Published online Dec 15, 2022. doi: 10.4239/wjd.v13.i12.1014
Peer-review started: August 19, 2022
First decision: September 26, 2022
Revised: October 7, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: December 15, 2022
Processing time: 117 Days and 21.1 Hours
Diabetic foot ulcers (DFUs) have become one of the important causes of mortality and morbidity in patients with diabetes, and they are also a common cause of hospitalization, which places a heavy burden on patients and society. The prevention and treatment of DFUs requires multidisciplinary management. By controlling various risk factors, such as blood glucose levels, blood pressure, lipid levels and smoking cessation, local management of DFUs should be strengthened, such as debridement, dressing, revascularization, stem cell decompression and oxygen therapy. If necessary, systemic anti-infection treatment should be administered. We reviewed the progress in the clinical practice of treating DFUs in recent years, such as revascularization, wound repair, offloading, stem cell transplantation, and anti-infection treatment. We also summarized and prospectively analyzed some new technologies and measurements used in the treatment of DFUs and noted the future challenges and directions for the development of DFU treatments.
Core Tip: Diabetes foot ulcer has become one of the important causes of mortality and morbidity of diabetes patients, and it is also a common cause of hospitalization, which brings a heavy burden to patients and society. The prevention and treatment of diabetes foot ulcer needs multidisciplinary management. We reviewed the progress in the clinical practice of diabetes foot ulcer in recent years, such as revascularization, wound repair, offloading, stem cell transplantation, anti-infection treatment. We also summarized and prospected some new technologies and measurements in the treatment of diabetes foot ulcer, and pointed out the future challenge and development direction of diabetes foot ulcer.
- Citation: Yang L, Rong GC, Wu QN. Diabetic foot ulcer: Challenges and future. World J Diabetes 2022; 13(12): 1014-1034
- URL: https://www.wjgnet.com/1948-9358/full/v13/i12/1014.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i12.1014
The prevalence of diabetes is gradually increasing: the global prevalence was estimated to be 9.3% (463 million people) in 2019 and is expected to increase to 10.2% (570 million people) in 2030 and 10.9% (700 million people) in 2045[1]. The prevalence rate of diabetes in Chinese people older than 18 years of age is 11.2%[2]. Diabetic foot ulcer (DFU) is one of the most serious and dreaded complications of diabetes. A total of 10%-15% of patients with diabetes may experience foot ulcers[3]. At least half of all amputations occur in patients with diabetes, and the most common cause is DFU infection. In a large cohort study of patients with DFU and patients with diabetes in China, the annual ulcer incidence rate among diabetic patients was 8.1%, the annual new ulcer incidence rate among patients with DFU was 31.6%, the amputation rate among patients with DFU was 5.1%, and the annual mortality rates among patients with diabetes and patients with DFU were 2.8% and 14.4%, respectively, during the 1-year follow-up period[4]. DFU is the main cause of hospitalization, amputation, deterioration of quality of life and disability of patients, which imposes a heavy economic burden on the medical and health system, and its economic burden ranks tenth among all diseases[5]. Therefore, the management of DFUs is particularly important. This article mainly introduces the risk factors and treatment of DFUs.
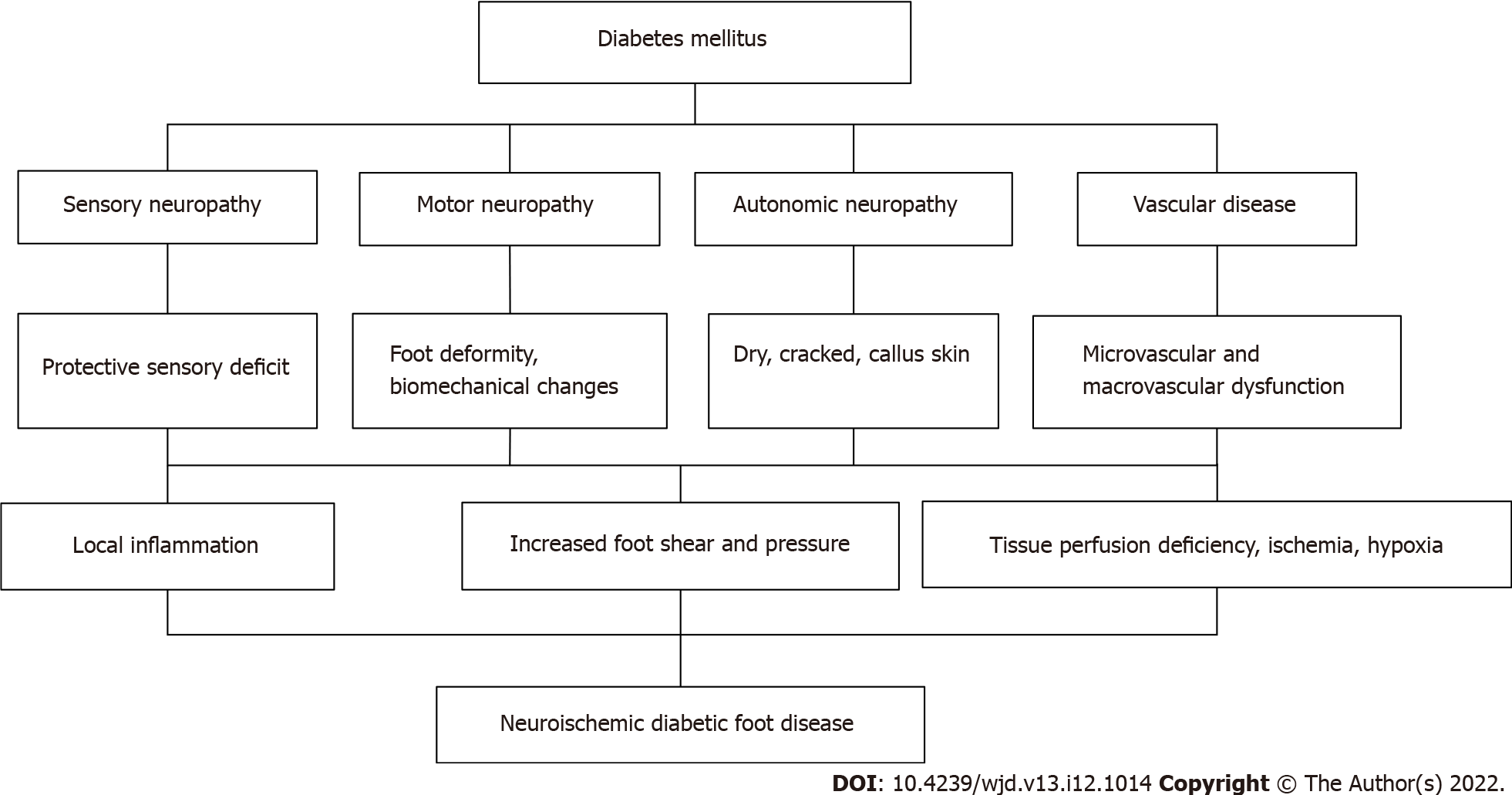
The World Health Organization and the International Diabetes Federation define DFU as a serious complication of diabetes, mainly due to foot tissue ulcers and wounds caused by hyperglycemia, diabetic peripheral vascular disease and/or diabetic peripheral neuropathy[1]. DFU results from multiple factors. The risk factors for DFU must be addressed to reduce the rates of foot ulcers and amputation (Figure 1).
Neuropathy is a common complication of diabetes that occurs in 50% of patients with type 2 diabetes. Neuropathy is an important cause of ulcers. Long-term hyperglycemia leads to peripheral nerve fiber damage. Distal sensor motor peripheral neuropathy is the most common type. It manifests as distal, symmetric, and length-dependent multiple neuropathy[6]. Usually, small nerve fibers are damaged earlier than larger nerve fibers[7]. The dysfunction of small-fiber nerves leads to sensory changes, such as sensory dullness, acupuncture sensations, numbness, burning sensations, abnormal pain and other clinical symptoms. Sensory defects, such as defects in pain perception and temperature perception, are clinically called protective sensory deficits. The loss of protective sensation leads to a loss of sensitivity to injury and stimulation of the lower limbs, thus leading to continuous unconscious trauma, which tends to form ulcers. Usually, diabetic ulcers are found when blood stains are observed on the socks and floor, which portends an untimely diagnosis and treatment of the ulcers and aggravates the disease. Compared with patients with diabetes presenting without neuropathy, patients with diabetes presenting protective sensory loss have a 7-fold increased risk of developing DFUs[8]. The autonomic nerves will also be damaged. Dysfunction of the peripheral sympathetic nerves may lead to reduced sweating, dry skin, cracking and an increased risk of calluses complicated with peripheral arterial disease, and the appearance of symptoms increased. In the absence of peripheral artery disease, the dorsal foot vein expands, the foot feels warm, and some edema occurs. This situation places the patient's foot at high risk of ulceration. Biomechanical changes occur in the early stage of diabetic neuropathy[9]. Motor neuropathy causes an imbalance of foot muscle tissue, muscle weakness and atrophy and changes the normal foot dynamics and pressure distribution, leading to the loss of joint stability and the development of foot deformities such as claw toe, hammer toe, horseshoe foot, Charcot’s ankle, arch changes, and plantar aponeurosis[10]. The increased shear stress and friction force increase the risk of foot ulcers, and when these factors are combined with the loss of protective sensation, the risk of foot ulcers is higher.
Lower extremity arterial disease is an important risk factor for DFUs, resulting in an insufficient blood supply, hypercoagulability of the lower extremities, and serious limb ischemia[11]. The clinical manifestations are malnutrition, muscle atrophy, decreased skin temperature, pigmentation, weakened or absent limb artery pulsation, and even intermittent claudication, resting pain and ulceration of the lower limbs. Long-term ischemia and hypoxia render the areas prone to tissue ulcers through the action of external forces, particularly ulcers at compressed parts of the heel or metatarsophalangeal joint, which are prone to secondary infection. Patients with diabetes usually have lower-limb arterial disease and neuropathy, which lead to difficult healing of neuropathic and ischemic ulcers. In 50%-75% of cases, peripheral arterial lesions lead to wound occurrence or a failure to heal[12,13].
The age and course of diabetes also affect ulcer healing. The risk of ulcer and amputation increases two to four times with increasing age and a prolonged disease course. Repeated minor injuries caused by neuropathic foot pressure and inappropriate footwear may increase the risk of ulceration. One study indicated that the overall risk of injury in patients with diabetes was 2% per year, the risk for patients with diabetes neuropathy increased to 7.5%, the risk for patients with a previous ulcer history increased to 40%, and the risk further increased to approximately 60% after 3 years and reached 75% after 5 years[14]. If the patient has eye diseases such as retinopathy and cataracts, resulting in decreased vision, the risk of a foreign body stabbing the foot is high, and the risk of ulceration is also increased. Some studies have suggested that dialysis patients with diabetic nephropathy have a very high risk of foot ulcers, and dialysis treatment is an independent risk factor for foot ulcers[15].
The ultimate goal of DFU treatment is to achieve healing and prevent wound infection, amputation and reduced quality of life. It mainly includes glycemic control, management of peripheral artery disease (PAD), and wound management, among others.
Glycated hemoglobin may be the best indicator to evaluate average blood glucose control. An HbA1c level ≥ 8% and fasting blood glucose level ≥ 7 mmol/L are associated with an increased risk of lower limb amputation in patients with DFUs[16]. Studies have recommended that the glycated hemoglobin level in patients with DFUs should be controlled at 7%-8%, which is helpful for ulcer healing and will not increase the mortality of patients[17]. In another study, the glycosylated hemoglobin level was related to the wound healing speed, which was more obvious in patients with neuropathy and lower-limb arterial disease[18]; however, the results were not repeated in another study[19]. However, an appropriate blood glucose level is undoubtedly beneficial to prevent and delay microvascular and macrovascular complications in patients with diabetes[20]. The ideal blood glucose control target is reached when the glycated hemoglobin level is less than 7% and the blood glucose level within 2 h after a meal is less than 11.1 mmol/L. However, the indicators should be appropriately relaxed for elderly patients and patients prone to hypoglycemia[21]. Regardless of the size of the initial ulcer area, early intensive blood glucose control may improve the prognosis of DFUs in the first 4 wk of treatment[22]. Intensive blood glucose control reduced the risk of amputation in patients with DFUs and contributed to wound healing[23,24]. However, in another systematic analysis, no evidence was obtained that strict control of blood glucose improved ulcer wound healing[25]. Many factors affect ulcer healing, and an increasing number of large samples randomized controlled trials (RCTs) are needed to indicate the effect of intensive blood glucose control on the prognosis of DFUs. According to the specific conditions of patients and blood glucose control objectives, appropriate hypoglycemic programs are formulated to avoid hypoglycemia. Fifty percent of patients with DFUs may have PAD, suggesting that they have atherosclerotic cardiovascular disease[26-28]. According to the latest recommendations of the American Diabetes Association for patients with type 2 diabetes complicated with cardiovascular disease, if blood sugar cannot be controlled by lifestyle changes and metformin, they should start taking a hypoglycemic drug that has been suggested to reduce adverse cardiovascular events and cardiovascular mortality[29], such as a sodium glucose cotransporter 2 inhibitor and a glucagon-like peptide-1 receptor agonist. Compared with the placebo, liraglutide did not increase the risk of DFUs but reduced the risk of DFU-related amputation in patients with type 2 diabetes mellitus and high-risk cardiovascular events[30]. However, the specific mechanism remains unclear. Studies have suggested that liraglutide may promote diabetic wound healing by inhibiting endothelial dysfunction induced by hyperglycemia[31]. Daggligin significantly reduced the level of inflammation and oxidative stress in diabetic animal models, which may contribute to the improvement of endothelial dysfunction and diabetic vascular complications[32]. However, some studies suggested that the amputation risk of patients who use canagliflozin is increased, particularly for patients with DFUs presenting lower limb atherosclerosis, neuropathy and amputation history[33]. However, the OBSERVE-4D study[34] indicated that although cagelin increases the risk of amputation, the risk islower than that reported in previous CANVAS and CANVAS-R trials[35], especially for patients undergoing timely monitoring, who have a lower risk. The results of a randomized controlled trial conducted by Marfella et al[36] suggested that the granulation score of wound granulation tissue and the rate of complete wound healing in the Vigliptin group (50 mg/dose, bid) were significantly better than those in the control group, and the incidence of ulcer-related adverse events (such as ulcer wound infection, osteomyelitis, honeycomb tissue inflammation, etc.) was also significantly reduced, suggesting that venagliptin may improve the healing rate of DFUs possibly by reducing oxidative stress, changing capillary density, increasing angiogenesis and promoting wound healing. Compared with the control group, the healing rate of foot ulcers in the saxagliptin (5 mg/time, qd) group was higher, and the healing time of ulcers was shorter. The main mechanism was that shagliptin directly and indirectly promoted the epithelial-mesenchymal transformation and reduced scarring to improve diabetic wound healing[37]. Dipeptidyl peptidase IV (DDP-IV) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists reduce inflammatory reactions and antioxidant activity, induce angiogenesis and tissue reconstruction, and may promote DFU healing[38]. These treatments represent new directions with potential effects on DFUs. Systemic insulin therapy improves wound healing of diabetic ulcers by increasing angiogenesis and granulation tissue formation and reducing the duration of the inflammatory phase[39]. Compared with patients with type 2 diabetes using insulin and insulin secretion-promoting drugs, patients with type 2 diabetes using insulin sensitizers have a lower incidence of PAD, suggesting that insulin sensitizers may reduce the incidence of PAD and its subsequent outcomes[40]. Therefore, when choosing hypoglycemic drugs, the appropriate hypoglycemic regimen should be selected according to the basic situation, blood glucose level, wound condition and other comprehensive factors of patients with DFUs.
Patients with diabetes are prone to PAD. According to a Chinese multicenter study, the proportion of lower-limb arterial disease in patients with diabetes over 50 years old is 19.5%[41]. For every 1% increase in the glycated hemoglobin level, the risk of peripheral vascular disease increases by 25%-28%[42]. For patients with DFUs, the vascular lesions are mainly located in the tibiofibular artery below the knee. The arterial lumen is narrow or even completely occluded, causing lower limb ischemia, hypoxia, infection, ulcer and even gangrene. More than 80% of patients with DFUs have lower limb ischemia[43], which is an important reason for the difficulty in wound healing. Adequate blood perfusion provides a good metabolic demand for the damaged tissue, while an insufficient blood supply may lead to insufficient nutrients available for wound healing and limited delivery of antibiotics, resulting in a decreased healing capacity and increased amputation risk. Therefore, the arterial blood supply of the lower extremities must be reconstructed to improve and restore the blood flow of the extremities, avoid limb ischemia and necrosis, reduce amputation, and improve the quality of life and survival rate of the patients[26,27]. Patients with PAD have a high risk of cardiovascular and cerebrovascular events. Even after revascularization, the incidence of cardiovascular disease is still high[44]. Therefore, patients should also receive active cardiovascular risk management, including smoking cessation, statins, antiplatelet drugs and intensive blood pressure therapy[28,45]. Smoking is a risk factor for atherosclerotic plaques. Severe peripheral atherosclerosis may lead to stenosis and occlusion of the vascular lumen with the progression of the disease, which may lead to foot tissue ischemia that causes tissue damage and postpones wound healing[46]. Smoking is also a risk factor affecting the degree of DFU lesions[47] and is an effective predictor of death and amputation in patients with DFUs[48]. Smoking cessation is recommended for all patients with PAD. Blood pressure should be controlled within 130/80 mmHg to reduce the risk of cardiovascular and cerebrovascular events[49]. However, another study suggested that the optimal mean systolic blood pressure of patients with PAD was 135–145 mmHg and diastolic blood pressure was 60–90 mmHg. Low blood pressure may increase the risk of cardiovascular events[50]. The use of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) by patients with PAD not only reduces blood pressure but also reduces major cardiovascular adverse events and mortality[51,52], but it does not reduce major adverse limb events and amputation risk in patients with PAD[52]. Treatments regulating lipid levels target low-density lipoprotein cholesterol. Researchers have recommended a low-density lipoprotein cholesterol (LDL-C) level < 1.4 mmol/L (< 55 mg/dL) or a decrease in the LDL-C level by 50%[53]. In patients with PAD, patients who took statins had an 18% reduction in the long-term risk of adverse prognosis of the lower limbs (such as symptom deterioration, peripheral vascular reconstruction and ischemic amputation) and a 17% reduction in the incidence of cardiovascular events compared with patients who did not take statins, indicating that statins not only reduce the risk of adverse cardiovascular events but also exert a positive effect on the limb prognosis of patients with PAD[54]. Therefore, patients with type 2 diabetes and PAD should be prescribed statins. Medications for improving the circulation of patients with PAD include vasodilator drugs, antiplatelet drugs and anticoagulant drugs. Vasodilator drugs include alprostadil injection, beraprost sodium, cilostazol, salgrel hydrochloride, buflomedil and pentoxifylline, which reduce blood viscosity and change hypercoagulability. Aspirin and clopidogrel are considered antiplatelet drugs. Current practice guidelines recommend the use of aspirin or clopidogrel alone as a method for the secondary prevention of cardiovascular events in patients with PAD[55,56]. Compared with aspirin alone, clopidogrel combined with aspirin significantly reduces all-cause mortality and cardiovascular events, but the risk of severe bleeding is increased[57]. The COMPASS study suggested that the absolute benefit of low-dose rivalsaban (2.5 mg bid) combined with aspirin (100 mg qd) in reducing the risk of cardiovascular events and all-cause mortality in patients with stable atherosclerosis seems to be greater than that of nondiabetic patients[58]. Compared with aspirin alone, low-dose rivalsaban combined with aspirin reduced major cardiovascular events and major limb adverse events. Rivalsaban alone only reduced major limb adverse events but did not significantly reduce major cardiovascular adverse events. However, the latter two schemes increased the risk of bleeding, mainly in the gastrointestinal tract, but the incidence of fatal bleeding or bleeding in key organs did not increase[59]. Routine use of proton pump inhibitors may reduce bleeding from gastroduodenal lesions[60]. Therefore, the combination of low-dose rivalsaban and aspirin provides a new therapeutic direction for patients with diabetes complicated with PAD, but further studies are necessary to determine which subgroups of patients may benefit.
Patients with diabetes have hypoimmunity, slow ulcer healing and a high infection rate. Infection often occurs when pus flows around the wound and the surrounding tissue is red and swollen. DFUs are usually chronic wounds. The bacteria on the wound surface produce biofilms that inhibit wound healing. Biofilms induce inflammation in the surrounding tissues and adversely affect the removal of bacteria by antibiotics or the host immune system[61]. Therefore, the wound must be thoroughly cleaned. Debridement is the first and foremost measurement of DFU treatment, which involves removing necrotic, inactivated or seriously polluted tissues from the wound surface to convert the wound into an acute wound and facilitate wound healing[62]. Debridement should be performed as soon as possible.
At present, many methods, such as surgical debridement, maggot debridement, high-pressure fluid irrigation and enzymatic treatment, have been developed, but surgical debridement is usually preferred. Amputation is necessary when the ulcer infection worsens or osteomyelitis occurs. For patients with lower limb ischemia, the time of debridement and revascularization must be evaluated. For patients with dry gangrene, the blood supply should be reconstructed first, and then wound debridement should be performed to promote wound healing. However, if wet gangrene or abscess formation is observed in the wound, debridement is preferred. More than half of patients with DFUs had wound infections at the time of the visit. Infection is an important reason for hospitalization of patients with diabetes and an important factor contributing to the nontraumatic amputation of the lower limbs[63].
In the initial stage of superficial DFU infection, gram-positive cocci are mainly detected, among which Staphylococcus aureus and Streptococcus are the most common organisms[64,65]. If chronic infection, extensive necrosis, wet gangrene, deep infection, long-term repeated use of antibiotics and other conditions exist on the wound surface, a mixed bacterial infection is usually present. Currently, the proportion of gram-negative bacterial infections is increasing, and the proportion of fungal infections is also increasing[66]. Due to the autoimmune status of the body, sanitary conditions, repeated hospitalization, frequent use and abuse of antibiotics, multiple microbial infections, insufficient arterial blood supply of the lower limbs and other reasons, the number of multidrug-resistant bacteria is increasing. The most resistant pathogen is methicillin-resistant Staphylococcus aureus[67]. Therefore, accurate identification of the pathogen causing the bacterial infection is essential for anti-infection treatment of DFUs. Once the infection is confirmed, pathogenic bacteria samples should be collected after the necrotic tissues are removed from the infected wound and before the use of antibacterial drugs. Pathogenic bacterial culture samples shall be obtained from deep tissues as far as possible and sent for culture immediately after the samples are collected. In addition, samples should be collected repeatedly during anti-infection treatment to identify pathogenic bacteria and guide the selection of antibiotics. Tissue biopsy is considered the most useful and standard technology, but it may cause the spread of infection and the loss of adjacent tissue structure of limbs; thus, it is not completely feasible. The collection of swab culture samples is easier, and any type of ulcer can be used. However, the cotton swab culture results usually include colonized bacteria, and the test results are not necessarily reliable[68]. Compared with the culture method, the molecular test method is more sensitive and reliable, with high accuracy and a fast test speed. It represents a powerful method or the identification of microbial colonies infecting chronic wounds and has a bright future in the convenient nursing and treatment of DFUs[69]. Molecular microbiological diagnostic techniques improve the prognosis of patients with chronic wounds[70].
The use of antibiotics should follow the principles of selectivity, timeliness, relatively narrow spectrum, shortest course of treatment, safety, minimal adverse reactions, high cost performance, and step-down. At present, the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (IWGDF) is used to score DFU infection, which is divided into mild (superficial with slight cellulitis), moderate (deeper or more extensive) or severe (with systemic sepsis signs), and the presence of osteomyelitis[71].
The course of antibiotic use is related to the severity of the wound and the presence of bone tissue involvement. The course of treatment ranges from 1 to 12 wk[61]. However, a comprehensive and individualized analysis is necessary to appropriately adjust the course of antibiotics according to the basic diseases of the whole body, nutritional status, liver and kidney functions, blood supply of the lower limbs, and other parameters. Oral antibiotics and intravenous antibiotics maybe selected, but the narrowest-spectrum antibiotic and the shortest course of treatment for pathogenic bacteria should be selected to prevent drug resistance. The IWGDF recommendations[72] for superficial ulcers with localized soft tissue infection (mild) are to start with empirical oral antibiotic treatment against Staphylococcus aureus and Streptococcus aureus (unless other pathogens should be considered). For deep or extensive infections (moderate or severe infections), a broad-spectrum antibiotic should initially be intravenously administered that mainly targets common gram-positive and gram-negative bacteria, including specific anaerobic bacteria, and the antibiotic program should be adjusted according to the clinical efficacy of empirical therapy, tissue culture and drug sensitivity results. Biofilms are polysaccharide layers formed by a variety of signal transduction mechanisms that delay the healing of DFUs. Therefore, inhibiting the formation of biofilms is a new direction of modern anti-infection treatment research. Studies have shown that acyl homoserine lactones (AHLs) regulate multiple factors during biofilm formation by Pseudomonas aeruginosa and play a fundamental role in regulating different genes involved in biofilm formation. Therefore, AHL can be used as a therapeutic target to provide a correct path for drug design targeting multidrug-resistant bacteria[73]. However, in practice, the abuse of antibiotics still frequently leads to the emergence of drug-resistant bacteria. Biological maggot debridement therapy (MDT) provides a new option for the treatment of DFUs.
MDT refers to a natural biological therapy that uses medical maggots to help clean ulcerated wounds by eating the necrotic tissue and bacteria that hinder wound healing[74]; it is used for wounds with unclear boundaries between necrotic tissue and normal tissue, gaps or deep sinuses. It has anti-infection functions, promoting wound growth and debridement. However, because maggots need a moist living environment, ischemic DFUs are not suitable. Currently, the most commonly used organism is the larva of the green silk fly, which is strictly saprophytic and will not cause damage to healthy tissues[75]. Maggots resist infection by draining bacteria from the wound[76,77], absorbing and digesting bacteria in the necrotic tissue of the wound[78], changing the pH of the wound, secreting a variety of bactericidal and antibacterial substances (such as allantoin, urea, phenylacetic acid, calcium bicarbonate, peptides, and bactericides)[79-81], hindering the formation of and degrading bacterial biofilms[82], improving tissue oxygenation at the wound[83], and stimulating the growth of human fibroblasts[84]. Maggots also activate inflammatory cells and other mechanisms to promote wound tissue growth. The necrotic tissue can be cleared through the proteolytic enzymes produced by maggots (such as chymotrypsin, trypsin and collagenase)[85,86] to achieve the debridement effect. Studies have suggested that MDT is effective against a variety of bacterial infections, especially Staphylococcus aureus[87], which is resistant to multiple antibiotics. MDT reduces the number of bacteria and decreases the antibiotic treatment time and the hospitalization cost.
In the local treatment of wounds, antiseptics have more extensive antibacterial activity than antibiotics, and they do not induce drug resistance[88]. Antiseptics are commonly used to reduce the bacterial load of wounds and prevent or treat infections[89]. However, the finding that the antiseptic may be toxic to wound-healing cells is a cause for concern, which limits its application. Therefore, the current guidelines recommend the use of clean water or saline to clean the DFUs as the standard of care. In vitro studies of povidone iodine have shown that it penetrates and reduces the formation of biofilms, and it seems to have no negative effect on wound healing[90].
After effective debridement of the wound, dressings are applied to keep the base of the wound moist, control the exudation of the wound, avoid normal skin impregnation around the wound, help clean the chronic wound, promote the formation of epithelium and heal the wound. The choice of wound dressings for patients with DFUs should be based on the patient's specific conditions (such as the wound appearance, depth, exudate, infection, compliance and economic status) and the cost and comfort of dressings. Hydrogel dressings, film dressings, foam dressings, hydrocolloid dressings, and alginate dressings are commonly used in clinical practice[91]; see Table 1 for detailed descriptions of various dressings. Hydrogel dressings are widely used in patients with DFUs. These dressings can expand and absorb water and exudate, maintain structural stability, and promote cell proliferation and differentiation and wound healing[92,93]. Some studies have shown that hydrogel dressings shorten the wound healing time by 7.28 d on average and improve the healing rate by 57%[94]. Alginate dressings absorb large amounts of water and can absorb 20 times their own weight. They are the best choice for highly exudative wounds[95]. Synthetic foam dressings can be selected for severe exudative wounds and concave wounds to fill cavities and eliminate potential cavities[96]. In an international, multicenter, double-blind, randomized, controlled, 20-week trial, the noninfectious diabetic neuroischemic foot ulcer had an area greater than 1 cm2 compared with the control group receiving the same standard of care. After the use of sucrose octasulfate dressing, the number of patients exhibiting wound closure was greater, the wound healing time was shorter, the wound closure rate was improved, and the safety was similar between the two groups[96]. In 2019, the international national guidelines for the prevention and treatment of diabetic foot recommended the use of asucrose octasulfate dressing to promote wound healing of noninfectious neuro-ischemic DFUs that are difficult to heal after standard care[72]. Different dressings have their own advantages. They are selected in the clinic according to the specific conditions of the patient's wound and the characteristics of various dressings (Table 1).
| Type of dressing | Character | Scope of application | Advantage | Shortcoming |
| Film dressing | The polyurethane film is used as a protective layer or a second layer of dressing | Clean and superficial wounds | Good air permeability, isolating bacteria and liquid, transparent film, easy to observe the wound, less immersion, no pain | Strong adhesiveness, non-absorption, easy accumulation of wound exudates, leading to easy growth of bacteria and infections, and impermeability of proteins and drugs |
| Foam dressing | It is composed of polyurethane or a silicone resin center with a semi-closed outer layer | Burns, chronic wounds, cavity wounds, deep ulcers | Strong water absorption, local humid environment, free from bacteria, easy to use and low cost | Strong adhesiveness, forming an opaque layer, hindering wound observation, unsuitable for dry wounds, and poor stability |
| Hydrogel dressing | The composition is 70%-90% water and cross-linked insoluble starch polymer; super absorbent resin | Most wound and burn types | Supplement water to maintain a humid environment, high exudation, poor adhesion, easy to remove, accelerate wound healing, reduces pain and inflammation, and low cost | Translucent, semipermeable to gas and water vapor, poor bacterial barrier, sometimes poor mechanical stability, frequent replacement needed, and may cause secondary damage to the wound |
| Alginate dressing | Alginate is composed of calcium alginate and a calcium–sodium complex, forming a gel on the wound surface to promote Hemostasis | Surgical wounds and burns | Strong water absorption, non-adhesion, high stability, easy to be removed by salt water, and good bacterial barrier | Expensive, smelly, scarce materials, difficult to handle |
| Hydrocolloid dressing | It is composed of viscous materials, hydrophilic colloids, artificial elastomers, and other components that contact wound exudates to form gels and exert its functions | Chronic ulcers and burns | Strong water absorption, salt water or sterilized water is easy to clean and remove, not easy to adhere, high density, good waterproof performance, and no pain | Slight cytotoxicity, unstable volume, easy leakage of exudate, delayed healing of dextran hydrocolloid, impermeable, unpleasant smell, and obstructing wound observation |
Growth factors play an important role in wound healing. Vascular growth factors promote the formation of vascular collateral circulation and improve the blood supply of the lower limbs; these factors include fibroblast growth factor and hepatocyte growth factor[97,98]. Platelet-derived growth factor, which is mainly released from platelets, is also released from other cells involved in wound healing, such as endothelial cells, macrophages, keratinocytes and fibroblasts. Platelet-derived growth factor stimulates the secretion of vascular endothelial growth factor and promotes angiogenesis, fibroblast activity, granulation tissue formation and endothelial cell migration. However, a risk of tumorigenesis has been noted, and its application is limited. A systematic analysis was performed to study the effects of 11 different growth factors on DFUs. The local application of growth factors may increase the possibility of a complete cure of foot ulcers in patients with diabetes, but the quality of evidence is low[99]. The safety of using growth factors and the overall reports of their adverse events are very poor, and a comparison of the time points at which various growth factors promote wound healing has not been performed. Further tests are needed to study the effects of growth factors on wound healing. Platelet-rich plasma (PRP) is an autogenous product containing a large amount of platelets, growth factors, fibrin and other substances necessary for wound healing[100]. It is often used to treat relatively sterile wounds after debridement, which potentially improves the proliferation of local granulation tissue in ischemic wounds and promotes wound healing. Compared with standard treatment, topical application of PRP increases the healing potential and promotes complete wound healing without significant adverse events, although the quality of evidence is low[100]. Although growth factors and PRP may promote the healing of DFUs from the perspective of the path-ophysiological mechanism, greater requirements are present on the wound surface. Usually, DFUs are complicated with infections and bacterial biofilms exist, which limits the therapeutic application of growth factors and PRP.
DFUs are often chronic wounds; as a continuous oxygen supply is essential for chronic and difficult wounds[101], oxygen therapy has emerged as a potential treatment. Currently, hyperbaric oxygen and local oxygen therapy are commonly used oxygen therapies. Hyperbaric oxygen therapy is applied via a hyperbaric oxygen chamber to reduce inflammatory reactions[102] and induce angiogenesis to promote wound healing, thereby reducing the amputation rate[103]. However, the efficacy of hyperbaric oxygen on DFUs is still controversial. In 1987, Diabetes Care published the first cohort study showing that hyperbaric oxygen significantly reduces the amputation rate of patients with DFUs[104]. Follow-up studies also found that when the traditional treatment method for chronic DFUs is not effective, hyperbaric oxygen treatment improves long-term wound healing[105,106]. However, in the study by Margolis, patients with DFUs who were treated with hyperbaric oxygen did not show significantly improved wound healing and amputation rates compared with the control group[107]. Dr. Fedorko et al[108] also confirmed that hyperbaric oxygen did not significantly improve the quality of life of patients with DFUs. At the same time, the results of two other RCTs evaluating the use of hyperbaric oxygen in the treatment of DFUs also showed that hyperbaric oxygen therapy did not reduce amputation or promote wound healing in patients with diabetes complicated with chronic DFUs through comprehensive wound care[109,110], but the results of these two studies were highly biased. At present, the number of studies assessing hyperbaric oxygen therapy for DFUs is small, the level of research evidence is low, the efficacy evaluation indicators are uneven, and many subjective factors are present. At the same time, hyperbaric oxygen is time-consuming, expensive, and cost-effective, which restricts the recommendation of hyperbaric oxygen therapy for DFUs. Local oxygen therapy directly delivers oxygen to the wound by pressurization. A multicenter randomized double-blind controlled study showed that standard treatment supplemented with local oxygen therapy increased the possibility of wound healing by more than 4.5 times[111], but more studies are needed to further confirm its efficacy.
Biological therapy has been used to promote diabetic wound healing. A study assessing the effect of biological scaffolds (chitosan polyvinyl alcohol and polycaprolactone chitosan polyvinyl alcohol nanofiber blend scaffolds) on the treatment of diabetic rats indicated that the scaffolds had higher biological performance. Compared with the control group, the ulcer area of diabetic rats treated with biological scaffolds was smaller at all time points, and the healing effect was significantly better. At the same time, more obvious granulation tissue was detected in the scaffold-treated wounds[112].
As a new technology for the treatment of DFUs, stem cell transplantation may promote neovascularization of the ischemic limb and improve and restore the blood flow of the limb to achieve the goal of treating limb ischemia and the ultimate goal of promoting ulcer healing. Stem cells that have been used in preclinical and clinical research include umbilical cord blood mesenchymal stem cells, umbilical cord mesenchymal stem cells, placental mesenchymal stem cells, adipose mesenchymal stem cells and bone marrow mesenchymal stem cells, among which adipose mesenchymal stem cells are the most widely used cell type. In addition, genetically engineered SCs that overexpress certain cytokines exhibit different characteristics from conventional stem cells in vivo, providing a new direction for future clinical applications[113]. The most frequently studied stem cells are mainly found in bone marrow, adipose tissue, cartilage and bone tissue, umbilical cord blood and placenta, of which bone marrow is the most abundant source and these stem cells have a multidirectional differentiation potential, differentiating into osteoblasts, chondroblasts, adipoblasts, muscle cells and nerve cells. Bone marrow mesenchymal stem cells rebuild the local microcirculation[114], improve the blood flow of chronic ischemic limbs[115], provide media and sufficient nutrition for wound repair and remove local metabolites. Pilot research on local transplantation of bone marrow mesenchymal cells for the treatment of patients with vascular disease involving DFUs and lower limbs that failed vascular reconstruction showed that the transplantation of these cells significantly improved the percutaneous oxygen partial pressure and toe brachial index of the patients, and the limb preservation rate was 81%[116]. Similarly, another study showed that bone marrow mesenchymal stem cell therapy significantly improves the painless walking distance and wound healing of patients with diabetes presenting lower limb vascular occlusion[117]. After 6 mo of treatment with adipose mesenchymal stem cells, approximately 2/3 of the clinical symptoms of lower limb ischemia in patients with diabetes were relieved (including resting pain and walking distance), and angiography showed a significant increase in collateral circulation[118]. A study of nondiabetic patients with lower limb ischemia who could not undergo vascular reconstruction also showed that adipose mesenchymal stem cell transplantation improved their lower limb percutaneous oxygen partial pressure and promoted the healing of local ulcers, proving that this therapy is also applicable to nondiabetic patients with lower limb ischemia[119]. After an intravascular injection of umbilical cord blood mesenchymal stem cells into rats with diabetic skin ulcers, neovascularization in the ulcer area was substantially increased on the third day, new granulation tissue appeared on the seventh day, and stratified squamous epithelial tissue appeared on the fourteenth day. Based on observations on the seventh and fourteenth days, the skin ulcer area was significantly reduced. The mechanism was that the injected umbilical cord blood mesenchymal stem cells promoted the secretion of keratin 19 by epithelial keratinocytes, participating in the formation of extracellular matrix[120]. Bone marrow mesenchymal stem cells not only improve vascular disease in the lower limbs of patients with diabetes but also promote the healing of ulcers[117,121,122]. Studies have shown that adipose mesenchymal stem cells also contribute to the healing of skin ulcers in diabetic mice[123,124]. At present, the mechanism of action of stem cells remains unclear. Generally, mesenchymal stem cells directly from new blood vessels through endocrine secretion from vascular endothelial cells and smooth muscle cells that participate in angiogenesis through paracrine vascular endothelial growth factor, basic fibroblast growth factor, hepatocyte growth factor, angiopoietin-2, angiopoietin-1 and other angiogenic factors[125]; improve the local microcirculation; increase the blood supply of the distal foot; and promote the healing of DFUs, but the mechanism still requires further study.
With the research and disclosure of the mechanism of action of stem cells, the development of stem cells or other cell derivatives with clearer mechanisms of action for DFU treatment has become a research hotspot. The application of these derivatives in DFU treatment shows efficacy and characteristics similar to those of stem cells. Among these derivatives, exosomes are the most popular. Exosomes are spherical or cup-shaped vesicles surrounded by double-layer membranes that are secreted by various cells. They exist in saliva, blood, milk, semen, blood and other tissue fluids. Exosomes carry a variety of signaling molecules and bioactive substances and participate in the occurrence and development of systemic immunity, intercellular communication, cell proliferation, cell migration, cell differentiation and metabolic diseases[126]. Previous studies have confirmed that mesenchymal stem cells exert therapeutic effects on DFU. They release exosomes through paracrine signaling, and exosomes, important mediators of intercellular communication, participate in the appellate cell process, which is one of the mechanisms by which mesenchymal stem cells exert their therapeutic functions. Exosomes secreted by mesenchymal stem cells are membrane vesicles with a diameter of 30-150 nm and a density of 1.10-1.18 g/mL[127]; they carry nucleic acid molecules such as miRNAs, cytosolic proteins and mRNAs and bind to receptors to mediate intracellular signal transduction and change cell functions. Exosomes secreted by adipose mesenchymal stem cells promote the proliferation of vascular endothelial stem cells, angiogenesis, wound granulation tissue formation, and growth factor expression and reduce the levels of inflammation- and oxidative stress-related proteins in a high-glucose environment[128]. Exosomes secreted by umbilical cord blood mesenchymal stem cells induce wound angiogenesis[129]. In a study that treated chronic wound skin of diabetic rats with exosomes secreted by PRP, exosomes effectively induced the proliferation and migration of fibroblasts and endothelial cells and improved the angiogenesis and re-epithelialization of chronic wounds[130]. Mesenchymal stem cell exosomes derived from menstrual blood also promote neovascularization and increase the amount of neovascularization in the skin of diabetic mice[131]. Exosomes are characterized by a simple structure, lack of replicability, lack of genetic material in the stem cell nucleus, shorter action time, smaller particle size for easy diffusion in vivo, and other properties. At the same time, in vivo studies have confirmed that they have similar biological activity to stem cells intreating DFUs. Therefore, stem cell derivatives with higher safety and simpler mechanisms of action will have better development prospects. However, exosomes are also associated with various problems, such as a high preparation cost, hampering large-scale production. Methods to produce uniform and reliable exocrine therapeutic drugs is an important research topic for the clinical application of exosomes in the future.
Due to the inconvenience caused by the characteristics of stem cells in preparations for the external use of stem cells, an increasing amount of research is devoted to the development of excipients that provide support for stem cells, such as collagen scaffolds and cell gels, to prolong the maintenance of the efficacy of preparations for the external use of stem cells. The extracellular matrix (ECM) is a noncellular three-dimensional polymer network composed of collagen, elastin, proteoglycan/glycosaminoglycan, laminin, fibronectin and other glycoproteins[132]. It provides extracellular scaffolds for cells and interacts with cells. The ECM directly or indirectly affects the shape, metabolism, migration, proliferation and apoptosis of cells. Studies have confirmed that it is closely related to immunity, inflammation, angiogenesis, wound healing and malignant transformation[133]; therefore, maintaining ECM homeostasis is very important for DFU healing. Hyaluronic acid is an important component of the skin ECM. It affects many processes, such as cell migration, proliferation, inflammatory reactions and angiogenesis, in the proliferation stage of wound healing and plays an important role in wound healing and tissue repair[134]. However, the hyaluronic acid content is reduced in DFU skin, resulting in delayed wound healing[135]. Collagen promotes myofibroblast differentiation and fibrosis to maintain the ECM structure and promote healing[136], but collagen deposition in DFU skin reduces the skin thickness and integrity[137]. In individuals with diabetes, the production of matrix metalloproteinases in the ECM increases, and the ratio of matrix metalloproteinases/tissue inhibitors of metalloproteinases increases, aggravating the inflammatory response and leading to an imbalance in ECM homeostasis[138]. In DFU wound tissues, the secretion of matrix metalloproteinases is increased, and the levels of matrix metalloprotease-hydrolyzed ECM fragments are increased. Studies have confirmed that the occurrence of inflammation in vivo is significantly related to the presence of a large number of ECM fragments and their receptors[139]. At the same time, the production of ECM fragments in the inflammatory process also activates immune cells, leading to the continuous occurrence of inflammatory reactions[140]. The interaction between the abnormally expressed ECM and the inflammatory response causes a high level of inflammation to persist in DFU wounds for a long time and makes wound healing difficult. In view of the complexity and safety of the stem cell therapy mechanism and the special disease characteristics of DFUs, the development of stem cell preparations for the external treatment of DFUs, such as those combined with ECMs caffolds, is another effective technical approach showing considerable application prospects and will certainly play an important role in future clinical applications.
Negative pressure wound therapy (NPWT) includes two modes: vacuum-assisted closure (VAC) and vacuum sealing drainage (VSD). The pipeline used by VAC has poor hydrophilicity and a high supporting force. The pipeline is placed on the surface of the dressing to form a device similar to a suction cup. Wound healing is promoted by adjusting the negative pressure level and selecting the intermittent mode[141]. The drainage tube adopted by VSD has high plasticity, good hydrophilicity and contains side holes. It covers the dressings and wound surface with a fully closed and translucent polyurethane film to form a closed space. The necrotic tissues and secretions on the wound surface are drained by negative pressure to promote wound cleaning; it is mainly used for drainage of deep wounds and body cavities. VAC focuses on the treatment of wounds on the body surface and exerts a good effect on treating DFUs, limb soft tissue lacerations, lower limb venous ulcers, deep pressure ulcers, and other wounds[142-144].
NPWT is widely used to treat DFUs as an acute and chronic wound treatment technology. Armstrong et al[142] suggested that, compared with standard treatment, VAC accelerated wound healing, improved wound healing ratio and reduced the re-amputation rate when treating complicated diabetic foot wounds. In the meta-analysis by Liu et al[145], compared with conventional dressing changes, VAC reduced the area and depth of DFU to a greater extent, improved the complete healing rate of ulcer, shortened the healing time of ulcer, reduced the amputation rate of patients, and improved cost-effectiveness.
The mechanism of NPWT is as follows: (1) Keep the wound moist and stabilize the wound environment pain[146]; and (2) inhibit bacterial growth. Weed et al[147] indicated that after treatment with negative pressure drainage technology, the number of bacteria in the wound, particularly gram-negative bacteria, was significantly reduced. Additional components of the mechanism include: (1) Improving wound blood perfusion and promoting wound healing[148]; (2) promoting cell proliferation, angiogenesis and wound tissue repair[149]; and (3) regulating the signaling pathway to modulate cytokine expression[150].
Before using NPWT, the necrotic tissue and dead bone on the wound surface should be completely removed. NPWT also has contraindications, such as deep wound infection, severe ischemia, eschar or necrosis, active bleeding, coagulation dysfunction, exposure of blood vessels or nerves or tendons or ligaments, untreated osteomyelitis, wet gangrene, and malignant tumors. At the same time, the use of NPWT may lead to tube plugging, poor drainage, residual dressings, wound maceration, residual dressings, and other complications.
Based on the limitations of NPWT, negative pressure wound therapy with installation (NEWTi) emerged at a historic moment. It combines negative pressure therapy with liquid perfusion technology to accelerate the cooperative use of wound water and promote the dissolution and clearance of deep necrotic tissues by intermittently or continuously perfusing solutions to closed wounds. The destruction of biofilms and autolytic debridement are the main factors contributing to the superiority of NPWTi to NPWT. However, a uniform standard for the selection of irrigation solution, irrigation time, irrigation speed, and irrigation frequency is unavailable when NPWTi is used to treat DFUs.
At present, the basic principle for the prevention and treatment of neurogenic DFUs is to redistribute the increased local pressure on the foot[151,152], reduce the plantar pressure and shear force, and promote wound healing. Therefore, the selection of a suitable decompression device according to the actual situation of the patient is very important to prevent foot ulcers[153]. Offloading is divided into nonsurgical offloading and surgical offloading. Nonsurgical offloading provides external decom-pression through the use of individually customized or prefabricated devices. The efficacy is determined by whether the patient can continue to wear the decompression device. Currently, the most commonly used pressure reducing devices are the total contact cast (TCC) and detachable cast. TCC is the most effective decompression technique for the treatment of neurogenic DFUs[154] and is even the gold standard for foot restraint and treatment of DFUs[14]. Studies have shown that TCC can reduce the pressure at the ulcer by 84%-92%[155], and it is effective for most nonischemic and noninfectious diabetic plantar ulcers, with an ulcer healing rate of 69.6%-73.9%[156,157]. A TCC can reduce local inflammation, help reduce or delay edema during wound healing, accelerate ulcer healing, and possibly protect the foot from infection because it is not easy to disassemble, which increases the patient's compliance with use and may be an important reason for its benefit[158,159]. However, as TCCs are not easy to disassemble and patients may easily fall while wearing them, their use limits patients’ daily activities. Moreover, inappropriately fitting braces may cause skin irritation, even skin ulceration and infection, and muscle weakness may occur after long-term use. At the same time, TCC use requires the cooperation of experienced doctors, technicians and patients, and consequently, the application of TCCs is limited. The detachable plaster branch has the advantages of easy disassembly, easy observation of wounds, convenient local treatment of wounds, and it can be used for the treatment of infected wounds and superficial ulcers. However, it also has the disadvantages of poor patient compliance and the reduction of the local decompression effect due to irregular wearing. Therefore, patients must be educated while repeatedly emphasizing the benefits of consistently wearing the device to achieve the therapeutic effect[160]. For patients who do not accept gypsum braces, felt-like foam pads and appropriate therapeutic shoes can also be used for decompression treatment[72]. Studies have confirmed that therapeutic shoes reduce plantar pressure and prevent ulcer recurrence[161]. Crutches, walking aids and wheelchairs may also be used for decompression, but some devices increase the pressure on the healthy foot during use, which will increase the incidence of new ulcers on the healthy side[155]. At the same time, the use of these devices is limited due to the lack of upper limb strength and perseverance to use these devices independently[162].
Surgical decompression treatment serves to redistribute the pressure or change the position of the pressure points through surgery, with the purpose of permanently changing the internal pressure point. When a patient does not exhibit complete local decompression after using the optimized shoes and tools and ulcers occur or the patient cannot decompress after ulcer healing and after using the optimized shoes and tools, then he or she can be decompressed during amputation. Surgical decompression usually includes Achilles tendon extension, metatarsal head resection, arthroplasty, and toe flexor tendon resection[163]. Patients with diabetes are prone to shortening of the calf gastrocnemius muscle, which will lead to a continuous increase in the plantar pressure of the forefoot. The extension of the Achilles tendon may reduce the plantar pressure of the forefoot[164]. Studies have confirmed that Achilles tendon lengthening promotes wound healing and reduces ulcer recurrence in patients with neuropathic plantar ulcers and horseshoe foot[165]. Diabetic neuropathy leads to a high pressure load on the plantar skin above the metatarsal head. Removing these biomechanical factors may reduce pressure and facilitate wound healing. For nerve plantar ulcers with difficult healing, early removal of the metatarsal head may be the key to promoting wound ulcer healing[166]. Compared with traditional conservative treatment, metatarsal head resection has a higher healing rate and lower infection rate and ulcer recurrence rate[167]. At the same time, metatarsal head resection promotes the healing of plantar ulcers, which is not related to sex, age, body mass index, height, weight, diabetes duration or the duration of preoperative ulcers[168]. Arthroplasty is an effective procedure for the treatment of recurrent or complex neurological DFUs. Using routine treatment and decompression, metatarsal finger arthroplasty results in a faster healing rate and a lower recurrence rate than the standard treatment[169]. The flexor pollicis longus and flexor digitorum longus can be used to decompress the toe and make the toe tip more flexible[170]; a systematic review confirmed that this operation exerts a good therapeutic effect on closing wounds and newly formed ulcers[171].
Low-level laser therapy (LLLT) uses low-energy light to stimulate the wound surface and produce a series of pathophysiological reactions mainly through photobiological regulation. It does not directly induce photothermal injury of the wound tissue and does not damage the normal tissue cells at the wound surface[172]. In the study by Kaviani et al[173], 8 patients in the LLLT group achieved complete healing after 20 wk, while only 3 patients in the control group achieved complete healing. Although the difference was not statistically significant, the average time of complete healing in patients receiving LLLT (11 wk) was less than that in the control group (14 wk), suggesting that LLLT might accelerate the healing process of chronic DFUs and shorten the time of complete healing, but the sample size of this experiment was small. Another analysis of the efficacy of LLLT in the process of chronic wound tissue repair of DFUs showed that the tissue repair index in the LLLT treatment group increased significantly, mainly because LLLT shortened the inflammatory period, promoted angiogenesis and the production of extracellular matrix components, and accelerated the healing process[173,174]. Percival et al[175] proposed that LLLT promotes wound healing by inhibiting the microbial membrane of chronic wounds, especially cocci and some gram-negative bacteria. Other studies have shown that LLLT promotes wound healing by improving the blood flow and autonomic nervous system regulation of DFUs[176]. A systematic review and meta-analysis of the efficacy of low-dose laser treatment of DFUs[177] found that the ulcer area in the LLLT treatment group was significantly reduced by 30.90% compared with the control group. Compared with the control group, the ulcer area in the treatment group decreased by 4.2 cm2. The probability of complete healing of DFUs was 4.65 times higher than that of the control group, indicating that LLLT may accelerate wound healing and reduce the area of DFUs. However, the review did not provide the best laser treatment parameters. However, in another review, LLLT was shown to be safe and effective in treating DFUs. The laser parameters were 632.8-685 nm, 50 mW/cm2, and 3–6 J/cm2; the irradiation time was 30–80 s, three times a week, and the duration of one month was beneficial for the prognosis of DFU wounds in patients[178]. Because the pathophysiological mechanism of DFUs is complex and the prognosis of the ulcer surface is different due to the diverse ulcer surfaces and different laser parameters, more rigorous, high-quality and large-sample RCTs are needed to determine the best treatment parameters for different types of ulcers.
The incidence of DFUs is high, and the amputation rate is high; moreover, ulcer healing is slow, and the treatment effect is relatively poor. Therefore, the prevention of DFUs is particularly important. However, people currently focus on treatment after the occurrence of DFUs. Researchers mainly focus on medical treatment rather than prevention. Cesare Miranda[179] suggested that comprehensive management should be implemented to prevent DFUs and provided a flow chart for the prevention of DFUs, including DFU education, blood glucose control, management of PAD, identification of risky feet, regular inspection of susceptible feet, long-term wearing of appropriate shoes, and treatment of ulcer risk factors. DFU education can reduce the incidence of DFUs and amputations. It encourages patients to conduct foot self-examinations, identify risk factors, provide appropriate self-care and treat feet with any signs of pre-ulceration[153]. However, the smooth performance of this examination is usually affected by the decrease in vision and limited movement of patients. Through regular foot screening and follow-up of patients with diabetes, the incidence of DFUs and the amputation rate have significantly decreased, but only 20%-30% of patients in China undergo regular foot screening[180]. Studies have shown that the use of diabetic foot treatment shoes and insoles may reduce the ulcer recurrence rate by 30%-50%, but the ulcer recurrence rate is still as high as 30%[181]. In the study by Frykberg et al[182], a new type of remote wireless intelligent temperature monitoring foot pad system was provided for patients with previous DFUs, which is a wireless temperature foot pad that can be used in daily life and senses changes in and asymmetry of foot temperature. The research results show that the intelligent detection system accurately predicts the experimental patients with recurrent DFUs. However, this experiment has its own limitations, including its nonintervention design, small sample size, short experimental time, lack of evaluation of other factors and costs that may affect the occurrence of DFUs, and artificial bias. The results thus require further confirmation. Nevertheless, the results of this study are still very meaningful, suggesting that more intelligent devices can be further developed for the prevention and treatment of DFUs and can thereby reduce the familial and social burdens related to patients with DFUs.
DFUs are one of the serious complications of diabetes. Many risk factors lead to the occurrence of the disease, and the amputation rate is high. Once diabetes is diagnosed, we should perform more work on the management of diabetes, including screening for high-risk factors for DFUs, such as neuropathy and arteriopathy. The high incidence of DFUs may be related to the lack of DFU risk education programs, the insufficient attention of patients, the low rate of foot examination, and the poor knowledge of medical personnel. Boulton et al[183] found that less than 20% of patients with diabetes received a foot examination provided by medical and health professionals. Therefore, foot care education should be provided to all patients with diabetes and the risk of DFU should be evaluated at a minimum of annually[184].
With the development of artificial intelligence, intelligent detection instruments and evaluation tools (such as intelligent insoles) can be applied to the prevention and treatment of DFUs. The management of DFUs requires multidisciplinary cooperation, mainly including endocrinologists, vascular surgeons, orthopedic doctors, wound specialists, shoe technicians, rehabilitation physicians, psychological consultants and specialized nurses. The correct evaluation and comprehensive management of DFUs by multidisciplinary teams are essential to protect the function and quality of life of patients. Optimizing diabetes management is still the most important step to prevent diabetes-related complications[185,186]. The effect of intensive treatment on the prognosis of DFUs requires further study. In patients with diabetes complicated with PAD, sodium glucose co-transporter 2 inhibitors or GLP-1 receptor agonists are recommended[145,187]. Both DDP-IV inhibitors and GLP-1 receptor agonists promote DFU healing[187]. When patients with DFUs choose hypoglycemic drugs, they should not only consider the hypoglycemic effect but also consider whether cardiovascular risk factors are present and whether these drugs can promote ulcer healing. Statins, antiplatelet agents, ACEIs and ARBs are effective in the secondary prevention of cardiovascular events in patients with PAD. For patients with PAD com-plicated with diabetes, the combination of low-dose rivarsaban and aspirin reduces major limb adverse events, including amputation[60], but the risk of bleeding must be monitored. Offloading relieves plantar pressure and shear force to promote wound healing. The value of decompression shoes lies in preventing ulcers, not in using them during the treatment of active ulcers[188]. Surgical offloading is mainly employed to treat specific foot ulcers, usually when other nonsurgical offloading interventions fail. Internal offloading and external offloading are used together to promote wound healing. Necrotic tissue and the microbial membrane are removed, and chronic wounds are transformed into acute wounds. A systematic review reviewed the effect of surgical debridement on DFU healing. The results indicated that the higher the application frequency of surgical debridement, the better the results[189]. However, excessive debridement is not conducive to ulcer healing. An appropriate debridement frequency and debridement method should be selected for different DFUs. The combined use of antibiotics, wound dressings and NPWT may accelerate wound healing. However, the efficacy of oxygen therapy must be confirmed in more high-quality studies. Additionally, the specific parameters of LLLT treatment for different DFUs also require strict, large-sample RCTs researches to provide data. Biological scaffolds, stem cells, exosomes, cell matrix, growth factors and PRP represent new approaches for the treatment of DFUs. The preliminary data seemed positive and revealed a potential effect, but the specific mechanisms of action of these therapies are not clear, and further clinical research may provide better suggestions. In summary, multidisciplinary combination treatment should be adopted in the treatment of DFUs.
The current situation is that the screening rate and follow-up rate of DFUs are low, the incidence rate and the amputation rate are high, and many treatment methods are available, but the effect is not satisfactory. However, with the development of the information age, people's understanding of diabetes and DFU has gradually improved, and various new technologies have been continuously developed, which provides opportunities for the management of DFUs. In the future, comprehensive prevention and treatment management of DFUs are needed to avoid the occurrence of DFUs, effectively shorten the healing time of DFUs, improve the clinical cure rate, reduce the amputation rate, improve the standard of living of patients with DFUs, and reduce the social burden. This task may be a complex, huge and meaningful project.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miranda C, Italy; Mostafavinia A, Iran; Mrozikiewicz-Rakowska B, Poland; Terabe Y, Japan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5867] [Article Influence: 977.8] [Reference Citation Analysis (8)] |
| 2. | Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1030] [Cited by in RCA: 990] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 3. | Petrova N, Edmonds M. Emerging drugs for diabetic foot ulcers. Expert Opin Emerg Drugs. 2006;11:709-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 4. | Jiang Y, Wang X, Xia L, Fu X, Xu Z, Ran X, Yan L, Li Q, Mo Z, Yan Z, Ji Q. A cohort study of diabetic patients and diabetic foot ulceration patients in China. Wound Repair Regen. 2015;23:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1537] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 6. | Vinik AI. CLINICAL PRACTICE. Diabetic Sensory and Motor Neuropathy. N Engl J Med. 2016;374:1455-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Won JC, Park TS. Recent Advances in Diagnostic Strategies for Diabetic Peripheral Neuropathy. Endocrinol Metab (Seoul). 2016;31:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ; North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 637] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 9. | Wu Q, Lei X, Chen L, Zheng Y, Huang H, Qian C, Liang Z. Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: a case report. Ann Transl Med. 2018;6:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Hazari A, Maiya A, Agouris I, Monteiro A, Shivashankara. Prediction of peak plantar pressure for diabetic foot: The regressional model. Foot (Edinb). 2019;40:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Dunyach-Remy C, Ngba Essebe C, Sotto A, Lavigne JP. Staphylococcus aureus Toxins and Diabetic Foot Ulcers: Role in Pathogenesis and Interest in Diagnosis. Toxins (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 672] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 13. | Ndip A, Jude EB. Emerging evidence for neuroischemic diabetic foot ulcers: model of care and how to adapt practice. Int J Low Extrem Wounds. 2009;8:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2301] [Article Influence: 287.6] [Reference Citation Analysis (2)] |
| 15. | Lavery LA, Lavery DC, Hunt NA, La Fontaine J, Ndip A, Boulton AJ. Amputations and foot-related hospitalisations disproportionately affect dialysis patients. Int Wound J. 2015;12:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Lane KL, Abusamaan MS, Voss BF, Thurber EG, Al-Hajri N, Gopakumar S, Le JT, Gill S, Blanck J, Prichett L, Hicks CW, Sherman RL, Abularrage CJ, Mathioudakis NN. Glycemic control and diabetic foot ulcer outcomes: A systematic review and meta-analysis of observational studies. J Diabetes Complications. 2020;34:107638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Xiang J, Wang S, He Y, Xu L, Zhang S, Tang Z. Reasonable Glycemic Control Would Help Wound Healing During the Treatment of Diabetic Foot Ulcers. Diabetes Ther. 2019;10:95-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol. 2011;131:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Fesseha BK, Abularrage CJ, Hines KF, Sherman R, Frost P, Langan S, Canner J, Likes KC, Hosseini SM, Jack G, Hicks CW, Yalamanchi S, Mathioudakis N. Association of Hemoglobin A1c and Wound Healing in Diabetic Foot Ulcers. Diabetes Care. 2018;41:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5314] [Cited by in RCA: 5266] [Article Influence: 309.8] [Reference Citation Analysis (0)] |
| 21. | American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care. 2006;29 Suppl 1:S4-42. [PubMed] |
| 22. | Dutta A, Bhansali A, Rastogi A. Early and Intensive Glycemic Control for Diabetic Foot Ulcer Healing: A Prospective Observational Nested Cohort Study. Int J Low Extrem Wounds. 2021;15347346211033458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Hasan R, Firwana B, Elraiyah T, Domecq JP, Prutsky G, Nabhan M, Prokop LJ, Henke P, Tsapas A, Montori VM, Murad MH. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63:22S-28S.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Dissanayake A, Vandal AC, Boyle V, Park D, Milne B, Grech R, Ng A. Does intensive glycaemic control promote healing in diabetic foot ulcers? BMJ Open. 2020;10:e029009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Fernando ME, Seneviratne RM, Tan YM, Lazzarini PA, Sangla KS, Cunningham M, Buttner PG, Golledge J. Intensive vs conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2016;CD010764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Hinchliffe RJ, Brownrigg JR, Andros G, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC; International Working Group on the Diabetic Foot. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev. 2016;32 Suppl 1:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Aiello A, Anichini R, Brocco E, Caravaggi C, Chiavetta A, Cioni R, Da Ros R, De Feo ME, Ferraresi R, Florio F, Gargiulo M, Galzerano G, Gandini R, Giurato L, Graziani L, Mancini L, Manzi M, Modugno P, Setacci C, Uccioli L; Italian Society of Diabetes; Italian Society of Radiology; Italian Society of Vascular Endovascular Surgery. Treatment of peripheral arterial disease in diabetes: a consensus of the Italian Societies of Diabetes (SID, AMD), Radiology (SIRM) and Vascular Endovascular Surgery (SICVE). Nutr Metab Cardiovasc Dis. 2014;24:355-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes--a review. Diabet Med. 2010;27:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S73-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 553] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 30. | Dhatariya K, Bain SC, Buse JB, Simpson R, Tarnow L, Kaltoft MS, Stellfeld M, Tornøe K, Pratley RE; LEADER Publication Committee on behalf of the LEADER Trial Investigators. The Impact of Liraglutide on Diabetes-Related Foot Ulceration and Associated Complications in Patients With Type 2 Diabetes at High Risk for Cardiovascular Events: Results From the LEADER Trial. Diabetes Care. 2018;41:2229-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Huang H, Wang L, Qian F, Chen X, Zhu H, Yang M, Zhang C, Chu M, Wang X, Huang X. Liraglutide via Activation of AMP-Activated Protein Kinase-Hypoxia Inducible Factor-1α-Heme Oxygenase-1 Signaling Promotes Wound Healing by Preventing Endothelial Dysfunction in Diabetic Mice. Front Physiol. 2021;12:660263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 32. | Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond). 2019;133:2415-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5372] [Article Influence: 671.5] [Reference Citation Analysis (0)] |
| 34. | Ryan PB, Buse JB, Schuemie MJ, DeFalco F, Yuan Z, Stang PE, Berlin JA, Rosenthal N. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab. 2018;20:2585-2597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 35. | Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 467] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 36. | Marfella R, Sasso FC, Rizzo MR, Paolisso P, Barbieri M, Padovano V, Carbonara O, Gualdiero P, Petronella P, Ferraraccio F, Petrella A, Canonico R, Campitiello F, Della Corte A, Paolisso G, Canonico S. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diabetes Res. 2012;2012:892706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Long M, Cai L, Li W, Zhang L, Guo S, Zhang R, Zheng Y, Liu X, Wang M, Zhou X, Wang H, Li X, Li L, Zhu Z, Yang G, Zheng H. DPP-4 Inhibitors Improve Diabetic Wound Healing via Direct and Indirect Promotion of Epithelial-Mesenchymal Transition and Reduction of Scarring. Diabetes. 2018;67:518-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Ku HC, Liang YJ. Incretin-based therapy for diabetic ulcers: from bench to bedside. Expert Opin Investig Drugs. 2018;27:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Vatankhah N, Jahangiri Y, Landry GJ, Moneta GL, Azarbal AF. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regen. 2017;25:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Althouse AD, Abbott JD, Sutton-Tyrrell K, Forker AD, Lombardero MS, Buitrón LV, Pena-Sing I, Tardif JC, Brooks MM; BARI 2D Study Group. Favorable effects of insulin sensitizers pertinent to peripheral arterial disease in type 2 diabetes: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Diabetes Care. 2013;36:3269-3275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Li YJ, Guan H, Ye W, Liu CW. [Pulse wave velocity a sensitive predicator for peripheral artery disease among diabetic patients]. Zhonghua Wai Ke Za Zhi. 2009;47:1487-1490. [PubMed] |
| 42. | Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1027] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 43. | Soyoye DO, Abiodun OO, Ikem RT, Kolawole BA, Akintomide AO. Diabetes and peripheral artery disease: A review. World J Diabetes. 2021;12:827-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (7)] |
| 44. | Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, Venermo M, Zierler RE, Hinchliffe RJ, Schaper NC. Effectiveness of revascularisation of the ulcerated foot in patients with diabetes and peripheral artery disease: A systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | American Diabetes Association. 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S86-S104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 366] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 46. | Xia N, Morteza A, Yang F, Cao H, Wang A. Review of the role of cigarette smoking in diabetic foot. J Diabetes Investig. 2019;10:202-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Sayiner ZA, Can FI, Akarsu E. Patients' clinical charecteristics and predictors for diabetic foot amputation. Prim Care Diabetes. 2019;13:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Zhang X, Ran X, Xu Z, Cheng Z, Shen F, Yu Y, Gao L, Chai S, Wang C, Liu J, Sun Z, Zhao J, Ji L; China DIA-LEAD Study Investigators. Epidemiological characteristics of lower extremity arterial disease in Chinese diabetes patients at high risk: a prospective, multicenter, cross-sectional study. J Diabetes Complications. 2018;32:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2620] [Cited by in RCA: 2419] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 50. | Bavry AA, Anderson RD, Gong Y, Denardo SJ, Cooper-Dehoff RM, Handberg EM, Pepine CJ. Outcomes Among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational VErapamil-SR/Trandolapril STudy. Hypertension. 2010;55:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Böhm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, Sliwa K, Weber MA, Williams B, Yusuf S. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017;389:2226-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 52. | Armstrong EJ, Chen DC, Singh GD, Amsterdam EA, Laird JR. Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use is associated with reduced major adverse cardiovascular events among patients with critical limb ischemia. Vasc Med. 2015;20:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2624] [Article Influence: 656.0] [Reference Citation Analysis (0)] |
| 54. | Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, Banerjee S, Creager MA, Bhatt DL; REACH Registry Investigators. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 55. | Society for Vascular Surgery Lower Extremity Guidelines Writing Group; Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, Moneta GL, Murad MH, Powell RJ, Reed AB, Schanzer A, Sidawy AN; Society for Vascular Surgery. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61:2S-41S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 574] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 56. | Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 57. | Wong PF, Chong LY, Mikhailidis DP, Robless P, Stansby G. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;CD001272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, Branch KRH, Probstfield J, Bosch J, Shestakovska O, Szarek M, Maggioni AP, Widimský P, Avezum A, Diaz R, Lewis BS, Berkowitz SD, Fox KAA, Ryden L, Yusuf S; COMPASS Steering Committee and Investigators. Role of Combination Antiplatelet and Anticoagulation Therapy in Diabetes Mellitus and Cardiovascular Disease: Insights From the COMPASS Trial. Circulation. 2020;141:1841-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 59. | Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O'Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 595] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 60. | Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, Leong D, Anand SS, Störk S, Branch KRH, Bhatt DL, Verhamme PB, O'Donnell M, Maggioni AP, Lonn EM, Piegas LS, Ertl G, Keltai M, Cook Bruns N, Muehlhofer E, Dagenais GR, Kim JH, Hori M, Steg PG, Hart RG, Diaz R, Alings M, Widimsky P, Avezum A, Probstfield J, Zhu J, Liang Y, Lopez-Jaramillo P, Kakkar A, Parkhomenko AN, Ryden L, Pogosova N, Dans A, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik T, Vinereanu D, Tonkin AM, Lewis BS, Felix C, Yusoff K, Metsarinne K, Fox KAA, Yusuf S; COMPASS Investigators. Pantoprazole to Prevent Gastroduodenal Events in Patients Receiving Rivaroxaban and/or Aspirin in a Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology. 2019;157:403-412.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 61. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1152] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 62. | Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 501] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 63. | Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the Infectious Diseases Society of America's diabetic foot infection classification system. Clin Infect Dis. 2007;44:562-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 64. | Younes NA, Bakri FG. Diabetic foot infection. Saudi Med J. 2006;27:596-603. [PubMed] |
| 65. | Akhi MT, Ghotaslou R, Asgharzadeh M, Varshochi M, Pirzadeh T, Memar MY, Zahedi Bialvaei A, Seifi Yarijan Sofla H, Alizadeh N. Bacterial etiology and antibiotic susceptibility pattern of diabetic foot infections in Tabriz, Iran. GMS Hyg Infect Control. 2015;10:Doc02. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 66. | Li X, Qi X, Yuan G, Ju S, Yu Z, Deng W, Liu Y, Li Y, Bu X, Ding M, Li Q, Guo X. Microbiological profile and clinical characteristics of diabetic foot infection in northern China: a retrospective multicentre survey in the Beijing area. J Med Microbiol. 2018;67:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Djahmi N, Messad N, Nedjai S, Moussaoui A, Mazouz D, Richard JL, Sotto A, Lavigne JP. Molecular epidemiology of Staphylococcus aureus strains isolated from inpatients with infected diabetic foot ulcers in an Algerian University Hospital. Clin Microbiol Infect. 2013;19:E398-E404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Sapico FL, Witte JL, Canawati HN, Montgomerie JZ, Bessman AN. The infected foot of the diabetic patient: quantitative microbiology and analysis of clinical features. Rev Infect Dis. 1984;6 Suppl 1:S171-S176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 115] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One. 2008;3:e3326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 398] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 70. | Wolcott RD, Cox SB, Dowd SE. Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. J Wound Care. 2010;19:272-278, 280. [PubMed] |
| 71. | Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 72. | Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA; IWGDF Editorial Board. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 73. | Srivastava P, Sivashanmugam K. Combinatorial Drug Therapy for Controlling Pseudomonas aeruginosa and Its Association With Chronic Condition of Diabetic Foot Ulcer. Int J Low Extrem Wounds. 2020;19:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Sherman RA. Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol. 2009;3:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Whitaker IS, Twine C, Whitaker MJ, Welck M, Brown CS, Shandall A. Larval therapy from antiquity to the present day: mechanisms of action, clinical applications and future potential. Postgrad Med J. 2007;83:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Beasley WD, Hirst G. Making a meal of MRSA-the role of biosurgery in hospital-acquired infection. J Hosp Infect. 2004;56:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Armstrong DG, Mossel J, Short B, Nixon BP, Knowles EA, Boulton AJ. Maggot debridement therapy: a primer. J Am Podiatr Med Assoc. 2002;92:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Lerch K, Linde HJ, Lehn N, Grifka J. Bacteria ingestion by blowfly larvae: an in vitro study. Dermatology. 2003;207:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Simmons SW. A Bactericidal Principle in Excretions of Surgical Maggots which Destroys Important Etiological Agents of Pyogenic Infections. J Bacteriol. 1935;30:253-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Evans R, Dudley E, Nigam Y. Detection and partial characterization of antifungal bioactivity from the secretions of the medicinal maggot, Lucilia sericata. Wound Repair Regen. 2015;23:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Altincicek B, Vilcinskas A. Metamorphosis and collagen-IV-fragments stimulate innate immune response in the greater wax moth, Galleria mellonella. Dev Comp Immunol. 2006;30:1108-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Bohova J, Majtan J, Majtan V, Takac P. Selective Antibiofilm Effects of Lucilia sericata Larvae Secretions/Excretions against Wound Pathogens. Evid Based Complement Alternat Med. 2014;2014:857360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Nigam Y, Dudley E, Bexfield A, Bond AE, Evans J, James J. The Physiology of Wound Healing by the Medicinal Maggot, Lucilia sericata. Adv In Insect Phys. 2010;39:39-81. [DOI] [Full Text] |
| 84. | Rayner K. Larval therapy in wound debridement. Prof Nurse. 1999;14:329-333. [PubMed] |
| 85. | Cazander G, Pritchard DI, Nigam Y, Jung W, Nibbering PH. Multiple actions of Lucilia sericata larvae in hard-to-heal wounds: larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection. Bioessays. 2013;35:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Sherman RA. Mechanisms of maggot-induced wound healing: what do we know, and where do we go from here? Evid Based Complement Alternat Med. 2014;2014:592419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 87. | Szczepanowski Z, Grabarek BO, Boroń D, Tukiendorf A, Kulik-Parobczy I, Miszczyk L. Microbiological effects in patients with leg ulcers and diabetic foot treated with Lucilia sericata larvae. Int Wound J. 2022;19:135-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Gottrup F, Apelqvist J, Bjarnsholt T, Cooper R, Moore Z, Peters EJ, Probst S. Antimicrobials and Non-Healing Wounds. Evidence, controversies and suggestions-key messages. J Wound Care. 2014;23:477-478, 480, 482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Scimeca CL, Bharara M, Fisher TK, Kimbriel H, Mills JL, Armstrong DG. An update on pharmacological interventions for diabetic foot ulcers. Foot Ankle Spec. 2010;3:285-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg. 2017;44:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 91. | Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8:217-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 927] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 92. | Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 667] [Cited by in RCA: 587] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 93. | Su J, Li J, Liang J, Zhang K. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 94. | Zhang L, Yin H, Lei X, Lau JNY, Yuan M, Wang X, Zhang F, Zhou F, Qi S, Shu B, Wu J. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front Bioeng Biotechnol. 2019;7:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 95. | Sackheim K, De Araujo TS, Kirsner RS. Compression modalities and dressings: their use in venous ulcers. Dermatol Ther. 2006;19:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing vs control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018;6:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 97. | Nikol S, Baumgartner I, Van Belle E, Diehm C, Visoná A, Capogrossi MC, Ferreira-Maldent N, Gallino A, Graham Wyatt M, Dinesh Wijesinghe L, Fusari M, Stephan D, Emmerich J, Pompilio G, Vermassen F, Pham E, Grek V, Coleman M, Meyer F. Therapeutic Angiogenesis With Intramuscular NV1FGF Improves Amputation-free Survival in Patients With Critical Limb Ischemia. Mol Ther. 2008;16:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 98. | Gu Y, Cui S, Wang Q, Liu C, Jin B, Guo W, Chu T, Shu C, Zhang F, Han C, Liu Y. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of Hepatocyte Growth Factor in the Treatment of Critical Limb Ischemia. Mol Ther. 2019;27:2158-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeño-Taborda J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2015;CD008548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 100. | Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, Garcia J, Zaror C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;CD006899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 101. | Gottrup F, Dissemond J, Baines C, Frykberg R, Jensen PØ, Kot J, Kröger K, Longobardi P. Use of Oxygen Therapies in Wound Healing. J Wound Care. 2017;26:S1-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 102. | Oyaizu T, Enomoto M, Yamamoto N, Tsuji K, Horie M, Muneta T, Sekiya I, Okawa A, Yagishita K. Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci Rep. 2018;8:1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | van Neck JW, Tuk B, Fijneman EMG, Redeker JJ, Talahatu EM, Tong M. Hyperbaric oxygen therapy for wound healing in diabetic rats: Varying efficacy after a clinically-based protocol. PLoS One. 2017;12:e0177766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Baroni G, Porro T, Faglia E, Pizzi G, Mastropasqua A, Oriani G, Pedesini G, Favales F. Hyperbaric oxygen in diabetic gangrene treatment. Diabetes Care. 1987;10:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 106. | Löndahl M, Katzman P, Hammarlund C, Nilsson A, Landin-Olsson M. Relationship between ulcer healing after hyperbaric oxygen therapy and transcutaneous oximetry, toe blood pressure and ankle-brachial index in patients with diabetes and chronic foot ulcers. Diabetologia. 2011;54:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 107. | Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M, Glick HA, Thom SR, Mitra N. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36:1961-1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 108. | Li G, Hopkins RB, Levine MAH, Jin X, Bowen JM, Thabane L, Goeree R, Fedorko L, O'Reilly DJ. Relationship between hyperbaric oxygen therapy and quality of life in participants with chronic diabetic foot ulcers: data from a randomized controlled trial. Acta Diabetol. 2017;54:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Fedorko L, Bowen JM, Jones W, Oreopoulos G, Goeree R, Hopkins RB, O'Reilly DJ. Hyperbaric Oxygen Therapy Does Not Reduce Indications for Amputation in Patients With Diabetes With Nonhealing Ulcers of the Lower Limb: A Prospective, Double-Blind, Randomized Controlled Clinical Trial. Diabetes Care. 2016;39:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 110. | Santema KTB, Stoekenbroek RM, Koelemay MJW, Reekers JA, van Dortmont LMC, Oomen A, Smeets L, Wever JJ, Legemate DA, Ubbink DT; DAMO2CLES Study Group. Hyperbaric Oxygen Therapy in the Treatment of Ischemic Lower- Extremity Ulcers in Patients With Diabetes: Results of the DAMO2CLES Multicenter Randomized Clinical Trial. Diabetes Care. 2018;41:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 111. | Frykberg RG, Franks PJ, Edmonds M, Brantley JN, Téot L, Wild T, Garoufalis MG, Lee AM, Thompson JA, Reach G, Dove CR, Lachgar K, Grotemeyer D, Renton SC; TWO2 Study Group. A Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers: The TWO2 Study. Diabetes Care. 2020;43:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 112. | Gholipour-Kanani A, Bahrami SH, Rabbani S. Effect of novel blend nanofibrous scaffolds on diabetic wounds healing. IET Nanobiotechnol. 2016;10:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T; Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1230] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 114. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15188] [Article Influence: 584.2] [Reference Citation Analysis (0)] |
| 115. | Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 116. | Procházka V, Gumulec J, Chmelová J, Klement P, Klement GL, Jonszta T, Czerný D, Krajca J. Autologous bone marrow stem cell transplantation in patients with end-stage chronical critical limb ischemia and diabetic foot. Vnitr Lek. 2009;55:173-178. [PubMed] |
| 117. | Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 118. | Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76:1750-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 119. | Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, Taurand M, Dupuis-Coronas S, Leobon B, Casteilla L. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 120. | Zhao QS, Xia N, Zhao N, Li M, Bi CL, Zhu Q, Qiao GF, Cheng ZF. Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. Int J Biol Sci. 2013;10:80-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 519] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 122. | Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 326] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 123. | Kim SW, Zhang HZ, Guo L, Kim JM, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One. 2012;7:e41105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 124. | Feng J, Doi K, Kuno S, Mineda K, Kato H, Kinoshita K, Kanayama K, Mashiko T, Yoshimura K. Micronized cellular adipose matrix as a therapeutic injectable for diabetic ulcer. Regen Med. 2015;10:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002;12:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of Exosome Composition. Cell. 2019;177:428-445.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 2051] [Article Influence: 341.8] [Reference Citation Analysis (1)] |
| 127. | Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Ayame H, Iwasaki K, Taki A, Oshima N, Morita I. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 128. | Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 129. | Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 573] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 130. | Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 131. | Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 132. | Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1579] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 133. | Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1592] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 134. | King IC, Sorooshian P. Hyaluronan in skin wound healing: therapeutic applications. J Wound Care. 2020;29:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Weindl G, Schaller M, Schäfer-Korting M, Korting HC. Hyaluronic acid in the treatment and prevention of skin diseases: molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol Physiol. 2004;17:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 136. | Seo BR, Chen X, Ling L, Song YH, Shimpi AA, Choi S, Gonzalez J, Sapudom J, Wang K, Andresen Eguiluz RC, Gourdon D, Shenoy VB, Fischbach C. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc Natl Acad Sci U S A. 2020;117:11387-11398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 137. | Blair MJ, Jones JD, Woessner AE, Quinn KP. Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv Wound Care (New Rochelle). 2020;9:127-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 138. | Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 139. | Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 289] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 140. | Wight TN, Kang I, Evanko SP, Harten IA, Chang MY, Pearce OMT, Allen CE, Frevert CW. Versican-A Critical Extracellular Matrix Regulator of Immunity and Inflammation. Front Immunol. 2020;11:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 141. | Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: A review. J Clin Orthop Trauma. 2019;10:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 142. | Armstrong DG, Lavery LA; Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 574] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 143. | Eneroth M, van Houtum WH. The value of debridement and Vacuum-Assisted Closure (V.A.C.) Therapy in diabetic foot ulcers. Diabetes Metab Res Rev. 2008;24 Suppl 1:S76-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Petkar KS, Dhanraj P, Kingsly PM, Sreekar H, Lakshmanarao A, Lamba S, Shetty R, Zachariah JR. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split-thickness skin grafts in burned patients. Burns. 2011;37:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 145. | Liu S, He CZ, Cai YT, Xing QP, Guo YZ, Chen ZL, Su JL, Yang LP. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:533-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 146. | Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery. 2009;146:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 147. | Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg. 2004;52:276-9; discussion 279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 148. | Horch RE, Ludolph I, Müller-Seubert W, Zetzmann K, Hauck T, Arkudas A, Geierlehner A. Topical negative-pressure wound therapy: emerging devices and techniques. Expert Rev Med Devices. 2020;17:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 149. | Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg. 1993;96:488-492. [PubMed] |
| 150. | Borys S, Hohendorff J, Frankfurter C, Kiec-Wilk B, Malecki MT. Negative pressure wound therapy use in diabetic foot syndrome-from mechanisms of action to clinical practice. Eur J Clin Invest. 2019;49:e13067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 151. | Waaijman R, de Haart M, Arts ML, Wever D, Verlouw AJ, Nollet F, Bus SA. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 152. | Bus SA. Priorities in offloading the diabetic foot. Diabetes Metab Res Rev. 2012;28 Suppl 1:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, van Netten JJ; International Working Group on the Diabetic Foot. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 154. | Messenger G, Masoetsa R, Hussain I. A Narrative Review of the Benefits and Risks of Total Contact Casts in the Management of Diabetic Foot Ulcers. J Am Coll Clin Wound Spec. 2017;9:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 155. | Lavery LA, Vela SA, Lavery DC, Quebedeaux TL. Reducing dynamic foot pressures in high-risk diabetic subjects with foot ulcerations. A comparison of treatments. Diabetes Care. 1996;19:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 156. | Lavery LA, Higgins KR, La Fontaine J, Zamorano RG, Constantinides GP, Kim PJ. Randomised clinical trial to compare total contact casts, healing sandals and a shear-reducing removable boot to heal diabetic foot ulcers. Int Wound J. 2015;12:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 157. | Faglia E, Caravaggi C, Clerici G, Sganzaroli A, Curci V, Vailati W, Simonetti D, Sommalvico F. Effectiveness of removable walker cast vs nonremovable fiberglass off-bearing cast in the healing of diabetic plantar foot ulcer: a randomized controlled trial. Diabetes Care. 2010;33:1419-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 158. | Morona JK, Buckley ES, Jones S, Reddin EA, Merlin TL. Comparison of the clinical effectiveness of different off-loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2013;29:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 159. | Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag. 2007;3:65-76. [PubMed] |
| 160. | Bus SA, Armstrong DG, Gooday C, Jarl G, Caravaggi C, Viswanathan V, Lazzarini PA; International Working Group on the Diabetic Foot (IWGDF). Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 161. | Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR; International Working Group on the Diabetic Foot. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32 Suppl 1:99-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 162. | Katz IA, Harlan A, Miranda-Palma B, Prieto-Sanchez L, Armstrong DG, Bowker JH, Mizel MS, Boulton AJ. A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care. 2005;28:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 163. | Ahluwalia R, Maffulli N, Lázaro-Martínez JL, Kirketerp-Møller K, Reichert I. Diabetic foot off loading and ulcer remission: Exploring surgical off-loading. Surgeon. 2021;19:e526-e535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 164. | Lazzarini PA, Jarl G, Gooday C, Viswanathan V, Caravaggi CF, Armstrong DG, Bus SA. Effectiveness of offloading interventions to heal foot ulcers in persons with diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 165. | Meshkin DH, Fagothaman K, Arneson J, Black CK, Episalla NC, Walters ET, Evans KK, Steinberg JS, Attinger CE, Kim PJ. Plantar Foot Ulcer Recurrence in Neuropathic Patients Undergoing Percutaneous Tendo-Achilles Lengthening. J Foot Ankle Surg. 2020;59:1177-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 166. | Yammine K, Kheir N, Assi C. A Meta-Analysis of the Outcomes of Metatarsal Head Resection for the Treatment of Neuropathic Diabetic Foot Ulcers. Adv Wound Care (New Rochelle). 2021;10:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 167. | Piaggesi A, Schipani E, Campi F, Romanelli M, Baccetti F, Arvia C, Navalesi R. Conservative surgical approach vs non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med. 1998;15:412-417. [PubMed] [DOI] [Full Text] |
| 168. | Kalantar Motamedi A, Kalantar Motamedi MA. Determinants of Success After Metatarsal Head Resection for the Treatment of Neuropathic Diabetic Foot Ulcers. J Foot Ankle Surg. 2020;59:909-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Tamir E, Tamir J, Beer Y, Kosashvili Y, Finestone AS. Resection Arthroplasty for Resistant Ulcers Underlying the Hallux in Insensate Diabetics. Foot Ankle Int. 2015;36:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 170. | van Netten JJ, Bril A, van Baal JG. The effect of flexor tenotomy on healing and prevention of neuropathic diabetic foot ulcers on the distal end of the toe. J Foot Ankle Res. 2013;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 171. | Bonanno DR, Gillies EJ. Flexor Tenotomy Improves Healing and Prevention of Diabetes-Related Toe Ulcers: A Systematic Review. J Foot Ankle Surg. 2017;56:600-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 172. | Wu X, Alberico S, Saidu E, Rahman Khan S, Zheng S, Romero R, Sik Chae H, Li S, Mochizuki A, Anders J. Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen. 2015;23:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 173. | Kaviani A, Djavid GE, Ataie-Fashtami L, Fateh M, Ghodsi M, Salami M, Zand N, Kashef N, Larijani B. A randomized clinical trial on the effect of low-level laser therapy on chronic diabetic foot wound healing: a preliminary report. Photomed Laser Surg. 2011;29:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 174. | de Alencar Fonseca Santos J, Campelo MBD, de Oliveira RA, Nicolau RA, Rezende VEA, Arisawa EÂL. Effects of Low-Power Light Therapy on the Tissue Repair Process of Chronic Wounds in Diabetic Feet. Photomed Laser Surg. 2018;36:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 175. | Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012;20:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 176. | Salvi M, Rimini D, Molinari F, Bestente G, Bruno A. Effect of low-level light therapy on diabetic foot ulcers: a near-infrared spectroscopy study. J Biomed Opt. 2017;22:38001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 177. | Zhou Y, Chia HWA, Tang HWK, Lim SYJ, Toh WY, Lim XL, Cheng LJ, Lau Y. Efficacy of low-level light therapy for improving healing of diabetic foot ulcers: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2021;29:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 178. | Santos CMD, Rocha RBD, Hazime FA, Cardoso VS. A Systematic Review and Meta-Analysis of the Effects of Low-Level Laser Therapy in the Treatment of Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2021;20:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 179. | Miranda C, Da Ros R, Marfella R. Update on prevention of diabetic foot ulcer. Arch Med Sci Atheroscler Dis. 2021;6:e123-e131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (4)] |
| 180. | D'Souza MS, Ruppert SD, Parahoo K, Karkada SN, Amirtharaj A, Jacob D, Balachandran S, Al Salmi NM. Foot care behaviors among adults with type 2 diabetes. Prim Care Diabetes. 2016;10:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 181. | Uccioli L, Faglia E, Monticone G, Favales F, Durola L, Aldeghi A, Quarantiello A, Calia P, Menzinger G. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care. 1995;18:1376-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 189] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 182. | Frykberg RG, Gordon IL, Reyzelman AM, Cazzell SM, Fitzgerald RH, Rothenberg GM, Bloom JD, Petersen BJ, Linders DR, Nouvong A, Najafi B. Feasibility and Efficacy of a Smart Mat Technology to Predict Development of Diabetic Plantar Ulcers. Diabetes Care. 2017;40:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 183. | Boulton AJ, Meneses P, Ennis WJ. Diabetic foot ulcers: A framework for prevention and care. Wound Repair Regen. 1999;7:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 184. | Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM; American Diabetes Association. Preventive foot care in diabetes. Diabetes Care. 2004;27 Suppl 1:S63-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 185. | Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:412-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 593] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 186. | Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127:P66-P145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 187. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S111-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 683] [Article Influence: 170.8] [Reference Citation Analysis (0)] |
| 188. | Chatzistergos PE, Gatt A, Formosa C, Farrugia K, Chockalingam N. Optimised cushioning in diabetic footwear can significantly enhance their capacity to reduce plantar pressure. Gait Posture. 2020;79:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 189. | Lebrun E, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |