Published online Jul 15, 2021. doi: 10.4239/wjd.v12.i7.975
Peer-review started: January 26, 2021
First decision: February 25, 2021
Revised: March 4, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: July 15, 2021
Processing time: 166 Days and 13.7 Hours
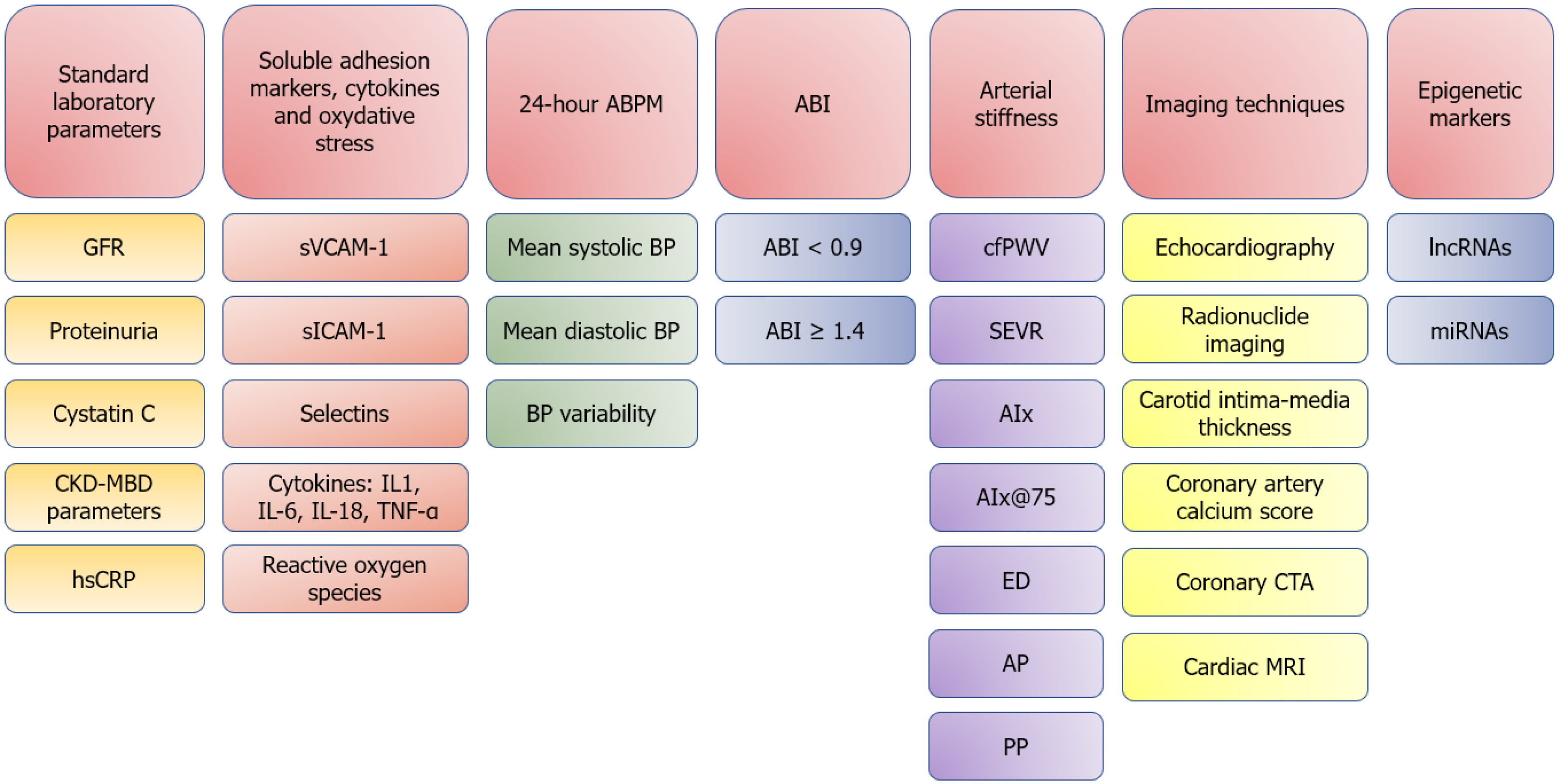
The prevalence and burden of diabetes mellitus and chronic kidney disease on global health and socioeconomic development is already heavy and still rising. Diabetes mellitus by itself is linked to adverse cardiovascular events, and the presence of concomitant chronic kidney disease further amplifies cardiovascular risk. The culmination of traditional (male gender, smoking, advanced age, obesity, arterial hypertension and dyslipidemia) and non-traditional risk factors (anemia, inflammation, proteinuria, volume overload, mineral metabolism abnormalities, oxidative stress, etc.) contributes to advanced atherosclerosis and increased cardiovascular risk. To decrease the morbidity and mortality of these patients due to cardiovascular causes, timely and efficient cardiovascular risk assessment is of huge importance. Cardiovascular risk assessment can be based on laboratory parameters, imaging techniques, arterial stiffness parameters, ankle-brachial index and 24 h blood pressure measurements. Newer methods include epigenetic markers, soluble adhesion molecules, cytokines and markers of oxidative stress. In this review, the authors present several non-invasive methods of cardiovascular risk assessment in patients with diabetes mellitus and chronic kidney disease.
Core Tip: The culmination of traditional and non-traditional atherosclerosis risk factors in patients with diabetes mellitus and chronic kidney disease leads to fulminant and advanced atherosclerosis, consequently resulting in cardiovascular morbidity and mortality. Non-invasive cardiovascular risk assessment should therefore be performed in all these patients and can be based on standard laboratory parameters, cytokines and markers of oxidative stress, 24 h blood pressure measurements, ankle-brachial index, arterial stiffness parameters, imaging techniques and epigenetic markers. In this review article, we present different methods of non-invasive cardiovascular risk assessment in patients with diabetes mellitus and chronic kidney disease.
- Citation: Piko N, Bevc S, Ekart R, Petreski T, Vodošek Hojs N, Hojs R. Diabetic patients with chronic kidney disease: Non-invasive assessment of cardiovascular risk. World J Diabetes 2021; 12(7): 975-996
- URL: https://www.wjgnet.com/1948-9358/full/v12/i7/975.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i7.975
The burden of diabetes mellitus (DM) on global public health and socioeconomic development is heavy and is escalating. The International Diabetes Foundation has stated that in 2017, 451 million people worldwide have lived with DM and that this number would soar in the next couple of years and decades[1].
DM is the number one cause of chronic kidney disease (CKD) worldwide and is inherently linked with increased mortality mostly due to cardiovascular causes[2]. CKD is another major public concern with rising prevalence, which is now estimated at 9.1%[3]. In 2017, CKD resulted in 1.2 million deaths and was the 12th leading cause of death globally. Additionally, 7.6% of all cardiovascular deaths were due to CKD[4].
Cardiovascular disease in patients with DM and CKD is attributed to advanced and fulminant atherosclerosis, due to the presence and interplay of traditional [male gender, smoking, advanced age, obesity, arterial hypertension (AH), dyslipidemia] and non-traditional, CKD-specific risk factors (anemia, inflammation, proteinuria, volume overload, mineral metabolism abnormalities, oxidative stress, etc.)[5].
Due to the increased rate of cardiovascular events, prompt and timely cardio
The diagnosis of CKD is based on functional and structural changes of the kidneys, with the former assessed by estimating or measuring the glomerular filtration rate (GFR) and albuminuria/proteinuria and the latter by using different imaging techniques and/or kidney biopsy. Multiple creatinine and/or cystatin C based equations are only an assessment of kidney function and have several limitations, but the alternative (measurements of GFR) is usually not available in routine clinical practice[7].
Reduced estimated GFR is an important marker of cardiovascular risk. Go et al[8] performed a study in which they estimated the longitudinal GFR among 1120295 adults in whom serum creatinine had been measured between 1996-2000 and who had not undergone dialysis or kidney transplantation. Of the included patients, 9.6% had concomitant DM. They found an independent, graded increase in cardiovascular deaths, events and hospitalization rates in those with reduced estimated GFR. The authors postulated that the relationship between reduced GFR and cardiovascular disease is partly due to the presence of several traditional atherosclerosis risk factors and partly due to the presence of several CKD-specific risk factors, such as increased levels of inflammation markers, hyperhomocysteinemia, abnormal apolipoprotein levels, enhanced coagulability, anemia, left ventricular hypertrophy (LVH), increased endothelial dysfunction, increased arterial stiffness and augmented arterial calcifications.
In a study by Wu et al[9], the authors found that reduced estimated GFR was an important hallmark of diabetic retinopathy, which is commonly associated with advanced diabetic nephropathy and increased cardiovascular risk as well. In a 1-year cross-sectional study by Babaliche et al[10], the authors found that reduced GFR in type 2 DM patients was significantly associated with a higher incidence of micro
DM exerts its negative effects on kidney function through negative glomerular hemodynamic effects, especially through glomerular hyperfiltration, which is defined as an estimated GFR more than two standard deviations above the mean estimated GFR of healthy individuals. In a study performed on healthy, middle-aged individuals by Dupuis et al[11], the authors found that glomerular hyperfiltration is independently associated with higher cardiovascular risk, similar to the risk observed in patients with CKD stage 3A. Similar findings have been observed in several studies on patients with type 1 and type 2 DM, with and without previous cardiovascular disease[12-14]. Glomerular hyperfiltration was also associated with an increased incidence of coronary artery calcification and LVH, both important cardiovascular risk markers[15].
Proteinuria (defined as urine protein excretion greater than 300 mg over 24 h) and moderately increased albuminuria (defined as urinary albumin excretion of 30-300 mg over 24 h) are usually signs of renal injury and can often be detected earlier than any tangible fall in GFR[16]. Both are strong and independent predictors of increased risk for all-cause and cardiovascular mortality in patients with and without DM. Additionally, both are risk factors for faster CKD progression as well and are therefore included in the KDIGO (kidney disease: Improving global outcomes) CKD staging system[16].
In a post-hoc analysis of the Reduction in Endpoint in Non-insulin dependent DM with the angiotensin II antagonist losartan trial, the authors found that proteinuria of 3 g or more per day was associated with a renal endpoint of doubling of creatinine or end-stage renal disease (ESRD) in 85% of patients and with a cardiovascular endpoint (defined as the composite of myocardial infarction, stroke, first hospitalization for heart failure or unstable angina, coronary or peripheral revascularization or cardiovascular death) in 44% of patients. In the same study, moderately increased albuminuria was associated with an increased likelihood of kidney disease progression and cardiovascular risk as well, even after adjusting for estimated GFR and comorbidities[17].
The Strong Heart Study showed that patients with type 2 DM and proteinuria had worse left ventricular function and impaired diastolic left ventricular filling compared with patients without proteinuria[18]. According to studies, diabetic patients with proteinuria are at increased risk for peripheral artery disease (PAD)[19] and incident stroke as well[20].
In a Heart Outcome Prevention Evaluation (HOPE) substudy, a linear relationship between moderately increased albuminuria and cardiovascular events, especially in patients aged 55 years or more with previous cardiovascular disease or DM, was proven. In the same study, a higher incidence of systolic and diastolic dysfunction was found in patients with moderately increased albuminuria[21]. A study of 308 patients showed that patients with angiographic evidence of coronary artery disease (CAD) had higher urinary albumin levels than disease-free individuals and that urinary albumin excretion increased progressively with CAD severity[22].
All the above-stated studies confirm the fact that the detection and early recognition of moderately increased albuminuria and proteinuria is crucial in managing cardiovascular risk and the rate of CKD progression[23,24].
The exact pathophysiologic pathways that would explain the mechanism behind increased cardiovascular risk and proteinuria are still not known. It is postulated that the proteins that leak through damaged glomerular capillary endothelium cause tubulointerstitial injury and inflammation and subsequently lead to parenchymal damage, renal fibrosis and progressive decline in renal function[25]. The steno hypothesis suggests that urinary protein excretion signals a subclinical renal disease and systemic endothelial dysfunction and systemic inflammation[26]. Additionally, several thrombogenic factors, for example, von Willebrand factor, fibrinogen, cell adhesion molecules and tissue plasminogen activator have also been connected with proteinuria. It has been suggested that high platelet adhesiveness and erythrocyte aggregation demonstrated in diabetic patients with proteinuria could indicate increased thrombosis risk. Insulin resistance has been proven in patients with proteinuria, implying the role of hyperinsulinism in explaining the increased cardiovascular risk in these patients[26].
Serum cystatin C is a low-molecular-weight, non-glycosylated protein from the family of cysteine protease inhibitors that closely approximates what could be considered an ideal marker of renal function because it is freely filtered by the glomerulus, reabsorbed and degraded completely by proximal tubule and is not secreted by the tubules. It is more sensitive than usual endogenous markers (serum creatinine and urea) used in kidney function assessment, especially in the early stages of CKD[27]. However, cystatin C can also be used as a cardiovascular risk marker having important prognostic implications in patients with different degrees of CKD[28].
According to a cross-sectional epidemiological study by Cepeda et al[29], elevated cystatin C was associated with an increased presence of several cardiovascular risk factors, such as DM, AH and CKD, along with higher levels of C-reactive protein (CRP), homocysteine and fibrinogen. Correa et al[30] performed a study on 4965 individuals after acute coronary syndrome in which they found a higher likelihood of adverse cardiovascular events in those with increased cystatin C, opening up a potential new role of cystatin C in risk stratification. Madero et al[31] performed an arterial stiffness study on 2468 individuals (24% diabetic) and found an association between cystatin C and increased arterial stiffness, especially in older patients. In two mortality studies, elevated cystatin C levels were associated with increased all-cause mortality, even in patients with normal renal function[32,33].
It has been suggested that cystatin C exerts its function through inhibition of lysosomal cathepsins, which leads to a reduction in atherosclerotic plaque degradation and increased risk of cardiovascular events[34].
CKD mineral and bone disorder (CKD-MBD) refers to the clinical syndrome of laboratory abnormalities, bone disease and extraskeletal calcification, including the arterial system. Among the earliest manifestations of CKD-MBD are vitamin D deficiency, disordered calcium and phosphate homeostasis, elevated levels of parathyroid hormone and fibroblast growth factor-23 (FGF-23). These alterations lead to an increased risk of ESRD, cardiovascular disease and mortality[35]. The amount of evidence is especially strong for elevated serum phosphate and FGF-23, both of which have direct negative cardiovascular effects through promoting LVH and consequent left ventricular dysfunction[36,37]. Several cross-sectional studies have demonstrated that vitamin D deficiency also increases the risk of developing AH, heart failure and sudden cardiac death, all through downregulation of the renin-angiotensin-aldosterone system, impaired insulin sensitivity, direct effects on the heart and vasculature and through worsened glycemic control[38,39].
The difference in CKD-MBD according to diabetes status has also been noticed. In a large descriptive study of patients with CKD stages 2-4, participants with DM had higher levels of serum phosphate, parathyroid hormone and FGF-23 and lower levels of vitamin D compared to patients without DM. Moreover, the elements of CKD-MBD evolved earlier in the course of CKD in diabetic patients, partly explaining the higher cardiovascular risk in diabetic CKD patients. An inverse relationship between the level of proteinuria and vitamin D was also observed, further confirming the importance of surveillance of mineral metabolism in diabetic CKD patients[40].
Chronic, low-grade inflammation plays a grand role in the initiation and progression of atherothrombosis, metabolic disorders, AH, DM and renal disease[41]. High-sensitivity CRP (hsCRP) is an established inflammatory biomarker and is one of the most widely used markers associated with risk of cardiovascular events[42]. In the study by Ridker et al[43], 27939 presumed healthy American women were followed up for a mean of 8 years for incident myocardial infarction, ischemic stroke, coronary revascularization or death from cardiovascular causes. The authors found that cardiovascular events were more common in those with higher hsCRP levels, independent of traditional atherosclerosis risk factors. In the Cardiovascular Health Study, nearly 6000 subjects were followed up for 3-4 years, and their inflammatory markers were measured before and after completed follow-up. The results of the study showed that those with higher baseline hsCRP values were more likely to develop DM in the follow-up period[44]. An association has also been found between higher values of glycated hemoglobin and hsCRP, suggesting the role of systemic inflammation in diminished insulin sensitivity and suboptimal glycemic control[45].
Interestingly, studies have not shown uniform results regarding the role of hsCRP as a marker of cardiovascular risk in diabetic patients. In a pooled analysis of 25797 patients from four different United Kingdom prospective cohort studies, hsCRP was linked to increased risk of cardiovascular events and mortality only in patients without DM[46]. Similar results were found in the Jager et al[47] study and in the Strong Heart Study as well[48]. In the Diabetes Heart Study, hsCRP was analyzed in 846 type 2 DM patients who had follow-ups for a mean period of 7.3 years. On the contrary to other studies, baseline hsCRP was a strong predictor of mortality in this group of patients[49].
Elevated hsCRP has been found to be an important marker of acute kidney injury, subclinical kidney injury, incident CKD and CKD progression[50,51]. Sinha et al[52] found that higher baseline hsCRP is associated with incident diabetic nephropathy. In a study by Jalal et al[53] in which 3166 elderly subjects were included (18% diabetics), high hsCRP was associated with a higher likelihood of major adverse cardiovascular events. In a meta-analysis by Li et al[54] on CKD and ESRD patients, hsCRP was associated with a significantly higher risk of cardiovascular morbidity and mortality. Besides being an important biomarker of cardiovascular risk, a change in hsCRP can be a sign of changed renal and consequently cardiovascular risk profile. Liu et al[55] found that a reduction in hsCRP favored kidney outcomes in patients with impaired glucose metabolism or DM, showing that serial measurements of hsCRP can be indicative of change in systemic inflammation and atherogenesis.
Inflammation underlies all stages of atherosclerotic plaque formation, even in the early stages where inflammatory cells adhere and infiltrate the subendothelium. The two most important adhesion molecules that play a major role in atherosclerosis are vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). Selectins are another group of adhesion molecules and are found on leukocytes, endothelial cells and thrombocytes[56]. Cell adhesion molecules and selectins can be broken off into the circulation and can be measured in the serum. Soluble adhesion molecules have shown potential as biomarkers of cardiovascular risk[57].
Increased levels of soluble VCAM-1 (sVCAM-1) and soluble ICAM-1 (sICAM-1) have been found in diabetics with increased stages of CKD[57,58]. In a study by Bavbek et al[56], increased serum levels of selectins were associated with microva
Another important component of the inflammatory process in atherosclerosis are cytokines and their receptors. Interleukin (IL)-2 receptor has been associated with increased carotid intima-media thickness (IMT) and advanced atherosclerosis in hemodialysis patients[62]. Population-based studies have shown that inflammatory cytokines are strong predictors of the development of DM and are also important in the development of micro- and macrovascular complications in diabetic patients[63]. IL-1, IL-6, IL-18 and tumor necrosis factor-alpha enhance the expression of ICAM-1, VCAM-1 and selectins, affect the dynamics of the mesangial matrix, lead to glomerular basal membrane thickening, alter glomerular hemodynamics and lead to renal toxicity[64,65]. Cytokines can also lead to dysregulation between antioxidative mechanisms and the formation of reactive oxygen species, such as superoxide anions, hydrogen peroxide and hydroxyl radicals. Ineffective antioxidant capacity or excess reactive oxygen species is implicated in the development and progression of renal and cardiovascular disease through several pathophysiologic mechanisms, including through direct tissue toxicity and promotion of further inflammation[66-68].
In patients with AH, multiple blood pressure measurements during longer periods of time [for example 24 h ambulatory blood pressure measurements (ABPM)] are more likely to predict cardiovascular risk and are therefore superior to those obtained in the doctor’s office for estimating the risk of future cardiovascular morbidity and mortality[69].
In a population-based prospective study on 1700 Danish people by Hansen et al[70], the authors found that 24 h ABPM was an important prognostic tool in assessing subjects’ cardiovascular risks and that isolated ambulatory systolic hypertension as well as blunted decrease in blood pressure during the night were both prognostically unfavorable. The percentage of DM was higher in the sustained high blood pressure group (4.3%) compared to the isolated ambulatory hypertension group (2.8%), isolated office hypertension group (1.3%) and normotensive group (1.2%).
In a cohort study by Grezzana et al[71] that included 569 hypertensive patients, the 24 h ABPM showed a predictive result for new cases of atrial fibrillation and a combination of cardiovascular outcomes, mortality and hospital admissions. In a study by Iqbal et al[72] in which 1187 individuals were included, significant associations were found between cerebrovascular events and absent nocturnal drop in blood pressure (≤ 10%), between high day time diastolic blood pressure, PAD and morning surge ≥ 20/15 mmHg and between cardiac arrhythmias, high day time and nighttime diastolic blood pressure.
In patients with and without DM, 24 h ABPM is superior to office recordings in terms of recognizing masked and white coat hypertension[73]. A lack of nocturnal dip in blood pressure is suggestive of autonomic neuropathy and is commonly observed in diabetic patients and can very commonly be a sign of concomitant obstructive sleep apnea, which is a known cardiovascular risk factor[74]. In a study by Eguchi[75] performed on patients with type 2 DM, 24 h systolic blood pressure was significantly correlated with silent cerebral infarcts and LVH, even more so than the values of glycosylated hemoglobin, indicating that perhaps uncontrolled hypertension is the main cause of accelerated atherosclerosis and increased cardiovascular risk in this population.
Like DM, CKD is also associated with a distinctive blood pressure profile, resulting in undiagnosed hypertension, which is a major factor in a continuing decline in kidney function. Manios et al[76] demonstrated that short-term blood pressure variability is more pronounced in CKD patients, rendering office measurements obsolete and imprecise in this population. Similar studies have confirmed these findings and additionally showed a larger presence of non-dippers and patients with masked hypertension in the CKD group as well[77]. Decreased diurnal blood pressure variation is independently associated with a faster decline in kidney function[78] and with increased cardiovascular mortality in CKD patients[79]. In the prospective African American study of kidney disease and hypertension cohort study of 617 CKD patients, Gabbai et al[80] demonstrated that 24 h systolic blood pressure predicted both kidney and cardiovascular outcomes. Wang et al[81] studied a large (n = 1219) cohort of diabetic and non-diabetic CKD patients and found that blood pressure load and ABPM levels were independently correlated with left ventricle mass index, estimated GFR and proteinuria in all groups of CKD patients. Besides CKD stages 2-4, studies have shown an adverse cardiovascular profile in ESRD patients with elevated 24 h systolic blood pressure and a non-dipping ABPM profile as well[82-84].
PAD is usually diagnosed by ankle-brachial index (ABI) < 0.9 and can be considered a clinical model for atherosclerosis. It is the result of structural and functional vessel wall aberrations, resulting in limb ischemia and changes in pulse wave propagation, ultimately impacting the myocardium as well[85]. PAD is an independent risk factor for stroke, myocardial infarction and cardiovascular death, and the risk is even more apparent in diabetic patients[86]. In a study by Li et al[87] that was performed on 1647 patients with type 2 DM, low ABI (≤ 0.9) was independently associated with a high risk of all-cause and cardiovascular mortality. In a retrospective study by Alves-Cabratosa et al[88] in which 34689 patients with type 2 DM were included, the authors found that even ABI in the lower normal range (0.91-1.00) was associated with significantly increased risk for nephropathy, retinopathy, acute myocardial infarction and mortality. In the same study, high ABI (> 1.3) was a marker of increased medial calcinosis and was associated with cardiovascular complications, most likely due to increased arterial stiffness, directly leading to increased cardiac workload, left ventricular hypertrophy, myocardial fibrosis, ischemia and arrhythmias[89].
CKD patients are especially prone to complications of atherosclerosis, PAD, calcifications of arterial walls and increased arterial stiffness[90]. According to the Cardiovascular Health Study, in which 4.513 community-living subjects aged 65 years or more were enrolled (15.3% diabetics), CKD was associated with both high (> 1.4) and low (< 0.9) extremes of ABI, which was explained by advanced atherosclerosis and increased vessel wall calcifications in this subgroup[90]. The prevalence of medial calcinosis has been shown to be even higher in patients on hemodialysis[91]. The results of the prospective NEFRONA study, in which 2445 CKD and 559 non-CKD subjects were included, showed higher prevalence of PAD in CKD patients and a rising prevalence of ABI > 1.4 in advanced stages of CKD. DM was the only factor predicting both pathological values of ABI in all CKD stages[92].
Both high and low ABI measurements play a role in assessing cardiovascular risk and renal outcome. According to a study by Chen et al[93] in which 436 CKD patients were enrolled (36.9% with DM type 2), reduced ABI (< 0.9) was associated with a more rapid decline in renal function and with a higher incidence of cardiovascular events. Similar association was found between renal function, cardiovascular events and high ABI[94]. In a study on 52 hemodialysis patients by Bevc et al[95], survival analysis showed higher risk for cardiovascular death in patients with ABI > 1.4. It appears that the systemic nature of atherosclerosis is only partly responsible for these effects of changed ABI and that increased arterial stiffness and consequent hemodynamic changes play an integral role as well[96].
Increased arterial stiffness is recognized as a surrogate endpoint for cardiovascular disease and is the result of several structural alterations in the arterial walls, leading to reduced distensibility and decreased buffering capacity of arteries to pulsatile cardiac ejection[97].
Applanation tonometry is a non-invasive, easily reproducible technique often used for measuring arterial stiffness. It enables us to perform pulse wave analysis on the radial artery, giving us information on indirect parameters of central arterial stiffness and blood supply to the endocardium of the heart[98]. It also allows us to measure carotid-femoral pulse wave velocity (cfPWV) on the carotid and femoral arteries, which is the most precise way to non-invasively determine central arterial stiffness[99]. All the applanation tonometry-derived arterial stiffness parameters are presented in Table 1.
| Arterial stiffness parameter | Definition |
| cfPWV (m/s) | Pulse wave distance between two measuring sites (carotid and femoral artery) divided by pulse transit time (measured by electrocardiographic monitoring) |
| PP (mmHg) | Difference between systolic and diastolic pressure |
| AP (mmHg) | Difference between systolic and inflection pressure |
| AIx (%) | AP divided by PP |
| AIx@75 (%) | AIx adjusted for heart rate at 75 beats per minute |
| ED (ms) | Duration of left ventricular systolic ejection |
| EDI (%) | The ratio of the duration of systolic ejection to the total duration of the heart cycle |
| SEVR (%) | The diastolic area under the curve divided by the systolic area under the curve, derived from the pulse wave curve |
Increased arterial stiffness is a well-known risk factor for major cardiovascular events. Weber et al[100] performed a prospective study on 465 male patients undergoing coronary angiography and found that augmentation pressure, augmentation index (AIx), and AIx@75 (AIx adjusted for heart rate 75/min) were strong predictors of obstructive CAD. Prskalo et al[101] performed a study on 160 patients with CAD undergoing elective coronary angiography and found that increased values of PWV and AIx were associated with a more advanced CAD, a higher likelihood of in-stent restenosis and left main CAD. An association between PAD and increased arterial stiffness has also been described[96]. A prospective Rotterdam study involving 2835 volunteers aged 55 years or more reported that subjects with higher values of cfPWV had a higher risk of coronary and cerebro
Briet et al[106] performed a study on 95 patients with CKD (GFR measured with 51Cr-EDTA clearance; 11% diabetics) and 121 hypertensive patients without CKD (GFR estimated with the use of Modification Diet in Renal Disease equation; 5% diabetics). They found that patients with CKD presented with increased arterial stiffness, determined by higher values of cfPWV. They explained their findings by the higher presence of DM and other traditional and non-traditional atherosclerosis risk factors in the CKD group. According to Sedaghat et al[107], the correlation between CKD and arterial stiffness is reciprocal, suggesting that besides being the result of CKD, increased arterial stiffness can lead to a faster CKD progression as well. A similar finding was found in a study by Fountoulakis et al[108], which was performed on diabetic patients with CKD. Proteinuria has also been linked to higher cfPWV and reduced SEVR and ejection duration[109,110].
Di Micco et al[111] performed a prospective, 3-year study involving 212 patients with CKD stages 3 and 4. During the study period, 34 patients died, 29 of them due to cardiovascular causes. Patients with lower SEVR had higher mortality. Post-mortem evaluation showed a higher degree of coronary artery calcification and a larger myocardial mass in patients with previously lower values of SEVR. Ekart et al[112] performed a study on non-dialysis CKD patients (27% diabetics) and found that SEVR < 130% predicted fatal and non-fatal cardiovascular events.
In a study by Kimoto et al[113], the authors found that the degree of CKD-associated increase in arterial stiffness varies among arterial regions in type 2 DM and is predominantly increased in the aorta. This has clinical implications because aortic stiffness is a strong and independent predictor of cardiovascular death, as shown in ESRD, DM, hypertensive and elderly patients.
Structural and functional changes of the heart muscle are pivotal in understanding the increased cardiovascular risk in patients with DM and CKD. Both entities are related to macrovascular and microvascular pathology, resulting in increased myocardial fibrosis and subsequently in systolic and diastolic dysfunction[114,115]. It appears as though these changes are even more pronounced in the uremic milieu and are most likely intensified through myocardial calcifications in CKD patients[116]. Several studies have shown an independent association between DM, LVH and reduced systolic function of the left ventricle, both of which are commonly linked to an increased likelihood of sudden cardiac death, mostly due to arrhythmias[114,117,118]. LVH is especially common in CKD patients as it is linked to common comorbidities in these patients (AH, DM) and to CKD-specific factors, for example, volume overload, hyperphosphatemia and elevated levels of FGF-23. The clinical significance of LVH is prognostically unfavorable and is linked to increased cardiovascular mortality in patients with different degrees of CKD, including those on hemodialysis[119,120]. An additional strength of echocardiography is shown in a study by Di Cori et al[121], in which the authors found important subclinical dysfunction in asymptomatic type 1 DM patients aged under 40 years by using strain, strain rate and integrated backscatter. A similar finding was demonstrated in a study by Ha et al[122], in which the authors presented the importance of tissue Doppler indexes for unmasking subclinical myocardial ischemia. The prevalence of diastolic dysfunction, left atrial fibrosis and left atrial enlargement is also higher in patients with DM and CKD. All of the mentioned changes are a reflection of structural myocardial disease and are markers of increased cardiovascular risk as well[123].
Regadenoson-stress single-photon emission computed tomography (SPECT) is particularly appealing for cardiovascular risk assessment in asymptomatic diabetic patients[124]. The cause of concern in this population is silent myocardial ischemia and the data on the prevalence of this condition have been disparate. In the prospective detection of ischemia in asymptomatic diabetics study, which included asymptomatic patients with type 2 DM, a 22% prevalence of any perfusion defect or left ventricle dysfunction by SPECT was detected, and in 6% of patients, a moderate to large myocardial ischemia was found[125]. In a more recent analysis of 1354 asymptomatic patients (302 without DM) a lower prevalence of myocardial ischemia was found (7.2%). An important finding of the study was a much higher prevalence of silent ischemia observed in diabetic patients (12.5% vs 5.6%)[126]. The Basel asymptomatic high-risk diabetes outcome trial prospectively recruited 400 asymptomatic patients with type 2 DM. In the study, nearly a quarter of asymptomatic patients had silent myocardial ischemia, which was associated with a worse outcome and a higher likelihood of major adverse cardiovascular events[127]. The yield of SPECT can be further improved by choosing patients with higher basal cardiovascular risk, especially patients with certain other comorbidities, such as CKD. According to studies, abnormal SPECT is a good indicator of future acute coronary events and cardiovascular disease in diabetic and non-diabetic CKD patients[128]. Conversely, a normal SPECT is associated with a fairly good cardiovascular outcome in CKD patients, but it should be noted that due to accelerated coronary atherosclerosis in patients with CKD stages 4 and 5, a normal SPECT testing is still linked to higher cardiovascular risk compared to patients with better renal function[129]. In these patients, continuous follow-up is pivotal in preventing major adverse cardiovascular events[130].
Carotid IMT is an ultrasound-based, non-invasive measurement of atherosclerosis burden. It is used to investigate the determinants and consequents of atherosclerosis and has been used as a surrogate end-point and a therapeutic target in some clinical trials as well[131,132].
Several clinical studies have demonstrated the association between the presence of atherosclerotic plaques in the carotid arteries, increased carotid IMT and atherosclerosis in other vascular territories[133]. Bots et al[134] have shown that patients with an increased carotid IMT have a higher likelihood of having advanced atherosclerotic plaques in the abdominal aorta. In a study by Ogata et al[135], a significant correlation between left main coronary atherosclerosis (determined by intravascular coronary ultrasound) and increased carotid IMT was observed. A positive association was also found for patients with symptomatic CAD, cerebrovascular disease and patients with PAD[136]. A descriptive, cross-sectional study was performed by Gómez-Marcos et al[137] in which they found an increased carotid IMT in patients with DM compared to patients without DM, and the difference was even greater in patients with advanced age. Signs of carotid damage were found in 23% of patients with DM. In a study by Matsagoura et al[138] that included patients with type 2 DM, an increased carotid IMT was observed in patients with moderately increased albuminuria or proteinuria compared to patients without proteinuria.
Kota et al[139] demonstrated a higher risk for ischemic stroke in type 2 DM patients with increased thickness of carotid intima-media. Sunil Kumar et al[140] performed a study on 30 patients with ESRD in which they found increased carotid IMT in these patients. They found the measurements to be an easy, non-invasive, easily-reproducible and cost-effective investigation in assessing cardiovascular risk in patients with chronic kidney failure. According to a study by Ekart et al[141], carotid IMT correlated with higher blood pressure in hemodialysis patients. In a study by Lawal et al[142], carotid IMT correlated with many cardiovascular risk factors among CKD patients, serving as a potential surrogate marker for cardiovascular disease in these patients. Roumeliotis et al[143] performed a study on 142 diabetic patients with different stages of CKD. Patients with increased carotid IMT had higher all-cause and cardiovascular mortality and had a higher degree of atherosclerosis in other vascular territories, further confirming the important role of carotid IMT measurements in recognizing patients with higher cardiovascular risk.
High coronary artery calcium (CAC) score is associated with advanced atherosclerosis and with a 4-10-fold increase in the incidence of cardiovascular disease, independent of other risk factors[144]. Diabetic patients harbor larger amounts of CAC than non-diabetic patients of similar age[145]. Additionally, asymptomatic diabetic patients have a similar CAC than non-diabetic patients with known CAD[146]. In the coronary artery calcification in type 1 diabetes study, 656 adult patients with type 1 DM had higher CAC compared to 764 age- and sex-matched individuals without DM, with no differences between genders. Extensive vascular calcifications were registered even in younger patients with type 1 DM (17-28-years-old)[147]. The extent of CAC has also shown an important positive association with SPECT-registered myocardial ischemia, cardiovascular events and mortality[148,149]. Anand et al[150] followed 392 patients with type 2 DM and found that CAC progression was among the best predictors of increased cardiovascular risk.
Patients with CKD exhibit a higher prevalence of vascular calcification than the general population. In a 10-year prospective study on 137 CKD patients, the authors found that severe CAC was an important predictor of cardiovascular mortality[151]. Krajnc et al[152] compared CAC score between patients on hemodialysis and diabetic patients without renal involvement and found higher CAC score in the hemodialysis group, with the difference between both groups especially evident in the very high risk CAC score category. Besides being an adverse prognostic sign, higher CAC has been associated with faster progression of CKD to ESRD[153]. According to a study by Cano-Megías et al[154], the synergistic effect of DM and CKD leads to an even higher CAC score, increased inflammation and higher mortality compared to patients without DM, showing the importance of higher CAC in cardiovascular risk assessment of diabetic CKD patients.
Coronary computed tomography angiography (CTA) is a non-invasive imaging modality that provides a detailed and comprehensive evaluation of the presence and the extent of CAD. According to a study by de Araújo Gonçalves et al[155], DM is an independent predictor of CAD and is associated with a more advanced CAD and a higher prevalence of atherosclerotic plaques in every anatomical subset of coronary arteries, all evaluated with the use of coronary CTA. An important obstructive CAD was observed even in asymptomatic diabetic patients[156]. An additional important advantage of coronary CTA was found in a study by Madaj et al[157] in which they studied the presence of CAD in younger patients with type 1 and 2 DM. They found that coronary CTA detects CAD even in patients with a normal CAC score, which can be explained by a higher percentage of non-calcified plaques in younger patients with diabetes, rendering the CAC score less useful in this context. In diabetic CKD patients, coronary CTA is useful in determining the extent of CAD and atherosclerotic plaque characteristics; in CKD patients, a trend towards non-obstructive calcified plaques has been noticed[158]. There is, however, a reason for cautious use of coronary CTA in patients with advanced stages of CKD because of the risk of contrast-induced nephropathy[159].
Cardiac magnetic resonance imaging (CMRI) provides important prognostic information and aids in risk stratification in most cardiovascular diseases. It is a non-invasive imaging modality that can visualize myocardial scarring, myocardial steatosis and triglyceride content, interstitial fibrosis and interstitial myocardial edema. It is also useful in the evaluation of coronary arteries, valvular pathology, and in the differentiation of cardiomyopathies[160]. Diabetic patients are at risk of developing severe cardiomyopathy, partly due to CAD and partly due to metabolic derangements. The presence of late gadolinium hyperenhancement as a marker of prior myocardial infarction is associated with a 4-fold increased risk of a major adverse cardiovascular event and with a 7-fold increased risk of mortality[161]. Late gadolinium hyperenhancement was demonstrated in 4.3% of asymptomatic type 1 DM patients in the diabetes control and complications trial/epidemiology of diabetes interventions and complications trial[162] and 17% of asymptomatic diabetic older patients in a community-based study in Iceland[163].
T1 mapping is useful in assessing the amount of extracellular volume and interstitial fibrosis. Wong et al[164] demonstrated high short-term mortality in non-diabetic patients with increased extracellular volume. Some studies have shown increased extracellular volume and diastolic dysfunction in diabetic patients as well[165,166]. By using spectroscopy, myocardial lipid content can be assessed, which is an important pathophysiological step in understanding left ventricular dysfunction in diabetic patients[167]. Adenosine stress MRI test has excellent characteristics for the detection of obstructive CAD and microvascular dysfunction in patients with DM[168] and CKD patients as well[169]. Myocardial fibrosis is a hallmark of progressive CKD, uremic cardiomyopathy and is a crucial cause of increased cardiovascular risk in these patients[170]. Concerns relating to an association between gadolinium contrast agents and nephrogenic systemic fibrosis have led to the use of lower doses, lower risk gadolinium agents and even native CMRI in CKD patients, which is an important step forward in the wider use of CMRI in this subgroup of patients[171]. In the Graham-Brown et al[172] study on hemodialysis patients, a native CMRI was performed. The authors found an increase in myocardial fibrosis and interstitial edema, mostly in the septal region of the heart. By applying a similar methodology, Rutherford et al[170] showed that these findings were independent of patient’s fluid status. In a study by Edwards et al[173] on CKD patients stages 2-4, a low dose gadolinium method was used. They found increased interstitial fibrosis and myocardial dysfunction in these patients, and the finding was not dependent on blood pressure. CMRI has a proven role in the understanding of uremic cardiomyopathy, ventricular dysfunction, myocardial fibrosis and cardiovascular risk assessment in patients with early and advanced CKD[171].
Epigenetics is the study of gene expression and involves phenotypic changes without changes in genotype. Several studies have confirmed that epigenetic regulations are crucial in the pathogenesis of atherosclerotic plaque formation, vessel wall inflammation and plaque rupture, in a cell-type and stage-specific manner[174,175]. Three epigenetic mechanisms are important in atherosclerosis: DNA methylation, post-translational modification of histones and the activity of non-coding ribonucleic acids (RNAs), most commonly long non-coding RNAs and micro RNAs (miR)[176]. The expression of non-coding RNAs can be measured in peripheral blood, urine and saliva using microarrays, indicating the potential of these markers as diagnostic tools and future therapeutic targets[177,178].
Several long non-coding RNAs are linked to LVH, myocardial infarction, heart failure and mortality[179]. Polymorphisms and increased expression of miR-124a, miR-375 and miR-146a are associated with obesity, insulin resistance and increased incidence of type 2 DM[180,181]. miR-27 exerts negative effects on adipogenesis and is associated with decreased incidence of DM[182]. In a study by Buraczynska et al[183], miR-196a2 has been linked to an increased likelihood of cardiovascular events in diabetic patients. miR-4513 is overly expressed in patients with metabolic syndrome and DM and is associated with major cardiovascular events, especially acute coronary syndrome[184]. Endothelial dysfunction is an important factor in CKD patients and according to a study by Kétszeri et al[178], miR-142-3p expression is linked to preventing endothelial dysfunction.
Non-coding RNAs are a marker of increased cardiovascular risk and can be employed to determine the risk of developing and/or progressing CKD in patients with DM. According to studies, several long non-coding RNAs (ERBB4-IR, MGC, ENSMUST00000147869) are centrally involved in the development and progression of CKD in patients with DM, either via direct pathogenic roles or as indirect mediators of different nephropathic and profibrotic pathways[185]. The upregulation of profibrotic miR-192 and miR-21 was confirmed to be linked to renal fibrosis, development and progression of CKD in diabetic patients[186]. Vice versa, reduced expression of anti-fibrotic miR-29 and miR-200 was also associated with more advanced kidney disease[187,188]. The downregulation of miR-30s, miR-124 and miR-93 has been shown to lead to increased podocyte injury and proteinuria[186]. In a study by Abdelsalam et al[189], miR-451 is a highly specific marker of CKD chronicity in patients with DM. In a study by Fourdinier et al[190], decreased serum levels of miR-126 and miR-223 were found in patients with advanced CKD with DM and were linked to increased mortality due to cardiovascular causes. It appears that the potential of non-coding RNAs is vast but currently of limited clinical importance due to high cost, limited availability and non-specificity of different molecules[176].
Due to the increasing burden of DM and CKD, the prevalence of the cardiovascular disease will continue to rise. To reduce morbidity, mortality and socioeconomic burden of these patients, immediate cardiovascular risk assessment is pivotal. Fulminant atherosclerosis, a hallmark of diabetic patients with CKD is a complex process, involving the interplay between traditional and non-traditional, CKD-specific risk factors, culminating in endothelial dysfunction, inflammation, plaque formation and ultimately target organ ischemia and damage. Due to the multifaceted process, it appears that a multimarker approach should be used to recognize patients with the highest risk for cardiovascular events. In the future, more attention should be given to the decrease in prevalence of DM and prevention of CKD development in diabetic patients.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kwon SK, Ong H S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (1)] |
| 2. | Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu XO, Chen Y, Gupta PC, Gu D, Tsugane S, Xiang YB, Gao YT, Yuan JM, Tamakoshi A, Irie F, Sadakane A, Tomata Y, Kanemura S, Tsuji I, Matsuo K, Nagata C, Chen CJ, Koh WP, Shin MH, Park SK, Wu PE, Qiao YL, Pednekar MS, He J, Sawada N, Li HL, Gao J, Cai H, Wang R, Sairenchi T, Grant E, Sugawara Y, Zhang S, Ito H, Wada K, Shen CY, Pan WH, Ahn YO, You SL, Fan JH, Yoo KY, Ashan H, Chia KS, Boffetta P, Inoue M, Kang D, Potter JD, Zheng W. Association of Diabetes With All-Cause and Cause-Specific Mortality in Asia: A Pooled Analysis of More Than 1 Million Participants. JAMA Netw Open. 2019;2:e192696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 3. | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4332] [Cited by in RCA: 3807] [Article Influence: 761.4] [Reference Citation Analysis (0)] |
| 4. | Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol. 2020;16:251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 231] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 5. | Lovre D, Shah S, Sihota A, Fonseca VA. Managing Diabetes and Cardiovascular Risk in Chronic Kidney Disease Patients. Endocrinol Metab Clin North Am. 2018;47:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Suckling R, Gallagher H. Chronic kidney disease, diabetes mellitus and cardiovascular disease: risks and commonalities. J Ren Care. 2012;38 Suppl 1:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8530] [Article Influence: 406.2] [Reference Citation Analysis (0)] |
| 9. | Wu J, Geng J, Liu L, Teng W, Chen L. The Relationship between Estimated Glomerular Filtration Rate and Diabetic Retinopathy. J Ophthalmol. 2015;2015:326209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Babaliche P, Nadpara RA, Maldar A. Association Between Estimated Glomerular Filtration Rate and Microvascular Complications in Type II Diabetes Mellitus Patients: A 1-Year Cross-Sectional Study. J Natl Med Assoc. 2019;111:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Dupuis ME, Nadeau-Fredette AC, Madore F, Agharazii M, Goupil R. Association of Glomerular Hyperfiltration and Cardiovascular Risk in Middle-Aged Healthy Individuals. JAMA Netw Open. 2020;3:e202377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K, Heikkilä O, Forsblom C; FinnDiane Study Group. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 13. | Davis TM, Chubb SA, Davis WA. The relationship between estimated glomerular filtration rate trajectory and all-cause mortality in type 2 diabetes: the Fremantle Diabetes Study. Eur J Endocrinol. 2016;175:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Luo Y, Wang X, Wang Y, Wang C, Wang H, Wang D, Liu L, Jia Q, Liu G, Zhao X, Wang Y; CNSR Investigators. Association of glomerular filtration rate with outcomes of acute stroke in type 2 diabetic patients: results from the China National Stroke Registry. Diabetes Care. 2014;37:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Eriksen BO, Løchen ML, Arntzen KA, Bertelsen G, Eilertsen BA, von Hanno T, Herder M, Jenssen TG, Mathisen UD, Melsom T, Njølstad I, Solbu MD, Toft I, Mathiesen EB. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int. 2014;86:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Agrawal V, Marinescu V, Agarwal M, McCullough PA. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009;6:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 563] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Liu JE, Robbins DC, Palmieri V, Bella JN, Roman MJ, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol. 2003;41:2022-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Wattanakit K, Folsom AR, Criqui MH, Kramer HJ, Cushman M, Shea S, Hirsch AT. Albuminuria and peripheral arterial disease: results from the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2008;201:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ninomiya T, Perkovic V, Verdon C, Barzi F, Cass A, Gallagher M, Jardine M, Anderson C, Chalmers J, Craig JC, Huxley R. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1728] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 22. | Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: An angiographic study. Am J Kidney Dis. 1999;34:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Weir MR. Microalbuminuria in type 2 diabetics: an important, overlooked cardiovascular risk factor. J Clin Hypertens (Greenwich). 2004;6:134-41; quiz 142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 559] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 26. | Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2013;7:13-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C as an endogenous marker of renal function in patients with mild to moderate impairment of kidney function. Nephrol Dial Transplant. 2006;21:1855-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Bevc S, Hojs N, Knehtl M, Ekart R, Hojs R. Cystatin C as a predictor of mortality in elderly patients with chronic kidney disease. Aging Male. 2019;22:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Cepeda J, Tranche-Iparraguirre S, Marín-Iranzo R, Fernández-Rodríguez E, Riesgo-García A, García-Casas J, Hevia-Rodríguez E. [Cystatin C and cardiovascular risk in the general population]. Rev Esp Cardiol. 2010;63:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Correa S, Morrow DA, Braunwald E, Davies RY, Goodrich EL, Murphy SA, Cannon CP, O'Donoghue ML. Cystatin C for Risk Stratification in Patients After an Acute Coronary Syndrome. J Am Heart Assoc. 2018;7:e009077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Madero M, Wassel CL, Peralta CA, Najjar SS, Sutton-Tyrrell K, Fried L, Canada R, Newman A, Shlipak MG, Sarnak MJ; Health ABC Study. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol. 2009;20:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Woitas RP, Kleber ME, Meinitzer A, Grammer TB, Silbernagel G, Pilz S, Tomaschitz A, Weihrauch G, Dobnig H, März W, Scharnagl H. Cystatin C is independently associated with total and cardiovascular mortality in individuals undergoing coronary angiography. The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis. 2013;229:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 840] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 36. | Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432-2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 856] [Cited by in RCA: 793] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 37. | Stöhr R, Schuh A, Heine GH, Brandenburg V. FGF23 in Cardiovascular Disease: Innocent Bystander or Active Mediator? Front Endocrinol (Lausanne). 2018;9:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338:40-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 39. | Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Wahl P, Xie H, Scialla J, Anderson CA, Bellovich K, Brecklin C, Chen J, Feldman H, Gutierrez OM, Lash J, Leonard MB, Negrea L, Rosas SE, Anderson AH, Townsend RR, Wolf M, Isakova T; Chronic Renal Insufficiency Cohort Study Group. Earlier onset and greater severity of disordered mineral metabolism in diabetic patients with chronic kidney disease. Diabetes Care. 2012;35:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Libby P. Inflammation in Atherosclerosis-No Longer a Theory. Clin Chem. 2021;67:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 42. | Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168:5126-5134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 43. | Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2540] [Cited by in RCA: 2496] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 44. | Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 377] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 45. | King DE, Mainous AG 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26:1535-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care. 2012;35:396-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:3071-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 206] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Best LG, Zhang Y, Lee ET, Yeh JL, Cowan L, Palmieri V, Roman M, Devereux RB, Fabsitz RR, Tracy RP, Robbins D, Davidson M, Ahmed A, Howard BV. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation. 2005;112:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Cox AJ, Agarwal S, M Herrington D, Carr JJ, Freedman BI, Bowden DW. C-reactive protein concentration predicts mortality in type 2 diabetes: the Diabetes Heart Study. Diabet Med. 2012;29:767-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Fu EL, Franko MA, Obergfell A, Dekker FW, Gabrielsen A, Jernberg T, Carrero JJ. High-sensitivity C-reactive protein and the risk of chronic kidney disease progression or acute kidney injury in post-myocardial infarction patients. Am Heart J. 2019;216:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Chuang HH, Lin RH, Li WC, Yeh WC, Lin YA, Chen JY. High-Sensitivity C-Reactive Protein Elevation Is Independently Associated with Subclinical Renal Impairment in the Middle-Aged and Elderly Population-A Community-Based Study in Northern Taiwan. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Sinha SK, Nicholas SB, Sung JH, Correa A, Rajavashisth TB, Norris KC, Lee JE. hs-CRP Is Associated With Incident Diabetic Nephropathy: Findings From the Jackson Heart Study. Diabetes Care. 2019;42:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Jalal D, Chonchol M, Etgen T, Sander D. C-reactive protein as a predictor of cardiovascular events in elderly patients with chronic kidney disease. J Nephrol. 2012;25:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Li WJ, Chen XM, Nie XY, Zhang J, Cheng YJ, Lin XX, Wu SH. Cardiac troponin and C-reactive protein for predicting all-cause and cardiovascular mortality in patients with chronic kidney disease: a meta-analysis. Clinics (Sao Paulo). 2015;70:301-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Liu L, Gao B, Wang J, Yang C, Wu S, Wu Y, Chen S, Li Q, Zhang H, Wang G, Chen M, Zhao MH, Zhang L. Reduction in Serum High-Sensitivity C-Reactive Protein Favors Kidney Outcomes in Patients with Impaired Fasting Glucose or Diabetes. J Diabetes Res. 2020;2020:2720905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Bavbek N, Kargili A, Kaftan O, Karakurt F, Kosar A, Akcay A. Elevated concentrations of soluble adhesion molecules and large platelets in diabetic patients: are they markers of vascular disease and diabetic nephropathy? Clin Appl Thromb Hemost. 2007;13:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with Type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med. 2000;17:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Güler S, Cakir B, Demirbas B, Yönem A, Odabasi E, Onde U, Aykut O, Gürsoy G. Plasma soluble intercellular adhesion molecule 1 Levels are increased in type 2 diabetic patients with nephropathy. Horm Res. 2002;58:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Becker A, van Hinsbergh VW, Jager A, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Why is soluble intercellular adhesion molecule-1 related to cardiovascular mortality? Eur J Clin Invest. 2002;32:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Kawamura T, Umemura T, Kanai A, Uno T, Matsumae H, Sano T, Sakamoto N, Sakakibara T, Nakamura J, Hotta N. The incidence and characteristics of silent cerebral infarction in elderly diabetic patients: association with serum-soluble adhesion molecules. Diabetologia. 1998;41:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Otsuki M, Hashimoto K, Morimoto Y, Kishimoto T, Kasayama S. Circulating vascular cell adhesion molecule-1 (VCAM-1) in atherosclerotic NIDDM patients. Diabetes. 1997;46:2096-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Bevc S, Sabic S, Hojs R. Atherosclerosis in hemodialysis patients--the role of microinflammation. Ren Fail. 2008;30:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Navarro JF, Mora C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. ScientificWorldJournal. 2006;6:908-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 65. | Hojs R, Ekart R, Bevc S, Hojs N. Biomarkers of Renal Disease and Progression in Patients with Diabetes. J Clin Med. 2015;4:1010-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Nuhu F, Bhandari S. Oxidative Stress and Cardiovascular Complications in Chronic Kidney Disease, the Impact of Anaemia. Pharmaceuticals (Basel). 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45-63. [PubMed] |
| 68. | Vodošek Hojs N, Bevc S, Ekart R, Hojs R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 69. | Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O'Brien E; Office vs Ambulatory Pressure Study Investigators. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 793] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 70. | Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 71. | Grezzana GB, Moraes DW, Stein AT, Pellanda LC. Impact of Different Normality Thresholds for 24-hour ABPM at the Primary Health Care Level. Arq Bras Cardiol. 2017;108:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Iqbal P, Stevenson L. Cardiovascular outcomes in patients with normal and abnormal 24-hour ambulatory blood pressure monitoring. Int J Hypertens. 2010;2011:786912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1207] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 74. | Parati G, Bilo G. Should 24-h ambulatory blood pressure monitoring be done in every patient with diabetes? Diabetes Care. 2009;32 Suppl 2:S298-S304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Eguchi K. Ambulatory blood pressure monitoring in diabetes and obesity-a review. Int J Hypertens. 2011;2011:954757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Manios E, Stamatelopoulos K, Tsivgoulis G, Barlas G, Koroboki E, Tsagalis G, Michas F, Vemmos K, Zakopoulos N. Time rate of blood pressure variation: a new factor associated with coronary atherosclerosis. J Hypertens. 2011;29:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Velasquez MT, Beddhu S, Nobakht E, Rahman M, Raj DS. Ambulatory Blood Pressure in Chronic Kidney Disease: Ready for Prime Time? Kidney Int Rep. 2016;1:94-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 79. | Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 80. | Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, Faulkner ML, Hiremath L, Jamerson KA, Lea JP, Lipkowitz MS, Pogue VA, Rostand SG, Smogorzewski MJ, Wright JT, Greene T, Gassman J, Wang X, Phillips RA; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Wang C, Zhang J, Deng W, Gong W, Liu X, Ye Z, Peng H, Lou T. Nighttime Systolic Blood-Pressure Load Is Correlated with Target-Organ Damage Independent of Ambulatory Blood-Pressure Level in Patients with Non-Diabetic Chronic Kidney Disease. PLoS One. 2015;10:e0131546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000;57:2485-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 83. | Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, Watanabe M, Toriyama T, Kawahara H, Matsuo S. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003;18:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 84. | Tripepi G, Fagugli RM, Dattolo P, Parlongo G, Mallamaci F, Buoncristiani U, Zoccali C. Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int. 2005;68:1294-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 85. | Catalano M, Scandale G, Carzaniga G, Cinquini M, Minola M, Antoniazzi V, Dimitrov G, Carotta M. Aortic augmentation index in patients with peripheral arterial disease. J Clin Hypertens (Greenwich). 2014;16:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Ugwu E, Anyanwu A, Olamoyegun M. Ankle brachial index as a surrogate to vascular imaging in evaluation of peripheral artery disease in patients with type 2 diabetes. BMC Cardiovasc Disord. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Li J, Luo Y, Xu Y, Yang J, Zheng L, Hasimu B, Yu J, Hu D. Risk factors of peripheral arterial disease and relationship between low ankle - brachial index and mortality from all-cause and cardiovascular disease in Chinese patients with type 2 diabetes. Circ J. 2007;71:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Alves-Cabratosa L, Comas-Cufí M, Ponjoan A, Garcia-Gil M, Martí-Lluch R, Blanch J, Elosua-Bayes M, Parramon D, Camós L, Guzmán L, Ramos R. Levels of ankle-brachial index and the risk of diabetes mellitus complications. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2637] [Article Influence: 659.3] [Reference Citation Analysis (0)] |
| 90. | Ix JH, Katz R, De Boer IH, Kestenbaum BR, Allison MA, Siscovick DS, Newman AB, Sarnak MJ, Shlipak MG, Criqui MH. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2009;54:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Nishimura H, Miura T, Minamisawa M, Ueki Y, Abe N, Hashizume N, Mochidome T, Harada M, Shimizu K, Shoin W, Yoshie K, Oguchi Y, Ebisawa S, Motoki H, Izawa A, Koyama J, Ikeda U, Kuwahara K. Clinical Characteristics and Outcomes of Patients with High Ankle-Brachial Index from the IMPACT-ABI Study. PLoS One. 2016;11:e0167150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Arroyo D, Betriu A, Valls J, Gorriz JL, Pallares V, Abajo M, Gracia M, Valdivielso JM, Fernandez E; Investigators from the NEFRONA study. Factors influencing pathological ankle-brachial index values along the chronic kidney disease spectrum: the NEFRONA study. Nephrol Dial Transplant. 2017;32:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Chen FA, Yang CY, Yang WC, Chen JY, Ng YY, Li SY, Liu WS, Cheng ST, Wang YJ, Lin CC. Ankle-brachial index is a powerful predictor of renal outcome and cardiovascular events in patients with chronic kidney disease. ScientificWorldJournal. 2012;2012:238494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Ix JH, Katz R, Kestenbaum B, Fried LF, Kramer H, Stehman-Breen C, Shlipak MG. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Bevc S, Purg D, Knehtl M, Hren M, Turnšek N, Hojs N, Zorman T, Dvoršak B, Ekart R, Hojs R. Ankle-Brachial Index and Long-Term (10 Years) Survival of Nondiabetic Hemodialysis Patients. Ther Apher Dial. 2016;20:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Piko N, Bevc S, Hojs R, Naji FH, Ekart R. The association between pulse wave analysis, carotid-femoral pulse wave velocity and peripheral arterial disease in patients with ischemic heart disease. BMC Cardiovasc Disord. 2021;21:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3382] [Cited by in RCA: 3085] [Article Influence: 205.7] [Reference Citation Analysis (0)] |
| 98. | Doupis J, Papanas N, Cohen A, McFarlan L, Horton E. Pulse Wave Analysis by Applanation Tonometry for the Measurement of Arterial Stiffness. Open Cardiovasc Med J. 2016;10:188-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Butlin M, Qasem A. Large Artery Stiffness Assessment Using SphygmoCor Technology. Pulse (Basel). 2017;4:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 100. | Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 781] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 101. | Prskalo Z, Brizić I, Markota D, Markota I, Boban M, Tomic M, Starcevic B. Arterial stiffness in patients with coronary artery disease: relation with in-stent restenosis following percutaneous coronary intervention. BMC Cardiovasc Disord. 2016;16:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1350] [Cited by in RCA: 1439] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 103. | Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Westerhof N, Stehouwer CD. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 104. | Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 105. | Laugesen E, Høyem P, Fleischer J, Kumarathas I, Knudsen ST, Hansen KW, Christiansen JS, Hansen TK, Poulsen PL. Reduced Subendocardial Viability Ratio Is Associated With Unfavorable Cardiovascular Risk Profile in Women With Short Duration of Type 2 Diabetes. Am J Hypertens. 2016;29:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 107. | Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A. Arterial Stiffness and Decline in Kidney Function. Clin J Am Soc Nephrol. 2015;10:2190-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 108. | Fountoulakis N, Thakrar C, Patel K, Viberti G, Gnudi L, Karalliedde J. Increased Arterial Stiffness is an Independent Predictor of Renal Function Decline in Patients With Type 2 Diabetes Mellitus Younger Than 60 Years. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Weir MR, Townsend RR, Fink JC, Teal V, Anderson C, Appel L, Chen J, He J, Litbarg N, Ojo A, Rahman M, Rosen L, Sozio SM, Steigerwalt S, Strauss L, Joffe MM. Hemodynamic correlates of proteinuria in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2403-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Ekart R, Bevc S, Hojs N, Knehtl M, Dvoršak B, Hojs R. Albuminuria is Associated With Subendocardial Viability Ratio in Chronic Kidney Disease Patients. Kidney Blood Press Res. 2015;40:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Di Micco L, Salvi P, Bellasi A, Sirico ML, Di Iorio B. Subendocardial viability ratio predicts cardiovascular mortality in chronic kidney disease patients. Blood Purif. 2013;36:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Ekart R, Bevc S, Hojs N, Hojs R. Derived Subendocardial Viability Ratio and Cardiovascular Events in Patients with Chronic Kidney Disease. Cardiorenal Med. 2019;9:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Kimoto E, Shoji T, Shinohara K, Hatsuda S, Mori K, Fukumoto S, Koyama H, Emoto M, Okuno Y, Nishizawa Y. Regional arterial stiffness in patients with type 2 diabetes and chronic kidney disease. J Am Soc Nephrol. 2006;17:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006;48:1548-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Budoff MJ, Raggi P, Beller GA, Berman DS, Druz RS, Malik S, Rigolin VH, Weigold WG, Soman P; Imaging Council of the American College of Cardiology. Noninvasive Cardiovascular Risk Assessment of the Asymptomatic Diabetic Patient: The Imaging Council of the American College of Cardiology. JACC Cardiovasc Imaging. 2016;9:176-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 116. | Marcassi AP, Yasbek DC, Pestana JO, Fachini FC, De Lira Filho EB, Cassiolato JL, Canziani ME. Ventricular arrhythmia in incident kidney transplant recipients: prevalence and associated factors. Transpl Int. 2011;24:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol. 1991;68:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 392] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 118. | Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 641] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 119. | Pecoits-Filho R, Barberato SH. Echocardiography in chronic kidney disease: diagnostic and prognostic implications. Nephron Clin Pract. 2010;114:c242-c247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Ekart R, Kanič V, Pečovnik Balon B, Bevc S, Hojs R. Prognostic value of 48-hour ambulatory blood pressure measurement and cardiovascular mortality in hemodialysis patients. Kidney Blood Press Res. 2012;35:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Di Cori A, Di Bello V, Miccoli R, Talini E, Palagi C, Delle Donne MG, Penno G, Nardi C, Bianchi C, Mariani M, Del Prato S, Balbarini A. Left ventricular function in normotensive young adults with well-controlled type 1 diabetes mellitus. Am J Cardiol. 2007;99:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Ha JW, Lee HC, Kang ES, Ahn CM, Kim JM, Ahn JA, Lee SW, Choi EY, Rim SJ, Oh JK, Chung N. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 123. | Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 124. | Hachamovitch R, Kang X, Amanullah AM, Abidov A, Hayes SW, Friedman JD, Cohen I, Thomson LE, Germano G, Berman DS. Prognostic implications of myocardial perfusion single-photon emission computed tomography in the elderly. Circulation. 2009;120:2197-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, Engel S, Ratner RE, Iskandrian AE; Detection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 544] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 126. | Malhotra S, Sharma R, Kliner DE, Follansbee WP, Soman P. Relationship between silent myocardial ischemia and coronary artery disease risk factors. J Nucl Cardiol. 2013;20:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Zellweger MJ, Maraun M, Osterhues HH, Keller U, Müller-Brand J, Jeger R, Pfister O, Burkard T, Eckstein F, von Felten S, Osswald S, Pfisterer M. Progression to overt or silent CAD in asymptomatic patients with diabetes mellitus at high coronary risk: main findings of the prospective multicenter BARDOT trial with a pilot randomized treatment substudy. JACC Cardiovasc Imaging. 2014;7:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 128. | Rabbat CG, Treleaven DJ, Russell JD, Ludwin D, Cook DJ. Prognostic value of myocardial perfusion studies in patients with end-stage renal disease assessed for kidney or kidney-pancreas transplantation: a meta-analysis. J Am Soc Nephrol. 2003;14:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 129. | Marie PY, Rossignol P. Stress myocardial perfusion gated-SPECT imaging in advanced chronic kidney disease. J Nucl Cardiol. 2019;26:1971-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 130. | Furuhashi T, Moroi M, Joki N, Hase H, Minakawa M, Masai H, Kunimasa T, Fukuda H, Sugi K. Prediction of cardiovascular events in pre-dialysis chronic kidney disease patients with normal SPECT myocardial perfusion imaging. J Cardiol. 2014;63:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | Centurión OA. Carotid Intima-Media Thickness as a Cardiovascular Risk Factor and Imaging Pathway of Atherosclerosis. Crit Pathw Cardiol. 2016;15:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Hojs R. Carotid intima-media thickness and plaques in hemodialysis patients. Artif Organs. 2000;24:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Akazawa S, Tojikubo M, Nakano Y, Nakamura S, Tamai H, Yonemoto K, Sadasima E, Kawasaki T, Koga N. Usefulness of carotid plaque (sum and maximum of plaque thickness) in combination with intima-media thickness for the detection of coronary artery disease in asymptomatic patients with diabetes. J Diabetes Investig. 2016;7:396-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 134. | Bots ML, Witteman JC, Grobbee DE. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis. 1993;102:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Ogata T, Yasaka M, Yamagishi M, Seguchi O, Nagatsuka K, Minematsu K. Atherosclerosis found on carotid ultrasonography is associated with atherosclerosis on coronary intravascular ultrasonography. J Ultrasound Med. 2005;24:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 136. | Dijk JM, van der Graaf Y, Bots ML, Grobbee DE, Algra A. Carotid intima-media thickness and the risk of new vascular events in patients with manifest atherosclerotic disease: the SMART study. Eur Heart J. 2006;27:1971-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 137. | Gómez-Marcos MA, Recio-Rodríguez JI, Rodríguez-Sánchez E, Patino-Alonso MC, Magallón-Botaya R, Martínez-Vizcaino V, Gómez Sánchez L, García-Ortiz L. [Carotid intima-media thickness in diabetics and hypertensive patients]. Rev Esp Cardiol. 2011;64:622-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Matsagoura M, Andreadis E, Diamantopoulos EJ, Vassilopoulos C, Tentolouris N, Katsilambros N. Carotid intima-media thickness in patients with type 2 diabetes: the significance of microalbuminuria and different risk factors for atherosclerosis. Diabetes Care. 2003;26:2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 139. | Kota SK, Mahapatra GB, Kota SK, Naveed S, Tripathy PR, Jammula S, Modi KD. Carotid intima media thickness in type 2 diabetes mellitus with ischemic stroke. Indian J Endocrinol Metab. 2013;17:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 140. | Sunil Kumar K, Lakshmi AY, Srinivasa Rao PV, Das GC, Siva Kumar V. Carotid intima-media thickness in patients with end-stage renal disease. Indian J Nephrol. 2009;19:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 141. | Ekart R, Hojs R, Pecovnik-Balon B, Bevc S, Dvorsak B. Blood pressure measurements and carotid intima media thickness in hemodialysis patients. Ther Apher Dial. 2009;13:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 142. | Lawal OM, Balogun MO, Akintomide AO, Ayoola OO, Mene-Afejuku TO, Ogunlade O, Okunola OO, Lawal AO, Akinsola A. Carotid Intima-Media Thickness: A Surrogate Marker for Cardiovascular Disease in Chronic Kidney Disease Patients. Clin Med Insights Cardiol. 2019;13:1179546819852941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 143. | Roumeliotis A, Roumeliotis S, Panagoutsos S, Theodoridis M, Argyriou C, Tavridou A, Georgiadis GS. Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren Fail. 2019;41:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 144. | Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC Jr, Psaty BM, Greenland P, Herrington DM. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 145. | Wong ND, Sciammarella MG, Polk D, Gallagher A, Miranda-Peats L, Whitcomb B, Hachamovitch R, Friedman JD, Hayes S, Berman DS. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 146. | Khaleeli E, Peters SR, Bobrowsky K, Oudiz RJ, Ko JY, Budoff MJ. Diabetes and the associated incidence of subclinical atherosclerosis and coronary artery disease: Implications for management. Am Heart J. 2001;141:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M; Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? Diabetes. 2003;52:2833-2839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 148. | Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, Friedman JD, Kang X, Polk D, Hachamovitch R, Shaw L, Rozanski A. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 149. | Mirbolouk M, Kianoush S, Dardari Z, Miedema MD, Shaw LJ, Rumberger JA, Berman DS, Budoff MJ, Rozanski A, Al-Mallah MH, McEvoy JW, Nasir K, Blaha MJ. The association of coronary artery calcium score and mortality risk among smokers: The coronary artery calcium consortium. Atherosclerosis. 2020;294:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 150. | Anand DV, Lim E, Darko D, Bassett P, Hopkins D, Lipkin D, Corder R, Lahiri A. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 151. | Cano-Megías M, Guisado-Vasco P, Bouarich H, de Arriba-de la Fuente G, de Sequera-Ortiz P, Álvarez-Sanz C, Rodríguez-Puyol D. Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: a prospective long-term follow-up study. BMC Nephrol. 2019;20:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 152. | Krajnc M, Pečovnik-Balon B, Hojs R, Rupreht M. Comparison of coronary artery calcification and some coronary artery calcification risk factors in patients on haemodialysis and in patients with type 2 diabetes. J Int Med Res. 2011;39:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 153. | Lamarche MC, Hopman WM, Garland JS, White CA, Holden RM. Relationship of coronary artery calcification with renal function decline and mortality in predialysis chronic kidney disease patients. Nephrol Dial Transplant. 2019;34:1715-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Cano-Megías M, Bouarich H, Guisado-Vasco P, Pérez Fernández M, de Arriba-de la Fuente G, Álvarez-Sanz C, Rodríguez-Puyol D. Coronary artery calcification in patients with diabetes mellitus and advanced chronic kidney disease. Endocrinol Diabetes Nutr. 2019;66:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | de Araújo Gonçalves P, Garcia-Garcia HM, Carvalho MS, Dores H, Sousa PJ, Marques H, Ferreira A, Cardim N, Teles RC, Raposo L, Gabriel HM, Almeida M, Aleixo A, Carmo MM, Machado FP, Mendes M. Diabetes as an independent predictor of high atherosclerotic burden assessed by coronary computed tomography angiography: the coronary artery disease equivalent revisited. Int J Cardiovasc Imaging. 2013;29:1105-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | Kamimura M, Moroi M, Isobe M, Hiroe M. Role of coronary CT angiography in asymptomatic patients with type 2 diabetes mellitus. Int Heart J. 2012;53:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 157. | Madaj PM, Budoff MJ, Li D, Tayek JA, Karlsberg RP, Karpman HL. Identification of noncalcified plaque in young persons with diabetes: an opportunity for early primary prevention of coronary artery disease identified with low-dose coronary computed tomographic angiography. Acad Radiol. 2012;19:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Deng W, Peng L, Yu J, Shuai T, Chen Z, Li Z. Characteristics of coronary artery atherosclerotic plaques in chronic kidney disease: evaluation with coronary CT angiography. Clin Radiol 2019; 74: 731.e1-731. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 159. | Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, Gill JS, Hlatky MA, Jardine AG, Landmesser U, Newby LK, Herzog CA, Cheung M, Wheeler DC, Winkelmayer WC, Marwick TH; Conference Participants. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1823-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 160. | Aljaroudi WA, Flamm SD, Saliba W, Wilkoff BL, Kwon D. Role of CMR imaging in risk stratification for sudden cardiac death. JACC Cardiovasc Imaging. 2013;6:392-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 161. | Manfrini O, Russo V, Ciavarella A, Ceroni L, Montalti M, Fattori R. Coronary plaque quantification and composition in asymptomatic patients with type II diabetes mellitus. J Cardiovasc Med (Hagerstown). 2012;13:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 162. | Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel K, Siu S, Brown KA. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 163. | Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 164. | Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 408] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 165. | Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011;4:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 166. | Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 439] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 167. | van der Meer RW, Lamb HJ, Smit JW, de Roos A. MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology. 2012;264:21-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 168. | Levelt E, Piechnik SK, Liu A, Wijesurendra RS, Mahmod M, Ariga R, Francis JM, Greiser A, Clarke K, Neubauer S, Ferreira VM, Karamitsos TD. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J Cardiovasc Magn Reson. 2017;19:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 169. | Shah R, Sree Raman K, Walls A, Woodman RJ, Faull R, Gleadle JM, Selvanayagam JB. Gadolinium-Free Cardiovascular Magnetic Resonance Stress T1 Mapping in Patients With Chronic Kidney Disease. JACC Cardiovasc Imaging. 2019;12:2083-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 170. | Rutherford E, Talle MA, Mangion K, Bell E, Rauhalammi SM, Roditi G, McComb C, Radjenovic A, Welsh P, Woodward R, Struthers AD, Jardine AG, Patel RK, Berry C, Mark PB. Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int. 2016;90:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 171. | Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014;7:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 172. | Graham-Brown MP, March DS, Churchward DR, Stensel DJ, Singh A, Arnold R, Burton JO, McCann GP. Novel cardiac nuclear magnetic resonance method for noninvasive assessment of myocardial fibrosis in hemodialysis patients. Kidney Int. 2016;90:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 173. | Edwards NC, Moody WE, Yuan M, Hayer MK, Ferro CJ, Townend JN, Steeds RP. Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol. 2015;115:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 174. | Valencia-Morales Mdel P, Zaina S, Heyn H, Carmona FJ, Varol N, Sayols S, Condom E, Ramírez-Ruz J, Gomez A, Moran S, Lund G, Rodríguez-Ríos D, López-González G, Ramírez-Nava M, de la Rocha C, Sanchez-Flores A, Esteller M. The DNA methylation drift of the atherosclerotic aorta increases with lesion progression. BMC Med Genomics. 2015;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 175. | Cao J, Ye B, Lin L, Tian L, Yang H, Wang C, Huang W, Huang Z. Curcumin Alleviates oxLDL Induced MMP-9 and EMMPRIN Expression through the Inhibition of NF-κB and MAPK Pathways in Macrophages. Front Pharmacol. 2017;8:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 176. | Shirodkar AV, Marsden PA. Epigenetics in cardiovascular disease. Curr Opin Cardiol. 2011;26:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 177. | Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118:703-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 504] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 178. | Kétszeri M, Kirsch A, Frauscher B, Moschovaki-Filippidou F, Mooslechner AA, Kirsch AH, Schabhuettl C, Aringer I, Artinger K, Pregartner G, Ekart R, Breznik S, Hojs R, Goessler W, Schilcher I, Müller H, Obermayer-Pietsch B, Frank S, Rosenkranz AR, Eller P, Eller K. MicroRNA-142-3p improves vascular relaxation in uremia. Atherosclerosis. 2019;280:28-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 179. | Hobuß L, Bär C, Thum T. Long Non-coding RNAs: At the Heart of Cardiac Dysfunction? Front Physiol. 2019;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 180. | Li Y, Li C, Yang M, Shi L, Tao W, Shen K, Li X, Wang X, Yang Y, Yao Y. Association of single nucleotide polymorphisms of miRNAs involved in the GLUT4 pathway in T2DM in a Chinese population. Mol Genet Genomic Med. 2019;7:e907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 181. | Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology. 2013;154:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 182. | Ciccacci C, Di Fusco D, Cacciotti L, Morganti R, D'Amato C, Greco C, Rufini S, Novelli G, Sangiuolo F, Spallone V, Borgiani P. MicroRNA genetic variations: association with type 2 diabetes. Acta Diabetol. 2013;50:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 183. | Buraczynska M, Zukowski P, Wacinski P, Ksiazek K, Zaluska W. Polymorphism in microRNA-196a2 contributes to the risk of cardiovascular disease in type 2 diabetes patients. J Diabetes Complications. 2014;28:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 184. | Li Q, Chen L, Chen D, Wu X, Chen M. Influence of microRNA-related polymorphisms on clinical outcomes in coronary artery disease. Am J Transl Res. 2015;7:393-400. [PubMed] |
| 185. | Guo J, Liu Z, Gong R. Long noncoding RNA: an emerging player in diabetes and diabetic kidney disease. Clin Sci (Lond). 2019;133:1321-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 186. | Chung AC. microRNAs in Diabetic Kidney Disease. Adv Exp Med Biol. 2015;888:253-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 187. | Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 188. | Howe EN, Cochrane DR, Richer JK. The miR-200 and miR-221/222 microRNA families: opposing effects on epithelial identity. J Mammary Gland Biol Neoplasia. 2012;17:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 189. | Abdelsalam M, Wahab AM, El Sayed Zaki M, Motawea M. MicroRNA-451 as an Early Predictor of Chronic Kidney Disease in Diabetic Nephropathy. Int J Nephrol. 2020;2020:8075376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 190. | Fourdinier O, Schepers E, Metzinger-Le Meuth V, Glorieux G, Liabeuf S, Verbeke F, Vanholder R, Brigant B, Pletinck A, Diouf M, Burtey S, Choukroun G, Massy ZA, Metzinger L; European Uremic Toxin Work Group-EUTox. Serum levels of miR-126 and miR-223 and outcomes in chronic kidney disease patients. Sci Rep. 2019;9:4477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |