, and the results were only reported for males.
This study first reports the dynamic effects of the EFV + 3TC + TDF regimen and the baseline CD4+ count on glucose metabolism parameters in male PLWH treated with a specific ART regimen. The biochemical and anthropometric parameters and virological and immunological indicators associated with glucose metabolism parameters were identified. The results showed that in male PLWH initially treated with the EFV + 3TC + TDF regimen for 3 years, regardless of whether glucose metabolism disorder was present at baseline, the FPG level increased continuously and gradually, the percentage of IFG and HbA1c level also increased gradually, there was no obvious insulin resistance, and there was significantly reduced islet β cell function. The main factors associated with the FPG level were the CD3+CD8+ cell count and VL, while those associated with the HOMA-β value were the ALT level, VL and CD3+CD8+ count.
The limitations of this study were small sample size, a single-center cohort study, only male patients and only the examination of the effect of the EFV + 3TC + TDF regimen. A large-sample, multicenter study of both male and female patients treated with additional ART regimens and a randomized controlled clinical trial are needed.
In the future, the application of more accurate and comprehensive glucose monitoring methods, such as glucose tolerance tests at baseline and at certain follow-up time points and a continuous glucose monitoring system[36], are necessary for long-term glucose monitoring to timely detect and intervene in glucose metabolism disorders and improve the prognosis of the disease[37]. The application of more in-depth analytical methods, such as deep Convolutional Neural Networks Model[38,39], is also needed for more accurate analysis of factors affecting glucose metabolism and timely intervention.
These findings provide guidance for clinicians who wish to monitor FPG levels closely and who are concerned about islet β cell dysfunction and IFG rate during long-term ART with the EFV + 3TC + TDF regimen; the focus is on the avoidance of the application of insulin secretagogues and protecting islet β cell function when hypoglycemic therapy is needed.
Antiretroviral therapy (ART) is currently the most effective treatment for acquired immune deficiency syndrome (AIDS), as it can prolong life expectancy and improve quality of life. However, non-AIDS-related diseases, such as metabolic abnormalities, osteoporosis and cardiovascular diseases, have become essential factors affecting the prognosis of AIDS patients. Disorders of glucose metabolism are common metabolic diseases and risk factors for cardiovascular disease. No reports about the long-term dynamic characteristics of glucose metabolism after ART with a specific regimen as the efavirenz (EFV) plus lamivudine (3TC) plus tenofovir (TDF) (EFV + 3TC + TDF) regimen were found in the literature.
As one of the first-line ART programs since the National Twelfth Five-Year Plan in China, the EFV + 3TC + TDF regimen has been used for more than ten years and has a reduced effect on metabolism, and there are few reports about its effect on glucose metabolism in the literature. Our previous study showed that the fasting plasma glucose (FPG) level increased within four weeks and then returned to the baseline level at 12 wk after ART with the EFV + 3TC + TDF regimen, especially in patients with CD4+ counts less than 350 cells/μL. However, the long-term dynamic characteristics of glucose metabolism and its contributing factors in such patients treated with the EFV + 3TC + TDF regimen are unclear and warrant further examination.
This study aimed at the long-term dynamic characteristics of glucose metabolism and its contributing factors in male patients living with human immunodeficiency virus (PLWH) who accepted primary treatment with the EFV + 3TC + TDF regimen for 156 wk.
This study was designed using a follow-up design. Sixty-one male treatment-naïve PLWH, including 50 cases with normal glucose tolerance and 11 cases with prediabetes, were treated with the EFV + 3TC + TDF regimen for 156 wk. The glucose metabolism dynamic characteristics, the main risk factors and the differences among the three CD4+ count groups were analyzed.
In treatment-naive male PLWH, regardless of whether glucose metabolism disorder was present at baseline, who accepted treatment with the EFV + 3TC + TDF regimen for 156 wk, a continuous increase in the FPG level, the rate of IFG and the HbA1c level were found. These changes were not due to insulin resistance but rather to significantly reduced islet β cell function, according to HOMA-β. Moreover, the lower the baseline CD4+ T-cell count was, the higher the FPG level and the lower the HOMA-β value. Furthermore, the main risk factors for the FPG levels were the CD3+CD8+ cell count and VL, and the factors contributing to the HOMA-β values were the alanine aminotransferase level, VL and CD3+CD8+ cell count.
These findings provide guidance to clinicians who are monitoring FPG levels closely and are concerned about IFG and decreased islet β cell function during ART with the EFV + 3TC + TDF regimen for long-term application.
To our knowledge, this prospective 3-year follow-up cohort study is the first to report the long-term dynamic effects of the TDF + 3TC + EFV regimen and the baseline CD4+ T cell count on glucose metabolism in male PLWH. The results showed that in male PLWH who were TDF + 3TC + EFV regimen treatment-naïve for 3 years, glucose metabolism continuously deteriorated, as shown by gradual increases in the FPG level, IFG rate and HbA1c level, regardless of whether glucose metabolism disorder was present at baseline, and the change was not due to insulin resistance but rather to significantly reduced islet β cell function. Moreover, the lower the baseline CD4+ T cell count was, the higher the FPG level and the lower the HOMA-β value were. These findings should encourage clinicians to monitor the FPG level closely and to be concerned about the IFG rate and decreased islet β cell function in patients who receive long-term TDF + 3TC + EFV regimen treatment.
We would like to thank Dr. Wang Y in the infectious disease department at the Public and Health Clinic Centre of Chengdu.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abu Yousuf M, Bangladesh; Cigrovski Berkovic M, Croatia S-Editor: Gao CC L-Editor: A P-Editor: Chen YX
| 1. | Tencent. "Fight AIDS, Share Health" -- AIDS prevention publicity in 2022. [cited 2 December 2022]. In: Tencent [Internet]. Available from: https://new.qq.com/rain/a/20221202A0648H00. |
| 2. | Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, Deeks S, Jacobson LP, Bosch RJ, Kitahata MM, Goedert JJ, Moore R, Gange SJ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1010] [Cited by in RCA: 1097] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 3. | May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, Chadwick D, Martin F, Hill T, Walsh J, Post F, Fisher M, Ainsworth J, Jose S, Leen C, Nelson M, Anderson J, Sabin C; UK Collaborative HIV Cohort (UK CHIC) Study. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 4. | Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205 Suppl 3:S383-S390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Paula AA, Schechter M, Tuboi SH, Faulhaber JC, Luz PM, Veloso VG, Moreira RI, Grinsztejn B, Harrison LH, Pacheco AG. Continuous increase of cardiovascular diseases, diabetes, and non-HIV related cancers as causes of death in HIV-infected individuals in Brazil: an analysis of nationwide data. PLoS One. 2014;9:e94636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Muyanja D, Muzoora C, Muyingo A, Muyindike W, Siedner MJ. High Prevalence of Metabolic Syndrome and Cardiovascular Disease Risk Among People with HIV on Stable ART in Southwestern Uganda. AIDS Patient Care STDS. 2016;30:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Steiniche D, Jespersen S, Erikstrup C, Krarup H, Handberg A, Østergaard L, Haraldsdottir T, Medina C, Gomes Correira F, Laursen AL, Bjerregaard-Andersen M, Wejse C, Hønge BL; Bissau HIV Cohort study group. Diabetes mellitus and impaired fasting glucose in ART-naïve patients with HIV-1, HIV-2 and HIV-1/2 dual infection in Guinea-Bissau: a cross-sectional study. Trans R Soc Trop Med Hyg. 2016;110:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Shankalala P, Jacobs C, Bosomprah S, Vinikoor M, Katayamoyo P, Michelo C. Risk factors for impaired fasting glucose or diabetes among HIV infected patients on ART in the Copperbelt Province of Zambia. J Diabetes Metab Disord. 2017;16:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Beraldo RA, Santos APD, Guimarães MP, Vassimon HS, Paula FJA, Machado DRL, Foss-Freitas MC, Navarro AM. Body fat redistribution and changes in lipid and glucose metabolism in people living with HIV/AIDS. Rev Bras Epidemiol. 2017;20:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Alvarez C, Salazar R, Galindez J, Rangel F, Castaãeda ML, Lopardo G, Cuhna CA, Roldan Y, Sussman O, Gutierrez G, Cure-Bolt N, Seas C, Carcamo C, Castrillo M. Metabolic syndrome in HIV-infected patients receiving antiretroviral therapy in Latin America. Braz J Infect Dis. 2010;14:256-263. [PubMed] |
| 12. | Han WM, Jiamsakul A, Kiertiburanakul S, Ng OT, Sim BL, Sun LP, Van Nguyen K, Choi JY, Lee MP, Wong WW, Kamarulzaman A, Kumarasamy N, Zhang F, Tanuma J, Do CD, Chaiwarith R, Merati TP, Yunihastuti E, Pujari S, Ditangco R, Khusuwan S, Ross J, Avihingsanon A; IeDEA Asia-Pacific. Diabetes mellitus burden among people living with HIV from the Asia-Pacific region. J Int AIDS Soc. 2019;22:e25236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Karamchand S, Leisegang R, Schomaker M, Maartens G, Walters L, Hislop M, Dave JA, Levitt NS, Cohen K. Risk Factors for Incident Diabetes in a Cohort Taking First-Line Nonnucleoside Reverse Transcriptase Inhibitor-Based Antiretroviral Therapy. Medicine (Baltimore). 2016;95:e2844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and Risk Factors for Prediabetes and Diabetes Mellitus Among HIV-infected Adults on Antiretroviral Therapy: A Systematic Review and Meta-analysis. Epidemiology. 2018;29:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Haldane V, Legido-Quigley H, Chuah FLH, Sigfrid L, Murphy G, Ong SE, Cervero-Liceras F, Watt N, Balabanova D, Hogarth S, Maimaris W, Buse K, McKee M, Piot P, Perel P. Integrating cardiovascular diseases, hypertension, and diabetes with HIV services: a systematic review. AIDS Care. 2018;30:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, Cooper DA, Emery S; INITIO Trial International Coordinating Committee. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21:2445-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Idiculla J, Ravindra'n GD, D'Souza J, Singh G, Furruqh S. Diabetes mellitus, insulin resistance, and metabolic syndrome in HIV-positive patients in South India. Int J Gen Med. 2011;4:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Sharma TS, Jacobson DL, Anderson L, Gerschenson M, Van Dyke RB, McFarland EJ, Miller TL; Pediatric HIV/AIDS Cohort Study (PHACS). Short communication: The relationship between mitochondrial dysfunction and insulin resistance in HIV-infected children receiving antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Liu D, Zhang X, Kang J, Gao F, He Y, He S. Gradual increasing dyslipidemia in treatment-naive male patients with human immunodeficiency virus and treated with tenofovir plus lamivudine plus efavirenz for 3 years. Diabetol Metab Syndr. 2021;13:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 20. | AIDS Group; Society of Infectious Diseases; Chinese Medical Association. [Third Edition of AIDS Diagnosis and Treatment Guidelines (2015 Edition)]. Zhonghua Linchuang Ganran Bing Zazhi. 2015;8:385-401. |
| 21. | Diabetes Society of Chinese Medical Association. Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2013 edition). Zhonghua Tangniaobing Zazhi. 2014;30:893-942. |
| 22. | Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3281] [Cited by in RCA: 3716] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 23. | Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 24. | Mayanja BN, Kasamba I, Levin J, Namakoola I, Kazooba P, Were J, Kaleebu P, Munderi P; CoLTART study team. COHORT PROFILE: The Complications of Long-Term Antiretroviral Therapy study in Uganda (CoLTART), a prospective clinical cohort. AIDS Res Ther. 2017;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Hejazi N, Rajikan R, Choong CL, Sahar S. Metabolic abnormalities in adult HIV infected population on antiretroviral medication in Malaysia: a cross-sectional survey. BMC Public Health. 2013;13:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Ataro Z, Ashenafi W, Fayera J, Abdosh T. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV-positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia. HIV AIDS (Auckl). 2018;10:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, Singano V, Akello H, Joshua M, Nyirenda MJ, Matengeni A, Berman J, Mallewa J, Chinomba GS, Kayange N, Allain TJ, Chan AK, Sodhi SK, van Oosterhout JJ. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health. 2016;16:1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1720] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 29. | Damouche A, Lazure T, Avettand-Fènoël V, Huot N, Dejucq-Rainsford N, Satie AP, Mélard A, David L, Gommet C, Ghosn J, Noel N, Pourcher G, Martinez V, Benoist S, Béréziat V, Cosma A, Favier B, Vaslin B, Rouzioux C, Capeau J, Müller-Trutwin M, Dereuddre-Bosquet N, Le Grand R, Lambotte O, Bourgeois C. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11:e1005153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 30. | Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3164] [Cited by in RCA: 3579] [Article Influence: 198.8] [Reference Citation Analysis (0)] |
| 31. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 3626] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 32. | Gao D, Madi M, Ding C, Fok M, Steele T, Ford C, Hunter L, Bing C. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab. 2014;307:E289-E304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166-E174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Takemoto JK, Miller TL, Wang J, Jacobson DL, Geffner ME, Van Dyke RB, Gerschenson M; Pediatric HIVAIDS Cohort Study. Insulin resistance in HIV-infected youth is associated with decreased mitochondrial respiration. AIDS. 2017;31:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Hester EK. HIV medications: an update and review of metabolic complications. Nutr Clin Pract. 2012;27:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Schierbauer JR, Günther S, Haupt S, Zimmer RT, Zunner BEM, Zimmermann P, Wachsmuth NB, Eckstein ML, Aberer F, Sourij H, Moser O. Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial. Sensors (Basel). 2022;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Xiao F, Zhou YC, Zhang MB, Chen D, Peng SL, Tang HN, Li L, Tang CY, Liu JY, Li B, Zhou HD. Hyperglycemia and blood glucose deterioration are risk factors for severe COVID-19 with diabetes: A two-center cohort study. J Med Virol. 2022;94:1967-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Aurna NF, Yousuf MA, Taher KA, Azad AKM, Moni MA. A classification of MRI brain tumor based on two stage feature level ensemble of deep CNN models. Comput Biol Med. 2022;146:105539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Faruqui N, Yousuf MA, Whaiduzzaman M, Azad AKM, Barros A, Moni MA. LungNet: A hybrid deep-CNN model for lung cancer diagnosis using CT and wearable sensor-based medical IoT data. Comput Biol Med. 2021;139:104961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |