Published online Jul 15, 2021. doi: 10.4239/wjd.v12.i7.997
Peer-review started: January 29, 2021
First decision: March 1, 2021
Revised: March 17, 2021
Accepted: May 22, 2021
Article in press: May 22, 2021
Published online: July 15, 2021
Processing time: 163 Days and 19.2 Hours
Bone loss associated with type 1 diabetes mellitus (T1DM) begins at the onset of the disease, already in childhood, determining a lower bone mass peak and hence a greater risk of osteoporosis and fractures later in life. The mechanisms underlying diabetic bone fragility are not yet completely understood. Hyper
Core Tip: Bone fragility is a well-known complication related to type 1 diabetes mellitus, and it can manifest from the disease onset, already in childhood. The mechanisms underlying this relationship, and the precise role of metabolic control in preventing bone impairment, are not yet fully understood. Future studies are needed to clarify better the factors responsible for bone damage in diabetic subjects, and to identify strategies for avoiding and managing osteopenia/osteoporosis in these subjects.
- Citation: Brunetti G, D'Amato G, De Santis S, Grano M, Faienza MF. Mechanisms of altered bone remodeling in children with type 1 diabetes. World J Diabetes 2021; 12(7): 997-1009
- URL: https://www.wjgnet.com/1948-9358/full/v12/i7/997.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i7.997
Type 1 diabetes mellitus (T1DM) is a common endocrine disease that affects approximately 500000 children and adolescents worldwide. Moreover, in the last decade the incidence has been rising and the age at onset dropping[1]. Micro and macrovascular T1DM-related complications may already have developed a few years after disease onset[2] and are correlated with the age at onset, diabetes duration, body mass index, and pubertal development[3-5]. Hyperglycemia is the main systemic risk factor for diabetic complications, although multiple biochemical pathways, such as the formation of advanced glycation end products, oxidative stress, endoplasmic reticulum stress, inflammatory cytokines, and kallikrein-bradykinin activation, link the adverse effects of hyperglycemia with the microvascular dysfunction[6]. Consequently, long-term glycemic control is considered the most important modifiable factor to delay the onset, as well as the progression of microvascular complications, as clearly demonstrated by The Diabetes Control and Complications Trial[7]. In addition, beyond the effect of glycemic control, the impairment of some protective factors, such as insulin and insulin-like growth factor-1 (IGF1), may contribute to the vascular damage over time[8].
Recently, it was recognized that autoimmune diabetic disease also affects the skeleton[9-11]. In T1DM, a reduced bone mass may be present at an early stage after diagnosis[11], but it is unclear whether it is the duration of diabetes or degree of glycemic control that may induce a lifelong increased risk of fractures[12]. The association between glucose metabolism and bone-fat tissue interactions[13,14], as well as muscle-bone crosstalk, has been clearly demonstrated[15]. In particular, the skeleton acts as an endocrine organ, by modulating glucose tolerance through the secretion of bone-specific proteins, in particular osteocalcin (OCN). Furthermore, proteins involved in bone remodeling, like osteoprotegerin (OPG), are associated with an impaired insulin function[16].
The aim of this review is to provide an overview of current knowledge of the mechanisms underlying altered bone remodeling in children affected by T1DM.
Childhood and adolescence are the critical ages for linear growth, bone mineral accrual, and the attainment of the peak bone mass, which is a key determinant of the lifelong risk of osteoporosis[17,18]. Therefore, osteoporosis prevention begins by improving bone mineral gains during an individual's years of growth[19]. During peripuberty, the bone mineral content and bone mineral density (BMD) in the lumbar spine and proximal femur increase by four-fold to six-fold. Furthermore, puberty is also the time when the main gender differences in bone growth emerge, particularly in terms of bone size and bone mass content. At the same time, the peak T1DM onset time ranges between the ages of 9 and 14 years[20], so children and adolescents affected by T1DM may be particularly predisposed to bone impairment.
Among the risk factors for osteoporosis, some factors are modifiable, such as a balanced diet and exercise, which have an important role already in childhood, whereas obvious non-modifiable factors include gender, age, genetic factors, diseases, and drugs[21,22].
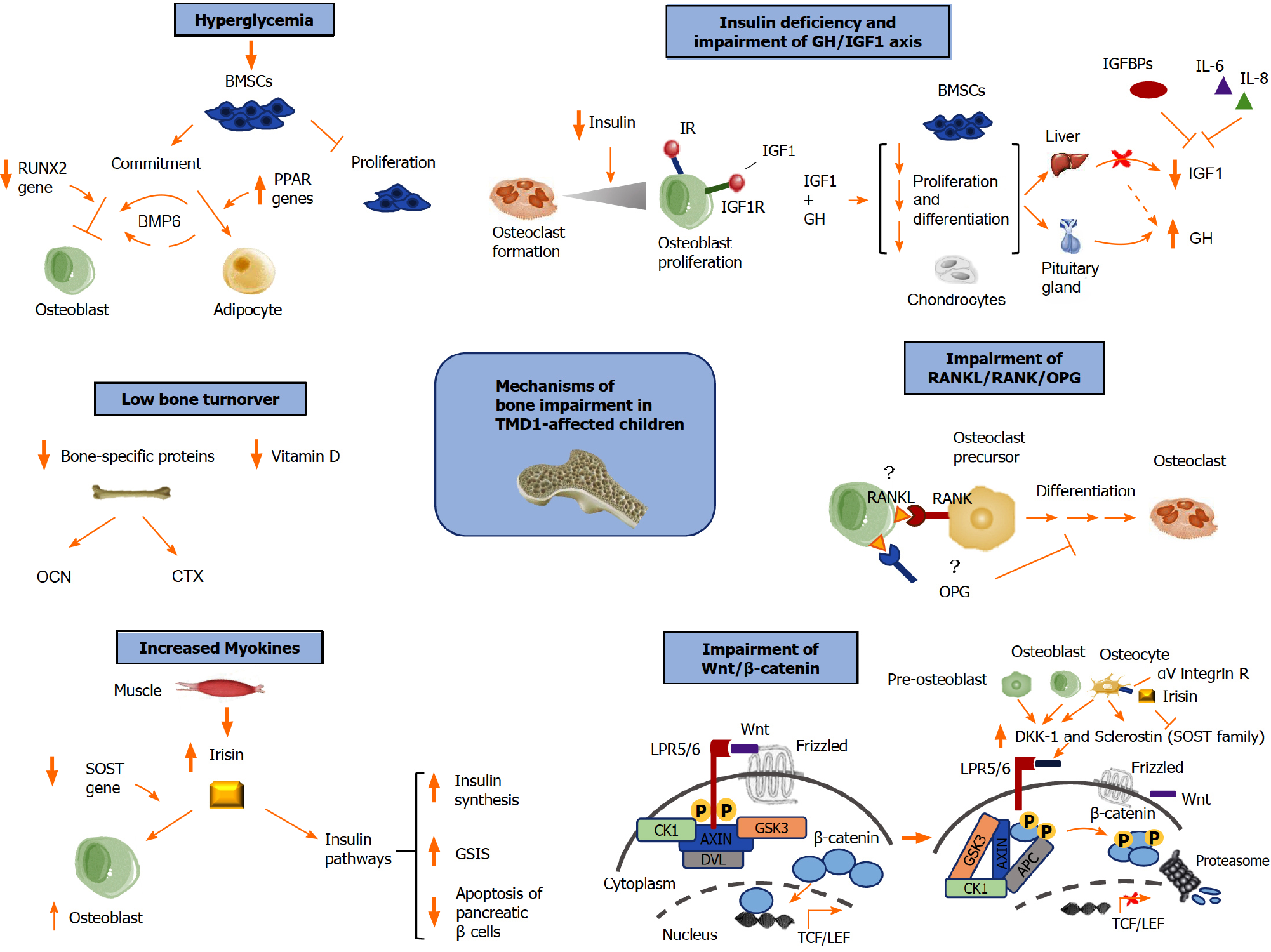
Impaired bone mass accrual (density and quality) in T1DM children has been attributed to multiple local factors in the bone marrow, as well as to systemic factors, which affect osteoblast (OB) differentiation and function (Figure 1).
Several studies have suggested that hyperglycemia impairs the biology and function of multipotent bone marrow-derived mesenchymal stem cells (BMSCs), which generate mesodermal tissues including cartilage, bone, muscle, tendon, ligament, and fat[23]. In particular, hyperglycemia both reduces the proliferation and increases the senescence of BMSCs in vitro[24,25] and also inhibits OB activities[26] (Figure 1). In addition, runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are downregulated in T1DM[27], suggesting that diabetic conditions may affect the BMSC fate commitment. Chronic hyperglycemia increases the expression of peroxisome proliferator-activated receptors (PPAR) genes, which also stimulate BMSCs differentiation in bone marrow adipocytes[28] (Figure 1). In addition to this, thiazolidinediones, antidiabetic PPARγ agonists, promote marrow adipogenesis, thus increasing the fracture risk[29]. The bone marrow adipose tissue (BMAT) is considered to be a single anatomical entity with a different distribution in the various skeletal sites. In an animal T1DM model, BMAT was significantly augmented, and bone formation was inversely associated with the adipocytes in the bone marrow[30]. BMAT also directly regulates osteoclastogenesis by producing receptor activator of nuclear factor-κB ligand (RANKL)[31]. Bone morphogenetic protein-6 (BMP6) is known to induce bone formation (Figure 1), and adipose-derived BMSCs overexpressing BMP6 have been shown to be capable of repairing bone defects in an animal model[32]. In addition, BMP6 can probably mitigate T1DM-associated bone loss by directing BMSC differentiation towards the osteogenic lineage. Recently, BMP6 supplementation in streptozotocin-induced diabetic mice has been demonstrated to directly restore BMD without influencing glucose levels[33], although a possible indirect role of BMP6 exerted through the modulation of glucose concentrations was observed[34].
This finding suggests that hyperglycemia may not be the main determinant of bone loss in T1DM patients, since other factors, like insulin and the growth hormone/ insulin-like growth factor 1 (GH/IGF-1) axis, could modulate BMSCs osteogenesis via BMP6 or other pathways.
Insulin, GH, and IGF-1 are anabolic hormones that directly affect bone cells (Figure 1). Insulin stimulates both osteoclast (OC) formation and OB proliferation, achieving a steady state in favor of bone formation[35]. Insulin signaling is essential for normal bone acquisition, as demonstrated in insulin receptor (IR) knockout mice[36], likely due to the role of insulin in the regulation of bone energy metabolism. Moreover, IR activation in the growth plate of mice fed with a hypercaloric diet stimulates skeletal growth as well as growth plate chondrogenesis[37]. OBs also express the IGF-1 receptor (IGF1R), and IGF-1 binds both to IGF1R and, with a lower affinity, to IR, thus triggering the insulin signaling pathway and exerting osteoanabolic activities.
Linear growth as well as BMD are critically affected by the GH/IGF-1 axis. Moreover, GH and IGF-1 play a key role at the growth plate, acting on chondrocyte proliferation, differentiation, and hypertrophy. Abnormalities in this axis have been reported in T1DM subjects, especially during puberty because of the increased insulin requirements due to physiological insulin resistance. In particular, T1DM patients exhibit GH hypersecretion, resulting from portal insulinopenia associated with a decreased hepatic output of IGF-1 together with pituitary hypersecretion of GH. The low IGF-1 serum levels are also related to increased levels of the inflammatory cytokines interleukin-6 and interleukin-8, which inhibit IGF-1 transcription[38] (Figure 1).
Several studies have established correlations between a low BMD, low IGF-1, and glycemic control in T1DM children and adolescents, also providing evidence of low BMD in subjects with poor glycemic control[39-42]. These data are correlated with reduced IGF1, IGF1R and transforming growth factor β1 gene expression in peripheral blood mononuclear cells in T1DM patients[43]. In addition, changes in the levels of the IGF-1 binding proteins that modulate IGF-1 bioactivity in serum and tissues have been observed in T1DM subjects[44].
Bone health depends on the balance between OCs, the bone-reabsorbing cells, and OBs, the bone-forming cells. In several pediatric diseases, bone impairment is due to an imbalance of OBs and OCs activity accomplishing the remodeling process[45]. OBs produce positive and negative regulators of osteoclastogenesis, such as the RANKL and the natural decoy receptor for RANKL, OPG, respectively[46]. Although OBs are a major source of RANKL, this cytokine is also expressed by osteocytes, fibroblasts, and immune system cells, including T cells and mature dendritic cells[47]. OCs differentiate under the control of RANKL, which binds to its receptor, RANK. OPG is the RANKL decoy receptor, thus acting as a negative regulator of osteoclastogenesis (Figure 1). OPG is produced not only by OBs but also by B lymphocytes and dendritic cells, as well as several cytokines[47,48]. In the last years, the impaired OB differentiation and function mechanisms in diabetic bone have been further elucidated, demonstrated by low serum levels of OB markers in T1DM subjects[49], and a decreased osteoblastic activity in streptozotocin-induced T1DM mice[50]. However, OC activity and bone resorption in T1DM are still debated. In diabetic animal models, an increase in OC numbers[51], as well as messenger RNA (mRNA levels of tartrate resistant acid phosphatase (TRAP) and cathepsin K, bone resorption markers, has been demonstrated[52,53]. By contrast, bone resorption was unaffected or even decreased in T1DM rodents[54]. In a recent study by Yang et al[55] the OC activity of trabecular bone was increased in diabetic mice at the early stage, accompanied by an augmented protein expression of RANKL. Remarkably, the RANKL mRNA levels remained unchanged, suggesting that the increased bone resorption in early-stage diabetic mice is induced by RANKL derived from BMAT rather than from the bone tissue itself[55]. This finding indicates that BMAT could be a key factor in regulating bone homeostasis in pathological conditions such as diabetes.
Data about OPG and RANKL levels in T1DM children and adolescents are conflicting. Chrysis et al[56] found that serum OPG levels were significantly increased in patients with T1DM compared with controls, whereas RANKL levels did not change. The low RANKL levels in T1DM patients are probably due to blockade of the RANKL signal by an OPG increase on the OPG/RANKL/RANK axis[56]. Con
The Wnt/β-catenin pathway is a signal transduction cascade that controls numerous processes during development. Thus, aberrant Wnt signaling underlies a broad range of diseases in humans.
The pathway is regulated at several levels, also by secreted Frizzled-related proteins and Wnt inhibitory protein, both of which inhibit interactions between Wnt and Wnt receptors[66] (Figure 1). Other Wnt inhibitors belong to the Dickkopf-1 (DKK-1) and the WISE/SOST families, which antagonize signaling by binding low-density lipoprotein-related receptor-5/6[67]. DKK-1 is expressed by preosteoblasts, OBs, and osteocytes, and acts as an antagonist of the canonical Wnt signaling by binding to low-density lipoprotein-related receptor-5/6 (Figure 1). Sclerostin is a secreted protein encoded by the SOST gene and produced by mature osteocytes, which antagonizes Wnt/β-catenin signaling by abrogating its bone anabolic actions[68,69] (Figure 1). DKK-1 and sclerostin are key regulators of bone mass, and high levels of these Wnt signaling inhibitors have been found in several bone diseases[70]. The important role of both molecules has also been demonstrated in a mouse model, showing that a bispecific antibody targeting sclerostin and DKK-1 supports bone mass accrual and fracture repair, exerting a greater effect compared to monotherapies[71]. In type 1 diabetic rats, an increased SOST mRNA and sclerostin expression has been observed[72]. Clinical studies and Homeostatic Model Assessment for Insulin Resistance have shown an inverse correlation between sclerostin and insulin levels, suggesting that sclerostin could modulate glucose homeostasis[73]. DKK-1 involvement has been demonstrated in a large cohort of T1DM children and adolescents affected by T1DM[74], in which DKK-1 levels were correlated with bone formation markers, the BMD-Z-score, sex, and pubertal stage[74]. Neumann et al[75] reported higher serum levels of sclerostin in T1DM subjects compared with controls but found no correlations between sclerostin levels and bone metabolism markers. On the contrary, Tsentidis et al[76] found comparable levels of sclerostin in T1DM children and controls. Recent data suggested that sclerostin levels are increased in pediatric T1MD patients and confirmed a relationship between sclerostin and the glucose metabolism[77]. In addition, in T1MD subjects’ bone-derived OCN, as well as fat-derived leptin, appear to modulate sclerostin support in metabolic regulation[77]. Future studies are needed to clarify the role of sclerostin in bone impairment associated with T1DM.
Bone and muscles are integrated organs that exert a mutual control and are in turn controlled by several factors, such as the GH-IGF-1 axis, sex steroids, adipokines (e.g., leptin, adiponectin, visfatin, resistin), and vitamin D[78,79]. In addition to mediating the muscle-bone crosstalk, muscles release myokines that affect other organs and tissues, including the liver, intestine, and adipose tissue, which in turn release cytokines and hormones responsible for regulating bone homeostasis. Among the myokines, irisin is a small peptide derived from the proteolytic cleavage of fibronectin III domain-containing protein 5, produced during physical exercise[80]. This myokine has been associated with the browning response and thermogenesis of white adipose tissue[80]. In addition, it has an essential role in the bone-muscle unit, and exerts anabolic effects on bone, both in vitro and in vivo[81]. Irisin acts through the activation of osteoblastic bone formation, the induction of the pro-osteoblastic genes, and the decrease of osteoblastogenesis inhibitors[81]. The direct effect of irisin on OBs is exerted by means of a downregulation of SOST expression, which negatively regulates bone formation[81] (Figure 1). In agreement with this finding, Zhang et al[82] demonstrated in vitro that treatment with recombinant irisin on OB precursors causes the accumulation of β-catenin in the nucleus, suggesting that irisin restores SOST-mediated inhibition of the Wnt/β-catenin pathway by directly inhibiting SOST. In addition, irisin interacts with osteocytes by directly binding to αV integrin receptors, thus protecting them from apoptosis, and inducing the secretion of SOST in vivo[83]. Regarding its metabolic effects, recombinant irisin has been shown to stimulate insulin biosynthesis and glucose-stimulated insulin secretion in a protein kinase A-dependent manner. It also prevents saturated fatty acid-induced apoptosis in human and rat pancreatic β cells, as well as in human and murine pancreatic islets, via the AKT/B-cell lymphoma 2 signaling pathway[84] (Figure 1). Studies in humans have elucidated the role of irisin both in healthy subjects and in patients affected by diseases related to bone metabolism, such as hyperparathyroidism and T1DM. In a recent study, irisin was demonstrated to be one of the main determinants of bone mineral status during childhood[85]. In addition, high irisin levels have been found in adult patients with long-lasting T1DM that were correlated with positivity for anti-glutamic acid decarboxylase antibodies, suggesting that autoimmunity can have a role in regulating the levels of this myokine[86,87].
In T1DM children and adolescents, elevated irisin levels have been found to be closely related to better metabolic control and an improved bone mass[88]. These findings are in agreement with the recent data showing that irisin can promote insulin synthesis as well as glucose-stimulated insulin secretion[84]. In addition, irisin overexpression enhanced insulin sensitivity in mice while reducing hyperlipidemia and hyperglycemia[89], suggesting that irisin could have a key role in diabetes man
Bone is considered to be an endocrine “gland,” and its modulation of glucose tolerance by the secretion of bone-specific proteins, in particular OCN, has been clearly demonstrated[16]. OCN is the main non-collagen protein secreted by the OBs and stored in the bone extracellular matrix. The carboxylated form of OCN shows a high affinity to hydroxyapatite, the mineral present in bone. Instead, the uncarboxylated form is free in the circulation and regulates glucose metabolism and insulin resistance[90]. Several data have suggested that serum levels of uncarboxylated OCN are negatively correlated with insulin resistance, obesity, diabetes, and markers of the metabolic syndrome[91-93].
T1DM subjects show a low bone turnover, which is another mechanism underlying bone fragility in these subjects[94]. Previous studies demonstrated that both markers of bone resorption, such as the C-terminal cross-link of collagen (CTX), and markers of bone formation, such as OCN, were decreased in T1DM compared to healthy controls[94]. Furthermore, while levels of TRAP and procollagen type 1 amino terminal propeptide (P1NP) were comparable in patients with T1DM and healthy subjects, low vitamin D levels were found in T1DM patients[94]. Similarly, reduced OCN levels were observed in children and adolescents with T1DM, while P1NP levels would seem to be lower and CTX levels higher in T1DM than in healthy subjects[95]. Chen et al[96] showed low levels of bone alkaline phosphatase and CTX in T1DM children as compared to controls.
A recent report by Madsen et al[97] investigated bone turnover markers in relation to BMD and metabolic control in T1DM children and adolescents. The results of this study demonstrated that markers of bone formation and resorption were significantly decreased in both sexes, and HbA1c levels were negatively correlated to the resorption marker CTX but not to any of the bone formation markers[97]. Another important finding of this study was that the decreased levels of both markers of bone formation and bone resorption were independent of the T1DM duration and Tanner stage. Thus, the impairment of bone health in T1DM begins in early childhood, independently of age and pubertal stage.
A possible explanation for the low bone turnover in diabetic subjects may be an insulin deficiency, which contributes to a low bone formation, as demonstrated by low bone turnover in a mouse model of insulinopenia and restoration following insulin treatment[98].
It is possible that the low bone turnover caused by insulin deficiency occurs over time and may not be detected in studies based on acute changes in insulin levels[99].
T1DM is the most frequent chronic disease in the pediatric population. It is associated with an increased bone fragility from childhood onward, and hence the risk of fractures later in life. Although a low BMD is documented in diabetic individuals, the precise mechanisms underlying bone loss are not yet fully understood. Hyperglycemia seems not to be the main cause of bone impairment in T1DM patients, but other factors, like insulin deficiency, the GH/IGF-1 axis, and low bone turnover, could contribute to the bone impairment observed in these subjects. The use of diabetes technologies, like the use of insulin pumps and continuous glucose monitors, to achieve glycemia control appears to be correlated with an improved bone health, although further studies are needed to confirm this finding. In addition, prospective studies should clarify the causal relationships among metabolic control, bone turnover markers, the RANKL/OPG ratio, Wnt-signaling inhibitors, myokine activity, and bone mineralization in T1DM subjects.
To date, no specific biomarkers are available to predict accurately fracture outcomes in T1DM patients. Additional large-scale prospective studies are needed to identify high-risk patients. In addition to dual x-ray absorptiometry, a fracture risk assessment tool and trabecular bone score could, in the future, offer additional technologies to evaluate better the bone quality of T1DM patients[100].
There is no clear evidence in support of early intervention to avoid the risk of osteoporosis or of the use of anti-osteoporotic drugs in diabetic subjects[101].
Preclinical studies indicated that denosumab, a human monoclonal antibody to RANKL approved for the treatment of osteoporosis or for patients at high fracture risk[102], may stimulate β-cell proliferation in humans[103] and improve liver insulin sensitivity[104].
No data are currently available on romosozumab, an anti-sclerostin antibody indicated to reduce the risk of clinical and vertebral fractures in postmenopausal women with osteoporosis[105]. In addition, there are still few data on the effects of vitamin D, calcium intake, and physical activity on bone health in T1DM subjects[106]. Recently, toll-like receptor-4 (TLR4) has been correlated with diabetic bone disorders via the nuclear factor-κB pathway[107,108]. It has been demonstrated that TLR4 deletion improves streptozotocin-induced diabetic osteoporosis in mice, so TLR4 may be a possible therapeutic target for the treatment of diabetic osteoporosis[109].
T1DM has a strong impact on bone health, and skeletal fragility is now recognized among the complications of diabetes. The fracture risk is greater in patients with T1DM and increases linearly with the disease duration. T1DM subjects show a decreased BMD already in childhood, possibly due to an absolute insulin deficiency and the inability of exogenous insulin to reflect endogenous insulin secretion. However, the reduction in BMD does not entirely explain the increase in bone fragility observed in these subjects. It is unclear whether reducing hypoglycemic events by means of continuous glucose monitoring and a closed-loop insulin delivery system can improve bone health in subjects with T1DM. Randomized clinical trials to evaluate the efficacy of anti-fracture drugs in diabetes are lacking, while some observational data have indicated an analogous efficacy in those with or without diabetes, so such drugs should be used according to existing indications.
Further studies are warranted to clarify better the factors responsible for bone damage in diabetic subjects and to identify efficacious strategies to prevent osteopenia/osteoporosis and the risk of fractures in these subjects.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Larkin J S-Editor: Liu M L-Editor: Filipodia P-Editor: Liu JH
| 1. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2503] [Article Influence: 312.9] [Reference Citation Analysis (0)] |
| 2. | Donaghue KC, Wadwa RP, Dimeglio LA, Wong TY, Chiarelli F, Marcovecchio ML, Salem M, Raza J, Hofman PL, Craig ME; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2014;15 Suppl 20:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Faienza MF, Acquafredda A, Tesse R, Luce V, Ventura A, Maggialetti N, Monteduro M, Giordano P, Cavallo L. Risk factors for subclinical atherosclerosis in diabetic and obese children. Int J Med Sci. 2013;10:338-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Demirel F, Tepe D, Kara O, Esen I. Microvascular complications in adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2013;5:145-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care. 2004;27:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW, Vinik AI, Casellini CM. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2017;102:4343-4410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16273] [Article Influence: 508.5] [Reference Citation Analysis (3)] |
| 8. | Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care. 2011;34:968-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Hough FS, Pierroz DD, Cooper C, Ferrari SL; IOF CSA Bone and Diabetes Working Group. MECHANISMS IN ENDOCRINOLOGY: Mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174:R127-R138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Weber DR, Schwartz G. Epidemiology of Skeletal Health in Type 1 Diabetes. Curr Osteoporos Rep. 2016;14:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 705] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 13. | Lecka-Czernik B, Rosen CJ. Energy Excess, Glucose Utilization, and Skeletal Remodeling: New Insights. J Bone Miner Res. 2015;30:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Abdalrahaman N, McComb C, Foster JE, Lindsay RS, Drummond R, McKay GA, Perry CG, Ahmed SF. The relationship between adiposity, bone density and microarchitecture is maintained in young women irrespective of diabetes status. Clin Endocrinol (Oxf). 2017;87:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112:12157-12162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 377] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 16. | Faienza MF, Luce V, Ventura A, Colaianni G, Colucci S, Cavallo L, Grano M, Brunetti G. Skeleton and glucose metabolism: a bone-pancreas loop. Int J Endocrinol. 2015;2015:758148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Sopher AB, Fennoy I, Oberfield SE. An update on childhood bone health: mineral accrual, assessment and treatment. Curr Opin Endocrinol Diabetes Obes. 2015;22:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 975] [Cited by in RCA: 846] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 19. | Carey DE, Golden NH. Bone Health in Adolescence. Adolesc Med State Art Rev. 2015;26:291-325. [PubMed] |
| 20. | Royal College of Paediatrics and Child Health. National Paediatric Diabetes Audit (NPDA) national reports; 2016 [cited 10 January 2021]. [Internet]. Available from: https://www.rcpch.ac.uk/sites/default/files/2018-03/npda_national_report_2013-14.pdf. |
| 21. | Stefania S, Clodoveo ML, Cariello M, D'Amato G, Franchini C, Faienza MF, Corbo F. Polyphenols and obesity prevention: critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit Rev Food Sci Nutr. 2020;1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Faienza MF, Lassandro G, Chiarito M, Valente F, Ciaccia L, Giordano P. How Physical Activity across the Lifespan Can Reduce the Impact of Bone Ageing: A Literature Review. Int J Environ Res Public Health. 2020;17:1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One. 2015;10:e0126537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, Schneider D, Limbert C, Seufert J, Kassem M, Schütze N, Jakob F, Ebert R. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun. 2007;363:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Cao B, Liu N, Wang W. High glucose prevents osteogenic differentiation of mesenchymal stem cells via lncRNA AK028326/CXCL13 pathway. Biomed Pharmacother. 2016;84:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Fowlkes JL, Bunn RC, Liu L, Wahl EC, Coleman HN, Cockrell GE, Perrien DS, Lumpkin CK Jr, Thrailkill KM. Runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology. 2008;149:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Kim TY, Schafer AL. Diabetes and Bone Marrow Adiposity. Curr Osteoporos Rep. 2016;14:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Motyl KJ, Raetz M, Tekalur SA, Schwartz RC, McCabe LR. CCAAT/enhancer binding protein β-deficiency enhances type 1 diabetic bone phenotype by increasing marrow adiposity and bone resorption. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1250-R1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, Baron R, Bronson RT, Horowitz MC, Wu JY, Bilezikian JP, Dempster DW, Rosen CJ, Lanske B. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017;25:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 32. | Sheyn D, Tawackoli W, Gazit Z, Pelled G. Allogeneic, BMP6 gene-modified, bone marrow MSCs induce vertebral fracture repair ina porcine model: A pilot study. Spine J. 2013;13:S71-S72. [DOI] [Full Text] |
| 33. | Wang JF, Lee MS, Tsai TL, Leiferman EM, Trask DJ, Squire MW, Li WJ. Bone Morphogenetic Protein-6 Attenuates Type 1 Diabetes Mellitus-Associated Bone Loss. Stem Cells Transl Med. 2019;8:522-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Pramojanee SN, Phimphilai M, Chattipakorn N, Chattipakorn SC. Possible roles of insulin signaling in osteoblasts. Endocr Res. 2014;39:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 574] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 37. | Wu S, Zhang Y, De Luca F. The effect of a high-calorie diet on bone growth is mediated by the insulin receptor. Bone. 2019;122:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Van Sickle BJ, Simmons J, Hall R, Raines M, Ness K, Spagnoli A. Increased circulating IL-8 is associated with reduced IGF-1 and related to poor metabolic control in adolescents with type 1 diabetes mellitus. Cytokine. 2009;48:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ. Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J Pediatr. 2004;144:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | de Souza KS, Ururahy MA, da Costa Oliveira YM, Loureiro MB, da Silva HP, Bortolin RH, Melo Dos Santos F, Luchessi AD, Neto JJ, Arrais RF, Hirata RD, das Graças Almeida M, Hirata MH, de Rezende AA. Low bone mineral density in patients with type 1 diabetes: association with reduced expression of IGF1, IGF1R and TGF B 1 in peripheral blood mononuclear cells. Diabetes Metab Res Rev. 2016;32:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Valerio G, del Puente A, Esposito-del Puente A, Buono P, Mozzillo E, Franzese A. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Horm Res. 2002;58:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Moyer-Mileur LJ, Dixon SB, Quick JL, Askew EW, Murray MA. Bone mineral acquisition in adolescents with type 1 diabetes. J Pediatr. 2004;145:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Raisingani M, Preneet B, Kohn B, Yakar S. Skeletal growth and bone mineral acquisition in type 1 diabetic children; abnormalities of the GH/IGF-1 axis. Growth Horm IGF Res. 2017;34:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Sharma A, Purohit S, Sharma S, Bai S, Zhi W, Ponny SR, Hopkins D, Steed L, Bode B, Anderson SW, She JX. IGF-Binding Proteins in Type-1 Diabetes Are More Severely Altered in the Presence of Complications. Front Endocrinol (Lausanne). 2016;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Brunetti G, D'Amato G, Chiarito M, Tullo A, Colaianni G, Colucci S, Grano M, Faienza MF. An update on the role of RANKL-RANK/osteoprotegerin and WNT-ß-catenin signaling pathways in pediatric diseases. World J Pediatr. 2019;15:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Brunetti G, Di Benedetto A, Posa F, Colaianni G, Faienza MF, Ballini A, Colucci S, Passeri G, Lo Muzio L, Grano M, Mori G. High expression of TRAIL by osteoblastic differentiated dental pulp stem cells affects myeloma cell viability. Oncol Rep. 2018;39:2031-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Soysa NS, Alles N, Aoki K, Ohya K. Osteoclast formation and differentiation: an overview. J Med Dent Sci. 2012;59:65-74. [PubMed] |
| 49. | Tsentidis C, Gourgiotis D, Kossiva L, Doulgeraki A, Marmarinos A, Galli-Tsinopoulou A, Karavanaki K. Higher levels of s-RANKL and osteoprotegerin in children and adolescents with type 1 diabetes mellitus may indicate increased osteoclast signaling and predisposition to lower bone mass: a multivariate cross-sectional analysis. Osteoporos Int. 2016;27:1631-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Peng J, Hui K, Hao C, Peng Z, Gao QX, Jin Q, Lei G, Min J, Qi Z, Bo C, Dong QN, Bing ZH, Jia XY, Fu DL. Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice. Connect Tissue Res. 2016;57:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Alharbi MA, Zhang C, Lu C, Milovanova TN, Yi L, Ryu JD, Jiao H, Dong G, O'Connor JP, Graves DT. FOXO1 Deletion Reverses the Effect of Diabetic-Induced Impaired Fracture Healing. Diabetes. 2018;67:2682-2694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Hie M, Tsukamoto I. Increased expression of the receptor for activation of NF-kappaB and decreased runt-related transcription factor 2 expression in bone of rats with streptozotocin-induced diabetes. Int J Mol Med. 2010;26:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Hie M, Shimono M, Fujii K, Tsukamoto I. Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone. 2007;41:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622-3631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Yang J, Chen S, Zong Z, Yang L, Liu D, Bao Q, Du W. The increase in bone resorption in early-stage type I diabetic mice is induced by RANKL secreted by increased bone marrow adipocytes. Biochem Biophys Res Commun. 2020;525:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Chrysis D, Efthymiadou A, Mermigka A, Kritikou D, Spiliotis BE. Osteoprotegerin, RANKL, ADMA, and Fetuin-A serum levels in children with type I diabetes mellitus. Pediatr Diabetes. 2017;18:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Galluzzi F, Stagi S, Salti R, Toni S, Piscitelli E, Simonini G, Falcini F, Chiarelli F. Osteoprotegerin serum levels in children with type 1 diabetes: a potential modulating role in bone status. Eur J Endocrinol. 2005;153:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Szymańska M, Michałus I, Kaszkowiak M, Wyka K, Chlebna-Sokół D, Fendler W, Jakubowska-Pietkiewicz E, Młynarski W, Szadkowska A, Zmysłowska A. Metabolic bone markers can be related to preserved insulin secretion in children with newly diagnosed type 1 diabetes. Pediatr Endocrinol Diabetes Metab. 2020;26:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Loureiro MB, Ururahy MA, Freire-Neto FP, Oliveira GH, Duarte VM, Luchessi AD, Brandão-Neto J, Hirata RD, Hirata MH, Maciel-Neto JJ, Arrais RF, Almeida MG, Rezende AA. Low bone mineral density is associated to poor glycemic control and increased OPG expression in children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2014;103:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Karalazou P, Ntelios D, Chatzopoulou F, Fragou A, Taousani M, Mouzaki K, Galli-Tsinopoulou A, Kouidou S, Tzimagiorgis G. OPG/RANK/RANKL signaling axis in patients with type I diabetes: Associations with parathormone and vitamin D. Ital J Pediatr. 2019;45:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Lambrinoudaki I, Tsouvalas E, Vakaki M, Kaparos G, Stamatelopoulos K, Augoulea A, Pliatsika P, Alexandrou A, Creatsa M, Karavanaki K. Osteoprotegerin, Soluble Receptor Activator of Nuclear Factor- κ B Ligand, and Subclinical Atherosclerosis in Children and Adolescents with Type 1 Diabetes Mellitus. Int J Endocrinol. 2013;2013:102120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Wang ST, Xu JM, Wang M, Chen FL, Ding G. Increased plasma osteoprotegerin concentrations in Type 1 diabetes with albuminuria. Clin Nephrol. 2013;79:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Singh DK, Winocour P, Summerhayes B, Viljoen A, Sivakumar G, Farrington K. Low serum osteoprotegerin levels in normoalbuminuric type 1 diabetes mellitus. Acta Diabetol. 2010;47 Suppl 1:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Abd El Dayem SM, El-Shehaby AM, Abd El Gafar A, Fawzy A, Salama H. Bone density, body composition, and markers of bone remodeling in type 1 diabetic patients. Scand J Clin Lab Invest. 2011;71:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4393] [Article Influence: 337.9] [Reference Citation Analysis (0)] |
| 67. | Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1271] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 68. | Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 836] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 69. | Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol. 2016;12:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 70. | Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1539] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 71. | Florio M, Gunasekaran K, Stolina M, Li X, Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li C, Sun B, Tan HL, Zhang L, Han CY, Case R, Duguay AN, Grisanti M, Stevens J, Pretorius JK, Pacheco E, Jones H, Chen Q, Soriano BD, Wen J, Heron B, Jacobsen FW, Brisan E, Richards WG, Ke HZ, Ominsky MS. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 72. | Pacicca DM, Brown T, Watkins D, Kover K, Yan Y, Prideaux M, Bonewald L. Elevated glucose acts directly on osteocytes to increase sclerostin expression in diabetes. Sci Rep. 2019;9:17353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Cipriani C, Colangelo L, Santori R, Renella M, Mastrantonio M, Minisola S, Pepe J. The Interplay Between Bone and Glucose Metabolism. Front Endocrinol (Lausanne). 2020;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 74. | Faienza MF, Ventura A, Delvecchio M, Fusillo A, Piacente L, Aceto G, Colaianni G, Colucci S, Cavallo L, Grano M, Brunetti G. High Sclerostin and Dickkopf-1 (DKK-1) Serum Levels in Children and Adolescents With Type 1 Diabetes Mellitus. J Clin Endocrinol Metab. 2017;102:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Neumann T, Sämann A, Lodes S, Kästner B, Franke S, Kiehntopf M, Hemmelmann C, Lehmann T, Müller UA, Hein G, Wolf G. Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with Type 1 diabetes. Diabet Med. 2011;28:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Tsentidis C, Gourgiotis D, Kossiva L, Marmarinos A, Doulgeraki A, Karavanaki K. Sclerostin distribution in children and adolescents with type 1 diabetes mellitus and correlation with bone metabolism and bone mineral density. Pediatr Diabetes. 2016;17:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Wędrychowicz A, Sztefko K, Starzyk JB. Sclerostin and its significance for children and adolescents with type 1 diabetes mellitus (T1D). Bone. 2019;120:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Laurent MR, Dubois V, Claessens F, Verschueren SM, Vanderschueren D, Gielen E, Jardí F. Muscle-bone interactions: From experimental models to the clinic? Mol Cell Endocrinol. 2016;432:14-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 79. | Lombardi G, Barbaro M, Locatelli M, Banfi G. Novel bone metabolism-associated hormones: the importance of the pre-analytical phase for understanding their physiological roles. Endocrine. 2017;56:460-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3682] [Cited by in RCA: 3517] [Article Influence: 270.5] [Reference Citation Analysis (0)] |
| 81. | Buccoliero C, Oranger A, Colaianni G, Pignataro P, Zerlotin R, Lovero R, Errede M, Grano M. The effect of Irisin on bone cells in vivo and in vitro. Biochem Soc Trans. 2021;49:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 82. | Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, Jiang H, Dard MM, Huang J, Xu Z, Tu Q, Chen J. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017;5:16056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 83. | Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Bouxsein ML, Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell. 2019;178:507-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Natalicchio A, Marrano N, Biondi G, Spagnuolo R, Labarbuta R, Porreca I, Cignarelli A, Bugliani M, Marchetti P, Perrini S, Laviola L, Giorgino F. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes. 2017;66:2849-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 85. | Colaianni G, Faienza MF, Sanesi L, Brunetti G, Pignataro P, Lippo L, Bortolotti S, Storlino G, Piacente L, D'Amato G, Colucci S, Grano M. Irisin serum levels are positively correlated with bone mineral status in a population of healthy children. Pediatr Res. 2019;85:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Espes D, Lau J, Carlsson PO. Increased levels of irisin in people with long-standing Type 1 diabetes. Diabet Med. 2015;32:1172-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Ates I, Arikan MF, Erdogan K, Kaplan M, Yuksel M, Topcuoglu C, Yilmaz N, Guler S. Factors associated with increased irisin levels in the type 1 diabetes mellitus. Endocr Regul. 2017;51:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Faienza MF, Brunetti G, Sanesi L, Colaianni G, Celi M, Piacente L, D'Amato G, Schipani E, Colucci S, Grano M. High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res Clin Pract. 2018;141:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 89. | Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, Li YH, Zhou YB, Han Y, Zhang F, Gao XY, Kang YM, Zhu GQ. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852:1867-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 90. | Pi M, Quarles LD. Novel bone endocrine networks integrating mineral and energy metabolism. Curr Osteoporos Rep. 2013;11:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellström D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 92. | Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, Hou X, Lu H, Bao Y, Jia W. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 94. | Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. MECHANISMS IN ENDOCRINOLOGY: Diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137-R157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (1)] |
| 95. | Madsen JOB, Jørgensen NR, Pociot F, Johannesen J. Bone turnover markers in children and adolescents with type 1 diabetes-A systematic review. Pediatr Diabetes. 2019;20:510-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Chen SC, Shepherd S, McMillan M, McNeilly J, Foster J, Wong SC, Robertson KJ, Ahmed SF. Skeletal Fragility and Its Clinical Determinants in Children With Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:3585-3594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 97. | Madsen JOB, Herskin CW, Zerahn B, Jørgensen NR, Olsen BS, Pociot F, Johannesen J. Decreased markers of bone turnover in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2020;21:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 98. | Thrailkill KM, Liu L, Wahl EC, Bunn RC, Perrien DS, Cockrell GE, Skinner RA, Hogue WR, Carver AA, Fowlkes JL, Aronson J, Lumpkin CK Jr. Bone formation is impaired in a model of type 1 diabetes. Diabetes. 2005;54:2875-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 99. | Basu R, Peterson J, Rizza R, Khosla S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. J Clin Endocrinol Metab. 2011;96:1450-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Jiang N, Xia W. Assessment of bone quality in patients with diabetes mellitus. Osteoporos Int. 2018;29:1721-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 101. | Anagnostis P, Paschou SA, Gkekas NN, Artzouchaltzi AM, Christou K, Stogiannou D, Vryonidou A, Potoupnis M, Goulis DG. Efficacy of anti-osteoporotic medications in patients with type 1 and 2 diabetes mellitus: a systematic review. Endocrine. 2018;60:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 102. | Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2264] [Cited by in RCA: 2277] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 103. | Kondegowda NG, Fenutria R, Pollack IR, Orthofer M, Garcia-Ocaña A, Penninger JM, Vasavada RC. Osteoprotegerin and Denosumab Stimulate Human Beta Cell Proliferation through Inhibition of the Receptor Activator of NF-κB Ligand Pathway. Cell Metab. 2015;22:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 104. | Lasco A, Morabito N, Basile G, Atteritano M, Gaudio A, Giorgianni GM, Morini E, Faraci B, Bellone F, Catalano A. Denosumab Inhibition of RANKL and Insulin Resistance in Postmenopausal Women with Osteoporosis. Calcif Tissue Int. 2016;98:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 105. | Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375:1532-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 1098] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 106. | Gil-Díaz MC, Raynor J, O'Brien KO, Schwartz GJ, Weber DR. Systematic review: associations of calcium intake, vitamin D intake, and physical activity with skeletal outcomes in people with Type 1 diabetes mellitus. Acta Diabetol. 2019;56:1091-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 107. | Szasz T, Wenceslau CF, Burgess B, Nunes KP, Webb RC. Toll-Like Receptor 4 Activation Contributes to Diabetic Bladder Dysfunction in a Murine Model of Type 1 Diabetes. Diabetes. 2016;65:3754-3764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Vijayan V, Khandelwal M, Manglani K, Gupta S, Surolia A. Methionine down-regulates TLR4/MyD88/NF-κB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br J Pharmacol. 2014;171:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Cao Y, Han X, Wang Z, Liu Y, Wang Y, Zhang R, Ye J, Zou L, Dai W. TLR4 knockout ameliorates streptozotocin-induced osteoporosis in a mouse model of diabetes. Biochem Biophys Res Commun. 2021;546:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |