Published online Dec 15, 2022. doi: 10.4239/wjd.v13.i12.1001
Peer-review started: August 21, 2022
First decision: October 21, 2022
Revised: October 26, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 15, 2022
Processing time: 111 Days and 2.1 Hours
Diabetic foot ulcer (DFU) and poor wound healing are chronic complications in patients with diabetes. The increasing incidence of DFU has resulted in huge pressure worldwide. Diagnosing and treating this condition are therefore of great importance to control morbidity and improve prognosis. Finding new markers with potential diagnostic and therapeutic utility in DFU has gathered increasing interest. Wound healing is a process divided into three stages: Inflammation, proliferation, and regeneration. Non-coding RNAs (ncRNAs), which are small protected molecules transcribed from the genome without protein translation function, have emerged as important regulators of diabetes complications. The deregulation of ncRNAs may be linked to accelerated DFU development and delayed wound healing. Moreover, ncRNAs can be used for therapeutic purposes in diabetic wound healing. Herein, we summarize the role of microRNAs, long ncRNAs, and circular RNAs in diverse stages of DFU wound healing and their potential use as novel therapeutic targets.
Core Tip: Non-coding RNAs (ncRNAs) have emerged as important regulators of diabetic foot and wound healing. NcRNAs can be used for therapeutic purposes in diabetic wound healing. In this study, we summarize the roles of microRNAs, long ncRNAs, and circular RNAs in diverse stages of diabetic foot ulcer wound healing and their potential use as novel therapeutic targets.
- Citation: Tang YB, Uwimana MMP, Zhu SQ, Zhang LX, Wu Q, Liang ZX. Non-coding RNAs: Role in diabetic foot and wound healing. World J Diabetes 2022; 13(12): 1001-1013
- URL: https://www.wjgnet.com/1948-9358/full/v13/i12/1001.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i12.1001
Diabetes mellitus (DM) is a chronic metabolic disease that is rapidly increasing worldwide. DM is a global public health burden with a negative impact on global health and socioeconomic development. Chronic hyperglycemia causes blood vessel inflammation, which leads to macroangiopathy and microangiopathy, particularly diabetic foot ulcer (DFU) and delayed wound healing. Delayed healing of chronic ulcer wounds in patients with diabetes is due to neuropathy, microangiopathy, and immune system dysfunction[1,2]. One of the leading causes of death in patients with diabetes is lower extremity amputation, which accounts for approximately 15% of DFU cases[3]. Different functional and structural microvascular changes in patients with diabetes increase the vulnerability of the skin and contribute to impaired wound healing[4]. DFU contributes to physical and psychological problems that hinder the health economy immensely. Conventional DFU treatments have an inefficient impact on reduction of the amputation rate; thus, a more efficient treatment is needed. Therefore, a better understanding of the molecular mechanisms and biomolecules involved in DFU development is necessary to provide better therapeutic options for wound healing.
Non-coding RNAs (ncRNAs) are potential novel biomarkers transcribed from the genome without protein translation function but can still perform specific biological functions. NcRNAs can be divided into two categories depending on the length of nucleotides; short-stranded RNAs or microRNAs (miRNAs) which are less than 200 nucleotides in length, and long ncRNAs (lncRNAs) which are greater than 200 nucleotides in length. Emerging evidence suggests that ncRNAs have an important regulatory role in various metabolic diseases, such as DM, based on the development of microarray and high-throughput sequencing[5]. In addition, some lncRNAs are covalently bound to the 3’-5’ end, forming circular RNAs (circRNAs)[6]. NcRNAs can be protected from the effects of RNA enzyme activity, temperature changes, and extreme pH values by binding to proteins or being packaged into ex-tracellular vesicles. In this way, ncRNAs can maintain a stable state in the extracellular environment and can be used as a potential biomarker for diagnosing and treating diseases[7-9]. NcRNAs regulate cellular chromatin rearrangements, histone modifications, variable splicing gene modifications, or gene expression; mediate different biological processes; and ultimately influence the development of certain diseases[10]. Exosome-cargoed ncRNAs have been reported as pivotal regulators of angiogenesis during wound closure[11]. This background confers the possible treatment of delayed wound healing using ncRNAs. In this study, we summarize the role and mechanism of miRNAs, lncRNAs, and circRNAs in the pathogenesis and process of wound healing in DFU and the research progress of ncRNAs in cell therapy.
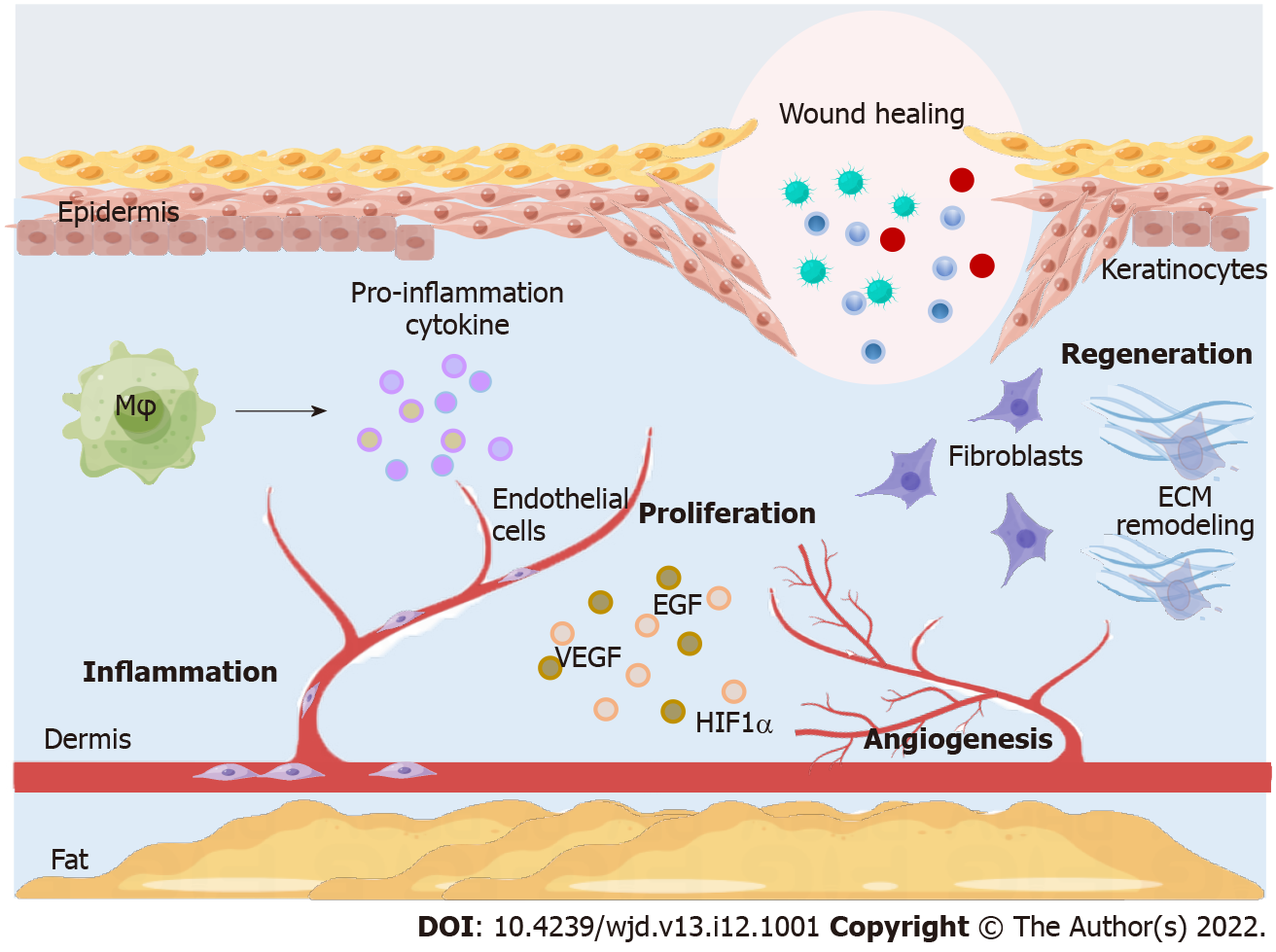
Wound healing is a complex and highly regulated process divided into three phases: Inflammation, proliferation, and regeneration[12]. Diabetic wound healing is widely associated with different cellular components and the extracellular matrix (ECM) in different parts of the skin[13]. The main effector cells in the inflammatory phase are macrophages. When normal skin is damaged, macrophages polarize to M1 phenotype, producing pro-inflammatory cytokines and stimulating endothelial cells and fibroblasts to release reactive oxygen species (ROS) to remove bacteria and debris from wounds. The subsequent shift to the M2 phenotype is correlated with remission of the inflammatory response and wound remodeling[14,15]. In diabetic wounds, the persistence of the M1 phenotype and the inability to subsequently polarize to the M2 phenotype are the key components delaying wound healing. Angiogenesis is the main basis of the proliferative phase of wound healing, cell proliferation, migration, and differentiation[14]. The integrity of the endothelial cell structure plays a very important role in maintaining normal blood circulation in the body. In healthy tissues, endothelial cells are in a quiescent phase. In diabetic patients, wound healing is slowed by decreased angiogenic growth factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and hypoxia-inducible factor (HIF)-1α[16-18]. An unfavorable diabetic wound environment promotes the dysregulation of key signaling pathways, such as Notch and PI3K/AKT/eNOS[19,20]. The regenerative phase of wound healing includes re-epithelialization and ECM remodeling. Reduced blood flow restricts the migration of leukocytes, keratinocytes, fibroblasts, and endothelial cells to the wound, which is detrimental to wound healing[21]. Fibroblasts proliferate and secrete ECM components, such as collagen fibers, which provide supportive structures for cell proliferation and migration to restore skin tissue function and integrity to maintain tissue elasticity and strength[22]. DFUs have collagen degeneration and deformation and reduced fibroblasts in the proliferation and migration stages[23]. Keratinocytes are the main constituent cells of the epidermis involved in skin wound healing through migration, proliferation, and differentiation[24]. In addition, epithelial-to-mesenchymal transition (EMT) plays a crucial role in DFU regeneration and wound healing[25]. Many studies have shown that ncRNAs regulate EMT involved in DFU and wound healing[26,27]. The wound healing process is shown in Figure 1.
MiRNAs are a class of endogenous small ncRNAs with a molecular length of 18–25 nucleotides that regulate gene and/or protein expression at the post-transcriptional level by specifically binding to the 3′-untranslated region of downstream target miRNAs. The increased prevalence of diabetes has prompted increasing research into the mechanisms of miRNAs as therapeutic targets in DFU and wound healing. A study showed that low miR-24 expression is an independent risk factor for DFU in multifactorial logistic regression analysis[28]. Furthermore, low miR-24 expression is negatively correlated with fasting blood glucose and glycated hemoglobin and positively correlated with inflammatory markers[28-30]. MiRNAs have been associated with DFU progression and severity; specific miRNAs, such as miR-26, increase DFU severity[31], whereas other miRNAs, such as miR-129 and miR-335, improve wound healing[26].
MiR-217 belongs to the group that increases DFU severity. A study showed that a dual luciferase reporter gene assay confirmed HIF-1α as a direct target gene of miR-217. MiR-217 expression was upregulated whereas HIF-1α/VEGF expression was downregulated in patients with DFU and in the serum of rats with DFU compared with DM and healthy controls[32]. MiR-23c is upregulated in the peripheral blood and wound tissue in DFU, targeting stromal cell-derived factor-1α and inhibiting wound angiogenesis by recruiting inflammatory cells, such as macrophages[33]. In a mouse DFU model, miR-497 expression was downregulated, which considerably increased the expression of pro-inflammatory factors, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, resulting in a prolonged inflammatory phase of wound healing[34]. MiR-155 regulates insulin sensitivity and blood glucose levels in mice[35]. MiR-155 is markedly upregulated in diabetic skin[36]. MiR-155 has pro-inflammatory effects; thus, miR-155 inhibition leads to reduced inflammation, increased macrophage M2 polarization, reduced IL-1β and TNF-α levels, more regular collagen fiber alignment, and faster diabetic wound healing[37-39]. MiR-217, miR-497, and miR-155 are effector molecules in the inflammatory phase of diabetic wound healing; however, a further exploration of their mechanisms might improve wound healing during the inflammatory phase.
Angiogenesis is an essential step in the proliferative phase associated with DFU prognosis and wound healing. Recent studies have focused on the mechanisms and applications of miRNAs in regulating angiogenesis during the proliferative phase[40-42]. A maggot therapeutic approach study found that miR-18a/19a is markedly upregulated and thrombospondin-1 (TSP-1) expression is downregulated in DFU wounds as a result of impaired angiogenesis. The target activation of miR-18a/19a transcript levels and the regulation of TSP-1 expression may be a novel strategy for DFU treatment[40]. MiR-15a-3p is upregulated in the blood exosomes of patients with diabetes[41]. In vivo and in vitro experiments showed that exosomes with low miR-15a-3p expression inhibited diabetic wound healing. By contrast, knockdown of circulating exosomal miR-15a-3p expression may accelerate wound healing through the activation of NADPH oxidase (NOX) 5 and increase ROS release[41]. NOX activates redox signaling pathways and promotes angiogenesis[43]. Phosphatase and tensin homolog (PTEN) expression is regulated by blood glucose concentrations, is mainly found in epithelial cells, and activates signaling cascades that affect angiogenesis[44]. MiR-152-3p is an upstream negative regulator of PTEN upregulated in diabetic wounds; hence, inhibiting the angiogenic function of PTEN leads to delayed wound healing[45]. MiR-195-5p and miR-205-5p carried by extracellular vesicles in DFU wound fluid negatively regulate angiogenesis and wound healing in DFU[42]. Increased miR-133b expression induces downregulation of EGF receptor (EGFR), affecting endothelial cell proliferation and angiogenesis in all diabetic wounds. In vitro experiments showed that miR-133b downregulation in human umbilical vein endothelial cells partially reverses impaired angiogenesis[46]. These findings imply that miR-133b negatively regulates angiogenesis during the proliferative phase of wound healing. Huang et al[47] found that miR-489-3p downregulation increases sirtuin (SIRT) 1 expression, promotes the PI3K/AKT/eNOS signaling pathway, improves cellular antioxidant capacity, and alleviates DFU. MiR-199a-5p has an important role in the development of diabetes and its complications[48,49]. Moreover, miR-199a-5p promotes apoptosis and ROS production within pancreatic β-cells in type 2 DM (T2DM)[50]. MiR-199a-5p sponge-adsorbed to hsa-circ-006040 inhibits macrophage-mediated inflammatory responses in type 1 DM (T1DM)[48]. Wang et al[49] found that downregulating miR-199a-3p in endothelial cells alleviates inhibition of the target VEGFA and Rho-related kinase 1, rescuing the cellular damage induced by high glucose and restoring angiogenic function. Therefore, these findings suggest that regulating miRNA expression during the proliferative phase of wound healing has great potential in DFU treatment and wound repair.
Recently, Moura et al[36] also found that the local inhibition of miR-155 in diabetic wounds increased the expression of its target, fibroblast growth factor (FGF) 7, which sequentially increased re-epithelialization and accelerated wound healing[36,51]. Yuan et al[52] found that miR-203 upregulation in DFU tissues may inhibit the EMT process and delay wound healing in a rat DFU model. On the contrary, miR-203 knockdown promoted wound healing by activating the target gene, IL-8, and IL-8/AKT downstream pathways. High miR-203 expression reduces keratinocyte proliferation and migration, partially explaining the development of DFU into chronic refractory wounds[52]. On the contrary, recent studies have found that negative pressure wound therapy can reverse the inhibition of keratinocytes as a result of high levels of miR-203 by reducing miR-203 in the peripheral blood and wound tissue and upregulating p63 expression[53]. Sprouty homolog (SPRY) 1, an antagonist of the FGF pathway, is expressed in fibroblasts, and its downregulation plays an important role in wound healing[54,55]. MiR-21-3p is downregulated in diabetic patients compared with healthy controls and in fibroblasts stimulated with D-glucose compared with control fibroblasts[56]. Enhanced miR-21-3p expression may inhibit SPRY1, stimulate fibroblast proliferation and migration, and accelerate wound healing[42]. MiR-146a is downregulated in DFU wound tissue. Bioinformatics analysis revealed that A-kinase-anchoring protein 12 (AKAP12) and Toll-like receptor 4 (TLR4) are the target genes of miR-146a. Peng et al[57] showed that miR-146a activates in the inflammatory phase of diabetic wound healing by inhibiting the TLR4/nuclear factor-kappaB axis involved in macrophage M2 polarization. In addition, Zhang et al[58] constructed an in vitro DFU model using human keratinocyte-derived HaCaT cells and demonstrated that miR-146a is activated during the tissue regeneration phase. In vivo and ex vivo results showed that miR-146a overexpression inhibited the angiogenic regulator AKAP12, activated the HIF-1α/Wnt3α/β-catenin signaling pathway, and promoted cell proliferation and migration[57]. MiRNAs have regulatory effects on a wide range of cells involved in tissue remodeling during the regeneration phase. MiRNAs are the most studied ncRNAs and act in various periods of DFU and wound healing, respectively, or continuously. We summarized some of the considerably altered miRNAs in diabetic patients as shown in Table 1. Notably, most of these pooled miRNAs have not been reported to have a clear therapeutic role in DFU and should therefore be evaluated in future studies.
| Name | Expression | Animal | Target gene | Pathway | Phase | Ref. |
| miRNA-217 | Up | Mouse | HIF-1α | VEGF | Inflammation | Lin et al[32], 2019 |
| miRNA-23c | Up | / | SDF-1α | SDF-1α/CXCL12 | Inflammation | Amin et al[33], 2020 |
| miRNA-497 | Down | Mouse | IL-1β, IL-6, TNF-α | NF-κB | Inflammation | Ban et al[34], 2020 |
| miRNA-155 | Up | Mouse | FGF7 | / | Inflammation/regeneration | Moura et al[36], 2019; Gondaliya et al[51], 2022 |
| miRNA-18a/19a | Up | / | TSP-1 | / | Proliferation | Wang et al[40], 2020 |
| miRNA-15a-3p | Up | Mouse | NOX5 | ROS | Proliferation | Xiong et al[41], 2020 |
| miRNA-152-3p | Up | Mouse | PTEN | / | Proliferation | Xu et al[45], 2020 |
| miRNA-133b | Up | Mouse | EGFR | / | Proliferation | Zhong et al[46], 2021 |
| miRNA-195-5p | Up | Rat | VEGFA | / | Proliferation | Liu et al[42], 2021 |
| miRNA-205-5p | Up | Rat | VEGFA | / | Proliferation | Liu et al[42], 2021 |
| miRNA-199a-5p | Up | Rat | VEGFA, ROCK1 | / | Proliferation | Wang et al[49], 2022 |
| miRNA-203 | Up | Rat | IL-8 | AKT | Regeneration | Yuan et al[52], 2019 |
| / | p63 | / | Regeneration | Liu et al[53], 2022 | ||
| miR-489-3p | Up | Rat | SIRT1 | PI3K/AKT/eNOS | Regeneration | Huang et al[47], 2022 |
| miRNA-21-3p | Down | Mouse | SPRY1 | FGF | Regeneration | Wu et al[56], 2020 |
| miRNA-146a | Down | / | AKAP12 | Wnt/β-catenin | Regeneration | Peng et al[57], 2022 |
| / | TLR4 | NF-κB | Inflammation | Zhang et al[58], 2022 |
LncRNAs are located in highly conserved genomic regions with spatially and temporally tightly regulated expression and dysregulated expression profiles as important markers of altered disease or developmental status. The main mechanism and function of lncRNAs are to act as competing endogenous RNAs (ceRNAs) for miRNAs, which interact with mRNA target base pairs to control various signaling pathways[59]. Another mechanism is by interacting with RNA-binding proteins[60]. Increasing evidence shows that lncRNAs play an important role in diabetic complications. LncRNA 3632454L22RiK can promote corneal epithelial wound healing in diabetic mice by sponging miR-181a-5p[61]. The regulatory role of lncRNA MIAT in diabetic cardiomyopathy has also been demonstrated[62]. These findings indicate an increased awareness of lncRNAs in diabetic complications.
The mechanism of lncRNAs in the inflammatory phase lacks enough evidence. LncRNA growth arrest specific 5 (GAS5) has been identified as a tumor suppressor that inhibits cell proliferation and promotes apoptosis[63]. GAS5 expression was markedly elevated in DFU wounds[64]. GAS5 promotes the M1 phenotypic polarization of macrophages through the upregulation of signal transducer and activator of transcription 1 (STAT1), leading to prolonged inflammatory phase and delayed wound remodeling and closure[64]. STAT1 signaling is exactly the central pathway that controls M1-M2 polarization in macrophages. Reduced GAS5 levels in wounds appear to promote healing by facilitating the conversion of M1 macrophages to M2 macrophages. Thus, targeting lncRNA GAS5 may contribute to efficient therapeutic interventions for impaired wound healing in diabetes.
GAS5 regulates the inflammatory process of wound healing and plays a part in the proliferative phase. During the proliferative phase, GAS5 activates the HIF-1α/VEGF pathway by binding to TATA box-binding protein associated factor 15, stimulating endothelial cell proliferation and angiogenesis and leading to accelerated DFU wound healing[65]. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a relatively well-studied transcript among lncRNAs. The role of MALAT1 has been reported in a variety of diseases, including renal tumors, osteosarcoma, and gestational diabetes[66-68]. MALAT1 protects endothelial cells from oxidative stress injury by activating the nuclear factor erythroid-2-related factor 2 (Nrf2) pathway. MALAT1 is markedly reduced in DFU-infected tissues, leading to insufficient HIF-1α/VEGF activation and impeding angiogenesis[69]. The exogenous uptake of exosome lnc01435 by vascular endothelial cells alters the subcellular localization of transcription factor yin yang 1 (YY1) and synergistically upregulates histone deacetylase (HDAC) 8 expression with YY1. HDACs are important components of the NOTCH signaling pathway with negatively regulated expression levels and thus affect endothelial cell function and angiogenesis[70,71]. In summary, targeting GAS5, MALAT1, and lnc01435 may help develop new therapeutic strategies to treat DFUs.
LncRNA H19, located on chromosome 11, exhibits negative regulation of diabetic wound healing. LncRNA H19 acts as a sponge for miR-29b and competitively represses miR-29b expression; therefore, it upregulates fibrillin 1 (FBN1), activates the transforming growth factor-β/Smad signaling pathway, and promotes ECM accumulation[72]. Connective tissue growth factor (CTGF) is a matricellular protein from the Cyr61/CTGF/Nov protein family, which interacts with ECM protein to mediate external signal transduction into cells through many subtypes of integrin receptors[73]. During the proliferative phase of diabetic wound healing, lncRNA H19 recruits the transcription factor SRF to the CTGF promoter region, activating CTGF and its downstream MAPK signaling pathway to accelerate fibroblast proliferation and wound healing[74]. These findings elaborate lncRNA H19 as a regulator in the regenerative phase of wound healing. A novel lncRNA MRAK052872, named lnc-upregulated in diabetic skin (URIDS), is involved in the mechanism of DFU wound healing. Lnc-URIDS is highly expressed in diabetic skin and dermal fibroblasts treated with advanced glycosylation end products. Lnc-URIDS binds to procollagen-lysine and 2-oxoglutarate 5-dioxygenase 1 (plod1), decreases plod1 protein stability, and leads to dysregulated collagen deposition and delayed wound healing[27]. LncRNA cancer susceptibility candidate 2 (CASC2) was originally discovered in an endometrial cancer study and is located on human chromosome 10q26[75]. Furthermore, CASC2 overexpression inhibited fibroblast migration and proliferation, suppressed apoptosis, and facilitated wound healing, especially in DFU mice. By contrast, miR-155 overexpression inhibited the function of CASC2[75]. Another study showed that HIF-1α inhibition reversed the effects of miR-155 downregulation on fibroblasts[76]. Evidently, lncRNAs have a considerable regulatory role in cellular functions during re-epithelialization and remodeling.
The mechanisms by which lncRNAs cause DFU and delayed wound healing are atypical inflammatory responses, impaired angiogenesis, impaired and abnormal ECM accumulation, and epithelial processes that regulate wound healing. The lncRNAs in DFU and delayed wound healing are listed in Table 2. These findings provide new information for the clinical treatment of diabetic chronic non-healing wounds.
| Name | Expression | Sponge | Animal | Target gene | Pathway | Phase | Ref. |
| GAS5 | Up | / | Mouse | STAT1 | / | Inflammation | Hu et al[64], 2020 |
| / | Mouse | TAF15 | HIF-1α/VEGF | Proliferation | Peng et al[65], 2021 | ||
| MALAT1 | Down | / | / | HIF-1α/Nrf2 | Proliferation | Jayasuriya et al[69], 2020 | |
| Lnc01435 | Up | / | Mouse | YY1, HDACs | Notch | Proliferation | Fu et al[70], 2022 |
| H19 | Up | miRNA-29b | Mouse | FBN1 | TGF-β/Smad | Regeneration | Li et al[72], 2021 |
| Up | / | Rat | CTGF, SRF | MAPK | Regeneration | Li et al[74], 2020 | |
| URIDS | Up | / | Rat | Plod1 | VEGF/TGF-β | Regeneration | Hu et al[27], 2020 |
| CASC2 | Down | miR-155 | Mouse | HIF-1α | / | Regeneration | He et al[76], 2022 |
CircRNAs are a unique type of ncRNA derived from exons, introns, or intergenic regions that are covalently linked to produce a closed loop structure in the absence of 50 caps and 30 tails. CircRNAs are conserved among species owing to their resistance properties to RNase R. CircRNAs are involved in a wide range of biological processes, such as transcription and mRNA splicing, RNA decay, and RNA translation; the dysregulation of circRNAs leads to abnormal cellular functions and human diseases[77,78]. CircRNAs can also act as a miRNA sponge to inhibit miRNA function, which plays a crucial role in the pathogenesis of diabetes and its vascular complications[79]. Circ-PNPT1 and has_circ_0046060 promote the development of gestational DM by regulating trophoblast cell function or causing insulin resistance[80,81]. Circ-ITCH improved renal inflammation and fibrosis in diabetic mice by regulating the miR-33a-5p/SIRT6 axis[82]. CircRNAs are closely related to the development of diabetes and its complications. Studies on the role and mechanism of circRNAs in DFU and delayed wound healing are relatively few. Existing studies evaluated the regulatory role of circRNAs on angiogenesis and re-epithelialization.
CircRNAs protein kinase, DNA-activated, catalytic subunit (circ_PRKDC, has-circ-0084443) is involved in the promotion of keratinocyte proliferation and the suppression of keratinocyte migration during wound healing[83]. Circ_PRKDC negatively regulates keratinocyte migration via the EGFR pathway, impeding re-epithelialization and angiogenesis[84]. However, circ_PRKDC knockdown promotes epidermal keratinocyte migration via the miR-31/FBN1 axis[83]. This finding shows that circ_PRKDC has therapeutic potential for skin wound healing. Shang et al[85] evaluated the effect of circ-Klhl8 in epithelial progenitor cells (EPCs) on diabetic wound closure by establishing an in vivo mouse model of total skin defect and found that circ-Klhl8 overexpression increases the therapeutic effect of EPCs to promote diabetic wound healing by targeting the miR-212-3p/SIRT5 axis. Altered circRNA expression can affect disease progression and wound healing in DFU (Table 3). Studies on circRNAs in various stages of DFU and wound healing are few and prompted the need for further research on functional circRNAs in the future to identify limitations in DFU treatment.
The standard treatment for DFUs includes optimizing blood flow, debridement, infection control, and offloading. In standard treatment, only 50% of patients heal within 20 wk and 50% relapse within 18 mo; thus, efficient treatment for DFUs are urgently needed[86]. Cell-based DFU therapy is a new treatment intervention therapy studied in the last few years. Stem cells can affect ulcer healing through various pathophysiological processes, such as stimulating tissue repair, increasing ECM synthesis, and promoting angiogenesis in ischemic tissues[87]. Soluble factors and extracellular vesicles secreted by stem cells are active factors in diabetic wound healing. Extracellular vesicles from mesenchymal stem cells (MSCs) are considered an alternative treatment for immune disorders, including diabetes. Emerging evidence suggests that MSC-derived exosomes applied to the wound surface can promote angiogenesis and tissue repair[88]. MSC regenerative therapy is a novel tissue regeneration modality that accelerates wound healing in DFU and identifies patients at high risk of amputation[89]. Adipose-derived stem cells (ADSCs) have become an alternative to cell therapy owing to their abundance, subcutaneous location, easy accessibility, and longer culture time than bone marrow mesenchymal cells (BMSCs) and thus exert greater proliferation and differentiation capacity. Previous studies found that ADSC transplantation can promote foot wound healing in diabetic rats whereas stem cell transplantation may have clinical application in DFU treatment[90]. EPCs are the precursor cells of vascular endothelial cells that can be directed to the site of ischemic injury and form new vessels through proliferation and differentiation to promote wound healing[91]. Cell-derived exosomes loaded with ncRNAs have a therapeutic effect on refractory DFUs.
Gondaliya et al[51] revealed the therapeutic potential of miR-155 inhibitor-loaded MSC-derived exosomes in diabetic wound healing and demonstrated that wrapping miRNA and antibiotics in MSC-derived exosomes improved the management of chronic, non-healing diabetic wounds. Studies found that miR-129 may promote diabetic wound healing by balancing ECM synthesis and degradation through the inhibition of Sp1-mediated matrix metalloproteinase 9 expression[26]. A recent study also showed that miR-129 loaded in MSC-derived extracellular vesicles promoted wound healing via the downregulation of tumor necrosis factor receptor-associated factor 6[92]. Evidently, miR-129 is an important regulator of the proliferative and regenerative phases of wound healing and may be a biologically active molecule in MSC for DFU treatment. Xu et al[93] showed that miR-221-3p in EPC-derived exosomes accelerated skin wound healing in normal and diabetic mouse models. The latest study further demonstrated the mechanism of miR-221-3p in diabetic wound treatment[94]. MiR-221-3p overexpression may inhibit the anti-angiogenic function of its direct targeted homeodomain-interacting protein kinase 2 (HIPK2) and promote endothelial cell proliferation[94].
Li et al[95] showed that the MSC-derived exosomal lncRNA, lncRNA H19, causes fibroblast inflammation and apoptosis by disrupting miR-152-3p-mediated PTEN inhibition, leading to a stimulated wound-healing process in DFU. MSCs have demonstrated a therapeutic effect in DFU by generating pro-angiogenesis factors, such as VEGF. Recent research shows that genetically modified MSCs have been used in therapy, and the depletion of miR-205-5p in human MSCs promotes VEGF-mediated therapeutic effects in DFU[96,97]. LncRNA MALAT1 is a ceRNA for miR205-5p but has a low expression in human MSCs. Ectopic MALAT1 expression in human MSCs considerably decreased miR-205-5p levels, resulting in the upregulation of VEGF production and improved in vitro endothelial cell tube formation. In an immunodeficient NOD/SCID mouse model of diabetic foot (DF), the transplantation of human miR-205p-depleted MSCs resulted in better therapeutic effects on DF recovery than control MSCs. Moreover, MALAT1-expressing MSCs showed even better therapeutic effects on DF recovery than miR-205-5p-depleted MSCs. This difference in DF recovery was associated with the levels of on-site vascularization. Overall, MALAT1 functions as a sponge RNA for miR-205-5p to increase the therapeutic effects of MSCs on DF[97]. As mentioned above, miR-205-5p is an anti-angiogenic factor that inhibits VEGFA expression at the post-transcriptional level in MSCs, and the inhibition of its expression leads to angiogenesis and considerably improves the therapeutic effect of MSCs on diabetic wounds[97,98]. BMSC-derived exosomes can encapsulate lncRNA Kruppel-like factor 3 antisense RNA 1 (KLF3-AS1); adequately promote vascular endothelial cell proliferation, migration, and tube formation; and inhibit high glucose-induced apoptosis[99]. Diabetic wound healing by lncRNA KLF3-AS1 encapsulated by MSC-derived exosomes was achieved by downregulating miR-383 and upregulating its target, VEGFA[99].
High-throughput sequencing revealed an abnormally reduced expression of mmu_circ_0000250 in diabetic mice[100]. Exosomes from mmu_circ_0000250-modified ADSCs promote wound healing in diabetic mice through the induction of miR-128-3p/SIRT1-mediated autophagy[100]. In the study by Shi et al[100], the exosomes of ADSCs exerted therapeutic effects by restoring vascular endothelial cell function under high-glucose conditions. Circ-0000250 expression may increase the effectiveness of exosome therapy. Circ_ARHGAP12 is a cyclic molecule that inhibits high glucose-induced cell apoptosis by enhancing cellular autophagy[101]. Circ_ARHGAP12 was able to directly interact with miR-301b-3p and subsequently stimulate miRNAs to regulate the expression of ATG16L1 and ULK2, the target genes of miR-301b-3p, as well as downstream signaling pathways[101]. These findings propose a prospective therapeutic strategy of targeting circ_ARHGAP12 in MSCs to promote diabetic wound healing. Recent studies have found that circRNAs HIPK three (circHIPK3)-rich exosomes derived from human umbilical cord-derived MSCs have promising therapeutic effects in DFU. Exosomal circHIPK3 significantly promotes revascularization and wound healing by sponging to miR-20b-5p and upregulating the Nrf2/VEGFA axis[102]. Some ncRNAs for the cell therapy of DFU are shown in Table 4. NcRNAs and vector exosomes are effector molecules with great potential among the cellular therapeutic approaches for DFU and are expected to be of clinical use in the near future.
| Name | Origin | Expression | Sponge | Target gene | Phase | Ref. |
| miRNA-155 | MSC | Up | / | FGF7 | Proliferation | Moura et al[36], 2019; Gondaliya et al[51], 2022 |
| miR-129 | MSC | Down | / | TRAF6 | Proliferation | Hu et al[92], 2022 |
| miRNA-221-3p | EPC | Down | / | HIPK2 | Proliferation | Yu et al[94], 2022 |
| LncRNA H19 | MSC | Up | miRNA-152-3P | PTEN | Proliferation | Li et al[95], 2020 |
| MALAT1 | MSC | Down | miR-205-5p | VEGF | Proliferation | Zhu et al[97], 2019 |
| Lnc KLF3-AS1 | BMSC | Down | miR-383 | VEGFA | Proliferation | Han et al[99], 2022 |
| Circ_0000250 | ADSC | Down | miR-128-3p | SIRT1 | Proliferation | Shi et al[100], 2020 |
| Circ_ARHGAP12 | MSC | Down | miR-301b-3p | ATG16L1, ULK2 | Proliferation | Meng et al[101], 2022 |
| Circ HIPK3 | MSC | Down | miR-20b-3p | Nrf2/VEGFA | Proliferation | Liang et al[102], 2022 |
This study summarized the role and intrinsic mechanisms of ncRNAs in diabetic wound healing and provided more potential targets for future studies on wound healing in patients with diabetes. NcRNAs are regulatory molecules that modify many physiological processes and aspects of human diseases. The inflammation, proliferation, and regeneration phases of diabetic wound healing overlap, and ncRNAs are biologically active during all three phases. NcRNAs have a crucial role in the pathogenesis and impairment of wound healing in patients with diabetes. NcRNAs activate certain signaling pathways by downregulating or upregulating certain genes. Some of these molecules may provide valuable information in the clinical setting and serve as diagnostic or screening tools to predict high-risk DFUs and provide a basis for early prevention. These findings suggest that cell therapy using ncRNAs for DFU has great potential in the field of regenerative medicine.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li H, China; Mostafavinia A, Iran; Terabe Y, Japan S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Choby B. Diabetes Update: Prevention and Management of Diabetes Complications. FP Essent. 2017;456:36-40. [PubMed] |
| 2. | Tentolouris N, Edmonds ME, Jude EB, Vas PRJ, Manu CA, Tentolouris A, Eleftheriadou I. Editorial: Understanding Diabetic Foot Disease: Current Status and Emerging Treatment Approaches. Front Endocrinol (Lausanne). 2021;12:753181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6:37-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 291] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (11)] |
| 4. | Sharma S, Schaper N, Rayman G. Microangiopathy: Is it relevant to wound healing in diabetic foot disease? Diabetes Metab Res Rev. 2020;36 Suppl 1:e3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Zhang TN, Yang N, Goodwin JE, Mahrer K, Li D, Xia J, Wen R, Zhou H, Zhang T, Song WL, Liu CF. Characterization of Circular RNA and microRNA Profiles in Septic Myocardial Depression: a Lipopolysaccharide-Induced Rat Septic Shock Model. Inflammation. 2019;42:1990-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3368] [Cited by in RCA: 4116] [Article Influence: 374.2] [Reference Citation Analysis (1)] |
| 7. | Faruq O, Vecchione A. microRNA: Diagnostic Perspective. Front Med (Lausanne). 2015;2:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1749] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 9. | Shi T, Gao G, Cao Y. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis Markers. 2016;2016:9085195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 10. | Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1422] [Article Influence: 203.1] [Reference Citation Analysis (0)] |
| 11. | Lou R, Chen J, Zhou F, Wang C, Leung CH, Lin L. Exosome-cargoed microRNAs: Potential therapeutic molecules for diabetic wound healing. Drug Discov Today. 2022;27:103323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Mulholland EJ, Dunne N, McCarthy HO. MicroRNA as Therapeutic Targets for Chronic Wound Healing. Mol Ther Nucleic Acids. 2017;8:46-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Verdi J, Shirian S, Saleh M, Khadem Haghighian H, Kavianpour M. Mesenchymal Stem Cells Regenerate Diabetic Foot Ulcers: A Review Article. World J Plast Surg. 2022;11:12-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2865] [Cited by in RCA: 2942] [Article Influence: 326.9] [Reference Citation Analysis (0)] |
| 15. | Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861-3885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 1064] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 16. | Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. 2008;173:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Abd El-Khalik SR, Hafez YM, Elkholy RA. The role of circulating soluble fms-like tyrosine kinase-1 in patients with diabetic foot ulcer: A possible mechanism of pathogenesis via a novel link between oxidative stress, inflammation and angiogenesis. Microvasc Res. 2020;130:103987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Li L, Wang M, Mei Z, Cao W, Yang Y, Wang Y, Wen A. lncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1α by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed Pharmacother. 2017;96:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Wang X, Pan J, Liu D, Zhang M, Li X, Tian J, Liu M, Jin T, An F. Nicorandil alleviates apoptosis in diabetic cardiomyopathy through PI3K/Akt pathway. J Cell Mol Med. 2019;23:5349-5359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Lim R, Sugino T, Nolte H, Andrade J, Zimmermann B, Shi C, Doddaballapur A, Ong YT, Wilhelm K, Fasse JWD, Ernst A, Kaulich M, Husnjak K, Boettger T, Guenther S, Braun T, Krüger M, Benedito R, Dikic I, Potente M. Deubiquitinase USP10 regulates Notch signaling in the endothelium. Science. 2019;364:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Li Q, Zhao H, Chen W, Huang P, Bi J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int J Biochem Cell Biol. 2019;114:105570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Li X, Li N, Li B, Feng Y, Zhou D, Chen G. Noncoding RNAs and RNA-binding proteins in diabetic wound healing. Bioorg Med Chem Lett. 2021;50:128311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle). 2014;3:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 906] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 25. | Singh K, Rustagi Y, Abouhashem AS, Tabasum S, Verma P, Hernandez E, Pal D, Khona DK, Mohanty SK, Kumar M, Srivastava R, Guda PR, Verma SS, Mahajan S, Killian JA, Walker LA, Ghatak S, Mathew-Steiner SS, Wanczyk KE, Liu S, Wan J, Yan P, Bundschuh R, Khanna S, Gordillo GM, Murphy MP, Roy S, Sen CK. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Wang W, Yang C, Wang XY, Zhou LY, Lao GJ, Liu D, Wang C, Hu MD, Zeng TT, Yan L, Ren M. MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes. 2018;67:1627-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 27. | Hu M, Wu Y, Yang C, Wang X, Wang W, Zhou L, Zeng T, Zhou J, Wang C, Lao G, Yan L, Ren M. Novel Long Noncoding RNA lnc-URIDS Delays Diabetic Wound Healing by Targeting Plod1. Diabetes. 2020;69:2144-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Li X, Tang Y, Jia Z, Zhao X, Chen M. Decreased expression of miR-24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair Regen. 2020;28:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr Diab Rep. 2018;18:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 30. | Xiang Y, Cheng J, Wang D, Hu X, Xie Y, Stitham J, Atteya G, Du J, Tang WH, Lee SH, Leslie K, Spollett G, Liu Z, Herzog E, Herzog RI, Lu J, Martin KA, Hwa J. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125:3377-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Icli B, Nabzdyk CS, Lujan-Hernandez J, Cahill M, Auster ME, Wara AK, Sun X, Ozdemir D, Giatsidis G, Orgill DP, Feinberg MW. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol. 2016;91:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Lin CJ, Lan YM, Ou MQ, Ji LQ, Lin SD. Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J Endocrinol Invest. 2019;42:1307-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Amin KN, Umapathy D, Anandharaj A, Ravichandran J, Sasikumar CS, Chandra SKR, Kesavan R, Kunka Mohanram R. miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc Res. 2020;127:103924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Ban E, Jeong S, Park M, Kwon H, Park J, Song EJ, Kim A. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed Pharmacother. 2020;121:109613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Lin X, Qin Y, Jia J, Lin T, Lin X, Chen L, Zeng H, Han Y, Wu L, Huang S, Wang M, Xie R, Liang L, Liu Y, Liu R, Zhang T, Li J, Wang S, Sun P, Huang W, Yao K, Xu K, Du T, Xiao D. MiR-155 Enhances Insulin Sensitivity by Coordinated Regulation of Multiple Genes in Mice. PLoS Genet. 2016;12:e1006308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Moura J, Sørensen A, Leal EC, Svendsen R, Carvalho L, Willemoes RJ, Jørgensen PT, Jenssen H, Wengel J, Dalgaard LT, Carvalho E. microRNA-155 inhibition restores Fibroblast Growth Factor 7 expression in diabetic skin and decreases wound inflammation. Sci Rep. 2019;9:5836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | van Solingen C, Araldi E, Chamorro-Jorganes A, Fernández-Hernando C, Suárez Y. Improved repair of dermal wounds in mice lacking microRNA-155. J Cell Mol Med. 2014;18:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Yang L, Liu L, Ying H, Yu Y, Zhang D, Deng H, Zhang H, Chai J. Acute downregulation of miR-155 leads to a reduced collagen synthesis through attenuating macrophages inflammatory factor secretion by targeting SHIP1. J Mol Histol. 2018;49:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Ye J, Kang Y, Sun X, Ni P, Wu M, Lu S. MicroRNA-155 Inhibition Promoted Wound Healing in Diabetic Rats. Int J Low Extrem Wounds. 2017;16:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Wang TY, Wang W, Li FF, Chen YC, Jiang D, Chen YD, Yang H, Liu L, Lu M, Sun JS, Gu DM, Wang J, Wang AP. Maggot excretions/secretions promote diabetic wound angiogenesis via miR18a/19a - TSP-1 axis. Diabetes Res Clin Pract. 2020;165:108140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Xiong Y, Chen L, Yu T, Yan C, Zhou W, Cao F, You X, Zhang Y, Sun Y, Liu J, Xue H, Hu Y, Chen D, Mi B, Liu G. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany NY). 2020;12:8968-8986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Liu J, Wang J, Fu W, Wang X, Chen H, Wu X, Lao G, Wu Y, Hu M, Yang C, Yan L, Ren M. MiR-195-5p and miR-205-5p in extracellular vesicles isolated from diabetic foot ulcer wound fluid decrease angiogenesis by inhibiting VEGFA expression. Aging (Albany NY). 2021;13:19805-19821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Chen W, Deng W, Goldys EM. Light-Triggerable Liposomes for Enhanced Endolysosomal Escape and Gene Silencing in PC12 Cells. Mol Ther Nucleic Acids. 2017;7:366-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Chen Y, Yu H, Zhu D, Liu P, Yin J, Liu D, Zheng M, Gao J, Zhang C, Gao Y. miR-136-3p targets PTEN to regulate vascularization and bone formation and ameliorates alcohol-induced osteopenia. FASEB J. 2020;34:5348-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Xu Y, Yu T, He L, Ouyang L, Qu Y, Zhou J, Han Y, Duan D. Inhibition of miRNA-152-3p enhances diabetic wound repair via upregulation of PTEN. Aging (Albany NY). 2020;12:14978-14989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Zhong H, Qian J, Xiao Z, Chen Y, He X, Sun C, Zhao Z. MicroRNA-133b Inhibition Restores EGFR Expression and Accelerates Diabetes-Impaired Wound Healing. Oxid Med Cell Longev. 2021;2021:9306760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | Huang L, Cai HA, Zhang MS, Liao RY, Huang X, Hu FD. Corrigendum to 'Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489e3p/Sirt1 axis'[Journal of Pharmacological Sciences 147 (2021) 271-283]. J Pharmacol Sci. 2022;149:173. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Yang L, Han X, Zhang C, Sun C, Huang S, Xiao W, Gao Y, Liang Q, Luo F, Lu W, Fu J, Zhou Y. Hsa_circ_0060450 Negatively Regulates Type I Interferon-Induced Inflammation by Serving as miR-199a-5p Sponge in Type 1 Diabetes Mellitus. Front Immunol. 2020;11:576903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Wang H, Wang X, Liu X, Zhou J, Yang Q, Chai B, Chai Y, Ma Z, Lu S. miR-199a-5p Plays a Pivotal Role on Wound Healing via Suppressing VEGFA and ROCK1 in Diabetic Ulcer Foot. Oxid Med Cell Longev. 2022;2022:4791059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Lin N, Li XY, Zhang HM, Yang Z, Su Q. microRNA-199a-5p mediates high glucose-induced reactive oxygen species production and apoptosis in INS-1 pancreatic β-cells by targeting SIRT1. Eur Rev Med Pharmacol Sci. 2017;21:1091-1098. [PubMed] |
| 51. | Gondaliya P, Sayyed AA, Bhat P, Mali M, Arya N, Khairnar A, Kalia K. Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol Pharm. 2022;19:1294-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 52. | Yuan L, Sun Y, Xu M, Zeng F, Xiong X. miR-203 Acts as an Inhibitor for Epithelial-Mesenchymal Transition Process in Diabetic Foot Ulcers via Targeting Interleukin-8. Neuroimmunomodulation. 2019;26:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Liu L, Chen R, Jia Z, Li X, Tang Y, Zhao X, Zhang S, Luo L, Fang Z, Zhang Y, Chen M. Downregulation of hsa-miR-203 in peripheral blood and wound margin tissue by negative pressure wound therapy contributes to wound healing of diabetic foot ulcers. Microvasc Res. 2022;139:104275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 54. | Li N, Wang Z, Gao F, Lei Y, Li Z. Melatonin ameliorates renal fibroblast-myofibroblast transdifferentiation and renal fibrosis through miR-21-5p regulation. J Cell Mol Med. 2020;24:5615-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 55. | Wang P, Zhou Y, Yang JQ, Landeck L, Min M, Chen XB, Chen JQ, Li W, Cai SQ, Zheng M, Man XY. The role of Sprouty1 in the proliferation, differentiation and apoptosis of epidermal keratinocytes. Cell Prolif. 2018;51:e12477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Wu Y, Zhang K, Liu R, Zhang H, Chen D, Yu S, Chen W, Wan S, Zhang Y, Jia Z, Chen R, Ding F. MicroRNA-21-3p accelerates diabetic wound healing in mice by downregulating SPRY1. Aging (Albany NY). 2020;12:15436-15445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Peng X, He F, Mao Y, Lin Y, Fang J, Chen Y, Sun Z, Zhuo Y, Jiang J. miR-146a promotes M2 macrophage polarization and accelerates diabetic wound healing by inhibiting the TLR4/NF-κB axis. J Mol Endocrinol. 2022;69:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 58. | Zhang HC, Wen T, Cai YZ. Overexpression of miR-146a promotes cell proliferation and migration in a model of diabetic foot ulcers by regulating the AKAP12 axis. Endocr J. 2022;69:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Huang YA, Chan KCC, You ZH. Constructing prediction models from expression profiles for large scale lncRNA-miRNA interaction profiling. Bioinformatics. 2018;34:812-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 60. | Mohamadkhani A. Long Noncoding RNAs in Interaction With RNA Binding Proteins in Hepatocellular Carcinoma. Hepat Mon. 2014;14:e18794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Chen X, Hu J. Long Noncoding RNA 3632454L22Rik Contributes to Corneal Epithelial Wound Healing by Sponging miR-181a-5p in Diabetic Mice. Invest Ophthalmol Vis Sci. 2021;62:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Xiao W, Zheng D, Chen X, Yu B, Deng K, Ma J, Wen X, Hu Y, Hou J. Long non-coding RNA MIAT is involved in the regulation of pyroptosis in diabetic cardiomyopathy via targeting miR-214-3p. iScience. 2021;24:103518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Pickard MR, Williams GT. Molecular and Cellular Mechanisms of Action of Tumour Suppressor GAS5 LncRNA. Genes (Basel). 2015;6:484-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 64. | Hu J, Zhang L, Liechty C, Zgheib C, Hodges MM, Liechty KW, Xu J. Long Noncoding RNA GAS5 Regulates Macrophage Polarization and Diabetic Wound Healing. J Invest Dermatol. 2020;140:1629-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 65. | Peng WX, He PX, Liu LJ, Zhu T, Zhong YQ, Xiang L, Peng K, Yang JJ, Xiang GD. LncRNA GAS5 activates the HIF1A/VEGF pathway by binding to TAF15 to promote wound healing in diabetic foot ulcers. Lab Invest. 2021;101:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 66. | Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205. Cancer Res. 2015;75:1322-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 468] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 67. | Li Q, Pan X, Wang X, Jiao X, Zheng J, Li Z, Huo Y. Long noncoding RNA MALAT1 promotes cell proliferation through suppressing miR-205 and promoting SMAD4 expression in osteosarcoma. Oncotarget. 2017;8:106648-106660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Zhang Y, Qu L, Ni H, Wang Y, Li L, Yang X, Wang X, Hou Y. Expression and function of lncRNA MALAT1 in gestational diabetes mellitus. Adv Clin Exp Med. 2020;29:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Jayasuriya R, Dhamodharan U, Karan AN, Anandharaj A, Rajesh K, Ramkumar KM. Role of Nrf2 in MALAT1/ HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic Biol Med. 2020;156:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 70. | Fu W, Liang D, Wu X, Chen H, Hong X, Wang J, Zhu T, Zeng T, Lin W, Chen S, Yan L, Ren M. Long noncoding RNA LINC01435 impedes diabetic wound healing by facilitating YY1-mediated HDAC8 expression. iScience. 2022;25:104006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Mobley RJ, Raghu D, Duke LD, Abell-Hart K, Zawistowski JS, Lutz K, Gomez SM, Roy S, Homayouni R, Johnson GL, Abell AN. MAP3K4 Controls the Chromatin Modifier HDAC6 during Trophoblast Stem Cell Epithelial-to-Mesenchymal Transition. Cell Rep. 2017;18:2387-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y, Zhai A, Bi C. Long noncoding RNA H19 acts as a miR-29b sponge to promote wound healing in diabetic foot ulcer. FASEB J. 2021;35:e20526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Pi L, Robinson PM, Jorgensen M, Oh SH, Brown AR, Weinreb PH, Trinh TL, Yianni P, Liu C, Leask A, Violette SM, Scott EW, Schultz GS, Petersen BE. Connective tissue growth factor and integrin αvβ6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology. 2015;61:678-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Li B, Zhou Y, Chen J, Wang T, Li Z, Fu Y, Bi C, Zhai A. Long non-coding RNA H19 contributes to wound healing of diabetic foot ulcer. J Mol Endocrinol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Rozzo C, Dessole S, Cherchi P, Gianfrancesco F, Pintus A, Carboni A, Deiana A, Tanda F, Palmieri G. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat. 2004;23:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | He M, Tu L, Shu R, Meng Q, Du S, Xu Z, Wang S. Long Noncoding RNA CASC2 Facilitated Wound Healing through miRNA-155/HIF-1α in Diabetic Foot Ulcers. Contrast Media Mol Imaging. 2022;2022:6291497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 77. | Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 78. | Cortés-López M, Miura P. Emerging Functions of Circular RNAs. Yale J Biol Med. 2016;89:527-537. [PubMed] |
| 79. | Zaiou M. circRNAs Signature as Potential Diagnostic and Prognostic Biomarker for Diabetes Mellitus and Related Cardiovascular Complications. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Zhang L, Zeng M, Tang F, Chen J, Cao D, Tang ZN. Circ-PNPT1 contributes to gestational diabetes mellitus (GDM) by regulating the function of trophoblast cells through miR-889-3p/PAK1 axis. Diabetol Metab Syndr. 2021;13:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Cao M, Bu C, Zhang J, Ren Y, Zhou G, Chen C, Han G, Jiang SW, Wen J. Exosomal Circular RNA hsa_circ_0046060 of Umbilical Cord Mesenchymal Stromal Cell Ameliorates Glucose Metabolism and Insulin Resistance in Gestational Diabetes Mellitus via the miR-338-3p/G6PC2 Axis. Int J Endocrinol. 2022;2022:9218113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Liu J, Duan P, Xu C, Xu D, Liu Y, Jiang J. CircRNA circ-ITCH improves renal inflammation and fibrosis in streptozotocin-induced diabetic mice by regulating the miR-33a-5p/SIRT6 axis. Inflamm Res. 2021;70:835-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 83. | Han D, Liu W, Li G, Liu L. Circ_PRKDC knockdown promotes skin wound healing by enhancing keratinocyte migration via miR-31/FBN1 axis. J Mol Histol. 2021;52:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Wang A, Toma MA, Ma J, Li D, Vij M, Chu T, Wang J, Li X, Xu Landén N. Circular RNA hsa_circ_0084443 Is Upregulated in Diabetic Foot Ulcer and Modulates Keratinocyte Migration and Proliferation. Adv Wound Care (New Rochelle). 2020;9:145-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 85. | Shang B, Xu T, Hu N, Mao Y, Du X. Circ-Klhl8 overexpression increased the therapeutic effect of EPCs in diabetic wound healing via the miR-212-3p/SIRT5 axis. J Diabetes Complications. 2021;35:108020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Gorecka J, Gao X, Fereydooni A, Dash BC, Luo J, Lee SR, Taniguchi R, Hsia HC, Qyang Y, Dardik A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen Med. 2020;15:1277-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Chiang KJ, Chiu LC, Kang YN, Chen C. Autologous Stem Cell Therapy for Chronic Lower Extremity Wounds: A Meta-Analysis of Randomized Controlled Trials. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 88. | Shi Y, Shi H, Nomi A, Lei-Lei Z, Zhang B, Qian H. Mesenchymal stem cell-derived extracellular vesicles: a new impetus of promoting angiogenesis in tissue regeneration. Cytotherapy. 2019;21:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Cao Y, Gang X, Sun C, Wang G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J Diabetes Res. 2017;2017:9328347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 90. | Shi R, Jin Y, Cao C, Han S, Shao X, Meng L, Cheng J, Zhang M, Zheng J, Xu J, Li M. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 91. | Cho H, Balaji S, Hone NL, Moles CM, Sheikh AQ, Crombleholme TM, Keswani SG, Narmoneva DA. Diabetic wound healing in a MMP9-/- mouse model. Wound Repair Regen. 2016;24:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Hu J, Liu X, Chi J, Che K, Ma X, Qiu M, Fu Z, Wang Y, Wang W. Resveratrol Enhances Wound Healing in Type 1 Diabetes Mellitus by Promoting the Expression of Extracellular Vesicle-Carried MicroRNA-129 Derived from Mesenchymal Stem Cells. J Proteome Res. 2022;21:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Xu J, Bai S, Cao Y, Liu L, Fang Y, Du J, Luo L, Chen M, Shen B, Zhang Q. miRNA-221-3p in Endothelial Progenitor Cell-Derived Exosomes Accelerates Skin Wound Healing in Diabetic Mice. Diabetes Metab Syndr Obes. 2020;13:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 94. | Yu Q, Liu L, Zhang X, Chang H, Ma S, Xie Z, Tang S, Ju X, Zhu H, Shen B, Zhang Q. MiR-221-3p targets HIPK2 to promote diabetic wound healing. Microvasc Res. 2022;140:104306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 95. | Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, Fu Y, Zhai A, Bi C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 96. | Zhu L, Wang G, Fischbach S, Xiao X. Suppression of microRNA-205-5p in human mesenchymal stem cells improves their therapeutic potential in treating diabetic foot disease. Oncotarget. 2017;8:52294-52303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Zhu L, Zhong Q, Yang T, Xiao X. Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205-5p. Aging (Albany NY). 2019;11:12236-12245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 98. | Zhao X, Zhou S, Wang D, He W, Li J, Zhang S. MicroRNA-205 is downregulated in hepatocellular carcinoma and inhibits cell growth and metastasis via directly targeting vascular endothelial growth factor A. Oncol Lett. 2018;16:2207-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX, Xu HL. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract. 2022;183:109126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 100. | Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, Zhao S, Yuan H, Yang X, Shi J, Zhao H. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318:C848-C856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 101. | Meng F, Shen F, Ling H, Jin P, Zhou D, Li Q. CircARHGAP12 Triggers Mesenchymal Stromal Cell Autophagy to Facilitate its Effect on Repairing Diabetic Wounds by Sponging miR-301b-3p/ATG16L1 and miR-301b-3p/ULK2. J Invest Dermatol. 2022;142:1976-1989.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Liang ZH, Lin SS, Pan NF, Zhong GY, Qiu ZY, Kuang SJ, Lin ZH, Zhang Z, Pan YC. UCMSCs-derived exosomal circHIPK3 promotes ulcer wound angiogenesis of diabetes mellitus via miR-20b-5p/Nrf2/VEGFA axis. Diabet Med. 2022;e14968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |