Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.279
Peer-review started: October 27, 2022
First decision: December 12, 2022
Revised: December 21, 2022
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: March 15, 2023
Processing time: 139 Days and 14 Hours
Microalbuminuria is an early and informative marker of diabetic nephropathy. Our study found that microalbuminuria developed in patients with newly diagnosed type 2 diabetes mellitus (T2DM).
To investigate the association between glucagon-like peptide 1 (GLP-1) and microalbuminuria in newly diagnosed T2DM patients.
In total, 760 patients were recruited for this cross-sectional study. The GLP-1 levels during a standard meal test and urinary albumin-creatinine ratio (UACR) were determined.
Patients with microalbuminuria exhibited lower GLP-1 levels at 30 min and 120 min during a standard meal test than patients with normal albuminuria (30 min GLP-1, 16.7 ± 13.3 pmol vs 19.9 ± 15.6 pmol, P = 0.007; 120 min GLP-1, 16.0 ± 14.1 pmol vs 18.4 ± 13.8 pmol, P = 0.037). The corresponding area under the curve for active GLP-1 (AUCGLP-1) was also lower in microalbuminuria patients (2257, 1585 to 3506 vs 2896, 1763 to 4726, pmol × min, P = 0.003). Postprandial GLP-1 levels at 30 min and 120 min and AUCGLP-1 were negatively correlated with the UACR (r = 0.159, r = 0.132, r = 0.206, respectively, P < 0.001). The prevalence of microalbuminuria in patients with newly diagnosed T2DM was 21.7%, which decreased with increasing quartiles of AUCGLP-1 levels (27.4%, 25.3%, 18.9% and 15.8%). After logistic regression analysis adjusted for sex, age, hemoglobin A1c, body mass index, systolic blood pressure, estimated glomerular filtration rate, homeostasis model assessment of insulin resistance, AUCglucose and AUCglucagon, patients in quartile 4 of the AUCGLP-1 presented a lower risk of microalbuminuria compared with the patients in quartile 1 (odds ratio = 0.547, 95% confidence interval: 0.325-0.920, P = 0.01). A consistent association was also found between 30 min GLP-1 or 120 min GLP-1 and microalbuminuria.
Postprandial GLP-1 levels were independently associated with microalbuminuria in newly diagnosed Chinese T2DM patients.
Core Tip: The association between the microalbuminuria and glucagon-like peptide 1 (GLP-1) response after a standard meal load in newly diagnosed Chinese type 2 diabetes mellitus patients was identified. Patients with microalbuminuria showed lower postprandial GLP-1 levels than those without microalbuminuria. The prevalence of microalbuminuria decreased with increasing quartiles of 30 min and 120 min and area under the curve for active GLP-1 levels after a standard meal. The highlights of our study are that the patients were newly diagnosed, which excluded the influence of glucose-lowering therapies. Furthermore, we assessed the fasting and postprandial GLP-1 levels in response to a standard meal, not oral glucose. Third, the GLP-1 determined in our study was active GLP-1, not total GLP-1.
- Citation: Song LL, Wang N, Zhang JP, Yu LP, Chen XP, Zhang B, Yang WY. Postprandial glucagon-like peptide 1 secretion is associated with urinary albumin excretion in newly diagnosed type 2 diabetes patients. World J Diabetes 2023; 14(3): 279-289
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/279.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.279
Microalbuminuria, defined as a urine albumin-creatinine ratio (UACR) of 30 to 300 mg/g, is a highly predictive marker of structural damage in the kidneys in the early stages of diabetic nephropathy when the glomerular filtration rate (GFR) is preserved (higher than 60 mL/min)[1]. In fact, microalbuminuria appears as early as the early stage of diabetes and even prediabetes. An increased prevalence of microalbuminuria has been observed in patients with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT). Compared with that in subjects with normal glucose tolerance, urinary albumin excretion is approximately 70% higher in obese subjects with IFG or IGT[2]. A German study reported that the prevalence of microalbuminuria in individuals with isolated IFG, isolated IGT, IFG + IGT and unknown type 2 diabetes mellitus (T2DM) was 5.3%, 9.7%, 5.8% and 13.2%, respectively[3]. The presence of microalbuminuria is associated with atherosclerotic vascular disease, cardiovascular events, ischemic stroke and premature mortality in both individuals with or without diabetes[4-7].
Multiple mechanisms are involved in the increase in glomerular basement membrane permeability, resulting in increased urinary albumin excretion[8,9]. It has been reported that endocrine hormones also participate in the pathogenesis of microalbuminuria[10-13]. The development of T2DM is accompanied by disordered secretion of endocrine hormones, such as insulin, incretins, glucagon, and leptin. Glucagon-like peptide 1 (GLP-1) has been reported to be an important hormone that regulates nutrition metabolism. Impairment in GLP-1 secretion is associated with abnormally elevated blood glucose levels and increased body weights. Decreased GLP-1 secretion not only accounts for diabetes development but also may take part in the development and progression of related microvascular complications.
However, there is a lack of evidence on the associations of active GLP-1 levels and GLP-1 response to a meal with microalbuminuria in T2DM patients. Newly diagnosed T2DM patients are good subjects for risk factor studies of microalbuminuria because the influence of glucose-lowering therapy is avoided and the impact of disease duration is minimized. In this cross-sectional study, we investigated the association of fasting and postprandial plasma GLP-1 levels with microalbuminuria in patients newly diagnosed with T2DM.
For this multicenter study, patients were recruited from 11 clinical centers. All patients had been diagnosed with T2DM within the past 12 mo. The major inclusion criteria included: Met World Health Organization 1999 T2DM diagnostic criteria; aged between 18 and 75 years; and were not treated with antidiabetic medicine or received treatment for less than 30 d and stopped three months before entering this study. The detailed criteria can be found in a previously published article[13]. The study flowchart is displayed in Supplementary Figure 1.
This study was reviewed and approved by China-Japan Friendship Hospital Institutional Review Board (Approval No. 2008-23). All patients provided informed consent prior to study enrollment and the trial was implemented in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. This trial registration was registered at ChiCTR (Registration No. ChiCTR-TRC-08000231).
The general clinical measurements included body weight, height, body mass index (BMI), waist circumference, and systolic/diastolic blood pressure (SBP/DBP). The glucose metabolism variables included hemoglobin A1c (HbA1c), fasting blood glucose (FBG) and postprandial glucose in a standard meal test. The lipid metabolism variables included low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). The indexes of insulin sensitivity and insulin secretion were calculated with the following equations: Homeostasis model assessment of insulin resistance (HOMA-IR) = fasting insulin (FINS) (μIU/mL) × FBG (mmol/L)/22.5; HOMA-B = 20 × FINS (μIU/mL)/[FBG (mmol/L)-3.5]; UACR = urinary albumin (mg/L)/urinary creatinine (g/L).
Levels of glucose, insulin, glucagon and GLP-1 were measured during a standard test at 0 min, 30 min, 120 min and 180 min. The area under the curve (AUC) during a standard meal test was calculated with the following equations: AUCglucose = (glucose0 min + glucose30 min) × 30/2 + (glucose30 min + glucose120 min) × 90/2 + (glucose120 min + glucose180 min) × 60/2; AUCglucagon = (glucagon0 min + glucagon30 min) × 30/2 + (glucagon30 min + glucagon120 min) × 90/2 + (glucagon120 min + glucagon180 min) × 60/2; AUCinsulin = (insulin0 min + insulin30 min) × 30/2 + (insulin30 min + insulin120 min) × 90/2 + (insulin120 min + insulin180 min) × 60/2; AUC for active GLP-1 (AUCGLP-1)= (GLP-10 min + GLP-130 min) × 30/2 + (GLP-130 min + GLP-1120 min) × 90/2 + (GLP-1120 min + GLP-1180 min) × 60/2.
Statistical analysis was performed using SPSS 25.0 software (SPSS Inc., Chicago, IL). Normally distributed variables are expressed as the mean and standard deviation, and the 2-tailed independent-sample t test was used to compare the parameters between patients with microalbuminuria and normal albuminuria. The Kruskal-Wallis test and the chi-squared test were used to compare variables between the two groups. Pearson’s correlation analysis was performed to identify the correlation between hormone levels and UACR. Then, multivariable linear regression analyses were used to detect the mean differences [B; 95% confidence interval (CI)] in natural logarithm of UACR (LnUACR) between patients with different quartiles of postprandial plasma GLP-1 levels, with the first quartile (Q1) set as the reference, to display the degree of influence of post plasma GLP-1 secretion on UACR. We performed multivariate logistic regression analyses to analyze the impact of postprandial GLP-1 levels on the risk of microalbuminuria, shown as the odds ratios [ORs (95%CIs)] for microalbuminuria in different postprandial GLP-1 levels. Confounding variables were adjusted in different models. P values < 0.05 indicated statistically significant differences.
There were 595 participants with a UACR of less than 30 mg/g (78.3%) and 165 with a UACR of 30 mg/g or higher (21.7%). There were no significant differences in age, sex, BMI, waist circumference, HbA1c, TG, HDL-C, LDL-c, estimated GFR (eGFR) or HOMA-β between participants with normal albuminuria and microalbuminuria. SBP and DBP were higher in the microalbuminuria group than in the normal albuminuria group. The calculated HOMA-IR was also higher in the microalbuminuria group (Table 1).
| Variable | Newly diagnosed type 2 diabetes | P value | ||
| Total | UACR < 30 mg/g | UACR ≥ 30 mg/g | ||
| Number | 760 | 595 | 165 | |
| Age, yr | 50.5 ± 9.1 | 50.3 ± 9.1 | 51.1 ± 9.6 | 0.306 |
| Sex, % | 0.234 | |||
| Female | 306 (40.1) | 232 (38.8) | 74 (44.3) | 0.212 |
| BMI, kg/m2 | 25.5 ± 2.6 | 25.4 ± 2.6 | 25.7 ± 2.7 | 0.281 |
| Waist circumference, cm | 89.4 ± 8.4 | 89.2 ± 8.4 | 90.1 ± 8.2 | 0.226 |
| SBP, mmHg | 123.7 ± 13.1 | 122.7 ± 12.8 | 127.2 ± 13.4 | < 0.001 |
| DBP, mmHg | 79.1 ± 8.5 | 78.4 ± 8.4 | 81.3 ± 8.7 | < 0.001 |
| eGFR, mL/min | 105.6 ± 54.8 | 103.5 ± 31.9 | 112.6 ± 98.3 | 0.052 |
| HbA1c, % | 7.5 ± 1.1 | 7.5 ± 1.2 | 7.6 ± 1.3 | 0.804 |
| TG, mmol/L | 2.4 ± 2.4 | 2.3 ± 2.2 | 2.7 ± 2.9 | 0.058 |
| HDL-C, mmol/L | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.812 |
| LDL-C, mmol/L | 3.1 ± 0.9 | 3.0 ± 0.9 | 3.1 ± 1.0 | 0.508 |
| HOMA-IR | 4.0 (2.5-6.3) | 3.8 (2.5-6.1) | 4.9 (3.1-7.4) | < 0.001 |
| HOMA-β | 49.2 (29.0-76.2) | 48.1 (28.1-74.4) | 53.0 (32.9-81.4) | 0.086 |
| LnUACR | 2.34 ± 1.51 | 1.82 ± 1.26 | 4.16 ± 0.65 | < 0.001 |
| RAS inhibitor/RASR blocker use | 34 (4.4) | 25 (4.2) | 9 (5.4) | 0.431 |
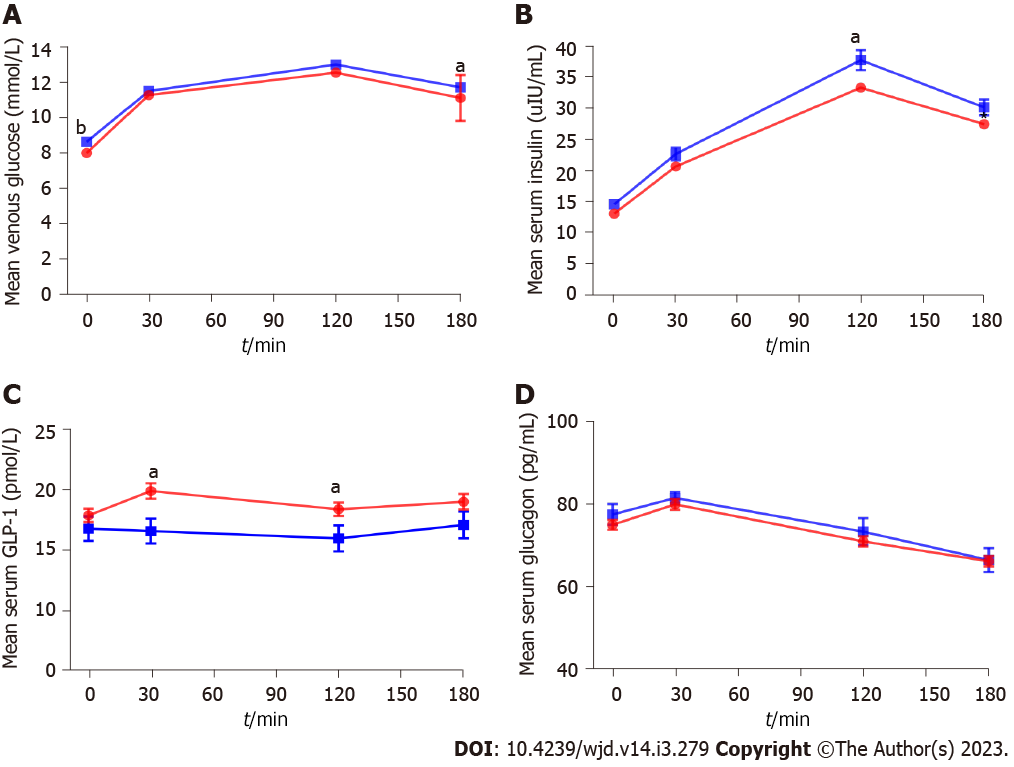
Glucose and hormone responses are shown in Figure 1 and Supplementary Table 1. Fasting and 180 min glucose levels were slightly increased in the microalbuminuria group compared with the normal albuminuria group (8.6 ± 1.4 mmol/L vs 8.3 ± 1.5 mmol/L, P = 0.004; 11.7 ± 2.9 mmol/L vs 11.1 ± 3.1 mmol/L, P = 0.026). FINS, GLP-1 and glucagon were not different between the microalbuminuria group and the normal albuminuria group. For postprandial insulin, the 120 min and 180 min insulin levels were higher in the microalbuminuria group than in the normal albuminuria group (38.0 ± 20.2 vs 33.6 ± 17.9, P = 0.016; 31.5 ± 17.2 μIU/mL vs 28.2 ± 16.5 μIU/mL, P = 0.027). For postprandial GLP-1, the 30 min and 120 min GLP-1 levels were lower in the microalbuminuria group than in the normal albuminuria group (16.7 ± 13.3 pmol vs 19.9 ± 15.6 pmol, P = 0.007; 16.0 ± 14.1 vs 18.4 ± 13.8, P = 0.037). Glucagon levels showed no significant difference at any time point during a standard meal test between the two groups. The AUCglucose was slightly higher in the microalbuminuria group than in the normal albuminuria group (2110, 1852 to 2405 vs 2027, 1767 to 2345 mmol/L × min, P = 0.036), while the AUCGLP-1 was lower in the microalbuminuria group (2257, 1585 to 3506 vs 2896, 1763 to 4726 pmol × min, P = 0.003).
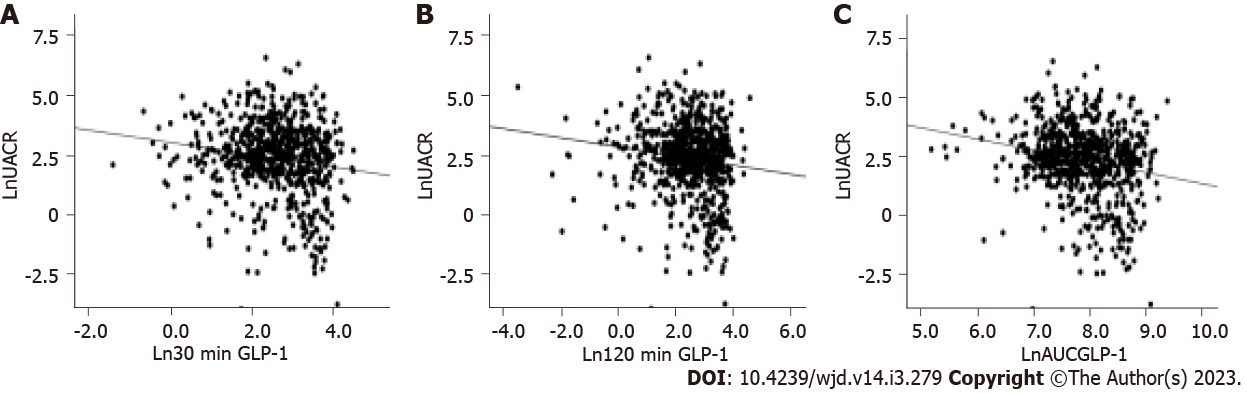
Figure 2 shows the correlations between postprandial GLP-1 levels and UACR, as analyzed by Pearson’s correlation test. Ln30 min GLP-1, Ln120 min GLP-1 and the corresponding LnAUCGLP-1 were negatively correlated with LnUACR: Ln30 min GLP-1 (r = -0.132, P < 0.001), Ln120 min GLP-1 (r = -0.159, P < 0.001) and LnAUCGLP-1 (r = -0.206, P < 0.001). There was no correlation between postprandial insulin or glucagon levels and UACR.
The UACR of the patients in Q4 of postprandial GLP-1 levels was significantly higher than the UACR of the patients in Q1. Since other clinical risk factors were adjusted, the adjusted mean change in the LnUACR of the patients in Q4 vs Q1 of 30 min plasma GLP-1 was -0.708 (95%CI: -1.017 to -0.399). The adjusted mean change in the LnUACR of the patients in Q4 vs Q1 of 120 min plasma GLP-1 was -0.431 (95%CI: -0.744 to -0.119), and the corresponding mean change in the LnUACR of the patients in Q4 vs Q1 of AUCGLP-1 was -0.860 (95%CI: -1.169 to -0.552) (Table 2).
| Variable | Q1 | Q2 | Q3 | Q4 | P value |
| 30 min GLP-1 | 0.24-7.8 | 7.81-14.3 | 14.33-27.24 | 27.31-89.48 | |
| Number | 191 | 196 | 187 | 186 | - |
| LnUACR | 2.58 ± 1.37 | 2.62 ± 1.30 | 2.34 ± 1.47 | 1.85 ± 1.75 | - |
| Model 0 | 0-reference | -0.036 (-0.333 to 0.261) | -0.214 (-0.516 to 0.088) | -0.746 (-1.046 to -0.445)a | < 0.001 |
| Model 1 | 0-reference | -0.022 (-0.314 to 0.271) | -0.213 (-0.510 to 0.084) | -0.772 (-1.069 to -0.476)a | < 0.001 |
| Model 2 | 0-reference | 0.086 (-0.223 to 0.395) | -0.152 (-0.460 to 0.155) | -0.708 (-1.017 to -0.399)a | < 0.001 |
| 120 min GLP-1 | 0.03-7.13 | 7.18-13.6 | 13.61-25.97 | 26.0-98.36 | |
| Number | 194 | 190 | 193 | 183 | - |
| LnUACR | 2.57 ± 1.54 | 2.59 ± 1.22 | 2.23 ± 1.52 | 2.01 ± 1.65 | - |
| Model 0 | 0-reference | -0.030 (-0.272 to 0.331) | -0.330 (-0.632 to -0.028)a | -0.517 (-0.822 to -0.213)a | 0.001 |
| Model 1 | 0-reference | -0.051 (-0.247 to 0.350) | -0.270 (-0.567 to 0.028) | -0.456 (-0/758 to -0.155)a | < 0.001 |
| Model 2 | 0-reference | 0.169 (-0.142 to 0.480) | -0.332 (-0.639 to -0.025)a | -0.431 (-0.744 to -0.119)a | < 0.001 |
| AUCGLP-1 | 175.1-734.2 | 313.7-1736.3 | 2817.0-4454.6 | 4510.2-11877.2 | |
| Number | 193 | 192 | 189 | 186 | - |
| LnUACR | 2.80 ± 1.24 | 2.49 ± 138 | 2.25 ± 1.51 | 1.86 ± 1.72 | - |
| Model 0 | 0-reference | -0.331 (-0.630 to -0.033) | -0.528 (-0.827 to -0.229) | -0.920 (-1.220 to -0.619)a | < 0.001 |
| Model 1 | 0-reference | -0.251 (-0.547 to 0.045) | -0.496 (-0.794 to -0.198)a | -0.869 (-1.168 to -0.569)a | < 0.001 |
| Model 2 | 0-reference | -0.231 (-0.539 to 0.077) | -0.446 (-0.757 to -0.135)a | -0.860 (-1.169 to -0.552)a | < 0.001 |
As shown in Table 3, the prevalence of microalbuminuria in these newly diagnosed T2DM patients was 21.7%, and the prevalence was 27.4%, 25.3%, 18.9% and 15.8% in Q1, Q2, Q3 and Q4 of AUCGLP-1, respectively (P < 0.05). Compared with the patients in Q1 of AUCGLP-1, those in Q4 presented a lower risk of microalbuminuria (OR = 0.498, 95%CI: 0.301 to 0.823, P < 0.01). In logistic regression analysis adjusted for sex, age, HbA1c, BMI, SBP, eGFR, HOMA-IR, AUCglucose and AUCglucagon, the OR for microalbuminuria of patients in Q4 vs those in Q1 of AUCGLP-1 was 0.547 (95%CI: 325 to 0.920, P = 0.01). A consistent association was also found between 30 min GLP-1 or 120 min GLP-1 and microalbuminuria (Table 3).
| Variable | Q1 | Q2 | Q3 | Q4 | P value |
| 30 min GLP-1 | |||||
| Microalbuminuria | 48 (25.4) | 47 (24.6) | 42 (23.2) | 29 (15.3) | |
| Model 0 | 1-reference | 0.959 (0.603-0.525) | 0.828 (0.516-1.330) | 0.529 (0.317-0.884)a | 0.014 |
| Model 1 | 1-reference | 0.962 (0.599-1.543) | 0.817 (0.505-1.322) | 0.517 (0.307-0.873)a | 0.012 |
| Model 2 | 1-reference | 0.967 (0.600-1.557) | 0.826 (0.507-1.346) | 0.534 (0.315-0.905)a | 0.018 |
| 120 min GLP-1 | |||||
| Microalbuminuria | 51 (26.8) | 42 (22.2) | 41 (21.4) | 32 (16.8) | |
| Model 0 | 1-reference | 0.779 (0.487-1.245) | 0.740 (0.642-1.186) | 0.552 (0.336-0.908)a | 0.022 |
| Model 1 | 1-reference | 0.798 (0.495-1.286) | 0.775 (0.480-1.251) | 0.585 (0.353-0.969)a | 0.044 |
| Model 2 | 1-reference | 0.826 (0.508-1.343) | 0.819 (0.504-1.331) | 0.592 (0.355-0.988)a | 0.056 |
| AUCGLP-1 | |||||
| Microalbuminuria | 52 (27.4) | 51 (25.3) | 37 (18.9) | 32 (15.8) | |
| Model 0 | 1-reference | 0.891 (0.568-1.417) | 0.620 (0.383-1.006) | 0.498 (0.301-0.823)a | 0.002 |
| Model 1 | 1-reference | 0.973 (0.610-1.552) | 0.640 (0.391-1.048) | 0.528 (0.316-0.883)a | 0.005 |
| Model 2 | 1-reference | 1.015 (0.632-1.630) | 0.704 (0.426-1.161) | 0.547 (0.325-0.920)a | 0.010 |
In this study, we identified an association between microalbuminuria and GLP-1 response after a standard meal load in newly diagnosed Chinese T2DM patients. Increased GLP-1 levels at 30 min and 120 min and AUCGLP-1 levels in a standard meal test are correlated with decreased UACR. The prevalence of microalbuminuria in patients with newly diagnosed T2DM was 21.7%, which showed a decreasing trend with increasing quartiles of the levels of GLP-1 at 30 min and 120 min and AUCGLP-1 levels. Logistic regression analysis revealed that after adjustment for other confounders, patients in Q4 of postprandial GLP-1 levels exhibited a decreased risk of microalbuminuria compared with those in Q1 by up to approximately 50%. The adjusted microalbuminuria risk for patients from Q4 of 30 min GLP-1 levels was 0.534-fold (95%CI: 0.315 to 0.905). This risk for patients from Q4 of 120 min GLP-1 levels was 0.592-fold (95%CI: 0.355 to 0.988), and this risk for patients from Q4 of AUCGLP-1 levels was 0.547-fold (95%CI: 0.325 to 0.920). In summary, postprandial GLP-1 levels were associated with a decreased risk of microalbuminuria in T2DM patients independent of metabolic indexes, including glucose metabolic status and blood pressure levels. The highlights of our study are that the patients were newly diagnosed, which excluded the influence of glucose-lowering therapies. Furthermore, we assessed the fasting and postprandial GLP-1 levels in response to a standard meal, not oral glucose. Third, the GLP-1 determined in our study was active GLP-1, not total GLP-1.
Evidence has revealed the relationship between GLP-1 and diabetic microvascular complications. Acute (5-d) or early-onset diabetes induces an overexpression of GLP-1, which is believed to be an antioxidant and transiently preserves retinal function in the early stage of diabetes progression[14]. Endogenously increased GLP-1 levels in dipeptidyl peptidase 4-deficient rats attenuated diabetic nephropathy[15]. In our study, a lower postprandial GLP-1 response to a standard meal was associated with a higher microalbuminuria risk. Renoprotective mechanisms of GLP-1 are likely complicated. In animal models, GLP-1 may attenuate renal tubular injury by inhibiting endoplasmic reticulum stress and apoptosis, dampening inflammatory reactions, regulating advanced glycation end product formation and other mechanisms[15-17]. GLP-1 secretion is impaired in patients with abnormal glucose metabolism and body weight gain. In adults and adolescents, impaired GLP-1 secretion may occur early in diabetes development. Compared with that in individuals with nasogastric tube (NGT), the GLP-1 response to an oral glucose tolerance test was lower in patients with prediabetes or T2DM, and this was more pronounced in women[18]. Reduced 120-min GLP-1 concentrations were independent of BMI and age[18]. Adolescents with obesity, IGT and T2DM had lower fasting GLP-1 and glicentin 1 levels than those with NGT[19]. The overall GLP-1 response is also reduced in pregnant women with gestational diabetes mellitus[20]. Lower postprandial GLP-1 levels were independently and significantly associated with liver lipid content[21]. Moreover, the incretin effect, including β-cell responses to GLP-1 and the inhibition of glucagon secretion, was also significantly decreased in T2DM patients. The response of insulin to physiological concentrations of GLP-1 was decreased significantly and even absent in people with impaired oral glucose tolerance, hyperglycemia, and diabetes compared with that in healthy volunteers[22,23]. A decrease in the incretin effect and gastrointestinal-mediated glucose disposal were also observed in women with prior gestational diabetes mellitus and prediabetes. Our study indicated that impaired postprandial GLP-1 secretion may be one of the mechanisms that contributes to microalbuminuria.
Lifestyle intervention is the first step in preventing diabetes and its complications. Compared to the use of GLP-1 agonists, the modification of eating habits has lower costs and fewer adverse reactions, so it is more easily accepted by people at high risk of diabetes or patients with early diabetes. Studies have shown that nutrients enhance GLP-1 secretion, thereby contributing to the prevention and progression of diabetes. Researchers have found that dietary proteins play a key role in triggering the postprandial GLP-1 response in the distal intestine[24]. It was reported that fiber-free feeding for 3 wk markedly reduced the total GLP-1 level by 37% in the ileum and 55% in the colon. It is believed that dietary fiber is necessary to preserve the secretion of incretins by intestinal L cells in mice[25]. Dietary resistant starch intake (4 wk of 40 g/d) significantly increased GLP-1 levels as well as early-phase insulin levels and reduced the intra-abdominal and subcutaneous fat mass. Dietary eriodyctiol modulated the production and release of GLP-1[26]. An increase in plasma GLP-1 levels induced by dietary furocoumarin imperatorin was also found in type 1-like diabetic rats[27]. The speed and sequence of eating also affect GLP-1 secretion. The dietary approach that slows digestion, including the addition of viscous dietary fiber and enzyme inhibitors of phytochemicals into the designed overall food matrix or encapsulation of nutrients, sustains the secretion of GLP-1 after a meal[28]. Intake of protein or glutamine before a carbohydrate or mixed meal can enhance GLP-1 and insulin secretion, delay gastric emptying and improve postprandial blood glucose elevation[29-31]. Mechanisms related to dietary changes in GLP-1 secretion are not very clear. Changing the abundance of intestinal short-chain fatty acids (SCFAs) is probably one of the mechanisms by which diet enhances GLP-1 secretion[32,33]. SCFAs maintain mucosal integrity in the colon, induce L cell numbers and promote the differentiation of L cells, which increase the production of GLP-1[34,35]. This is thought to be mediated through SCFA binding to the free fatty acid receptors 2 and 3 (GPR41 and GPR43) located on L-cells[35]. A dietary fiber-rich diet not only provides raw materials for SCFA production but also improves the ratio of SCFA-producing microbiota.
This study has several limitations. First, it was a cross-sectional study; thus, prospective studies are warranted to confirm that measures that increase postprandial GLP-1 levels, including dietary strategies involving adjusting diet structure and meal sequence, are beneficial for preventing and alleviating diabetic nephropathy by increasing GLP-1 secretion. Second, a mixed meal containing a variety of nutrients may be more likely to mimic the GLP-1 secretion pattern induced by daily diet, but a standard meal test was competent to illustrate the association between postprandial GLP-1 levels and UACR.
In conclusion, our study showed that higher postprandial GLP-1 levels after a standard meal were independently associated with microalbuminuria in newly diagnosed T2DM patients. This finding adds clinical evidence for the renoprotective effect of GLP-1 in newly diagnosed T2DM patients.
The increase in urinary albumin excretion appeared in the early stage of type 2 diabetes mellitus (T2DM) independent of blood glucose and diabetic duration, which suggests that there may be other mechanisms involved in glomerular basement membrane damage during the progression of abnormal glucose metabolism. Identifying related factors and understanding the underlying mechanisms are helpful for the prevention of diabetic nephropathy.
Metabolic hormones have been confirmed to play an important role in the development of diabetes. Evidence that metabolic hormones also have renoprotective effects is needed to develop prevention measures.
This research intends to find the relationship between glucagon-like peptide 1 (GLP-1) secretion and microalbuminuria in untreated new type 2 diabetes patients.
Newly diagnosed T2DM patients were recruited for this cross-sectional study. The urinary albumin-creatinine ratio (UACR) and active GLP-1 levels at 0 min, 30 min, 120 min and 180 min during a standard meal test were determined. We used multivariable linear regression analyses to detect the mean differences [B; 95% confidence interval (CI)] in LnUACR between patients with different quartiles of postprandial plasma GLP-1 levels, with the first quartile (Q1) set as the reference, to display the degree of influence of post plasma GLP-1 secretion on UACR. Multivariate logistic regression analyses were performed to analyze the impact of postprandial GLP-1 levels on the risk of microalbuminuria, which is shown as the odds rations (95%CIs) for microalbuminuria in different postprandial GLP-1 levels.
Ln30 min GLP-1, Ln120 min GLP-1 and the corresponding Ln [area under the curve for active GLP-1 (AUCGLP-1)] were negatively correlated with natural logarithm of UACR . The UACR of the patients in Q4 of postprandial GLP-1 levels was significantly higher than the UACR of the patients in Q1. The prevalence of microalbuminuria decreased with increasing quartiles of 30 min and 120 min and AUCGLP-1 levels. Logistic regression analysis revealed that after adjustment for other confounders, patients in Q4 of postprandial GLP-1 levels exhibited a decreased risk of microalbuminuria compared with those in Q1 by up to approximately 50%. The adjusted microalbuminuria risk for patients from Q4 of AUCGLP-1 levels was 0.547-fold (95%CI: 0.325 to 0.920).
Our study showed for the first time that higher postprandial GLP-1 levels after a standard meal were negatively associated with microalbuminuria in newly diagnosed T2DM patients independent of metabolic status. This finding adds clinical evidence for the renoprotective effect of GLP-1 in newly diagnosed T2DM patients.
Prospective studies should clarify the effect of measures that increase postprandial GLP-1 levels, including dietary strategies involving adjusting diet structure and meal sequence, on preventing and alleviating diabetic nephropathy in the early stage of diabetes.
We thank all investigators for their effort in this clinical trial.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: AASD, CDS_406.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar S, Malaysia; Surani S, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Looker HC, Mauer M, Saulnier PJ, Harder JL, Nair V, Boustany-Kari CM, Guarnieri P, Hill J, Esplin CA, Kretzler M, Nelson RG, Najafian B. Changes in Albuminuria But Not GFR are Associated with Early Changes in Kidney Structure in Type 2 Diabetes. J Am Soc Nephrol. 2019;30:1049-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Tsuda A, Ishimura E, Uedono H, Ochi A, Nakatani S, Morioka T, Mori K, Uchida J, Emoto M, Nakatani T, Inaba M. Association of Albuminuria With Intraglomerular Hydrostatic Pressure and Insulin Resistance in Subjects With Impaired Fasting Glucose and/or Impaired Glucose Tolerance. Diabetes Care. 2018;41:2414-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Kirthi V, Zuckerman BP, Alam U, Bunce C, Hopkins D, Jackson TL. Associations between dysglycemia, retinal neurodegeneration, and microalbuminuria in prediabetes and type 2 diabetes. Retina. 2022;42:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Shin DI, Seung KB, Yoon HE, Hwang BH, Seo SM, Shin SJ, Kim PJ, Chang K, Baek SH. Microalbuminuria is independently associated with arterial stiffness and vascular inflammation but not with carotid intima-media thickness in patients with newly diagnosed type 2 diabetes or essential hypertension. J Korean Med Sci. 2013;28:252-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Currie GE, von Scholten BJ, Mary S, Flores Guerrero JL, Lindhardt M, Reinhard H, Jacobsen PK, Mullen W, Parving HH, Mischak H, Rossing P, Delles C. Urinary proteomics for prediction of mortality in patients with type 2 diabetes and microalbuminuria. Cardiovasc Diabetol. 2018;17:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Cao JJ, Biggs ML, Barzilay J, Konen J, Psaty BM, Kuller L, Bleyer AJ, Olson J, Wexler J, Summerson J, Cushman M. Cardiovascular and mortality risk prediction and stratification using urinary albumin excretion in older adults ages 68-102: the Cardiovascular Health Study. Atherosclerosis. 2008;197:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Elyas S, Shore AC, Kingwell H, Keenan S, Boxall L, Stewart J, James MA, Strain WD. Microalbuminuria could improve risk stratification in patients with TIA and minor stroke. Ann Clin Transl Neurol. 2016;3:678-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol. 2003;23:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Nishad R, Mukhi D, Singh AK, Motrapu M, Chintala K, Tammineni P, Pasupulati AK. Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria. Cell Death Dis. 2021;12:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Nishad R, Mukhi D, Tahaseen SV, Mungamuri SK, Pasupulati AK. Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy. J Biol Chem. 2019;294:16109-16122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Bankir L, Roussel R, Bouby N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am J Physiol Renal Physiol. 2015;309:F2-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Bacci S, De Cosmo S, Garruba M, Placentino G, Liuzzi A, Barbano F, Di Giorgio A, Trischitta V, Viberti GC. Role of insulin-like growth factor (IGF)-1 in the modulation of renal haemodynamics in Type I diabetic patients. Diabetologia. 2000;43:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, Weng J, Jia W, Lu J, Xu Y, Yang Z, Chen W. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2014;2:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 14. | Adeghate JO, D'Souza C, Kántor O, Tariq S, Souid AK, Adeghate E. Early (5-Day) Onset of Diabetes Mellitus Causes Degeneration of Photoreceptor Cells, Overexpression of Incretins, and Increased Cellular Bioenergetics in Rat Retina. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Sarker MK, Lee JH, Lee DH, Chun KH, Jun HS. Attenuation of diabetic kidney injury in DPP4-deficient rats; role of GLP-1 on the suppression of AGE formation by inducing glyoxalase 1. Aging (Albany NY). 2020;12:593-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Hussien NI, Sorour SM, El-Kerdasy HI, Abdelrahman BA. The glucagon-like peptide-1 receptor agonist Exendin-4, ameliorates contrast-induced nephropathy through suppression of oxidative stress, vascular dysfunction and apoptosis independent of glycaemia. Clin Exp Pharmacol Physiol. 2018;45:808-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Færch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Sandbæk A, Holst JJ, Jørgensen ME. GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes. 2015;64:2513-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 19. | Manell H, Staaf J, Manukyan L, Kristinsson H, Cen J, Stenlid R, Ciba I, Forslund A, Bergsten P. Altered Plasma Levels of Glucagon, GLP-1 and Glicentin During OGTT in Adolescents With Obesity and Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Foghsgaard S, Vedtofte L, Andreasen C, Andersen ES, Bahne E, Bagger JI, Svare JA, Holst JJ, Clausen TD, Mathiesen ER, Damm P, Knop FK, Vilsbøll T. Women with prior gestational diabetes mellitus and prediabetes are characterised by a decreased incretin effect. Diabetologia. 2017;60:1344-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Bozzetto L, Annuzzi G, Ragucci M, Di Donato O, Della Pepa G, Della Corte G, Griffo E, Anniballi G, Giacco A, Mancini M, Rivellese AA. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2016;26:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 23. | Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 24. | Hira T, Sekishita M, Hara H. Blood Sampling From Rat Ileal Mesenteric Vein Revealed a Major Role of Dietary Protein in Meal-Induced GLP-1 Response. Front Endocrinol (Lausanne). 2021;12:689685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Hunt JE, Hartmann B, Schoonjans K, Holst JJ, Kissow H. Dietary Fiber Is Essential to Maintain Intestinal Size, L-Cell Secretion, and Intestinal Integrity in Mice. Front Endocrinol (Lausanne). 2021;12:640602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Guo J, Tan L, Kong L. Impact of dietary intake of resistant starch on obesity and associated metabolic profiles in human: a systematic review of the literature. Crit Rev Food Sci Nutr. 2021;61:889-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Wang LY, Cheng KC, Li Y, Niu CS, Cheng JT, Niu HS. The Dietary Furocoumarin Imperatorin Increases Plasma GLP-1 Levels in Type 1-Like Diabetic Rats. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Qin W, Ying W, Hamaker B, Zhang G. Slow digestion-oriented dietary strategy to sustain the secretion of GLP-1 for improved glucose homeostasis. Compr Rev Food Sci Food Saf. 2021;20:5173-5196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Rao M, Zumbro EL, Broughton KS, LeMieux MJ. Whey protein preload enhances the active GLP-1 response and reduces circulating glucose in women with polycystic ovarian syndrome. Nutr Res. 2021;92:84-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Kubota S, Liu Y, Iizuka K, Kuwata H, Seino Y, Yabe D. A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Tricò D, Frascerra S, Baldi S, Mengozzi A, Nesti L, Mari A, Natali A. The insulinotropic effect of a high-protein nutrient preload is mediated by the increase of plasma amino acids in type 2 diabetes. Eur J Nutr. 2019;58:2253-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1535] [Article Influence: 219.3] [Reference Citation Analysis (68)] |
| 33. | De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1611] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 34. | Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, Brown IL, Birkett A, Newman SS, Skaf J, Hegsted M, Tulley RT, Blair E, Zhou J. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: a microarray study. J Nutrigenet Nutrigenomics. 2012;5:26-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Bodinham CL, Al-Mana NM, Smith L, Robertson MD. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br J Nutr. 2013;110:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |