Published online Jul 15, 2022. doi: 10.4239/wjd.v13.i7.566
Peer-review started: December 20, 2021
First decision: April 18, 2022
Revised: April 29, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: July 15, 2022
Processing time: 203 Days and 5.6 Hours
The prevalence of glucolipid metabolic disorders (GLMDs) in children and adolescents has a recognized association with cardiovascular diseases and type 2 diabetes mellitus in adulthood. Therefore, it is important to enhance our under-standing of the risk factors for GLMD in childhood and adolescence.
To explore the relationship between quality of life (QoL) and adolescent GLMD.
This study included 1956 samples in 2019 from a cohort study established in 2014. The QoL scale and glycolipid indexes were collected during follow-up; other covariates of perinatal factors, physical measures, and socioeconomic indicators were collected and adjusted. A generalized linear regression model and logistic regression model were used to analyse the correlation between QoL and GLMD.
Higher scores of QoL activity opportunity, learning ability and attitude, attitude towards doing homework, and living convenience domains correlated negatively with insulin and homeostasis model assessment insulin resistance (IR) levels. Psychosocial factors, QoL satisfaction factors, and total QoL scores had significant protective effects on insulin and IR levels. Activity opportunity, learning ability and attitude, attitude towards doing homework domains of QoL, psychosocial factor, and total score of QoL correlated positively with high density lipoprotein. In addition, the attitude towards doing homework domain was a protective factor for dyslipidaemia, IR > 3, and increased fasting blood glucose; four factors, QoL and total QoL score correlated significantly negatively with IR > 3. In subgroup analyses of sex, more domains of QoL correlated with insulin and triglyceride levels, dyslipidaemia, and IR > 3 in females. Poor QoL was associated with an increased prevalence of GLMD, and the effect was more pronounced in males than in females. Measures to improve the QoL of adolescents are essential to reduce rates of GLMD.
Our study revealed that QoL scores mainly correlate negatively with the prevalence of GLMD in adolescents of the healthy population. The independent relationship between QoL and GLMD can be illustrated by adjusting for multiple covariates that may be associated with glycaemic index. In addition, among females, more QoL domains are associated with glycaemic index.
Core Tip: Persistent abnormalities of glucose and lipid metabolism in childhood have a well-established association with adulthood cardiovascular diseases. Previous conclusions about the association between quality of life (QoL) and glycolipid metabolism disorder (GLMD) were almost all based on adults with type 2 diabetes or dyslipidaemia, whereas there is limited evidence for the association between QoL and GLMD in healthy children and adolescents. This study found that a poor QoL score was associated with increased insulin, triglyceride, and IR levels, and the association was more significant in males than in females. In addition, seven domains, four factors, and total QoL score were negatively associated with abnormalities in glucose and lipid metabolism. Measures to improve the QoL of adolescents are essential to reduce the prevalence of GLMD.
- Citation: Liang XH, Ren YL, Liang XY, Chen JY, Qu P, Tang X. Relationship between quality of life and adolescent glycolipid metabolism disorder: A cohort study. World J Diabetes 2022; 13(7): 566-580
- URL: https://www.wjgnet.com/1948-9358/full/v13/i7/566.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i7.566
The increased prevalence of glucolipid metabolic disorders (GLMDs) in children and adolescents has a well-established association with cardiovascular diseases (CVDs) and type 2 diabetes (T2D) in adulthood[1]. Therefore, it is important to increase our understanding of the risk factors for GLMD during childhood and adolescence. Previous studies have illustrated that risk factors for GLMD in children include unhealthy dietary habits[2], genetic factors[3,4], poor prenatal exposure to high maternal fasting blood glucose (FBG) levels[5], and gestational diabetes. Overall, conclusions about insulin resistance (IR) and quality of life (QoL) are controversial. The results of Schlotz et al[6] showed that IR is associated with lower health-related QoL only in physical health domains[6]. However, a cohort study reported that participants with IR had deteriorated health-related QoL involving physical functioning, emotional role limitations, social functioning, pain, and general health perception, and a more significant correlation was found in males[7]. Several previous studies[8,9] have found that limited trials have reported health-related QoL (HRQoL) in diabetes mellitus, and diabetes affects several dimensions of QoL, such as physical, social well-being, and emotional, compared with a control group[10,11]. Additionally, the primary intervention of pravastatin plus intensive dietary advice might improve the QoL of patients with hyperlipidaemia[12,13]. Several intervention trials[10,14,15] of patients with T2D found disease special-QoL and HRQoL to be improved after treatment, accompanied by decreased FPG, triglyceride (TG), and insulin levels. A systematic review also illustrated that diabetes self-management education may improve the QoL of diabetes by decreasing glycosylated haemoglobin (HbA1c) levels[11]. Therefore, previous conclusions suggest that hyperlipidaemia and impaired fasting glycaemia may impact QoL. Moreover, a study showed that lower QoL impacts the ability to achieve a good HbA1c level[16]. Diverse QoL survey tools have been used in previous studies, with most of these assessment tools being focused on disease-specific QoL[11,17], whereas there are few scales for measuring the global health or general health of healthy subjects[7]. QoL includes multidimensional terms, which represent satisfaction with life status and describe a subject’s functioning in physical, emotional, and social domains. Little evidence about the relationship between QoL and GLMD has been reported, especially in children and adolescents, which is an important stage of growth.
To our knowledge, there are no studies exploring the correlation between QoL and GLMD in healthy children aged 10-14 years from a rural-urban cohort study. The hypothesis of this study is that QoL affect GLMD in children and adolescents. The aim of this cohort study was to explore the correlation of QoL scores with GLMD in adolescents, providing an excellent opportunity to identify independent risk factors for GLMD after adjusting for multiple variables, such as perinatal variables, socioeconomic status (SES), anthropometric measures, and other biochemical indexes.
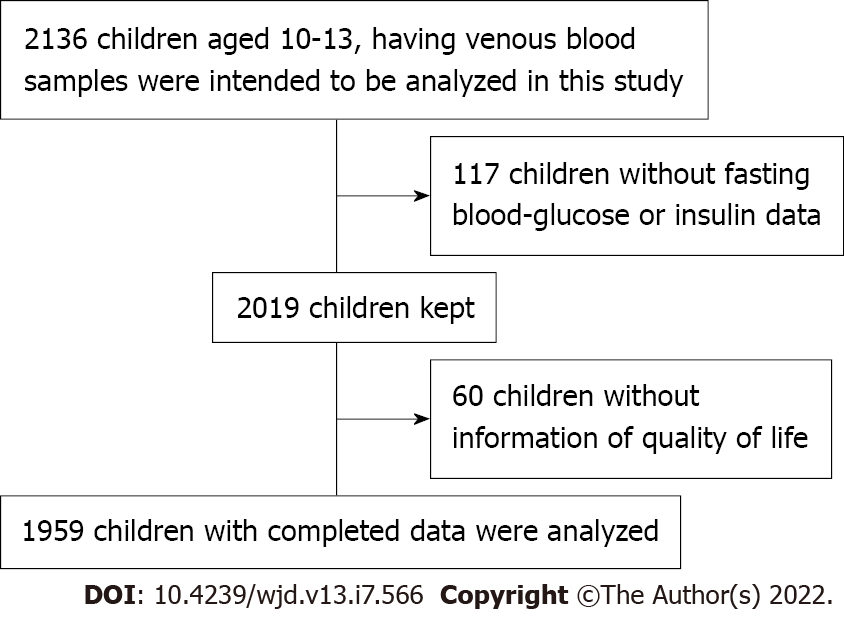
Two-stage stratified cluster sampling was used to select children from urban and rural areas of Chongqing; then, two regions per county were randomly chosen; and finally, all children living in the selected region were informed and included if they were satisfied the inclusion criteria. In addition, a bidirectional cohort in which retrospective and prospective variables were adjusted was used to evaluate the relationship between QoL and GLMD. At baseline, children who met all the following criteria were recruited: (1) Aged 6-9 years in 2014; (2) Resided in the selected areas for more than 6 mo; (3) Did not have serious diseases (e.g., nephropathy, CVD, or cancer); and (4) Consent both from the parents and children for participation. At baseline, all participants in grades 1 and 2 were recruited mainly from two elementary schools. The class head teacher delivered questionnaires to children who signed informed consent forms, and the children took the questionnaires home and completed them with their parents, after which the teacher collected the questionnaires. Two thousand one hundred and thirty-six children with venous blood samples were analysed in this study. After excluding 117 children without FBG or insulin data and 60 children without QoL information, 1959 children with complete data were analysed (Figure 1). This study was approved by the Institutional Review Board of the Children’s Hospital of Chongqing Medical University, and all subjects and their parents/guardians signed informed consent forms.
Demographic information and SES (parents' occupation and education level, and family income) were collected. Bachelor’s and master’s degrees were combined, as there were few parents with the latter. Therefore, parental education level was measured on a four-point scale [≤ 9 years (elementary and middle school), 9-12, 12-15, and > 15 years]. Perinatal variables included maternal obesity and maternal increased weight during pregnancy. Family history of obesity or CVD was investigated using a self-filled questionnaire. In addition, sleeping quality and dietary intake of vegetables, red meat, and salt were surveyed; the detailed protocol was published in a previous paper[18,19].
The questionnaire is valid and reliable, has been used in more than 20000 children, has been modified several times after each survey, and has been described in detail in our previous publications[19-21]. The questionnaire included information on demographics, perinatal status, SES, dietary intake, physical activity, and sleep quality; it was completed both by the children and their parents or guardians according to the protocol.
Anthropometric indexes of height, weight, and waist circumference were measured by well-trained paediatric nurses, and the detailed protocol for these measurements has been introduced in our previous papers[19,22,23].
Venous blood (3 mL) was drawn in the morning after at least 12 h of fasting from subjects who provided informed consent[24]. FBG, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), TGs, fasting insulin (FI), and glycosylated haemoglobin were measured within 2 h after blood draw; details are provided in other publications[19]. In 2019, FI was measured using a Siemens Centaur XP.
The QoL Scale for Children and Adolescents (QLSCA) with 49 items, which is suitable for children aged 7-18 years old, was used to investigate the QoL of children[25]. QLSCA includes four factors and 13 domains, which have been introduced in our previous publication[25]. A four-point scale was used with the QLSCA, with a randomized bidirectional response (positive or negative) item by item to limit the bias of the response. Individual item values were recorded in the same direction prior to analysis. Response values were summed and normalized to the age-, sex-, and urban-rural-specific norms of Chinese individuals into a score ranging from 0-100 (normalized with a mean of 50 and SD of 10), whereby higher scores represent better QoL[26]. The domain scores and factor scores between males and females are displayed in Table 1.
| Variable | Male | Female | F | P value |
| Anthropometric measures | ||||
| Age, yr | 11.21 ± 0.64 | 11.13 ± 0.68 | 7.55 | 0.006 |
| Height, cm | 151.52 ± 8.53 | 152.08 ± 7.30 | 2.41 | 0.121 |
| Weight, kg | 45.31 ± 11.87 | 43.46 ± 10.00 | 13.81 | < 0.001 |
| Waist circumference, cm | 68.17 ± 11.07 | 63.76 ± 8.52 | 96.30 | < 0.001 |
| FBG, mmol/L | 4.49 ± 0.45 | 4.42 ± 0.41 | 14.81 | < 0.001 |
| HbA1c, % | 5.37 ± 0.20 | 5.37 ± 0.19 | 0.04 | 0.843 |
| Insulin, pmol/L | 82.91 ± 81.09 | 85.30 ± 70.01 | 0.48 | 0.486 |
| Insulin resistance index | 2.42 ± 2.65 | 2.41 ± 2.13 | 0.01 | 0.952 |
| Creatinine, mmol/L | 52.86 ± 10.60 | 53.96 ± 28.20 | 1.26 | 0.261 |
| Uric acid, μmol/L | 333.51 ± 83.13 | 305.13 ± 66.72 | 65.16 | < 0.001 |
| TG, mean, mmol/L | 1.04 ± 0.52 | 1.09 ± 0.49 | 4.41 | 0.036 |
| HDL-C, mmol/L | 1.43 ± 0.32 | 1.43 ± 0.30 | 0.00 | 0.994 |
| LDL-C, mmol/L | 1.84 ± 0.43 | 1.84 ± 0.44 | 0.02 | 0.893 |
| Physical activity, min/day | 3.52 ± 0.62 | 3.52 ± 0.57 | 0.01 | 0.939 |
| Sleep score | 45.08 ± 5.85 | 45.97± 6.38 | 10.36 | 0.001 |
| Dietary intake, g/day | ||||
| Cereals and potatoes | 183.48 ± 173.6 | 160.72 ± 164.5 | 8.86 | 0.003 |
| Vegetables | 207.71 ± 197.7 | 216.41 ± 213.8 | 0.88 | 0.349 |
| Red meat | 159.26 ± 199.9 | 152.11 ± 204.2 | 0.61 | 0.434 |
| Nutritional supplements | 20.05 ± 32.04 | 19.29 ± 32.57 | 0.27 | 0.603 |
| Increased BMI during pregnancy, kg/m2 | 1.82 ± 0.75 | 1.82 ± 0.75 | 0.05 | 0.829 |
| 13 domains of QoL | ||||
| Self-satisfaction | 50.83 ± 11.09 | 49.33 ± 11.48 | 8.60 | 0.003 |
| Relationship of teacher and pupil | 53.62 ± 10.20 | 53.81 ± 9.64 | 0.19 | 0.665 |
| Physical feeling | 50.32 ± 10.53 | 49.60 ± 11.00 | 2.16 | 0.142 |
| Companionship | 54.34 ± 9.88 | 53.14 ± 10.96 | 6.49 | 0.011 |
| Parenthood | 52.13 ± 10.83 | 50.73 ± 11.76 | 7.43 | 0.007 |
| Physical activity ability | 50.11 ± 10.96 | 50.13 ± 10.23 | 0.01 | 0.966 |
| Learning ability and attitude | 52.34 ± 9.92 | 51.41 ± 10.33 | 4.14 | 0.042 |
| Self-esteem | 51.18 ± 11.20 | 49.75 ± 10.84 | 8.21 | 0.004 |
| Negative emotion | 48.23 ± 10.79 | 47.14 ± 11.57 | 4.61 | 0.032 |
| Attitude towards doing homework | 51.44 ± 9.23 | 51.59 ± 9.00 | 0.13 | 0.716 |
| Activity opportunity | 54.99 ± 9.40 | 54.39 ± 9.64 | 1.88 | 0.170 |
| Living convenience | 54.41 ± 7.88 | 54.54 ± 7.47 | 0.14 | 0.704 |
| Other | 50.99 ± 10.01 | 50.60 ± 10.13 | 0.71 | 0.401 |
| Four factors of QoL | ||||
| Psychosocial factor | 64.71 ± 10.26 | 65.25 ± 10.17 | 1.36 | 0.244 |
| Physical and mental health factor | 36.00 ± 6.02 | 35.77 ± 5.90 | 0.73 | 0.393 |
| Living environment factor | 24.36 ± 4.20 | 23.73 ± 4.28 | 10.58 | 0.001 |
| Quality of life satisfaction factor | 25.13 ± 4.31 | 24.72 ± 4.42 | 4.38 | 0.037 |
| Mother’s education, yr, n (%) | ||||
| Approximately 9 | 314 (31.15) | 325 (34.17) | 2.35 | 0.308 |
| Approximately 12 | 360 (35.71) | 315 (33.12) | ||
| ≥ 15 | 334 (33.13) | 311 (32.70) | ||
| Father’s education, yr, n (%) | ||||
| Approximately 9 | 277 (27.48) | 250 (26.29) | 6.22 | 0.045 |
| Approximately 12 | 338 (33.53) | 369 (38.80) | ||
| ≥ 15 | 393 (38.99) | 332 (34.91) | ||
| Income, Yuan/year, n (%) | ||||
| Approximately 25000 | 155 (15.38) | 141 (14.83) | 2.74 | 0.741 |
| Approximately 50000 | 166 (16.47) | 168 (17.67) | ||
| Approximately 100000 | 236 (23.41) | 245 (25.76) | ||
| Approximately 150000 | 190 (18.85) | 163 (17.14) | ||
| Approximately 200000 | 117 (11.61) | 106 (11.15) | ||
| > 200000 | 144 (14.29) | 128 (13.46) | ||
| Region | ||||
| Urban, n (%) | 764 (75.79) | 728 (76.55) | 0.16 | 0.694 |
| Rural, n (%) | 244 (24.21) | 223 (23.45) |
Children were diagnosed with increased blood glucose if their FBG was ≥ 5.6 mmol/L[27]. Dyslipidaemia was indicated if any one of the following criteria were met[28]: (1) TC ≥ 200 mg/dL; (2) TG ≥ 130 mg/dL; (3) LDL-C ≥ 130 mg/dL; and (4) HDL-C ≤ 40 mg/dL. Moreover, IR was defined by homeostasis model assessment (HOMA)-IR > 3.0[29], which was calculated by the formula (FI mU/L) × (FBG mmol/L)/22.5. Maternal overweight/obesity was defined as a body mass index greater than 24 kg/m2[30]. Maternal pregnancy weight gain was defined by the guidelines of the institute of medicine[31], as gaining 12.5-18.0 kg, 11.5-16.0 kg, 7.0-11.5 kg, and 5.0-9.0 kg for underweight, normal weight, overweight, and obesity, respectively.
Continuous variables, such as insulin, HOMA-IR, and TG, which did not conform to a normal distribution, were subjected to natural logarithmic transformation before analyses. The relationship between QoL and GLMD was analysed with a generalized linear model. Two models were used to adjust for covariates: Model 1 adjusted for age and sex, and Model 2 adjusted for covariates of height, weight, vegetable intake, red meat intake, salt intake, sleeping quality, father's education, mother's education, household income, urban-rural areas, maternal increased weight during pregnancy, and maternal obesity, which may reflect the independent effect of QoL on blood glucose and lipid indexes. In addition, a logistic regression model was used to analyse the relationship between QoL and GLMD with two models to adjust for covariates.
The results were analysed with SAS 9.4 software (Copyright© 2020 SAS Institute Inc. Cary, NC, United States). An α level of 0.05 was defined as a significant difference.
The general characteristics of the subjects are presented in Table 1. A total of 1956 samples were included. The mean age was 11.21 ± 0.64 years, and 51.53% (1008/1956) were males. The 13 domains, four factors, and total score of QoL, biochemical indexes, and anthropometric, perinatal, and SES variables between males and females are shown in Table 1.
Tables 2 and 3 display the effect of QoL on glycolipid metabolism in adolescents. Adolescents with higher domain scores of living convenience had lower FBG than their counterparts (P < 0.05). TG and HDL-C were higher in adolescents who had a negative attitude towards doing homework (P < 0.05), and the impact of living convenience and attitude towards doing homework on glycolipid indexes (TG and HDL-C) was also significant after adjusting for multiple factors. However, the impact of QoL factors on LDL-C and TC was not significant (P > 0.05) (Supplementary Tables 1 and 2). Levels of insulin and IR were lower in adolescents with a higher factor score of psychosocial, living environment, and QoL satisfaction than their counterparts (P < 0.05). Moreover, adolescents with higher psychosocial factor scores and total QoL scores had decreased TGs and increased HDL-C compared with their counterparts after adjusting for covariates (P < 0.05).
| Variable | FI | IR | TG | HDL | |||||
| β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | ||
| Model 1 (domains of QoL) | Self-satisfaction | -0.399 (-0.696, -0.102) | 0.009 | -0.011 (-0.021, -0.001) | 0.028 | -0.003 (-0.005, -0.001) | 0.004 | 0.000 (-0.001, 0.002) | 0.509 |
| Relationship of teacher and pupil | -0.272 (-0.607, 0.063) | 0.112 | -0.006 (-0.017, 0.005) | 0.252 | -0.004 (-0.006, -0.001) | 0.002 | 0.001 (-0.000, 0.002) | 0.177 | |
| Activity opportunity | -0.452 (-0.761, -0.143) | 0.004 | -0.013 (-0.023, -0.003) | 0.014 | -0.003 (-0.005, -0.000) | 0.017 | 0.001 (-0.000, 0.002) | 0.098 | |
| Physical activity ability | -0.608 (-0.920, -0.295) | < 0.001 | -0.014 (-0.024, -0.004) | 0.008 | -0.004 (-0.007, -0.002) | < 0.001 | 0.002 (0.001, 0.003) | 0.098 | |
| Learning ability and attitude | -0.391 (-0.721, -0.061) | 0.020 | -0.010 (-0.021, 0.001) | 0.075 | -0.002 (-0.004, 0.000) | 0.109 | 0.002 (0.001, 0.003) | 0.002 | |
| Attitude towards doing homework | -0.684 (-1.05, | <0.001 | -0.018 (-0.030, -0.006) | 0.003 | -0.005 (-0.008, -0.003) | < 0.001 | 0.002 (0.000, 0.003) | 0.033 | |
| Living convenience | -0.469 (-0.905, -0.034) | 0.035 | -0.016 (-0.030, -0.001) | 0.030 | -0.003 (-0.005, 0.000) | 0.093 | 0.001 (-0.001, 0.003) | 0.311 | |
| Model 2 (domains of QoL) | Self-satisfaction | -0.352 (-0.641, -0.063) | 0.017 | -0.009 (-0.019, 0.000) | 0.054 | -0.003 (-0.005, -0.001) | 0.006 | 0.001 (-0.001, 0.002) | 0.330 |
| Relationship of teacher and pupil | -0.327 (-0.646, -0.007) | 0.045 | -0.008 (-0.019, 0.002) | 0.127 | -0.003 (-0.006, -0.001) | 0.003 | 0.001 (-0.000, 0.002) | 0.203 | |
| Activity opportunity | -0.421 (-0.719, -0.123) | 0.006 | -0.012 (-0.022, -0.002) | 0.018 | -0.003 (-0.005, -0.001) | 0.011 | 0.001 (0.000, 0.003) | 0.027 | |
| Physical activity ability | -0.394 (-0.696, -0.091) | 0.011 | -0.008 (-0.018, 0.002) | 0.113 | -0.003 (-0.005, -0.001) | 0.005 | 0.001 (-0.000, 0.002) | 0.192 | |
| Learning ability and attitude | -0.442 (-0.758, -0.126) | 0.006 | -0.011 (-0.021, -0.000) | 0.046 | -0.001 (-0.004, 0.001) | 0.190 | 0.002 (0.001, 0.004) | 0.001 | |
| Attitude towards doing homework | -0.720 (-1.07, | < 0.001 | -0.019 (-0.031, -0.008) | 0.001 | -0.005 (-0.008, -0.003) | < 0.001 | 0.002 (0.001, 0.004) | 0.005 | |
| Living convenience | -0.413 (-0.825, -0.001) | 0.049 | -0.014 (-0.028, -0.000) | 0.043 | -0.002 (-0.005, 0.001) | 0.127 | 0.001 (-0.001, 0.003) | 0.186 | |
| Variable | FI | IR | TG | HDL | |||||
| β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | β (95%CI) | P value | ||
| Model 1 (four factors of QoL) | Physical and mental health factor | -0.222 (-0.547, 0.104) | 0.181 | -0.005 (-0.016, 0.006) | 0.364 | -0.003 (-0.005, -0.000) | 0.017 | 0.001 (-0.000, 0.003) | 0.064 |
| Psychosocial factor | -0.835 (-1.390, -0.278) | 0.003 | -0.021 (-0.039, -0.002) | 0.026 | -0.006 (-0.010, -0.002) | 0.002 | 0.002 (-0.000, 0.005) | 0.052 | |
| Living environment factor | -0.821 (-1.610, -0.036) | 0.040 | -0.014 (-0.039, 0.012) | 0.298 | -0.007 (-0.012, -0.002) | 0.012 | 0.004 (0.001, 0.008) | 0.008 | |
| Quality of life satisfaction factor | -0.848 (-1.610, -0.082) | 0.030 | -0.023 (-0.048, 0.002) | 0.074 | -0.006 (-0.011, -0.001) | 0.031 | 0.001 (-0.002, 0.004) | 0.507 | |
| Total score of QoL | -0.440 (-0.710, -0.169) | 0.002 | -0.012 (-0.020, -0.003) | 0.011 | -0.004 (-0.006, -0.002) | < 0.001 | 0.001 (0.000, 0.002) | 0.040 | |
| Model 2 (four factors of QoL) | Physical and mental health factor | -0.272 (-0.588, 0.044) | 0.092 | -0.006 (-0.017, 0.004) | 0.229 | -0.002 (-0.005, -0.000) | 0.039 | 0.001 (-0.000, 0.002) | 0.122 |
| Psychosocial factor | -0.906 (-1.450, -0.367) | 0.001 | -0.023 (-0.041, -0.005) | 0.011 | -0.007 (-0.010, -0.003) | 0.001 | 0.003 (0.001, 0.005) | 0.008 | |
| Living environment factor | -0.916 (-1.690, -0.143) | 0.020 | -0.018 (-0.044, 0.008) | 0.172 | -0.005 (-0.011, 0.000) | 0.067 | 0.003 (-0.001, 0.006) | 0.126 | |
| Quality of life satisfaction factor | -0.965 (-1.710, -0.217) | 0.012 | -0.027 (-0.052, -0.002) | 0.035 | -0.006 (-0.011, -0.001) | 0.027 | 0.002 (-0.001, 0.005) | 0.239 | |
| Total score of QoL | -0.441 (-0.705, -0.177) | 0.001 | -0.011 (-0.020, -0.003) | 0.010 | -0.004 (-0.006, -0.002) | 0.001 | 0.001 (0.000, 0.002) | 0.021 | |
The effect of QoL on glycolipid metabolism indexes by sex is shown in Supplementary Tables. The results in Supplementary Tables 3 and 4 illustrate the relationship between QoL and indexes (FI, IR, TG, and HDL). Scores of attitude towards doing homework and living convenience were negative for FI, IR, and TG (P < 0.05 or P < 0.001), and the association of attitude towards doing homework with IR/TG was also significant after adjusting for covariates in Model 2 (P < 0.05). Moreover, the relationship between total QoL score and FI/TG was negative (P < 0.05). Higher scores of activity opportunity, physical activity ability, learning ability, and attitude and lower levels of FI, TG, and HDL were found for females (P < 0.05); in Model 2, the score of attitude towards doing homework correlated negatively with IR level (P = 0.018).
The results showed that the total score of QoL was a negative factor for FI [β (95%CI): -0.441 (-0.705, -0.177)], IR [β (95%CI): -0.011 (-0.020, -0.003)], and TG [β (95%CI): -0.004 (-0.006, -0.002)] but a positive factor for HDL [β (95%CI): 0.001 (0.000, 0.002)].
In addition, the association between the four factors of QoL and the prevalence of glycolipid metabolism indexes by sex is shown in Supplementary Tables 5 and 6. The relationship between the living convenience score and FBG was negative (P < 0.05). However, significant relationships for females were only found in Model 1.
The results in Tables 4 and 5 indicated the relationship between QoL and GLMD in adolescents. The attitude towards doing homework domain score was a protective factor for dyslipidaemia [OR (95%CI): 0.984 (0.972, 0.995); P = 0.004], and the relationship was significant even after adjusting for covariates [OR (95%CI): 0.982 (0.970, 0.994); P = 0.003]. This relationship was also statistically significant in males [OR (95%CI): 0.983 (0.968, 0.998); P = 0.031]. However, among females, there were other factors of significance, such as relationship of teacher and pupil [OR (95%CI): 0.985 (0.969, 1.001); P = 0.060], activity opportunity [OR (95%CI): 0.984 (0.970, 0.998); P = 0.026], and learning ability and attitude [OR (95%CI): 0.984 (0.969, 1.000); P = 0.046]. After adjusting for covariates in Model 1 and Model 2, self-satisfaction, the relationship of teacher and pupil, activity opportunity, physical activity ability, learning ability and attitude, and attitude towards doing homework were protective factors for IR > 3 in all participants and in females, but only the physical activity ability domain score was significant in males [OR (95%CI): 0.984 (0.970, 0.999); P = 0.037] in Model 1. Attitude towards the homework domain was a protective factor for FBG in Model 2 for all subjects and females (P < 0.05) (Table 4).
| Variable | Dyslipidemia | IR > 3 | Increased FBG | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| Total | |||||||
| Model 1 (domains of QoL) | Self-satisfaction | 0.994 (0.984, 1.003) | 0.177 | 0.987 (0.978, 0.997) | 0.009 | 1.009 (0.991, 1.027) | 0.344 |
| Relationship of teacher and pupil | 0.991 (0.981, 1.002) | 0.095 | 0.987 (0.977, 0.998) | 0.025 | 1.012 (0.991, 1.032) | 0.264 | |
| Activity opportunity | 0.989 (0.980, 0.999) | 0.031 | 0.988 (0.978, 0.998) | 0.023 | 1.006 (0.988, 1.025) | 0.527 | |
| Physical activity ability | 0.988 (0.978, 0.998) | 0.020 | 0.981 (0.971, 0.992) | 0.001 | 1.013 (0.994, 1.031) | 0.184 | |
| Learning ability and attitude | 0.990 (0.979, 1.000) | 0.057 | 0.989 (0.978, 1.000) | 0.047 | 1.003 (0.984, 1.023) | 0.736 | |
| Attitude towards doing homework | 0.984 (0.972, 0.995) | 0.004 | 0.986 (0.974, 0.998) | 0.026 | 0.978 (0.959, 0.997) | 0.022 | |
| Living convenience | 0.997(0.984,1.011) | 0.712 | 0.990(0.976,1.004) | 0.158 | 0.993(0.970,1.018) | 0.587 | |
| Model 2 (domains of QoL) | Self-satisfaction | 0.993 (0.984, 1.003) | 0.183 | 0.986 (0.975, 0.996) | 0.007 | 1.008 (0.989, 1.026) | 0.408 |
| Relationship of teacher and pupil | 0.992 (0.981, 1.003) | 0.154 | 0.984 (0.972, 0.996) | 0.008 | 1.007 (0.987, 1.029) | 0.480 | |
| Activity opportunity | 0.988 (0.978, 0.998) | 0.017 | 0.986 (0.975, 0.997) | 0.016 | 1.007 (0.988, 1.026) | 0.497 | |
| Physical activity ability | 0.994 (0.984, 1.005) | 0.268 | 0.985 (0.973, 0.997) | 0.012 | 1.013 (0.994, 1.033) | 0.184 | |
| Learning ability and attitude | 0.990 (0.979, 1.001) | 0.072 | 0.986 (0.974, 0.998) | 0.018 | 1.003 (0.983, 1.023) | 0.770 | |
| Attitude towards doing homework | 0.982 (0.970, 0.994) | 0.003 | 0.983 (0.971, 0.996) | 0.012 | 0.978 (0.958, 0.997) | 0.024 | |
| Living convenience | 0.997 (0.983, 1.011) | 0.685 | 0.990 (0.975, 1.005) | 0.195 | 0.996 (0.972, 1.021) | 0.765 | |
| Male | |||||||
| Model 1 (domains of QoL) | Self-satisfaction | 0.996 (0.983, 1.009) | 0.538 | 0.990 (0.977, 1.004) | 0.173 | 1.001 (0.980, 1.023) | 0.927 |
| Relationship of teacher and pupil | 0.998 (0.983, 1.012) | 0.759 | 0.994 (0.978, 1.009) | 0.414 | 1.003 (0.979, 1.027) | 0.836 | |
| Activity opportunity | 0.995 (0.981, 1.009) | 0.478 | 0.993 (0.978, 1.008) | 0.370 | 0.999 (0.977, 1.022) | 0.942 | |
| Physical activity ability | 0.993 (0.980, 1.007) | 0.341 | 0.984 (0.970, 0.999) | 0.037 | 1.006 (0.984, 1.028) | 0.623 | |
| Learning ability and attitude | 0.996 (0.981, 1.011) | 0.597 | 0.999 (0.983, 1.016) | 0.915 | 0.992 (0.969, 1.016) | 0.503 | |
| Attitude towards doing homework | 0.983 (0.968, 0.998) | 0.031 | 0.996 (0.979, 1.013) | 0.636 | 0.980 (0.957, 1.004) | 0.102 | |
| Living convenience | 1.008 (0.988, 1.027) | 0.444 | 0.988 (0.968, 1.008) | 0.229 | 0.982 (0.955, 1.010) | 0.211 | |
| Model 2 (domains of QoL) | Self-satisfaction | 0.996 (0.982, 1.010) | 0.570 | 0.991 (0.976, 1.006) | 0.241 | 1.003 (0.981, 1.026) | 0.766 |
| Relationship of teacher and pupil | 0.999 (0.984, 1.014) | 0.888 | 0.991 (0.975, 1.008) | 0.313 | 1.000 (0.976, 1.025) | 0.982 | |
| Activity opportunity | 0.993 (0.978, 1.008) | 0.365 | 0.992 (0.976, 1.008) | 0.332 | 1.004 (0.981, 1.027) | 0.762 | |
| Physical activity ability | 1.002 (0.988, 1.017) | 0.745 | 0.991 (0.975, 1.007) | 0.282 | 1.009 (0.986, 1.033) | 0.438 | |
| Learning ability and attitude | 0.996 (0.981, 1.012) | 0.645 | 0.998 (0.980, 1.015) | 0.793 | 0.994 (0.970, 1.018) | 0.604 | |
| Attitude towards doing homework | 0.980 (0.964, 0.996) | 0.017 | 0.996 (0.977, 1.014) | 0.650 | 0.983 (0.959, 1.008) | 0.180 | |
| Living convenience | 1.013 (0.992, 1.034) | 0.222 | 0.994 (0.973, 1.016) | 0.603 | 0.987 (0.958, 1.016) | 0.363 | |
| Female | |||||||
| Model 1 (domains of QoL) | Self-satisfaction | 0.991 (0.978, 1.004) | 0.195 | 0.985 (0.972, 0.998) | 0.022 | 1.024 (0.992, 1.056) | 0.146 |
| Relationship of teacher and pupil | 0.983 (0.968, 0.999) | 0.035 | 0.980 (0.965, 0.996) | 0.012 | 1.029 (0.991, 1.068) | 0.139 | |
| Activity opportunity | 0.984 (0.971, 0.998) | 0.021 | 0.984 (0.970, 0.998) | 0.021 | 1.017 (0.986, 1.050) | 0.292 | |
| Physical activity ability | 0.982 (0.967, 0.997) | 0.016 | 0.977 (0.962, 0.993) | 0.004 | 1.025 (0.993, 1.058) | 0.133 | |
| Learning ability and attitude | 0.984 (0.969, 0.999) | 0.032 | 0.980 (0.965, 0.995) | 0.009 | 1.023 (0.991, 1.057) | 0.164 | |
| Attitude towards doing homework | 0.984 (0.968, 1.001) | 0.062 | 0.977 (0.960, 0.994) | 0.007 | 0.972 (0.942, 1.004) | 0.084 | |
| Living convenience | 0.986 (0.967, 1.006) | 0.174 | 0.991 (0.971, 1.012) | 0.387 | 1.018 (0.972, 1.067) | 0.448 | |
| Model 2 (domains of QoL) | Self-satisfaction | 0.991 (0.978, 1.005) | 0.230 | 0.981 (0.966, 0.996) | 0.011 | 1.014 (0.981, 1.049) | 0.404 |
| Relationship of teacher and pupil | 0.985 (0.969, 1.001) | 0.060 | 0.975 (0.958, 0.992) | 0.005 | 1.017 (0.977, 1.058) | 0.420 | |
| Activity opportunity | 0.984 (0.970, 0.998) | 0.026 | 0.982 (0.967, 0.997) | 0.022 | 1.009 (0.977, 1.043) | 0.573 | |
| Physical activity ability | 0.986 (0.971, 1.002) | 0.097 | 0.976 (0.959, 0.994) | 0.007 | 1.019 (0.983, 1.056) | 0.307 | |
| Learning ability and attitude | 0.984 (0.969, 1.000) | 0.046 | 0.974 (0.958, 0.991) | 0.003 | 1.016 (0.982, 1.051) | 0.362 | |
| Attitude towards doing homework | 0.983 (0.966, 1.000) | 0.052 | 0.972 (0.954, 0.991) | 0.003 | 0.965 (0.934, 0.997) | 0.031 | |
| Living convenience | 0.983 (0.963, 1.004) | 0.106 | 0.984 (0.963, 1.007) | 0.167 | 1.013 (0.964, 1.064) | 0.613 | |
| Variables | Dyslipidemia | IR | Increased FBG | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| Total | |||||||
| Model 1 (four factors of QoL) | Physical and mental health factor | 0.992 (0.982, 1.002) | 0.120 | 0.991 (0.980, 1.002) | 0.113 | 1.020 (1.000, 1.041) | 0.049 |
| Psychosocial factor | 0.976 (0.959, 0.994) | 0.008 | 0.980 (0.962, 0.998) | 0.034 | 0.995 (0.964, 1.027) | 0.767 | |
| Living environment factor | 0.977 (0.952, 1.001) | 0.065 | 0.979 (0.954, 1.006) | 0.121 | 1.077 (1.026, 1.130) | 0.003 | |
| Quality of life satisfaction factor | 0.992 (0.968, 1.017) | 0.531 | 0.972 (0.948, 0.997) | 0.028 | 1.033 (0.986, 1.082) | 0.168 | |
| Total score of QoL | 0.990 (0.982, 0.999) | 0.025 | 0.988 (0.979, 0.997) | 0.007 | 1.009 (0.993, 1.025) | 0.267 | |
| Model 2 (four factors of QoL) | Physical and mental health factor | 0.994 (0.983, 1.005) | 0.296 | 0.988 (0.976, 1.000) | 0.047 | 1.015 (0.994, 1.036) | 0.154 |
| Psychosocial factor | 0.973 (0.953, 0.993) | 0.010 | 0.973 (0.953, 0.993) | 0.010 | 0.992 (0.960, 1.025) | 0.620 | |
| Living environment factor | 0.988 (0.962, 1.015) | 0.387 | 0.971 (0.942, 1.000) | 0.053 | 1.060 (1.008, 1.115) | 0.024 | |
| Quality of life satisfaction factor | 0.992 (0.966, 1.017) | 0.520 | 0.961 (0.934, 0.988) | 0.005 | 1.024 (0.977, 1.074) | 0.320 | |
| Total score of QoL | 0.991 (0.982, 1.000) | 0.043 | 0.985 (0.976, 0.995) | 0.004 | 1.009 (0.992, 1.026) | 0.317 | |
| Male | |||||||
| Model 1 (four factors of QoL) | Physical and mental health factor | 0.999 (0.985, 1.014) | 0.899 | 0.999 (0.983, 1.015) | 0.913 | 1.016 (0.992, 1.042) | 0.196 |
| Psychosocial factor | 0.981 (0.957, 1.005) | 0.124 | 0.998 (0.972, 1.025) | 0.889 | 0.982 (0.945, 1.021) | 0.355 | |
| Living environment factor | 1.001 (0.966, 1.038) | 0.935 | 0.987 (0.950, 1.026) | 0.514 | 1.060 (0.998, 1.125) | 0.057 | |
| Quality of life satisfaction factor | 1.002 (0.968, 1.037) | 0.916 | 0.986 (0.950, 1.023) | 0.459 | 1.018 (0.962, 1.077) | 0.537 | |
| Total score of QoL | 0.995 (0.983, 1.008) | 0.470 | 0.995 (0.982, 1.009) | 0.482 | 1.003 (0.983, 1.023) | 0.799 | |
| Model 2 (four factors of QoL) | Physical and mental health factor | 1.002 (0.986, 1.017) | 0.824 | 0.999 (0.982, 1.016) | 0.895 | 1.013 (0.988, 1.039) | 0.301 |
| Psychosocial factor | 0.994 (0.965, 1.024) | 0.686 | 0.994 (0.965, 1.024) | 0.686 | 0.983 (0.945, 1.024) | 0.414 | |
| Living environment factor | 1.021 (0.982, 1.062) | 0.299 | 0.989 (0.947, 1.033) | 0.633 | 1.047 (0.983, 1.114) | 0.154 | |
| Quality of life satisfaction factor | 1.001 (0.964, 1.039) | 0.969 | 0.982 (0.943, 1.022) | 0.374 | 1.016 (0.959, 1.077) | 0.580 | |
| Total score of QoL | 0.997 (0.984, 1.010) | 0.645 | 0.996 (0.982, 1.011) | 0.601 | 1.005 (0.984, 1.026) | 0.631 | |
| Female | |||||||
| Model 1 (four factors of QoL) | Physical and mental health factor | 0.984 (0.970, 0.999) | 0.037 | 0.983 (0.968, 0.998) | 0.026 | 1.025 (0.990, 1.061) | 0.160 |
| Psychosocial factor | 0.971 (0.946, 0.996) | 0.024 | 0.962 (0.936, 0.988) | 0.004 | 1.020 (0.965, 1.079) | 0.477 | |
| Living environment factor | 0.952 (0.919, 0.987) | 0.008 | 0.970 (0.935, 1.006) | 0.105 | 1.102 (1.016, 1.195) | 0.019 | |
| Quality of life satisfaction factor | 0.983 (0.950, 1.017) | 0.329 | 0.958 (0.925, 0.992) | 0.016 | 1.056 (0.976, 1.143) | 0.173 | |
| Total score of QoL | 0.985 (0.973, 0.997) | 0.014 | 0.980 (0.968, 0.993) | 0.002 | 1.020 (0.992, 1.049) | 0.156 | |
| Model 2 (four factors of QoL) | Physical and mental health factor | 0.987 (0.971, 1.003) | 0.103 | 0.976 (0.960, 0.993) | 0.007 | 1.011 (0.974, 1.049) | 0.565 |
| Psychosocial factor | 0.956 (0.929, 0.984) | 0.003 | 0.956 (0.929, 0.984) | 0.003 | 1.006 (0.950, 1.065) | 0.846 | |
| Living environment factor | 0.962 (0.925, 1.000) | 0.048 | 0.951 (0.912, 0.992) | 0.019 | 1.072 (0.982, 1.171) | 0.120 | |
| Quality of life satisfaction factor | 0.984 (0.948, 1.021) | 0.393 | 0.941 (0.905, 0.978) | 0.002 | 1.025 (0.942, 1.115) | 0.568 | |
| Total score of QoL | 0.985 (0.972, 0.998) | 0.024 | 0.975 (0.961, 0.989) | 0.001 | 1.012 (0.982, 1.043) | 0.445 | |
The relationship between the four factors of QoL and the prevalence of GLMD was not significant in males (P > 0.05). Psychosocial factor [OR (95%CI): 0.976 (0.959, 0.994); P = 0.008] [OR (95%CI): 0.980 (0.962, 0.998); P = 0.034] and total score of QoL [OR (95%CI): 0.990 (0.982, 0.999); P = 0.025] [OR (95%CI): 0.988 (0.979, 0.997); P = 0.007] were protective factors for dyslipidaemia and IR > 3, respectively, with statistical significance in the total cohort in Model 1. In addition, higher score of psychosocial factor [OR (95%CI): 0.971 (0.946, 0.996); P = 0.024], living environment factor [OR (95%CI): 0.952 (0.919, 0.987); P = 0.008], and total score of QoL [OR (95%CI): 0.985 (0.973, 0.997); P = 0.014] in females was related to a lower prevalence of dyslipidaemia in Model 2. In Model 2, adjusted for covariates, all factors of QoL were protective factors for IR > 3 (P < 0.05) (Table 5).
The association between QoL and the prevalence of GLMD was illustrated using a large-sample-size childhood health cohort study. By adjusting for multiple covariates that may correlate with glycolipid indexes, the independent relationship between QoL and GLMD was demonstrated. In addition, more domains of QoL correlated with glycolipid indexes in females.
Our study revealed that QoL scores mainly correlate negatively with the prevalence of GLMD in adolescents. Research on the relationship between QoL and GLMD in the healthy population is limited. According to a previous cross-sectional study that included 74 diabetic adolescents[32], no significant relationship between QoL and HbA1c levels was observed. However, a cross-sectional study found that QoL scores correlated with an increase in the components of MS, with the physical health domain of QoL having the most significant association[33]. In our study, six domains, four factors, and the total QoL score were correlated significantly negatively with glycolipid indexes, and the effect was independent of obesity. To our knowledge, this is the first cohort study with a large sample size to explore the relationship of QoL with GLMD in adolescents.
The association between QoL and GLMD may be impacted by sex. Scores on several domains of QoL are reportedly lower in females than in males[34-37]. Longitudinal studies have shown a significant relationship between weight and QoL only in females[38]. However, one study found that females and males have similar psychological characteristics[39]. Overall, numerous studies have detected significant sex differences in awareness and mental health[40,41]. For instance, in terms of personality, males score higher than females in self-acceptance and autonomy, whereas females score higher than males in personal growth and positive relationships with others[42]. Our study adds more evidence about the sex difference in the association between QoL and GLMD; overall, more domains of QoL correlated with GLMD in females.
Several mechanisms may explain why QoL may impact GLMD. Physical and psychological health and social well-being are encompassed in HRQoL[43]. Previous study results show that an increase in total HbA1c is related to a decrease in QoL[44]. In addition, research has found that better QoL is associated with better healthy dietary patterns and behaviours in children and adolescents[45]. Irrational diets may induce FBG increases. For example, a high-fat diet induces IR, triggering accumulation of diacylglycerol and ceramide levels in the liver and inhibiting the insulin signalling pathway[46]. Some studies have suggested that physical activity and mental health are positively associated with QoL[44]; it is well known that exercise enhances insulin signalling independent of PI3K and that glucose transport and GLUT4 translocation are enhanced as skeletal muscle contraction is stimulated by insulin[47]. Similarly, better education may lead to greater confidence, a sense of security, and building better relationships with others, contributing to mental health[48]. The mechanisms through which IR influences emotional regulation are being revealed by animal and human studies, and the brain requires glucose as an essential energy source[49,50].
In conclusion, GLMD prevalence and high glycolipid levels are elevated in adolescents with low QoL scores. To our knowledge, this is the first study to explore the relationship of QoL with glycolipid indexes from a large-sample-size cohort study of adolescents, and the correlation was significant after adjusting for multiple covariates. Our study emphasizes the importance of improving QoL in children and adolescents and provides scientific evidence for educational institutions to improve the educational model to enhance the QoL of school-age children. However, our study illustrates the relationship between QoL and glycolipid indexes from a nearly cross-sectional perspective, and a further well-designed large-sample-size cohort study or randomized controlled trial study should be conducted to examine the causal relationships.
Our study reveals that QoL scores mainly correlate negatively with the prevalence of GLMD in adolescents of the healthy population. The independent relationship between QoL and GLMD can be illustrated by adjusting for multiple covariates that may be associated with glycaemic index. In addition, more QoL domains are associated with glycaemic index in females.
The prevalence of glucolipid metabolic disorders (GLMDs) in children and adolescents has a recognized association with cardiovascular diseases and type 2 diabetes mellitus in adulthood. Therefore, it is important to increase our understanding of the risk factors for GLMD in childhood and adolescence.
Quality of life (QoL) includes multidimensional terms, which represent satisfaction with life status and describe a subject’s functioning in physical, emotional, and social domains. Little evidence about the relationship between QoL and GLMD has been reported, especially in children and adolescents, which is an important stage of growth.
The aim of this cohort study was to explore the correlation of QoL scores and personality traits with GLMD in adolescents, providing an excellent opportunity to identify independent risk factors for GLMD after adjusting for multiple variables, such as perinatal variables, socioeconomic status, anthropometric measures, and other biochemical indexes.
Two-stage stratified cluster sampling was used to select children from urban and rural areas of Chongqing; two regions per county were randomly chosen; and finally, all children living in the selected region were informed and included if they met the inclusion criteria.
Our study revealed that QoL scores mainly correlate negatively with the prevalence of GLMD in adolescents.
The prevalence of GLMD and high glycolipid levels are increased in adolescents with features of low QoL scores. Our study adds more evidence about sex difference in the association between QoL and GLMD, and more domains of QoL correlate with GLMD in females.
Our study illustrates the relationship between QoL and glycolipid indexes from a nearly cross-sectional perspective, and a further well-designed cohort study with a large sample size or randomized controlled trial should be conducted to explore the causal relationships.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kuru Çolak T, Turkey; Sibiya N, South Africa; Teixeira KN, Brazil; Xiao JB, Spain S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Koskinen JS, Kytö V, Juonala M, Viikari JSA, Nevalainen J, Kähönen M, Lehtimäki T, Hutri-Kähönen N, Laitinen T, Tossavainen P, Jokinen E, Magnussen CG, Raitakari OT. Childhood risk factors and carotid atherosclerotic plaque in adulthood: The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2020;293:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Lorzadeh E, Sangsefidi ZS, Mirzaei M, Hosseinzadeh M. Dietary Habits and their Association with Metabolic Syndrome in a sample of Iranian adults: A population-based study. Food Sci Nutr. 2020;8:6217-6225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Brown AE, Walker M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr Cardiol Rep. 2016;18:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 4. | Zhu X, Chen D, Xiu M, Li S, Zhang XY. Serum BDNF levels, glycolipid metabolism in deficit schizophrenia: A case-control study. Asian J Psychiatr. 2022;69:103003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Scholtens DM, Metzger BE. Response to Comment on Scholtens et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019;42:381-392. Diabetes Care. 2019;42:e128-e129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Schlotz W, Ambery P, Syddall HE, Crozier SR, Sayer AA, Cooper C, Phillips DI; Hertfordshire Cohort Study Group. Specific associations of insulin resistance with impaired health-related quality of life in the Hertfordshire Cohort Study. Qual Life Res. 2007;16:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Kazukauskiene N, Podlipskyte A, Varoneckas G, Mickuviene N. Health-related quality of life and insulin resistance over a 10-year follow-up. Sci Rep. 2021;11:24294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;CD007170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12:CD003054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Tajaddini A, Roshanravan N, Mobasseri M, Aeinehchi A, Sefid-Mooye Azar P, Hadi A, Ostadrahimi A. Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: A double-blind, placebo-controlled, randomised clinical trial. Int J Clin Pract. 2021;75:e14334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Cunningham AT, Crittendon DR, White N, Mills GD, Diaz V, LaNoue MD. The effect of diabetes self-management education on HbA1c and quality of life in African-Americans: a systematic review and meta-analysis. BMC Health Serv Res. 2018;18:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;CD004816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 13. | Lindholm LH, Ekbom T, Dash C, Isacsson A, Scherstén B. Changes in cardiovascular risk factors by combined pharmacological and nonpharmacological strategies: the main results of the CELL Study. J Intern Med. 1996;240:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Florez H, Pan Q, Ackermann RT, Marrero DG, Barrett-Connor E, Delahanty L, Kriska A, Saudek CD, Goldberg RB, Rubin RR; Diabetes Prevention Program Research Group. Impact of lifestyle intervention and metformin on health-related quality of life: the diabetes prevention program randomized trial. J Gen Intern Med. 2012;27:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, Abolhassanzadeh Z, Iraji A, Vojoud M, Heydari M, Shams M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Cochran J, Conn VS. Meta-analysis of quality of life outcomes following diabetes self-management training. Diabetes Educ. 2008;34:815-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Peña-Purcell NC, Jiang L, Ory MG, Hollingsworth R. Translating an evidence-based diabetes education approach into rural african-american communities: the "wisdom, power, control" program. Diabetes Spectr. 2015;28:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Tong J, Ren Y, Liu F, Liang F, Tang X, Huang D, An X, Liang X. The Impact of PM2.5 on the Growth Curves of Children's Obesity Indexes: A Prospective Cohort Study. Front Public Health. 2022;10:843622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Wang Q, Qu P, Chen J, Tang X, Hao G, Liang X. Associations Between Physical Activity and Hypertension in Chinese Children: A Cross-Sectional Study From Chongqing. Front Med (Lausanne). 2021;8:771902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Liang X, Chen J, An X, Liu F, Liang F, Tang X, Qu P. The impact of PM2.5 on children's blood pressure growth curves: A prospective cohort study. Environ Int. 2022;158:107012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Liang X, Xiao L, Luo Y, Xu J. Prevalence and risk factors of childhood hypertension from birth through childhood: a retrospective cohort study. J Hum Hypertens. 2020;34:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Liang X, Tang X, Xi B, Qu P, Ren Y, Hao G. Abdominal obesity-related lipid metabolites may mediate the association between obesity and glucose dysregulation. Pediatr Res. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Liang X, Hao G, Xiao L, Luo S, Zhang G, Tang X, Qu P, Li R. Association Between Extraversion Personality With the Blood Pressure Level in Adolescents. Front Cardiovasc Med. 2022;9:711474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Liang X, Chen M, Wang D, Wen J, Chen J. Vitamin A deficiency indicating as low expression of LRAT may be a novel biomarker of primary hypertension. Clin Exp Hypertens. 2021;43:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Liang X, Zhang P, Luo S, Zhang G, Tang X, Liu L. The association of quality of life and personality characteristics with adolescent metabolic syndrome: a cohort study. Health Qual Life Outcomes. 2021;19:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Wu HR, Liu PL, Meng H. Norm, Reliability and Validity of Children and Adolescents’ QOL scale. Zhongguo Xuexiao Weisheng. 2006;27:4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1255] [Cited by in RCA: 1546] [Article Influence: 128.8] [Reference Citation Analysis (5)] |
| 28. | Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1578] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 29. | Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, Mi J. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Hu L, Huang X, You C, Li J, Hong K, Li P, Wu Y, Wu Q, Wang Z, Gao R, Bao H, Cheng X. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS One. 2017;12:e0183934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 31. | Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, Varner MW, Wapner RJ, Thorp JM Jr, Mercer BM, Peaceman AM, Ramin SM, Samuels P, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network*. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol. 2013;121:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Afshar M, Mohammadi MR. Investigating the relationship between quality of life, self-care capability and HbA1c level in diabetic adolescents. J Kashan Univer Med Sci. 2014;18:8. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Amiri P, Deihim T, Taherian R, Karimi M, Gharibzadeh S, Asghari-Jafarabadi M, Shiva N, Azizi F. Factors Affecting Gender Differences in the Association between Health-Related Quality of Life and Metabolic Syndrome Components: Tehran Lipid and Glucose Study. PLoS One. 2015;10:e0143167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68:714-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1038] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 35. | Mond JM, Hay PJ. Functional impairment associated with bulimic behaviors in a community sample of men and women. Int J Eat Disord. 2007;40:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Striegel RH, Bedrosian R, Wang C, Schwartz S. Why men should be included in research on binge eating: results from a comparison of psychosocial impairment in men and women. Int J Eat Disord. 2012;45:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Bentley C, Mond J, Rodgers B. Sex differences in psychosocial impairment associated with eating-disordered behavior: what if there aren't any? Eat Behav. 2014;15:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Garner RE, Feeny DH, Thompson A, Bernier J, McFarland BH, Huguet N, Kaplan MS, Orpana H, Ross NA, Blanchard C. Bodyweight, gender, and quality of life: a population-based longitudinal study. Qual Life Res. 2012;21:813-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Hyde JS. Gender similarities and differences. Annu Rev Psychol. 2014;65:373-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 613] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 40. | Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, Parkinson J, Secker J, Stewart-Brown S. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2543] [Cited by in RCA: 2270] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 41. | Matud MP, Bethencourth JM, Ibáñez I, Fortes D. Gender and psychological well-being in older adults. Int Psychogeriatr. 2020;32:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Matud MP, López-Curbelo M, Fortes D. Gender and Psychological Well-Being. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Solans M, Pane S, Estrada MD, Serra-Sutton V, Berra S, Herdman M, Alonso J, Rajmil L. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. Value Health. 2008;11:742-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 405] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 44. | Parsa P, Ahmadinia-Tabesh R, Mohammadi Y, Khorami N. Investigating the relationship between quality of life with lipid and glucose levels in Iranian diabetic patients. Diabetes Metab Syndr. 2017;11 Suppl 2:S879-S883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Wu XY, Zhuang LH, Li W, Guo HW, Zhang JH, Zhao YK, Hu JW, Gao QQ, Luo S, Ohinmaa A, Veugelers PJ. The influence of diet quality and dietary behavior on health-related quality of life in the general population of children and adolescents: a systematic review and meta-analysis. Qual Life Res. 2019;28:1989-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 46. | Zabielski P, Hady HR, Chacinska M, Roszczyc K, Gorski J, Blachnio-Zabielska AU. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci Rep. 2018;8:7249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S; Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611-619. [PubMed] |
| 48. | Iwanowicz-Palus G, Zarajczyk M, Bień A. The relationship between health-related quality of life, acceptance of illness and characteristics of pregnant women with hyperglycemia. Health Qual Life Outcomes. 2020;18:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 1050] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 50. | Hopkins DF, Williams G. Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet Med. 1997;14:1044-1050. [PubMed] [DOI] [Full Text] |